Abstract
Hydrogen peroxide (H2O2) detection in both liquid and gas phases has garnered significant attention due to its importance in various biological and industrial processes. Monitoring H2O2 levels is essential for understanding its effects on biology, industry, and the environment. Significant advancements in the physical dimensions and performance of biosensors for H2O2 detection have been made, mainly through the integration of fluorescence techniques and nanotechnology. These advancements have resulted in more sensitive, selective, and versatile detection systems, enhancing our ability to monitor H2O2 in both liquid and gas phases effectively. However, limited comprehensive reviews exist on the detection of vaporized H2O2, which is used in disinfection and the production of explosive agents, making its detection vital. This review provides an overview of recent progress in nanostructured fluorescence sensors for H2O2 detection, covering both liquid and gas phases. It examines various fluorescence-based detection methods and focuses on emerging nanomaterials for sensor development. Additionally, it discusses the dual applications of H2O2 detection in biomedical and non-biomedical fields, offering insights into the current state of the field and future directions. Finally, the challenges and perspectives for developing novel nanostructured fluorescence sensors are presented to guide future research in this rapidly evolving area.
1. Introduction
Hydrogen peroxide (H2O2) is a vital molecule with diverse roles in cellular signaling, environmental processes, and safety applications [1]. As a reactive oxygen species (ROS), H2O2 plays a critical role in physiological functions such as wound healing, immune responses, and cellular signaling pathways [1,2]. However, its overproduction can lead to oxidative stress, implicated in various diseases and conditions. Accurate detection is essential in biomedical research to study diseases like cancer and neurodegeneration [3,4]. Monitoring H2O2 is also important in medical diagnostics, food safety, waste water treatment, etc. [5,6,7]. For instance, H2O2 reacts with Fe2+ in water, producing highly reactive hydroxyl radicals (•OH), which can cause oxidative damage in biological and environmental systems. Detecting H2O2 is crucial to prevent excessive radical formation in biological and industrial settings. In addition, H2O2 can interfere with chlorine-based disinfection, affecting water purification. Measuring H2O2 ensures proper disinfection in drinking water treatment. On the other hand, H2O2 in its vaporized form (vH2O2) also carries significant implications for health and safety. In fact, the detection of vH2O2 is important not only as an indicator of potential terrorist activities and peroxide-based explosives but also due to its role in various physiological and pathological processes [5,6]. Excessive inhalation of vH2O2 can cause oxidative stress, leading to diseases such as pneumonia, atherosclerosis, and diabetes. It also serves as a biomarker for respiratory illnesses like lung cancer and asthma, as it is produced by inflammatory cells in response to these conditions [6].
In the field of H2O2 detection, two prominent methods have gained significant attention: electrochemical and optical sensing. Optical methods stand out for their precision and sensitivity in detecting H2O2 [7,8]. Among these methods, fluorescence-based sensors stand out because they provide better selectivity, sensitivity, and the ability to monitor in real time. However, traditional fluorescence sensors face issues like interference from background signals and trouble achieving high sensitivity in complex samples. To address these limitations, new methods have been developed, resulting in the use of nanomaterials in fluorescence sensing techniques [9,10].
Nanomaterials have become essential in improving the capabilities of fluorescence sensors thanks to their unique properties like high surface-to-volume ratio and tunable physicochemical characteristics. These characteristics help overcome the limitations of traditional fluorescence sensors, leading to improved overall performance [10,11].
Despite these major benefits, it is crucial to assess how well nanomaterials can reduce interference from background signals. Although nanomaterials provide improved fluorescence properties and greater sensitivity, not all of them are equally effective at reducing background signal interference. The effectiveness of background signal reduction depends on the specific type of nanomaterial and the design of the sensor. For instance, quantum dots (QDs) and certain metal nanoparticles (metal NPs) can provide high brightness and photostability, which helps distinguish the signal from the background. However, issues like non-specific binding and environmental factors can still present challenges [10,12,13].
Nanotechnology-based sensors use a variety of nanomaterials, from zero-dimensional nanostructures like QDs to three-dimensional nanostructures. Each of these materials has distinct properties that enhance signal amplification [14]. This inherent capability is especially beneficial for fluorescence sensing, as the optical properties of nanomaterials enhance detection intensity and provide long-term stability. Notably, using nanomaterials not only improves sensitivity but also maintains sample integrity, thereby reducing the risk of contamination.
This review provides a detailed analysis of fluorescence-based sensors that use nanomaterials to detect H2O2 in various applications, including the detection of vH2O2—a topic that, though not new, is still relatively underexplored. The review explores the fundamental sensing mechanisms, the types of nanomaterials used, and their practical applications (Scheme 1). By addressing both traditional and novel aspects of H2O2 detection, it provides a comprehensive overview of the current state and future directions in this rapidly evolving field. The dual focus on various applications sets this review article apart from existing literature, making it a valuable resource for advancing the development of next-generation fluorescence sensors for more effective and versatile H2O2 detection.
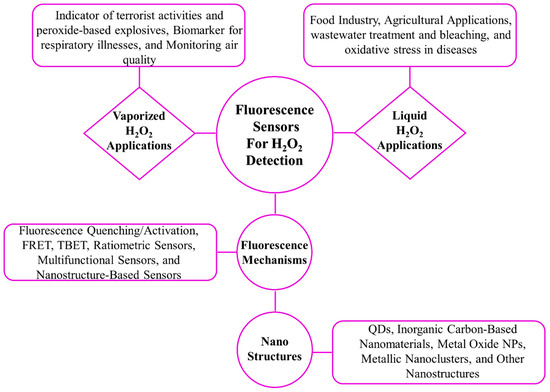
Scheme 1.
Fluorescence mechanisms and nanostructures used in H2O2 detection, with applications for liquid and vaporized H2O2 detection.
2. Evolution of Fluorescence Sensors for H2O2 Detection: From Inception to AI Integration
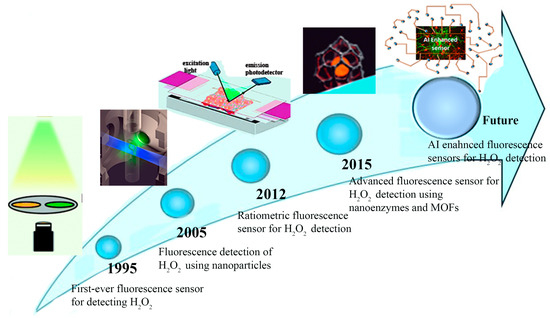
As shown in Scheme 2, fluorescence sensors for H2O2 detection have evolved from inception in 1995 to advanced nanoparticle-based ratiometric sensors with AI integration. The evolution of fluorescence sensors for H2O2 detection has progressed significantly since the first-ever sensor was introduced in 1995. Early methods relied on traditional fluorescence detection mechanisms, providing a basic yet effective tool for monitoring H2O2 in various environments. By 2005, researchers began exploring the use of nanoparticles to enhance sensor performance. Nanoparticles improved sensitivity, accuracy, and stability, making a key advancement in fluorescence detection technology. These innovations addressed the limitations of earlier sensors and opened the door for more complex applications, especially in biological and environmental monitoring.
In 2012, ratiometric methods were first used for H2O2 detection, providing better accuracy by reducing interference and offering more reliable data. The development of nanozymes and metal-organic frameworks (MOFs) by 2015 further revolutionized the field, enabling the creation of advanced fluorescence sensors with superior catalytic properties and versatility. Looking ahead, the integration of ratiometric fluorescence sensors with nanoparticles presents a cost-effective and highly sensitive approach. Using artificial intelligence (AI) for real-time analysis could greatly improve sensor performance, opening up new uses in medical tests, environmental monitoring, and industry.
Scheme 2.
Timeline of key advances in fluorescence sensors for H2O2 detection: from early innovations to future AI-enhanced approaches [15,16,17,18].
3. Various Types of Fluorescence Sensors
The sensing mechanisms in fluorescence sensors involve various strategies that utilize the interaction between sensor components and fluorophores. These strategies can be categorized by their mechanisms and design strategies. Based on their mechanisms, they can be divided into fluorescence quenching/activation, Förster resonance energy transfer (FRET), and Through Bond Energy Transfer (TBET) (Figure 1). Based on their design strategies, they can be divided into ratiometric fluorescence sensors, multifunctional fluorescence-based sensors and nanostructure-based fluorescence sensors.
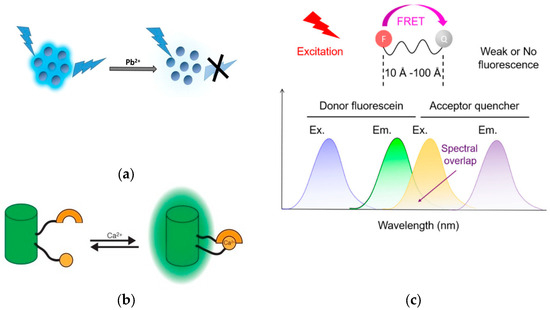
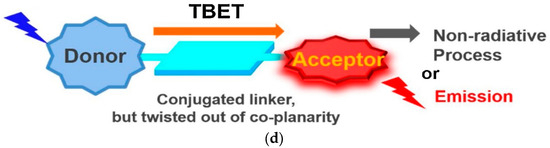
Figure 1.
Various Types of Fluorescence Sensors based on their mechanisms (a) Fluorescence quenching (reprinted with permission from ref. [19]). Copyright 2018 ELSEVIER. (b) Fluorescence activation (reprinted with permission from ref. [20]). Copyright 2020 ELSEVIER. (c) Fluorescence Resonance Energy Transfer (FRET) (reprinted with permission from ref. [21]). Copyright 2022 Wiley Online Library. (d) Through Bond Energy Transfer (TBET) (reprinted with permission from ref. [22]). Copyright 2020 Elsevier.
3.1. Fluorescence Quenching/Activation
Fluorescence quenching or turn-off sensor refers to the reduction in fluorescence intensity of a fluorophore by facilitating non-radiative pathways for its transition from the excited state to the ground state (Figure 1a) [23]. This process can occur through several mechanisms, including energy transfer, electron transfer, excited-state reactions, molecular conformational changes, and the formation of ground-state complexes or interactions with quenchers. In many bioassays, quenching typically happens when a quencher molecule interacts with a fluorophore, either through static or dynamic mechanisms (Figure 2) [23,24].
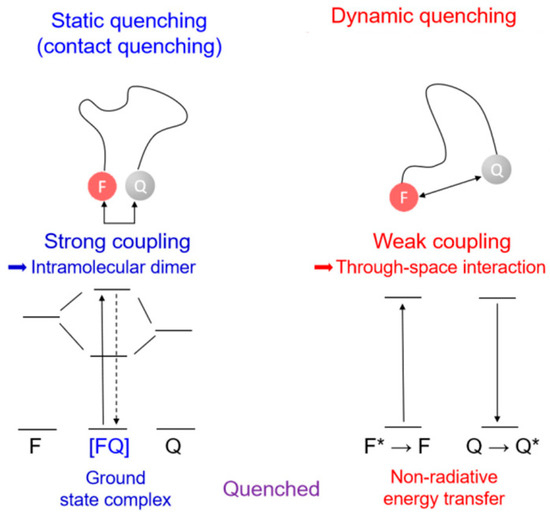
Figure 2.
Comparison of static and dynamic quenching mechanisms. Static quenching: donor and acceptor form a ground-state complex; dynamic quenching: involves energy transfer through molecular overlap at close distances. (* refers the excited state) (reprinted with permission from ref. [21]). Copyright 2022 Wiley Online Library.
Static quenching occurs when the quencher and fluorophore form a complex in the ground state, preventing the fluorophore from absorbing light and moving to the excited state. Conversely, dynamic quenching happens through collisions between the excited-state fluorophore and the quencher, leading to the fluorophore returning to the ground state. The decrease in fluorescence emission intensity can also be described by the Stern–Volmer equation, which is influenced by factors like temperature and solvent viscosity, as it is driven by diffusion [23].
where I0 and I represent the relative fluorescence intensities before and after the addition of the quencher, respectively, Ksv is the constant associated with quenching and [Q] indicates the concentration of the quencher [25,26].
Although fluorescence quenching is a common mechanism in fluorescence-based sensors, the number of materials capable of acting as quenchers is limited. This limitation can be addressed by attaching a quencher to the same molecule, thereby improving the sensor’s ability to detect analytes with quenching properties [27].
Turn-on fluorescence sensors offer a reliable and effective way to detect specific analytes by increasing luminescence when the target is present (Figure 1b). This approach is preferred over turn-off sensors because the bright signal produced is easier to detect and less prone to interference from other species, which can cause false positives. In biological systems, the strong emission of turn-on sensors stands out against a dark background, making them especially useful [28,29].
Fluorescence enhancement in turn-on sensors occurs through the correction of a structural defect and when an analyte corrects or modulates the fluorophore’s structure or electronic state [30]. Aggregation-Induced Emission Enhancement (AIEE), which reduces non-radiative decay by restricting molecular rotation, and Crosslink-Enhanced Emission (CEE), which stabilizes the fluorophore to minimize vibrational relaxation, are important mechanisms. Additionally, Photoinduced Electron Transfer (PET) and Intramolecular Charge Transfer (ICT) alter the electronic distribution within the fluorophore, further enhancing fluorescence. Common strategies such as Chelation-Enhanced Fluorescence (CHEF) are employed to design highly effective turn-on probes [31].
3.2. Fluorescence Resonance Energy Transfer (FRET)
FRET is a powerful technique in biosensing, valued for its high sensitivity and specificity. FRET operates through energy transfer between two closely positioned fluorescent chromophores—a donor and an acceptor—resulting in a measurable shift in fluorescence (Figure 1c). This technology is particularly effective in detecting a variety of analytes, including ions, small molecules, and biological macromolecules. It works by using changes in fluorescence that happen when the distance between the donor and acceptor falls within a range of 2–10 nm [32,33].
The efficiency of FRET, which is crucial for biosensors, depends on several key factors. First, there must be good spectral overlap between the donor’s emission and the acceptor’s excitation spectra; more overlap leads to better energy transfer. Second, the physical distance between the donor and acceptor affects efficiency: transfer rates decrease sharply as the distance increases, following an inverse sixth power relationship. Lastly, the orientation of their dipoles needs to be optimal, as misalignment can reduce energy transfer [33,34]. Theodor Förster first established the theoretical foundation for FRET in 1948, which emphasized these factors ensuring the technique’s high selectivity and sensitivity. FRET is widely used to monitor molecular interactions and conformational changes in biomolecules without the need for direct labeling or modification, making it a safer and more specific alternative to other techniques [32].
Advances in nanotechnology have further enhanced FRET’s capabilities with the integration of photoluminescent NPs such as QDs and quenchers such as graphene oxide, which offer high photostability and sensitivity [35,36]. These materials, alongside traditional fluorophores like organic dyes and fluorescent proteins, allow for the precise design of FRET-based biosensors. The technique’s ability to visualize molecular interactions in real time, along with its strong anti-interference and signal amplification features, makes FRET a top tool in biological research and diagnostics [36].
3.3. Through Bond Energy Transfer (TBET)
TBET-based fluorescent sensors consist of three main components: an energy donor, an energy acceptor, and a rigid conjugated linker (Figure 1d). Unlike FRET, TBET does not require spectral overlap between the donor’s emission and the acceptor’s absorption, which allows for greater flexibility in selecting donor–acceptor pairs [22].
In FRET, energy transfer occurs through space via a non-conjugated spacer, requiring substantial spectral overlap. TBET, on the other hand, involves energy transfer through electronically conjugated linkers, such as phenyl rings or triple bonds. This mechanism results in faster and more efficient energy transfer, even with minimal spectral overlap, leading to larger pseudo-Stokes shifts and improved resilience against environmental factors [22,37,38].
Despite these advantages, for designing TBET-based fluorescent probes, it is necessary to have donors or acceptors with multiple active reaction sites to simplify the synthesis process, which involves the use of multi-step reactions. Furthermore, unlike FRET, modifying the response site for an analyte is more challenging, making the fabrication of fluorescence probes more difficult [22]. Despite the limited choice of fluorophores, TBET chemosensors are valued for their high efficiency and strong performance in dual-channel fluorescence imaging. TBET chemosensors are usually classified by the structure of their energy acceptors, with rhodamine derivatives being a common choice [37,39].
3.4. Ratiometric Fluorescence Sensors
Ratiometric fluorescence sensors offer a reliable way to detect analytes with high specificity and sensitivity by measuring the ratio of fluorescence at two different wavelengths (Figure 3) [40]. This method adjusts for environmental and instrumental variations, ensuring accurate readings and minimizing signal interference. The dual-emission mechanism of ratiometric sensors greatly improves their performance over traditional fluorescence techniques, making them particularly effective for applications requiring precise and reliable detection [41].
These sensors utilize various mechanisms like ICT, FRET, and excited-state intramolecular proton transfer (ESIPT) to achieve their functionality. The integration of nanomaterials, such as QDs and metal–organic frameworks (MOFs), has further advanced the capabilities of these sensors, particularly in biosensing and cell imaging [40,42]. The development of sensors with multiple emission centers, enhanced by nanotechnology, has improved their sensitivity and selectivity, making them a powerful tool for real-time analysis. As technology advances, ratiometric sensors are expected to play an increasingly important role in various applications, providing even more precise detection capabilities [43,44].
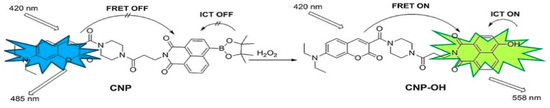
Figure 3.
The operational process of the innovative ratiometric fluorescent probe CNP in the presence of H2O2 (reproduced with authorization from Reference [45]). Copyright 2024 Wiley Online Library.
3.5. Multifunctional Fluorescence Sensors
Multifunctional fluorescent probes offer advantages over traditional single-analyte sensors by detecting multiple substances simultaneously and producing distinct emission changes. This enhances efficiency by reducing costs and analysis time. These probes incorporate multiple recognition sites within a single molecule, allowing for simultaneous detection. They can be categorized to reaction-type probes or coordination-type probes [46,47].
Reaction-type probes are known for their selectivity and quick response, making them effective at capturing target analytes. By integrating multiple detection functions into a single molecule, these probes improve both sensitivity and versatility. Bifunctional probes, which produce two different color changes, are a notable example of this design strategy. Coordination-type probes are widely used, especially for metal ion detection. Coordination of metal ions with organic dyes results in a strong optical response, which can be achieved through interactions with phenolic hydroxyl groups, imine nitrogen atoms, and nitrogen atoms in conjugated heterocyclic compounds such as piperidine [46,48].
The ability of multifunctional probes to simultaneously detect and differentiate multiple substances is crucial for understanding complex biological and environmental systems. They are promising candidates in rapidly analyzing food, biological processes, and environmental conditions, offering a foundation for future research [47].
3.6. Nanostructure-Based Fluorescence Sensors
The integration of nanotechnology has revolutionized biosensors, particularly fluorescence-based systems, which are now preferred for their sensitivity, simplicity, and non-destructive nature [49]. While organic dyes were traditionally used as reporters, limitations like low quantum yield and poor photostability have led to the adoption of fluorescent NPs. These nanomaterials offer unique optical properties and larger surface areas, which can be finely tuned during synthesis [12].
Nanomaterials serve multiple roles in fluorescence biosensors—as tags, quenchers, and signal amplifiers. Advances in molecular probes have facilitated the precise targeting and quantification of molecules, often using hybrid or doped nanomaterials as fluorescence tags [50]. Furthermore, over the past two decades, fluorescence detection platforms based on nanomaterials have been developed, with noble metals like gold (Au) NPs and silver (Ag) NPs being particularly effective as quenchers due to their strong plasmonic resonance and surface chemistry. Carbon nanomaterials, such as graphene, are also highly effective at quenching fluorescence. Besides labeling, nanomaterials like silica amplify fluorescence signals and support bioassays [12].
3.7. Comparative Analysis of Fluorescence Sensors
Fluorescence sensors for H2O2 detection exhibit varying levels of sensitivity, selectivity, response speed, and detection limits, influenced by their design and the nanomaterials used (Table 1). Fluorescence quenching and activation sensors are relatively simple and have moderate response times, ranging from 1 to 30 min. With detection limits between 10 µM and 1 mM, they are commonly used for environmental monitoring and intracellular H2O2 detection where low cost and reliability are beneficial [51,52].
FRET-based sensors use energy transfer mechanisms to achieve high sensitivity, with detection limits between 0.87 µM and 10 µM. However, their response times vary from 10 min to 1 h, making them suitable for applications like studying protein interactions and tracking H2O2 levels in live cells, where precise detection is more important than speed [53,54,55,56,57,58].
TBET sensors, which rely on through-bond energy transfer, offer quick response times from 10 s to 10 min, with detection limits between 1 µM and 100 µM. These features make TBET sensors ideal for rapid bioimaging, although their complex design and higher cost may limit widespread use [33,47,48,49].
Ratiometric fluorescence sensors are versatile and have detection limits as low as 7.7 ppb to 26.9 nM, with moderate response times of 1 to 30 min. They are effective in a range of applications, including intracellular pH sensing, food safety, and water quality testing, where accuracy and adaptability are crucial.

Table 1.
Comparison of fluorescence-based sensors: response speed, LOD, system complexity, and cost [51,52,59,60,61].
Table 1.
Comparison of fluorescence-based sensors: response speed, LOD, system complexity, and cost [51,52,59,60,61].
| Sensor Type | Response Time | Limit of Detection (LOD) | Excitation and Emission | Applications |
|---|---|---|---|---|
| Fluorescence Quenching/ Activation | 1 to 30 min | 10 µM to 1 mM | 300–700 nm | Environmental monitoring, Intracellular H2O2 detection |
| FRET (Fluorescence Resonance Energy Transfer) | 10 min to 1 h | 0.87 µM to 10 µM | 400–700 nm | Protein-protein interaction, live cell H2O2 sensing |
| TBET (Through Bond Energy Transfer) | 10 s to 10 min | 1 µM to 100 µM | 450–750 nm | Bioimaging |
| Ratiometric Fluorescence | 1 to 30 min | 7.7 ppb to 26.9 nM | 400–800 nm | Intracellular pH sensing, food and water analysis |
Notes: LOD Values: The ranges given represent typical detection limits for each sensor type. Specific applications may require different LODs based on analytical needs. Practical Applicability: The effectiveness of each sensor type is contingent upon whether its LOD meets the requirements for practical applications in specific analytical problems.
4. Nanomaterials Utilized in Fluorescence Sensors for H2O2 Detection
The utilization of nanomaterials presents numerous opportunities for enhancing the development of sensitive and effective sensors. When employed at the nanoscale, these materials exhibit advantages such as rapid response, durability, and selectivity compared to their microscopic counterparts. A diverse array of nanomaterials, including QDs, inorganic carbon-based nanomaterials, metal oxide NPs, metallic nanoclusters, and various other nanostructures, find application in fluorescence systems for the rapid detection of biomarkers [62].
4.1. QDs Used in Fluorescence Sensors for Detecting H2O2
QDs are currently under exploration as promising tools for detecting H2O2, potentially engaging in PET or FRET processes. QDs represent a promising advancement in fluorescence-based sensors for detecting H2O2, owing to their remarkable optical characteristics. Although their potential is significant, the use of QD-based sensing membranes for optical H2O2 detection remains relatively unexplored. Conventional fluorescence sensors that utilize dyes like ruthenium complexes, HP green fluorescence probes, and europium tetracycline complexes encounter issues such as inconsistent dye distribution and sensitivity to variations in light sources and detectors. In contrast, QDs provide notable benefits for H2O2 detection, including superior brightness, enhanced photostability, and adjustable emission spectra. When incorporated into sensing membranes, QDs can significantly improve the accuracy and reliability of measurements. Additionally, employing ratiometric fluorescence with QDs, which involves analyzing two separate fluorescence signals, can effectively address problems like uneven dye distribution and fluctuations in light sources, resulting in more precise H2O2 detection. Ratiometric fluorescence sensors based on CdTe QDs for H2O2 detection were prepared by Cao et al. They introduced a ratiometric fluorescence platform using CdTe QDs, carbon dots (CDs), and horseradish peroxidase-labeled hepatitis B core antibody (HBcAb-HRP) for detecting H2O2. HBcAb-HRP enhances the fluorescence of CdTe QDs, which is reduced when H2O2 is present, while the CDs remain stable. The platform has a detection limit of 2.9 μM for H2O2, and under UV light, a color change from orange-yellow to blue enables easy visual detection, successfully applied to commercial contact lens solutions [63].
Carbon quantum dots (CQDs) serve as crucial sensors components due to their unique features such as outstanding water solubility, distinct optical properties, and minimal harm to living organisms. Zhao et al. devised an inventive fluorescent probe for detecting H2O2 with high sensitivity, using an amide interaction between amino-functional CQDs and carboxyl-modified AuNCs. This nanoaster, combining CQD-AuNCs, emitted dual bands when excited by a single wavelength, facilitating energy resonance transfer to improve sensitivity and selectivity. Using a ratiometric probe helps decrease environmental impact and increase accuracy. Additionally, their study introduced a method for detecting H2O2 in milk and toothpaste as well as live cell imaging [58].
In another study, Zhou et al. found a new method to detect H2O2 with a hybrid of CdSe@ZnS/AgNCs. DHLA-AgNCs were utilized as the responsive element to H2O2 due to their high sensitivity to H2O2-induced etching. The addition of H2O2 initiated the oxidation of AgNCs, leading to the formation of Ag+ ions, which subsequently decreased the fluorescence of CdSe@ZnS QDs. This approach facilitated the accurate detection of H2O2 with a calculated LOD of 0.3 μM, surpassing many other H2O2 sensors based on fluorescent nanomaterials [64].
4.2. Inorganic Carbon-Based Nanomaterials for H2O2 Detection
Inorganic carbon-based nanomaterials are widely used in developing fluorescence sensors for H2O2 detection because of their unique features like large surface area, good electrical conductivity, and biocompatibility. Previous studies have underscored the efficiency of single-walled carbon nanotubes (SWCNTs) in biosensing applications, particularly emphasizing the chirality-dependent near-infrared (NIR) fluorescence of semiconducting SWCNTs [65]. Ledesma et al. presented a method for attaching proteins to SWCNTs to create effective sensors for biological detection (Figure 4). Using HRP, a sensor for H2O2 was developed, showing a clear fluorescence response that depends on the concentration, with a detection limit of 31 µm and good selectivity for H2O2. The azide-based triazine chemistry used ensures that both the SWCNT fluorescence and the enzyme function remain intact. This adaptable approach can be applied to other proteins, making it useful for sensors in bioimaging and theranostics [66]. Mu et al. used an organic–inorganic hybrid made of silica nanoparticles to speed up deprotonation reactions, resulting in noticeable fluorescence reduction in just 5 s at room temperature. The estimated detection limit was 184 ppt (parts per trillion) which could be visualized as 184 s in 31,700 years, ranking among the most sensitive. This study introduced an ultrafast, highly sensitive organic–inorganic hybrid probe for H2O2 vapor, presenting a new design strategy for promising H2O2 probes [67].
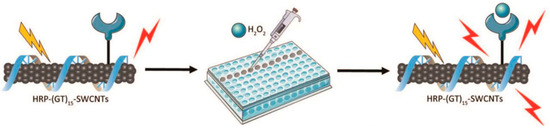
Figure 4.
The HRP-(GT)15-SWCNT nanosensor reacts to H2O2 in a solution-phase screening test. The diagram illustrates the response screening setup, where adding the analyte to a 384-well plate containing the nanosensor triggers a significant increase in NIR fluorescence emission (reproduced with authorization from reference [66]). Copyright 2024 Wiley Online Library.
Graphitic carbon nitride (g-C3N4) nanosheets, which are similar to graphene, have high fluorescence and large surface areas, making them ideal for biosensors. Liu et al. introduced a novel approach for the sensitive detection of H2O2 and glucose using a ratiometric fluorescence sensing strategy. The method involved the assembly of oxidized o-phenylenediamine (OPD) on g-C3N4 nanosheets, inducing fluorescence quenching and a new emission peak. Reliable detection of H2O2 and glucose was achieved, with detection limits of 50 nM and 0.4 μM, respectively, and has been successfully applied to measure these substances in human serum [68].
4.3. Metal Oxide NPs in Fluorescence Sensors
Metal oxide NPs, particularly iron oxide (Fe3O4), zinc oxide (ZnO), and titanium dioxide (TiO2), have undergone extensive investigation for their utility in fluorescence sensors for H2O2 detection. Using metal oxide nanoparticles in fluorescence sensors offers potential for various applications, such as medical diagnostics, environmental monitoring, and industrial processes, because of their sensitivity and selectivity [69].
In a chemical approach, Zheng et al. created an aligned nanoreactor sensor, NR-Fe3O4, with Fe3O4 nanoparticles in anodized aluminum oxide channels, improving H2O2 detection sensitivity to 10–5000 nM and a detection limit of 0.9 nM. The sensor worked well at neutral pH, remained stable for over 8 h, and filtered out larger background molecules in complex water samples. This design enhanced sensitivity by allowing efficient production and capture of hydroxyl radicals (•OH), with future efforts focused on reducing interference [70].
Organic–inorganic hybrids are popular for versatile properties. Anatase TiO2 offers high UV photocatalytic efficiency, refractive index, and UV absorption. The TiO2 nanosheets/graphene QD (TiO2 NS/GQD) composite detects H2O2 through fluorescence (Figure 5a). The TiO2 NSs act as a photocatalyst, enhancing the fluorescence emission from the graphene QDs. Upon exposure to H2O2, the fluorescence changes, allowing for detection. This composite has a low detection limit of 10–14 M and a broad detection range from 10–14 M to 10–2 M, making it highly sensitive for H2O2 detection [71].
Another approach was developed using a hyperbranched pyrenyl–fluorene copolymer (S1) combined with a ZnO nanorod array. The hyperbranched polymer S1, noted for its steric hindrance, elevated HOMO level, and enhanced fluorescence yield, was particularly sensitive to H2O2 vapor. When integrated with the ZnO nanorod array, this combination significantly improved detection sensitivity. Specifically, S1 with the ZnO nanorod array achieved approximately 60% fluorescence quenching for H2O2 within 300 s. The detection limit for H2O2 was found to be 1.6 ppb, demonstrating a highly sensitive method for detecting peroxide explosives [72].
4.4. Metallic Nanoclusters Used in Fluorescence Sensors
Extensive research has been conducted on metallic nanoclusters (NCs) with fluorescence properties, highlighting their great potential for detecting biomolecules and chemicals. They have been used in fluorescence sensors to detect H2O2, taking advantage of their unique fluorescence properties [73].
Gold nanoclusters (AuNCs) are fluorescent, low-toxicity nanostructures with unique optical and catalytic properties, ideal for sensing applications. Zhang et al. developed photoelectrochemical sensors using low-toxicity fluorescent AuNCs for H2O2 and glucose detection. They detailed sensor fabrication and performance, presenting a model based on AuNCs’ energy levels and electron tunneling. The sensors, which require no extra modifications, effectively sense H2O2 through fluorescence and catalytic properties of AuNCs. The sensitivity and LOD of AuNCs at −500 mV were 4.33 nA/mM and 35 μM, respectively [74].
However, as AuNCs are not stable in solution, they require stabilization. This is achieved by using ligands such as dendrimers, peptides, DNA, or proteins. Lee et al. developed horseradish peroxidase-encapsulated fluorescent bio-nanoparticles (HEFBNPs) which combine bovine serum albumin and horseradish peroxidase-stabilized AuNCs (Figure 5b). These NPs use a two-step fluorescence quenching mechanism and allow rapid reaction events, detecting H2O2 as low as 0.5 nM with high selectivity. For practical use, they created a glass-based microfluidic device for visual detection, making HEFBNPs ideal for point-of-care testing [75].
Ratiometric fluorescence probes for H2O2 detection fall into three categories: (1) those using small molecule probes that react with H2O2, (2) those exploiting H2O2 as an electron acceptor in peroxidase reactions, and (3) those utilizing H2O2 to etch silver NPs into silver ions. A highly sensitive ratiometric fluorescence sensor using blue carbon dots, silver nanoprisms, and o-phenylenediamine (B-CDs-Ag NPRs-OPD) was developed for detecting H2O2 and glucose by Li et al. The sensor detects H2O2 by etching Ag NPs into Ag+ ions, which oxidize OPD into DAP, emitting yellow fluorescence and quenching the blue fluorescence of B-CDs through the inner filter effect with detection limits of 0.12 μM for H2O2. The method’s simplicity and sensitivity make it promising for broader applications [76].
Bimetallic nanoclusters are more effective than monometallic ones because the combination of different metals enhances their properties. In a study, Mi et al. developed and purified Au/Ag bimetallic nanoclusters (GSH-Lys@Ag-AuNCs) using isoelectric point precipitation. The purified nanoclusters showed enhanced sensitivity for detecting H2O2 with a wide linear range (0.2–500 μM) and a low detection limit (0.05 μM). They were effective in glucose and uric acid assays and monitored •OH levels in cells. The nanoclusters had two emission peaks at 440 and 640 nm, with improved fluorescence sensitivity after purification [77]. The concept of combining the synergistic effects of different metal species has been extended to other types of metallic NCs, such as functionalized bimetallic IrPt NCs [78] and FePt-Au ternary metallic NCs [79].
4.5. Other Nanostructures Used in Fluorescence Sensors
Hybrid nanostructures with special fluorescence properties have played a significant role in enhancing the fluorescent signal in H2O2 sensing. Polymer-based biosensors are popular for H2O2 sensing because they can be adjusted for specific needs and are biocompatible. Their stability and ability to be modified make polymer nanomaterials good choices for detecting H2O2 [80].
Sun et al. developed a fluorescence sensor (CTS-HP) by integrating naphthalene fluorophores with borate into chitosan (CTS), offering low toxicity and biocompatibility. CTS-HP showed a 21-fold fluorescence increase, a detection limit of 8.98 nM, and a fast response time of 16 min. It successfully imaged H2O2 in zebrafish, HeLa cells, and water samples, with recovery rates of 90.93–102.9%. This positions CTS as a valuable material for sensitive and safe detection of oxidative stress markers [81].
Nanozymes, a novel enzyme mimic, have broad applications in disease diagnosis, food testing, and sample preparation. A bifunctional Ni-MOF nanozyme was developed for label-free fluorescent detection of H2O2 (0.1–20 mM) by Guo et al. (Figure 5c). It exhibited peroxidase-like activity, generating hydroxyl radicals that triggered fluorescence, enabling sensitive detection with a low limit of 4.0 × 10−5 M. This method demonstrated good reproducibility and accuracy in detecting H2O2 in various samples, offering a green and economical approach for monitoring this biomolecule [82].
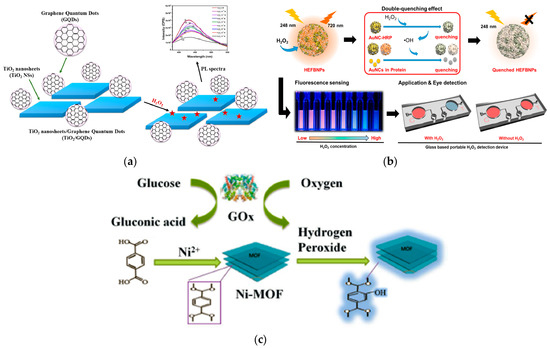
Figure 5.
(a) An illustration of a novel, low-cost, and eco-friendly method for fabricating TiO2 nanosheets/graphene quantum dot (TiO2 NS/GQD) composites in a simple step. The generated TiO2 NSs/GQDs were used as catalysts for H2O2 detection (reproduced with authorization from reference [71]). Copyright 2022 ELSEVIER. (b) Schematic representation of the fluorescence quenching mechanism of HEFBNPs upon H2O2 addition and its potential applications. (•OH = hydroxyl radical) (reproduced with authorization from reference [75]). Copyright 2023 MDPI. (c) Schematic representation of the label-free fluorescence sensing platform based on the bifunctional Ni-MOF nanozyme for detecting H2O2 and glucose (reproduced with authorization from reference [82]). Copyright 2022 Springer Nature.
Hybrid nanofibers represent a significant category of nanomaterials employed within fluorescence sensor systems to detect H2O2 across various states, including the gas phase, with significantly high sensitivity. For instance, Nazari et al. created low-cost optical sensors for H2O2 using photochromic (R/S)-2-(3′,3′-dimethyl-6-nitro-3′H-spiro[chromene-2,2′-indole]-1′-yl) ethanol (SPOH) and acrylic terpolymer films or nanofibers. The terpolymer, poly(methyl methacrylate-butyl acrylate-hydroxyethyl methacrylate) (MBH), was made and combined with SPOH. The sensors showed color changes when exposed to H2O2, with detection limits of 11 mg/dL for the film and 2.3 mg/dL for the nanofibers, which responded faster (50 min). The MBH/SPOH@NF sensor effectively detected H2O2 in real water samples, showing its potential for monitoring industrial waste [83].
5. Applications of Detecting Liquid H2O2 by Using Nanostructured Fluorescence Sensors
Fluorescence and nanostructured fluorescence sensors for H2O2 detection are widely used in different fields. In biomedical research, they are essential for tracking oxidative stress in cells. In environmental monitoring, they help detect H2O2 as a pollutant or a sign of water quality. Additionally, in industrial processes where precise measurement of H2O2 concentrations is critical, these sensors are essential. Moreover, the integration of nanotechnology with fluorescence sensing techniques not only enhances sensitivity but also facilitates the development of portable and cost-effective devices. This integration supports new solutions in non-biomedical and biomedical applications.
5.1. Non-Biomedical Applications for Liquid H2O2 Detection
Fluorescence and nanostructured fluorescence sensors are now used in many non-biomedical fields. Their unique features, such as high sensitivity, selectivity, and quick response times, make them valuable tools for various applications, including the food industry, plant science, water and air quality assessment, etc.
In the food industry, the use of H2O2 for its antibacterial properties raises concerns regarding residual amounts. Regulatory standards, such as 0.05% in dairy products and 60 mg/kg as an antimicrobial agent, highlight the necessity for rapid H2O2 detection to ensure food safety [84]. Lu et al. developed a dual-signal method using sulfur QDs to simultaneously detect iron (II) (Fe2+) and H2O2 in food samples. The interaction of Fe2+ with sulfur QDs results in fluorescence quenching and a color shift from yellow to green. Upon H2O2 addition, fluorescence is restored, turning the solution colorless. This method demonstrates linear correlations between Fe2+ and H2O2 concentrations and fluorescence intensity/absorbance within specific ranges, with detection limits of 1.41 μM and 0.54 μM for Fe2+ and 0.03 μM and 0.06 μM for H2O2, respectively. Using sulfur QDs in a dual-signal sensing system allows accurate detection within the given concentration ranges [85].
Plants use H2O2 within their leaves to send signals that trigger leaf cells to produce protective compounds against predators like insects and aid in repair [86]. Wu et al. prepared an engineered NIR fluorescent SWCNT embedded in Arabidopsis thaliana leaves acting as sensors for H2O2, an important signaling molecule linked to plant stress. The NIR sensor fluorescence responds to H2O2 within the plant’s physiological range, enabling remote imaging of plant responses to stresses like UV-B light, high light, and pathogen-related peptides. Minimal leaf cell death and comparable photosynthetic rates to controls without SWCNT indicate the high biocompatibility of these nanosensors. They offer early stress detection in plants, enhancing understanding of plant stress communication for precision agriculture and optimized agrochemical use [87].
H2O2 plays a major role in industrial activities, affecting water and air quality, making its detection essential for environmental management. A novel fluorescent nanoprobe for rapid H2O2 detection was developed by Ali et al., comprising fluorescein dye and MnO2 nanosheets. The nanoprobe demonstrates a linear detection range of 0.04–30 μM, a low limit of detection of 7.5 nM, and a limit of quantitation of 21 nM. MnO2 nanosheets act as efficient nano-quencher and recognition agents for H2O2, facilitating selective recovery of fluorescence intensity. The method’s applicability was validated in water and cosmetic samples, with a low mean relative standard deviation of 2.6% [88].
H2O2 is widely used in industries like wastewater treatment and bleaching because of its oxidizing properties, gas release upon decomposition, role as an energy source, influence on free radicals, and impact on biological processes. However, its widespread use has turned H2O2 into a significant contaminant in industrial waste and products, raising concerns about higher exposure levels. Monitoring H2O2 levels allows for early detection of potential contaminants, helping with quality control and prompt corrective actions [89]. Duong et al. developed ratiometric fluorescence sensors for H2O2 detection in wastewater, utilizing CdSe/ZnS QDs and amino fluorescein (AF) as a reference dye. Immobilized in a sol–gel matrix, the sensors exhibit linear detection range and corresponding detection limits, with enhanced sensitivity achieved by incorporating HRP on the membrane. The sensor shows strong selectivity, making it very effective for monitoring H2O2 levels in wastewater treatment [90].
5.2. Biomedical Applications for Liquid H2O2 Detection
ROS are byproducts of cellular oxidative metabolism, playing crucial roles in various physiological processes, including cell survival, differentiation, signal transduction, and inflammation-mediated factor production. However, elevated ROS levels can cause significant cellular damage by oxidizing biopolymers such as nucleic acids, lipids, and carbohydrates, eventually leading to cell death through necrosis or apoptosis. Furthermore, ROS are involved in various human diseases, including cancer, neurodegenerative disorders, diabetes, and cardiovascular conditions [91,92]. Maintaining optimal ROS levels is critical for cellular homeostasis. Disruptions can harm physiological functions: low ROS levels can affect cellular proliferation and defense mechanisms, while high concentrations can damage cellular components and activate pathways linked to aging and age-related diseases. Consequently, monitoring ROS status provides invaluable insights into the pathophysiology of diseased cells and tissues. Compared to other ROS, H2O2 is a stable biomarker that is particularly important in oxidative stress across various diseases because of its longer lifespan and greater stability.
Recently, nanomaterial-based fluorescence methods with unique optical properties have shown excellent sensitivity and spatiotemporal resolution for detecting H2O2 in biological samples. These probes can be customized for H2O2 detection in solution, cells, and in vivo, or for fluorescence imaging of intracellular H2O2 in cells and animal models [93]. Pioneering researchers have successfully visualized H2O2 in living cells, including macrophages, cervical cancer cells, gastric cancer cells, and liver cancer cells, as well as in mouse and angelfish models, by designing and synthesizing fluorescent small-molecular probes.
Jain et al. successfully developed BSA-AuNCs for detecting H2O2 within mammalian cells using a fluorescence microscope. Their studies revealed that these BSA-AuNCs emit a bright red fluorescence. However, exposure to H2O2 results in a notable reduction in the fluorescence intensity of these nanoclusters. Moreover, these nanoclusters exhibit exceptional biocompatible and are actively internalized by human liver cells [92].
In recent years, hybrid fluorescent nanostructures comprising CQDs and metal NCs have gained attraction due to their unique optical properties and biocompatibility, finding applications in biosensing and imaging [94]. Li et al. synthesized a CQD-AuNCs nanosatellite by combining dihydrolipoic acid-protected AuNCs (DHLA-AuNCs) with branched poly(ethylenimine)-capped CQDs (BPEI-CQDs) through a carbodiimide-activated coupling reaction. This nanosatellite exhibited dual-emission fluorescence with a 40% FRET efficiency between CQDs and AuNCs. Additionally, it could detect H2O2, with the red emission from DHLA-AuNCs being quenched and the blue emission from BPEI-CQDs serving as a reference signal, resulting in a ratiometric fluorescence probe sensitive to H2O2 in the range of 5.0 nM to 80 nM with a detection limit of 2.9 nM. The CQD-AuNC nanosatellite enabled visual detection and imaging of H2O2 within living cells [95].
Many intracellular H2O2 probes rely on irreversible reactions between H2O2 and indicator molecules, causing changes in optical properties, typically seen as increases or decreases in fluorescence intensity [7,96]. In a study, Hu et al. achieved highly sensitive H2O2 quantification in cells using CIS/ZnS/ZnS QDs modified with ZnO shells for self-passivation. Self-passivation, unlike traditional methods, improved fluorescence emission and stability. Upon exposure to H2O2, the ZnO and ZnS shells decomposed, resulting in fluorescence quenching via exciton energy transfer. This fluorescence-based H2O2 sensor offers a wide measurement range and a low limit of detection, 0.46 nM. The enhanced selectivity and sensitivity of the ZnO shells enabled quantifying H2O2 variations within cells, offering a promising approach for sensitive intracellular H2O2 detection [97].
Upconversion NPs (UCNPs) convert NIR light to shorter wavelengths, making them suitable for biological applications due to NIR’s minimal sample damage and deep penetration [98,99,100]. Wang et al. designed UCNPs-MoS2 nanoflake assemblies for sensitive tracking of ROS in biological systems. UCNPs emitted fluorescence while MoS2 nanoflakes served as quenchers and ROS detectors. The assemblies enabled quantification and detection of ROS levels in living cells, and they were utilized for in vivo ROS visualization in zebrafish owing to their low autofluorescence and deep penetration properties [101].
Most optical detection methods for H2O2 in published literature face significant challenges, including irreversibility, interference from other analytes like different ROS or pH changes, instability, temperature sensitivity, and limitations to specific testing environments [102,103]. To make nanomaterials practical as H2O2 sensors, key challenges need to be addressed. This includes conducting extensive long-term research to transition H2O2 nanosensors from the lab to commercial use, simplifying and cost-effectively fabricating these sensors with label-free methods, and ensuring they have reproducible optical characteristics and high quantum yields. These improvements are crucial for enhancing the utility of H2O2 nanosensors in both in vivo and in vitro studies, ensuring accuracy and reliability for various applications [44,91].
6. Detection of Vaporized H2O2 (vH2O2) by Using Nanostructured Fluorescence Sensors
Detecting vH2O2 is crucial for ensuring safety, environmental protection, and health monitoring across various industrial and medical settings. Monitoring vH2O2 levels is crucial for workplace safety in industries that use it, such as disinfection and chemical manufacturing, to minimize potential hazards related to inhalation [60]. Health monitoring systems can offer early warnings of vH2O2 exposure in settings like laboratories or medical facilities, where respiratory irritation and other health issues may occur [104]. Furthermore, vH2O2 serves as an important marker of oxidative stress in conditions like asthma and chronic obstructive pulmonary disease (COPD). It increases in breath due to production by white blood cells during inflammation [105].
Traditional vH2O2 analysis methods are costly and impractical for everyday use, requiring lab equipment and trained staff. In contrast, fluorescent sensors are portable, easy to use, and highly sensitive, making them ideal for real-time gas detection in various environments.
The detection of peroxide-based explosives (PEs), such as TATP and hexamethylene triperoxide diamine (HMTD), has become a critical concern for law enforcement agencies globally due to their widespread use in terrorist activities. H2O2 emerges as a key target in this effort, acting both as a precursor and a decomposition product of PEs. Despite the challenges presented by the unique properties of PEs, such as their lack of quenching abilities and limited solubility, the urgent need for reliable and sensitive detection of these explosives in various scenarios—including luggage, cars, and aircraft—highlights the importance of continued research and innovation in this area.
Existing explosive detection methods like gas sensing and chromatography are accurate but hindered by bulky equipment and complex sample preparation. Fluorescent detection, including luminescence and colorimetric methods, is favored for on-site use due to its quick, visual, and specific results with simpler operation [106].
Recent advancements in this field largely result from the development of new fluorophores with improved properties, such as higher quantum yield and better photostability. Researchers have explored the utilization of QDs, organic dyes, and other nanomaterials to design fluorescence sensors with improved performance. However, understanding the various sensing mechanisms used by fluorescence sensors to detect vH2O2 is essential. This understanding allows for the development of fluorescence sensors customized to specific design needs.
The thin-film fluorescence probe, known for its durability and sensitivity, is gaining attention in explosive detection. Rare-earth-doped vanadate is particularly suitable for detecting vH2O2 due to fluorescence quenching, despite its poor photostability. In this regard, Ambard et al. demonstrated a microwave-assisted sol–gel synthesis to enhance the photostability of EuVO4 films, significantly improving sensitivity to H2O2 vapor with a detection limit of 100 ppb. The surface chemistry of the nanoparticles, analyzed using photoelectron spectroscopy, highlights their sensitivity to H2O2. The improved synthesis process produces photostable films and efficient H2O2 sensors, offering a reliable method for detecting vH2O2 [107].
Detecting vH2O2 after UV exposure provides a useful indirect sensing method for PEs. Many studies on PEs and vH2O2 sensing have used various strategies, with most involving solid-state gas sensors. Caron et al. synthesized a fluorescent dioxazaborocane for vH2O2 sensing, demonstrating strong fluorescence quenching upon exposure to vH2O2. The study also reports the synthesis and characterization of dioxazaborocane 2, emphasizing an understanding of its solid-state H2O2 sensing properties. Challenges at the solid–gas interface led to the use of indirect methods, such as IR and UV spectroscopy, to analyze reactions in solution in related studies [108].
Although current studies are mostly conducted in the laboratory and focus on determining detection limits and sensitivity response times through data fitting, efforts are being made to develop a practical device for detecting trace amounts of vH2O2. Chen et al. introduced a practical fluorescent sensor for detecting trace amounts of vH2O2, enhancing fluorescence analysis performance and optimizing the C6NIB (naphthalimide-based fluorescence turn-on sensor) fluorescent molecule for engineering applications. Their efficient field-test prototype achieved a detection limit of 2 ppb and a response time of <0.5 s, addressing challenges in packaging and storage. The vacuum-sealed test paper prevents oxidation, increasing the practical value of fluorescence analysis studies for real-world peroxide detection [109].
Fluorescence quenching or enhancement is commonly utilized for detecting vH2O2. However, certain probes encounter challenges such as long response times, moderate sensitivity, photobleaching, or the requirement for complex custom synthesis. Garreffi et al. introduced a boronate ester quinoline (QIB) fluorophore for highly sensitive and selective detection of vH2O2. QIB exhibits significant fluorescence enhancement upon exposure to H2O2 vapors in solid thin films or QIB-porous silicon (Si) nanocomposites, achieving a detection limit of 150 ppt (Figure 6a). This approach offers direct, fast, and selective trace detection of vH2O2, with QIB films resisting photobleaching. The exceptional sensitivity, which depends on the amount of QIB infiltrated inside nanoporous Si, reduces the need for complex synthesis since QIB is commercially available (Figure 6b,c) [110].
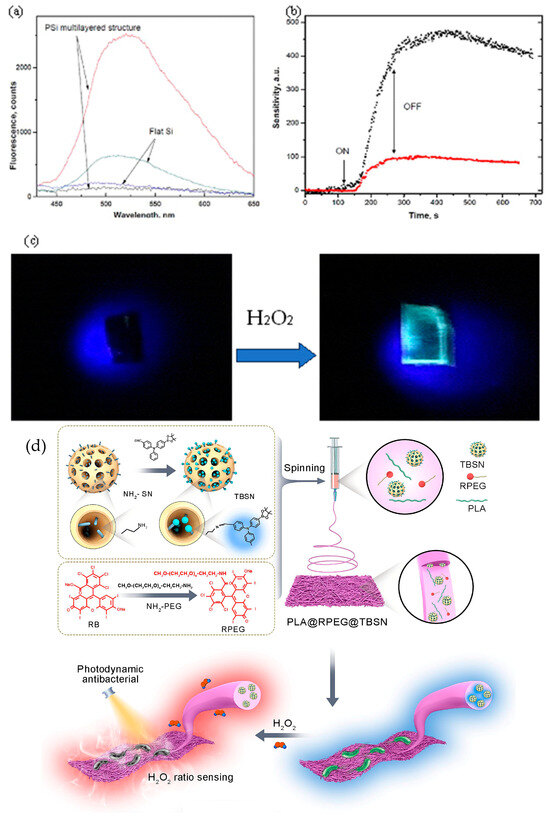
Figure 6.
(a) Fluorescence spectra of PSMS infiltrated with QIB and QIB spin-coated on flat Si before (black, blue) and after (red, navy) exposure to H2O2 vapors (214 ppm) for 120 s, λexit = 365 nm; (b) Time traces of sensitivity, R, for PSMS-QIB and QIB on flat Si upon H2O2 vapors exposure, arrows indicating the start (ON) and end (OFF) of H2O2 exposure, λexit = 365 nm, λdet = 520 nm. (c) Image of PSMS-QIB composite film before and after 120 s exposure to H2O2 vapors (214 ppm), with the fluorescence quantum yield of the PSMS-QIB film determined as zero and 0.23 before and after vH2O2 exposure (reprinted with permission from ref. [108]) [110]. Copyright 2018 ELSEVIER. (d) Diagram of the PLA@RPEG@TBSN film construction for sensing and photodynamic antibacterial applications (reprinted with permission from ref. [6]). Copyright 2024 Elsevier.
Detecting vH2O2 is crucial for diagnosing respiratory diseases and preventing harm from inhalation. A wearable sensor, such as one in a mask, can effectively monitor this vapor. With the rise of flexible sensors, there is an increasing need for enhanced health protection along with sensing capabilities. Additionally, bacterial contamination on these sensors presents infection risks, making the development of antibacterial wearable sensors essential. In a study, An et al. created a flexible, wearable film sensor (PLA@RPEG@TBSN) using electrospinning to monitor vH2O2 with high accuracy and visual feedback (Figure 6d). This sensor combines aggregation-induced emission NPs for fluorescence detection and Rose Bengal-modified PEG for reference, enabling precise ratiometric sensing. It shows excellent vH2O2 detection with a low limit of 7 ppb and significant antibacterial properties, killing 84% of bacteria. When used in a mask, it offers both gas sensing and bacterial resistance, making it ideal for complex applications [6].
The Inner Filter Effect (IFE)-based dual-mode sensing system is widely recognized for its simple fabrication, easy modification, and broad applicability. Cao et al. proposed a carbon dot (CD)/TiOSO4 sensing system for dual-mode peroxide detection, utilizing the IFE. It achieves colorimetric and fluorescent detection limits of 31.3 µM and 0.2 µM, respectively, for vH2O2. With a hydrogel platform, the system universally detects peroxide-based explosives in liquid, solid, and vapor phases, with a vapor sensing limit of 0.15 ppb. The work introduces an innovative strategy for on-site dual-mode sensing using IFE [111]. Table 2 lists the reported fluorescence sensors used for detecting vH2O2.

Table 2.
Fluorescence sensors for detecting vH2O2: A comparative overview of sensing materials, mechanisms, detection limits, and applications [112,113,114,115,116,117,118,119,120,121].
7. Current Challenges and Future Perspectives
As the field of nanostructured fluorescence sensors for H2O2 detection continues to evolve, several promising directions and challenges are emerging, guiding the future of this field. This section outlines the challenges in H2O2 detection and offers future perspectives for advancement. It begins by addressing general challenges affecting H2O2 detection, such as single-peak fluorescence detection, sensitivity, selectivity, and response time [122]. Next, it focuses on the specific difficulties associated with liquid H2O2 detection, including autofluorescence interference and biocompatibility. The discussion then shifts to the unique challenges of detecting vH2O2, emphasizing the need for real-time monitoring solutions. Finally, future perspectives are presented, highlighting the potential of nanomaterials and the integration of machine learning technologies to fluorescence sensors to enhance detection capabilities.
Many fluorescent probes depend on single-peak fluorescence detection, which can complicate quantitative measurements due to factors like photobleaching and variations in instrument efficiency. Ratiometric fluorescence, which measures intensity changes at two different wavelengths, offers a solution by correcting these issues and improving accuracy. However, traditional methods for developing ratiometric sensors tend to be complex and costly. Thus, there is an urgent need for a straightforward, sensitive, and affordable H2O2 ratiometric fluorescence sensor [52,63,123].
Regarding selectivity, many of the currently reported probes lack specificity for H2O2 and may also react with other ROS such as HClO, ONOO−, and NO. This lack of specificity is a significant limitation, as it can lead to false positives. Additionally, the reaction rates and response speeds of current probes are too slow to detect the trace amount of H2O2 in environmental and living systems, making it necessary to develop faster reaction mechanisms.
Autofluorescence interference has been a constant challenge in traditional fluorescence detection of liquid H2O2 in biological systems. Most fluorescent probes for H2O2 detection emit in the visible region, making them susceptible to background interference. NIR probes minimize this interference and are better suited for H2O2 detection [124]. However, NIR wavelength adjustments have only partly resolved autofluorescence issues, and making autofluorescence-free H2O2 probes is still difficult. Persistent luminescent nanoparticles (PLNPs) emit light even after the excitation source is removed. These NPs provide autofluorescence-free imaging but face challenges with biocompatibility and size uniformity, which limits their use in biological systems [125].
Commonly used fluorescent dyes such as BODIPY, rhodamine, fluorescein, and coumarin have been modified with receptor units that specifically react to liquid H2O2, causing a noticeable change in fluorescence. However, these hydrophobic dyes often suffer from poor water solubility, high cytotoxicity, and leakage from cells. Despite their excellent photophysical properties, there is a need for new dyes with high excitation and emission wavelengths, strong extinction coefficients, and better biocompatibility. Researchers address these issues by modifying probes to improve water solubility using ionic groups or self-assembly. Yet, these approaches can sometimes impact fluorescence performance and cellular entry [81,126]. Furthermore, to prevent foreign body reaction, the hydrogel must have nonionic, hydrophilic, and low-protein adsorption affinity [127].
Biocompatibility is a key factor, as all biosensors, regardless of their placement in the body, initially encounter protein adhesion. When a sensor is inserted into a blood vessel, blood proteins adhere to its surface, facilitating cell attachment and leading to clot formation. To improve the body’s response and reduce cell adhesion, strategies to prevent or lessen protein buildup have been suggested [128]. In addition, occasionally, fluorescence detection must penetrate cells, enable real-time monitoring of analytes, and accurately target specific organelles for detection [129]. The most common method to improve the biocompatibility of fluorescent probes uses the coupling of fluorophores with biocompatible molecules [81]. Neutral hydrophilic polymers such as polyethylene glycol (PEG), polyethylene oxide (PEO), and polyamides are known for their ability to resist protein buildup better than other polymers. PEG is the most studied of these because it is easy to make and has low fouling properties. However, its use in long-term implantable sensors is limited by its degradation and oxidation inside the body [128].
Since H2O2 can break down into water and oxygen and its instabilities are caused by temperature, pH value, etc., it must be carefully considered when evaluating a sensor’s sensitivity and accuracy. The instability of H2O2 makes both sensitivity and accuracy challenging but manageable through fast-response sensors, real-time monitoring, proper calibration, and correction strategies. Since H2O2 can be present at very low concentrations, sensors must have a low detection limit for biological and environmental applications. In addition, dual-sensing mechanisms (e.g., ratiometric fluorescence) can help improve measurement accuracy. For instance, ratiometric fluorescence sensors have detection limits as low as 7.7 ppb to 26.9 nM, [37,38,39,40,41,42,43,44,45,46]. Moreover, sensitivity should not be compromised by interference from other reactive oxygen species (ROS) or background noise. Since H2O2 degrades over time, standard solutions must be freshly prepared and verified before use. Instead of single-time-point measurements, continuous monitoring systems provide more reliable data by tracking H2O2 dynamics in real time [43,44,45,46]. Real-time or fast response sensors could also address rapid decomposition of H2O2. The microenvironment within cells or organelles can also affect probe performance, given the low steady-state concentrations of H2O2 in living systems. Additionally, the limited penetration depth of light and the instability of probes further constrain their imaging utility. Fluorescent dye biosensors are often susceptible to photodegradation, and their sensitivity can be influenced by environmental conditions such as pH. Thus, sometimes there is a need to develop pH-compatible fluorescent sensors for detecting H2O2 in biological systems [130]. Detecting vH2O2 presents several significant challenges compared to its detection in liquid forms. One major issue is the limited availability of effective sensor materials that can not only selectively and sensitively respond to vH2O2 but also efficiently collect and accumulate it from the vapor phase, especially in open environments [131]. The solid–vapor interface is more complex to engineer than the solid–liquid interface, making it difficult for gases to be absorbed by solid substrates [132]. This complexity hampers the development of reliable sensors capable of providing accurate readings of vH2O2 concentrations in various settings.
Another challenge lies in the current focus of research, which is primarily on detecting low concentrations of H2O2 in solutions (below 10 ppm), with limited information on vapor phase detection. This is crucial in medical or pharmaceutical isolators, where monitoring low H2O2 concentrations at room temperature ensures effective sterilization. After decontamination, an aeration phase reduces residual H2O2 to below 1 ppm. Existing commercial gas detectors, while used for monitoring, are costly and inadequate for multi-dimensional mapping due to their size and single-point measurement capability. Therefore, there is a need for miniaturized, cost-effective H2O2 sensors that can cover the concentration range of 100 to 1000 ppm and enable three-dimensional mapping in pharmaceutical isolators [133,134].
Finally, humidity presents a major challenge for detecting vH2O2. The oxidizing properties of vH2O2 make it unstable in the presence of moisture, which can interfere with sensor performance. Current detection methods often have slow response times, lack selectivity, and involve bulky equipment [104,135,136]. Although some fluorescence methods have been suggested, they encounter issues like long detection times and interference from environmental factors, making real-time detection difficult. Therefore, creating a reliable and efficient sensor for vH2O2 continues to be a significant challenge that requires more research and innovation [137].
It is likely that future research will focus on developing ratiometric fluorescent NPs that are both sensitive and cost-effective. Additionally, designing NPs that function in the NIR region can help reduce autofluorescence interference in biological systems. Improving nanoparticle biocompatibility through advanced surface chemistry or new materials is also crucial for enhancing stability and reducing cytotoxicity. Persistent luminescent NPs offer potential but need refinement in size and biocompatibility to be more effective for in vivo applications.
Furthermore, it is essential to explore mechanisms beyond oxidation, such as specific binding or capturing strategies, to enhance the specificity and reaction speed of H2O2 probes. Chemical modifications to improve the solubility, cell permeability, targetability, and photostability of probes will make them more suitable for real-time and microscale monitoring of H2O2.
Regarding vH2O2 detection, electrochemical sensors for vH2O2 face challenges such as humidity interference, slow response times, and complex sampling techniques that impact sensitivity. Detecting trace levels is difficult due to the need for selective materials, and many sensors are costly and bulky, limiting their practicality in confined spaces like medical isolators. Nanostructured materials enhance selectivity and sensitivity for H2O2 by improving binding and minimizing humidity interference. Fluorescence sensors provide faster response times and lower detection limits using techniques like FRET, while their optical nature resists environmental interference. Miniaturization allows for portable applications, and combining fluorescence with other sensing methods can create effective dual-mode sensors.
Future sensors need to address issues related to high selectivity and sensitivity, low auto-oxidation, high optical stability, low cytotoxicity, good solubility, and cell permeability to be practical in various biological environments. Since redox reactions are crucial for cellular functions and health, developing probes that can monitor changes in reversible fluorescence over time will be a key focus. This will improve our understanding of cellular redox biology and the roles of H2O2 in health and disease. However, only a few sensors can reversibly monitor H2O2 inside cells [138,139].
Combining fluorescence sensing with techniques like electrochemical or photoacoustic methods can offer deeper insights and expand the range of applications. This approach could create versatile sensors that work well in different environments and sample types. Additionally, combining AI and machine learning with sensing technology holds great potential to boost performance. AI and ML algorithms can help sensors adapt, learn from data, and improve accuracy and efficiency, allowing for better pattern recognition, complex data analysis, and smarter decision-making in various situations.
By addressing these limitations and pursuing these future directions, the field can advance towards developing highly effective and reliable nanostructured fluorescence sensors for H2O2 detection. These advancements will have significant impacts on biomedical research, environmental monitoring, and a wide range of industrial applications.
8. Conclusions
In summary, advances in nanostructured fluorescence sensors for H2O2 detection highlight a range of optical methods and sensing techniques, including fluorescence quenching/activation, FRET, TBET, ratiometric sensors, and multifunctional nanostructure-based systems. Nanomaterials have greatly improved the potential of these sensors for various applications in both biomedical and non-biomedical fields. Liquid H2O2 detection is useful in the food industry, agriculture, wastewater treatment, and studying oxidative stress in diseases. However, challenges like autofluorescence interference, slow response times, photostability issues, biocompatibility, and limited water solubility continue to affect performance. Overcoming these challenges is important for developing more reliable and effective liquid-phase H2O2 sensors.
For vH2O2, fluorescence detection is still developing but shows promise for air quality monitoring, detecting respiratory illness biomarkers, and identifying peroxide-based explosives used in terrorism. Despite its potential, vH2O2 detection faces challenges such as a lack of suitable materials, difficulties with solid–vapor interfaces, interference from humidity, slow real-time detection, and bulky sensor designs. Addressing these issues is crucial for improving sensor performance. Further exploration of new nanomaterials and sensing strategies is essential to enhancing both liquid and vH2O2 detection, driving innovation and expanding applications in various industries.
Author Contributions
H.P.: Investigation, Validation, Methodology, Formal Analysis, Writing—Original Draft. R.P.: Investigation, Validation, Writing—Original Draft. P.B.: Investigation, writing part of original draft and revising the manuscript. J.F.: Methodology, writing and editing the manuscript, funding acquisition and supervision. J.Z.: Conceptualization, Methodology, Funding Acquisition Supervision, Writing—Review and Editing. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by Natural Sciences and Engineering Research Council of Canada (NSERC). P.B. and J.F. are thankful for the financial support by U.S. National Science Foundation (NSF) under grant DMR-2347030.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| AF | amino fluorescein |
| AgNCs | Ag nanoclusters |
| AI | artificial intelligence |
| AIEE | aggregation-induced emission enhancement |
| AuNCs | gold nanoclusters |
| BPEI-CQDs | branched poly(ethylenimine)-capped CQDs |
| BSA | bovine serum albumin |
| CL | chemiluminescence |
| CQDs | carbon quantum dots |
| DAT-B | 2,5-bis((((4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)benzyl)oxy)carbonyl)amino)terephthalate |
| DAT-N | diethyl 2,5-diaminoterephthalate |
| DHLA | dihydrolipoic acid |
| DHLA-AuNCs | dihydrolipoic acid-protected AuNCs |
| Fe3O4 | iron oxide |
| FRET | Förster resonance energy transfer |
| g-C3N4 | graphitic carbon nitride |
| H2O2 | hydrogen peroxide |
| HMTD | hexamethylene triperoxide diamine |
| HRP | horseradish peroxidase |
| IFE | inner filter effect |
| LOD | limit of detection |
| ML | machine learning |
| MnO2-rGO | manganese oxide on reduced graphene oxide nanosheets |
| MNPs | magnetite nanoparticles |
| MOFs | metal–organic frameworks |
| NCs | metallic nanoclusters |
| NIR | near-infrared |
| NPs | nanoparticles |
| NRs | nanorods |
| OPD | oxidized o-phenylenediamine |
| PEs | peroxide-based explosives |
| PET | photoinduced electron transfer |
| PVA | polyvinyl alcohol |
| QDs | quantum dots |
| ROS | reactive oxygen species |
| SEM | scanning electron microscopy |
| SERS | surface-enhanced Raman spectroscopy |
| SWCNTs | single-walled carbon nanotubes |
| TATP | triacetone triperoxide |
| TiO2 | titanium dioxide |
| TNT | 2,4,6-trinitrotoluene |
| UCNPs | upconversion nanoparticles |
| vH2O2 | vaporized H2O2 |
| ZnO | zinc oxide |
References
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Xu, X.; Leng, X.; He, M.; Wang, J.; Cheng, S.; Wu, H. Roles of reactive oxygen species in cell signaling pathways and immune responses to viral infections. Arch. Virol. 2017, 162, 603–610. [Google Scholar] [CrossRef] [PubMed]
- Rababa’h, A.M.; Guillory, A.N.; Mustafa, R.; Hijjawi, T. Oxidative stress and cardiac remodeling: An updated edge. Curr. Cardiol. Rev. 2018, 14, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Tian, R.; She, Z.; Cai, J.; Li, H. Role of oxidative stress in the pathogenesis of nonalcoholic fatty liver disease. Free Radic. Biol. Med. 2020, 152, 116–141. [Google Scholar] [CrossRef]
- Moßhammer, M.; Kühl, M.; Koren, K. Possibilities and Challenges for Quantitative Optical Sensing of Hydrogen Peroxide. Chemosensors 2017, 5, 28. [Google Scholar] [CrossRef]
- Duanghathaipornsuk, S.; Farrell, E.J.; Alba-Rubio, A.C.; Zelenay, P.; Kim, D.S. Detection technologies for reactive oxygen species: Fluorescence and electrochemical methods and their applications. Biosensors 2021, 11, 30. [Google Scholar] [CrossRef]
- Fang, X.; Zheng, Y.; Duan, Y.; Liu, Y.; Zhong, W. Recent advances in design of fluorescence-based assays for high-throughput screening. Anal. Chem. 2018, 91, 482–504. [Google Scholar] [CrossRef]
- Wang, W.; Li, H.; Huang, W.; Chen, C.; Xu, C.; Ruan, H.; Li, B.; Li, H. Recent development and trends in the detection of peroxide-based explosives. Talanta 2023, 264, 124763. [Google Scholar] [CrossRef]
- An, X.; Liu, Y.; Sun, Y.; Zhang, X.; Liu, Y.; Tao, Y.; Guo, L.; Jiang, X.; Gao, M. Portable multifunctional sensing platform for ratiometric H2O2 detection and photodynamic anti-bacteria using an AIE-featured electrospinning film. Chem. Eng. J. 2024, 487, 150675. [Google Scholar] [CrossRef]
- Demchenko, A.P. Nanoparticles and nanocomposites for fluorescence sensing and imaging. Methods Appl. Fluoresc. 2013, 1, 022001. [Google Scholar] [CrossRef]
- Ng, S.M.; Koneswaran, M.; Narayanaswamy, R. A review on fluorescent inorganic nanoparticles for optical sensing applications. RSC Adv. 2016, 6, 21624–21661. [Google Scholar] [CrossRef]
- Sharma, A.; Majdinasab, M.; Khan, R.; Li, Z.; Hayat, A.; Marty, J.L. Nanomaterials in fluorescence-based biosensors: Defining key roles. Nano-Struct. Nano-Objects 2021, 27, 100774. [Google Scholar] [CrossRef]
- Zhong, W. Nanomaterials in fluorescence-based biosensing. Anal. Bioanal. Chem. 2009, 394, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Malik, S.; Singh, J.; Goyat, R.; Saharan, Y.; Chaudhry, V.; Umar, A.; Ibrahim, A.A.; Akbar, S.; Ameen, S.; Baskoutas, S. Nanomaterials-based biosensor and their applications: A review. Heliyon 2023, 9, e19929. [Google Scholar] [CrossRef]
- Schubert, F.; Wang, F.; Rinneberg, H. Fibre optic fluorometric enzyme sensors for hydrogen peroxide and lactate, based on horseradish peroxidase and lactate oxidase. Microchim. Acta 1995, 121, 237–247. [Google Scholar] [CrossRef]
- Liang, A.-H.; Zhou, S.-M.; Jiang, Z.-L. A simple and sensitive resonance scattering spectral method for determination of hydroxyl radical in Fenton system using rhodamine S and its application to screening the antioxidant. Talanta 2006, 70, 444–448. [Google Scholar] [CrossRef]
- Qian, Y.-Y.; Xue, L.; Hu, D.-X.; Li, G.-P.; Jiang, H. Quinoline-based fluorescent probe for ratiometric detection of hydrogen peroxide in aqueous solution. Dye. Pigment. 2012, 95, 373–376. [Google Scholar] [CrossRef]
- Gong, C.; Chen, J.; Shen, Y.; Song, Y.; Song, Y.; Wang, L. Microperoxidase-11/metal–organic framework/macroporous carbon for detecting hydrogen peroxide. RSC Adv. 2016, 6, 79798–79804. [Google Scholar]
- Bandi, R.; Dadigala, R.; Gangapuram, B.R.; Guttena, V. Green synthesis of highly fluorescent nitrogen-doped carbon dots from Lantana camara berries for effective detection of lead (II) and bioimaging. J. Photochem. Photobiol. B Biol. 2018, 178, 330–338. [Google Scholar] [CrossRef]
- O’Banion, C.P.; Yasuda, R. Fluorescent sensors for neuronal signaling. Curr. Opin. Neurobiol. 2020, 63, 31–41. [Google Scholar] [CrossRef]
- Fang, B.; Shen, Y.; Peng, B.; Bai, H.; Wang, L.; Zhang, J.; Hu, W.; Fu, L.; Zhang, W.; Li, L. Small-Molecule Quenchers for Förster Resonance Energy Transfer: Structure, Mechanism, and Applications. Angew. Chem. 2022, 134, e202207188. [Google Scholar] [CrossRef]
- Cao, D.; Zhu, L.; Liu, Z.; Lin, W. Through bond energy transfer (TBET)-based fluorescent chemosensors. J. Photochem. Photobiol. C Photochem. Rev. 2020, 44, 100371. [Google Scholar] [CrossRef]
- Battisti, A.; Samal, S.K.; Puppi, D. Biosensing systems based on graphene oxide fluorescence quenching effect. Micromachines 2023, 14, 1522. [Google Scholar] [CrossRef]
- Genovese, D.; Cingolani, M.; Rampazzo, E.; Prodi, L.; Zaccheroni, N. Static quenching upon adduct formation: A treatment without shortcuts and approximations. Chem. Soc. Rev. 2021, 50, 8414–8427. [Google Scholar] [CrossRef] [PubMed]
- Molaei, M.J. Principles, mechanisms, and application of carbon quantum dots in sensors: A review. Anal. Methods 2020, 12, 1266–1287. [Google Scholar]
- Ashley, J.; Manikova, P. Fluorescent sensors. Fundam. Sens. Technol. 2023, 2023, 147–161. [Google Scholar] [CrossRef]
- Sargazi, S.; Fatima, I.; Kiani, M.H.; Mohammadzadeh, V.; Arshad, R.; Bilal, M.; Rahdar, A.; Díez-Pascual, A.M.; Behzadmehr, R. Fluorescent-based nanosensors for selective detection of a wide range of biological macromolecules: A comprehensive review. Int. J. Biol. Macromol. 2022, 206, 115–147. [Google Scholar] [CrossRef]
- Karmakar, A.; Samanta, P.; Dutta, S.; Ghosh, S.K. Fluorescent “turn-on” sensing based on metal–organic frameworks (MOFs). Chem.–Asian J. 2019, 14, 4506–4519. [Google Scholar] [CrossRef]
- Germain, M.E.; Knapp, M.J. Optical explosives detection: From color changes to fluorescence turn-on. Chem. Soc. Rev. 2009, 38, 2543–2555. [Google Scholar] [CrossRef]
- Olenin, A.Y.; Yagov, V. Using the Turn-On Fluorescence Effect in Chemical and Biochemical Analysis. J. Anal. Chem. 2022, 77, 1082–1110. [Google Scholar] [CrossRef]
- Li, P.; Li, S.F. Recent advances in fluorescence probes based on carbon dots for sensing and speciation of heavy metals. Nanophotonics 2020, 10, 877–908. [Google Scholar] [CrossRef]
- Yang, W.C.; Li, S.Y.; Ni, S.; Liu, G. Advances in FRET-based biosensors from donor-acceptor design to applications. Aggregate 2024, 5, 460. [Google Scholar] [CrossRef]
- Zuo, Y.; Gou, Z.; Lan, Y.; Yan, M. Design strategies of logic gate sensors based on FRET mechanism. TrAC Trends Anal. Chem. 2023, 167, 117271. [Google Scholar] [CrossRef]
- Verma, A.K.; Noumani, A.; Yadav, A.K.; Solanki, P.R. FRET Based Biosensor: Principle Applications Recent Advances and Challenges. Diagnostics 2023, 13, 1375. [Google Scholar] [CrossRef]
- Fang, C.; Huang, Y.; Zhao, Y. Review of FRET biosensing and its application in biomolecular detection. Am. J. Transl. Res. 2023, 15, 694. [Google Scholar]
- Bhupathi, P.; Elhassan A-Elgadir, T.M.; Mohammed Ali, R.H.; Sanaan Jabbar, H.; Gulnoza, D.; Joshi, S.; Kadhem Abid, M.; Ahmed Said, E.; Alawadi, A.; Alsaalamy, A. Fluorescence Resonance Energy Transfer (FRET)-Based Sensor for Detection of Foodborne Pathogenic Bacteria: A Review. Crit. Rev. Anal. Chem. 2023, 55, 233–250. [Google Scholar] [CrossRef]
- Shen, S.-L.; Ning, J.-Y.; Zhang, X.-F.; Miao, J.-Y.; Zhao, B.-X. Through-bond energy transfer-based ratiometric fluorescent probe for the imaging of HOCl in living cells. Sens. Actuators B Chem. 2017, 244, 907–913. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, W.; Cai, Y.; Kwok, R.T.; Hu, Y.; Lam, J.W.; Gu, X.; He, Z.; Zhao, Z.; Zheng, X. AIEgens for dark through-bond energy transfer: Design, synthesis, theoretical study and application in ratiometric Hg2+ sensing. Chem. Sci. 2017, 8, 2047–2055. [Google Scholar] [CrossRef]
- Guo, Y.; Lu, G.; Zhuo, J.; Wang, J.; Li, X.; Zhang, Z. A visible-near-infrared fluorescent probe for peroxynitrite with large pseudo-Stokes and emission shift via through-bond energy and charge transfers controlled by energy matching. J. Mater. Chem. B 2018, 6, 2489–2496. [Google Scholar] [CrossRef]
- Liu, L.; Ga, L.; Ai, J. Ratiometric fluorescence sensing with logical operation: Theory, design and applications. Biosens. Bioelectron. 2022, 213, 114456. [Google Scholar] [CrossRef]
- Wen, Y.; Sun, D.; Zhang, Y.; Zhang, Z.; Chen, L.; Li, J. Molecular imprinting-based ratiometric fluorescence sensors for environmental and food analysis. Analyst 2023, 148, 3971–3985. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Min, H.; Shi, W.; Cheng, P. Multicenter metal–organic framework-based ratiometric fluorescent sensors. Adv. Mater. 2020, 32, 1805871. [Google Scholar] [CrossRef]
- Han, Y.; Yang, W.; Luo, X.; He, X.; Zhao, H.; Tang, W.; Yue, T.; Li, Z. Carbon dots based ratiometric fluorescent sensing platform for food safety. Crit. Rev. Food Sci. Nutr. 2022, 62, 244–260. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Hou, D.; Wang, Z.; Cai, N.; Au, C. Nanomaterial-based dual-emission ratiometric fluorescent sensors for biosensing and cell imaging. Polymers 2021, 13, 2540. [Google Scholar] [CrossRef]
- Liang, X.; Xu, X.; Qiao, D.; Yin, Z.; Shang, L. Dual Mechanism of an Intramolecular Charge Transfer (ICT)–FRET-Based Fluorescent Probe for the Selective Detection of Hydrogen Peroxide. Chem. Asian J. 2017, 12, 3187–3194. [Google Scholar] [CrossRef]
- Duan, N.; Yang, S. Research progress on multifunctional fluorescent probes for biological imaging, food and environmental detection. Crit. Rev. Anal. Chem. 2024, 54, 775–817. [Google Scholar] [CrossRef]
- Jiang, X.; Yang, R.; Lei, X.; Xue, S.; Wang, Z.; Zhang, J.; Yan, L.; Xu, Z.; Chen, Z.; Zou, P. Design, synthesis, application and research progress of fluorescent probes. J. Fluoresc. 2024, 34, 965–975. [Google Scholar] [CrossRef]
- Ashafaq, M.; Khalid, M.; Raizada, M.; Ahmad, M.S.; Khan, M.S.; Shahid, M.; Ahmad, M. A Zn-based fluorescent coordination polymer as bifunctional sensor: Sensitive and selective aqueous-phase detection of picric acid and heavy metal ion. J. Inorg. Organomet. Polym. Mater. 2020, 30, 4496–4509. [Google Scholar] [CrossRef]
- Zhang, Y.; Tang, H.; Chen, W.; Zhang, J. Nanomaterials used in fluorescence polarization based biosensors. Int. J. Mol. Sci. 2022, 23, 8625. [Google Scholar] [CrossRef]
- Wolfbeis, O.S. An overview of nanoparticles commonly used in fluorescent bioimaging. Chem. Soc. Rev. 2015, 44, 4743–4768. [Google Scholar] [CrossRef]
- Chen, M.; Liang, Z.; Fan, X.; Qu, R.; Wang, H.; Chen, T. A ratiometric ESIPT fluorescent probe for detection of anticancer-associated H2O2 level in vitro and in vivo. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2022, 276, 121163. [Google Scholar] [CrossRef]
- Chen, X.; He, D.; Shentu, J.; Yang, S.; Yang, Y.; Wang, Y.; Zhang, R.; Wang, K.; Qian, J.; Long, L. Smartphone-assisted colorimetric and near-infrared ratiometric fluorescent sensor for on-spot detection of H2O2 in food samples. Chem. Eng. J. 2023, 472, 144900. [Google Scholar] [CrossRef]
- Chawre, Y.; Satnami, M.L.; Kujur, A.B.; Ghosh, K.K.; Nagwanshi, R.; Karbhal, I.; Pervez, S.; Deb, M.K. Forster Resonance Energy Transfer between Multicolor Emissive N-Doped Carbon Quantum Dots and Gold Nanorods for the Detection of H2O2, Glucose, Glutathione, and Acetylcholinesterase. ACS Appl. Nano Mater. 2023, 6, 8046–8058. [Google Scholar] [CrossRef]
- Liu, F.-T.; Wang, S.; Wang, Y.-P.; Jiang, P.-F.; Miao, J.-Y.; Zhao, B.-X.; Lin, Z.-M. A near-infrared fluorescent probe based FRET for ratiometric sensing of H2O2 and viscosity in live cells. Talanta 2024, 275, 126135. [Google Scholar] [CrossRef] [PubMed]
- Velusamy, N.; Thirumalaivasan, N.; Bobba, K.N.; Podder, A.; Wu, S.P.; Bhuniya, S. FRET-based dual channel fluorescent probe for detecting endogenous/exogenous H2O2/H2S formation through multicolor images. J. Photochem. Photobiol. B Biol. 2019, 191, 99–106. [Google Scholar] [CrossRef]
- Wan, P.; Fu, H.; Zhang, Y.; Liao, C.; Lu, Q.; Xu, H.; Mei, Q. Engineering a polymer-encapsulated manganese dioxide/upconversion nanoprobe for FRET-based hydrogen peroxide detection. Anal. Bioanal. Chem. 2023, 415, 4333–4341. [Google Scholar] [CrossRef]
- Wang, H.; Li, Y.; Yang, M.; Wang, P.; Gu, Y. FRET-based upconversion nanoprobe sensitized by Nd3+ for the ratiometric detection of hydrogen peroxide in vivo. ACS Appl. Mater. Interfaces. 2019, 11, 7441–7449. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhou, C.; Yang, Q.; Chu, Z.; Jia, N. A FRET-based fluorescent probe for hydrogen peroxide based on the use of carbon quantum dots conjugated to gold nanoclusters. Microchim. Acta. 2019, 186, 294. [Google Scholar] [CrossRef]
- Li, Y.; Gu, X.; Zhao, J.; Xi, F. Fabrication of a ratiometric fluorescence sensor based on carbon dots as both luminophores and nanozymes for the sensitive detection of hydrogen peroxide. Molecules 2022, 27, 7379. [Google Scholar] [CrossRef]
- Li, Z.; Xiao, L.; Sun, X.; Luo, C.; Li, R.; Zhang, W.; Wang, Z.; Xiao, H.; Shu, W. An ESIPT-based ratiometric fluorescent probe for detecting H2O2 in water environment and biosystems. Sci. Total Environ. 2023, 867, 161609. [Google Scholar] [CrossRef]
- Wang, C.; Wang, Y.; Wang, G.; Huang, C.; Jia, N. A new mitochondria-targeting fluorescent probe for ratiometric detection of H2O2 in live cells. Anal. Chim. Acta 2020, 1097, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Burmistrova, N.A.; Kolontaeva, O.A.; Duerkop, A. New nanomaterials and luminescent optical sensors for detection of hydrogen peroxide. Chemosensors 2015, 3, 253–273. [Google Scholar] [CrossRef]
- Cao, J.; Jiang, H.; Wu, Y.; Yu, X. Visual detection of H2O2 and glucose by HBcAb-HRP fluorescence-enhanced CdTe QDs/CDs ratiometric fluorescence sensing platform. Colloids Surf. B Biointerfaces 2024, 235, 113774. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Yang, L.; Huang, L.; Liao, Y.; Liu, Y.; Xiao, Q. A novel fluorescent probe for H2O2detection based on CdSe@ ZnS quantum dots/Ag nanocluster hybrid. Anal. Chim. Acta. 2020, 1106, 176–182. [Google Scholar] [CrossRef]
- Dai, L.; Chang, D.W.; Baek, J.B.; Lu, W. Carbon nanomaterials for advanced energy conversion and storage. Small 2012, 8, 1130–1166. [Google Scholar] [CrossRef]
- Ledesma, F.; Nishitani, S.; Cunningham, F.J.; Hubbard, J.D.; Yim, D.; Lui, A.; Chio, L.; Murali, A.; Landry, M.P. Covalent Attachment of Horseradish Peroxidase to Single-Walled Carbon Nanotubes for Hydrogen Peroxide Detection. Adv. Funct. Mater. 2024, 34, 2316028. [Google Scholar] [CrossRef]
- Mu, H.; Zhang, Y.; Zheng, P.; Zhang, M. Ultrafast fluorescence probe to H2O2 vapor based on organic-inorganic hybrid silica nanoparticles. Talanta 2022, 237, 122914. [Google Scholar] [CrossRef]
- Liu, J.-W.; Luo, Y.; Wang, Y.-M.; Duan, L.-Y.; Jiang, J.-H.; Yu, R.-Q. Graphitic carbon nitride nanosheets-based ratiometric fluorescent probe for highly sensitive detection of H2O2 and glucose. ACS Appl. Mater. Interfaces. 2016, 8, 33439–33445. [Google Scholar] [CrossRef]
- Falcaro, P.; Ricco, R.; Yazdi, A.; Imaz, I.; Furukawa, S.; Maspoch, D.; Ameloot, R.; Evans, J.D.; Doonan, C.J. Application of metal and metal oxide nanoparticles@ MOFs. Coord. Chem. Rev. 2016, 307, 237–254. [Google Scholar] [CrossRef]
- Zheng, J.; Li, X.; Li, Y.; Zhang, S. Engineered Fe3O4 nanoreactor as a high-performance hydrogen peroxide sensor. Sens. Actuators B Chem. 2024, 402, 135116. [Google Scholar] [CrossRef]
- Quyen, T.T.B.; My, N.N.T.; Pham, D.T.; Thien, D.V.H. Synthesis of TiO2 nanosheets/graphene quantum dots and its application for detection of hydrogen peroxide by photoluminescence spectroscopy. Talanta Open 2022, 5, 100103. [Google Scholar] [CrossRef]
- Chen, L.; Gao, Y.; Fu, Y.; Zhu, D.; He, Q.; Cao, H.; Cheng, J. Borate ester endcapped fluorescent hyperbranched conjugated polymer for trace peroxide explosive vapor detection. RSC Adv. 2015, 5, 29624–29630. [Google Scholar] [CrossRef]
- Mody, V.V.; Siwale, R.; Singh, A.; Mody, H.R. Introduction to metallic nanoparticles. J. Pharm. Bioallied Sci. 2010, 2, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Tu, L.; Zhao, S.; Liu, G.; Wang, Y.; Wang, Y.; Yue, Z. Fluorescent gold nanoclusters based photoelectrochemical sensors for detection of H2O2 and glucose. Biosens. Bioelectron. 2015, 67, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.-J.; Song, J.-A.; Choi, J.-H.; Shin, J.-H.; Myeong, J.-W.; Lee, K.-P.; Kim, T.; Park, K.-E.; Oh, B.-K. Horseradish peroxidase-encapsulated fluorescent bio-nanoparticle for ultra-sensitive and easy detection of hydrogen peroxide. Biosensors 2023, 13, 289. [Google Scholar] [CrossRef]
- Li, Y.; Chen, M.; Lu, H.; Xu, S. Smartphone-based ratiometric fluorescence sensor for sensitive visual detection of H2O2 and glucose based on B-CDs-Ag NPRs-OPD ternary system. J. Lumin. 2024, 275, 120755. [Google Scholar] [CrossRef]
- Mi, W.; Tang, S.; Jin, Y.; Shao, N. Au/Ag bimetallic nanoclusters stabilized by glutathione and lysozyme for ratiometric sensing of H2O2 and hydroxyl radicals. ACS Appl. Nano Mater. 2021, 4, 1586–1595. [Google Scholar] [CrossRef]
- Tran, V.-K.; Gupta, P.K.; Park, Y.; Son, S.E.; Hur, W.; Lee, H.B.; Park, J.Y.; Kim, S.N.; Seong, G.H. Functionalized bimetallic IrPt alloy nanoparticles: Multi-enzyme mimics for colorimetric and fluorometric detection of hydrogen peroxide and glucose. J. Taiwan Inst. Chem. Eng. 2021, 120, 336–343. [Google Scholar] [CrossRef]
- Ding, Y.; Yang, B.; Liu, H.; Liu, Z.; Zhang, X.; Zheng, X.; Liu, Q. FePt-Au ternary metallic nanoparticles with the enhanced peroxidase-like activity for ultrafast colorimetric detection of H2O2. Sens. Actuators B Chem. 2018, 259, 775–783. [Google Scholar] [CrossRef]
- Lu, X.-Y.; Wu, D.-C.; Li, Z.-J.; Chen, G.-Q. Polymer nanoparticles. Prog. Mol. Biol. Transl. Sci. 2011, 104, 299–323. [Google Scholar] [CrossRef]
- Sun, H.; Xu, Q.; Ren, M.; Kong, F. A biocompatible chitosan-based fluorescent polymer for efficient H2O2 detection in living cells and water samples. Int. J. Biol. Macromol. 2024, 257, 128760. [Google Scholar] [CrossRef]
- Guo, J.; Liu, Y.; Mu, Z.; Wu, S.; Wang, J.; Yang, Y.; Zhao, M.; Wang, Y. Label-free fluorescence detection of hydrogen peroxide and glucose based on the Ni-MOF nanozyme–induced self-ligand emission. Microchim. Acta 2022, 189, 219. [Google Scholar] [CrossRef]
- Nazari, A.; Raeesi, M.; Salehi-Mobarakeh, H.; Mahdavian, A.R. Ready-to-use optical H2O2 sensor based on stimuli-responsive polyacrylic film and nanofibers containing spiropyran. Dye. Pigment. 2022, 204, 110399. [Google Scholar] [CrossRef]
- Xing, L.; Zhang, W.; Fu, L.; Lorenzo, J.M.; Hao, Y. Fabrication and application of electrochemical sensor for analyzing hydrogen peroxide in food system and biological samples. Food Chem. 2022, 385, 132555. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Wang, Y.; Xu, B.; Zhang, W.; Xie, Y.; Chen, Y.; Wang, L.; Wang, X. A colorimetric and fluorescence dual-signal determination for iron (II) and H2O2 in food based on sulfur quantum dots. Food Chem. 2022, 366, 130613. [Google Scholar] [CrossRef]
- Johnson, M.S.; Sajeev, S.; Nair, R.S. Role of Nanosensors in agriculture. In Proceedings of the 2021 International Conference on Computational Intelligence and Knowledge Economy (ICCIKE), Dubai, United Arab Emirates, 17–18 March 2021; pp. 58–63. [Google Scholar]
- Wu, H.; Nißler, R.; Morris, V.; Herrmann, N.; Hu, P.; Jeon, S.-J.; Kruss, S.; Giraldo, J.P. Monitoring plant health with near-infrared fluorescent H2O2 nanosensors. Nano Lett. 2020, 20, 2432–2442. [Google Scholar] [CrossRef]
- Ali, H.R.H.; Hassan, A.I.; Hassan, Y.F.; El-Wekil, M.M. One pot fabrication of fluorescein functionalized manganese dioxide for fluorescence “Turn OFF–ON” sensing of hydrogen peroxide in water and cosmetic samples. RSC Adv. 2020, 10, 17506–17514. [Google Scholar] [CrossRef]
- Kyomuhimbo, H.D.; Feleni, U.; Haneklaus, N.H.; Brink, H. Recent Advances in Applications of Oxidases and Peroxidases Polymer-Based Enzyme Biocatalysts in Sensing and Wastewater Treatment: A Review. Polymers 2023, 15, 3492. [Google Scholar] [CrossRef]
- Duong, H.D.; Rhee, J.I. Development of ratiometric fluorescence sensors based on CdSe/ZnS quantum dots for the detection of hydrogen peroxide. Sensors 2019, 19, 4977. [Google Scholar] [CrossRef]
- Kailasa, S.K.; Vajubhai, G.N.; Koduru, J.R.; Park, T.J. Recent progress of nanomaterials for colorimetric and fluorescence sensing of reactive oxygen species in biological and environmental samples. Trends Environ. Anal. Chem. 2023, 37, e00196. [Google Scholar] [CrossRef]
- Jain, V.; Bhagat, S.; Singh, S. Bovine serum albumin decorated gold nanoclusters: A fluorescence-based nanoprobe for detection of intracellular hydrogen peroxide. Sens. Actuators B Chem. 2021, 327, 128886. [Google Scholar] [CrossRef]
- Yan, K.C.; Sedgwick, A.C.; Zang, Y.; Chen, G.R.; He, X.P.; Li, J.; Yoon, J.; James, T.D. Sensors, Imaging Agents, and Theranostics to Help Understand and Treat Reactive Oxygen Species Related Diseases. Small Methods 2019, 3, 190003. [Google Scholar] [CrossRef]
- Sobhanan, J.; Rival, J.V.; Anas, A.; Shibu, E.S.; Takano, Y.; Biju, V. Luminescent Quantum Dots: Synthesis, Optical Properties, Bioimaging and Toxicity. Adv. Drug Delivery Rev. 2023, 197, 114830. [Google Scholar] [CrossRef]
- Li, Z.; Guo, S.; Yuan, Z.; Lu, C. Carbon quantum dot-gold nanocluster nanosatellite for ratiometric fluorescence probe and imaging for hydrogen peroxide in living cells. Sens. Actuators B Chem. 2017, 241, 821–827. [Google Scholar] [CrossRef]
- Geng, Y.; Wang, Z.; Zhou, J.; Zhu, M.; Liu, J.; James, T.D. Recent progress in the development of fluorescent probes for imaging pathological oxidative stress. Chem. Soc. Rev. 2023, 52, 3873–3926. [Google Scholar] [CrossRef]
- Hu, X.; Liu, J.; Jin, H.; Huang, F.; Wang, Z.; Wang, F.; Dai, Z. Ultrasensitive determination of intracellular hydrogen peroxide by equipping quantum dots with a sensing layer via self-passivation. Nano Res. 2022, 15, 4350–4356. [Google Scholar] [CrossRef]
- Chen, B.; Wang, F. Emerging frontiers of upconversion nanoparticles. Trends Chem. 2020, 2, 427–439. [Google Scholar] [CrossRef]
- Wu, S.; Butt, H.J. Near-infrared-sensitive materials based on upconverting nanoparticles. Adv. Mater. 2016, 28, 1208–1226. [Google Scholar] [CrossRef]
- Lu, C.; Joulin, E.; Tang, H.; Pouri, H.; Zhang, J. Upconversion nanostructures applied in theranostic systems. Int. J. Mol. Sci. 2022, 23, 9003. [Google Scholar] [CrossRef]
- Wang, F.; Qu, X.; Liu, D.; Ding, C.; Zhang, C.; Xian, Y. Upconversion nanoparticles-MoS2 nanoassembly as a fluorescent turn-on probe for bioimaging of reactive oxygen species in living cells and zebrafish. Sens. Actuators B Chem. 2018, 274, 180–187. [Google Scholar] [CrossRef]
- Alnadari, F.; Xue, Y.; Almakas, A.; Mohedein, A.; Samie, A.; Abdel-Shafi, M.; Abdin, M. Large batch production of galactooligosaccharides using β-glucosidase immobilized on chitosan-functionalized magnetic nanoparticle. J. Food Biochem. 2021, 45, e13589. [Google Scholar] [CrossRef] [PubMed]
- Star, B.G.; Shahlaei, M.; Karami, C. A novel fluorescent turn-on probe for hydrogen peroxide based on carbon dots. J. Mater.Sci. Mater. Electron. 2021, 32, 5615–5623. [Google Scholar]
- Maier, D.; Laubender, E.; Basavanna, A.; Schumann, S.; Guder, F.; Urban, G.A.; Dincer, C. Toward continuous monitoring of breath biochemistry: A paper-based wearable sensor for real-time hydrogen peroxide measurement in simulated breath. ACS Sens. 2019, 4, 2945–2951. [Google Scholar] [CrossRef] [PubMed]
- Quimbar, M.E.; Davis, S.Q.; Al-Farra, S.T.; Hayes, A.; Jovic, V.; Masuda, M.; Lippert, A.R. Chemiluminescent measurement of hydrogen peroxide in the exhaled breath condensate of healthy and asthmatic adults. Anal. Chem. 2020, 92, 14594–14600. [Google Scholar] [CrossRef]
- Li, H.; Wu, Y.; Xu, Z.; Wang, Y. In situ anchoring Cu nanoclusters on Cu-MOF: A new strategy for a combination of catalysis and fluorescence toward the detection of H2O2 and 2, 4-DNP. Chem. Eng. J. 2024, 479, 147508. [Google Scholar] [CrossRef]
- Ambard, C.; Duée, N.; Pereira, F.; Portehault, D.; Méthivier, C.; Pradier, C.-M.; Sanchez, C. Improvements in photostability and sensing properties of EuVO4 nanoparticles by microwave-assisted sol–gel route for detection of H2O2 vapors. J. Sol-Gel Sci. Technol. 2016, 79, 381–388. [Google Scholar] [CrossRef]
- Caron, T.; Palmas, P.; Frénois, C.; Méthivier, C.; Pasquinet, E.; Pradier, C.-M.; Serein-Spirau, F.; Hairault, L.; Montméat, P. Detection of hydrogen peroxide using dioxazaborocanes: Elucidation of the sensing mechanism at the molecular level by NMR and XPS measurements. New J. Chem. 2020, 44, 4114–4121. [Google Scholar] [CrossRef]
- Chen, Q.; Yang, L.; Guo, K.; Yang, J.; Han, J.M. Expedite Fluorescent Sensor Prototype for Hydrogen Peroxide Detection with Long-Life Test Substrates. ACS Omega 2021, 6, 11447–11457. [Google Scholar] [CrossRef]
- Garreffi, B.P.; Guo, M.; Tokranova, N.; Cady, N.C.; Castracane, J.; Levitsky, I.A. Highly sensitive and selective fluorescence sensor based on nanoporous silicon-quinoline composite for trace detection of hydrogen peroxide vapors. Sens. Actuators B Chem. 2018, 276, 466–471. [Google Scholar] [CrossRef]
- Hui-Yu, C.; Zhen-Zhen, C.; Yu-Shu, L.; Guang-Fa, W.; Xin-Cun, D. Colorimetric-fluorescent dual-mode sensing of peroxide explosives based on inner filter effect with boosted sensitivity and selectivity. Chin. J. Anal. Chem. 2022, 50, 4–12. [Google Scholar] [CrossRef]
- Zheng, P.; Abdurahman, A.; Zhang, Z.; Feng, Y.; Zhang, Y.; Ai, X.; Li, F.; Zhang, M. A simple organic multi-analyte fluorescent prober: One molecule realizes the detection to DNT, TATP and Sarin substitute gas. J. Hazard. Mater. 2021, 409, 124500. [Google Scholar] [CrossRef]
- Fu, Y.; Yao, J.; Xu, W.; Fan, T.; Jiao, Z.; He, Q.; Zhu, D.; Cao, H.; Cheng, J. Schiff Base Substituent-Triggered Efficient Deboration Reaction and Its Application in Highly Sensitive Hydrogen Peroxide Vapor Detection. Anal. Chem. 2016, 88, 5507–5512. [Google Scholar] [CrossRef]
- Larsson, K.; Aldén, M.; Bood, J. Simultaneous visualization of hydrogen peroxide and water concentrations using photofragmentation laser-induced fluorescence. Appl. Spectrosc. 2017, 71, 2118–2127. [Google Scholar] [CrossRef]
- Matsumoto, A.; Nishiyabu, R.; Kubo, Y. Synthesis of a borylated boron–dibenzopyrromethene dye enabling the visual detection of H2O2 vapor. RSC Adv. 2014, 4, 37973–37978. [Google Scholar] [CrossRef]
- Sakakibara, K.; Takahashi, Y.; Nishiyabu, R.; Kubo, Y. A Zn2+-coordinated boronate dipyrrin as a chemodosimeter toward hydrogen peroxide. J. Mater. Chem. 2017, 5, 3684–3691. [Google Scholar] [CrossRef]
- Sanchez, J.C.; Trogler, W.C. Polymerization of a boronate-functionalized fluorophore by double transesterification: Applications to fluorescence detection of hydrogen peroxide vapor. J. Mater. Chem. 2008, 18, 5134–5141. [Google Scholar] [CrossRef]
- Xu, M.; Han, J.M.; Zhang, Y.; Yang, X.; Zang, L. A selective fluorescence turn-on sensor for trace vapor detection of hydrogen peroxide. Chem. Commun. 2013, 49, 11779–11781. [Google Scholar] [CrossRef]
- Yuan, M.; Xu, W.; Zhang, W.; Zhao, J.; Li, H.; He, Q.; Huang, W.; Cheng, J.; Fu, Y. Efficient fluorescent vapour sensing induced by ZnO buffer. Dyes Pigm. 2023, 218, 111420. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, L.; Han, J.-M. Catalyst-integrated dual-fluorescent sensor array for highly efficient detection of triacetone triperoxide via simplified quadrantal pattern recognition. Sens. Actuators B Chem. 2023, 385, 133680. [Google Scholar] [CrossRef]
- Zhu, D.; Hua, K.; He, Q.; Cheng, J.; Cao, H. Quantum dots/polymer composite system for turn-on fluorescent detection of peroxide hydrogen. In Proceedings of the SENSORS, 2013 IEEE, Baltimore, MD, USA, 3–6 November 2013; pp. 1–4. [Google Scholar]
- Wu, Y.; Huang, H.; Jing, F.; Wang, Y.; Chen, S.; Wang, L.; Li, Y.; Hou, S. A fluorescent probe based on the ESIPT (excited state intramolecular proton transfer) mechanism for rapid detection of endogenous and exogenous H2O2 (hydrogen peroxide) in cells. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2024, 304, 123394. [Google Scholar] [CrossRef]
- Bai, W.; Zhang, K.; Yu, S.; Zhang, J.; Jin, L. The preparation of MnO2/BSA/CdTe quantum dots complex for ratiometric fluorescence/T1-weighted MRI detection of H2O2. Talanta 2023, 252, 123774. [Google Scholar] [CrossRef]
- Zhu, X.; Chen, X.; Liu, H.; Sun, B. Amino-Acid-Encoded Supramolecular Self-Assembly Architectures: Near-Infrared Fluorescence–Photothermal Temperature Dual-Signal Sensing of Hydrogen Peroxide. ACS Sustain. Chem. Eng. 2024, 12, 4803–4812. [Google Scholar] [CrossRef]
- Wang, L.; Shi, J.; Wang, P.; Rong, R. High-sensitive detection of H2O2 in biological systems by persistent luminescent nanoprobes. Chem. Eng. J. 2024, 486, 150291. [Google Scholar] [CrossRef]
- Saygili, E.; Ersoz-Gulseven, E.; Kıbrıs, E.; Cakan-Akdogan, G.; Ucuncu, M. A novel 2-aminophenalenone-based fluorescent probe designed for monitoring H2O2 for in vitro and in vivo bioimaging. Talanta 2024, 271, 125669. [Google Scholar] [CrossRef]
- Sawayama, J.; Takeuchi, S. Long-term continuous glucose monitoring using a fluorescence-based biocompatible hydrogel glucose sensor. Adv. Healthc. Mater. 2021, 10, 2001286. [Google Scholar] [CrossRef]
- Soto, R.J.; Hall, J.R.; Brown, M.D.; Taylor, J.B.; Schoenfisch, M.H. In vivo chemical sensors: Role of biocompatibility on performance and utility. Anal. Chem. 2017, 89, 276–299. [Google Scholar] [CrossRef]
- Yan, Q.; Yao, X.; Li, Y.; Zhong, K.; Tang, L.; Yan, X. A red fluorescence probe for reversible detection of HSO3−/H2O2 and its application in food samples and bioimaging. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2023, 299, 122882. [Google Scholar] [CrossRef]
- Du, W.; Shen, Z.; Liang, Y.; Gong, S.; Meng, Z.; Li, M.; Wang, Z.; Wang, S. A highly effective “naked eye” colorimetric and fluorimetric curcumin-based fluorescent sensor for specific and sensitive detection of H2O2 in vivo and in vitro. Analyst 2023, 148, 1824–1837. [Google Scholar] [CrossRef]
- Cao, Y.; Shi, H.; Zheng, Y.; Tan, Z.; Xie, Z.; Zhang, C.; Chen, Z. Polyaniline/Prussian blue nanolayer enhanced electrochemical sensing of H2O2 in EBC using an integrated condensation facemask. Sens. Actuators B Chem. 2023, 393, 134189. [Google Scholar] [CrossRef]
- Xu, M.; Bunes, B.R.; Zang, L. Paper-Based Vapor Detection of Hydrogen Peroxide: Colorimetric Sensing with Tunable Interface. ACS Appl. Mater. Interfaces 2011, 3, 642–647. [Google Scholar] [CrossRef]
- Vahidpour, F.; Alghazali, Y.; Akca, S.; Hommes, G.; Schöning, M.J. An enzyme-based Interdigitated electrode-type biosensor for detecting low concentrations of H2O2 vapor/aerosol. Chemosensors 2022, 10, 202. [Google Scholar] [CrossRef]
- Riaz, M.A.; Chen, Y. Electrodes and electrocatalysts for electrochemical hydrogen peroxide sensors: A review of design strategies. Nanoscale Horiz. 2022, 7, 463–479. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Gao, N.; Zhu, L.; Hunter, M.; Chen, S.; Zang, L. PEDOT: PSS/PEDOT film chemiresistive sensors for hydrogen peroxide vapor detection under ambient conditions. Chemosensors 2023, 11, 124. [Google Scholar] [CrossRef]
- Lee, J.-S.; Jeong, D.-W.; Byun, Y.T. Porphyrin nanofiber/single-walled carbon nanotube nanocomposite-based sensors for monitoring hydrogen peroxide vapor. Sens. Actuators B Chem. 2020, 306, 127518. [Google Scholar] [CrossRef]
- Xie, X.; Gao, N.; Hunter, M.; Zhu, L.; Yang, X.; Chen, S.; Zang, L. PEDOT Films Doped with Titanyl Oxalate as Chemiresistive and Colorimetric Dual-Mode Sensors for the Detection of Hydrogen Peroxide Vapor. Sensors 2023, 23, 3120. [Google Scholar] [CrossRef]
- Tian, L.; Sun, X.; Zhou, L.; Zhong, K.; Li, S.; Yan, X.; Tang, L. Reversible colorimetric and NIR fluorescent probe for sensing SO2/H2O2 in living cells and food samples. Food Chem. 2023, 407, 135031. [Google Scholar] [CrossRef]
- Li, Y.; Huang, Y.; Sun, X.; Zhong, K.; Tang, L. An AIE mechanism-based fluorescent probe for relay recognition of HSO3−/H2O2 and its application in food detection and bioimaging. Talanta 2023, 258, 124412. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).