Tailoring of Albumin Nanoparticles Modified with Mannose for Effective Targeting in Immunosuppressive Tumor Microenvironment
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Cell Lines
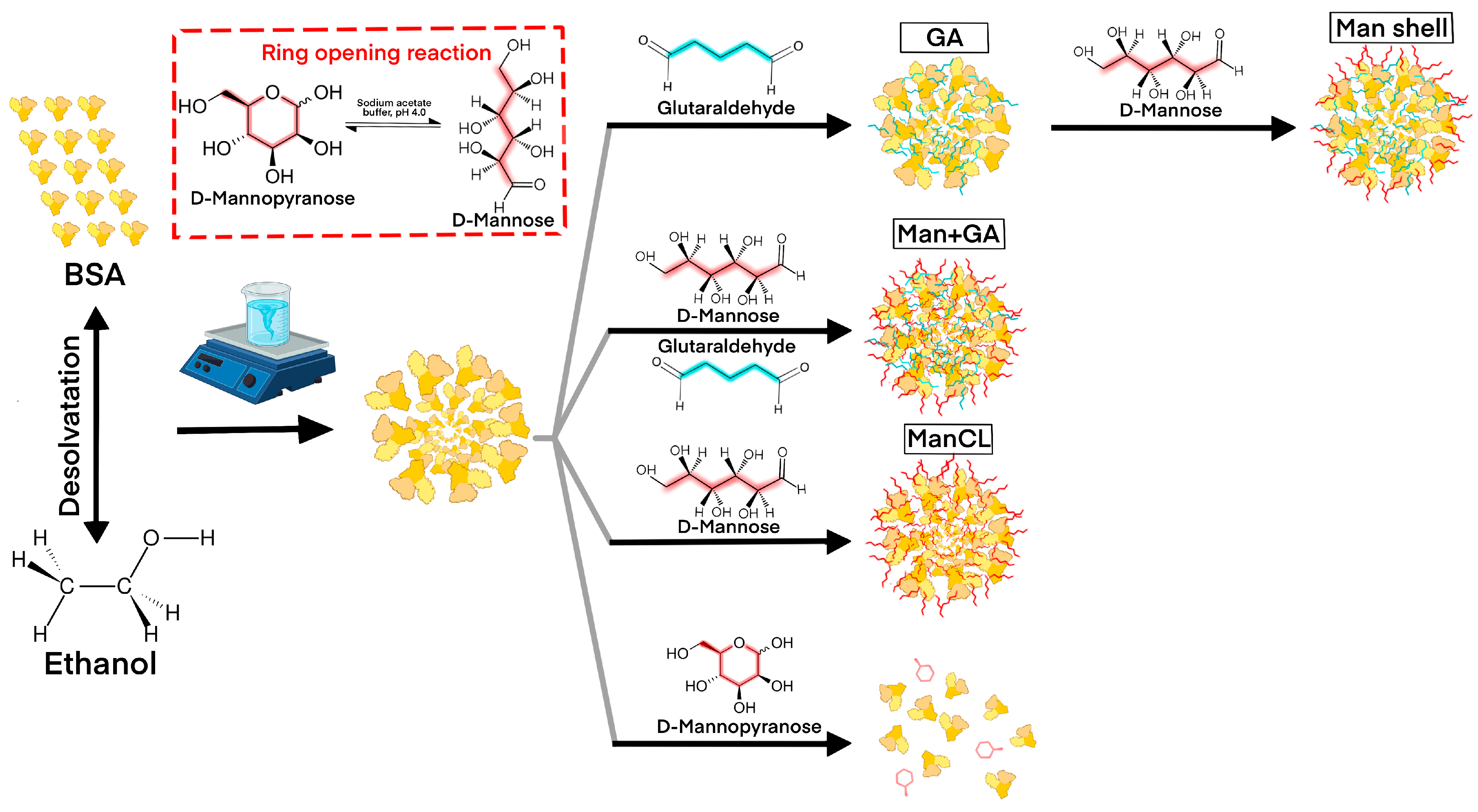
2.2. Fabrication of Mannosylated Albumin Nanoparticles Using Desolvation Method
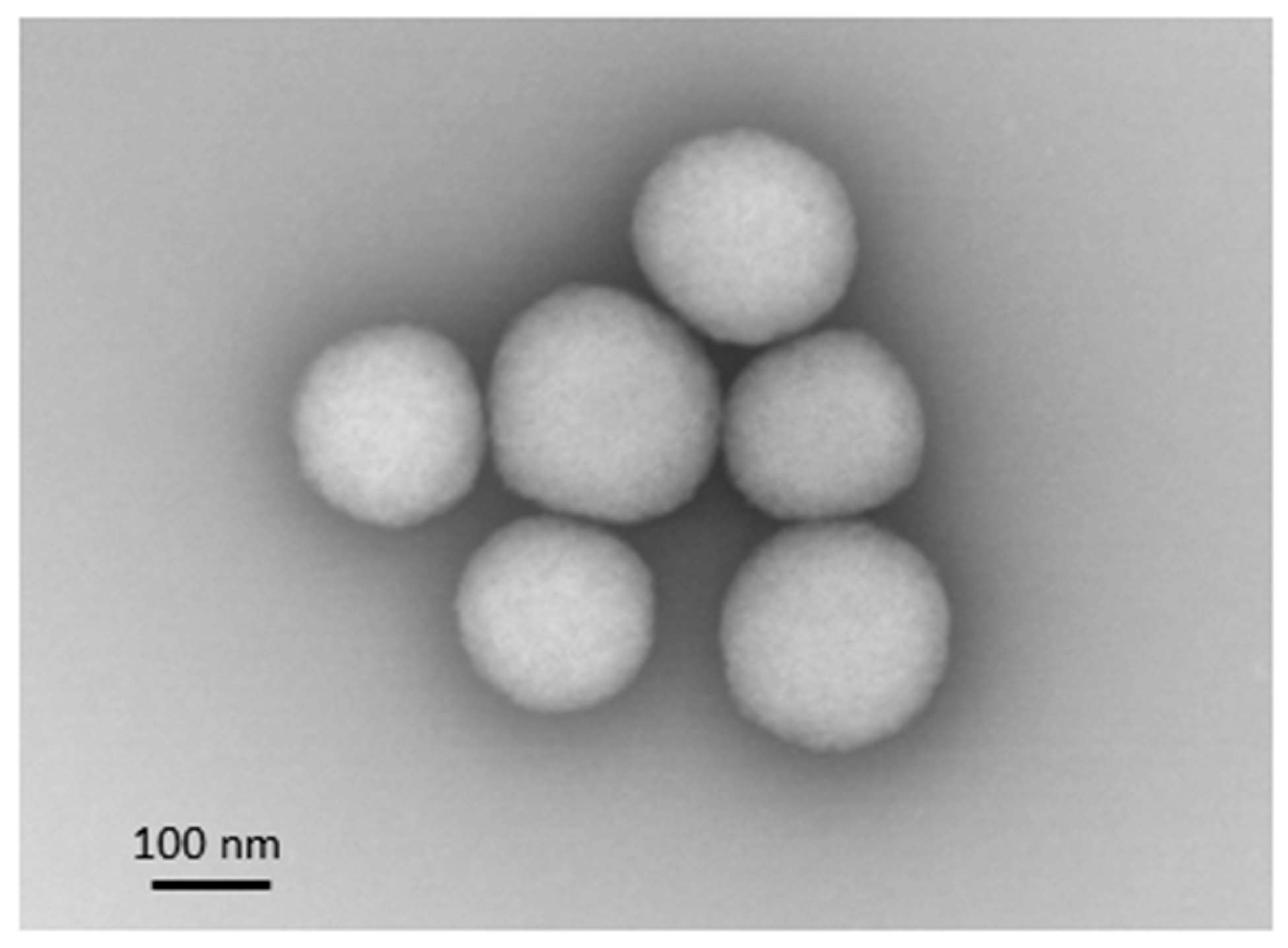
2.3. Nanoparticle Characterization
2.4. Cell Lines
2.5. Evaluation of the Internalization Efficiency of Cy 5.5-Labeled Mannosylated ANPs into M1 and M2 Macrophages
2.6. Evaluation of the Internalization Efficiency of Mannosylated ANP Man0.03+GA and Control ANPs Encapsulating FAM-Labeled Scramble siRNA in M1 and M2 Macrophages and in Cancer Cells
2.7. Evaluation of Localization of FAM-Labeled siRNA Within the M2 Cells
2.8. Determination of ANP Internalization Pathway
3. Results and Discussion
3.1. Mannosylated ANP Synthesis and Characterization
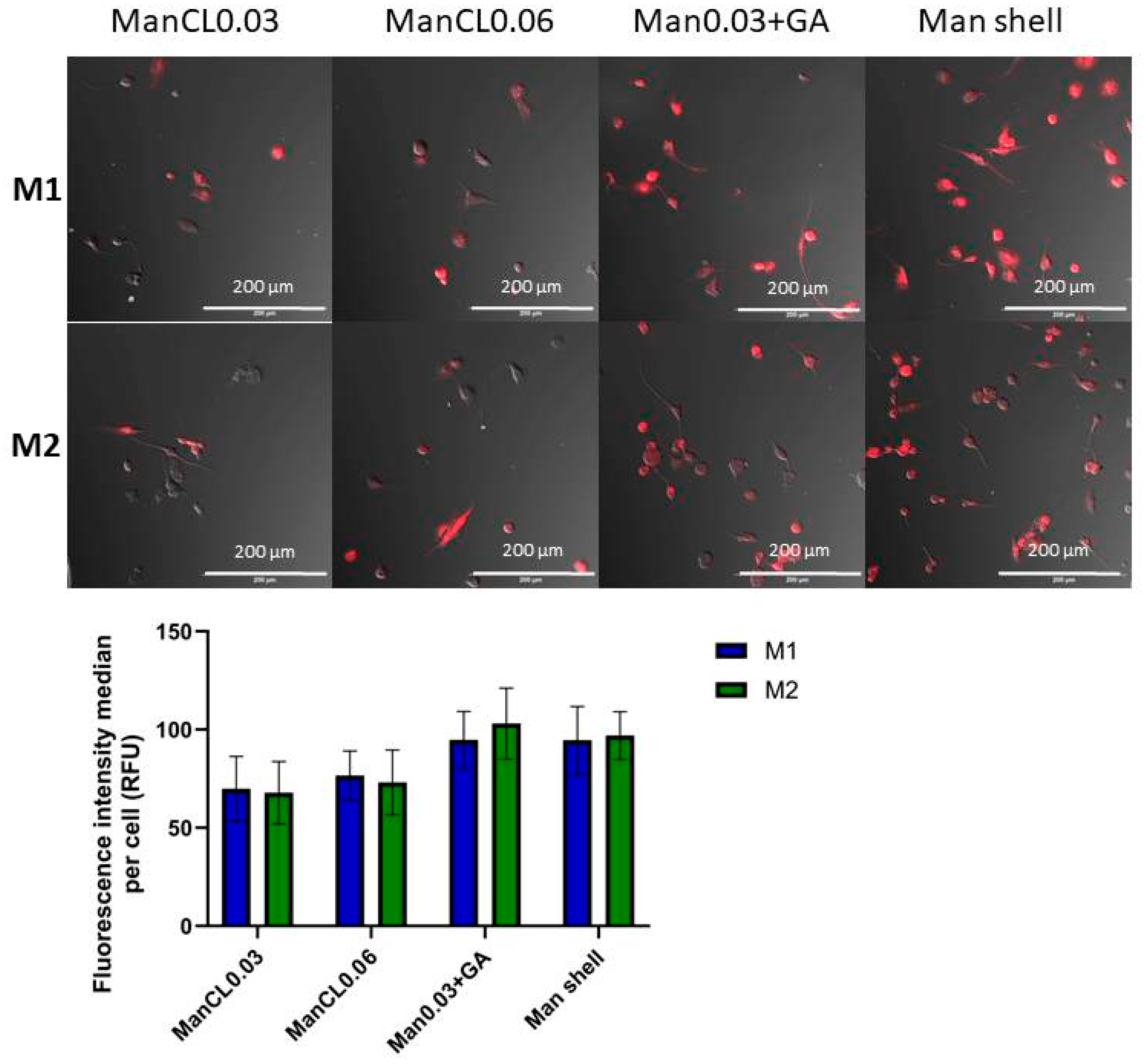
3.2. Efficiency of the Internalization of Mannosylated ANPs into M1 and M2 Macrophages
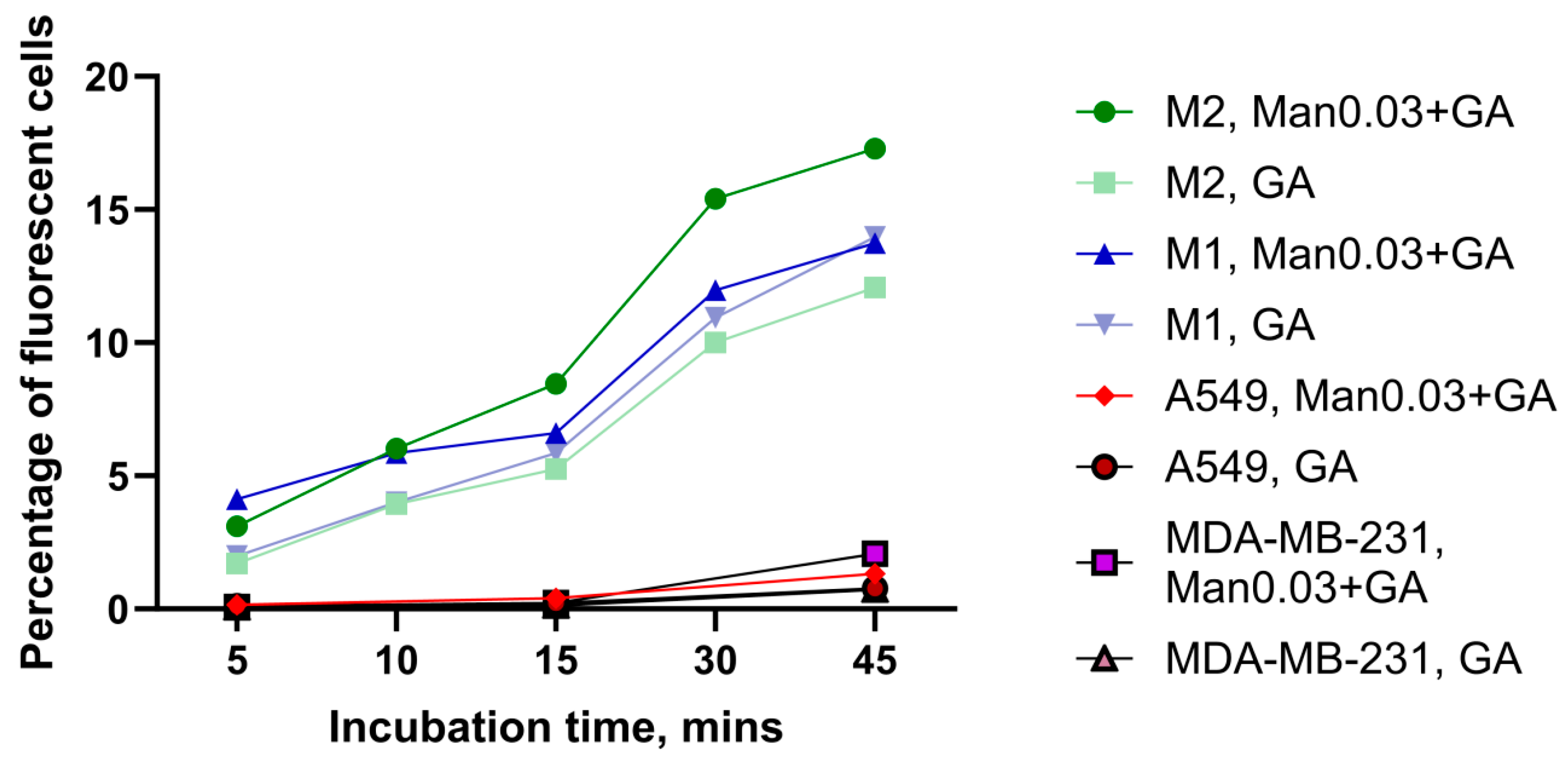
3.3. Comparison of the Internalization Efficiency of Mannosylated ANPs Man0.03+GA and Control ANPs in M1 and M2 Macrophages and in Cancer Cells
3.4. Analysis of Localization of FAM-Labeled siRNA Inside the M2 Cells
3.5. Determination of Endocytosis Pathway of Mannosylated ANPs Man0.03+GA and Control ANPs in M2 Macrophages
4. Limitations of the Study and Future Perspectives
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| TAM | Tumor-associated macrophage |
| TME | Tumor microenvironment |
| CCR7 | C-C chemokine receptor type 7 |
| CCL3 | C-C chemokine ligand 3 |
| CD206 | Mannose receptor (Cluster of Differentiation 206) |
| VSIG4 | V-set and immunoglobulin domain containing protein 4 |
| ANP | Albumin nanoparticle |
| DLS | Dynamic light scattering |
| PI | Polydispersity index |
| STEM | Scanning transmission electron microscopy |
| PMA | Phorbol 12-myristate 13-acetate |
| LPS | Lipopolysaccharide |
| ATRA | All-trans-retinoic acid |
| GA | Glutaraldehyde |
| EIPA | 5-(N-ethyl-N-isopropyl)-amiloride |
| PBS | Phosphate-buffered saline |
| siRNA | Small interfering RNA |
| FAM | Fluorescein |
| SD | Standard deviation |
References
- Kamat, K.; Krishnan, V.; Dorigo, O. Macrophage-derived CCL23 upregulates expression of T-cell exhaustion markers in ovarian cancer. Br. J. Cancer 2022, 127, 1026–1033. [Google Scholar] [CrossRef] [PubMed]
- Kuznetsova, A.B.; Kolesova, E.P.; Parodi, A.; Zamyatnin, A.A., Jr.; Egorova, V.S. Reprogramming Tumor-Associated Macrophage Using Nanocarriers: New Perspectives to Halt Cancer Progression. Pharmaceutics 2024, 16, 636. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.; Wang, H.W.; Bowman, R.L.; Joyce, J.A. STAT3 and STAT6 Signaling Pathways Synergize to Promote Cathepsin Secretion from Macrophages via IRE1α Activation. Cell Rep. 2016, 16, 2914–2927. [Google Scholar] [CrossRef] [PubMed]
- Deryugina, E.I.; Zajac, E.; Juncker-Jensen, A.; Kupriyanova, T.A.; Welter, L.; Quigley, J.P. Tissue-infiltrating neutrophils constitute the major in vivo source of angiogenesis-inducing MMP-9 in the tumor microenvironment. Neoplasia 2014, 16, 771–788. [Google Scholar] [CrossRef]
- Zhao, J.; Chen, L.; Shu, B.; Tang, J.; Zhang, L.; Xie, J.; Qi, S.; Xu, Y. Granulocyte/macrophage colony-stimulating factor influences angiogenesis by regulating the coordinated expression of VEGF and the Ang/Tie system. PLoS ONE 2014, 9, e92691. [Google Scholar] [CrossRef]
- Song, Y.; Tang, C.; Yin, C. Combination antitumor immunotherapy with VEGF and PIGF siRNA via systemic delivery of multi-functionalized nanoparticles to tumor-associated macrophages and breast cancer cells. Biomaterials 2018, 185, 117–132. [Google Scholar] [CrossRef]
- Guo, X.; Zhang, H.; He, C.; Qin, K.; Lai, Q.; Fang, Y.; Chen, Q.; Li, W.; Wang, Y.; Wang, X.; et al. RUNX1 promotes angiogenesis in colorectal cancer by regulating the crosstalk between tumor cells and tumor associated macrophages. Biomark. Res. 2024, 12, 29. [Google Scholar] [CrossRef]
- Grabowska, M.M.; Day, M.L. Soluble E-cadherin: More than a symptom of disease. Front. Biosci. (Landmark Ed). 2012, 17, 1948–1964. [Google Scholar] [CrossRef]
- Mitrović, A.; Pečar Fonović, U.; Kos, J. Cysteine cathepsins B and X promote epithelial-mesenchymal transition of tumor cells. Eur. J. Cell Biol. 2017, 96, 622–631. [Google Scholar] [CrossRef]
- Häuselmann, I.; Roblek, M.; Protsyuk, D.; Huck, V.; Knopfova, L.; Grässle, S.; Bauer, A.T.; Schneider, S.W.; Borsig, L. Monocyte Induction of E-Selectin-Mediated Endothelial Activation Releases VE-Cadherin Junctions to Promote Tumor Cell Extravasation in the Metastasis Cascade. Cancer Res. 2016, 76, 5302–5312. [Google Scholar] [CrossRef]
- Lu, Y.; Han, G.; Zhang, Y.; Zhang, L.; Li, Z.; Wang, Q.; Chen, Z.; Wang, X.; Wu, J. M2 macrophage-secreted exosomes promote metastasis and increase vascular permeability in hepatocellular carcinoma. Cell Commun. Signal. 2023, 21, 299. [Google Scholar] [CrossRef] [PubMed]
- Gadde, M.; Mehrabi-Dehdezi, M.; Debeb, B.G.; Woodward, W.A.; Rylander, M.N. Influence of Macrophages on Vascular Invasion of Inflammatory Breast Cancer Emboli Measured Using an In Vitro Microfluidic Multi-Cellular Platform. Cancers 2023, 15, 4883. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhang, J.; Zhang, Z.; Gao, Z.; Qi, Y.; Qiu, W.; Pan, Z.; Guo, Q.; Li, B.; Zhao, S.; et al. Hypoxic glioma-derived exosomes promote M2-like macrophage polarization by enhancing autophagy induction. Cell Death Dis. 2021, 12, 373. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Lan, Y.; Li, Y.; Li, Z.; Pu, J.; Wei, L. Hypoxic Tumor-Derived Exosomes Induce M2 Macrophage Polarization via PKM2/AMPK to Promote Lung Cancer Progression. Cell Transplant. 2022, 31, 9636897221106998. [Google Scholar] [CrossRef]
- Zhou, H.; Yao, J.; Zhong, Z.; Wei, H.; He, Y.; Li, W.; Hu, K. Lactate-Induced CCL8 in Tumor-Associated Macrophages Accelerates the Progression of Colorectal Cancer through the CCL8/CCR5/mTORC1 Axis. Cancers 2023, 15, 5795. [Google Scholar] [CrossRef]
- Gomez-Roca, C.; Cassier, P.; Zamarin, D.; Machiels, J.P.; Perez Gracia, J.L.; Stephen Hodi, F.; Taus, A.; Martinez Garcia, M.; Boni, V.; Eder, J.P.; et al. Anti-CSF-1R emactuzumab in combination with anti-PD-L1 atezolizumab in advanced solid tumor patients naïve or experienced for immune checkpoint blockade. J. Immunother. Cancer 2022, 10, e004076. [Google Scholar] [CrossRef]
- Cassier, P.A.; Italiano, A.; Gomez-Roca, C.; Le Tourneau, C.; Toulmonde, M.; D’Angelo, S.P.; Weber, K.; Loirat, D.; Jacob, W.; Jegg, A.M.; et al. Long-term clinical activity, safety and patient-reported quality of life for emactuzumab-treated patients with diffuse-type tenosynovial giant-cell tumour. Eur. J. Cancer 2020, 141, 162–170. [Google Scholar] [CrossRef]
- Nywening, T.M.; Wang-Gillam, A.; Sanford, D.E.; Belt, B.A.; Panni, R.Z.; Cusworth, B.M.; Toriola, A.T.; Nieman, R.K.; Worley, L.A.; Yano, M.; et al. Targeting tumour-associated macrophages with CCR2 inhibition in combination with FOLFIRINOX in patients with borderline resectable and locally advanced pancreatic cancer: A single-centre, open-label, dose-finding, non-randomised, phase 1b trial. Lancet Oncol. 2016, 17, 651–662. [Google Scholar] [CrossRef]
- Reid, T.; Oronsky, B.; Caroen, S.; Quinn, M.; Williams, J.; Cabrales, P.; Abrouk, N. Phase 1 pilot study of RRx-001 + nivolumab in patients with advanced metastatic cancer (PRIMETIME). Front. Immunol. 2023, 14, 1104753. [Google Scholar] [CrossRef]
- Byrne, K.T.; Betts, C.B.; Mick, R.; Sivagnanam, S.; Bajor, D.L.; Laheru, D.A.; Chiorean, E.G.; O’Hara, M.H.; Liudahl, S.M.; Newcomb, C.; et al. Neoadjuvant Selicrelumab, an Agonist CD40 Antibody, Induces Changes in the Tumor Microenvironment in Patients with Resectable Pancreatic Cancer. Clin. Cancer Res. 2021, 27, 4574–4586. [Google Scholar] [CrossRef]
- Zhao, S.; Ren, S.; Jiang, T.; Zhu, B.; Li, X.; Zhao, C.; Jia, Y.; Shi, J.; Zhang, L.; Liu, X.; et al. Low-Dose Apatinib Optimizes Tumor Microenvironment and Potentiates Antitumor Effect of PD-1/PD-L1 Blockade in Lung Cancer. Cancer Immunol. Res. 2019, 7, 630–643. [Google Scholar] [CrossRef] [PubMed]
- Rajan, A.; Abdul Sater, H.; Rahma, O.; Agajanian, R.; Lassoued, W.; Marté, J.L.; Tsai, Y.T.; Donahue, R.N.; Lamping, E.; Bailey, S.; et al. Efficacy, safety, and biomarker analyses of bintrafusp alfa, a bifunctional fusion protein targeting TGF-β and PD-L1, in patients with advanced non-small cell lung cancer. J. Immunother. Cancer 2024, 12, e008480. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Qi, Y.H.; Cheng, C.T.; Yang, W.B.; Malhotra, A.; Zhou, Q. Potential of siRNA-albumin complex against cancer. Chem. Biol. Interact. 2018, 295, 93–96. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, S.; Yang, X.; Zhang, S.; Cui, C. Preparation Optimization of Bovine Serum Albumin Nanoparticles and Its Application for siRNA Delivery. Drug Des. Dev. Ther. 2021, 15, 1531–1547. [Google Scholar] [CrossRef]
- Mehta, A.; Dalle Vedove, E.; Isert, L.; Merkel, O.M. Targeting KRAS Mutant Lung Cancer Cells with siRNA-Loaded Bovine Serum Albumin Nanoparticles. Pharm. Res. 2019, 36, 133. [Google Scholar] [CrossRef]
- Singh, A.; Chakraborty, S.; Wong, S.W.; Hefner, N.A.; Stuart, A.; Qadir, A.S.; Mukhopadhyay, A.; Bachmaier, K.; Shin, J.W.; Rehman, J.; et al. Nanoparticle targeting of de novo profibrotic macrophages mitigates lung fibrosis. Proc. Natl. Acad. Sci. USA 2022, 119, e2121098119. [Google Scholar] [CrossRef]
- Gu, G.J.; Chung, H.; Park, J.Y.; Yoo, R.; Im, H.J.; Choi, H.; Lee, Y.S.; Seok, S.H. Mannosylated-serum albumin nanoparticle imaging to monitor tumor-associated macrophages under anti-PD1 treatment. J. Nanobiotechnol. 2023, 21, 31. [Google Scholar] [CrossRef]
- Wang, C.; Xu, X.; Zhang, P.; Xiong, S.; Yuan, J.; Gao, X.; Guan, W.; Wang, F.; Li, X.; Dou, H.; et al. Lipid-coated albumin-paclitaxel nanoparticles loaded with sorcin-siRNA reverse cancer chemoresistance via restoring intracellular calcium ion homeostasis. J. Nanobiotechnol. 2022, 20, 319. [Google Scholar] [CrossRef]
- Fu, Z.; Wang, X.; Lu, X.; Yang, Y.; Zhao, L.; Zhou, L.; Wang, K.; Fu, H. Mannose-decorated ginsenoside Rb1 albumin nanoparticles for targeted anti-inflammatory therapy. Front. Bioeng. Biotechnol. 2022, 10, 962380. [Google Scholar] [CrossRef]
- Flannagan, R.S.; Jaumouillé, V.; Grinstein, S. The cell biology of phagocytosis. Annu. Rev. Pathol. 2012, 7, 61–98. [Google Scholar] [CrossRef]
- Kolesova, E.P.; Egorova, V.S.; Syrocheva, A.O.; Frolova, A.S.; Kostyushev, D.; Kostyusheva, A.; Brezgin, S.; Trushina, D.B.; Fatkhutdinova, L.; Zyuzin, M.; et al. Proteolytic Resistance Determines Albumin Nanoparticle Drug Delivery Properties and Increases Cathepsin B, D, and G Expression. Int. J. Mol. Sci. 2023, 24, 10245. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Tiruppathi, C.; Minshall, R.D.; Malik, A.B. Size and dynamics of caveolae studied using nanoparticles in living endothelial cells. ACS Nano 2009, 3, 4110–4116. [Google Scholar] [CrossRef] [PubMed]
- Allavena, P.; Chieppa, M.; Monti, P.; Piemonti, L. From pattern recognition receptor to regulator of homeostasis: The double-faced macrophage mannose receptor. Crit. Rev. Immunol. 2004, 24, 179–192. [Google Scholar] [CrossRef] [PubMed]
- Mo, Y.; Lim, L.Y. Mechanistic study of the uptake of wheat germ agglutinin-conjugated PLGA nanoparticles by A549 cells. J. Pharm. Sci. 2004, 93, 20–28. [Google Scholar] [CrossRef]
- Lin, H.P.; Singla, B.; Ghoshal, P.; Faulkner, J.L.; Cherian-Shaw, M.; O’Connor, P.M.; She, J.X.; Belin de Chantemele, E.J.; Csányi, G. Identification of novel macropinocytosis inhibitors using a rational screen of Food and Drug Administration-approved drugs. Br. J. Pharmacol. 2018, 175, 3640–3655. [Google Scholar] [CrossRef]
- Patel, P.C.; Giljohann, D.A.; Daniel, W.L.; Zheng, D.; Prigodich, A.E.; Mirkin, C.A. Scavenger receptors mediate cellular uptake of polyvalent oligonucleotide-functionalized gold nanoparticles. Bioconjugate Chem. 2010, 21, 2250–2256. [Google Scholar] [CrossRef]



| Inhibitor | Working Concentration |
|---|---|
| Filipin complex | 2 µM |
| EIPA | 24 µM |
| Bafilomycin A | 50 µM |
| Sodium azide | 1 µM |
| Hydroxychloroquine | 200 µM |
| Type of Man-ANP | ManCL0.03 | ManCL0.06 | Man+GA | Man Shell | ANP |
|---|---|---|---|---|---|
| Hydrodynamic diameter, nm | 202.1 | 158 | 130.5 | 154 | 109.3 |
| PI | 0.1203 | 0.2115 | 0.094 | 0.0799 | 0.124 |
| Zeta potential, mV | −8.9 | −15.8 | −14.3 | 12.67 | −47 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuznetsova, A.B.; Gorbacheva, V.I.; Kolesova, E.P.; Egorova, V.S. Tailoring of Albumin Nanoparticles Modified with Mannose for Effective Targeting in Immunosuppressive Tumor Microenvironment. Micro 2025, 5, 30. https://doi.org/10.3390/micro5020030
Kuznetsova AB, Gorbacheva VI, Kolesova EP, Egorova VS. Tailoring of Albumin Nanoparticles Modified with Mannose for Effective Targeting in Immunosuppressive Tumor Microenvironment. Micro. 2025; 5(2):30. https://doi.org/10.3390/micro5020030
Chicago/Turabian StyleKuznetsova, Alyona B., Valentina I. Gorbacheva, Ekaterina P. Kolesova, and Vera S. Egorova. 2025. "Tailoring of Albumin Nanoparticles Modified with Mannose for Effective Targeting in Immunosuppressive Tumor Microenvironment" Micro 5, no. 2: 30. https://doi.org/10.3390/micro5020030
APA StyleKuznetsova, A. B., Gorbacheva, V. I., Kolesova, E. P., & Egorova, V. S. (2025). Tailoring of Albumin Nanoparticles Modified with Mannose for Effective Targeting in Immunosuppressive Tumor Microenvironment. Micro, 5(2), 30. https://doi.org/10.3390/micro5020030








