Abstract
CRISPR-Cas systems are best known as adaptive immune defenses in prokaryotes, but they also function as versatile regulators bridging bacterial immunity with host-related processes. Beyond neutralizing invasive phages and plasmids, these systems influence core aspects of bacterial physiology, such as modulating gene expression, stress responses, biofilm formation, quorum sensing, and virulence. Notably, CRISPR-mediated regulation can facilitate immune evasion at the host-pathogen interface, underscoring these systems as central orchestrators of microbial survival and host interactions. In addition, CRISPR-Cas has rapidly become a cornerstone of synthetic biology and microbiome engineering. Recent strategies repurpose native and engineered CRISPR systems to precisely modulate microbiome composition or deliver sequence-specific antimicrobials, underscoring the expanding translational potential of this system. Collectively, emerging insights highlight both the canonical immune function and non-canonical regulatory roles of CRISPR-Cas, as well as their broad biological and biotechnological relevance. This review provides a critical synthesis of these developments, illustrating how CRISPR-Cas bridges adaptive immunity and microbial physiology, and outlines future directions for harnessing this duality to deepen understanding of microbial physiology and inform new translational applications.
1. Introduction
1.1. Overview of CRISPR-Cas Systems
The CRISPR-Cas system functions as an adaptive immune mechanism in prokaryotes, consisting of DNA loci known as clustered regularly interspaced short palindromic repeat (CRISPR) and adjacent Cas (CRISPR-associated) genes, which encode specialized proteins [1]. A CRISPR locus comprises short repetitive DNA sequences interspersed with unique “spacer” segments, which are derived from viruses or plasmids. These spacers serve as genetic records of previous infections. Concurrently, Cas genes encode nucleases, helicases, and other proteins that facilitate the immune response [2,3]. CRISPR-based immunity is characterized by three distinct stages: adaptation, which involves the integration of a new spacer from foreign DNA into the CRISPR array; expression, which encompasses the transcription and processing of the array into CRISPR RNAs (crRNAs); and interference, which entails the formation of an effector complex, wherein a crRNA directs Cas proteins to identify and cleave complementary nucleic acids [4,5]. Through this mechanism, bacteria and archaea achieve sequence-specific defense against invasive genetic elements, similar to an immune “memory” that targets recurring viruses for elimination [5,6].
CRISPR sequences were first identified in bacteria in 1987. However, their functions remained enigmatic until the mid-2000s. A pivotal insight emerged in 2005, when researchers observed that numerous CRISPR spacers corresponded to sequences from bacteriophages. This observation led to the hypothesis that CRISPR may serve as a prokaryotic antiviral system [1]. In 2007, experimental evidence confirmed that a Streptococcus thermophilus strain acquired phage resistance by incorporating new spacers following phage exposure. This finding provides direct evidence that the CRISPR-Cas system confers adaptive immunity against viruses [7]. The discovery that bacteria and archaea possess an adaptive, heritable immune system has fundamentally transformed our understanding of microbe–virus interactions. In CRISPR-based immunity, the microorganism effectively “remembers” previous infections by storing fragments of invader DNA, subsequently utilizing this memory to identify and eliminate invading pathogens with remarkable sequence specificity [8].
Researchers have identified a diverse array of CRISPR-Cas systems, which has led to their formal classification into several types and two broad classes. Class 1 CRISPR systems, which are prevalent in most CRISPR-bearing bacteria and nearly all archaea, utilize multi-protein effector complexes, such as the cascade complex with Cas3 nuclease. In contrast, class 2 systems depend on a single large Cas protein, such as Cas9, Cas12, or Cas13, to function as the effector responsible for cleaving target nucleic acids [9]. CRISPR-Cas systems are categorized into six primary types (Types I–VI), each characterized by a distinct signature Cas nuclease. These types encompass numerous subtypes, with Cas3, Cas9, Cas10, Cas12, and Cas13 serving as signature effectors for Types I, II, III, V, and VI, respectively [10]. As of 2020, a revised classification identified two classes, six types, and at least 33 subtypes of CRISPR-Cas systems (Table 1), highlighting the considerable diversity of this adaptive immunity mechanism in prokaryotes [9]. Beyond their structural classification, these systems are now recognized as key players influencing bacterial adaptation and host interactions, which are explored in the following sections.

Table 1.
Classification of CRISPR-Cas systems.
1.2. Historical Context
1.2.1. Evolutionary Development in Bacteria and Archaea
CRISPR-Cas defense mechanisms are ancient and appear to have co-evolved with the persistent threat of viruses within microbial ecosystems. Genomic analyses indicate that approximately 40% of sequenced bacteria and over 80% of archaea possess at least one CRISPR-Cas system [11,12], indicating this adaptive immunity occurred early and has been retained in many lineages. Phylogenetic analyses indicate that the multi-component Class 1 systems may constitute the original form of CRISPR-Cas immunity. For example, the complex Type III systems, which are capable of targeting both DNA and RNA, are considered a common ancestral branch from which other types have evolved [13,14]. In contrast, Class 2 systems, such as the Cas9-based Type II, likely emerged at a later stage and are predominantly found in bacteria. It is noteworthy that the distribution of CRISPR-Cas systems is uneven. Many bacterial taxa, as well as some archaeal groups, completely lack these systems. This suggests that there may be fitness costs or alternative anti-phage strategies that render CRISPR-Cas systems unnecessary in certain ecological niches [9,15]. In environments characterized by high viral diversity, microbes frequently possess multiple distinct CRISPR-Cas systems within a single genome. This phenomenon reflects the intense evolutionary “arms race” between hosts and their viruses. For example, nearly all known hyperthermophilic archaea encode several CRISPR-Cas variants, likely due to the strong selective pressure exerted by diverse co-existing viruses, which favors the retention of a broad array of immune defense modules [16,17].
1.2.2. From Microbial Immunity to Genome Editing
Over the past decade, the CRISPR-Cas system has evolved significantly from a simple bacterial defense mechanism to a transformative tool in biotechnology. A pivotal moment occurred in 2012–2013, when researchers successfully re-engineered the Type II CRISPR-Cas9 system from Streptococcus into a streamlined two-component format. This innovation involved the integration of the Cas9 enzyme with a synthetic single-guide RNA, enabling precise targeting and cleavage of DNA at specified sequences [18,19]. This significant advancement involved the repurposing of a bacterial immune nuclease for precise genome editing, representing a transformative technological development that initiated a new era in genetic engineering [1]. The CRISPR-Cas9 genome editing technology, renowned for its ease and precision, has been rapidly demonstrated across a variety of cell types and organisms, thereby catalyzing a proliferation of applications ranging from fundamental research to agricultural enhancement and gene therapy. Moreover, the CRISPR toolkit continues to expand as researchers have adapted additional Cas enzymes, such as Cas12 (Type V) for alternative DNA editing capabilities and Cas13 (Type VI) for RNA targeting and editing, thereby extending CRISPR’s applicability beyond DNA cleavage [20].
Recent comprehensive analyses have expanded on this evolutionary transition, emphasizing how CRISPR-Cas systems have progressed from bacterial immune mechanisms to pivotal tools in modern therapeutic and translational biotechnology. Saeed et al. (2025) [21] highlighted the rapid diversification of CRISPR platforms, including the discovery of novel Cas variants (CasΦ, CasX/Cas12f), precision editing approaches such as base and prime editing, and their applications in treating neurodegenerative, cardiovascular, viral, and inherited disorders. It further underscores the transformative trajectory of CRISPR from microbial defense to precision medicine, aligning with the growing body of work linking basic bacterial immunity to advanced gene therapy and diagnostic technologies. Likewise, CRISPR technology has entered a new decade of innovation, transforming not only biomedical research but also agriculture and environmental engineering. CRISPR innovations highlighted the progression from RNA-guided genome editors to precision tools enabling base and prime editing, the development of high-fidelity Cas variants, and the translation of these systems into clinical therapies such as CRISPR-based treatments for sickle-cell disease and beta-thalassemia. These perspectives provide a comprehensive overview of how CRISPR’s foundational microbial functions now underpin transformative applications across multiple domains of life [22]. In conclusion, what initially functioned as a microbial defense mechanism has now become a fundamental component of contemporary biotechnology. This transition from microbial defense to biotechnology also underscores CRISPR’s broader biological relevance, paving the way for understanding its emerging roles in bacterial physiology and host interactions.
2. CRISPR Role in Host-Pathogen Interaction
2.1. Adaptive Immunity Against Foreign Genetic Elements
CRISPR-Cas systems function as advanced adaptive immune mechanisms in bacteria and archaea, safeguarding these prokaryotic hosts against invasive genetic elements such as bacteriophages and plasmids [23]. These systems comprise a CRISPR locus, an array of short repetitive DNA sequences interspersed with unique “spacer” sequences derived from previous invaders, and adjacent Cas genes that encode the protein machinery necessary for defense. During the adaptation (spacer acquisition) phase of immunity, specialized Cas1–Cas2 integrase complexes capture short fragments of foreign DNA (protospacers) from an infecting virus or plasmid and integrate them as new spacers at the CRISPR locus [24]. The integration of spacers derived from invaders establishes a heritable genetic record of the pathogen.
During the subsequent interference phase, the CRISPR array undergoes transcription and is processed into small CRISPR RNAs (crRNAs). These crRNAs then assemble with Cas nucleases to form a ribonucleoprotein complex, known as the effector complex. This complex is guided by base-pair complementarity between the crRNA spacer sequence and the target protospacer within the invader genome, enabling it to bind and cleave the foreign nucleic acid, thereby neutralizing the threat. It is noteworthy that most DNA-targeting CRISPR systems necessitate the presence of a short protospacer-adjacent motif (PAM) flanking the target sequence for efficient recognition, which aids in distinguishing non-self DNA from self DNA [8,24,25]. Through the mechanism of spacer acquisition and targeted interference, the CRISPR-Cas system functions as an adaptive, sequence-specific immune system. This system enables prokaryotes to withstand recurrent attacks by identical phages or mobile genetic elements. The effectiveness of this immune response is demonstrated by the direct communication of numerous CRISPR spacers to phage or plasmid sequences, as well as by experimental evidence indicating that the acquisition of phage-derived spacers informs heritable phage resistance to the host cell [26,27]. Such CRISPR-Cas defenses play a pivotal role in host-pathogen interactions, shaping the co-evolutionary arms race between bacteria and their genetic invaders. Interestingly, recent studies show that the molecular machinery responsible for antiviral protection is not limited to defense alone. Several bacteria have adapted the same CRISPR-Cas components to regulate cellular communication, metabolic pathways, and stress responses that contribute to survival within the host environment.
2.2. Regulation of Endogenous Gene Expression
CRISPR-Cas systems in bacteria serve functions beyond the defense against foreign DNA. They also play a role in regulating the host organism’s gene expression and physiological processes [28]. These systems can target the bacterial genome or transcripts, thereby modulating genes involved in metabolism, virulence, and stress responses. The mechanism often involves direct interference with transcription or cleavage of specific mRNA transcripts [29]. For example, in Salmonella typhi, a CRISPR-Cas system regulates an outer membrane protein regulator (OmpR), altering the synthesis of outer membrane proteins (Omp) (Figure 1) [30]. Likewise, in Francisella novicida, the Cas9 protein uses a small CRISPR-associated RNA (scaRNA) to repress an endogenous immunostimulatory lipoprotein gene, effectively silencing its transcription [31].
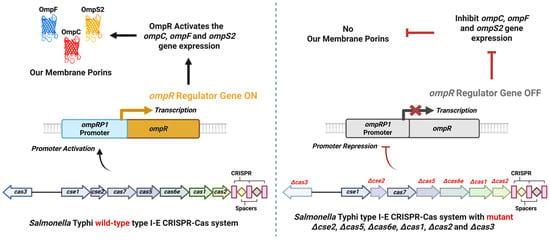
Figure 1.
CRISPR-Cas–mediated regulation of the OmpR-dependent porin system in Salmonella Typhi. In the wild-type strain with an active CRISPR-Cas locus, the coordinated activity of Cas3, Cse2, Cas5, Cas6e, Cas1, and Cas2 activates the ompRP1 promoter, leading to ompR transcription. OmpR, in turn, upregulates ompC, ompF, and ompS2, promoting proper porin synthesis (Left panel). In CRISPR-Cas mutants (Δcse2, Δcas5, Δcas6e, Δcas1, Δcas2, Δcas3), the ompRP1 promoter is repressed, resulting in loss of OmpR production and consequent inhibition of ompC, ompF, and ompS2 expression. This repression leads to impaired envelope function and altered stress resistance (Right panel). The diagram summarizes findings from Medina-Aparicio et al., 2021 [30]. (Created using Biorender.com).
Another crucial aspect is the control of mobile genetic elements (MGEs) like plasmids and transposons, which help maintain genomic integrity and optimize fitness [32]. CRISPR-Cas systems often carry spacers matching plasmid or prophage sequences, indicating the ability to recognize and neutralize these elements if they become active [31]. For instance, in Acinetobacter baumannii, a type I-Fb CRISPR-Cas system was shown to prevent uptake of an antibiotic resistance plasmid and thereby reduce virulence, highlighting how CRISPR limits horizontal gene transfer to benefit the host cell [33]. This regulatory role over MGEs ensures that bacteria do not acquire genetic elements that could be deleterious or energetically costly. In addition, CRISPR-Cas system activity in modulating metabolic operations as well as stress responses further substantiates their plasticity in roles beyond simple protection at an immune level [34]. Under nutrient-deficient conditions, these systems can downregulate non-essential metabolic pathways to conserve resources for vital processes. Conversely, when nutrients are plentiful, CRISPR-Cas may allow for full metabolic activity.
During environmental challenges, such as oxidative stress or DNA damage, CRISPR-Cas systems are frequently activated to confer cellular protection. For instance, evidence indicates that CRISPR-Cas transcription is upregulated under such stress conditions, thereby mitigating stress-induced damage to the bacterium [28]. In A. baumannii, the deletion of the cas3 gene not only impacted virulence but also modified the regulation of carbon metabolism and oxidative phosphorylation pathways [35], suggesting that a functional CRISPR-Cas system plays a role in coordinating metabolic responses to stress. By specifically targeting stress-response genes, such as those involved in DNA repair, membrane stabilization, or the production of heat-shock proteins, the CRISPR-Cas system can ensure that the cell allocates energy towards functions critical for survival. These multifaceted regulatory roles of CRISPR-Cas underscore their versatility in bacterial survival and adaptation beyond simple immune defense [28]. Table 2 summarizes experimental evidence of the role of CRISPR-Cas in endogenous gene expression across bacteria.

Table 2.
Roles of CRISPR-Cas in endogenous gene expression in bacteria.
2.3. Influence on Biofilm Formation
Biofilms are structured communities of bacteria encapsulated within a self-produced extracellular matrix, which adhere to surfaces. This mode of existence significantly enhances bacterial persistence by providing protection against antibiotics, desiccation, and host immune responses [45,46]. Bacteria in biofilms can be up to 1000 times more resistant to antibiotics than free-living cells [46]. CRISPR-Cas systems play a significant role in modulating biofilm formation by regulating the expression of genes associated with biofilm production, including those encoding exopolysaccharides, adhesins, and matrix enzymes [47]. Additionally, these systems influence the signaling pathways that govern biofilm dynamics [47,48]. Under specific conditions, bacteria employ the CRISPR-Cas system to mitigate excessive biofilm accumulation. The overproduction of the biofilm matrix can occasionally impede nutrient access or result in energy wastage; thus, CRISPR-Cas functions to inhibit critical factors that promote biofilm formation. This process frequently involves the enhanced degradation of RNA transcripts or signaling molecules that trigger matrix production [49]. For example, when researchers employed a CRISPR-Cas9 system to target the quorum-sensing regulator sdiA in Salmonella, they noted a reduction in cell adhesion and biofilm formation [46]. This implies that the native CRISPR-Cas can similarly interfere with biofilm-promoting signals. By moderating transcription of biofilm-associated genes, CRISPR-Cas helps avoid hyper-biofilm formation that could impede growth or resource uptake.
In certain contexts, the activity of CRISPR-Cas systems can actually promote the development of biofilms, particularly when these biofilms function as protective environments against threats such as antibiotics or bacteriophages. A study was observed in S. enterica, where the deletion of the cas3 gene, which encodes the principal nuclease of a Type I-E CRISPR-Cas system, resulted in a marked reduction in biofilm formation. In contrast, the wild-type CRISPR-Cas system facilitated substantial biofilm development and enhanced virulence [35,39]. Transcriptomic analysis has demonstrated that the CRISPR-Cas system in wild-type Salmonella specifically targets the lsr operon, which is involved in the uptake and processing of the AI-2 quorum-sensing signal. By partially inhibiting the lsr genes, such as lsrF and lsrG, which are responsible for the degradation of AI-2, Cas3 facilitates the accumulation of AI-2. This accumulation subsequently inactivates the repressor LsrR and leads to the upregulation of the entire biofilm matrix production pathway [39]. In essence, Salmonella’s CRISPR-Cas boosts quorum-sensing signals to induce biofilm formation when it is beneficial for survival. Another study showed that a Campylobacter jejuni strain with an intact Type II-C CRISPR-Cas formed stronger biofilms than a CRISPR-deficient mutant, suggesting that without CRISPR control, biofilm formation was attenuated [42]. This dynamic regulation ensures that the biofilm maintains an optimal density, which is essential for providing adequate protection. It also prevents the biofilm from becoming excessively abundant, thereby allowing resources to be allocated more efficiently to other areas. In C. jejuni, the CRISPR-Cas system also interacts dynamically with bacteriophages. Certain phages encode Cas4-like proteins that promote the acquisition of host-derived CRISPR spacers, linking phage infection to bacterial immune adaptation. During this carrier state, bacteria and phages coexist in equilibrium, allowing for spacer renewal while maintaining phage tolerance. This adaptive phage-CRISPR interplay may indirectly enhance biofilm persistence and stress resilience, complementing CRISPR’s regulatory role in C. jejuni biofilm formation [50,51].
In addition to their direct impact on biofilm-related genes, CRISPR-Cas systems play a role in biofilm formation through their interactions with bacteriophages. Numerous bacterial genomes contain lysogenic phages (prophages), the activation of which can disrupt biofilms by either lysing host cells or modifying gene expression [52]. The CRISPR-Cas system serves as a regulatory mechanism for prophages. Bacteria frequently possess CRISPR spacers that correspond to prophage DNA, suggesting that the CRISPR system can target and sustain prophage dormancy. By regulating lysogenic phages, CRISPR-Cas contributes to the stability of biofilms and prevents abrupt phage-induced lysis within the microbial community. In the case of Francisella, both the Cas9 and Cas12a systems contain spacers against a resident prophage, ensuring its latency unless activation is required [31]. CRISPR-Cas helps protect biofilms from harmful viruses called lytic phages. If a phage enters a biofilm, bacteria with CRISPR-Cas can destroy the phage’s DNA. This stops the biofilm from being destroyed [53]. In this way, CRISPR-Cas systems are critically important for bacterial resilience, particularly in the regulation of biofilms and interactions with lysogenic phages within specific environments. Phage defense is essential in environments where phages are prevalent. CRISPR-Cas systems offer a dual benefit to biofilms: they modulate the extent and architecture of the biofilm and function as a collective immune system against phage incursions. This ensures that bacterial communities form biofilms that are optimally robust, sufficiently strong to provide protection, yet not excessively overgrown to become detrimental, and remain resilient against external threats.
2.4. Interaction with Quorum-Sensing Mechanisms
Quorum sensing (QS) is a bacterial communication mechanism that coordinates population-wide behaviors through the release of signaling molecules known as autoinducers. When these signaling molecules reach a critical concentration, they trigger changes in gene expression, leading to collective behaviors such as biofilm formation, virulence factor production, and bioluminescence, among others [54]. By targeting and modulating key components of quorum sensing, CRISPR-Cas systems have the capacity to either enhance or suppress bacterial community behaviors [55]. The CRISPR-Cas system has the capability to bind to or cleave the DNA/RNA of key quorum-sensing (QS) regulators, thereby modulating the production or response of QS signals. By degrading the messenger RNAs of transcriptional regulators within QS circuits, CRISPR-Cas can attenuate or amplify QS signals. For instance, a CRISPR interference strategy that targeted the luxS gene, responsible for synthesizing the AI-2 autoinducer in E. coli, resulted in a significant reduction in biofilm formation (Figure 2) [46]. This demonstrates the principle that interfering with QS genes leads to altered group behavior. In natural environments, a bacterium’s endogenous CRISPR-Cas system may similarly identify and cleave QS regulator mRNAs. This mechanism enables the system to suppress quorum-sensing signals and subsequent behaviors when such actions would be disadvantageous. Modulating QS can be beneficial to prevent premature expression of virulence factors or to remain undetected at low population densities [56].
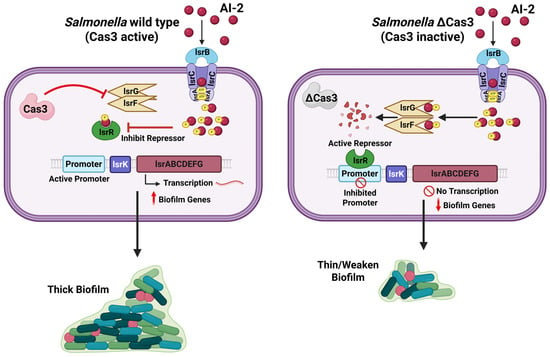
Figure 2.
Role of Cas3 in regulating quorum sensing and biofilm formation in Salmonella. In wild Salmonella, Cas3 interferes with the activity of the lsrF/G genes, reducing degradation of the quorum-sensing signal autoinducer-2 (AI-2). This leads to the accumulation of AI-2, inhibition of the repressor LsrR, activation of the lsr operon promoter, and transcription of the lsr operon and other biofilm-associated genes, resulting in robust and thick biofilm formation. In the absence of Cas3, lsrF/G genes remain active, causing AI-2 degradation. The active repressor LsrR inhibits the lsr operon promoter, suppressing transcription of other lsr operon and associated biofilm-associated genes and leading to weakened, thin biofilm architecture (Created using Biorender.com).
QS is strongly linked to virulence in many pathogens. Pathogenic bacteria often use QS to turn on virulence genes only when they have a sufficient population to overwhelm the host [57]. By targeting QS pathways, CRISPR-Cas has the potential to modulate virulence in a density-dependent manner. The implication of this finding is that CRISPR-Cas systems influence quorum sensing within the framework of biofilm dynamics and pathogen production [48]. QS supports the production and dispersal of extracellular polymeric matrix components, such as those involved in bioluminescence-associated biofilm formation [58]. Meanwhile, CRISPR-Cas systems can indirectly influence these processes by modulating quorum sensing signals, for example, by targeting quorum sensing-regulatory mRNAs, thereby affecting matrix synthesis and dispersal through an indirect regulatory pathway [59]. Moreover, pathogenic bacteria regulate the expression of virulence genes through quorum-sensing systems. By targeting these signaling pathways, CRISPR-Cas systems hold the potential to modulate bacterial virulence.
2.5. Modulation of Virulence Factors
CRISPR-Cas systems have emerged as significant modulators of bacterial virulence [29]. These systems can regulate the expression of virulence factor genes, thereby enabling pathogens to fine-tune their pathogenicity in response to environmental cues. This regulation can occur through the direct targeting of virulence gene transcripts. When a CRISPR-Cas system possesses a spacer that matches an endogenous virulence gene (or a regulator of virulence genes), the resulting crRNA-Cas complex can bind to and cause degradation or transcriptional repression of that mRNA, thus reducing the production of the virulence factor [60]. This mechanism enables bacteria to downregulate specific toxins or surface proteins when their expression may be disadvantageous, such as in scenarios where they could elicit a robust immune response under adverse conditions. Conversely, under conditions favorable for infection, relieving that CRISPR-based repression can quickly ramp up virulence factor production.
A well-documented instance is the Cas9-based CRISPR system in F. novicida, which primarily does not serve an immune function but instead targets one of the bacterium’s own genes. Specifically, Cas9 represses an endogenous bacterial lipoprotein that is highly immunostimulatory to the host. By silencing the expression of this lipoprotein, the bacterium effectively conceals one of its molecular signatures from the host immune system. Consequently, this results in enhanced virulence: mutants lacking Cas9 (and thus unable to repress the lipoprotein) exhibit significantly reduced virulence due to increased detection by the host. A Δcas9 strain of F. novicida exhibits significant attenuation in animal models; however, the deletion of the immunostimulatory lipoprotein gene results in the restoration of virulence [31]. This finding underscores the critical role of Cas9 in regulating a specific virulence factor gene, which is essential for the pathogenicity of F. novicida.
Beyond the regulation of individual genes, CRISPR-Cas systems have the capacity to modulate entire virulence regulons. In the previously mentioned study on Salmonella, the presence of Cas3 was found to influence the Salmonella Pathogenicity Island-1 (SPI-1) regulon, which comprises a cluster of genes responsible for encoding a Type III secretion system and effectors necessary for host cell invasion. Notably, the majority of these invasion-related genes were markedly downregulated in the cas3-null mutant, suggesting that a functional CRISPR-Cas system is essential for the full activation of Salmonella’s invasion machinery [39]. In Enterococcus faecalis, strains possessing a CRISPR-Cas system have been observed to result in higher mortality rates in mice compared to strains lacking CRISPR, indicating a potential association between CRISPR loci and virulence [61]. Researchers hypothesize that the CRISPR-Cas system may modulate virulence by interacting with global regulatory elements or by detecting signals that enable the activation or repression of virulence genes as required [28].
2.6. Evasion of Host Immune Responses
Pathogenic microorganisms must effectively evade or withstand the host’s immune response to ensure their survival. Bacteria have developed several CRISPR-Cas–mediated mechanisms to circumvent immune detection and destruction. CRISPR-Cas systems facilitate bacterial evasion of the immune system by downregulating the expression of highly immunogenic proteins on their surfaces. For instance, F. novicida employs Cas9 to suppress a surface lipoprotein that would otherwise elicit a robust immune response [31]. By degrading the mRNA of these proteins or otherwise inhibiting their expression, the CRISPR-Cas system reduces the number of target molecules available for recognition by immune cells. This mechanism of immune evasion enables the bacterium to establish an initial presence within the host without immediately triggering the host’s immune defenses.
Within a host organism, bacteria are subjected to reactive oxygen species (ROS), antimicrobial peptides, and various other stressors imposed by immune cells. The CRISPR-Cas system plays a crucial role in enhancing stress resistance, thereby facilitating immune evasion. Under conditions of oxidative stress, such as those encountered within a macrophage’s phagolysosome, bacteria possessing active CRISPR-Cas systems exhibit improved capacity to manage cellular damage. In certain instances, the CRISPR-Cas system is upregulated in response to oxidative or envelope stress, thereby contributing to the prevention of DNA damage and other forms of cellular injury [28]. In Staphylococcus aureus, the Type III-A CRISPR-Cas system is activated in response to phage-induced oxidative stress, indicating its potential function in mitigating such adverse conditions [62]. Similarly, Streptococcus anginosus strains possessing CRISPR-Cas systems exhibited distinct stress survival profiles compared to those lacking such systems, suggesting a trade-off between immune function and stress tolerance [63].
As previously discussed, numerous bacteria employ QS to regulate virulence, often increasing the production of toxins or biofilm upon reaching high cell densities. Nevertheless, an intense QS response can also activate the immune system, for example, by prompting the bacteria to produce molecules that host cells recognize as foreign or harmful. The CRISPR-Cas system provides bacteria with a mechanism to modulate QS when a more hidden approach is required. An illustrative example is in Pseudomonas aeruginosa, where the deletion of its cas3 gene resulted in elevated levels of the QS regulator LasR, which consequently rendered the infection more detectable by the host immune system through TLR4 recognition [38]. Under normal circumstances, the CRISPR-Cas system likely regulates QS-controlled virulence, maintaining it in check until the bacteria are prepared for a comprehensive attack. This concept is consistent with observations indicating that isolates from chronic infections frequently possess functional CRISPR systems and demonstrate reduced virulence, thereby facilitating persistent colonization rather than acute disease manifestation. Through mechanisms such as reducing antigenicity, enhancing stress resilience, and modifying communication signals, CRISPR-Cas systems play a crucial role in enabling bacteria to evade host immune responses. This dual functionality of CRISPR-Cas, offering both immune defense against phages and immune evasion from the host, positions it as a significant factor in pathogenesis.
3. Host Defense Against Horizontal Gene Transfer (HGT)
3.1. CRISPR-Cas Systems as Barriers to HGT
CRISPR-Cas systems are highly specialized adaptive immune systems of bacteria and archaea that are essential to protect genomic integrity by mitigating horizontal gene transfer (HGT) [64]. HGT, known as the movement of genetic material between organisms independent of classical reproduction, is one of the main methods by which bacteria attain beneficial characteristics like antibiotic resistance or virulence factors [65]. Although HGT allows for genetic diversity, it comes at a considerable threat to bacterial populations by introducing potentially harmful or disruptive genetic material. CRISPR-Cas systems act as a barrier to protect bacteria, specifically by targeting and inactivating foreign genetic material [66]. Mobile genetic elements (MGEs), such as plasmids, transposons, and bacteriophages, represent significant vectors for HGT. CRISPR-Cas systems recognize these elements through PAMs [67]. Once recognized, the Cas effector complex binds to the target DNA, initiating its cleavage and degradation. For example, in Escherichia coli, the Type I-E CRISPR-Cas system employs a multi-subunit Cascade complex to recognize and bind the target DNA, while the Cas3 nuclease degrades the DNA in a processive manner [68]. This targeted degradation prevents the replication and integration of MGEs, effectively halting the spread of foreign genetic material within the bacterial population.
Plasmids and bacteriophages are key vectors for gene exchange within microbial populations. Plasmids usually encode genes for antibiotic resistance or metabolic benefit, whereas bacteriophages enable lysogenic conversion and stimulate the horizontal dissemination of virulence factors [69]. The CRISPR-Cas systems are remarkable for their efficacy at combating these issues. Studies have shown that those of Streptococcus thermophilus have a powerful capacity to recognize and cleave phage DNA, to give protection against phage infections [64]. As a result, the acquisition of plasmids is limited by site-specific cleavage at replication origins or essential maintenance genes. The restriction ensures that only those plasmids lacking CRISPR-targeting sequences are able to survive and thus control influxes of potentially harmful genetic material.
Beyond single bacterial cells, the activity of CRISPR-Cas systems shapes the genetic landscape of whole microbial communities. By selectively restricting the propagation of MGEs, these systems shape the gene pool available for HGT, affecting microbial diversity and evolutionary paths [70]. For example, the inhibition of conjugative plasmids by CRISPR-Cas systems in Enterococcus faecalis has been correlated with decreased spread of antibiotic resistance genes in clinical settings [71]. Although CRISPR-Cas systems are effective, they encounter some challenges. Certain mobile genetic elements (MGEs) have evolved mechanisms to counteract CRISPR systems, including the production of anti-CRISPR proteins that inhibit the activity of CRISPR effector complexes [72]. Furthermore, the energetic burden associated with CRISPR-Cas system maintenance and function may result in their disappearance in environments where the likelihood of HGT is minimal [73]. Elucidating these dynamics is important for harnessing CRISPR-Cas systems for biotechnological and clinical purposes.
3.2. Prevention of Lysogenic Conversion
Lysogenic conversion occurs when bacteriophages insert their genetic material into bacterial chromosomes in the form of prophages. While this process can sometimes bring adaptive benefits, e.g., the acquisition of toxin genes or stress-resistance traits. However, it poses substantial dangers at the same time [74]. Prophage integration can disrupt the regulation of host genes, reduce genome stability, destabilize cellular processes, and even cause the lysis of host cells under unfavorable conditions. The CRISPR-Cas systems work to mitigate these risks by blocking prophage integration and actively targeting integrated prophage sequences for degradation. These systems recognize specific sequences within the phage genome during or shortly after the infection process, thereby inhibiting the successful integration of phage DNA into the host chromosome (Figure 3) [75]. For example, Type I and Type II CRISPR-Cas systems have been shown to inhibit phage lysogeny by degrading the phage DNA before it can recombine with the bacterial genome [76,77]. This mechanism guarantees that only phages devoid of CRISPR-targeted sequences can establish lysogeny, thereby restricting the spread of potentially harmful prophages. By preventing prophage integration, CRISPR-Cas systems play a vital role in maintaining bacterial genome stability.
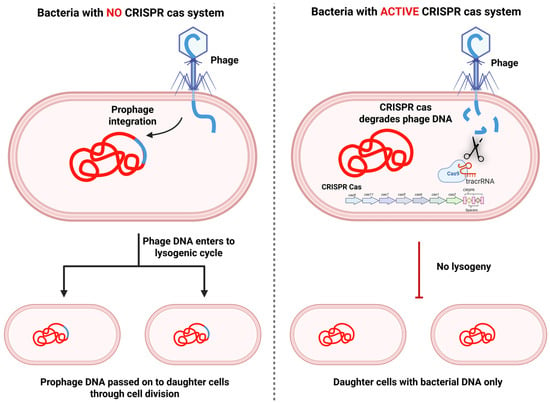
Figure 3.
Role of the CRISPR-Cas system in preventing lysogenic conversion by bacteriophages. In bacteria without a CRISPR-Cas system, the infecting phage injects its DNA, which integrates into the bacterial chromosome to form a prophage. The prophage enters the lysogenic cycle, and its DNA is stably inherited by daughter cells following division, perpetuating lysogeny (Left panel). In bacteria with an active CRISPR-Cas system, phage DNA is recognized and degraded by Cas nucleases (e.g., Cas9 guided by crRNA/tracrRNA). This prevents prophage integration, effectively blocking lysogeny. As a result, daughter cells inherit only the bacterial genome, maintaining genome stability and preventing phage propagation (Right panel). (Created using Biorender.com).
The inhibition of lysogenic conversion significantly impacts the broader dynamics between phages and their bacterial hosts. By restricting the capacity of temperate phages to establish lysogeny, CRISPR-Cas systems compel these phages to either adopt a lytic lifestyle or develop escape mutations. This interaction influences the co-evolutionary processes between bacteria and phages, thereby affecting the structure and stability of microbial communities. Furthermore, CRISPR-mediated suppression of lysogeny diminishes the horizontal transfer of prophage-associated genes, such as those encoding virulence factors or antibiotic resistance determinants, thereby enhancing the protection of the microbial genome [66,78]. Despite their effectiveness, the inhibitory role of CRISPR-Cas systems in lysogeny is not fully explored. Some bacteriophages have evolved mechanisms to evade CRISPR targeting, including mutating their target sequences or carrying anti-CRISPR proteins [79]. Future research could focus on enhancing CRISPR-Cas activity through synthetic biology approaches, potentially creating engineered systems capable of broader or more precise targeting of lysogenic phages.
3.3. Influence on Antibiotic Resistance Spread
CRISPR-Cas systems are essential for preventing the spread of antibiotic resistance genes, a significant public health issue. HGT mechanisms, including conjugation, transformation, and transduction, commonly mediate the transfer of resistance genes among bacterial species [80]. By targeting and degrading MGEs that carry antibiotic resistance genes, CRISPR-Cas systems restrict their spread and ensure the sustained effectiveness of antibiotics. Plasmids and integrative conjugative elements (ICEs) are common vectors for resistance genes. CRISPR-Cas systems are able to recognize these MGEs based on sequence-specific recognition and degrade their DNA, preventing their horizontal transfer [81,82]. For example, research has shown that CRISPR-Cas systems in Enterococcus faecalis target plasmids that bear vancomycin resistance genes, successfully inhibiting the spread of resistance in microbial populations [83]. Type I and II CRISPR systems in other bacterial species have also been found to prevent the conjugative transfer of resistance genes, indicating their significance in antimicrobial resistance control [84].
In addition to limiting the dissemination of existing resistance genes, CRISPR-Cas systems are involved in preventing the development of novel resistance mechanisms. By mitigating the acquisition of foreign DNA, these systems limit the genetic diversity available for selection under antibiotic pressure. This restriction slows the emergence of multidrug-resistant strains, particularly in environments with high antibiotic use, such as hospitals and agricultural setups [85]. Engineered CRISPR-Cas systems have also demonstrated potential in the battle against resistance. For example, synthetic CRISPR constructs targeting β-lactamase genes have been successfully delivered to bacterial populations via conjugative plasmids, which selectively kill resistant strains without harming susceptible bacteria [81,82]. Such applications represent promising approaches to addressing the global problem of antimicrobial resistance.
The activity of CRISPR-Cas systems has a profound impact on microbial population dynamics by altering competitive interactions among species. Bacteria that have effective CRISPR-Cas systems are better at excluding their competitors from picking up resistance genes, which in turn alters the balance of microbial communities. This selective pressure can cause the overall prevalence of resistance genes to reduce in a population, thus promoting a healthier ecosystem where antibiotic therapies remain effective [86]. In addition, the capacity of CRISPR-Cas systems to prevent the spread of resistance genes may help to delay the development of resistance hotspots in environments where there is high exposure to antibiotics, including wastewater treatment plants or hospital effluent systems [87]. These ecological effects highlight the important role played by CRISPR-Cas systems in maintaining microbial diversity and mitigating the global antimicrobial resistance crisis.
Despite their potential, natural CRISPR-Cas systems exhibit certain limitations in addressing antibiotic resistance. Some bacterial species completely lack CRISPR-Cas systems, while others may possess inactive or less effective variants [88]. Additionally, MGE has developed mechanisms to avoid CRISPR-Cas targeting, such as sequence mutations or the production of anti-CRISPR proteins, which can compromise the efficiency of these systems [78]. Future research should prioritize the development of engineered CRISPR-Cas systems to directly target and eliminate resistance genes across diverse bacterial populations. Integrating CRISPR-based strategies with existing antimicrobial stewardship programs and environmental interventions could significantly enhance efforts to mitigate the spread of antibiotic resistance. Moreover, investigating the co-evolution of CRISPR-Cas systems and MGEs may uncover novel strategies to strengthen bacterial defenses against HGT.
3.4. Balancing Genetic Diversity and Stability
CRISPR-Cas systems play a dual role in bacterial populations, balancing the need for genetic stability with the necessity for genetic diversity. This balance is essential for microbial persistence, allowing for adaptation to changing environments while protecting against the disruptive effects of uncontrolled HGT [89]. One of the main roles of CRISPR-Cas systems is to restrict gene flow by detecting and degrading foreign DNA. This activity ensures genomic stability by reducing the integration of potentially harmful MGEs, such as plasmids, transposons, and prophages [90]. For example, by blocking the uptake of antibiotic resistance genes or virulence factors, CRISPR-Cas systems protect bacterial populations from traits that could otherwise compromise their fitness in certain environments [91]. However, this limitation on gene flow also limits the genetic innovation opportunities, which is essential for adaptation. The uptake of genes via HGT can provide bacteria with novel traits that enhance survival under stressful conditions, such as exposure to antibiotics or environmental toxins [92]. Some studies suggest that bacteria with less active CRISPR-Cas systems may have greater potential for taking up adaptive traits through HGT, thus providing them with a competitive advantage in rapidly changing environments [93,94]. This trade-off highlights the evolutionary pressures on CRISPR-Cas systems to balance defensive functions and flexibility. While stringent targeting of MGEs prevents harmful effects, overly restrictive CRISPR activity could prevent the acquisition of beneficial traits, thus reducing long-term adaptability.
The influence of CRISPR-Cas systems extends beyond individual organisms, affecting entire microbial communities. By regulating the spread of MGE, these systems control the exchange of genetic material within and among bacterial populations. This regulatory function has profound effects on microbial ecology, including the structuring of populations, niche differentiation, and resilience of communities [11,95,96]. For example, in environments where there is intense predation by bacteriophages, bacteria possessing active CRISPR-Cas systems can dominate due to their resistance to phage infection. In contrast, the environments where genetic exchange is beneficial, e.g., biofilms or microbiomes, bacteria with less active or no CRISPR-Cas systems can dominate owing to their greater potential for genetic exchange [97].
In addition, CRISPR-Cas systems contribute significantly to microbial adaptation by shaping the genetic diversity available for evolutionary selection. Through the allowance of some genetic elements while precluding others, these systems create a filter that directs the evolutionary trajectory of bacterial populations. This filtering effect has been observed in natural environments, where the diversity of CRISPR arrays is linked to the range of environmental challenges faced by bacterial communities [11]. Although CRISPR-Cas systems play a crucial role in maintaining a balance between genetic diversity and stability, they are not devoid of limitations. The metabolic cost of maintaining these systems can be significant, leading to some bacteria to lose CRISPR-Cas loci in environments where HGT is not a significant threat [98]. Additionally, the specificity of CRISPR targeting may be manipulated by MGEs that develop strategies to evade detection, such as through the mutation of target sequences or the expression of anti-CRISPR proteins [99]. Future research should focus on understanding the ecological and evolutionary factors that drive the diversity of CRISPR-Cas systems in microbial populations. Investigating the interplay between CRISPR activity, environmental pressures, and microbial community dynamics could provide insights into how these systems shape bacterial evolution. It is also important to note that CRISPR-Cas systems present evolutionary trade-offs. While they protect against harmful mobile genetic elements, they can limit the acquisition of beneficial genes through horizontal gene transfer. Moreover, maintaining an active CRISPR-Cas system imposes metabolic costs, which may explain its uneven distribution among bacterial taxa. These unresolved questions continue to shape ongoing debates about the adaptive value of CRISPR systems across diverse ecological niches.
3.5. Acquisition of CRISPR-Cas Systems via HGT
The acquisition of CRISPR-Cas systems through HGT is an interesting aspect of bacterial evolution that underscores the dynamic nature of microbial genomes. Despite the fact that CRISPR-Cas systems are largely considered barriers to HGT, they themselves can be horizontally transferred between species, leading to the patchy distribution of these systems in bacterial populations [66]. Comparative genomics studies have uncovered several cases in which CRISPR-Cas loci have been transferred horizontally between bacterial species. These transfers tend to happen via MGE, like plasmids or transposons, that acquire and spread CRISPR-associated genes [90,100,101]. For example, researchers have reported the horizontal transfer of Type I and Type III CRISPR-Cas loci within and among varied members of the family Enterobacteriaceae, suggesting that these systems can become broadly disseminated across phylogenetic divides [102,103].
Horizontal acquisition of CRISPR-Cas loci may encode unique spacer sequences that enable recipient bacteria to quickly acquire adaptive immunity against phages and other MGEs, providing immediate survival benefits under hostile conditions. It is especially beneficial in ecosystems with high phage diversity or elevated HGT rates, where bacterial populations need to continuously evolve to prevent extinction [27]. Such events can lead to rapid shifts in microbial community dynamics, influencing competition, adaptation, and overall ecosystem stability [104]. The horizontal transfer of CRISPR-Cas systems helps explain their uneven distribution among bacterial populations. Although some species have highly diverse and active CRISPR arrays, others do not have these systems at all. This uneven distribution is likely the result of a mixture of ecological pressures, including phage pressure, and evolutionary trade-offs, such as the metabolic burden of CRISPR-Cas locus maintenance [11].
While horizontal transfer enables the dissemination of CRISPR-Cas systems, it also presents challenges. The integration of foreign CRISPR loci into a recipient genome can disrupt existing regulatory networks or impose metabolic burdens, potentially reducing fitness [105]. Moreover, the functionality of horizontally acquired CRISPR-Cas systems may be compromised if essential components are not co-transferred or if incompatibility arises with the host’s existing genetic machinery [106]. The horizontal transfer of CRISPR-Cas systems also points to their double function as both barriers and promoters of HGT. By taking part in their own spread, CRISPR-Cas systems play a role in the wider evolutionary context of microbial life by facilitating the rapid adaptation to environmental pressures. This duality underscores the complexity of bacterial evolution and the multifaceted interaction between defense mechanisms and genetic exchange in the establishment of microbial diversity and robustness.
4. Future Directions in CRISPR-Cas Research and Host Interaction
4.1. Expanding CRISPR Applications Beyond Immunity
Overall, these observations highlight that immune defense and host interaction are interconnected facets of the CRISPR-Cas system. The same nucleic-acid-targeting machinery that protects bacteria from phages also modulates host-related processes such as quorum sensing, stress adaptation, and immune evasion. Recognizing this overlap provides a clearer framework for future studies exploring CRISPR’s dual defensive and regulatory roles. Recent research has uncovered non-canonical functions of CRISPR-Cas systems that affect bacterial physiology and interactions with hosts. These “moonlighting” activities encompass roles in stress tolerance, virulence, biofilm formation, DNA repair, and other cellular processes, suggesting that CRISPR components may engage in gene regulation and signaling functions beyond their established role in immunity [107]. In addition, future studies should also address unresolved controversies, such as the evolutionary balance between CRISPR-mediated protection and horizontal gene exchange, as well as the energetic and ecological costs of maintaining these systems. Clarifying these aspects will be essential for interpreting CRISPR’s broader biological significance.
4.2. Development of CRISPR-Based Antimicrobials
CRISPR-based antimicrobials are being developed to specifically target pathogens, offering a precise strategy against multidrug-resistant bacteria. By programming CRISPR-Cas nucleases to cleave antibiotic resistance genes or essential bacterial sequences, researchers can selectively eliminate or neutralize drug-resistant strains. For example, the CRISPR/Cas9 system has been employed to eradicate plasmids harboring resistance genes in S. aureus and E. coli, thereby reinstating their susceptibility to antibiotics. Furthermore, CRISPR interference (CRISPRi) strategies have been utilized to disrupt biofilm-associated infections. Specifically, the silencing of QS and adhesion genes via CRISPR has demonstrated remarkable efficacy in reducing biofilm formation and enhancing the treatment of persistent infections. These advancements underscore CRISPR’s potential as a novel class of “smart” antibiotics, specifically designed to address antibiotic resistance and biofilm-related diseases [108,109].
4.3. Understanding CRISPR–Host Co-Evolution
Bacteria and bacteriophages are engaged in a continuous evolutionary arms race, with the CRISPR-Cas system serving as a crucial defense mechanism that drives mutual adaptation. As bacteria acquire new spacers to defend against phages, viruses counter-evolve through escape mutations or the production of anti-CRISPR proteins that neutralize the bacterial immune system. This reciprocal evolution fosters rapid genetic change and sustains diversity within microbial populations. Indeed, bacteria–phage coevolution is a significant driver of microbial diversity and community dynamics, influencing which bacterial strains persist and how microbial communities evolve over time [110]. Studying these CRISPR-mediated interactions offers valuable insights into the adaptive evolution of both microorganisms and their viral counterparts, highlighting a continuous “evolutionary arms race” that influences microbial ecosystems.
4.4. CRISPR-Cas in Host–Microbiome Engineering
Utilizing CRISPR technology for microbiome engineering represents a growing area of research with significant implications for health, agriculture, and disease prevention. Researchers are investigating CRISPR-based methodologies to selectively edit or eliminate specific microbes within complex microbial communities, with the objective of beneficially modulating the microbiome [111,112]. For example, a Phase 2 clinical trial utilizing CRISPR-enhanced bacteriophages (phage therapy augmented with CRISPR technology) demonstrated a significant reduction in pathogenic E. coli among patients with urinary tract infections, resulting in improved symptoms. In a separate study, CRISPR gene-editing was applied to modify the gut microbiota composition in mice, effectively preventing the manifestation of a disease phenotype, such as reducing the risk of asthma by reshaping the microbiome [113]. These findings underscore the potential of CRISPR technology to precisely manipulate microbiomes for health benefits. Similar approaches are anticipated in agriculture, such as modifying soil or plant microbiomes to enhance crop resilience, and in precision medicine to address conditions linked to the microbiome. Nevertheless, CRISPR-based microbiome editing is still in its early stages and faces challenges related to delivery, stability, and safety that must be addressed before it can be widely applied. Ongoing initiatives, such as the BIOME project, are actively engaged in the development of safer and more efficient CRISPR microbiome editors, highlighting the potential of this approach for future therapeutic applications and ecosystem management [113].
4.5. Synthetic Biology and CRISPR Innovations
Synthetic biology is advancing CRISPR-Cas innovations by designing systems tailored for specific applications and integrating CRISPR with other technologies. One approach involves the creation or discovery of novel Cas variants engineered for specialized tasks. For example, the introduction of Cas12 and Cas13 enzymes has expanded the CRISPR toolkit beyond Cas9, enabling multiplex DNA edits and direct RNA targeting for host applications that necessitate these capabilities [114,115]. Researchers are integrating CRISPR with complementary biotechnological tools to develop multifunctional platforms. This approach includes employing nanotechnology to enhance the delivery of CRISPR components, incorporating CRISPR-based gene circuits into cells, and utilizing machine learning to optimize guide RNA design and predict off-target effects [114,116,117]. The integration of CRISPR with other advanced technologies seeks to enhance both precision and versatility. For instance, the development of high-fidelity Cas proteins aims to minimize off-target effects, while coupling CRISPR with transcriptional regulators facilitates the creation of programmable cellular “devices.” These endeavors in synthetic biology suggest the potential for custom-designed CRISPR systems that can be precisely tailored for various host organisms and complex tasks, thereby advancing next-generation genome engineering and therapeutic applications [118,119,120].
5. Conclusions
CRISPR-Cas systems serve not only as prokaryotic immune defenses but also as pivotal regulators of bacterial adaptation and survival in complex environments. By orchestrating immunity against phages, regulating endogenous genes, modulating biofilm formation and quorum sensing, and fine-tuning virulence, these systems exemplify the intricate interplay between microbes and their hosts. Their evolutionary plasticity, coupled with emerging synthetic and therapeutic applications, positions CRISPR-Cas as a cornerstone of both microbial ecology and biotechnology. Continued exploration of their roles in host-pathogen interactions will not only deepen our understanding of bacterial physiology but also unlock new strategies for antimicrobial innovation and microbiome-based interventions.
Author Contributions
Conceptualization, C.E.J.; writing—original draft preparation, C.E.J., A.J., M.O.Y. and L.K.E.; writing—review and editing, C.E.J., A.J., M.O.Y. and L.K.E.; supervision, L.K.E. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Data provided in the manuscript are from published studies; no new data were generated.
Acknowledgments
The authors are grateful to the American Society of Microbiology (ASM) Future Leader Mentor-ship Fellowship (FLMF) Program. The authors would like to acknowledge the use of Paperpal for language editing assistance, which helped improve the clarity and readability of this manuscript. All final content was reviewed, verified, and endorsed in full by all the authors.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| CRISPR | Clustered Regularly Interspaced Short Palindromic Repeats |
| Cas | CRISPR-associated protein |
| AI-2 | Autoinducer-2 (universal quorum-sensing molecule) |
| AHL | Acyl-homoserine lactone |
| BLP | Bacterial lipoprotein |
| EPS | Extracellular polymeric substances (biofilm matrix) |
| MGE | Mobile genetic element |
| QS | Quorum sensing |
| ROS | Reactive oxygen species |
| SPI-1 | Salmonella pathogenicity island 1 |
| TLR2 | Toll-like receptor 2 |
| TLR4 | Toll-like receptor 4 |
| WT | Wild type |
References
- Barrangou, R. Diversity of CRISPR-Cas Immune Systems and Molecular Machines. Genome Biol. 2015, 16, 247. [Google Scholar] [CrossRef]
- Yuan, B.; Yuan, C.; Li, L.; Long, M.; Chen, Z. Application of the CRISPR/Cas System in Pathogen Detection: A Review. Molecules 2022, 27, 6999. [Google Scholar] [CrossRef]
- Mengstie, M.A.; Wondimu, B.Z. Mechanism and Applications of Crispr/Cas-9-Mediated Genome Editing. Biologics 2021, 15, 353–361. [Google Scholar] [CrossRef]
- Kumar, P.; Malik, Y.S.; Ganesh, B.; Rahangdale, S.; Saurabh, S.; Natesan, S.; Srivastava, A.; Sharun, K.; Yatoo, M.I.; Tiwari, R.; et al. CRISPR-Cas System: An Approach With Potentials for COVID-19 Diagnosis and Therapeutics. Front. Cell. Infect. Microbiol. 2020, 10, 576875. [Google Scholar] [CrossRef]
- Zhang, L.; Li, S.; Li, R.; Qiu, X.; Fan, T.; Wang, B.; Zhang, B.; Zhang, Y. Advances in Application of CRISPR-Cas13a System. Front. Cell. Infect. Microbiol. 2024, 14, 1291557. [Google Scholar] [CrossRef] [PubMed]
- Tao, S.; Chen, H.; Li, N.; Liang, W. The Application of the CRISPR-Cas System in Antibiotic Resistance. Infect. Drug Resist. 2022, 15, 4155–4168. [Google Scholar] [CrossRef] [PubMed]
- Sinkunas, T.; Gasiunas, G.; Waghmare, S.P.; Dickman, M.J.; Barrangou, R.; Horvath, P.; Siksnys, V. In Vitro Reconstitution of Cascade-Mediated CRISPR Immunity in Streptococcus Thermophilus. EMBO J. 2013, 32, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Rath, D.; Amlinger, L.; Rath, A.; Lundgren, M. The CRISPR-Cas Immune System: Biology, Mechanisms and Applications. Biochimie 2015, 117, 119–128. [Google Scholar] [CrossRef]
- Makarova, K.S.; Wolf, Y.I.; Iranzo, J.; Shmakov, S.A.; Alkhnbashi, O.S.; Brouns, S.J.J.; Charpentier, E.; Cheng, D.; Haft, D.H.; Horvath, P.; et al. Evolutionary Classification of CRISPR–Cas Systems: A Burst of Class 2 and Derived Variants. Nat. Rev. Microbiol. 2020, 18, 67–83. [Google Scholar] [CrossRef]
- Murugan, K.; Babu, K.; Sundaresan, R.; Rajan, R.; Sashital, D.G. The Revolution Continues: Newly Discovered Systems Expand the CRISPR-Cas Toolkit. Mol. Cell 2017, 68, 15–25. [Google Scholar] [CrossRef]
- Pinilla-Redondo, R.; Russel, J.; Mayo-Muñoz, D.; Shah, S.A.; Garrett, R.A.; Nesme, J.; Madsen, J.S.; Fineran, P.C.; Sørensen, S.J. CRISPR-Cas Systems Are Widespread Accessory Elements across Bacterial and Archaeal Plasmids. Nucleic Acids Res. 2022, 50, 4315–4328. [Google Scholar] [CrossRef]
- Makarova, K.S.; Wolf, Y.I.; Alkhnbashi, O.S.; Costa, F.; Shah, S.A.; Saunders, S.J.; Barrangou, R.; Brouns, S.J.J.; Charpentier, E.; Haft, D.H.; et al. An Updated Evolutionary Classification of CRISPR-Cas Systems. Nat. Rev. Microbiol. 2015, 13, 722–736. [Google Scholar] [CrossRef] [PubMed]
- Kolesnik, M.V.; Fedorova, I.; Karneyeva, K.A.; Artamonova, D.N.; Severinov, K.V. Type III CRISPR-Cas Systems: Deciphering the Most Complex Prokaryotic Immune System. Biochemistry 2021, 86, 1301–1314. [Google Scholar] [CrossRef]
- Koonin, E.V.; Makarova, K.S. Origins and Evolution of CRISPR-Cas Systems. Philos. Trans. R. Soc. B Biol. Sci. 2019, 374, 20180087. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.; Weissman, J.L.; Johnson, P.L.F. Ecological Drivers of CRISPR Immune Systems. mSystems 2024, 9, e0056824. [Google Scholar] [CrossRef]
- Antequera-Zambrano, L.; Parra-Sánchez, Á.; González-Paz, L.; Fernandez, E.; Martinez-Navarrete, G. Distribution of Genetic Determinants Associated with CRISPR-Cas Systems and Resistance to Antibiotics in the Genomes of Archaea and Bacteria. Microorganisms 2025, 13, 1321. [Google Scholar] [CrossRef] [PubMed]
- Medvedeva, S.; Liu, Y.; Koonin, E.V.; Severinov, K.; Prangishvili, D.; Krupovic, M. Virus-Borne Mini-CRISPR Arrays Are Involved in Interviral Conflicts. Nat. Commun. 2019, 10, 5204. [Google Scholar] [CrossRef]
- Jinek, M.; Chylinski, K.; Fonfara, I.; Hauer, M.; Doudna, J.A.; Charpentier, E. A Programmable Dual RNA-Guided DNA Endonuclease in Adaptive Bacterial Immunity One-Sentence Summary. Science 2012, 337, 816–821. [Google Scholar] [CrossRef]
- Jinek, M.; East, A.; Cheng, A.; Lin, S.; Ma, E.; Doudna, J. RNA-Programmed Genome Editing in Human Cells. eLife 2013, 2, e00471. [Google Scholar] [CrossRef]
- Jia, F.; Li, X.; Zhang, C.; Tang, X. The Expanded Development and Application of CRISPR System for Sensitive Nucleotide Detection. Protein Cell 2020, 11, 624–629. [Google Scholar] [CrossRef]
- Saeed, K.; Ayub, F.; Durrani, M.A.; Mujahid, M. CRISPR Cas Systems: From Bacterial Defense Mechanisms to Revolutionary Tools Reshaping Genetic Research and Translation Therapeutics. Microbe 2025, 7, 100344. [Google Scholar] [CrossRef]
- Wang, J.Y.; Doudna, J.A. CRISPR Technology: A Decade of Genome Editing Is Only the Beginning. Science 2023, 379, eadd8643. [Google Scholar] [CrossRef]
- Loureiro, A.; Da Silva, G.J. Crispr-Cas: Converting a Bacterial Defence Mechanism into a State-of-the-Art Genetic Manipulation Tool. Antibiotics 2019, 8, 18. [Google Scholar] [CrossRef]
- Li, Y.; Peng, N. Endogenous CRISPR-Cas System-Based Genome Editing and Antimicrobials: Review and Prospects. Front. Microbiol. 2019, 10, 2471. [Google Scholar] [CrossRef]
- Crawley, A.B.; Henriksen, E.D.; Stout, E.; Brandt, K.; Barrangou, R. Characterizing the Activity of Abundant, Diverse and Active CRISPR-Cas Systems in Lactobacilli. Sci. Rep. 2018, 8, 11544. [Google Scholar] [CrossRef] [PubMed]
- Pyenson, N.C.; Marraffini, L.A. Co-Evolution within Structured Bacterial Communities Results in Multiple Expansion of CRISPR Loci and Enhanced Immunity. eLife 2020, 9, e53078. [Google Scholar] [CrossRef] [PubMed]
- Newsom, S.; Parameshwaran, H.P.; Martin, L.; Rajan, R. The CRISPR-Cas Mechanism for Adaptive Immunity and Alternate Bacterial Functions Fuels Diverse Biotechnologies. Front. Cell. Infect. Microbiol. 2021, 10, 619763. [Google Scholar] [CrossRef] [PubMed]
- Haider, D.; Bauer, R.; Grempels, A.; Roscher, R.; Aslan, C.C.; Mauerer, S.; Spellerberg, B. The Stress of Carrying CRISPR-Cas. Virulence 2025, 16, 2541701. [Google Scholar] [CrossRef]
- Louwen, R.; Staals, R.H.J.; Endtz, H.P.; van Baarlen, P.; van der Oost, J. The Role of CRISPR-Cas Systems in Virulence of Pathogenic Bacteria. Microbiol. Mol. Biol. Rev. 2014, 78, 74–88. [Google Scholar] [CrossRef]
- Medina-Aparicio, L.; Rodriguez-Gutierrez, S.; Rebollar-Flores, J.E.; Martínez-Batallar, Á.G.; Mendoza-Mejía, B.D.; Aguirre-Partida, E.D.; Vázquez, A.; Encarnación, S.; Calva, E.; Hernández-Lucas, I. The CRISPR-Cas System Is Involved in OmpR Genetic Regulation for Outer Membrane Protein Synthesis in Salmonella Typhi. Front. Microbiol. 2021, 12, 657404. [Google Scholar] [CrossRef]
- Ratner, H.K.; Weiss, D.S. Francisella novicida CRISPR-Cas Systems Can Functionally Complement Each Other in DNA Defense While Providing Target Flexibility. J. Bacteriol. 2020, 202, e00670-19. [Google Scholar] [CrossRef] [PubMed]
- Nyerges, Á.; Bálint, B.; Cseklye, J.; Nagy, I.; Pál, C.; Feher, T. CRISPR-Interference-Based Modulation of Mobile Genetic Elements in Bacteria. Synth. Biol. 2019, 4, ysz008. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, J.; Sun, X.; Li, M.; Zhang, P.; Zhu, Z.; Jiao, H.; Guo, T.; Li, G. CRISPR-Cas in Acinetobacter baumannii Contributes to Antibiotic Susceptibility by Targeting Endogenous AbaI. Microbiol. Spectr. 2022, 10, e0082922. [Google Scholar] [CrossRef]
- Sampson, T.R.; Weiss, D.S. CRISPR-Cas Systems: New Players in Gene Regulation and Bacterial Physiology. Front. Cell. Infect. Microbiol. 2014, 4, 37. [Google Scholar] [CrossRef]
- Guo, T.; Yang, J.; Zhou, N.; Sun, X.; Huan, C.; Lin, T.; Bao, G.; Hu, J.; Li, G. Cas3 of Type I-Fa CRISPR-Cas System Upregulates Bacterial Biofilm Formation and Virulence in Acinetobacter baumannii. Commun. Biol. 2025, 8, 750. [Google Scholar] [CrossRef]
- Sampson, T.R.; Saroj, S.D.; Llewellyn, A.C.; Tzeng, Y.L.; Weiss, D.S. A CRISPR/Cas System Mediates Bacterial Innate Immune Evasion and Virulence. Nature 2013, 497, 254–257. [Google Scholar] [CrossRef] [PubMed]
- Sampson, T.R.; Weiss, D.S. Alternative Roles for CRISPR/Cas Systems in Bacterial Pathogenesis. PLoS Pathog. 2013, 9, e1003621. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Fang, L.; Tan, S.; Yu, M.; Li, X.; He, S.; Wei, Y.; Li, G.; Jiang, J.; Wu, M. Type I CRISPR-Cas Targets Endogenous Genes and Regulates Virulence to Evade Mammalian Host Immunity. Cell Res. 2016, 26, 1273–1287. [Google Scholar] [CrossRef]
- Cui, L.; Wang, X.; Huang, D.; Zhao, Y.; Feng, J.; Lu, Q.; Pu, Q.; Wang, Y.; Cheng, G.; Wu, M.; et al. CRISPR-Cas3 of Salmonella Upregulates Bacterial Biofilm Formation and Virulence to Host Cells by Targeting Quorum-Sensing Systems. Pathogens 2020, 9, 53. [Google Scholar] [CrossRef]
- Gao, N.J.; Al-Bassam, M.M.; Poudel, S.; Wozniak, J.M.; Gonzalez, D.J.; Olson, J.; Zengler, K.; Nizet, V.; Valderrama, J.A. Functional and Proteomic Analysis of Streptococcus pyogenes Virulence upon Loss of Its Native Cas9 Nuclease. Front. Microbiol. 2019, 10, 1967. [Google Scholar] [CrossRef]
- Dugar, G.; Leenay, R.T.; Eisenbart, S.K.; Bischler, T.; Aul, B.U.; Beisel, C.L.; Sharma, C.M. CRISPR RNA-Dependent Binding and Cleavage of Endogenous RNAs by the Campylobacter jejuni Cas9. Mol. Cell 2018, 69, 893–905.e7. [Google Scholar] [CrossRef]
- Shabbir, M.A.B.; Tang, Y.; Xu, Z.; Lin, M.; Cheng, G.; Dai, M.; Wang, X.; Liu, Z.; Yuan, Z.; Hao, H. The Involvement of the Cas9 Gene in Virulence of Campylobacter jejuni. Front. Cell. Infect. Microbiol. 2018, 8, 285. [Google Scholar] [CrossRef]
- Tang, B.; Gong, T.; Zhou, X.; Lu, M.; Zeng, J.; Peng, X.; Wang, S.; Li, Y. Deletion of Cas3 Gene in Streptococcus mutans Affects Biofilm Formation and Increases Fluoride Sensitivity. Arch. Oral Biol. 2019, 99, 190–197. [Google Scholar] [CrossRef]
- Spencer, B.L.; Deng, L.; Patras, K.A.; Burcham, Z.M.; Sanches, G.F.; Nagao, P.E.; Doran, K.S. Cas9 Contributes to Group b Streptococcal Colonization and Disease. Front. Microbiol. 2019, 10, 1930. [Google Scholar] [CrossRef] [PubMed]
- Hickok, N.J. What Are Biofilms? Spine 2018, 43, S7–S8. [Google Scholar]
- Afrasiabi, S.; Partoazar, A. Targeting Bacterial Biofilm-Related Genes with Nanoparticle-Based Strategies. Front. Microbiol. 2024, 15, 1387114. [Google Scholar] [CrossRef]
- Sharma, N.; Das, A.; Raja, P.; Marathe, S.A. The CRISPR-Cas System Differentially Regulates Surface-Attached and Pellicle Biofilm in Salmonella enterica Serovar Typhimurium. Microbiol. Spectr. 2022, 10, e0020222. [Google Scholar] [CrossRef] [PubMed]
- Saffari Natanzi, A.; Poudineh, M.; Karimi, E.; Khaledi, A.; Haddad Kashani, H. Innovative Approaches to Combat Antibiotic Resistance: Integrating CRISPR/Cas9 and Nanoparticles against Biofilm-Driven Infections. BMC Med. 2025, 23, 486. [Google Scholar] [CrossRef]
- Bano, S.; Hassan, N.; Rafiq, M.; Hassan, F.; Rehman, M.; Iqbal, N.; Ali, H.; Hasan, F.; Kang, Y.Q. Biofilms as Battlefield Armor for Bacteria against Antibiotics: Challenges and Combating Strategies. Microorganisms 2023, 11, 2595. [Google Scholar] [CrossRef]
- Hooton, S.P.T.; Brathwaite, K.J.; Connerton, I.F. The Bacteriophage Carrier State of Campylobacter jejuni Features Changes in Host Non-Coding RNAs and the Acquisition of New Host-Derived CRISPR Spacer Sequences. Front. Microbiol. 2016, 7, 355. [Google Scholar] [CrossRef] [PubMed]
- Hooton, S.P.T.; Connerton, I.F. Campylobacter jejuni Acquire New Host-Derived CRISPR Spacers When in Association with Bacteriophages Harboring a CRISPR-like Cas4 Protein. Front. Microbiol. 2015, 6, 744. [Google Scholar] [CrossRef]
- Ali, Q.; Wahl, L.M. Mathematical Modelling of CRISPR-Cas System Effects on Biofilm Formation. J. Biol. Dyn. 2017, 11, 264–284. [Google Scholar] [CrossRef]
- Liu, S.; Lu, H.; Zhang, S.; Shi, Y.; Chen, Q. Phages against Pathogenic Bacterial Biofilms and Biofilm-Based Infections: A Review. Pharmaceutics 2022, 14, 427. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, C.; Li, S.; Liang, S.; Xu, X.; Zhao, Z. Quorum Sensing Positively Regulates CPS-Dependent Autographiviridae Phage Infection in Vibrio Alginolyticus. Appl. Environ. Microbiol. 2024, 90, e0221023. [Google Scholar] [CrossRef] [PubMed]
- Patterson, A.G.; Jackson, S.A.; Taylor, C.; Evans, G.B.; Salmond, G.P.C.; Przybilski, R.; Staals, R.H.J.; Fineran, P.C. Quorum Sensing Controls Adaptive Immunity through the Regulation of Multiple CRISPR-Cas Systems. Mol. Cell 2016, 64, 1102–1108. [Google Scholar] [CrossRef]
- Devi, V.; Harjai, K.; Chhibber, S. CRISPR-Cas Systems: Role in Cellular Processes beyond Adaptive Immunity. Folia Microbiol. 2022, 67, 837–850. [Google Scholar] [CrossRef]
- Maura, D.; Hazan, R.; Kitao, T.; Ballok, A.E.; Rahme, L.G. Evidence for Direct Control of Virulence and Defense Gene Circuits by the Pseudomonas aeruginosa Quorum Sensing Regulator, MvfR. Sci. Rep. 2016, 6, 34083. [Google Scholar] [CrossRef]
- Solano, C.; Echeverz, M.; Lasa, I. Biofilm Dispersion and Quorum Sensing. Curr. Opin. Microbiol. 2014, 18, 96–104. [Google Scholar] [CrossRef]
- Juszczuk-Kubiak, E. Molecular Aspects of the Functioning of Pathogenic Bacteria Biofilm Based on Quorum Sensing (QS) Signal-Response System and Innovative Non-Antibiotic Strategies for Their Elimination. Int. J. Mol. Sci. 2024, 25, 2655. [Google Scholar] [CrossRef]
- Wu, Q.; Cui, L.; Liu, Y.; Li, R.; Dai, M.; Xia, Z.; Wu, M. CRISPR-Cas Systems Target Endogenous Genes to Impact Bacterial Physiology and Alter Mammalian Immune Responses. Mol. Biomed. 2022, 3, 22. [Google Scholar] [CrossRef]
- Bourgogne, A.; Garsin, D.A.; Qin, X.; Singh, K.V.; Sillanpaa, J.; Yerrapragada, S.; Ding, Y.; Dugan-Rocha, S.; Buhay, C.; Shen, H.; et al. Large Scale Variation in Enterococcus faecalis Illustrated by the Genome Analysis of Strain OG1RF. Genome Biol. 2008, 9, R110. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, C.; Cao, Y.; Chen, X.; Tang, Y.; Zhou, X.; Ingmer, H.; Jiao, X.; Li, Q. Oxidative Stress Elicited by Phage Infection Induces Staphylococcal Type III-A CRISPR–Cas System. Nucleic Acids Res. 2025, 53, gkaf541. [Google Scholar] [CrossRef]
- Serbanescu, M.A.; Cordova, M.; Krastel, K.; Flick, R.; Beloglazova, N.; Latos, A.; Yakunin, A.F.; Senadheera, D.B.; Cvitkovitch, D.G. Role of the Streptococcus mutans CRISPR-Cas Systems in Immunity and Cell Physiology. J. Bacteriol. 2015, 197, 749–761. [Google Scholar] [CrossRef]
- Barrangou, R.; Marraffini, L.A. CRISPR-Cas Systems: Prokaryotes Upgrade to Adaptive Immunity. Mol. Cell 2014, 54, 234–244. [Google Scholar] [CrossRef]
- Emamalipour, M.; Seidi, K.; Zununi Vahed, S.; Jahanban-Esfahlan, A.; Jaymand, M.; Majdi, H.; Amoozgar, Z.; Chitkushev, L.T.; Javaheri, T.; Jahanban-Esfahlan, R.; et al. Horizontal Gene Transfer: From Evolutionary Flexibility to Disease Progression. Front. Cell Dev. Biol. 2020, 8, 229. [Google Scholar] [CrossRef]
- Watson, B.N.J.; Staals, R.H.J.; Fineran, P.C. CRISPR-Cas-Mediated Phage Resistance Enhances Horizontal Gene Transfer by Transduction. mBio 2018, 9, e02406-17. [Google Scholar] [CrossRef]
- Faure, G.; Shmakov, S.A.; Yan, W.X.; Cheng, D.R.; Scott, D.A.; Peters, J.E.; Makarova, K.S.; Koonin, E.V. CRISPR–Cas in Mobile Genetic Elements: Counter-Defence and Beyond. Nat. Rev. Microbiol. 2019, 17, 513–525. [Google Scholar] [CrossRef]
- Liu, T.Y.; Doudna, J.A. Chemistry of Class 1 CRISPR-Cas Effectors: Binding, Editing, and Regulation. J. Biol. Chem. 2020, 295, 14473–14487. [Google Scholar] [CrossRef]
- Pfeifer, E.; Bonnin, R.A.; Rocha, E.P.C. Phage-Plasmids Spread Antibiotic Resistance Genes through Infection and Lysogenic Conversion. mBio 2022, 13, e01851-22. [Google Scholar] [CrossRef]
- Westra, E.R.; Levin, B.R. It Is Unclear How Important CRISPR-Cas Systems Are for Protecting Natural Populations of Bacteria against Infections by Mobile Genetic Elements. Proc. Natl. Acad. Sci. USA 2020, 117, 27777–27785. [Google Scholar] [CrossRef]
- Price, V.J.; McBride, S.W.; Hullahalli, K.; Chatterjee, A.; Duerkop, B.A.; Palmer, K.L. Enterococcus faecalis CRISPR-Cas Is a Robust Barrier to Conjugative Antibiotic Resistance Dissemination in the Murine Intestine. mSphere 2019, 4, e00464-19. [Google Scholar] [CrossRef]
- Sontheimer, E.J.; Davidson, A.R. Inhibition of CRISPR-Cas Systems by Mobile Genetic Elements. Curr. Opin. Microbiol. 2017, 37, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Zaayman, M.; Wheatley, R.M. Fitness Costs of CRISPR-Cas Systems in Bacteria. Microbiology 2022, 168, 001209. [Google Scholar] [CrossRef]
- Gummalla, V.S.; Zhang, Y.; Liao, Y.T.; Wu, V.C.H. The Role of Temperate Phages in Bacterial Pathogenicity. Microorganisms 2023, 11, 541. [Google Scholar] [CrossRef]
- Khambhati, K.; Bhattacharjee, G.; Gohil, N.; Dhanoa, G.K.; Sagona, A.P.; Mani, I.; Bui, N.L.; Chu, D.T.; Karapurkar, J.K.; Jang, S.H.; et al. Phage Engineering and Phage-Assisted CRISPR-Cas Delivery to Combat Multidrug-Resistant Pathogens. Bioeng. Transl. Med. 2023, 8, e10381. [Google Scholar] [CrossRef]
- Osuna, B.A.; Karambelkar, S.; Mahendra, C.; Christie, K.A.; Garcia, B.; Davidson, A.R.; Kleinstiver, B.P.; Kilcher, S.; Bondy-Denomy, J. Listeria Phages Induce Cas9 Degradation to Protect Lysogenic Genomes. Cell Host Microbe 2020, 28, 31–40.e9. [Google Scholar] [CrossRef] [PubMed]
- Pasechnek, A.; Rabinovich, L.; Stadnyuk, O.; Azulay, G.; Mioduser, J.; Argov, T.; Borovok, I.; Sigal, N.; Herskovits, A.A. Active Lysogeny in Listeria monocytogenes Is a Bacteria-Phage Adaptive Response in the Mammalian Environment. Cell Rep. 2020, 32, 107956. [Google Scholar] [CrossRef]
- Watson, B.N.J.; Steens, J.A.; Staals, R.H.J.; Westra, E.R.; van Houte, S. Coevolution between Bacterial CRISPR-Cas Systems and Their Bacteriophages. Cell Host Microbe 2021, 29, 715–725. [Google Scholar] [CrossRef] [PubMed]
- Landsberger, M.; Gandon, S.; Meaden, S.; Rollie, C.; Chevallereau, A.; Chabas, H.; Buckling, A.; Westra, E.R.; van Houte, S. Anti-CRISPR Phages Cooperate to Overcome CRISPR-Cas Immunity. Cell 2018, 174, 908–916.e12. [Google Scholar] [CrossRef]
- Mayorga-Ramos, A.; Zúñiga-Miranda, J.; Carrera-Pacheco, S.E.; Barba-Ostria, C.; Guamán, L.P. CRISPR-Cas-Based Antimicrobials: Design, Challenges, and Bacterial Mechanisms of Resistance. ACS Infect. Dis. 2023, 9, 1283–1302. [Google Scholar] [CrossRef]
- Tao, S.; Chen, H.; Li, N.; Wang, T.; Liang, W. The Spread of Antibiotic Resistance Genes In Vivo Model. Can. J. Infect. Dis. Med. Microbiol. 2022, 2022, 3348695. [Google Scholar] [CrossRef]
- Tao, S.; Chen, H.; Li, N.; Fang, Y.; Zhang, H.; Xu, Y.; Chen, L.; Liang, W. Elimination of Bla KPC–2-Mediated Carbapenem Resistance in Escherichia coli by CRISPR-Cas9 System. BMC Microbiol. 2023, 23, 310. [Google Scholar] [CrossRef] [PubMed]
- Huo, W.; Price, V.J.; Sharifi, A.; Zhang, M.Q.; Palmer, K.L. Enterococcus faecalis Strains with Compromised CRISPR-Cas Defense Emerge under Antibiotic Selection for a CRISPR-Targeted Plasmid. Appl. Environ. Microbiol. 2023, 89, e0012423. [Google Scholar] [CrossRef] [PubMed]
- Pursey, E.; Sünderhauf, D.; Gaze, W.H.; Westra, E.R.; van Houte, S. CRISPR-Cas Antimicrobials: Challenges and Future Prospects. PLoS Pathog. 2018, 14, e1006990. [Google Scholar] [CrossRef]
- Kadkhoda, H.; Gholizadeh, P.; Samadi Kafil, H.; Ghotaslou, R.; Pirzadeh, T.; Ahangarzadeh Rezaee, M.; Nabizadeh, E.; Feizi, H.; Aghazadeh, M. Role of CRISPR-Cas Systems and Anti-CRISPR Proteins in Bacterial Antibiotic Resistance. Heliyon 2024, 10, e34692. [Google Scholar] [CrossRef]
- Zakrzewska, M.; Burmistrz, M. Mechanisms Regulating the CRISPR-Cas Systems. Front. Microbiol. 2023, 14, 1060337. [Google Scholar] [CrossRef]
- Gunawardana, W.; Kalupahana, R.S.; Kottawatta, S.A.; Gamage, A.; Merah, O. A Review of the Dissemination of Antibiotic Resistance through Wastewater Treatment Plants: Current Situation in Sri Lanka and Future Perspectives. Life 2024, 14, 1065. [Google Scholar] [CrossRef]
- Duan, C.; Cao, H.; Zhang, L.H.; Xu, Z. Harnessing the CRISPR-Cas Systems to Combat Antimicrobial Resistance. Front. Microbiol. 2021, 12, 716064. [Google Scholar] [CrossRef]
- Lam, T.J.; Mortensen, K.; Ye, Y. Diversity and Dynamics of the CRISPR-Cas Systems Associated with Bacteroides Fragilis in Human Population. BMC Genom. 2022, 23, 573. [Google Scholar] [CrossRef]
- McDonald, N.D.; Regmi, A.; Morreale, D.P.; Borowski, J.D.; Fidelma Boyd, E. CRISPR-Cas Systems Are Present Predominantly on Mobile Genetic Elements in Vibrio Species. BMC Genom. 2019, 20, 105. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Battalapalli, D.; Hakeem, M.J.; Selamneni, V.; Zhang, P.; Draz, M.S.; Ruan, Z. Engineered CRISPR-Cas Systems for the Detection and Control of Antibiotic-Resistant Infections. J. Nanobiotechnol. 2021, 19, 401. [Google Scholar] [CrossRef]
- Deng, Y.; Xu, H.; Su, Y.; Liu, S.; Xu, L.; Guo, Z.; Wu, J.; Cheng, C.; Feng, J. Horizontal Gene Transfer Contributes to Virulence and Antibiotic Resistance of Vibrio harveyi 345 Based on Complete Genome Sequence Analysis. BMC Genom. 2019, 20, 761. [Google Scholar] [CrossRef]
- Zheng, Z.; Zhang, Y.; Liu, Z.; Dong, Z.; Xie, C.; Bravo, A.; Soberón, M.; Mahillon, J.; Sun, M.; Peng, D. The CRISPR-Cas Systems Were Selectively Inactivated during Evolution of Bacillus cereus Group for Adaptation to Diverse Environments. ISME J. 2020, 14, 1479–1493. [Google Scholar] [CrossRef]
- Wheatley, R.M.; MacLean, R.C. CRISPR-Cas Systems Restrict Horizontal Gene Transfer in Pseudomonas aeruginosa. ISME J. 2021, 15, 1420–1433. [Google Scholar] [CrossRef]
- Bonsma-Fisher, M.; Soutiere, D.; Goyal, S. How Adaptive Immunity Constrains the Composition and Fate of Large Bacterial Populations. Proc. Natl. Acad. Sci. USA 2018, 115, E7462–E7468. [Google Scholar] [CrossRef]
- López-Beltrán, A.; Botelho, J.; Iranzo, J. Dynamics of CRISPR-Mediated Virus-Host Interactions in the Human Gut Microbiome. ISME J. 2024, 18, wrae134. [Google Scholar] [CrossRef]
- Eghbalpoor, F.; Gorji, M.; Alavigeh, M.Z.; Moghadam, M.T. Genetically Engineered Phages and Engineered Phage-Derived Enzymes to Destroy Biofilms of Antibiotics Resistance Bacteria. Heliyon 2024, 10, e35666. [Google Scholar] [CrossRef] [PubMed]
- Bier, E.; Nizet, V. Driving to Safety: CRISPR-Based Genetic Approaches to Reducing Antibiotic Resistance. Trends Genet. 2021, 37, 745–757. [Google Scholar] [CrossRef]
- Deng, X.; Sun, W.; Li, X.; Wang, J.; Cheng, Z.; Sheng, G.; Wang, Y. An Anti-CRISPR That Represses Its Own Transcription While Blocking Cas9-Target DNA Binding. Nat. Commun. 2024, 15, 1806. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, P.; Han, S.R.; Lee, J.H.; Park, H.; Oh, T.J. A Computational Approach to Identify CRISPR-Cas Loci in the Complete Genomes of the Lichen-Associated Burkholderia sp. PAMC28687 and PAMC26561. Genomics 2021, 113, 881–888. [Google Scholar] [CrossRef] [PubMed]
- Karginov, F.V.; Hannon, G.J. The CRISPR System: Small RNA-Guided Defense in Bacteria and Archaea. Mol. Cell 2010, 37, 7–19. [Google Scholar] [CrossRef]
- Medina-Aparicio, L.; Dávila, S.; Rebollar-Flores, J.E.; Calva, E.; Hernández-Lucas, I. The CRISPR-Cas System in Enterobacteriaceae. Pathog. Dis. 2018, 76, fty002. [Google Scholar] [CrossRef] [PubMed]
- Xue, C.; Sashital, D.G. Mechanisms of Type I-E and I-F CRISPR-Cas Systems in Enterobacteriaceae. EcoSal Plus 2019, 8. [Google Scholar] [CrossRef]
- Zhang, Y.; Heidrich, N.; Ampattu, B.J.; Gunderson, C.W.; Seifert, H.S.; Schoen, C.; Vogel, J.; Sontheimer, E.J. Processing-Independent CRISPR RNAs Limit Natural Transformation in Neisseria Meningitidis. Mol. Cell 2013, 50, 488–503. [Google Scholar] [CrossRef]
- Varble, A.; Meaden, S.; Barrangou, R.; Westra, E.R.; Marraffini, L.A. Recombination between Phages and CRISPR–cas Loci Facilitates Horizontal Gene Transfer in Staphylococci. Nat. Microbiol. 2019, 4, 956–963. [Google Scholar] [CrossRef]
- Ramamurthy, T.; Ghosh, A.; Chowdhury, G.; Mukhopadhyay, A.K.; Dutta, S.; Miyoshi, S.I. Deciphering the Genetic Network and Programmed Regulation of Antimicrobial Resistance in Bacterial Pathogens. Front. Cell. Infect. Microbiol. 2022, 12, 952491. [Google Scholar] [CrossRef] [PubMed]
- Ratner, H.K.; Sampson, T.R.; Weiss, D.S. I Can See CRISPR Now, Even When Phage Are Gone: A View on Alternative CRISPR-Cas Functions from the Prokaryotic Envelope. Curr. Opin. Infect. Dis. 2015, 28, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Zuberi, A.; Ahmad, N.; Ahmad, H.; Saeed, M.; Ahmad, I. Beyond Antibiotics: CRISPR/Cas9 Triumph over Biofilm-Associated Antibiotic Resistance Infections. Front. Cell. Infect. Microbiol. 2024, 14, 1408569. [Google Scholar] [CrossRef]
- Amen, R.A.; Hassan, Y.M.; Essmat, R.A.; Ahmed, R.H.; Azab, M.M.; Shehata, N.R.; Elgazzar, M.M.; El-Sayed, W.M. Harnessing the Microbiome: CRISPR-Based Gene Editing and Antimicrobial Peptides in Combating Antibiotic Resistance and Cancer. Probiotics Antimicrob. Proteins 2025, 17, 1938–1968. [Google Scholar] [CrossRef]
- Koskella, B.; Brockhurst, M.A. Bacteria-Phage Coevolution as a Driver of Ecological and Evolutionary Processes in Microbial Communities. FEMS Microbiol. Rev. 2014, 38, 916–931. [Google Scholar] [CrossRef]
- Ali, N.; Vora, C.; Mathuria, A.; Kataria, N.; Mani, I. Chapter Four—Advances in CRISPR-Cas Systems for Gut Microbiome. In CRISPR-Cas-Based Genome Editing for Treating Human Diseases-Part A; Singh, V., Ed.; Progress in Molecular Biology and Translational Science; Academic Press: Cambridge, MA, USA, 2024; Volume 208, pp. 59–81. [Google Scholar]
- Echavarria Galindo, M.; Lai, Y. CRISPR-Based Genetic Tools for the Study of Host-Microbe Interactions. Infect. Immun. 2025, 93, e0051024. [Google Scholar] [CrossRef]
- Yaqub, M.O.; Jain, A.; Joseph, C.E.; Edison, L.K. Microbiome-Driven Therapeutics: From Gut Health to Precision Medicine. Gastrointest. Disord. 2025, 7, 7. [Google Scholar] [CrossRef]
- Chen, F.; Chen, L.; Yan, Z.; Xu, J.; Feng, L.; He, N.; Guo, M.; Zhao, J.; Chen, Z.; Chen, H.; et al. Recent Advances of CRISPR-Based Genome Editing for Enhancing Staple Crops. Front. Plant Sci. 2024, 15, 1478398. [Google Scholar] [CrossRef] [PubMed]
- Tao, J.; Bauer, D.E.; Chiarle, R. Assessing and Advancing the Safety of CRISPR-Cas Tools: From DNA to RNA Editing. Nat. Commun. 2023, 14, 212. [Google Scholar] [CrossRef]
- Duan, L.; Ouyang, K.; Xu, X.; Xu, L.; Wen, C.; Zhou, X.; Qin, Z.; Xu, Z.; Sun, W.; Liang, Y. Nanoparticle Delivery of CRISPR/Cas9 for Genome Editing. Front. Genet. 2021, 12, 673286. [Google Scholar] [CrossRef] [PubMed]
- Chuai, G.; Ma, H.; Yan, J.; Chen, M.; Hong, N.; Xue, D.; Zhou, C.; Zhu, C.; Chen, K.; Duan, B.; et al. DeepCRISPR: Optimized CRISPR Guide RNA Design by Deep Learning. Genome Biol. 2018, 19, 80. [Google Scholar] [CrossRef]
- Pandelakis, M.; Delgado, E.; Ebrahimkhani, M.R. CRISPR-Based Synthetic Transcription Factors In Vivo: The Future of Therapeutic Cellular Programming. Cell Syst. 2020, 10, 1–14. [Google Scholar] [CrossRef]
- Matsumoto, D.; Matsugi, E.; Kishi, K.; Inoue, Y.; Nigorikawa, K.; Nomura, W. SpCas9-HF1 Enhances Accuracy of Cell Cycle-Dependent Genome Editing by Increasing HDR Efficiency, and by Reducing off-Target Effects and Indel Rates. Mol. Ther. Nucleic Acids 2024, 35, 102124. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.C.W.; Gaidukov, L.; Lai, Y.; Wu, M.R.; Cao, J.; Gutbrod, M.J.; Choi, G.C.G.; Utomo, R.P.; Chen, Y.C.; Wroblewska, L.; et al. A Synthetic Transcription Platform for Programmable Gene Expression in Mammalian Cells. Nat. Commun. 2022, 13, 6167. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).