Abstract
The gut microbiota, a dynamic and metabolically active microbial ecosystem, plays a pivotal role in regulating host digestion, immune homeostasis, metabolism, and hormone signaling. Among its specialized functions, the estrobolome (a collection of bacterial genes involved in estrogen metabolism) has emerged as a key regulator of systemic estrogen levels. Through microbial β-glucuronidase activity, estrogens undergo deconjugation and reabsorption, influencing the pathogenesis of hormone-receptor-positive breast cancers. Disruption of the gut microbial balance, termed dysbiosis, can result from dietary changes, antibiotic use, environmental toxins, and psychosocial stress. Dysbiosis alters intestinal permeability, immune responses, and microbial metabolite profiles, contributing to chronic inflammation and endocrine disruption. Mechanistic links between gut microbiota and breast cancer include altered estrogen recirculation, immunomodulation, shifts in microbial metabolites (e.g., SCFAs, bile acids, tryptophan derivatives), and stress-mediated signaling through the microbiota–gut–brain axis. Accumulating preclinical and clinical evidence reveals distinct microbial signatures in breast cancer patients, supporting a causal or contributory role of gut dysbiosis in tumorigenesis. In parallel, biotics (including probiotics, prebiotics, synbiotics, and postbiotics) offer promising avenues for modulating the microbiota. Certain strains of Lactobacillus (L.) and Bifidobacterium (B.) exhibit anti-inflammatory and estrogen-modulating effects, while dietary fibers and microbial metabolites may enhance epithelial integrity and immunocompetence. This review critically examines the interplay between gut microbiota and breast cancer, elucidates the mechanistic pathways involved, and evaluates the current evidence on microbiota-targeted interventions. We also highlight research gaps, safety considerations, and the potential for integrating microbiome modulation into personalized oncologic care. This review uniquely integrates mechanistic pathways with those supported by preclinical and clinical evidence on biotics, highlighting microbiome-based precision strategies for breast cancer prevention and management.
1. Introduction
Breast cancer remains the most frequently diagnosed malignancy among women globally and is a leading cause of cancer-related mortality. According to the Global Cancer Observatory (GLOBOCAN), over 2.3 million new cases of breast cancer were reported worldwide in 2020, accounting for 11.7% of all cancer cases and surpassing lung cancer as the most common cancer in women [1]. Mortality remains disproportionately high in low- and middle-income countries due to limited access to early detection and effective treatment. The etiology of breast cancer is complex and multifactorial, involving genetic predisposition, hormonal influences, environmental exposures, and lifestyle factors. While high-penetrance mutations, such as those in BRCA1/2, are well known, most cases are sporadic and influenced by modifiable risk factors, including obesity, alcohol consumption, physical inactivity, and reproductive history [2,3,4,5]. Recent decades have witnessed an increasing focus on the role of systemic inflammation, metabolic dysfunction, and hormonal imbalances in breast carcinogenesis. Amidst this evolving paradigm, the gut microbiota has emerged as a novel player in modulating these risk factors, potentially influencing both the onset and progression of breast cancer. Consequently, understanding gut microbiota dynamics may open new avenues for prevention, risk stratification, and therapy.
The human body is home to an astonishing array of microorganisms, collectively known as the microbiota, which outnumber our own cells and collectively contribute to essential biological functions [6]. Among these, the gut microbiota has emerged as a central player in maintaining systemic health, orchestrating complex interactions across metabolic, immunologic, and endocrine systems [7,8]. What was once considered a passive bystander is now recognized as a dynamic ecosystem with the potential to shape disease trajectories, including those of cancer. Recent years have witnessed a paradigm shift in oncology: the realization that microbial ecosystems, particularly those residing in the gastrointestinal tract, may influence cancer initiation, progression, and response to therapy [9,10,11]. In the context of breast cancer, the most prevalent malignancy among women worldwide, emerging evidence suggests that microbial dysbiosis, estrogen metabolism, immune signaling, and systemic inflammation may converge through the gut axis to influence tumor biology. The human gut harbors a dense and dynamic microbial ecosystem, comprising trillions of microorganisms collectively referred to as the gut microbiota. This community is dominated by four major bacterial phyla: Firmicutes, Bacteroidetes, Actinobacteria, and Proteobacteria, with species from Lactobacillus (L.), Bifidobacterium, and Bacteroides being especially prevalent in a healthy individual [12].
The gut microbiota plays a multifaceted role in host physiology, extending far beyond the gastrointestinal tract. In terms of digestion, gut microbes aid in the breakdown of complex polysaccharides and fibers, producing short-chain fatty acids (SCFAs) such as acetate, propionate, and butyrate, which serve as energy sources for colonocytes and modulate inflammation [13,14]. Beyond nutrient metabolism, these commensal microbes profoundly influence the immune system, training innate and adaptive responses and contributing to immune tolerance [15]. A pivotal yet less recognized role of the gut microbiota is in hormonal regulation, especially the metabolism of estrogens through a subset of microbial genes known as the estrobolome. The estrobolome comprises bacteria capable of producing β-glucuronidase, an enzyme that deconjugates estrogen metabolites in the gut, facilitating their reabsorption into systemic circulation [16]. This recirculation of estrogens has significant implications for hormone-driven conditions, including breast cancer. As such, the gut microbiota can be considered a critical endocrine organ, influencing not only digestion and immunity but also systemic hormonal balance. The systemic impact of the gut microbiota is now well-established, with mounting evidence linking microbial imbalance referred to as dysbiosis to a spectrum of chronic conditions such as inflammatory bowel disease (IBD), type-2 diabetes (T2D), type-1 diabetes (T1D), cardiovascular disease (CVD), and various cancers [17,18,19,20]. Dysbiosis can arise from multiple factors, including the use of antibiotics, dietary imbalances, environmental toxins, chronic stress, and aging, which disrupt microbial diversity and function. In cancer biology, the gut microbiota is believed to modulate several oncogenic processes through immune modulation, metabolite production, and hormonal regulation [21]. For example, bacterial fermentation products, such as SCFAs, have been shown to exert anti-inflammatory and anti-proliferative effects.
In contrast, others, including secondary bile acids and lipopolysaccharides (LPS), may promote tumorigenesis under dysbiosis conditions [22,23]. Specific to breast cancer, mechanistic links have been proposed through the estrobolome, gut-associated immune responses, and microbial metabolites that influence cellular proliferation, DNA damage, angiogenesis, and apoptosis [24]. Additionally, the microbiota-gut–brain axis is emerging as a potential modulator of stress responses and neuroimmune interactions that may indirectly influence tumor progression and metastasis.
Given the growing interest in the intersection of gut microbiota and breast cancer, this review aims to synthesize current knowledge regarding the mechanistic and clinical interplay between these two entities. It will explore the composition and functional roles of gut microbiota in health, including its role in digestion, immune modulation, metabolism, and estrogen regulation through the estrobolome. The review will then delve into the concept of dysbiosis, elucidating its causes and systemic consequences, especially in relation to breast cancer risk. We will further dissect mechanistic links between gut microbiota and breast cancer, focusing on four major domains: estrogen metabolism and the estrobolome, immune modulation, microbial metabolite production, and the microbiota–gut–brain axis. These pathways collectively underscore the potential for microbial communities to influence breast tumor biology, particularly hormone-receptor-positive subtypes. Moreover, we will present preclinical and clinical evidence supporting the association between gut dysbiosis and breast cancer, including microbial signatures observed in patients with cancer. A dedicated section will review the therapeutic potential of biotics, including probiotics, prebiotics, synbiotics, and postbiotics, in restoring gut microbial balance and mitigating cancer risk or progression. The review will also assess clinical and preclinical studies on biotics in breast cancer contexts, evaluating their safety, efficacy, and mechanisms of action. Finally, we will address existing challenges and future directions, highlighting gaps in the current literature, the need for personalized approaches, integration with conventional cancer therapies, and regulatory considerations for microbiota-based interventions. So, this review explores the intricate relationship between gut microbiota and breast cancer, with a focus on underlying mechanisms, clinical implications, and emerging interventions. By unpacking the science of microbial endocrinology, immunomodulation, and metabolite signaling, we aim to position the gut microbiota not merely as a biomarker but as a modifiable factor in breast cancer pathogenesis and treatment.
Although recent reviews have explored microbial dysbiosis in breast cancer, most have focused primarily on descriptive associations or estrogen-centered mechanisms. In contrast, this review integrates a multi-axis mechanistic framework encompassing the estrobolome, immune pathways, microbial metabolites (SCFAs, bile acids, and tryptophan derivatives), and responses to therapy. Significantly, we extend beyond observational findings to evaluate the translational potential of biotics, including probiotics, prebiotics, synbiotics, and postbiotics, as modifiable therapeutic strategies supported by the preclinical and clinical evidence. This systems-level approach highlights microbial signatures as emerging diagnostic and prognostic tools, contextualizing the gut microbiota as a clinically actionable component within precision oncology frameworks.
2. Gut Microbiota: Composition and Functions
Human gut microbiota has emerged as a critical determinant of systemic health, influencing a range of physiological processes that extend well beyond the gastrointestinal tract. In the context of breast cancer, a growing body of evidence suggests that the microbial communities residing in the gut play an integral role in regulating hormonal balance, immune surveillance, and inflammatory factors that collectively modulate cancer susceptibility and progression [25,26]. This section examines the taxonomic composition and functional roles of the gut microbiota, with a particular emphasis on its metabolic, immunological, and endocrine aspects, laying the groundwork for a mechanistic understanding of how microbial imbalances may contribute to breast cancer development.
2.1. Microbial Composition in a Healthy Human Gut
The human gut harbors a diverse and densely populated ecosystem of microorganisms, primarily bacteria, as well as viruses, fungi, archaea, and protozoa, collectively referred to as the gut microbiota. This microbial community consists of an estimated 1014 cells and encodes over 100 times more genes than the human genome. In healthy individuals, the gut microbiota is predominantly composed of bacterial phyla Firmicutes and Bacteroidetes, which collectively account for over 90% of the total bacterial population [27]. Other consistently observed phyla include Actinobacteria (e.g., Bifidobacterium spp.), Proteobacteria, Verrucomicrobia (e.g., Akkermansia [A.] muciniphila), and Fusobacteria. At the genus level, Bacteroides, Faecalibacterium (F.), Ruminococcus, Lactobacillus, and Bifidobacterium represent core taxa with established roles in host physiology [28]. Notably, F. prausnitzii and A. muciniphila have been identified as next-generation probiotics due to their potent anti-inflammatory properties [29,30,31]. The richness and diversity of these microbial communities are considered hallmarks of a healthy gut ecosystem, contributing to microbial resilience and functional redundancy [32]. Perturbations in this balanced ecosystem, as explored in later sections, may predispose individuals to chronic inflammation and hormone dysregulation, creating a pro-oncogenic milieu.
2.2. Functional Capacities of the Gut Microbiota
The gut microbiota fulfills an expansive array of physiological functions that are indispensable to host health and homeostasis. These can be broadly categorized into four domains: digestion and nutrient assimilation, immune modulation, metabolic regulation, and endocrine control, each of which has implications for breast cancer biology. The gut microbiota facilitates the breakdown of dietary polysaccharides, fibers, and resistant starches that are otherwise indigestible by human enzymes. This microbial fermentation yields SCFAs, namely, acetate, propionate, and butyrate, that serve as energy substrates for colonocytes, reinforce intestinal barrier integrity, and exhibit immunomodulatory and anti-neoplastic effects [33]. Butyrate has been shown to inhibit histone deacetylases, thereby regulating gene expression and suppressing tumorigenesis [34,35]. Additionally, microbial activity contributes to the synthesis of essential micronutrients such as vitamin K and B-complex vitamins, further influencing host metabolic health [36,37]. The gut microbiota plays a foundational role in shaping the host immune system from infancy through adulthood [38]. Commensal bacteria promote the development of gut-associated lymphoid tissue (GALT), prime innate immune responses, and regulate the balance between pro-inflammatory helper T cell (Th)17 and anti-inflammatory regulatory T cell (Treg) populations [39]. Through molecular patterns recognized by pattern recognition receptors (PRRs), such as Toll-like receptors (TLRs), gut microbes can modulate cytokine production and immune cell trafficking [40,41]. Notably, this immune crosstalk extends systemically, influencing distant tissues, including the breast, through the circulation of cytokines, chemokines, and immune effector cells. By regulating bile acid metabolism, SCFA production, and LPS levels, the gut microbiota has a significant impact on host metabolic pathways [42]. For instance, secondary bile acids derived from microbial metabolism act as ligands for nuclear receptors, such as farnesoid X receptor (FXR) and TGR5, influencing lipid and glucose homeostasis [43]. In parallel, microbial-derived LPS can induce chronic, low-grade inflammation, a known driver of tumorigenesis through the activation of the TLR4/ Nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) signaling pathway [44]. Thus, dysregulated microbial metabolism may establish a pro-inflammatory, insulin-resistant state that fuels both metabolic syndromes and cancer progression. Perhaps one of the most compelling roles of gut microbiota in breast cancer biology lies in its regulation of systemic estrogen levels. This endocrine function is primarily mediated by a microbial subcommunity known as the estrobolome.
2.3. The Estrobolome: Microbial Gatekeeper of Estrogen Homeostasis
The estrobolome is a term coined to describe the collection of gut microbial genes capable of metabolizing estrogens, primarily through the production of the enzyme β-glucuronidase [16]. This enzymatic activity plays a pivotal role in the enterohepatic circulation of estrogens, a process with direct implications for hormonal homeostasis and hormone-sensitive diseases such as breast cancer. Under physiological conditions, estrogens primarily include estrone, estradiol, and estriol, which are metabolized in the liver through phase II conjugation reactions, where they are bound to glucuronide or sulfate groups [45]. This conjugation renders them water-soluble, facilitating their excretion via bile into the intestinal lumen. However, once in the gut, estrogens can be deconjugated by microbial β-glucuronidase enzymes, especially those produced by bacteria in the phyla Bacteroidetes, Firmicutes, and Proteobacteria. Deconjugated, or “free,” estrogens can then be reabsorbed into the bloodstream via the intestinal wall, thereby increasing systemic estrogen levels [46]. In premenopausal women, ovarian secretion remains the primary source of circulating estrogens, whereas in postmenopausal women, estrogen is produced mainly through peripheral aromatization in adipose tissue. The estrobolome does not replace these primary sources; instead, it modulates the fraction of estrogen undergoing enterohepatic recirculation. This regulatory function may have greater clinical relevance in settings of obesity, metabolic dysfunction, or postmenopausal, where circulating estrogens are more dependent on peripheral conversion.
This microbial-mediated reactivation of estrogen constitutes a critical regulatory checkpoint in estrogen metabolism. A balanced estrobolome contributes to hormonal homeostasis, ensuring that circulating estrogen levels remain within a physiological range. However, disruptions in the gut microbiota, such as those caused by antibiotics, western dietary patterns, environmental toxins, or stress, can disturb the functional capacity of the estrobolome, either enhancing or impairing β-glucuronidase activity [47]. Both scenarios are potentially harmful. On one hand, excessive β-glucuronidase activity, often a hallmark of dysbiosis, can lead to abnormally high levels of circulating estrogens, which have been implicated in the development and progression of estrogen receptor-positive (ER+) breast cancers [46].
On the other hand, microbial depletion may lower estrobolome activity, reducing the enterohepatic recirculation of estrogens and possibly disrupting feedback regulation in the hypothalamic–pituitary–gonadal axis. Recent studies have demonstrated that women with higher systemic estrogen levels tend to have distinct gut microbial signatures, characterized by an increased relative abundance of Escherichia (E.), Clostridium (C.), and Bacteroides species, which are known to produce β-glucuronidase. A pivotal study by Fuhrman et al. (2014) found that postmenopausal women with higher urinary estrogen levels had a more diverse gut microbiome and higher microbial gene counts related to estrogen metabolism, particularly β-glucuronidase activity [48]. Moreover, reduced microbial diversity and estrobolome gene expression have been associated with obesity, metabolic syndrome, and inflammation, all of which are recognized breast cancer risk factors [49]. Importantly, not all β-glucuronidase activity is harmful. The context, intensity, and location of the enzyme’s action are key. While moderate levels of β-glucuronidase contribute to regular estrogen recycling and homeostasis, unregulated or excessive activity may shift the hormonal milieu toward a pro-oncogenic state [16]. This underlines the need for precision modulation of the estrobolome, rather than indiscriminate suppression. Overall, the estrobolome acts as a critical microbial-endocrine interface, regulating estrogen availability through microbial enzymatic actions. Its modulation offers a novel, microbiota-targeted avenue for breast cancer prevention and therapy, particularly in estrogen-dependent subtypes. As research advances, the estrobolome may become a biomarker of hormonal risk and a therapeutic target in precision oncology.
3. Dysbiosis: Definition and Mechanisms
The human gut microbiota, a complex and dynamic microbial ecosystem, plays a crucial role in numerous physiological functions, including nutrient metabolism, immune regulation, and endocrine signaling. Its balance and diversity are critical for sustaining host health. However, when the composition, density, or functionality of the microbiota becomes disrupted, a condition termed “dysbiosis” arises. Gut dysbiosis reflects a pathological imbalance in the microbial community, and growing evidence implicates it as a key contributor to a broad spectrum of chronic diseases, including metabolic syndrome, autoimmune conditions, neurodegenerative disorders, and various cancers, notably breast cancer [50,51].
3.1. What Is Dysbiosis?
Dysbiosis refers to any qualitative or quantitative perturbation in the gut microbiome that disturbs the equilibrium between commensal and pathogenic organisms. It is characterized by:
- ▪
- A loss of microbial diversity;
- ▪
- A reduction in beneficial bacteria (e.g., Lactobacillus, Bifidobacterium);
- ▪
- An overgrowth of potentially pathogenic taxa (e.g., E. coli, C. difficile);
- ▪
- A shift in microbial metabolic activity that leads to the production of deleterious compounds.
Dysbiosis may be transient or chronic, and its consequences depend on the degree of microbial imbalance and the host’s resilience [52]. While a healthy microbiota is resilient to perturbation, sustained dysbiosis undermines barrier integrity, disrupts immune tolerance, and alters systemic metabolic and hormonal signals, establishing a microenvironment conducive to tumorigenesis.
3.2. Causes of Gut Dysbiosis
Several endogenous and exogenous factors can trigger dysbiosis. In the context of modern lifestyles, four primary categories are implicated and presented in Figure 1.
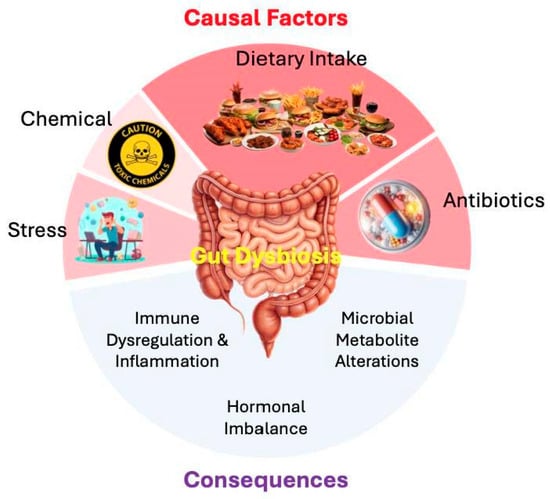
Figure 1.
Causal factors and consequences of gut dysbiosis in breast cancer etiology and management. The diagram summarizes how primary exogenous and endogenous triggers disturb the gut microbiota and the downstream effects relevant to breast cancer. The upper semicircle depicts causal factors: (i) dietary intake like western-style diets rich in saturated fats, refined sugars, and processed foods with low fiber reduce microbial diversity and SCFA production; (ii) antibiotics like broad-spectrum antimicrobials diminish commensal populations and colonization resistance; (iii) chemical exposures like environmental toxins such as pesticides, heavy metals, and plasticizers disrupt microbial ecology and interact with estrogenic pathways; and (iv) psychosocial stress like activation of the gut–brain axis alters motility, barrier integrity, and immune signaling, fostering dysbiosis. At the center, gut dysbiosis represents the net loss of microbial homeostasis. The lower semicircle illustrates its consequences: immune dysregulation and chronic low-grade inflammation; impaired endocrine homeostasis, including the metabolism of estrogen, androgen, progesterone, and cortisol; and shifts in microbial metabolites (e.g., reduced butyrate, increased secondary bile acids, and altered tryptophan derivatives) that influence epigenetic, immunomodulatory, and metabolic pathways. Collectively, these alterations create a tumor-permissive microenvironment that may facilitate breast cancer initiation, progression, and therapeutic resistance.
3.2.1. Diet
One of the most influential modulators of gut microbiota is dietary intake. A diet low in fiber and high in refined sugars, saturated fats, and processed foods, the hallmark of Western nutritional patterns, has been associated with reduced microbial diversity and an increased abundance of pro-inflammatory species. These diets deprive commensal bacteria of fermentable substrates, particularly non-digestible fibers (prebiotics), resulting in decreased production of SCFAs, especially butyrate, a key metabolite essential for maintaining mucosal integrity and promoting anti-inflammatory responses. Moreover, diets high in animal protein and fat stimulate the proliferation of bile-tolerant bacteria (e.g., Bilophila wadsworthia) while reducing beneficial saccharolytic bacteria [53]. The resultant shifts not only promote inflammation but may also affect estrogen metabolism via the estrobolome, thereby influencing breast cancer risk.
3.2.2. Antibiotic Use
Antibiotics are indispensable in modern medicine, yet their broad-spectrum activity can indiscriminately eliminate both harmful and beneficial bacteria. Even short courses of antibiotics can cause long-lasting changes in the gut microbiota composition, which sometimes persist for months or even years. This microbial vacuum can be exploited by opportunistic and pathogenic organisms, leading to reduced colonization resistance, metabolic dysregulation, and impaired immunological responses. In the context of estrogen metabolism, antibiotic-induced depletion of estrobolome taxa reduces β-glucuronidase activity, potentially impairing estrogen recycling and hormonal balance [54]. This has been observed in animal studies where antibiotic treatment altered estrogen plasma levels and impacted estrogen-responsive tissues. Although antibiotic exposure can transiently decrease microbial β-glucuronidase activity, epidemiological investigations do not demonstrate a corresponding reduction in breast cancer risk. This apparent paradox likely reflects the broader consequences of antibiotic-induced dysbiosis, including the depletion of SCFA-producing taxa, impaired gut barrier function, alterations in bile acid signaling, and dysregulated immune activity. Thus, while one estrogen-modulating enzyme may be suppressed, the net effect of disrupted microbial ecology may favor pro-inflammatory or pro-tumorigenic conditions.
3.2.3. Environmental Toxins
Chronic exposure to environmental toxins, such as pesticides, heavy metals (e.g., arsenic, lead), plasticizers (e.g., BPA, phthalates), and air pollutants, has been shown to alter gut microbial profiles [55]. These xenobiotics may exert direct antimicrobial effects or modulate host-microbe interactions via endocrine-disrupting properties. For example, bisphenol A (BPA), a known estrogen mimic, can disrupt microbial community structure and potentially synergize with dysbiosis microbiota to amplify estrogenic signaling, thereby increasing the likelihood of estrogen-receptor-positive breast tumor development [54].
3.2.4. Psychosocial and Physiological Stress
The gut–brain axis represents a bidirectional communication system between the central nervous system and the gut microbiota. Chronic stress, through the release of glucocorticoids and catecholamines, alters gut motility, mucosal permeability, and immune function, which impacts microbial ecology [56]. Stress-induced dysbiosis is associated with a reduced abundance of beneficial commensals and an increased presence of pro-inflammatory bacteria, which in turn stimulate immune dysregulation and low-grade systemic inflammation [57], two hallmarks of the cancer-promoting microenvironment.
3.3. Consequences of Dysbiosis on Host Physiology
Dysbiosis has a profound impact on host physiology through multiple interrelated pathways. In the context of breast cancer, three principal domains warrant special attention: a balanced microbiota plays an essential role in promoting immune tolerance and preventing excessive inflammation [15]. Dysbiosis skews this balance by enhancing gut permeability, also known as “leaky gut,” allowing microbial products, such as LPS, to enter systemic circulation [50]. This leads to chronic low-grade inflammation, which can fuel tumorigenesis by promoting angiogenesis, inhibiting apoptosis, and facilitating immune evasion by emerging tumor cells [58]. In addition, dysbiosis impairs Treg activity and disrupts antigen presentation, further impairing anti-tumor immune surveillance [59]. This is particularly critical in breast cancer, where immune infiltration patterns and inflammatory profiles are key determinants of prognosis and therapeutic response. The gut microbiota, especially the estrobolome, is integral to estrogen regulation. Dysbiosis can either augment or suppress β-glucuronidase-mediated deconjugation of estrogens, leading to hormonal imbalances that are directly implicated in estrogen-dependent tumor proliferation [16,46,47]. Elevated systemic estrogen levels, secondary to microbial dysregulation, have been observed in postmenopausal women with increased breast cancer risk [60]. Furthermore, gut bacteria influence the metabolism of other hormones such as androgens, progesterone, and cortisol [61,62,63,64,65,66,67]. Such disruptions in the endocrine landscape can synergize with oncogenic mutations, driving carcinogenesis and progression in hormone-responsive breast cancers. Dysbiosis alters microbial metabolic outputs, including the synthesis of SCFAs, bile acids, and tryptophan metabolites. These compounds exert potent epigenetic, immunomodulatory, and metabolic effects. For instance, butyrate, known for its anti-inflammatory and anti-proliferative properties, inhibits histone deacetylases (HDACs), thereby influencing gene expression relevant to tumor suppression [22,68,69,70]. Secondary bile acids, when elevated due to dysbiosis, can be cytotoxic, pro-inflammatory, and genotoxic [71]. Tryptophan metabolites such as indole derivatives modulate immune responses through the aryl hydrocarbon receptor (AhR), influencing cancer immunity [72,73,74,75]. Collectively, these shifts in microbial metabolite profiles can alter host cell signaling, disrupt DNA repair mechanisms, and create a pro-carcinogenic epigenetic landscape conducive to breast cancer initiation and progression.
4. Mechanistic Links Between Gut Microbiota and Breast Cancer
The interplay between gut microbiota and breast cancer development is multifaceted, involving hormonal modulation, immune regulation, and metabolic signaling (Figure 2). Emerging evidence suggests that specific microbial functions and metabolites orchestrate complex interactions with the host endocrine and immune systems, directly impacting tumorigenesis [76]. This section explores the mechanistic underpinnings that link gut microbial activity to breast cancer pathogenesis.
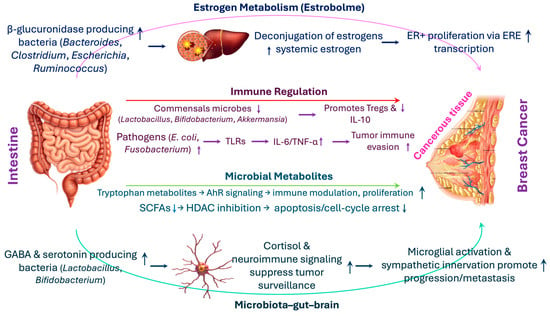
Figure 2.
Mechanistic links between gut microbiota and breast cancer: hormonal, immune, metabolic, and neuroimmune pathways. The schematic illustrates how specific microbial activities connect gut health to breast cancer pathogenesis. (i) Estrogen metabolism (Estrobolome): β-glucuronidase-producing bacteria (Bacteroides, Clostridium, Escherichia, Ruminococcus) deconjugate estrogens in the gut, enabling reabsorption and elevating systemic estrogen, which enhances proliferation of estrogen receptor-positive (ER+) cells through estrogen response element (ERE)-mediated transcription. (ii) Immune regulation: Commensals such as Lactobacillus, Bifidobacterium, and Akkermansia maintain mucosal tolerance by inducing Tregs and IL-10, whereas pathobionts (e.g., E. coli, Fusobacterium [Fu.] nucleatum) activate toll like receptors (TLRs), producing interleukin (IL)-6 and tumor necrosis factor (TNF)-α and fostering tumor immune evasion. (iii) Microbial metabolites: Short-chain fatty acids (SCFAs) primarily butyrate promote apoptosis and cell-cycle arrest via histone deacetylase (HDAC) inhibition, while secondary bile acids (e.g., Deoxycholic acid [DCA]) and tryptophan derivatives (e.g., indole-3-aldehyde, kynurenine) influence immune modulation, proliferation, and angiogenesis through aryl hydrocarbon receptor (AhR), Phosphatidylinositol 3-kinase/(PI3K)/Protein kinase B (Akt)/mammalian target of rapamycin (mTOR), and hypoxia-inducible factor 1-alpha (HIF-1α) pathways. (iv) Microbiota–gut–brain axis: Neuroactive metabolites (gamma-aminobutyric acid [GABA], serotonin) from Lactobacillus and Bifidobacterium regulate the hypothalamic–pituitary–adrenal (HPA) axis and cortisol release, shaping immune surveillance and facilitating tumor progression and metastasis via microglial activation and sympathetic innervation.
4.1. Estrogen Metabolism and the Estrobolome
One of the most studied mechanistic pathways linking gut microbiota to breast cancer is the regulation of systemic estrogen levels by the estrobolome, a subset of the gut microbiota capable of metabolizing estrogens. Estrogens undergo hepatic conjugation and are excreted into the bile as inactive glucuronides. In the gut, bacterial β-glucuronidase enzymes, predominantly from Bacteroides, Clostridium, Escherichia, and Ruminococcus species, deconjugate these estrogens, allowing them to be reabsorbed into the enterohepatic circulation [16,46,77]. Elevated β-glucuronidase activity has been associated with increased systemic estrogen levels, a known risk factor for hormone-receptor-positive breast cancers [78,79]. Higher systemic estrogen levels can enhance the proliferation of estrogen receptor-positive (ER+) breast cancer cells via estrogen response element (ERE)-mediated transcription. Studies have shown that postmenopausal women with reduced gut microbial diversity and increased abundance of β-glucuronidase-producing bacteria exhibit elevated plasma estrogen levels [48]. These findings suggest that dysbiosis of the estrobolome can potentially increase breast cancer risk or influence treatment outcomes in ER+ subtypes.
4.2. Immune System Modulation
The gut microbiota modulates immune responses through antigen presentation, cytokine production, and the maintenance of mucosal integrity. These immunological changes can have downstream effects on systemic inflammation and tumor surveillance. Commensal bacteria, such as Lactobacillus, Bifidobacterium, and A. muciniphila, promote the differentiation of Tregs and the production of anti-inflammatory cytokines, including IL-10, thereby maintaining immune homeostasis [80]. Conversely, pathogenic taxa such as E. coli and Fu. nucleatum can activate TLRs, induce pro-inflammatory cytokines (e.g., IL-6, TNF-α), and drive chronic inflammation conditions that favor tumor development and progression [81,82,83,84]. Chronic inflammation induced by microbial dysbiosis contributes to immune evasion by increasing the number of myeloid-derived suppressor cells (MDSCs) and Treg infiltration in the tumor microenvironment [85]. This suppressive milieu inhibits the activity of cytotoxic T lymphocytes, thereby facilitating tumor survival. Moreover, bacterial components, such as LPS, can potentiate NF-κB signaling and Cyclooxygenase-2 (COX-2) expression, thereby further amplifying the inflammatory cascades associated with breast cancer progression [81].
4.3. Microbial Metabolites
Microbial fermentation and metabolism yield bioactive compounds, including SCFAs, secondary bile acids, and tryptophan metabolites, that significantly influence host cell signaling pathways. SCFAs, primarily acetate, propionate, and butyrate, are produced by bacterial fermentation of dietary fibers. Butyrate exhibits anti-tumorigenic properties by inhibiting HDAC, thereby regulating the epigenetic expression of genes involved in apoptosis and cell cycle arrest . Secondary bile acids, such as DCA, derived from the microbial metabolism of primary bile acids, have been implicated in carcinogenesis due to their pro-inflammatory and DNA-damaging properties [71]. Tryptophan-derived metabolites, such as indole-3-aldehyde and kynurenine, activate the AhR signaling pathway, which influences the differentiation of immune cells and the function of the intestinal barrier. Dysregulated AhR signaling has been observed in breast cancer tissues and is thought to contribute to immune evasion and tumor progression [86]. These microbial metabolites can directly impact breast epithelial cells by modulating signaling pathways involved in proliferation (e.g., PI3K/Akt/mTOR), apoptosis (e.g., B-cell lymphoma 2 [Bcl-2]/BCL2-associated X [Bax] ratio), and angiogenesis (e.g., Vascular Endothelial Growth Factor [VEGF] expression). Butyrate, for instance, has been shown to suppress angiogenesis and metastasis in breast cancer models by downregulating HIF-1α [87,88].
4.4. Microbiota–Gut–Brain Axis
The bidirectional communication between the gut and brain, modulated by microbial metabolites and neuroactive compounds, also plays a role in cancer biology. Psychological stress is an established risk factor for cancer progression and can be modulated by gut microbiota through the microbiota–gut–brain axis. Microbes such as Lactobacillus and Bifidobacterium produce GABA, serotonin, and other neuromodulators that influence the HPA axis. Stress-induced activation of the HPA axis leads to increased cortisol levels, which can suppress immune surveillance and facilitate tumor growth and development [89,90,91,92]. Microbial dysbiosis has also been associated with neuroinflammation and alterations in microglial function, contributing to a permissive environment for cancer metastasis, particularly to the brain. In breast cancer models, microbiota-induced neuroimmune signaling has been implicated in regulating the sympathetic innervation of tumors, thereby influencing tumor growth and dissemination [93]. Overall, the microbiota-gut–brain axis emerges as a critical mediator linking psychological stress, neuroimmune signaling, and cancer progression. By shaping HPA axis activity, immune surveillance, and neuroinflammatory responses, gut microbes influence both tumor growth and metastatic potential. These insights highlight the gut–brain axis as a promising frontier for integrative cancer prevention and therapeutic strategies. Although preclinical evidence suggests that microbial metabolites and vagal signaling may influence neuroimmune pathways relevant to tumor progression, this concept remains hypothetical in human breast cancer. Current data provide biological plausibility, but causal relationships have not been demonstrated in clinical populations. Therefore, the microbiota–gut–brain axis should be regarded as an emerging mechanistic model that requires confirmation in longitudinal and interventional human studies.
5. Microbial Signatures of Breast Cancer: Linking Gut Health to Hormonal and Immune Pathways
In continuation of the previous section, gut dysbiosis refers to a disruption in the normal composition and function of the gut microbiome, characterized by reduced microbial diversity, loss of beneficial commensals, and an expansion of pathogenic or pro-inflammatory taxa [94]. This imbalance can profoundly alter systemic homeostasis, contributing to chronic inflammation, oxidative stress, and hormonal imbalances, key risk factors implicated in breast cancer pathophysiology. The mechanisms through which gut dysbiosis may promote breast cancer include: (i) modulation of estrogen metabolism via the estrobolome [95]; (ii) activation of immune signaling cascades promoting a pro-tumorigenic microenvironment [96]; and (iii) production of microbial metabolites that influence host epigenetics and cell proliferation [97]. These pathways have been validated through a spectrum of experimental approaches, including preclinical models and human studies, reinforcing the concept that gut microbiota may function as both a mediator and a marker of breast cancer risk [98]. The interplay between the gut microbiota and systemic host physiology has emerged as a focal point in understanding the etiopathogenesis of breast cancer. Mounting evidence from both experimental and clinical investigations suggests that disruptions in the gut microbial ecosystem, commonly referred to as gut dysbiosis, can modulate the host’s hormonal milieu and immune function in ways that may promote the development of mammary tumors. In this context, the identification of distinct microbial signatures associated with breast cancer has offered new avenues for both diagnostic and therapeutic innovation. This section synthesizes the current understanding of the evidence linking gut dysbiosis to breast cancer, drawing from preclinical models, epidemiological investigations, and microbiome profiling studies.
Advancements in high-throughput sequencing technologies and integrative metagenomic analyses have significantly enhanced our ability to characterize the gut microbiota in relation to breast cancer in both clinical and pre-clinical studies [99]. These methodologies have uncovered distinct microbial taxa and functional gene profiles that are consistently associated with the presence, progression, and subtype specificity of breast cancer [100]. Notably, a consistent pattern of compositional and functional dysbiosis has emerged in breast cancer cohorts, characterized by a relative depletion of SCFA-producing commensals, such as Roseburia and Eubacterium rectale, alongside an enrichment of pro-inflammatory genera, including Prevotella, Desulfovibrio, and specific Clostridium spp. [101,102]. This shift in microbial ecology contributes to a state of chronic, low-grade systemic inflammation. It is further implicated in the disruption of mucosal immunity and mucin layer integrity, thereby fostering a microenvironment conducive to tumorigenesis [103,104,105,106,107,108]. Metagenomic profiling has revealed functional alterations in microbial pathways that may mechanistically link gut dysbiosis to breast cancer biology [109]. These include upregulated microbial gene networks involved in xenobiotic degradation, estrogen metabolism, and LPS biosynthesis, each of which can influence host immune modulation and hormonal homeostasis [101]. Elevated microbial conversion of primary to secondary bile acids, such as deoxycholic acid, has been shown to induce oxidative stress, DNA damage, and increased epithelial proliferation, all of which are recognized as contributors to carcinogenesis [110,111]. Importantly, these microbial alterations are not confined to the gut milieu. Recent findings suggest that microbial DNA and metabolites may translocate into systemic circulation and localize to extraintestinal tissues, including the breast tumor microenvironment. Here, they can modulate the phenotypes of immune cells, cytokine production, and the behavior of tumor cells, thereby acting as functional modulators of cancer progression [112,113,114].
A growing body of literature supports the existence of breast cancer-associated microbial signatures, distinct constellations of microbial taxa and metabolic functions that correlate with disease presence, subtype differentiation, and clinical outcomes. Among these, the estrobolome, a subset of gut microbial genes capable of metabolizing estrogens, has garnered particular attention [46,115]. Dysregulation of the estrobolome, often characterized by increased β-glucuronidase activity, enhances enterohepatic recirculation of deconjugated estrogens, thereby elevating systemic estrogen levels [46,116]. This hormonal reactivation is especially relevant for estrogen receptor-positive (ER+) breast cancer, where increased estrogen bioavailability can directly stimulate tumor growth [49]. Higher relative abundances of Enterobacteriaceae, Clostridium, and Bacteroides spp. have been associated with elevated estrobolome activity and increased breast cancer risk, particularly among postmenopausal women [117]. In addition to hormonal modulation, immune-related microbial alterations have also been implicated in the pathophysiology of breast cancer [117]. Enrichment of pro-inflammatory taxa, such as Desulfovibrio, Bilophila wadsworthia, and LPS-producing Gammaproteobacteria, can activate innate immune receptors, including TLR4 and NOD2, driving chronic inflammation and immune cell recruitment to the tumor microenvironment [102,118,119,120]. Conversely, the depletion of immunoregulatory commensals such as Lactobacillus, Roseburia, and Faecalibacterium undermines Treg induction and mucosal barrier integrity, thereby exacerbating inflammation and promoting neoplastic transformation [121]. As illustrated in Figure 3, environmental and lifestyle stressors, including diet, antibiotics, psychological stress, and toxin exposure, serve as upstream disruptors of the gut microbiome and estrobolome, driving β-glucuronidase overactivity, metabolic imbalance (reduced SCFAs, bile acids, and tryptophan metabolites), endocrine dysfunction, and chronic immune activation. This multi-axis disruption not only amplifies estrogen-driven carcinogenesis but also creates a pro-tumorigenic microenvironment that fosters tumor initiation, progression, and therapeutic resistance.
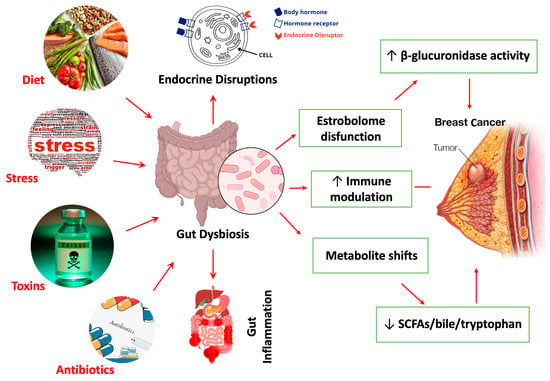
Figure 3.
Environment and lifestyle drivers of gut microbiome dysbiosis and estrobolome dysfunction contributing to breast Cancer pathogenesis. Dietary composition, psychological stress, environmental toxins, and indiscriminate antibiotic exposure act as key modulators of gut microbial ecology. These factors disrupt gut microbial homeostasis and the estrobolome, specifically β-glucuronidase-producing taxa, leading to impaired estrogen deconjugation, altered enterohepatic recycling, and endocrine perturbation. Dysbiosis further drives metabolite imbalance characterized by reduced SCFAs, bile acids, and tryptophan derivatives, accompanied by immune dysregulation and inflammatory signaling. Collectively, these perturbations promote a pro-carcinogenic microenvironment that increases breast cancer susceptibility, tumor progression, and hormonal therapy resistance. Red arrows indicate directionality of disruption across host–microbe–immune axes.
Emerging evidence further suggests that microbial signatures may differ by breast cancer subtype. ER+ tumors are frequently associated with heightened β-glucuronidase activity and increased abundance of estrogen-metabolizing microbes. In contrast, triple-negative breast cancer (TNBC) is more commonly linked to severe dysbiosis, pro-inflammatory microbial profiles, and diminished SCFA production [122,123]. These subtype-specific microbial patterns hold significant diagnostic and prognostic potential. Indeed, machine learning models that leverage microbiome-derived features have demonstrated robust accuracy in distinguishing between breast cancer subtypes and predicting therapeutic responses, underscoring the potential of gut microbial signatures as both non-invasive biomarkers and actionable targets in personalized oncology.
6. Role of Biotics in Modulating Gut Microbiota
Biotics comprising probiotics, prebiotics, synbiotics, and postbiotics have emerged as promising modulators of gut microbial composition and function, offering novel strategies to mitigate breast cancer risk by restoring microbial homeostasis. These interventions target specific axes of gut–host communication, including microbial metabolite production, mucosal immunity, endocrine signaling, and intestinal barrier function [124,125]. As evidence continues to reveal microbiota’s critical influence on estrogen metabolism, immune homeostasis, and systemic inflammation, biotics represent a rational, modifiable approach to alter breast cancer susceptibility and progression. To illustrate how distinct classes of microbiome-modulating interventions act upon gut–immune–endocrine axes implicated in breast cancer, Figure 4 provides a systems-level overview of probiotics, prebiotics, synbiotics, and postbiotics, highlighting their shared and complementary mechanisms, including immune modulation, improved barrier function, reduced β-glucuronidase activity, and enhanced SCFA production. This mechanistic framework supports the emerging rationale for biotics as adjuncts in breast cancer prevention and therapy.
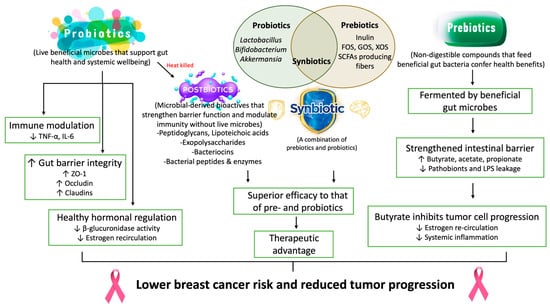
Figure 4.
Probiotic, prebiotic, and synbiotic interventions targeting the gut–breast axis: mechanisms and therapeutic advantages in breast cancer prevention and management. Probiotics (e.g., Lactobacillus, Bifidobacterium, Akkermansia) and prebiotics (e.g., inulin, FOS, GOS, XOS, SCFA-producing fibers) modulate gut microbial composition and function, thereby improving hormonal homeostasis and restoring immune regulatory balance. Prebiotic substrates are selectively fermented by beneficial microbes, thereby increasing the production of short-chain fatty acids (butyrate, acetate, propionate) and reducing intestinal permeability, LPS leakage, and pathobiont expansion. Synbiotics, which combine live beneficial microbes with targeted fermentable substrates, exhibit superior efficacy by enhancing microbial engraftment, SCFA yield, and gut barrier fortification. Butyrate, a major microbial metabolite, exerts direct anticancer effects by suppressing estrogen re-circulation, inhibiting tumor cell proliferation, and attenuating systemic inflammation. By synergistically modulating the microbiota–immune–endocrine axis, these microbiome-directed therapies offer a promising therapeutic advantage for breast cancer prevention, disease progression control, and optimization of treatment response.
6.1. Probiotics
Probiotics, defined as live microorganisms that confer health benefits when administered in adequate amounts, have been extensively studied for their immunomodulatory and anti-carcinogenic effects [126,127]. Among the most investigated genera are Lactobacillus and Bifidobacterium, which exert pleiotropic actions through multiple host–microbiota interactions [128,129]. Certain Lactobacillus strains inhibit bacterial β-glucuronidase activity in the gut, thus reducing enterohepatic recirculation of estrogens and lowering systemic estrogen exposure, a key hormonal factor implicated in hormone receptor-positive breast cancer [47,117,130]. Concurrently, probiotics can modulate immune function by enhancing mucosal immunity, increasing regulatory T-cell populations, promoting the production of anti-inflammatory cytokines (e.g., IL-10), and reducing the activity of pro-inflammatory mediators such as IL-6 and TNF-α [131,132,133]. Significantly, probiotics also strengthen gut barrier integrity by upregulating tight junction proteins such as Zo1, occludin, and claudins, thereby preventing microbial translocation and systemic endotoxemia, which are known to contribute to chronic inflammation and cancer progression [21,132,134,135,136]. Preclinical studies in mammary tumor models have shown that administering L. casei or B. longum can reduce tumor burden and promote antitumor immune responses [21,132,134,135,136]. Although clinical data remain limited, early-phase trials suggest that probiotic supplementation may improve systemic inflammation and enhance tolerance to anticancer therapies in breast cancer patients.
6.2. Prebiotics
Prebiotics, in contrast, are defined as selectively fermentable substrates that promote the growth and activity of beneficial gut microbes [137,138,139]. Commonly studied prebiotics include inulin, fructooligosaccharides (FOSs), and galactooligosaccharides (GOSs), all of which escape digestion in the upper gastrointestinal tract and undergo fermentation in the colon by commensal bacteria [140,141]. This process results in the production of SCFAs, notably butyrate, acetate, and propionate, which exert anti-inflammatory, immunoregulatory, and epigenetic effects. Butyrate acts as a histone deacetylase inhibitor, modulating gene expression, promoting apoptosis in cancer cells, and reinforcing epithelial barrier function [142,143,144]. Through the selective stimulation of beneficial taxa, such as Bifidobacterium and Lactobacillus, prebiotics contribute to a microbiota configuration associated with a reduced cancer risk. Moreover, by influencing microbial β-glucuronidase activity and mucosal immune responses, prebiotics indirectly impact estrogen metabolism and immune surveillance [46,145]. Although experimental models show that prebiotics can modulate tumorigenesis, especially when consumed with a high-fiber diet, robust clinical evidence in breast cancer remains limited [146,147,148,149].
Extending the concept of diet-driven microbial modulation, polyphenol-rich compounds, such as EGCG, exhibit prebiotic-like activity with both microbiota-dependent and direct anticancer effects. Green tea supplementation increases A. muciniphila and improves gut barrier and immune function in mice [150]. Similarly, human studies show that green tea polyphenols modulate microbial composition and immune responses [151]. EGCG also directly suppresses HER2/neu, NF-κB, and VEGF signaling, promoting apoptosis [152,153,154,155]. Although green tea polyphenols reduce mammary tumorigenesis in HER2/neu mice [156], A. muciniphila has not been confirmed as the mediator. Importantly, no clinical trial has yet evaluated microbiome changes, including those of A. muciniphila, in conjunction with breast cancer outcomes. Overall, prebiotics, including polyphenol-based modulators such as EGCG, show promise for reshaping gut ecology and attenuating oncogenic pathways, but causal links remain to be established.
6.3. Synbiotics
Synbiotics, which combine both probiotics and prebiotics, are designed to leverage the complementary benefits of microbial supplementation and substrate support [157,158,159,160]. This synergistic approach enhances colonization efficiency of probiotics, augments beneficial metabolite production, and stabilizes the microbial ecosystem. Synbiotics have demonstrated superior efficacy compared to individual biotics in modulating inflammatory markers, improving mucosal integrity, and restoring microbial diversity [161,162,163,164,165,166]. Clinical studies, particularly in gastrointestinal malignancies, suggest that synbiotics can reduce chemotherapy-induced gastrointestinal toxicity and systemic inflammation. Although specific evidence in breast cancer patients is limited, these findings indicate that synbiotics may offer therapeutic advantages by modulating host–microbe interactions implicated in breast carcinogenesis. For example, randomized trials involving synbiotics formulations combining L. rhamnosus GG with inulin have shown reduced gut permeability and improved systemic inflammatory profiles that are mechanistically relevant to breast cancer [167,168,169,170,171,172].
6.4. Postbiotics
Postbiotics represent a novel and increasingly recognized class of biotics, encompassing non-viable microbial cells, cellular components, and metabolites that confer biological activity in the host [173,174,175,176]. Unlike probiotics, postbiotics do not require microbial viability, offering advantages in terms of safety and stability. Key bioactive components, including SCFAs (particularly butyrate), bacteriocins, lipoteichoic acids, and extracellular polysaccharides, can directly influence host immune responses, modulate epithelial regeneration, and exert anti-proliferative effects on tumor cells [177,178,179,180,181,182]. Butyrate, for example, promotes apoptosis in breast cancer cell lines and regulates gene expression through epigenetic mechanisms [183,184,185,186]. Lipoteichoic acids and peptidoglycans derived from Lactobacillus species have been shown to enhance mucosal immunity and protect against inflammation-driven tumorigenesis [187]. Although the application of postbiotics in breast cancer remains in early stages, emerging studies suggest that they may potentiate the effects of immunotherapy, reduce cancer stem cell phenotypes, and serve as safe, standardized alternatives to live microbial administration.
Collectively, biotics represents a versatile toolkit for modulating the gut microbiota in ways that are increasingly understood to impact breast cancer biology. Through their influence on microbial composition, metabolite production, immune tone, and hormonal metabolism, biotics may alter the systemic milieu in a manner conducive to cancer prevention and improved treatment outcomes. The integration of biotic-based interventions into personalized medicine frameworks guided by microbial, metabolomic, and host-response profiling could unlock novel avenues for preventing and managing breast cancer and breast cancer-relevant outcomes, as summarized in Table 1 and Table 2. Future research must aim to elucidate strain-specific effects, define optimal dosing strategies, and validate clinical efficacy through rigorously designed mechanistic and interventional studies.

Table 1.
Preclinical Evidence of Biotics Modulating the Gut Microbiome in Breast-Cancer-Relevant Outcomes.

Table 2.
Clinical Evidence of Biotics Modulating the Gut Microbiome in Breast-Cancer-Relevant Outcomes.
7. Preclinical and Clinical Evidence on Biotics in Breast Cancer
Recent advances in microbiome science have revealed that breast cancer is not merely a localized disease but may be intricately linked with systemic microbial ecology, particularly the gut microbiota. Biotics encompassing probiotics, prebiotics, synbiotics, postbiotics, and next-generation microbial therapeutics offer a compelling frontier for modulating host immunity, metabolism, and estrogen regulation. In this section, we examine the current preclinical and clinical evidence for biotics in breast cancer prevention and therapy, detailing molecular mechanisms, translational efforts, and safety considerations.
7.1. Preclinical Evidence: In Vivo and In Vitro Models
Preclinical studies have provided critical insights into the therapeutic potential of biotics, including probiotics, prebiotics, synbiotics, and postbiotics, in the context of breast cancer. Using both in vitro and in vivo models, these investigations have elucidated key mechanisms by which gut microbial modulation influences tumor biology, including immunomodulation, induction of apoptosis, modulation of oncogenic signaling pathways, and alterations in microbial-derived metabolites, such as SCFAs. While these studies vary in terms of microbial species, delivery methods, and tumor models, they collectively support a strong rationale for exploring biotic interventions as adjuncts in breast cancer prevention and treatment [216]. Although murine models support the biological plausibility of microbiota-mediated modulation of tumor growth and immunity, direct extrapolation to humans should be made with caution. Significant differences in intestinal physiology, immune function, microbial composition, and estrogen metabolism indicate that animal data provide mechanistic hypothesis-generating evidence. Still, this evidence must be confirmed in controlled clinical studies.
7.2. In Vitro Studies
In vitro models have been instrumental in uncovering direct anti-tumor effects of bacterial strains and their metabolites on breast cancer cell lines. Cell-free supernatants derived from probiotic strains, such as L. rhamnosus GG, have demonstrated significant cytotoxicity against both estrogen receptor-positive (MCF-7) and TNBC (MDA-MB-231) cell lines, primarily through caspase-dependent apoptosis and proliferation suppression [217]. These effects are frequently associated with alterations in the expression of pro- and anti-apoptotic genes, including the upregulation of Bax and cleaved caspase-3, and the downregulation of Bcl-2 [218].
Beyond whole organisms, bacterial metabolites, particularly SCFAs such as butyrate, acetate, and propionate, exert potent anticancer effects [219]. Butyrate, for instance, acts as an HDAC inhibitor, leading to epigenetic reprogramming of cancer cells and upregulation of tumor suppressor genes, such as p21 and p53 [220]. Propionibacterium freudenreichii-derived SCFAs induced apoptosis in MCF-7 cells via both intrinsic (mitochondrial) and extrinsic (death receptor-mediated) pathways, with involvement of caspase-8 and caspase-9 [221]. These metabolites also interfere with cancer-associated signaling cascades, including the Wnt/β-catenin and MAPK pathways, contributing to reduced proliferation and increased apoptotic cell death [222]. Moreover, postbiotic components such as bacteriocins, peptidoglycans, and exopolysaccharides have demonstrated immunostimulatory and cytostatic properties [223]. Although mechanistic clarity remains incomplete, emerging evidence suggests that bacterial products can also modulate the expression of genes involved in cell cycle arrest and DNA repair, positioning them as promising non-viable alternatives to live biotherapeutic products (LBPs).
7.3. In Vivo Studies
Rodent models of breast cancer have corroborated the anti-tumor potential of biotics in vivo, offering crucial insights into host–microbiota–tumor interactions. In a seminal study by Lakritz et al. (2014), oral administration of L. reuteri to MMTV-HER2/neu transgenic mice significantly suppressed spontaneous mammary tumorigenesis [188]. This effect was mechanistically linked to systemic immune modulation, characterized by increased CD4+CD25+FoxP3+ regulatory T cells and suppression of pro-inflammatory cytokines such as IL-6 and TNF-α [224]. These findings suggest that microbial reprogramming may enhance anti-tumor immunity while tempering chronic inflammation, both of which are hallmarks of tumor suppression [225]. Another murine study demonstrated that L. plantarum effectively inhibited tumor growth in a breast cancer model [226,227]. Tumor-bearing mice treated with the probiotic exhibited reduced tumor volumes and downregulation of the PI3K/Akt/mTOR pathway in tumor tissues [228]. Concurrently, the probiotic intervention led to increased fecal abundance of SCFA-producing taxa, notably Faecalibacterium and Roseburia, indicating that microbial metabolic reprogramming contributes to systemic anti-tumor effects [229]. Prebiotics have also shown promise in breast cancer models. In a DMBA-induced carcinogenesis model, rats supplemented with inulin exhibited decreased tumor incidence and multiplicity, likely mediated through enhanced colonic butyrate production and immune activation [230]. Moreover, dietary fructooligosaccharides (FOS) reduced both primary tumor size and pulmonary metastases in a 4T1 mouse model [231]. These effects correlated with increased SCFA levels, restoration of gut microbial diversity, and a shift in the cytokine milieu toward a Th1-dominant, anti-tumor phenotype, as evidenced by elevated IFN-γ and IL-12 expression in splenic lymphocytes [25,232]. Synbiotic formulations, which combine probiotics and prebiotics, have demonstrated synergistic benefits. Even administration of a synbiotic comprising B. longum and inulin significantly attenuated tumor burden in 4T1-bearing mice [233,234]. The synbiotic regimen not only restored microbiota diversity and SCFA levels but also downregulated tumor angiogenesis and invasion markers, including VEGF and MMP-9 [148,235]. These results indicate that synbiotics can simultaneously enhance microbial stability and exert multi-level effects on tumor progression.
Emerging interest in postbiotics has added another dimension to preclinical strategies. Although in vivo studies in breast cancer remain limited, there is growing evidence that the direct administration of SCFAs or microbially derived metabolites could recapitulate many of the benefits observed with live bacterial interventions, potentially with improved safety profiles [236,237,238,239,240,241,242]. Butyrate supplementation in mouse models has demonstrated tumor-suppressive effects through HDAC inhibition and modulation of immune cells. At the same time, tryptophan-derived indole metabolites, such as indole-3-propionic acid (IPA), have shown antioxidant and anti-inflammatory properties in other cancer contexts, warranting further exploration in breast cancer [236]. Taken together, in vitro and in vivo studies underscore the multifaceted mechanisms by which biotics may modulate breast cancer risk and progression. These findings collectively establish a strong biological foundation for translational research, while also highlighting the complexity of host–microbiome–tumor interactions and the need for precise, context-specific interventions.
Several mechanistic pathways described in this review, such as microbial modulation of the gut–brain–cancer axis, metabolite-mediated neurotransmitter signaling, and vagal-immune interactions, remain conceptual and are supported primarily by preclinical or associative human data. While these frameworks are compelling from a systems biology perspective, they should be interpreted as hypotheses rather than established causal mechanisms. Future studies integrating neuroimaging, metabolomics, and microbial profiling are required to determine whether these interactions influence tumor behavior in patients.
8. Clinical Studies: From Pilot Trials to Emerging Translational Evidence
Corroborating insights from preclinical animal models, a growing body of human studies has substantiated the role of gut microbiota in the etiology and progression of breast cancer. Case–control analyses consistently report significant dysbiosis in breast cancer patients, characterized by reduced microbial α-diversity (both richness and evenness), and a notable shift in taxonomic composition compared to healthy controls [186,243,244,245,246,247,248,249,250,251,252]. This diminished diversity reflects a less resilient, less functionally redundant gut ecosystem, which may undermine host metabolic and immune homeostasis. Specifically, there is a recurring depletion of anti-inflammatory and metabolically beneficial taxa, including F. prausnitzii, Bifidobacterium spp., Lactobacillus spp., and A. muciniphila, alongside an enrichment of potentially pathogenic or pro-inflammatory genera such as Escherichia/Shigella, C. hathewayi, and various Enterobacteriaceae [24,253,254,255,256]. Beyond these cross-sectional associations, longitudinal cohort data are beginning to reveal temporal dynamics that link microbial profiles to breast cancer risk. Microbiota-based analyses from the American Gut Project and the Breast Cancer and the Environment Research Program (BCERP) have demonstrated that specific microbial signatures may precede clinical diagnosis [257,258]. Notably, fecal samples collected before diagnosis exhibited elevated abundances of Collinsella, Eggerthella, and Clostridium spp. microorganisms with known roles in estrogen metabolism, oxidative stress, and inflammation [259,260]. These taxa are enriched in genes encoding β-glucuronidase and sulfotransferases, enzymes that deconjugate estrogens in the gut lumen, facilitating enterohepatic recirculation and potentially increasing systemic estrogen levels [49,261,262]. This functional signature of the microbiota, often referred to as the estrobolome, is particularly relevant for hormone receptor-positive breast cancer subtypes [130,163]. Metagenomic and metabolomic investigations further illuminate distinct functional attributes of the gut microbiome in breast cancer patients. Comparative analyses reveal that microbiota in these patients harbor enriched gene pathways involved in estrogen reactivation, bile acid metabolism, and LPS biosynthesis, all of which are implicated in tumor-promoting systemic effects [101,252,263,264]. Elevated microbial β-glucuronidase activity has been detected in fecal metatranscriptomes of postmenopausal breast cancer patients [261], correlating with higher systemic levels of free estrogens. Moreover, increased microbial production of secondary bile acids (e.g., deoxycholic acid), indole derivatives, and phenolic compounds has been reported in serum and fecal metabolomic profiles of breast cancer cohorts [265,266]. These metabolites interact with host nuclear receptors such as the ER, AhR, and FXR, modulating cellular proliferation, apoptosis, and immune response in ways conducive to tumor growth.
The impact of gut microbiota on systemic hormone regulation is especially pronounced in postmenopausal women, in whom endogenous estrogen production shifts primarily to peripheral sources. In this demographic, alterations in the estrobolome may exert outsized effects. For example, higher β-glucuronidase activity observed in the gut microbiota of postmenopausal breast cancer patients has been associated with elevated circulating estradiol levels, suggesting a microbiota-mediated mechanism of hormonal dysregulation [46,145]. These findings suggest a bidirectional crosstalk between the microbial and endocrine axes, with implications for both disease prediction and intervention. Despite robust observational data linking dysbiosis with breast cancer risk, interventional studies evaluating the therapeutic modulation of the microbiota in clinical breast cancer settings remain limited, though recent trials offer encouraging findings. In a pilot randomized controlled trial, Kirtishanti et al. (2025) evaluated the effects of a multispecies probiotic formulation (comprising L. acidophilus, B. bifidum, and Streptococcus thermophilus) in patients with early-stage breast cancer undergoing chemotherapy [267,268,269]. During the intervention period, probiotic administration resulted in improved gut microbial diversity, attenuation of gastrointestinal side effects, and increased peripheral natural killer (NK) cell activity, suggesting an immunostimulatory role for microbial support during cytotoxic therapy [269,270,271]. Similarly, Toi et al. (2021) evaluated the effects of L. casei Shirota (Yakult) in breast cancer patients receiving adjuvant therapy [272]. The probiotic intervention was associated with reduced systemic inflammatory markers, including C-reactive protein (CRP) and IL-6, and a significantly lower incidence of neutropenia, highlighting a potential role for probiotics in mitigating treatment-associated immunosuppression and inflammation [273,274,275]. In addition to probiotic supplementation, observational evidence suggests that diet-induced modulation of the microbiome is a relevant factor in breast cancer risk. A landmark analysis from the Nurses’ Health Study II demonstrated that high dietary fiber intake during adolescence and early adulthood was inversely associated with breast cancer risk later in life [276]. The proposed mechanism centers on the fiber’s capacity to support SCFA-producing taxa and reduce estrogen reabsorption by modulating β-glucuronidase activity.
A follow-up clinical intervention explored the effects of a plant-based, high-fiber diet in breast cancer survivors [273,277]. Participants experienced significant increases in the relative abundance of F. prausnitzii and A. muciniphila, along with improvements in urinary estrogen metabolite ratios (i.e., increased 2-OHE1:16α-OHE1), suggesting enhanced estrogen detoxification capacity and favorable shifts in host-microbiota interactions [145,277,278]. Extending beyond probiotics and diet, recent trials have explored the impact of synbiotic formulations. A double-blind, randomized controlled trial by Khazaei et al. (2023) investigated the effects of multiple Lactobacillus species in postmenopausal breast cancer patients during a 12-week intervention [207]. Recent interventional studies using symbiotic formulations, including multi-species Lactobacillus consortia, demonstrated increased microbial alpha-diversity, enrichment of SCFAs-producing taxa, and elevated circulating or fecal levels of butyrate and propionate [279]. The synbiotic group showed significant improvements in microbial diversity and butyrate production, along with enhanced patient-reported outcomes, including reduced fatigue and improved quality of life [207]. Although oncologic endpoints (e.g., tumor progression or recurrence) were not directly assessed, the observed immune-metabolic benefits underscore the potential for synbiotics as supportive interventions during survivorship and therapy [207]. These metabolic shifts correlate with reduced inflammatory markers and immune modulation in breast cancer survivors and related oncology cohorts [207]. Improvements in patient-reported outcomes, including fatigue, sleep quality, gastrointestinal comfort, and overall quality of life, have also been reported following synbiotic or probiotic interventions [213,280]. Currently, no clinical trials have evaluated the direct administration of postbiotics in the treatment of breast cancer. However, observational studies suggest that reduced levels of SCFAs, particularly butyrate and propionate, are characteristic of the breast cancer microbiome [214,281]. A prospective study further demonstrated that fecal microbiota signatures predictive of butyrate production correlated inversely with systemic inflammation and circulating hormone levels associated with oncogenesis [21,129,282]. These data suggest that postbiotic restoration, whether through diet, probiotics, or direct supplementation, is a promising but underexplored therapeutic avenue. A detailed schematic representation of how the gut microbiome helps maintain a healthy breast and how its imbalance contributes to hormone-positive breast cancer is shown in Figure 5.
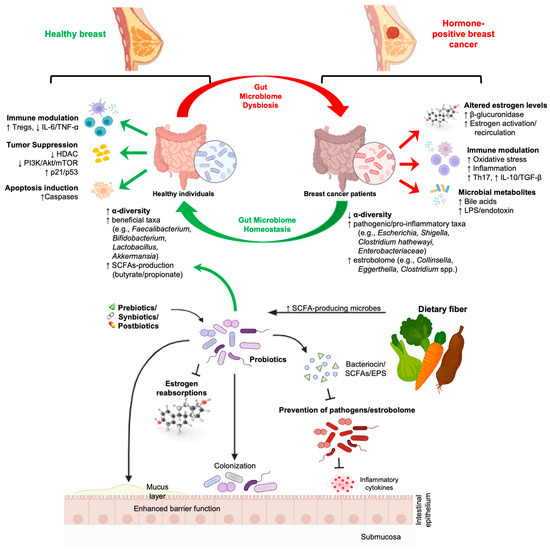
Figure 5.
Gut Microbiome dysbiosis and Hormone-positive breast cancer: bidirectional interactions, microbial metabolites, and microbiome-targeted interventions. The figure illustrates the contrasting microbial and immunometabolism profiles between healthy and hormone-positive breast cancer patients. Healthy individuals exhibit greater α-diversity, enrichment of beneficial taxa (e.g., Faecalibacterium, Bifidobacterium, Lactobacillus, Akkermansia), increased production of short-chain fatty acids (SCFAs), and enhanced anti-inflammatory and tumor-suppressive pathways, including Treg induction, caspase-mediated apoptosis, decreased HDAC activity, and downregulation of PI3K/Akt/mTOR signaling. In contrast, breast cancer patients exhibit reduced microbial diversity, the expansion of pathogenic and pro-inflammatory taxa (Escherichia, Shigella, Clostridium hathewayi, Enterobacteriaceae), elevated β-glucuronidase activity, altered estrogen metabolism, increased oxidative stress, and heightened systemic inflammatory signaling (Th17, IL-17, IL-10/TGF-β). Dysbiosis-driven microbial metabolites (bile acids, LPS/endotoxin) further promote tumor-supportive microenvironments. The figure also highlights how prebiotics, synbiotics, postbiotics, and probiotics restore gut homeostasis by producing SCFAs, modulating estrogen reabsorption, competitively excluding pathogens and estrobolome-producing bacteria, enhancing the mucosal barrier, and improving immune balance.
Collectively, these findings delineate a multifaceted interplay between gut microbiota composition, metabolic function, host hormonal status, and breast cancer risk and progression. The convergence of taxonomic, functional, and metabolic disruptions in the breast cancer microbiome highlights the need for integrative, multi-omics approaches in future clinical research. Longitudinal and interventional studies targeting the microbiota represent a compelling frontier in breast cancer prevention, prognosis, and therapy.
9. Safety, Efficacy, and Clinical Translation
The safety of biotics, especially probiotics, in immunocompromised individuals remains a key concern. While generally recognized as safe (GRAS), probiotics can pose rare risks of bacteremia, sepsis, or fungemia in critically ill or neutropenic patients [283]. Hence, clinical deployment necessitates rigorous safety screening, strain-level characterization, and patient stratification. Prebiotics and postbiotics present fewer safety concerns due to the absence of live organisms, although high intake may lead to bloating, gas, or osmotic diarrhea [284,285,286]. Importantly, postbiotics, such as SCFAs and bacteriocins, may offer therapeutic benefits without the risks associated with live microbes [285,287,288]. Clinical efficacy of biotics is highly variable and influenced by individual microbiome composition, genetic background, and treatment history. Stratified or personalized approaches potentially informed by baseline microbiota profiling and machine learning algorithms are likely required to identify responders.
The timing and context of biotic administration also matter. Some preclinical studies suggest that probiotics may enhance the efficacy of chemotherapeutic agents (e.g., paclitaxel, doxorubicin), while others caution against potential interference with drug metabolism [289,290,291,292]. A nuanced understanding of host–drug–microbe interactions is essential for optimized biotic deployment. Regulatory ambiguities hamper the translation of biotics into clinical practice. Most probiotics and prebiotics are classified as food supplements rather than therapeutics, resulting in a lack of standardized dosing, strain documentation, and clinical endpoints. Regulatory frameworks must evolve to accommodate next-generation biotics and microbiome-modifying agents, potentially under new classifications such as “live biotherapeutic products” (LBPs).
Standardizing biotic interventions remains a significant challenge due to high interindividual variability in microbiota composition, genetics, diet, and comorbidities. Emerging strategies emphasize: (A) strain-level characterization with documented functional and immunological effects; (B) baseline microbiome profiling to identify responder’s vs. non-responder’s; (C) dose and formulation standardization under LBPs’ regulatory frameworks; and (D) integration of machine-learning models to predict clinical response based on microbial features. Ultimately, precision-microbiome approaches, not universal supplementation, will be required to ensure reproducible clinical outcomes.
Microbiota modulation in hormonally sensitive breast cancer may carry theoretical risks. Certain probiotics influencing β-glucuronidase activity could, in principle, increase enterohepatic estrogen recirculation in susceptible individuals. Additionally, abrupt shifts in the microbial ecosystem can alter immune signaling or bile acid metabolism in unpredictable ways. Although these risks have not been documented in clinical trials to date, careful strain selection, monitoring of estrogen-responsive biomarkers, and patient stratification (e.g., ER-positive vs. triple-negative subtypes) are essential in future therapeutic applications.
10. Limitations and Future Challenges
Although the current review offers a mechanistic and translational synthesis, highlighting estrobolome activity, immune modulation, microbial metabolites, and the emerging therapeutic landscape, critical scientific and clinical gaps remain. Despite mounting preclinical, clinical, and molecular evidence linking dysbiosis to breast cancer initiation, progression, and treatment response, the translational pipeline is still emerging. Most human microbiome–breast cancer studies remain cross-sectional, limiting causal inference. Cohorts are often small and heterogeneous, and dietary habits, medication use, ethnicity, metabolic status, and environmental exposures frequently confound observed associations. Additionally, sequencing platforms, sample processing, taxonomic thresholds, and bioinformatic pipelines lack standardization, resulting in poor reproducibility across studies and making cross-study comparisons challenging. Functional interpretation remains another barrier: many reported microbial signatures are based on taxonomic abundance, while mechanistic validation via metabolomics, transcriptomics, and in situ modeling is still limited.
The clinical translation of microbiota-modulating strategies is further constrained by interindividual variability, strain-specific effects, the lack of standardized probiotic doses and delivery systems, and regulatory ambiguity that distinguishes dietary supplements from live biotherapeutic products. Even promising microbial therapies must address unresolved questions regarding optimal treatment windows, durability of microbial engraftment, interaction with conventional therapies, and long-term safety, especially in hormonally sensitive breast cancer subtypes.
Advancing the field requires targeted, mechanistically focused investigation and harmonized methodology. Future priorities include:
- Large-scale longitudinal cohorts integrating metagenomics with metabolomics, epigenomics, and host immune profiling to map temporal host-microbe interactions and identify causal pathways.
- Randomized interventional trials testing dietary modulation, probiotics, prebiotics, synbiotics, and fecal microbiota transplantation (FMT) with well-defined clinical endpoints relevant to tumor burden, recurrence, inflammation, and quality of life.
- Mechanistic studies using gnotobiotic models, CRISPR-engineered microbial strains, and metabolite supplementation to delineate causal roles of specific taxa, enzymes (e.g., β-glucuronidase), and microbial metabolites such as SCFAs, secondary bile acids, and indole derivatives.
- Standardization of sequencing methodologies, biotic strain documentation, dosing strategies, and regulatory pathways to enable reproducible science and safe implementation.
Together, these challenges define a critical agenda for translating microbiome science into precision breast oncology.
11. Conclusions
The gut microbiota has evolved from being viewed as a passive bystander to an active and clinically actionable regulator of breast cancer biology. Dysbiosis influences systemic estrogen availability through the estrobolome, shapes anti-tumor immunity, modulates inflammation, and generates a broad spectrum of bioactive metabolites that can accelerate or suppress tumorigenesis. These mechanistic insights position the gut–breast axis as a promising frontier for the prevention, diagnosis, and treatment of breast cancer. Importantly, microbial signatures may soon serve as non-invasive biomarkers for early detection, subtype differentiation, prediction of therapeutic response, or assessment of recurrence risk. Biotics, including probiotics, prebiotics, synbiotics, and postbiotics, represent modifiable interventions that can restore microbial balance, enhance immune tone, and optimize hormonal and metabolic homeostasis. Although current clinical evidence is still maturing, early trials demonstrate improved microbial diversity, reduced inflammation, enhanced treatment tolerance, and favorable metabolic shifts.
As research moves toward integrative, multi-omics, and precision medicine approaches, microbiome-informed oncology has the potential to transform clinical care. Ultimately, bridging gut microbial science with breast cancer treatment may yield personalized, mechanism-driven, and minimally invasive strategies that complement existing therapies and improve long-term outcomes. The next decade promises a transition from correlation to causation and from discovery to clinical application as the microbiota becomes an integral component of breast cancer prevention and management.
Author Contributions
P.M., S.G., A.P., S.S. and A.G. performed the literature search and wrote the manuscript. S.P.M., S.S. and J.B. prepared the figures. P.M., S.P.M. and S.S.C. had the idea for the article. A.R.S., J.B., S.S., S.S.C. and S.P.M. reviewed and revised the manuscript text critically for important intellectual content. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
No datasets were generated or analyzed in the present study.
Acknowledgments
We gratefully acknowledge the library and information services staff for their valuable assistance with literature searches and access to scientific resources. We also extend our sincere appreciation to colleagues whose insightful feedback helped refine the manuscript during its early stages of drafting.
Conflicts of Interest
The authors declare that there are no commercial or financial relationships that could be construed as a potential conflict of interest regarding the content of this review article.
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Admoun, C.; Mayrovitz, H.N. The Etiology of Breast Cancer. In Breast Cancer; Mayrovitz, H.N., Ed.; Exon Publications: Brisbane, Australia, 2022. [Google Scholar]
- Xu, H.; Xu, B. Breast cancer: Epidemiology, risk factors and screening. Chin. J. Cancer Res. 2023, 35, 565–583. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.; Zheng, L.W.; Ding, Y.; Chen, Y.F.; Cai, Y.W.; Wang, L.P.; Huang, L.; Liu, C.C.; Shao, Z.M.; Yu, K.D. Breast cancer: Pathogenesis and treatments. Signal Transduct. Target. Ther. 2025, 10, 49. [Google Scholar] [CrossRef] [PubMed]
- Dorling, L.; Carvalho, S.; Allen, J.; González-Neira, A.; Luccarini, C.; Wahlström, C.; Pooley, K.A.; Parsons, M.T.; Fortuno, C.; Wang, Q.; et al. Breast Cancer Risk Genes—Association Analysis in More than 113,000 Women. N. Engl. J. Med. 2021, 384, 428–439. [Google Scholar] [CrossRef]
- Gilbert, J.A.; Blaser, M.J.; Caporaso, J.G.; Jansson, J.K.; Lynch, S.V.; Knight, R. Current understanding of the human microbiome. Nat. Med. 2018, 24, 392–400. [Google Scholar] [CrossRef]
- Schoultz, I.; Claesson, M.J.; Dominguez-Bello, M.G.; Fåk Hållenius, F.; Konturek, P.; Korpela, K.; Laursen, M.F.; Penders, J.; Roager, H.; Vatanen, T.; et al. Gut microbiota development across the lifespan: Disease links and health-promoting interventions. J. Intern. Med. 2025, 297, 560–583. [Google Scholar] [CrossRef]
- Ahmadi, S.; Wang, S.; Nagpal, R.; Wang, B.; Jain, S.; Razazan, A.; Mishra, S.P.; Zhu, X.; Wang, Z.; Kavanagh, K.; et al. A human-origin probiotic cocktail ameliorates aging-related leaky gut and inflammation via modulating the microbiota/taurine/tight junction axis. JCI Insight 2020, 5, e132055. [Google Scholar] [CrossRef]
- Trosvik, P.; de Muinck, E.J. Ecology of bacteria in the human gastrointestinal tract--identification of keystone and foundation taxa. Microbiome 2015, 3, 44. [Google Scholar] [CrossRef]
- Goodman, B.; Gardner, H. The microbiome and cancer. J. Pathol. 2018, 244, 667–676. [Google Scholar] [CrossRef]
- El Tekle, G.; Garrett, W.S. Bacteria in cancer initiation, promotion and progression. Nat. Rev. Cancer 2023, 23, 600–618. [Google Scholar] [CrossRef]
- Hou, K.; Wu, Z.-X.; Chen, X.-Y.; Wang, J.-Q.; Zhang, D.; Xiao, C.; Zhu, D.; Koya, J.B.; Wei, L.; Li, J.; et al. Microbiota in health and diseases. Signal Transduct. Target. Ther. 2022, 7, 135. [Google Scholar] [CrossRef]
- Kim, C.H. Complex regulatory effects of gut microbial short-chain fatty acids on immune tolerance and autoimmunity. Cell. Mol. Immunol. 2023, 20, 341–350. [Google Scholar] [CrossRef]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Bäckhed, F. From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef]
- Zheng, D.; Liwinski, T.; Elinav, E. Interaction between microbiota and immunity in health and disease. Cell Res. 2020, 30, 492–506. [Google Scholar] [CrossRef] [PubMed]
- Ervin, S.M.; Li, H.; Lim, L.; Roberts, L.R.; Liang, X.; Mani, S.; Redinbo, M.R. Gut microbial β-glucuronidases reactivate estrogens as components of the estrobolome that reactivate estrogens. J. Biol. Chem. 2019, 294, 18586–18599. [Google Scholar] [CrossRef] [PubMed]
- Morgan, X.C.; Tickle, T.L.; Sokol, H.; Gevers, D.; Devaney, K.L.; Ward, D.V.; Reyes, J.A.; Shah, S.A.; LeLeiko, N.; Snapper, S.B.; et al. Dysfunction of the intestinal microbiome in inflammatory bowel disease and treatment. Genome Biol. 2012, 13, R79. [Google Scholar] [CrossRef]
- Dixon, S.A.; Mishra, S.; Dietsche, K.B.; Jain, S.; Mabundo, L.; Stagliano, M.; Krenek, A.; Courville, A.; Yang, S.; Turner, S.A.; et al. The effects of prebiotics on gastrointestinal side effects of metformin in youth: A pilot randomized control trial in youth-onset type 2 diabetes. Front. Endocrinol. 2023, 14, 1125187. [Google Scholar] [CrossRef]
- Mishra, S.; Wang, S.; Nagpal, R.; Miller, B.; Singh, R.; Taraphder, S.; Yadav, H. Probiotics and prebiotics for the amelioration of type 1 diabetes: Present and future perspectives. Microorganisms 2019, 7, 67. [Google Scholar] [CrossRef]
- Vich Vila, A.; Hu, S.; Andreu-Sánchez, S.; Collij, V.; Jansen, B.H.; Augustijn, H.E.; Bolte, L.A.; Ruigrok, R.; Abu-Ali, G.; Giallourakis, C.; et al. Faecal metabolome and its determinants in inflammatory bowel disease. Gut 2023, 72, 1472–1485. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Song, S.; Liu, J.; Chen, F.; Li, X.; Wu, G. Gut microbiota as a new target for anticancer therapy: From mechanism to means of regulation. NPJ Biofilms Microbiomes 2025, 11, 43. [Google Scholar] [CrossRef]
- Nshanian, M.; Gruber, J.J.; Geller, B.S.; Chleilat, F.; Lancaster, S.M.; White, S.M.; Alexandrova, L.; Camarillo, J.M.; Kelleher, N.L.; Zhao, Y.; et al. Short-chain fatty acid metabolites propionate and butyrate are unique epigenetic regulatory elements linking diet, metabolism and gene expression. Nat. Metab. 2025, 7, 196–211. [Google Scholar] [CrossRef]
- Nomura, M.; Nagatomo, R.; Doi, K.; Shimizu, J.; Baba, K.; Saito, T.; Matsumoto, S.; Inoue, K.; Muto, M. Association of Short-Chain Fatty Acids in the Gut Microbiome With Clinical Response to Treatment With Nivolumab or Pembrolizumab in Patients With Solid Cancer Tumors. JAMA Netw. Open 2020, 3, e202895. [Google Scholar] [CrossRef]
- Arnone, A.A.; Tsai, Y.-T.; Cline, J.M.; Wilson, A.S.; Westwood, B.; Seger, M.E.; Chiba, A.; Howard-McNatt, M.; Levine, E.A.; Thomas, A.; et al. Endocrine-targeting therapies shift the breast microbiome to reduce estrogen receptor-α breast cancer risk. Cell Rep. Med. 2025, 6, 101880. [Google Scholar] [CrossRef]
- Farhadi Rad, H.; Tahmasebi, H.; Javani, S.; Hemati, M.; Zakerhamidi, D.; Hosseini, M.; Alibabaei, F.; Banihashemian, S.Z.; Oksenych, V.; Eslami, M. Microbiota and Cytokine Modulation: Innovations in Enhancing Anticancer Immunity and Personalized Cancer Therapies. Biomedicines 2024, 12, 2776. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, W.; Liu, L.; He, Y.; Luo, H.; Fang, C. Causal effect of gut microbiota on the risk of cancer and potential mediation by inflammatory proteins. World J. Surg. Oncol. 2025, 23, 163. [Google Scholar] [CrossRef]
- Mariat, D.; Firmesse, O.; Levenez, F.; Guimarăes, V.; Sokol, H.; Doré, J.; Corthier, G.; Furet, J.P. The Firmicutes/Bacteroidetes ratio of the human microbiota changes with age. BMC Microbiol. 2009, 9, 123. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Zuo, T.; Frey, N.; Rangrez, A.Y. A systematic framework for understanding the microbiome in human health and disease: From basic principles to clinical translation. Signal Transduct. Target. Ther. 2024, 9, 237. [Google Scholar] [CrossRef] [PubMed]
- Effendi, R.; Anshory, M.; Kalim, H.; Dwiyana, R.F.; Suwarsa, O.; Pardo, L.M.; Nijsten, T.E.C.; Thio, H.B. Akkermansia muciniphila and Faecalibacterium prausnitzii in Immune-Related Diseases. Microorganisms 2022, 10, 2382. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.; Lee, A.R.; Kim, H.; Jhun, J.; Lee, S.Y.; Choi, J.W.; Jeong, Y.; Park, M.S.; Ji, G.E.; Cho, M.L.; et al. Faecalibacterium prausnitzii alleviates inflammatory arthritis and regulates IL-17 production, short chain fatty acids, and the intestinal microbial flora in experimental mouse model for rheumatoid arthritis. Arthritis Res. Ther. 2023, 25, 130. [Google Scholar] [CrossRef]
- Li, H.-B.; Xu, M.-L.; Xu, X.-D.; Tang, Y.-Y.; Jiang, H.-L.; Li, L.; Xia, W.-J.; Cui, N.; Bai, J.; Dai, Z.-M.; et al. Faecalibacterium prausnitzii Attenuates CKD via Butyrate-Renal GPR43 Axis. Circ. Res. 2022, 131, e120–e134. [Google Scholar] [CrossRef]
- Ley, R.E.; Peterson, D.A.; Gordon, J.I. Ecological and Evolutionary Forces Shaping Microbial Diversity in the Human Intestine. Cell 2006, 124, 837–848. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.P.; Jain, S.; Wang, B.; Wang, S.; Miller, B.C.; Lee, J.Y.; Borlongan, C.V.; Jiang, L.; Pollak, J.; Taraphder, S.; et al. Abnormalities in microbiota/butyrate/FFAR3 signaling in aging gut impair brain function. JCI Insight 2024, 9, e168443. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Cheung, J.; Cheung, S.W.M.; Chin, K.T.C.; Leung, R.W.K.; Lam, R.S.T.; Sharma, R.; Yiu, J.H.C.; Woo, C.W. Butyrate acts as a positive allosteric modulator of the 5-HT transporter to decrease availability of 5-HT in the ileum. Br. J. Pharmacol. 2024, 181, 1654–1670. [Google Scholar] [CrossRef]
- Mishra, S.P.; Karunakar, P.; Taraphder, S.; Yadav, H. Free Fatty Acid Receptors 2 and 3 as Microbial Metabolite Sensors to Shape Host Health: Pharmacophysiological View. Biomedicines 2020, 8, 154. [Google Scholar] [CrossRef] [PubMed]
- Grant, E.T.; Parrish, A.; Boudaud, M.; Hunewald, O.; Hirayama, A.; Ollert, M.; Fukuda, S.; Desai, M.S. Dietary fibers boost gut microbiota-produced B vitamin pool and alter host immune landscape. Microbiome 2024, 12, 179. [Google Scholar] [CrossRef] [PubMed]
- Rowland, I.; Gibson, G.; Heinken, A.; Scott, K.; Swann, J.; Thiele, I.; Tuohy, K. Gut microbiota functions: Metabolism of nutrients and other food components. Eur. J. Nutr. 2018, 57, 1–24. [Google Scholar] [CrossRef]
- Gao, H.; Nepovimova, E.; Adam, V.; Heger, Z.; Valko, M.; Wu, Q.; Kuca, K. Age-associated changes in innate and adaptive immunity: Role of the gut microbiota. Front. Immunol. 2024, 15, 1421062. [Google Scholar] [CrossRef]
- Yoo, J.Y.; Groer, M.; Dutra, S.V.O.; Sarkar, A.; McSkimming, D.I. Gut Microbiota and Immune System Interactions. Microorganisms 2020, 8, 1587. [Google Scholar] [CrossRef]
- Spindler, M.P.; Siu, S.; Mogno, I.; Li, Z.; Yang, C.; Mehandru, S.; Britton, G.J.; Faith, J.J. Human gut microbiota stimulate defined innate immune responses that vary from phylum to strain. Cell Host Microbe 2022, 30, 1481–1498.e1485. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, L.; Hua, H.; Liu, L.; Mao, Y.; Wang, R. Interactions between toll-like receptors signaling pathway and gut microbiota in host homeostasis. Immun. Inflamm. Dis. 2024, 12, e1356. [Google Scholar] [CrossRef]
- Martin, A.M.; Sun, E.W.; Rogers, G.B.; Keating, D.J. The Influence of the Gut Microbiome on Host Metabolism Through the Regulation of Gut Hormone Release. Front. Physiol. 2019, 10, 428. [Google Scholar] [CrossRef]
- Jyoti; Dey, P. Mechanisms and implications of the gut microbial modulation of intestinal metabolic processes. npj Metab. Health Dis. 2025, 3, 24. [Google Scholar] [CrossRef]
- Luo, R.; Yao, Y.; Chen, Z.; Sun, X. An examination of the LPS-TLR4 immune response through the analysis of molecular structures and protein–protein interactions. Cell Commun. Signal. 2025, 23, 142. [Google Scholar] [CrossRef]
- Chabi, K.; Sleno, L. Estradiol, Estrone and Ethinyl Estradiol Metabolism Studied by High Resolution LC-MS/MS Using Stable Isotope Labeling and Trapping of Reactive Metabolites. Metabolites 2022, 12, 931. [Google Scholar] [CrossRef]
- Hu, S.; Ding, Q.; Zhang, W.; Kang, M.; Ma, J.; Zhao, L. Gut microbial beta-glucuronidase: A vital regulator in female estrogen metabolism. Gut Microbes 2023, 15, 2236749. [Google Scholar] [CrossRef]
- Simpson, J.B.; Walker, M.E.; Sekela, J.J.; Ivey, S.M.; Jariwala, P.B.; Storch, C.M.; Kowalewski, M.E.; Graboski, A.L.; Lietzan, A.D.; Walton, W.G.; et al. Gut microbial β-glucuronidases influence endobiotic homeostasis and are modulated by diverse therapeutics. Cell Host Microbe 2024, 32, 925–944.e910. [Google Scholar] [CrossRef] [PubMed]
- Fuhrman, B.J.; Feigelson, H.S.; Flores, R.; Gail, M.H.; Xu, X.; Ravel, J.; Goedert, J.J. Associations of the fecal microbiome with urinary estrogens and estrogen metabolites in postmenopausal women. J. Clin. Endocrinol. Metab. 2014, 99, 4632–4640. [Google Scholar] [CrossRef] [PubMed]
- Larnder, A.H.; Manges, A.R.; Murphy, R.A. The estrobolome: Estrogen-metabolizing pathways of the gut microbiome and their relation to breast cancer. Int. J. Cancer 2025, 157, 599–613. [Google Scholar] [CrossRef]
- Shen, Y.; Fan, N.; Ma, S.X.; Cheng, X.; Yang, X.; Wang, G. Gut Microbiota Dysbiosis: Pathogenesis, Diseases, Prevention, and Therapy. MedComm 2025, 6, e70168. [Google Scholar] [CrossRef]
- Glaros, S.B.; Mishra, S.P.; Jain, S.; Davis, F.S.; Gabel, S.A.; Mueller, G.A.; Jarmusch, A.K.; Mabundo, L.; Courville, A.B.; Walter, M.F.; et al. Systemic and gut microbiome changes with metformin and liraglutide in youth-onset type 2 diabetes: The MIGHTY study. Gut Microbes 2025, 17, 2558071. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Tang, L.; Fenton, K.A.; Song, X. Exploring and evaluating microbiome resilience in the gut. FEMS Microbiol. Ecol. 2025, 101, fiaf046. [Google Scholar] [CrossRef]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef]
- Baker, J.M.; Al-Nakkash, L.; Herbst-Kralovetz, M.M. Estrogen-gut microbiome axis: Physiological and clinical implications. Maturitas 2017, 103, 45–53. [Google Scholar] [CrossRef]
- Chiu, K.; Warner, G.; Nowak, R.A.; Flaws, J.A.; Mei, W. The Impact of Environmental Chemicals on the Gut Microbiome. Toxicol. Sci. 2020, 176, 253–284. [Google Scholar] [CrossRef] [PubMed]
- Beurel, E. Stress in the microbiome-immune crosstalk. Gut Microbes 2024, 16, 2327409. [Google Scholar] [CrossRef]
- Anand, N.; Gorantla, V.R.; Chidambaram, S.B. The Role of Gut Dysbiosis in the Pathophysiology of Neuropsychiatric Disorders. Cells 2022, 12, 54. [Google Scholar] [CrossRef]
- Zhang, S.; Xiao, X.; Yi, Y.; Wang, X.; Zhu, L.; Shen, Y.; Lin, D.; Wu, C. Tumor initiation and early tumorigenesis: Molecular mechanisms and interventional targets. Signal Transduct. Target. Ther. 2024, 9, 149. [Google Scholar] [CrossRef]
- Lin, N.Y.-T.; Fukuoka, S.; Koyama, S.; Motooka, D.; Tourlousse, D.M.; Shigeno, Y.; Matsumoto, Y.; Yamano, H.; Murotomi, K.; Tamaki, H.; et al. Microbiota-driven antitumour immunity mediated by dendritic cell migration. Nature 2025, 644, 1058–1068. [Google Scholar] [CrossRef] [PubMed]
- Peters, B.A.; Lin, J.; Qi, Q.; Usyk, M.; Isasi, C.R.; Mossavar-Rahmani, Y.; Derby, C.A.; Santoro, N.; Perreira, K.M.; Daviglus, M.L.; et al. Menopause Is Associated with an Altered Gut Microbiome and Estrobolome, with Implications for Adverse Cardiometabolic Risk in the Hispanic Community Health Study/Study of Latinos. mSystems 2022, 7, e0027322. [Google Scholar] [CrossRef]
- Tao, J.; Dai, W.; Lyu, Y.; Liu, H.; Le, J.; Sun, T.; Yao, Q.; Zhao, Z.; Jiang, X.; Li, Y. Role of intestinal testosterone-degrading bacteria and 3/17β-HSD in the pathogenesis of testosterone deficiency-induced hyperlipidemia in males. npj Biofilms Microbiomes 2024, 10, 123. [Google Scholar] [CrossRef] [PubMed]
- Bui, N.-N.; Li, C.-Y.; Wang, L.-Y.; Chen, Y.-A.; Kao, W.-H.; Chou, L.-F.; Hsieh, J.-T.; Lin, H.; Lai, C.-H. Clostridium scindens metabolites trigger prostate cancer progression through androgen receptor signaling. J. Microbiol. Immunol. Infect. 2023, 56, 246–256. [Google Scholar] [CrossRef]
- McCurry, M.D.; D’Agostino, G.D.; Walsh, J.T.; Bisanz, J.E.; Zalosnik, I.; Dong, X.; Morris, D.J.; Korzenik, J.R.; Edlow, A.G.; Balskus, E.P.; et al. Gut bacteria convert glucocorticoids into progestins in the presence of hydrogen gas. Cell 2024, 187, 2952–2968.e2913. [Google Scholar] [CrossRef]
- Chen, M.J.; Chou, C.H.; Hsiao, T.H.; Wu, T.Y.; Li, C.Y.; Chen, Y.L.; Chao, K.H.; Lee, T.H.; Gicana, R.G.; Shih, C.J.; et al. Clostridium innocuum, an opportunistic gut pathogen, inactivates host gut progesterone and arrests ovarian follicular development. Gut Microbes 2024, 16, 2424911. [Google Scholar] [CrossRef]
- Arp, G.; Jiang, A.K.; Dufault-Thompson, K.; Levy, S.; Zhong, A.; Wassan, J.T.; Grant, M.R.; Li, Y.; Hall, B.; Jiang, X. Identification of gut bacteria reductases that biotransform steroid hormones. Nat. Commun. 2025, 16, 6285. [Google Scholar] [CrossRef] [PubMed]
- Tofani, G.S.S.; Leigh, S.-J.; Gheorghe, C.E.; Bastiaanssen, T.F.S.; Wilmes, L.; Sen, P.; Clarke, G.; Cryan, J.F. Gut microbiota regulates stress responsivity via the circadian system. Cell Metab. 2025, 37, 138–153.e135. [Google Scholar] [CrossRef]
- Mishra, S.P.; Jain, S.; Taraphder, S.; Yadav, H. New horizons in microbiota and metabolic health research. J. Clin. Endocrinol. Metab. 2021, 106, e1052–e1059. [Google Scholar] [CrossRef]
- Eshleman, E.M.; Rice, T.; Potter, C.; Waddell, A.; Hashimoto-Hill, S.; Woo, V.; Field, S.; Engleman, L.; Lim, H.-W.; Schumacher, M.A.; et al. Microbiota-derived butyrate restricts tuft cell differentiation via histone deacetylase 3 to modulate intestinal type 2 immunity. Immunity 2024, 57, 319–332.e316. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, G.; Ma, J.; Duan, Y.; Sharma, S.A.; Oladejo, S.; Ma, X.; Arellano, G.; Orchard, R.C.; Reese, T.A.; et al. HDAC3 integrates TGF-β and microbial cues to program tuft cell biogenesis and diurnal rhythms in mucosal immune surveillance. Sci. Immunol. 2024, 9, eadk7387. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Budina, E.; Raczy, M.M.; Solanki, A.; Nguyen, M.; Beckman, T.N.; Reda, J.W.; Hultgren, K.; Ang, P.S.; Slezak, A.J.; et al. A serine-conjugated butyrate prodrug with high oral bioavailability suppresses autoimmune arthritis and neuroinflammation in mice. Nat. Biomed. Eng. 2024, 8, 611–627. [Google Scholar] [CrossRef]
- Cong, J.; Liu, P.; Han, Z.; Ying, W.; Li, C.; Yang, Y.; Wang, S.; Yang, J.; Cao, F.; Shen, J.; et al. Bile acids modified by the intestinal microbiota promote colorectal cancer growth by suppressing CD8+ T cell effector functions. Immunity 2024, 57, 876–889.e811. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yang, M.; Xu, P.; Du, M.; Li, S.; Shi, J.; Li, Q.; Yuan, J.; Pang, Y. Kynurenine-AhR reduces T-cell infiltration and induces a delayed T-cell immune response by suppressing the STAT1-CXCL9/CXCL10 axis in tuberculosis. Cell. Mol. Immunol. 2024, 21, 1426–1440. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Wang, Z.; Guo, Z.; Zhang, H.; Liu, Y.; Zhang, H.; Jin, H.; Xu, F.; Wang, X.; Xie, C.; et al. Tryptophan Metabolite Indole-3-Aldehyde Induces AhR and c-MYC Degradation to Promote Tumor Immunogenicity. Adv. Sci. 2025, 12, e09533. [Google Scholar] [CrossRef] [PubMed]
- Vaaben, T.H.; Lützhøft, D.O.; Koulouktsis, A.; Dawoodi, I.M.; Stavnsbjerg, C.; Kvich, L.; Gögenur, I.; Vazquez-Uribe, R.; Sommer, M.O.A. Modulating tumor immunity using advanced microbiome therapeutics producing an indole metabolite. EMBO Rep. 2025, 26, 1688–1708. [Google Scholar] [CrossRef]
- Wang, G.; Fan, Y.; Zhang, G.; Cai, S.; Ma, Y.; Yang, L.; Wang, Y.; Yu, H.; Qiao, S.; Zeng, X. Microbiota-derived indoles alleviate intestinal inflammation and modulate microbiome by microbial cross-feeding. Microbiome 2024, 12, 59. [Google Scholar] [CrossRef]
- Nandi, D.; Parida, S.; Sharma, D. The gut microbiota in breast cancer development and treatment: The good, the bad, and the useful! Gut Microbes 2023, 15, 2221452. [Google Scholar] [CrossRef]
- Beaud, D.; Tailliez, P.; Anba-Mondoloni, J. Genetic characterization of the beta-glucuronidase enzyme from a human intestinal bacterium, Ruminococcus gnavus. Microbiology 2005, 151, 2323–2330. [Google Scholar] [CrossRef]
- Plottel, C.S.; Blaser, M.J. Microbiome and malignancy. Cell Host Microbe 2011, 10, 324–335. [Google Scholar] [CrossRef] [PubMed]
- Kwa, M.; Plottel, C.S.; Blaser, M.J.; Adams, S. The Intestinal Microbiome and Estrogen Receptor-Positive Female Breast Cancer. J. Natl. Cancer Inst. 2016, 108, djw029. [Google Scholar] [CrossRef]
- Belkaid, Y.; Hand, T.W. Role of the microbiota in immunity and inflammation. Cell 2014, 157, 121–141. [Google Scholar] [CrossRef]
- Naqash, A.R.; Kihn-Alarcón, A.J.; Stavraka, C.; Kerrigan, K.; Maleki Vareki, S.; Pinato, D.J.; Puri, S. The role of gut microbiome in modulating response to immune checkpoint inhibitor therapy in cancer. Ann. Transl. Med. 2021, 9, 1034. [Google Scholar] [CrossRef]
- Yamane, T.; Kanamori, Y.; Sawayama, H.; Yano, H.; Nita, A.; Ohta, Y.; Hinokuma, H.; Maeda, A.; Iwai, A.; Matsumoto, T.; et al. Iron accelerates Fusobacterium nucleatum-induced CCL8 expression in macrophages and is associated with colorectal cancer progression. JCI Insight 2022, 7, e156802. [Google Scholar] [CrossRef]
- Zheng, X.; Gong, T.; Luo, W.; Hu, B.; Gao, J.; Li, Y.; Liu, R.; Xie, N.; Yang, W.; Xu, X.; et al. Fusobacterium nucleatum extracellular vesicles are enriched in colorectal cancer and facilitate bacterial adhesion. Sci. Adv. 2024, 10, eado0016. [Google Scholar] [CrossRef]
- Dadgar-Zankbar, L.; Elahi, Z.; Shariati, A.; Khaledi, A.; Razavi, S.; Khoshbayan, A. Exploring the role of Fusobacterium nucleatum in colorectal cancer: Implications for tumor proliferation and chemoresistance. Cell Commun. Signal 2024, 22, 547. [Google Scholar] [CrossRef]
- Jacobson, R.; Dineen, S.; Mullinax, J.; Martin, R.; Mishra, S.; Maurin, M.; Soundararajan, R.; Nywening, T.; Karachristos, A.; Yadav, H.; et al. Collagenolytic Enterococcus faecalis induces DDR1 signaling, proliferation and altered immune infiltrate in colorectal peritoneal metastases. Surg. Open Sci. 2025, 28, 65–72. [Google Scholar] [CrossRef]
- Bernstein, H.; Bernstein, C.; Payne, C.M.; Dvorak, K. Bile acids as endogenous etiologic agents in gastrointestinal cancer. World J. Gastroenterol. 2009, 15, 3329–3340. [Google Scholar] [CrossRef] [PubMed]
- Rothhammer, V.; Quintana, F.J. The aryl hydrocarbon receptor: An environmental sensor integrating immune responses in health and disease. Nat. Rev. Immunol. 2019, 19, 184–197. [Google Scholar] [CrossRef] [PubMed]
- Davie, J.R. Inhibition of histone deacetylase activity by butyrate. J. Nutr. 2003, 133, 2485s–2493s. [Google Scholar] [CrossRef]
- Foster, J.A.; McVey Neufeld, K.A. Gut-brain axis: How the microbiome influences anxiety and depression. Trends Neurosci. 2013, 36, 305–312. [Google Scholar] [CrossRef]
- He, X.Y.; Gao, Y.; Ng, D.; Michalopoulou, E.; George, S.; Adrover, J.M.; Sun, L.; Albrengues, J.; Daßler-Plenker, J.; Han, X.; et al. Chronic stress increases metastasis via neutrophil-mediated changes to the microenvironment. Cancer Cell 2024, 42, 474–486.e412. [Google Scholar] [CrossRef]
- Hu, J. Stress-induced metastasis: The NET effect. Cancer Cell 2024, 42, 335–337. [Google Scholar] [CrossRef]
- Lu, Y.; Cheng, D.; Pang, J.; Peng, Y.; Jin, S.; Zhang, X.; Li, Y.; Zuo, Y. Chronic stress promotes gastric cancer progression via the adrenoceptor beta 2/PlexinA1 pathway. Cell Stress. Chaperones 2024, 29, 201–215. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zheng, Q.; Lu, Z.; Wang, L.; Ding, L.; Xia, L.; Zhang, H.; Wang, M.; Chen, Y.; Li, G. Role of the nervous system in cancers: A review. Cell Death Discov. 2021, 7, 76. [Google Scholar] [CrossRef]
- Mishra, S.; Jain, S.; Agadzi, B.; Yadav, H. A Cascade of Microbiota-Leaky Gut-Inflammation- Is it a Key Player in Metabolic Disorders? Curr. Obes. Rep. 2025, 14, 32. [Google Scholar] [CrossRef]
- Parida, S.; Sharma, D. The Microbiome–Estrogen Connection and Breast Cancer Risk. Cells 2019, 8, 1642. [Google Scholar] [CrossRef] [PubMed]
- Toumazi, D.; El Daccache, S.; Constantinou, C. An unexpected link: The role of mammary and gut microbiota on breast cancer development and management (Review). Oncol. Rep. 2021, 45, 80. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, N.; Madyan, E.F.; Yasser, A.; Attia, M.G.; Abdel-Ghany, S.; Sabit, H. Gut microbiota—A key player in breast cancer initiation and progression: A narrative review. Cancer Res. Stat. Treat. 2024, 7, 447–465. [Google Scholar] [CrossRef]
- Álvarez-Mercado, A.I.; del Valle Cano, A.; Fernández, M.F.; Fontana, L. Gut Microbiota and Breast Cancer: The Dual Role of Microbes. Cancers 2023, 15, 443. [Google Scholar] [CrossRef]
- Mishra, S.; Mishra, C.; Mishra, D.; Bhuyan, C.; Rosalin, B. Application of advanced molecular marker technique for improvement of animal: A critical review. J. Entomol. Zool. Stud. 2017, 5, 1283–1295. [Google Scholar]
- Jiang, Y.Z.; Ma, D.; Suo, C.; Shi, J.; Xue, M.; Hu, X.; Xiao, Y.; Yu, K.D.; Liu, Y.R.; Yu, Y.; et al. Genomic and Transcriptomic Landscape of Triple-Negative Breast Cancers: Subtypes and Treatment Strategies. Cancer Cell 2019, 35, 428–440.e425. [Google Scholar] [CrossRef]
- Zhu, J.; Liao, M.; Yao, Z.; Liang, W.; Li, Q.; Liu, J.; Yang, H.; Ji, Y.; Wei, W.; Tan, A.; et al. Breast cancer in postmenopausal women is associated with an altered gut metagenome. Microbiome 2018, 6, 136. [Google Scholar] [CrossRef]
- Hong, W.; Huang, G.; Wang, D.; Xu, Y.; Qiu, J.; Pei, B.; Qian, D.; Meng, X. Gut microbiome causal impacts on the prognosis of breast cancer: A Mendelian randomization study. BMC Genom. 2023, 24, 497. [Google Scholar] [CrossRef] [PubMed]
- Francescone, R.; Hou, V.; Grivennikov, S.I. Microbiome, inflammation, and cancer. Cancer J. 2014, 20, 181–189. [Google Scholar] [CrossRef]
- Ivleva, E.A.; Grivennikov, S.I. Microbiota-driven mechanisms at different stages of cancer development. Neoplasia 2022, 32, 100829. [Google Scholar] [CrossRef]
- Gubernatorova, E.O.; Gorshkova, E.A.; Bondareva, M.A.; Podosokorskaya, O.A.; Sheynova, A.D.; Yakovleva, A.S.; Bonch-Osmolovskaya, E.A.; Nedospasov, S.A.; Kruglov, A.A.; Drutskaya, M.S. Akkermansia muciniphila—Friend or foe in colorectal cancer? Front. Immunol. 2023, 14, 1303795. [Google Scholar] [CrossRef]
- Qu, S.; Zheng, Y.; Huang, Y.; Feng, Y.; Xu, K.; Zhang, W.; Wang, Y.; Nie, K.; Qin, M. Excessive consumption of mucin by over-colonized Akkermansia muciniphila promotes intestinal barrier damage during malignant intestinal environment. Front. Microbiol. 2023, 14, 1111911. [Google Scholar] [CrossRef]
- Dutta, D.; Lim, S.H. Bidirectional interaction between intestinal microbiome and cancer: Opportunities for therapeutic interventions. Biomark. Res. 2020, 8, 31. [Google Scholar] [CrossRef]
- He, R.; Qi, P.; Shu, L.; Ding, Y.; Zeng, P.; Wen, G.; Xiong, Y.; Deng, H. Dysbiosis and extraintestinal cancers. J. Exp. Clin. Cancer Res. 2025, 44, 44. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xie, Q.; Huo, X.; Liu, Z.; Da, M.; Yuan, M.; Zhao, Y.; Shen, G. Impact of intestinal dysbiosis on breast cancer metastasis and progression. Front. Oncol. 2022, 12, 1037831. [Google Scholar] [CrossRef]
- Bernstein, C.; Holubec, H.; Bhattacharyya, A.K.; Nguyen, H.; Payne, C.M.; Zaitlin, B.; Bernstein, H. Carcinogenicity of deoxycholate, a secondary bile acid. Arch. Toxicol. 2011, 85, 863–871. [Google Scholar] [CrossRef] [PubMed]
- Pérez Escriva, P.; Correia Tavares Bernardino, C.; Letellier, E. De-coding the complex role of microbial metabolites in cancer. Cell Rep. 2025, 44, 115358. [Google Scholar] [CrossRef]
- Urbaniak, C.; Gloor, G.B.; Brackstone, M.; Scott, L.; Tangney, M.; Reid, G. The Microbiota of Breast Tissue and Its Association with Breast Cancer. Appl. Environ. Microbiol. 2016, 82, 5039–5048. [Google Scholar] [CrossRef]
- Hieken, T.J.; Chen, J.; Hoskin, T.L.; Walther-Antonio, M.; Johnson, S.; Ramaker, S.; Xiao, J.; Radisky, D.C.; Knutson, K.L.; Kalari, K.R.; et al. The Microbiome of Aseptically Collected Human Breast Tissue in Benign and Malignant Disease. Sci. Rep. 2016, 6, 30751. [Google Scholar] [CrossRef]
- Costantini, L.; Magno, S.; Albanese, D.; Donati, C.; Molinari, R.; Filippone, A.; Masetti, R.; Merendino, N. Characterization of human breast tissue microbiota from core needle biopsies through the analysis of multi hypervariable 16S-rRNA gene regions. Sci. Rep. 2018, 8, 16893. [Google Scholar] [CrossRef]
- Samami, E.; Starkweather, A.; Lyon, D.E.; Kelly, D.L. Associations between dietary fiber, the gut microbiota, and health outcomes in breast cancer survivors: A scoping review. Clin. Nutr. Open Sci. 2025, 61, 174–189. [Google Scholar] [CrossRef]
- Nannini, G.; Cei, F.; Amedei, A. Unraveling the Contribution of Estrobolome Alterations to Endometriosis Pathogenesis. Curr. Issues Mol. Biol. 2025, 47, 502. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Murga, M.L.; Gil-Ortiz, F.; Serrano-García, L.; Llombart-Cussac, A. A New Paradigm in the Relationship between Gut Microbiota and Breast Cancer: β-glucuronidase Enzyme Identified as Potential Therapeutic Target. Pathogens 2023, 12, 1086. [Google Scholar] [CrossRef]
- Lin, W.; Gu, C.; Chen, Z.; Xue, S.; Wu, H.; Zeng, L. Exploring the relationship between gut microbiota and breast cancer risk in European and East Asian populations using Mendelian randomization. BMC Cancer 2024, 24, 970. [Google Scholar] [CrossRef] [PubMed]
- Arnone, A.A.; Wilson, A.S.; Soto-Pantoja, D.R.; Cook, K.L. Diet Modulates the Gut Microbiome, Metabolism, and Mammary Gland Inflammation to Influence Breast Cancer Risk. Cancer Prev. Res. 2024, 17, 415–428. [Google Scholar] [CrossRef]
- Terrisse, S.; Derosa, L.; Iebba, V.; Ghiringhelli, F.; Vaz-Luis, I.; Kroemer, G.; Fidelle, M.; Christodoulidis, S.; Segata, N.; Thomas, A.M.; et al. Intestinal microbiota influences clinical outcome and side effects of early breast cancer treatment. Cell Death Differ. 2021, 28, 2778–2796. [Google Scholar] [CrossRef]
- Eslami, M.; Naderian, R.; Bahar, A.; Babaeizad, A.; Rezanavaz Gheshlagh, S.; Oksenych, V.; Tahmasebi, H. Microbiota as diagnostic biomarkers: Advancing early cancer detection and personalized therapeutic approaches through microbiome profiling. Front. Immunol. 2025, 16, 1559480. [Google Scholar] [CrossRef]
- Huang, M.; Zhang, Y.; Chen, Z.; Yu, X.; Luo, S.; Peng, X.; Li, X. Gut microbiota reshapes the TNBC immune microenvironment: Emerging immunotherapeutic strategies. Pharmacol. Res. 2025, 215, 107726. [Google Scholar] [CrossRef] [PubMed]
- Hillege, L.E.; Barnett, D.J.M.; Ziemons, J.; Aarnoutse, R.; de Vos-Geelen, J.; van Geel, R.; de Boer, M.; van Riet, Y.E.A.; Vincent, J.; Penders, J.; et al. The gut microbiota during tamoxifen therapy in patients with breast cancer. Sci. Rep. 2025, 15, 7874. [Google Scholar] [CrossRef]
- Arnone, A.A.; Ansley, K.; Heeke, A.L.; Howard-McNatt, M.; Cook, K.L. Gut microbiota interact with breast cancer therapeutics to modulate efficacy. EMBO Mol. Med. 2025, 17, 219–234. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tian, L.; Fabi, J.P.; de Vos, P. The potential of prebiotics, probiotics, and synbiotics for ameliorating intestinal barrier dysfunction and modulating inflammatory responses as dietary supplements in diabetes mellitus management. Food Biosci. 2025, 72, 107539. [Google Scholar] [CrossRef]
- Garavaglia, B.; Vallino, L.; Ferraresi, A.; Visciglia, A.; Amoruso, A.; Pane, M.; Munteanu, C.; Isidoro, C. The Anti-Inflammatory, Immunomodulatory, and Pro-Autophagy Activities of Probiotics for Colorectal Cancer Prevention and Treatment: A Narrative Review. Biomedicines 2025, 13, 1554. [Google Scholar] [CrossRef]
- Thoda, C.; Touraki, M. Molecular Mechanisms of Probiotic Action Against Gastrointestinal Cancers. Int. J. Mol. Sci. 2025, 26, 7857. [Google Scholar] [CrossRef]
- Pei, B.; Peng, S.; Huang, C.; Zhou, F. Bifidobacterium modulation of tumor immunotherapy and its mechanism. Cancer Immunol. Immunother. 2024, 73, 94. [Google Scholar] [CrossRef]
- Xie, M.; Li, X.; Lau, H.C.-H.; Yu, J. The gut microbiota in cancer immunity and immunotherapy. Cell. Mol. Immunol. 2025, 22, 1012–1031. [Google Scholar] [CrossRef]
- Mir, R.; Albarqi, S.A.; Albalawi, W.; Alatwi, H.E.; Alatawy, M.; Bedaiwi, R.I.; Almotairi, R.; Husain, E.; Zubair, M.; Alanazi, G.; et al. Emerging Role of Gut Microbiota in Breast Cancer Development and Its Implications in Treatment. Metabolites 2024, 14, 683. [Google Scholar] [CrossRef]
- Barchelouei, N.K.; Yazdi, M.H.; Haghighat, S. Probiotic intervention alters immune gene expression and tumor characteristics in experimental breast cancer. Mol. Biol. Rep. 2025, 52, 809. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Hua, D.; Wu, D.; Hong, W.; Kang, Y.; Tang, L.; Yang, Q.; Wang, X.; Li, B.; Li, R.; et al. Oral Combined Probiotics Clostridium butyricum and Akkermansia muciniphila Inhibits the Progression of 4T1 Breast Cancer by Activating Bcl-2/Bax Pathway. Cancer Med. 2025, 14, e70987. [Google Scholar] [CrossRef]
- Jiang, S.; Ma, W.; Ma, C.; Zhang, Z.; Zhang, W.; Zhang, J. An emerging strategy: Probiotics enhance the effectiveness of tumor immunotherapy via mediating the gut microbiome. Gut Microbes 2024, 16, 2341717. [Google Scholar] [CrossRef]
- Matar, A.; Damianos, J.A.; Jencks, K.J.; Camilleri, M. Intestinal Barrier Impairment, Preservation, and Repair: An Update. Nutrients 2024, 16, 3494. [Google Scholar] [CrossRef]
- Mishra, S.P.; Wang, B.; Jain, S.; Ding, J.; Rejeski, J.; Furdui, C.M.; Kitzman, D.W.; Taraphder, S.; Brechot, C.; Kumar, A.; et al. A mechanism by which gut microbiota elevates permeability and inflammation in obese/diabetic mice and human gut. Gut 2023, 72, 1848–1865. [Google Scholar] [CrossRef]
- Khan, A.A.; Chaudhary, A.A.; Singh, H.; Baig, M.S. Probiotics integrated to cancer vaccines and their potential for cancer management. Microb. Pathog. 2025, 206, 107742. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef]
- Wang, S.; Xiao, Y.; Tian, F.; Zhao, J.; Zhang, H.; Zhai, Q.; Chen, W. Rational use of prebiotics for gut microbiota alterations: Specific bacterial phylotypes and related mechanisms. J. Funct. Foods 2020, 66, 103838. [Google Scholar] [CrossRef]
- Smolinska, S.; Popescu, F.-D.; Zemelka-Wiacek, M. A Review of the Influence of Prebiotics, Probiotics, Synbiotics, and Postbiotics on the Human Gut Microbiome and Intestinal Integrity. J. Clin. Med. 2025, 14, 3673. [Google Scholar] [CrossRef]
- Lordan, C.; Thapa, D.; Ross, R.P.; Cotter, P.D. Potential for enriching next-generation health-promoting gut bacteria through prebiotics and other dietary components. Gut Microbes 2020, 11, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Carlson, J.L.; Erickson, J.M.; Lloyd, B.B.; Slavin, J.L. Health Effects and Sources of Prebiotic Dietary Fiber. Curr. Dev. Nutr. 2018, 2, nzy005. [Google Scholar] [CrossRef] [PubMed]
- Matheus, V.A.; Oliveira, R.B.; Maschio, D.A.; Tada, S.F.S.; Soares, G.M.; Mousovich-Neto, F.; Costa, R.G.; Mori, M.A.; Barbosa, H.C.L.; Collares-Buzato, C.B. Butyrate restores the fat/lean mass ratio balance and energy metabolism and reinforces the tight junction-mediated intestinal epithelial barrier in prediabetic mice independently of its anti-inflammatory and epigenetic actions. J. Nutr. Biochem. 2023, 120, 109409. [Google Scholar] [CrossRef] [PubMed]
- Alberge, J.; Mussard, E.; Al-Ayoubi, C.; Lencina, C.; Marrauld, C.; Cauquil, L.; Achard, C.S.; Mateos, I.; Alassane-Kpembi, I.; Oswald, I.P.; et al. Butyrate reduces epithelial barrier dysfunction induced by the foodborne mycotoxin deoxynivalenol in cell monolayers derived from pig jejunum organoids. Gut Microbes 2024, 16, 2430424. [Google Scholar] [CrossRef] [PubMed]
- Ozkan, A.D.; Eskiler, G.G.; Kazan, N.; Turna, O. Histone deacetylase inhibitor sodium butyrate regulates the activation of toll-like receptor 4/interferon regulatory factor-3 signaling pathways in prostate cancer cells. J. Cancer Res. Ther. 2023, 19, 1812–1817. [Google Scholar] [CrossRef] [PubMed]
- Kang, L.; Chen, H.; He, C.; Li, J. Effects of gut microbiota in breast cancer. Front. Oncol. 2025, 15, 1617410. [Google Scholar] [CrossRef]
- Wu, H.; Witt, B.L.; van der Pol, W.J.; Morrow, C.D.; Duck, L.W.; Tollefsbol, T.O. Combined Phytochemical Sulforaphane and Dietary Fiber Inulin Contribute to the Prevention of ER-Negative Breast Cancer via PI3K/AKT/MTOR Pathway and Modulating Gut Microbial Composition. Nutrients 2025, 17, 2023. [Google Scholar] [CrossRef]
- Sampsell, K.; Wang, W.; Ohland, C.; Mager, L.F.; Pett, N.; Lowry, D.E.; Sales, K.M.; McNeely, M.L.; McCoy, K.D.; Culos-Reed, S.N.; et al. Exercise and Prebiotic Fiber Provide Gut Microbiota-Driven Benefit in a Survivor to Germ-Free Mouse Translational Model of Breast Cancer. Cancers 2022, 14, 2722. [Google Scholar] [CrossRef]
- Boucher, E.; Plazy, C.; Richard, M.L.; Suau, A.; Mangin, I.; Cornet, M.; Aldebert, D.; Toussaint, B.; Hannani, D. Inulin prebiotic reinforces host cancer immunosurveillance via ɣδ T cell activation. Front. Immunol. 2023, 14, 1104224. [Google Scholar] [CrossRef]
- Turati, F.; Esposito, G.; Concina, F.; Fiori, F.; Parpinel, M.; Parazzini, F.; Crispo, A.; Negri, E.; Serraino, D.; La Vecchia, C. Fiber-type prebiotics and gynecological and breast cancers risk: The PrebiotiCa study. Am. J. Epidemiol. 2024, 193, 1693–1700. [Google Scholar] [CrossRef]
- Jeong, H.W.; Kim, J.K.; Kim, A.Y.; Cho, D.; Lee, J.H.; Choi, J.K.; Park, M.; Kim, W. Green Tea Encourages Growth of Akkermansia muciniphila. J. Med. Food 2020, 23, 841–851. [Google Scholar] [CrossRef]
- Zhang, S.; Xu, M.; Sun, X.; Shi, H.; Zhu, J. Green tea extract alters gut microbiota and their metabolism of adults with metabolic syndrome in a host-free human colonic model. Food Res. Int. 2022, 160, 111762. [Google Scholar] [CrossRef]
- Pianetti, S.; Guo, S.; Kavanagh, K.T.; Sonenshein, G.E. Green tea polyphenol epigallocatechin-3 gallate inhibits Her-2/neu signaling, proliferation, and transformed phenotype of breast cancer cells. Cancer Res. 2002, 62, 652–655. [Google Scholar]
- Masuda, M.; Suzui, M.; Lim, J.T.; Weinstein, I.B. Epigallocatechin-3-gallate inhibits activation of HER-2/neu and downstream signaling pathways in human head and neck and breast carcinoma cells. Clin. Cancer Res. 2003, 9, 3486–3491. [Google Scholar]
- Tang, Y.; Zhao, D.Y.; Elliott, S.; Zhao, W.; Curiel, T.J.; Beckman, B.S.; Burow, M.E. Epigallocatechin-3 gallate induces growth inhibition and apoptosis in human breast cancer cells through survivin suppression. Int. J. Oncol. 2007, 31, 705–711. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.W.; Makey, K.L.; Tucker, K.B.; Chinchar, E.; Mao, X.; Pei, I.; Thomas, E.Y.; Miele, L. EGCG, a major green tea catechin suppresses breast tumor angiogenesis and growth via inhibiting the activation of HIF-1α and NFκB, and VEGF expression. Vasc. Cell 2013, 5, 9. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wu, H.; Tollefsbol, T.O. Combined Broccoli Sprouts and Green Tea Polyphenols Contribute to the Prevention of Estrogen Receptor-Negative Mammary Cancer via Cell Cycle Arrest and Inducing Apoptosis in HER2/neu Mice. J. Nutr. 2021, 151, 73–84. [Google Scholar] [CrossRef]
- Swanson, K.S.; Gibson, G.R.; Hutkins, R.; Reimer, R.A.; Reid, G.; Verbeke, K.; Scott, K.P.; Holscher, H.D.; Azad, M.B.; Delzenne, N.M.; et al. The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of synbiotics. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 687–701. [Google Scholar] [CrossRef]
- Hosseini, F.; Pourjam, M.; Mirzaeian, S.; Karimifar, M.; Feizi, A.; Entezari, M.H.; Saraf-Bank, S. Appetite sensation improvement by synbiotic supplementation in patients with metabolic syndrome: A randomized controlled clinical trial. Food Sci. Nutr. 2024, 12, 4772–4782. [Google Scholar] [CrossRef]
- Zolghadrpour, M.-A.; Jowshan, M.-R.; Heidari Seyedmahalleh, M.; Karimpour, F.; Imani, H.; Asghari, S. The effect of a new developed synbiotic yogurt consumption on metabolic syndrome components in adults with metabolic syndrome: A randomized controlled clinical trial. Nutr. Diabetes 2024, 14, 97. [Google Scholar] [CrossRef]
- Basafa-Roodi, P.; Jazayeri, S.; Hadi, F.; Paghaleh, S.J.; Khosravi-darani, K.; Malakouti, S.K. Effects of synbiotic supplementation on the components of metabolic syndrome in patients with schizophrenia: A randomized, double-blind, placebo-controlled trial. BMC Psychiatry 2024, 24, 669. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Hong, J.; Zhang, Y.; Gao, Y.; Liang, L. The effects of synbiotics surpass prebiotics in improving inflammatory biomarkers in children and adults: A systematic review, meta-analysis, and meta-evidence of data from 5207 participants in 90 randomized controlled trials. Pharmacol. Res. 2025, 218, 107832. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, Q.; Zhang, X.; Du, S.; Zhang, Y.; Wang, X.; Liu, Y.; Fang, B.; Chen, J.; Liu, R.; et al. Effects of synbiotics surpass probiotics alone in improving type 2 diabetes mellitus: A randomized, double-blind, placebo-controlled trial. Clin. Nutr. 2025, 44, 248–258. [Google Scholar] [CrossRef]
- Kadia, B.M.; Allen, S.J. Effect of Pre-, Pro-, and Synbiotics on Biomarkers of Systemic Inflammation in Children: A Scoping Review. Nutrients 2024, 16, 336. [Google Scholar] [CrossRef] [PubMed]
- Ramadan, M.E.; Mostafa, T.M.; Ghali, A.A.; El-Afify, D.R. Randomized controlled trial evaluating synbiotic supplementation as an adjuvant therapy in the treatment of Parkinson’s disease. Inflammopharmacology 2025, 33, 3897–3908. [Google Scholar] [CrossRef]
- Ghorbani, Z.; Shoaibinobarian, N.; Noormohammadi, M.; Taylor, K.; Kazemi, A.; Bonyad, A.; Khoshdooz, S.; Löber, U.; Forslund-Startceva, S.K. Reinforcing gut integrity: A systematic review and meta-analysis of clinical trials assessing probiotics, synbiotics, and prebiotics on intestinal permeability markers. Pharmacol. Res. 2025, 216, 107780. [Google Scholar] [CrossRef]
- Neurath, M.F.; Artis, D.; Becker, C. The intestinal barrier: A pivotal role in health, inflammation, and cancer. Lancet Gastroenterol. Hepatol. 2025, 10, 573–592. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Tandorost, A.; Moludi, J.; Dey, P. Prebiotics Plus Probiotics May Favorably Impact on Gut Permeability, Endocannabinoid Receptors, and Inflammatory Biomarkers in Patients with Coronary Artery Diseases: A Clinical Trial. Food Sci. Nutr. 2024, 12, 1207–1217. [Google Scholar] [CrossRef] [PubMed]
- Rafter, J.; Bennett, M.; Caderni, G.; Clune, Y.; Hughes, R.; Karlsson, P.C.; Klinder, A.; O’Riordan, M.; O’Sullivan, G.C.; Pool-Zobel, B.; et al. Dietary synbiotics reduce cancer risk factors in polypectomized and colon cancer patients. Am. J. Clin. Nutr. 2007, 85, 488–496. [Google Scholar] [CrossRef]
- Costabile, A.; Bergillos-Meca, T.; Rasinkangas, P.; Korpela, K.; de Vos, W.M.; Gibson, G.R. Effects of Soluble Corn Fiber Alone or in Synbiotic Combination with Lactobacillus rhamnosus GG and the Pilus-Deficient Derivative GG-PB12 on Fecal Microbiota, Metabolism, and Markers of Immune Function: A Randomized, Double-Blind, Placebo-Controlled, Crossover Study in Healthy Elderly (Saimes Study). Front. Immunol. 2017, 8, 1443. [Google Scholar] [CrossRef]
- Cosier, D.J.; Lambert, K.; Neale, E.P.; Probst, Y.; Charlton, K. The effect of oral synbiotics on the gut microbiota and inflammatory biomarkers in healthy adults: A systematic review and meta-analysis. Nutr. Rev. 2025, 83, e4–e24. [Google Scholar] [CrossRef]
- Shrout, M.R.; Madison, A.A.; Renna, M.E.; Alfano, C.M.; Povoski, S.P.; Lipari, A.M.; Agnese, D.M.; Carson, W.E., 3rd; Malarkey, W.B.; Bailey, M.T.; et al. The gut connection: Intestinal permeability as a pathway from breast cancer survivors’ relationship satisfaction to inflammation across treatment. Brain Behav. Immun. 2022, 100, 145–154. [Google Scholar] [CrossRef]
- Wilkie, T.; Verma, A.K.; Zhao, H.; Charan, M.; Ahirwar, D.K.; Kant, S.; Pancholi, V.; Mishra, S.; Ganju, R.K. Lipopolysaccharide from the commensal microbiota of the breast enhances cancer growth: Role of S100A7 and TLR4. Mol. Oncol. 2022, 16, 1508–1522. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Wang, S.; Wang, D.; Li, Y.; Yip, R.C.S.; Chen, H. Postbiotics-peptidoglycan, lipoteichoic acid, exopolysaccharides, surface layer protein and pili proteins-Structure, activity in wounds and their delivery systems. Int. J. Biol. Macromol. 2024, 274, 133195. [Google Scholar] [CrossRef]
- Nazarinejad, Z.; Molani-Gol, R.; Roozbeh Nasiraie, L.; Ebrahimzadeh-Attari, V. Postbiotics as a novel intervention for obesity management and improving metabolic parameters: A systematic review and meta-analysis of animal studies. J. Transl. Med. 2025, 23, 913. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Y.; E, Q.; Naveed, M.; Wang, X.; Liu, Y.; Li, M. The biological activity and potential of probiotics-derived extracellular vesicles as postbiotics in modulating microbiota-host communication. J. Nanobiotechnol. 2025, 23, 349. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Ahmadi, S.; Nagpal, R.; Jain, S.; Mishra, S.P.; Kavanagh, K.; Zhu, X.; Wang, Z.; McClain, D.A.; Kritchevsky, S.B.; et al. Lipoteichoic acid from the cell wall of a heat killed Lactobacillus paracasei D3-5 ameliorates aging-related leaky gut, inflammation and improves physical and cognitive functions: From C. elegans to mice. GeroScience 2020, 42, 333–352. [Google Scholar] [CrossRef]
- Jeong, H.; Lee, B.; Cho, S.Y.; Lee, Y.; Kim, J.; Hur, S.; Cho, K.; Kim, K.H.; Kim, S.-H.; Nam, K.T. Microbiota-derived short-chain fatty acids determine stem cell characteristics of gastric chief cells. Dev. Cell 2025, 60, 599–612.e596. [Google Scholar] [CrossRef]
- Mohammadi, A.; Kazemipour, N.; Ghorbankhani, G.A.; Morovati, S.; Hashempour Sadeghian, M. Glycated nisin enhances nisin’s cytotoxic effects on breast cancer cells. Sci. Rep. 2024, 14, 17808. [Google Scholar] [CrossRef]
- Assega, B.G.; Tsegaye, K.N.; Mitiku, T.; Tsehai, B.A. Bacteriocins as natural weapon against cancer: In vitro, in vivo, and in silico perspectives. Discov. Appl. Sci. 2025, 7, 940. [Google Scholar] [CrossRef]
- Liu, Y.; Mei, L.; Wang, L.; Tian, P.; Jin, X.; Guo, M.; Lu, J.; Chen, W.; Zhang, H.; Wang, G. The Immunomodulatory Effects of Lipoteichoic Acid from Lactobacillus reuteri L1 on RAW264.7 Cells and Mice Vary with Dose. J. Agric. Food Chem. 2024, 72, 20930–20943. [Google Scholar] [CrossRef]
- Silva de Oliveira, R.; Shupe, A.; Krause, T.; Richardo, T.; Ohland, C.; Sabachvili, M.; Bucher, K.; Hetzer, J.; Hörner, S.; Dauch, D.; et al. Bifidobacteria-derived exopolysaccharide promotes anti-tumor immunity. Cell Rep. 2025, 44, 116223. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Liu, L.; Yan, W.; Li, Y.; Li, Y.; Cui, K.; Yu, P.; Gu, Z.; Zhang, W.; Feng, J.; et al. The anticancer mechanisms of exopolysaccharide from Weissella cibaria D-2 on colorectal cancer via apoptosis induction. Sci. Rep. 2023, 13, 21117. [Google Scholar] [CrossRef]
- Zhang, L.; Huang, S.; Yuan, Y. Butyrate inhibits the malignant biological behaviors of breast cancer cells by facilitating cuproptosis-associated gene expression. J. Cancer Res. Clin. Oncol. 2024, 150, 287. [Google Scholar] [CrossRef]
- Sun, J.; Chen, S.; Zang, D.; Sun, H.; Sun, Y.; Chen, J. Butyrate as a promising therapeutic target in cancer: From pathogenesis to clinic (Review). Int. J. Oncol. 2024, 64, 44. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Martinez, V.D.; León-Del-Río, A.; Camacho-Luis, A.; Ayala-Garcia, V.M.; Lopez-Rodriguez, A.M.; Ruiz-Baca, E.; Meneses-Morales, I. Uncovering a novel mechanism: Butyrate induces estrogen receptor alpha activation independent of estrogen stimulation in MCF-7 breast cancer cells. Genet. Mol. Biol. 2024, 47, e20230110. [Google Scholar] [CrossRef] [PubMed]
- Kharazi, D.M.; Karam, L.; El Boustany, C.; Ibrahim, J.-N. Sodium butyrate and sodium propionate inhibit breast cancer cell migration and invasion through regulation of epithelial-to-mesenchymal transition and suppression of MEK/ERK signaling pathway. Front. Cell Dev. Biol. 2025, 13, 1535563. [Google Scholar] [CrossRef]
- Kadioglu, I.S.; Karaduman Yesildal, T. A non-immunotoxic exopolysaccharide with anticancer effect in colorectal cancer isolated from Lacticaseibacillus rhamnosus ACS5 strain. J. Radiat. Res. Appl. Sci. 2024, 17, 100891. [Google Scholar] [CrossRef]
- Lakritz, J.R.; Poutahidis, T.; Levkovich, T.; Varian, B.J.; Ibrahim, Y.M.; Chatzigiagkos, A.; Mirabal, S.; Alm, E.J.; Erdman, S.E. Beneficial bacteria stimulate host immune cells to counteract dietary and genetic predisposition to mammary cancer in mice. Int. J. Cancer 2014, 135, 529–540. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zhao, J.; Li, D.; Yang, H.; Chen, C.; Qin, M.; Wen, Z.; He, Z.; Xu, L. Akkermansia muciniphila: A potential target and pending issues for oncotherapy. Pharmacol. Res. 2023, 196, 106916. [Google Scholar] [CrossRef]
- Justino, P.F.C.; Melo, L.F.M.; Nogueira, A.F.; Morais, C.M.; Mendes, W.O.; Franco, A.X.; Souza, E.P.; Ribeiro, R.A.; Souza, M.H.L.P.; Soares, P.M.G. Regulatory role of Lactobacillus acidophilus on inflammation and gastric dysmotility in intestinal mucositis induced by 5-fluorouracil in mice. Cancer Chemother. Pharmacol. 2015, 75, 559–567. [Google Scholar] [CrossRef]
- Singh, A.; Zapata, R.C.; Pezeshki, A.; Reidelberger, R.D.; Chelikani, P.K. Inulin fiber dose-dependently modulates energy balance, glucose tolerance, gut microbiota, hormones and diet preference in high-fat-fed male rats. J. Nutr. Biochem. 2018, 59, 142–152. [Google Scholar] [CrossRef]
- Thompson, H.J.; Neuhouser, M.L.; Lampe, J.W.; McGinley, J.N.; Neil, E.S.; Schwartz, Y.; McTiernan, A. Effect of low or high glycemic load diets on experimentally induced mammary carcinogenesis in rats. Mol. Nutr. Food Res. 2016, 60, 1416–1426. [Google Scholar] [CrossRef]
- Liao, M.; Zhang, Y.; Qiu, Y.; Wu, Z.; Zhong, Z.; Zeng, X.; Zeng, Y.; Xiong, L.; Wen, Y.; Liu, R. Fructooligosaccharide supplementation alleviated the pathological immune response and prevented the impairment of intestinal barrier in DSS-induced acute colitis mice. Food Funct. 2021, 12, 9844–9854. [Google Scholar] [CrossRef]
- Chaiyasut, C.; Pattananandecha, T.; Sirilun, S.; Suwannalert, P.; Peerajan, S.; Sundaram Sivamaruthi, B. Synbiotic preparation with Lactic acid bacteria and inulin as a functional food: In vivo evaluation of microbial activities, and preneoplastic aberrant crypt foci. Ciência Tecnol. Aliment. 2017, 37, 328–336. [Google Scholar] [CrossRef]
- Salimi, V.; Shahsavari, Z.; Safizadeh, B.; Hosseini, A.; Khademian, N.; Tavakoli-Yaraki, M. Sodium butyrate promotes apoptosis in breast cancer cells through reactive oxygen species (ROS) formation and mitochondrial impairment. Lipids Health Dis. 2017, 16, 208. [Google Scholar] [CrossRef]
- Eladwy, R.A.; Alsherbiny, M.A.; Chang, D.; Fares, M.; Li, C.-G.; Bhuyan, D.J. The postbiotic sodium butyrate synergizes the antiproliferative effects of dexamethasone against the AGS gastric adenocarcinoma cells. Front. Nutr. 2024, 11, 1372982. [Google Scholar] [CrossRef] [PubMed]
- Tiptiri-Kourpeti, A.; Spyridopoulou, K.; Santarmaki, V.; Aindelis, G.; Tompoulidou, E.; Lamprianidou, E.E.; Saxami, G.; Ypsilantis, P.; Lampri, E.S.; Simopoulos, C.; et al. Lactobacillus casei Exerts Anti-Proliferative Effects Accompanied by Apoptotic Cell Death and Up-Regulation of TRAIL in Colon Carcinoma Cells. PLoS ONE 2016, 11, e0147960. [Google Scholar] [CrossRef] [PubMed]
- Escamilla, J.; Lane, M.A.; Maitin, V. Cell-Free Supernatants from Probiotic Lactobacillus casei and Lactobacillus rhamnosus GG Decrease Colon Cancer Cell Invasion In Vitro. Nutr. Cancer 2012, 64, 871–878. [Google Scholar] [CrossRef] [PubMed]
- Remadevi, V.; Jaikumar, V.S.; Vini, R.; Krishnendhu, B.; Azeez, J.M.; Sundaram, S.; Sreeja, S. Urolithin A, induces apoptosis and autophagy crosstalk in Oral Squamous Cell Carcinoma via mTOR /AKT/ERK1/2 pathway. Phytomedicine 2024, 130, 155721. [Google Scholar] [CrossRef]
- Ehrlich, A.M.; Pacheco, A.R.; Henrick, B.M.; Taft, D.; Xu, G.; Huda, M.N.; Mishchuk, D.; Goodson, M.L.; Slupsky, C.; Barile, D.; et al. Indole-3-lactic acid associated with Bifidobacterium-dominated microbiota significantly decreases inflammation in intestinal epithelial cells. BMC Microbiol. 2020, 20, 357. [Google Scholar] [CrossRef]
- Wekking, D.; Ende, T.v.d.; Bijlsma, M.F.; Vidal-Itriago, A.; Nieuwdorp, M.; van Laarhoven, H.W.M. Fecal microbiota transplantation to enhance cancer treatment outcomes across different cancer types: A systematic literature review. Cancer Treat. Rev. 2025, 140, 103025. [Google Scholar] [CrossRef]
- Honda, S.; Tominaga, Y.; Espadaler-Mazo, J.; Huedo, P.; Aguiló, M.; Perez, M.; Ueda, T.; Sawashita, J. Supplementation with a Probiotic Formula Having β-Glucuronidase Activity Modulates Serum Estrogen Levels in Healthy Peri- and Postmenopausal Women. J. Med. Food 2024, 27, 720–727. [Google Scholar] [CrossRef]
- Bongiovanni, T.; Santiago, M.; Zielinska, K.; Scheiman, J.; Barsa, C.; Jäger, R.; Pinto, D.; Rinaldi, F.; Giuliani, G.; Senatore, T.; et al. A Lactobacillus consortium provides insights into the sleep-exercise-microbiome nexus in proof of concept studies of elite athletes and in the general population. Microbiome 2025, 13, 1. [Google Scholar] [CrossRef]
- Napier, B.A.; Allegretti, J.R.; Feuerstadt, P.; Kelly, C.R.; Van Hise, N.W.; Jäger, R.; Kassam, Z.; Reid, G. Multi-Species Synbiotic Supplementation Enhances Gut Microbial Diversity, Increases Urolithin A and Butyrate Production, and Reduces Inflammation in Healthy Adults: A Randomized, Placebo-Controlled Trial. Nutrients 2025, 17, 2734. [Google Scholar] [CrossRef] [PubMed]
- Mineva, N.D.; Paulson, K.E.; Naber, S.P.; Yee, A.S.; Sonenshein, G.E. Epigallocatechin-3-gallate inhibits stem-like inflammatory breast cancer cells. PLoS ONE 2013, 8, e73464. [Google Scholar] [CrossRef] [PubMed]
- Baruch, E.N.; Youngster, I.; Ben-Betzalel, G.; Ortenberg, R.; Lahat, A.; Katz, L.; Adler, K.; Dick-Necula, D.; Raskin, S.; Bloch, N.; et al. Fecal microbiota transplant promotes response in immunotherapy-refractory melanoma patients. Science 2021, 371, 602–609. [Google Scholar] [CrossRef]
- Khazaei, Y.; Basi, A.; Fernandez, M.L.; Foudazi, H.; Bagherzadeh, R.; Shidfar, F. The effects of synbiotics supplementation on reducing chemotherapy-induced side effects in women with breast cancer: A randomized placebo-controlled double-blind clinical trial. BMC Complement. Med. Ther. 2023, 23, 339. [Google Scholar] [CrossRef]
- Dong, H.; Rowland, I.; Yaqoob, P. Comparative effects of six probiotic strains on immune function in vitro. Br. J. Nutr. 2012, 108, 459–470. [Google Scholar] [CrossRef] [PubMed]
- Kaga, C.; Takagi, A.; Kano, M.; Kado, S.; Kato, I.; Sakai, M.; Miyazaki, K.; Nanno, M.; Ishikawa, F.; Ohashi, Y.; et al. Lactobacillus casei Shirota enhances the preventive efficacy of soymilk in chemically induced breast cancer. Cancer Sci. 2013, 104, 1508–1514. [Google Scholar] [CrossRef]
- Scales, T.Q.; Smith, B.; Blanchard, L.M.; Wixom, N.; Tuttle, E.T.; Altman, B.J.; Peppone, L.J.; Munger, J.; Campbell, T.M.; Campbell, E.K.; et al. A whole food, plant-based diet reduces amino acid levels in patients with metastatic breast cancer. Cancer Metab. 2024, 12, 38. [Google Scholar] [CrossRef]
- McRae, M.P. The Benefits of Dietary Fiber Intake on Reducing the Risk of Cancer: An Umbrella Review of Meta-analyses. J. Chiropr. Med. 2018, 17, 90–96. [Google Scholar] [CrossRef]
- Hajjar, J.; Mendoza, T.; Zhang, L.; Fu, S.; Piha-Paul, S.A.; Hong, D.S.; Janku, F.; Karp, D.D.; Ballhausen, A.; Gong, J.; et al. Associations between the gut microbiome and fatigue in cancer patients. Sci. Rep. 2021, 11, 5847. [Google Scholar] [CrossRef]
- Otto-Dobos, L.D.; Strehle, L.D.; Loman, B.R.; Seng, M.M.; Sardesai, S.D.; Williams, N.O.; Gatti-Mays, M.E.; Stover, D.G.; Sudheendra, P.K.; Wesolowski, R.; et al. Baseline gut microbiome alpha diversity predicts chemotherapy-induced gastrointestinal symptoms in patients with breast cancer. npj Breast Cancer 2024, 10, 99. [Google Scholar] [CrossRef]
- Goedert, J.J.; Hua, X.; Bielecka, A.; Okayasu, I.; Milne, G.L.; Jones, G.S.; Fujiwara, M.; Sinha, R.; Wan, Y.; Xu, X.; et al. Postmenopausal breast cancer and oestrogen associations with the IgA-coated and IgA-noncoated faecal microbiota. Br. J. Cancer 2018, 118, 471–479. [Google Scholar] [CrossRef]
- Campbell, T.M.; Campbell, E.K.; Culakova, E.; Blanchard, L.M.; Wixom, N.; Guido, J.J.; Fetten, J.; Huston, A.; Shayne, M.; Janelsins, M.C.; et al. A whole-food, plant-based randomized controlled trial in metastatic breast cancer: Weight, cardiometabolic, and hormonal outcomes. Breast Cancer Res. Treat. 2024, 205, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Ruo, S.W.; Alkayyali, T.; Win, M.; Tara, A.; Joseph, C.; Kannan, A.; Srivastava, K.; Ochuba, O.; Sandhu, J.K.; Went, T.R.; et al. Role of Gut Microbiota Dysbiosis in Breast Cancer and Novel Approaches in Prevention, Diagnosis, and Treatment. Cureus 2021, 13, e17472. [Google Scholar] [CrossRef] [PubMed]
- Salemi, R.; Vivarelli, S.; Ricci, D.; Scillato, M.; Santagati, M.; Gattuso, G.; Falzone, L.; Libra, M. Lactobacillus rhamnosus GG cell-free supernatant as a novel anti-cancer adjuvant. J. Transl. Med. 2023, 21, 195. [Google Scholar] [CrossRef]
- Naseri, M.H.; Mahdavi, M.; Davoodi, J.; Tackallou, S.H.; Goudarzvand, M.; Neishabouri, S.H. Up regulation of Bax and down regulation of Bcl2 during 3-NC mediated apoptosis in human cancer cells. Cancer Cell Int. 2015, 15, 55. [Google Scholar] [CrossRef]
- Chen, J.; Zhao, K.N.; Vitetta, L. Effects of Intestinal Microbial–Elaborated Butyrate on Oncogenic Signaling Pathways. Nutrients 2019, 11, 1026. [Google Scholar] [CrossRef]
- Bishop, K.S.; Xu, H.; Marlow, G. Epigenetic Regulation of Gene Expression Induced by Butyrate in Colorectal Cancer: Involvement of MicroRNA. Genet. Epigenet. 2017, 9, 1179237x17729900. [Google Scholar] [CrossRef] [PubMed]
- Jan, G.; Belzacq, A.S.; Haouzi, D.; Rouault, A.; Métivier, D.; Kroemer, G.; Brenner, C. Propionibacteria induce apoptosis of colorectal carcinoma cells via short-chain fatty acids acting on mitochondria. Cell Death Differ. 2002, 9, 179–188. [Google Scholar] [CrossRef]
- Vallée, A.; Lecarpentier, Y.; Vallée, J.N. The Key Role of the WNT/β-Catenin Pathway in Metabolic Reprogramming in Cancers under Normoxic Conditions. Cancers 2021, 13, 5557. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Tu, H.; Chen, T. Postbiotics in Human Health: A Narrative Review. Nutrients 2023, 15, 291. [Google Scholar] [CrossRef]
- Zang, X.; Jiang, Y.; Li, X.; Du, Y.; Niu, J. Characteristics of CD4+CD25+Foxp3+ regulatory T cells in patients with multiple organ dysfunction syndrome. Exp. Ther. Med. 2016, 11, 1908–1912. [Google Scholar] [CrossRef] [PubMed]
- Tufail, M.; Jiang, C.-H.; Li, N. Tumor dormancy and relapse: Understanding the molecular mechanisms of cancer recurrence. Mil. Med. Res. 2025, 12, 7. [Google Scholar] [CrossRef] [PubMed]
- Budu, O.; Mioc, A.; Soica, C.; Caruntu, F.; Milan, A.; Oprean, C.; Lighezan, D.; Rotunjanu, S.; Ivan, V.; Banciu, C. Lactiplantibacillus plantarum Induces Apoptosis in Melanoma and Breast Cancer Cells. Microorganisms 2024, 12, 182. [Google Scholar] [CrossRef]
- Hong, C.; Wang, A.; Xia, J.; Liang, J.; Zhu, Y.; Wang, D.; Zhan, H.; Feng, C.; Jiang, X.; Pan, J.; et al. Ginsenoside Rh2-Based Multifunctional Liposomes for Advanced Breast Cancer Therapy. Int. J. Nanomed. 2024, 19, 2879–2888. [Google Scholar] [CrossRef]
- Mohseni, A.H.; Casolaro, V.; Bermúdez-Humarán, L.G.; Keyvani, H.; Taghinezhad, S.S. Modulation of the PI3K/Akt/mTOR signaling pathway by probiotics as a fruitful target for orchestrating the immune response. Gut Microbes 2021, 13, 1886844. [Google Scholar] [CrossRef]
- Geng, P.; Zhao, N.; Zhou, Y.; Harris, R.S.; Ge, Y. Faecalibacterium prausnitzii regulates carbohydrate metabolic functions of the gut microbiome in C57BL/6 mice. Gut Microbes 2025, 17, 2455503. [Google Scholar] [CrossRef]
- Ghali, E.; Pranav; Chauhan, S.C.; Yallapu, M.M. Inulin-based formulations as an emerging therapeutic strategy for cancer: A comprehensive review. Int. J. Biol. Macromol. 2024, 259, 129216. [Google Scholar] [CrossRef]
- Kim, E.J.; Choi, M.R.; Park, H.; Kim, M.; Hong, J.E.; Lee, J.Y.; Chun, H.S.; Lee, K.W.; Yoon Park, J.H. Dietary fat increases solid tumor growth and metastasis of 4T1 murine mammary carcinoma cells and mortality in obesity-resistant BALB/c mice. Breast Cancer Res. 2011, 13, R78. [Google Scholar] [CrossRef]
- Lu, Y.; Yuan, X.; Wang, M.; He, Z.; Li, H.; Wang, J.; Li, Q. Gut microbiota influence immunotherapy responses: Mechanisms and therapeutic strategies. J. Hematol. Oncol. 2022, 15, 47. [Google Scholar] [CrossRef]
- Rodríguez-Sorrento, A.; Castillejos, L.; López-Colom, P.; Cifuentes-Orjuela, G.; Moreno-Muñoz, J.A.; Martín-Orúe, S.M. Assessment of the Effects of the Synbiotic Combination of Bifidobacterium longum subsp. infantis CECT 7210 and Oligofructose-Enriched Inulin Against Digestive Bacterial Infections in a Piglet Model. Front. Microbiol. 2022, 13, 831737. [Google Scholar] [CrossRef] [PubMed]
- Nazari, F.; Jafari, P.; Nomanpour, B.; Varmira, K.; Reissi, F. Preventive effects of microvesicles isolated from Bifidobacterium bifidum on 4T1-induced breast cancer in BALB/c mice. Physiol. Pharmacol. 2022, 26, 333–344. [Google Scholar] [CrossRef]
- Vafa, S.; Haghighat, S.; Janani, L.; Totmaj, A.S.; Navaei, M.; Amirinejad, A.; Emamat, H.; Salehi, Z.; Zarrati, M. The effects of synbiotic supplementation on serum inflammatory markers and edema volume in breast cancer survivors with lymphedema. Excli J. 2020, 19, 1–15. [Google Scholar] [CrossRef]
- Feitelson, M.A.; Arzumanyan, A.; Medhat, A.; Spector, I. Short-chain fatty acids in cancer pathogenesis. Cancer Metastasis Rev. 2023, 42, 677–698. [Google Scholar] [CrossRef]
- Luu, M.; Riester, Z.; Baldrich, A.; Reichardt, N.; Yuille, S.; Busetti, A.; Klein, M.; Wempe, A.; Leister, H.; Raifer, H.; et al. Microbial short-chain fatty acids modulate CD8+ T cell responses and improve adoptive immunotherapy for cancer. Nat. Commun. 2021, 12, 4077. [Google Scholar] [CrossRef]
- Zhu, X.; Li, K.; Liu, G.; Wu, R.; Zhang, Y.; Wang, S.; Xu, M.; Lu, L.; Li, P. Microbial metabolite butyrate promotes anti-PD-1 antitumor efficacy by modulating T cell receptor signaling of cytotoxic CD8 T cell. Gut Microbes 2023, 15, 2249143. [Google Scholar] [CrossRef]
- Hu, Z.; Wei, F.; Su, Y.; Wang, Y.; Shen, Y.; Fang, Y.; Ding, J.; Chen, Y. Histone deacetylase inhibitors promote breast cancer metastasis by elevating NEDD9 expression. Signal Transduct. Target. Ther. 2023, 8, 11. [Google Scholar] [CrossRef]
- Sári, Z.; Mikó, E.; Kovács, T.; Jankó, L.; Csonka, T.; Lente, G.; Sebő, É.; Tóth, J.; Tóth, D.; Árkosy, P.; et al. Indolepropionic Acid, a Metabolite of the Microbiome, Has Cytostatic Properties in Breast Cancer by Activating AHR and PXR Receptors and Inducing Oxidative Stress. Cancers 2020, 12, 2411. [Google Scholar] [CrossRef]
- Xiao, H.-w.; Cui, M.; Li, Y.; Dong, J.-l.; Zhang, S.-q.; Zhu, C.-c.; Jiang, M.; Zhu, T.; Wang, B.; Wang, H.-C.; et al. Gut microbiota-derived indole 3-propionic acid protects against radiation toxicity via retaining acyl-CoA-binding protein. Microbiome 2020, 8, 69. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Cao, Z.; Shi, Z.; Lei, H.; Chen, C.; Yuan, P.; Wu, F.; Liu, C.; Dong, M.; Song, Y.; et al. Microbiome analysis combined with targeted metabolomics reveal immunological anti-tumor activity of icariside I in a melanoma mouse model. Biomed. Pharmacother. 2021, 140, 111542. [Google Scholar] [CrossRef]
- McCune, E.; Sharma, A.; Johnson, B.; O’Meara, T.; Theiner, S.; Campos, M.; Heditsian, D.; Brain, S.; Gilbert Jack, A.; Esserman, L.; et al. Gut and oral microbial compositional differences in women with breast cancer, women with ductal carcinoma in situ, and healthy women. mSystems 2024, 9, e01237-24. [Google Scholar] [CrossRef]
- Zeber-Lubecka, N.; Kulecka, M.; Jagiełło-Gruszfeld, A.; Dąbrowska, M.; Kluska, A.; Piątkowska, M.; Bagińska, K.; Głowienka, M.; Surynt, P.; Tenderenda, M.; et al. Breast cancer but not the menopausal status is associated with small changes of the gut microbiota. Front. Oncol. 2024, 14, 1279132. [Google Scholar] [CrossRef]
- Mahno, N.E.; Tay, D.D.; Khalid, N.S.; Yassim, A.S.M.; Alias, N.S.; Termizi, S.A.; Kasian, J.; Mokhtar, N.M.; Ahmad, H.F. The Relationship Between Gut Microbiome Estrobolome and Breast Cancer: A Systematic Review of Current Evidences. Indian. J. Microbiol. 2024, 64, 1–19. [Google Scholar] [CrossRef]
- Caleça, T.; Ribeiro, P.; Vitorino, M.; Menezes, M.; Sampaio-Alves, M.; Mendes, A.D.; Vicente, R.; Negreiros, I.; Faria, A.; Costa, D.A. Breast Cancer Survivors and Healthy Women: Could Gut Microbiota Make a Difference?—“BiotaCancerSurvivors”: A Case-Control Study. Cancers 2023, 15, 594. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Wang, Z.; Peng, Q.; Lian, W.; Chen, D. Comparison of the Gut Microbiota in Patients with Benign and Malignant Breast Tumors: A Pilot Study. Evol. Bioinform. Online 2021, 17, 11769343211057573. [Google Scholar] [CrossRef] [PubMed]
- Bose, C.; Uczkowski, N.G.; Sukla, K.; Raval, N.; Haque, M.M.; Zhang, Y.; Varma, B.; Satagopan, J.M. Breast cancer and microbiome: A systematic review highlighting challenges for clinical translation. BMC Womens Health 2025, 25, 416. [Google Scholar] [CrossRef]
- Gamba, G.; Colonetti, T.; Uggioni, M.L.R.; Elibio, L.U.; Balbinot, E.L.; Heinzen, R.; Macedo, A.C.L.; Grande, A.J.; da Rosa, M.I. Gut microbiota and breast cancer: Systematic review and meta-analysis. Breast Cancer 2025, 32, 242–257. [Google Scholar] [CrossRef] [PubMed]
- Ullern, A.; Holm, K.; Røssevold, A.H.; Andresen, N.K.; Bang, C.; Lingjærde, O.C.; Naume, B.; Hov, J.R.; Kyte, J.A. Gut microbiota diversity is prognostic and associated with benefit from chemo-immunotherapy in metastatic triple-negative breast cancer. Mol. Oncol. 2025, 19, 1229–1243. [Google Scholar] [CrossRef]
- Kibria, M.K.; Sifat, I.K.; Hossen, M.B.; Hasan, F.; Mosharaf, M.P.; Hassan, M.Z. Identification of bacterial key genera associated with breast cancer using machine learning techniques. Microbe 2025, 6, 100228. [Google Scholar] [CrossRef]
- Peng, Y.; Gu, J.; Liu, F.; Wang, P.; Wang, X.; Si, C.; Gong, J.; Zhou, H.; Qin, A.; Song, F. Integrated analysis of microbiota and gut microbial metabolites in blood for breast cancer. mSystems 2024, 9, e0064324. [Google Scholar] [CrossRef]
- Dikeocha, I.J.; Al-Kabsi, A.M.; Chiu, H.T.; Alshawsh, M.A. Faecalibacterium prausnitzii Ameliorates Colorectal Tumorigenesis and Suppresses Proliferation of HCT116 Colorectal Cancer Cells. Biomedicines 2022, 10, 1128. [Google Scholar] [CrossRef]
- Abdelqader, E.M.; Mahmoud, W.S.; Gebreel, H.M.; Kamel, M.M.; Abu-Elghait, M. Correlation between gut microbiota dysbiosis, metabolic syndrome and breast cancer. Sci. Rep. 2025, 15, 6652. [Google Scholar] [CrossRef] [PubMed]
- Bilenduke, E.; Sterrett, J.D.; Ranby, K.W.; Borges, V.F.; Grigsby, J.; Carr, A.L.; Kilbourn, K.; Lowry, C.A. Impacts of breast cancer and chemotherapy on gut microbiome, cognitive functioning, and mood relative to healthy controls. Sci. Rep. 2022, 12, 19547. [Google Scholar] [CrossRef] [PubMed]
- Altinok Dindar, D.; Chun, B.; Palma, A.; Cheney, J.; Krieger, M.; Kasschau, K.; Stagaman, K.; Mitri, Z.I.; Goodyear, S.M.; Shannon, J.; et al. Association between Gut Microbiota and Breast Cancer: Diet as a Potential Modulating Factor. Nutrients 2023, 15, 4628. [Google Scholar] [CrossRef]
- Wong, A.Y.W.; Bicchieraro, G.; Palumbo, I.; Ciabattoni, A.; Aristei, C.; Spaccapelo, R. Microbial Signatures in Breast Cancer: Exploring New Potentials Across Body Niches. Int. J. Mol. Sci. 2025, 26, 8654. [Google Scholar] [CrossRef]
- Cardona, B.; Rudel, R.A. Application of an in Vitro Assay to Identify Chemicals That Increase Estradiol and Progesterone Synthesis and Are Potential Breast Cancer Risk Factors. Env. Environ. Health Perspect. 2021, 129, 77003. [Google Scholar] [CrossRef]
- Byrd, D.A.; Vogtmann, E.; Wu, Z.; Han, Y.; Wan, Y.; Clegg-Lamptey, J.N.; Yarney, J.; Wiafe-Addai, B.; Wiafe, S.; Awuah, B.; et al. Associations of fecal microbial profiles with breast cancer and nonmalignant breast disease in the Ghana Breast Health Study. Int. J. Cancer 2021, 148, 2712–2723. [Google Scholar] [CrossRef] [PubMed]
- Bobin-Dubigeon, C.; Luu, H.T.; Leuillet, S.; Lavergne, S.N.; Carton, T.; Le Vacon, F.; Michel, C.; Nazih, H.; Bard, J.M. Faecal Microbiota Composition Varies between Patients with Breast Cancer and Healthy Women: A Comparative Case-Control Study. Nutrients 2021, 13, 2705. [Google Scholar] [CrossRef]
- Sui, Y.; Wu, J.; Chen, J. The Role of Gut Microbial β-Glucuronidase in Estrogen Reactivation and Breast Cancer. Front. Cell Dev. Biol. 2021, 9, 631552. [Google Scholar] [CrossRef]
- Kumari, N.; Kumari, R.; Dua, A.; Singh, M.; Kumar, R.; Singh, P.; Duyar-Ayerdi, S.; Pradeep, S.; Ojesina, A.I.; Kumar, R. From Gut to Hormones: Unraveling the Role of Gut Microbiota in (Phyto)Estrogen Modulation in Health and Disease. Mol. Nutr. Food Res. 2024, 68, e2300688. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Dong, W.; Sun, D.; Zhao, X.; Huang, Z.; Liu, C.; Sheng, Y. Bacterial metabolites: Effects on the development of breast cancer and therapeutic efficacy (Review). Oncol. Lett. 2025, 29, 210. [Google Scholar] [CrossRef]
- Manzoor, H.; Kayani, M.U.R. Insights into the gut microbiome-metabolite dynamics in breast cancer. Gut Microbes Rep. 2025, 2, 2483446. [Google Scholar] [CrossRef]
- Liu, J.; Shi, J.; Zhang, T.; Chen, M.; Li, Z.; Lu, C.; Wang, F. Serum and Fecal Metabolite Profiles Linking With Gut Microbiome in Triple-Negative Breast Cancer Patients. Breast Cancer 2024, 18, 11782234241285645. [Google Scholar] [CrossRef] [PubMed]
- Perl, M.; Fante, M.A.; Herfeld, K.; Scherer, J.N.; Poeck, H.; Thiele Orberg, E. Microbiota-derived metabolites: Key modulators of cancer immunotherapies. Med 2025, 6, 100773. [Google Scholar] [CrossRef]
- Kirtishanti, A.; Wijono, H.; Kok, T.; Setiawan, E.; Tanggo, V.V.C.M.; Zahara, G.S.; Davina, W.; Presley, B. Effect of multi-strain probiotics supplementation on chemotherapy-related side effects among patients with breast cancer: A pilot trial. Pharmacia 2025, 72, 1–9. [Google Scholar] [CrossRef]
- Ahmad, M.F.; Ahmad, F.A.; Alsayegh, A.A.; Zeyaullah, M.; Babalghith, A.O.; Faidah, H.; Ahmed, F.; Khanam, A.; Mozaffar, B.; Kambal, N.; et al. Probiotics and Cancer: Mechanistic Insights and Organ-Specific Impact. Biomolecules 2025, 15, 879. [Google Scholar] [CrossRef]
- Kaufman, P.; O’Meara, K.E.; Hawrelak, J. Preventing chemotherapy-induced diarrhea and microbiota imbalances with prebiotics and probiotics in breast cancer treatment: A case report. Gut Microbes Rep. 2024, 1, 2379475. [Google Scholar] [CrossRef]
- Juan, Z.; Chen, J.; Ding, B.; Yongping, L.; Liu, K.; Wang, L.; Le, Y.; Liao, Q.; Shi, J.; Huang, J.; et al. Probiotic supplement attenuates chemotherapy-related cognitive impairment in patients with breast cancer: A randomised, double-blind, and placebo-controlled trial. Eur. J. Cancer 2022, 161, 10–22. [Google Scholar] [CrossRef]
- Zhao, L.-Y.; Mei, J.-X.; Yu, G.; Lei, L.; Zhang, W.-H.; Liu, K.; Chen, X.-L.; Kołat, D.; Yang, K.; Hu, J.-K. Role of the gut microbiota in anticancer therapy: From molecular mechanisms to clinical applications. Signal Transduct. Target. Ther. 2023, 8, 201. [Google Scholar] [CrossRef]
- Toi, M.; Hirota, S.; Tomotaki, A.; Sato, N.; Hozumi, Y.; Anan, K.; Nagashima, T.; Tokuda, Y.; Masuda, N.; Ohsumi, S.; et al. Probiotic Beverage with Soy Isoflavone Consumption for Breast Cancer Prevention: A Case-control Study. Curr. Nutr. Food Sci. 2013, 9, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Sempach, L.; Doll, J.P.K.; Limbach, V.; Marzetta, F.; Schaub, A.-C.; Schneider, E.; Kettelhack, C.; Mählmann, L.; Schweinfurth-Keck, N.; Ibberson, M.; et al. Examining immune-inflammatory mechanisms of probiotic supplementation in depression: Secondary findings from a randomized clinical trial. Transl. Psychiatry 2024, 14, 305. [Google Scholar] [CrossRef]
- Romero-Ferreiro, V.; García-Fernández, L.; Biscaia, J.M.; Romero, C.; González-Soltero, R.; De la Fuente, M.; Álvarez-Mon, M.A.; Wynn, R.; Rodriguez-Jimenez, R. Effect of probiotics on C-reactive protein levels in schizophrenia: Evidence from a systematic review and meta-analysis. Complement. Ther. Med. 2025, 89, 103126. [Google Scholar] [CrossRef]
- Yu, X.; Yan, L.; Chen, L.; Shen, X.; Zhang, W. Alleviating effects of probiotic supplementation on biomarkers of inflammation and oxidative stress in non-communicable diseases: A systematic review and meta-analysis using the GRADE approach. BMC Pharmacol. Toxicol. 2025, 26, 124. [Google Scholar] [CrossRef]
- Farvid, M.S.; Eliassen, A.H.; Cho, E.; Liao, X.; Chen, W.Y.; Willett, W.C. Dietary Fiber Intake in Young Adults and Breast Cancer Risk. Pediatrics 2016, 137, e20151226. [Google Scholar] [CrossRef]
- Campbell, E.K.; Campbell, T.M.; Culakova, E.; Blanchard, L.; Wixom, N.; Guido, J.J.; Fetten, J.; Huston, A.; Shayne, M.; Janelsins, M.C.; et al. A whole food, plant-based randomized controlled trial in metastatic breast cancer: Feasibility, nutrient, and patient-reported outcomes. Breast Cancer Res. Treat. 2024, 206, 273–283. [Google Scholar] [CrossRef]
- Li, Z.; Yin, Z.; Chen, W.; Wang, Z. Impact of Gut and Reproductive Tract Microbiota on Estrogen Metabolism in Endometriosis. Am. J. Reprod. Immunol. 2025, 93, e70109. [Google Scholar] [CrossRef]
- Abolhassani, A.; Esmaili, H.; Rahati, S.; Jafarnejad, S. The Role of Lactobacillus Strain Probiotics in Breast Cancer: Strain-Specific Mechanisms and Therapeutic Potential Beyond Probiotics. Probiotics Antimicrob. Proteins 2025. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Cheng, L.; Cheng, X.; Wang, Y.; Lin, X.; Xia, S. The Mediating Role of Gut Microbiota on the Association Between Dietary Quality and Cancer-Related Fatigue Among Breast Cancer Patients: A Cross-Sectional Study. Nutrients 2024, 16, 4371. [Google Scholar] [CrossRef] [PubMed]
- Goedert, J.J.; Jones, G.; Hua, X.; Xu, X.; Yu, G.; Flores, R.; Falk, R.T.; Gail, M.H.; Shi, J.; Ravel, J.; et al. Investigation of the association between the fecal microbiota and breast cancer in postmenopausal women: A population-based case-control pilot study. J. Natl. Cancer Inst. 2015, 107, djv147. [Google Scholar] [CrossRef]
- Adlakha, Y.K.; Chhabra, R. The human microbiome: Redefining cancer pathogenesis and therapy. Cancer Cell Int. 2025, 25, 165. [Google Scholar] [CrossRef] [PubMed]
- Venugopalan, V.; Shriner, K.A.; Wong-Beringer, A. Regulatory oversight and safety of probiotic use. Emerg. Infect. Dis. 2010, 16, 1661–1665. [Google Scholar] [CrossRef] [PubMed]
- Martyniak, A.; Wójcicka, M.; Rogatko, I.; Piskorz, T.; Tomasik, P.J. A Comprehensive Review of the Usefulness of Prebiotics, Probiotics, and Postbiotics in the Diagnosis and Treatment of Small Intestine Bacterial Overgrowth. Microorganisms 2025, 13, 57. [Google Scholar] [CrossRef]
- Calvanese, C.M.; Villani, F.; Ercolini, D.; De Filippis, F. Postbiotics versus probiotics: Possible new allies for human health. Food Res. Int. 2025, 217, 116869. [Google Scholar] [CrossRef]
- Guo, S.; Ma, T.; Kwok, L.-Y.; Quan, K.; Li, B.; Wang, H.; Zhang, H.; Menghe, B.; Chen, Y. Effects of postbiotics on chronic diarrhea in young adults: A randomized, double-blind, placebo-controlled crossover trial assessing clinical symptoms, gut microbiota, and metabolite profiles. Gut Microbes 2024, 16, 2395092. [Google Scholar] [CrossRef]
- Pattapulavar, V.; Ramanujam, S.; Kini, B.; Christopher, J.G. Probiotic-derived postbiotics: A perspective on next-generation therapeutics. Front. Nutr. 2025, 12, 1624539. [Google Scholar] [CrossRef]
- Hamdi, A.; Lloyd, C.; Eri, R.; Van, T.T.H. Postbiotics: A Promising Approach to Combat Age-Related Diseases. Life 2025, 15, 1190. [Google Scholar] [CrossRef]
- Cuozzo, M.; Castelli, V.; Avagliano, C.; Cimini, A.; d’Angelo, M.; Cristiano, C.; Russo, R. Effects of Chronic Oral Probiotic Treatment in Paclitaxel-Induced Neuropathic Pain. Biomedicines 2021, 9, 346. [Google Scholar] [CrossRef]
- Cheng, C.-F.; Nguyen, T.K.N.; Shen, S.-C.; Chen, B.-Y.; Wu, Y.-B.; Liang, H.-J.; Wu, C.-H. Synbiotic Supplementation Attenuates Doxorubicin-Induced Oxidative Stress and Inflammation in the Gut-Heart Axis of Chemotherapy-Treated Mice. Int. J. Mol. Sci. 2025, 26, 5136. [Google Scholar] [CrossRef]
- Huang, C.; Li, X.; Li, H.; Chen, R.; Li, Z.; Li, D.; Xu, X.; Zhang, G.; Qin, L.; Li, B.; et al. Role of gut microbiota in doxorubicin-induced cardiotoxicity: From pathogenesis to related interventions. J. Transl. Med. 2024, 22, 433. [Google Scholar] [CrossRef] [PubMed]
- Mafe, A.N.; Büsselberg, D. Microbiome Integrity Enhances the Efficacy and Safety of Anticancer Drug. Biomedicines 2025, 13, 422. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).