Comparative Analysis of the Biomass Production and Nutritional Profiles of Two Wild-Type Strains of Yarrowia lipolytica
Abstract
1. Introduction
2. Materials and Methods
2.1. Microorganism
2.2. Culture Media
2.3. Preparation of Inoculum and Cultivation
2.4. Analytical Methods
3. Results
3.1. Biomass Production
3.2. Comparison of Biomass Composition
3.3. Comparison of Amino Acid Composition
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AAA | Aromatic amino acid (tryptophan + phenylalanine + tyrosine) |
| DHA | Docosahex-aenoic acid |
| DPA | Docosapentaenoic acid |
| EPA | Eicosapentaenoic acid |
| FA | Fatty acids |
| SSA | Sulfur amino acid (cysteine + methionine) |
References
- Lokko, Y.; Heijde, M.; Schebesta, K.; Scholtès, P.; Van Montagu, M.; Giacca, M. Biotechnology and the bioeconomy—Towards inclusive and sustainable industrial development. New Biotechnol. 2018, 40, 5–10. [Google Scholar] [CrossRef]
- FAO. Declaración de la FAO Sobre Biotecnología. Organización de las Naciones Unidas para la Alimentación y la Agricultura. Available online: https://www.fao.org/biotech/fao-statement-on-biotechnology/es/ (accessed on 20 October 2023).
- Madzak, C. Yarrowia lipolytica Strains and Their Biotechnological Applications: How Natural Biodiversity and Metabolic Engineering Could Contribute to Cell Factories Improvement. J. Fungi 2021, 7, 548. [Google Scholar] [CrossRef] [PubMed]
- Tullio, V. Yeast Genomics and Its Applications in Biotechnological Processes: What Is Our Present and Near Future? J. Fungi 2022, 8, 752. [Google Scholar] [CrossRef] [PubMed]
- Karaalioğlu, O.; Yüceer, Y.K. Nonconventional yeasts to produce aroma compounds by using agri-food waste materials. FEMS Yeast Res. 2021, 21, foab063. [Google Scholar] [CrossRef] [PubMed]
- Zieniuk, B.; Fabiszewska, A. Yarrowia lipolytica: A beneficious yeast in biotechnology as a rare opportunistic fungal pathogen: A minireview. World J. Microbiol. Biotechnol. 2018, 35, 10. [Google Scholar] [CrossRef]
- Timoumi, A.; Guillouet, S.E.; Molina-Jouve, C.; Fillaudeau, L.; Gorret, N. Impacts of environmental conditions on product formation and morphology of Yarrowia lipolytica. Appl. Microbiol. Biotechnol. 2018, 102, 3831–3848. [Google Scholar] [CrossRef] [PubMed]
- Vandermies, M.; Fickers, P. Bioreactor-Scale Strategies for the Production of Recombinant Protein in the Yeast Yarrowia lipolytica. Microorganisms 2019, 7, 40. [Google Scholar] [CrossRef]
- Park, Y.-K.; Ledesma-Amaro, R. What makes Yarrowia lipolytica well suited for industry? Trends Biotechnol. 2023, 41, 242–254. [Google Scholar] [CrossRef]
- Groenewald, M.; Boekhout, T.; Neuvéglise, C.; Gaillardin, C.; van Dijck, P.W.M.; Wyss, M. Yarrowia lipolytica: Safety assessment of an oleaginous yeast with a great industrial potential. Crit. Rev. Microbiol. 2014, 40, 187–206. [Google Scholar] [CrossRef]
- Yan, Y.; Zhang, X.; Chen, D. Enhanced catalysis of Yarrowia lipolytica lipase LIP2 immobilized on macroporous resin and its application in enrichment of polyunsaturated fatty acids. Bioresour. Technol. 2013, 131, 179–187. [Google Scholar] [CrossRef]
- Li, L.; Wu, W.; Deng, Z.; Zhang, S.; Guan, W. Improved thermostability of lipase Lip2 from Yarrowia lipolytica through disulfide bond design for preparation of medium-long-medium structured lipids. LWT 2022, 166, 113786. [Google Scholar] [CrossRef]
- Ping, L.; Yuan, X.; Zhang, M.; Chai, Y.; Shan, S. Improvement of extracellular lipase production by a newly isolated Yarrowia lipolytica mutant and its application in the biosynthesis of L-ascorbyl palmitate. Int. J. Biol. Macromol. 2018, 106, 302–311. [Google Scholar] [CrossRef]
- Moujehed, E.; Zarai, Z.; Khemir, H.; Miled, N.; Bchir, M.S.; Gablin, C.; Bessueille, F.; Bonhommé, A.; Leonard, D.; Carrière, F.; et al. Cleaner degreasing of sheepskins by the Yarrowia lipolytica LIP2 lipase as a chemical-free alternative in the leather industry. Colloids Surf. B Biointerfaces 2022, 211, 112292. [Google Scholar] [CrossRef]
- He, Y.; Li, K.; Bo, G.; Wang, J.; Xu, L.; Yan, J.; Yang, M.; Yan, Y. Enhancing biodiesel production via liquid Yarrowia lipolytica lipase 2 in deep eutectic solvents. Fuel 2022, 316, 123342. [Google Scholar] [CrossRef]
- Posso Mendoza, H.; Pérez Salinas, R.; Tarón Dunoyer, A.; Tatis, C.C.; Morgado-Gamero, W.B.; Castillo Ramírez, M.; Parody, A. Evaluation of Enzymatic Extract with Lipase Activity of Yarrowia lipolytica. In An Application of Data Mining for the Food Industry Wastewater Treatment; Springer: Cham, Switzerland, 2020; pp. 304–313. [Google Scholar]
- Guieysse, D.; Sandoval, G.; Faure, L.; Nicaud, J.-M.; Monsan, P.; Marty, A. New efficient lipase from Yarrowia lipolytica for the resolution of 2-bromo-arylacetic acid esters. Tetrahedron Asymmetry 2004, 15, 3539–3543. [Google Scholar] [CrossRef]
- Turki, S.; Mrabet, G.; Jabloun, Z.; Destain, J.; Thonart, P.; Kallel, H. A highly stable Yarrowia lipolytica lipase formulation for the treatment of pancreatic exocrine insufficiency. Biotechnol. Appl. Biochem. 2010, 57, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Sandoval, G.; Rivera, I.; Barrera-Rivera, K.A.; Martínez-Richa, A. Biopolymer Synthesis Catalyzed by Tailored Lipases. Macromol. Symp. 2010, 289, 135–139. [Google Scholar] [CrossRef]
- EFSA Panel on Nutrition, Novel Foods, Food Allergens (NDA); Turck, D.; Bohn, T.; Castenmiller, J.; De Henauw, S.; Hirsch-Ernst, K.I.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; Naska, A.; et al. Safety of an extension of use of Yarrowia lipolytica yeast biomass as a novel food pursuant to Regulation (EU) 2015/2283. EFSA Panel on Nutrition, Novel Foods, Food Allergens. EFSA J. 2022, 20, e07450. [Google Scholar] [CrossRef]
- Pesantes-Munoz, M.; Ledesma-Amaro, R. Pathway Engineering for Beta-Carotene and Carotenoid Biosynthesis in Y. lipolytica. In Yarrowia lipolytica: Methods and Protocols; Wheeldon, I., Blenner, M., Eds.; Springer: New York, NY, USA, 2021; pp. 191–204. [Google Scholar]
- Zieniuk, B.; Wołoszynowska, M.; Białecka-Florjańczyk, E.; Fabiszewska, A. Application of freeze-dried Yarrowia lipolytica biomass in the synthesis of lipophilic antioxidants. Biotechnol. Lett. 2021, 43, 601–612. [Google Scholar] [CrossRef]
- Kolhe, N.; Zinjarde, S.; Acharya, C. Removal of uranium by immobilized biomass of a tropical marine yeast Yarrowia lipolytica. J. Environ. Radioact. 2020, 223–224, 106419. [Google Scholar] [CrossRef] [PubMed]
- Fickers, P.; Benetti, P.-H.; Waché, Y.; Marty, A.; Mauersberger, S.; Smit, M.S.; Nicaud, J.-M. Hydrophobic substrate utilisation by the yeast Yarrowia lipolytica, and its potential applications. FEMS Yeast Res. 2005, 5, 527–543. [Google Scholar] [CrossRef]
- Ravindiran, G.; Ganapathy, G.P.; Josephraj, J.; Alagumalai, A. A critical insight into biomass derived biosorbent for bioremediation of dyes. ChemistrySelect 2019, 4, 9762–9775. [Google Scholar] [CrossRef]
- Carsanba, E.; Papanikolaou, S.; Erten, H. Production of oils and fats by oleaginous microorganisms with an emphasis given to the potential of the nonconventional yeast Yarrowia lipolytica. Crit. Rev. Biotechnol. 2018, 38, 1230–1243. [Google Scholar] [CrossRef] [PubMed]
- Lopes, M.; Miranda, S.M.; Costa, A.R.; Pereira, A.S.; Belo, I. Yarrowia lipolytica as a biorefinery platform for effluents and solid wastes valorization—Challenges and opportunities. Crit. Rev. Biotechnol. 2022, 42, 163–183. [Google Scholar] [CrossRef]
- Rakicka-Pustułka, M.; Miedzianka, J.; Jama, D.; Kawalec, S.; Liman, K.; Janek, T.; Skaradziński, G.; Rymowicz, W.; Lazar, Z. High value-added products derived from crude glycerol via microbial fermentation using Yarrowia clade yeast. Microb. Cell Fact. 2021, 20, 195. [Google Scholar] [CrossRef]
- Retcheski, M.C.; Maximowski, L.V.; Escorsin, K.J.S.; Kurosaki, J.K.d.A.R.; Romão, S.; Bitencourt, T.B.; Parra, J.E.G.; Cazarolli, L.H. Yarrowia lipolytica biomass—A potential additive to boost metabolic and physiological responses of Nile tilapia. Fish Physiol. Biochem. 2023, 49, 655–670. [Google Scholar] [CrossRef]
- Singh, A.; Vidakovic, A.; Singh, A.; Dicksved, J.; Schnurer, A.; Lundh, T. Yarrowia lipolytica yeast as a dietary supplement for rainbow trout (Oncorhynchus mykiss): Effects on gut microbiota, health and immunity. Aquaculture 2024, 590, 741065. [Google Scholar] [CrossRef]
- Niehus, X.; Casas-Godoy, L.; Vargas-Sánchez, M.; Sandoval, G. A Fast and Simple Qualitative Method for Screening Oleaginous Yeasts on Agar. J. Lipids 2018, 2018, 5325804. [Google Scholar] [CrossRef]
- Hata, D.J.; Hall, L.; Fothergill, A.W.; Larone, D.H.; Wengenack, N.L. Multicenter evaluation of the new VITEK 2 advanced colorimetric yeast identification card. J. Clin. Microbiol. 2007, 45, 1087–1092. [Google Scholar] [CrossRef]
- Niehus, X.; Crutz-Le Coq, A.M.; Sandoval, G.; Nicaud, J.M.; Ledesma-Amaro, R. Engineering Yarrowia lipolytica to enhance lipid production from lignocellulosic materials. Biotechnol. Biofuels 2018, 11, 11. [Google Scholar] [CrossRef] [PubMed]
- ATCC. Yarrowia lipolytica (Wickerham et al.) van der Walt et von Arx 9773™. Available online: https://www.atcc.org/products/9773 (accessed on 1 June 2025).
- Niehus, X.; Casas-Godoy, L.; Rodríguez-Valadez, F.J.; Sandoval, G. Evaluation of Yarrowia lipolytica Oil for Biodiesel Production: Land Use Oil Yield, Carbon, and Energy Balance. J. Lipids 2018, 2018, 6393749. [Google Scholar] [CrossRef]
- NMX-F-608-NORMEX-2011; Alimentos—Determinación de Proteínas en Alimentos—Método de Ensayo (Prueba). Secretaría-de-Economía: Mexico City, Mexico, 2011.
- AOAC. Association of Official Analytical Chemists Official Method 982.30: Amino Acid Profiling of Food and Feed Ingredients; AOAC: Rockville, MD, USA, 1982. [Google Scholar]
- FAO. Food Energy—Methods of Analysis and Conversion Factors: Report of a Technical Workshop; FAO Food and Nutrition Paper 77; FAO: Rome, Italy, 2003. [Google Scholar]
- NOM-086-SSA1-1994; Bienes y Servicios. Alimentos y Bebidas no Alcohólicas con Modificaciones en su Composición. Especificaciones Nutrimentales. Apéndice Normativo C, Numeral 1. Secretaría-de-Salud: Mexico City, Mexico, 1994.
- NMX-F-607-NORMEX-2020; Alimentos—Determinación de Cenizas en Alimentos—Método de Prueba. Secretaría-de-Economía: Mexico City, Mexico, 2020.
- Cosío-Cuadros, R.; Núñez-López, G.; Campo, M.F.M.d.; Rodríguez, J.A.; Mateos-Díaz, J.C.; Sandoval, G. Agro-Industrial Wastes to Sustainable Bio-Oil Fuels, Enzymes and Biobased Chemicals in Yeast-Biorefineries. In Microbiology of Green Fuels; Yousuf, A., Tomás-Pejó, E., Eds.; CRC Press: Boca Raton, FL, USA, 2023. [Google Scholar]
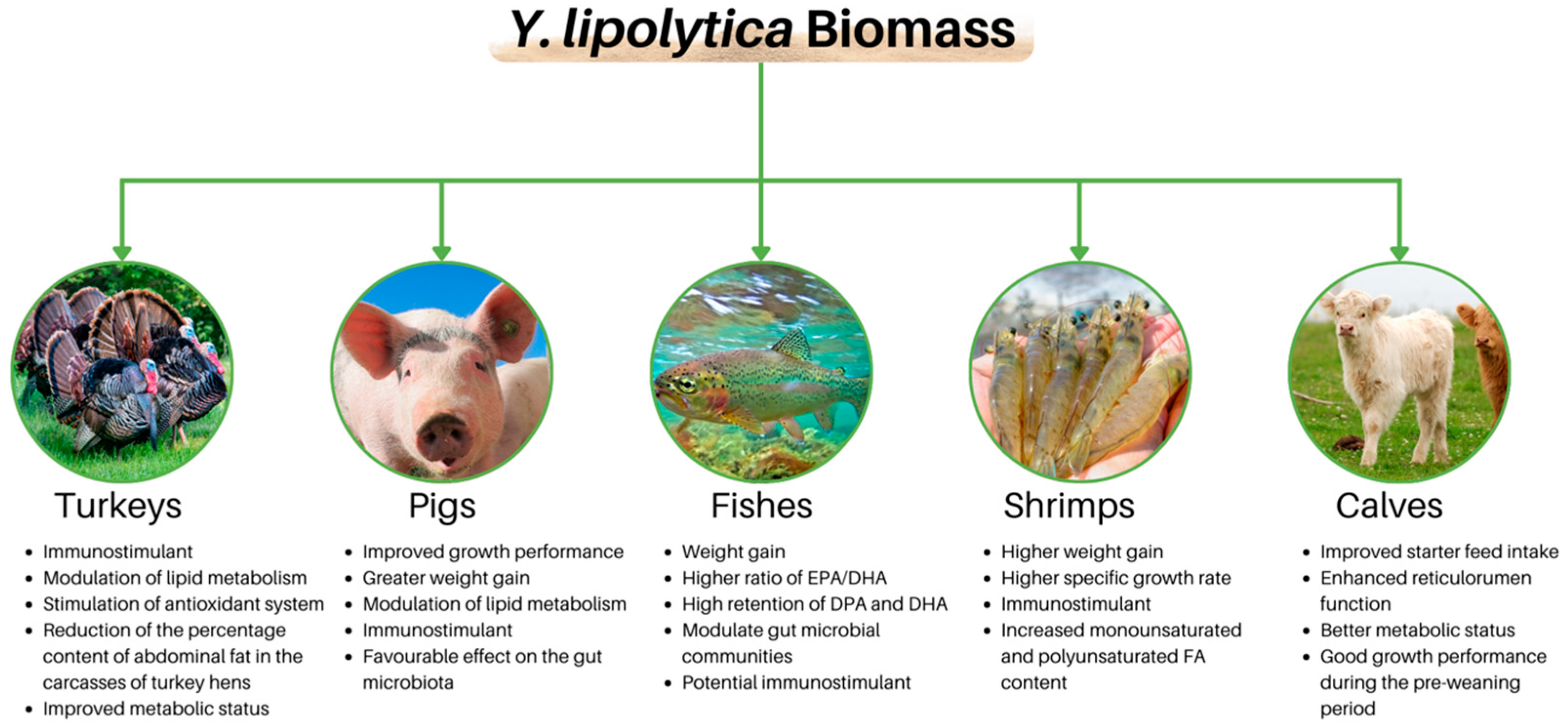
- Merska, M.; Czech, A.; Ognik, K. The effect of yeast Yarrowia lipolytica on the antioxidant indices and macro-and microelements in blood plasma of turkey hens. Pol. J. Vet. Sci. 2015, 18, 709–714. [Google Scholar] [CrossRef][Green Version]
- Merska, M.; Czech, A.; Ognik, K. The effect of different doses of dried yeast Yarrowia lipolytica on production effects of turkey hens and hematological indicators of blood. Ann. Univ. Mariae Curie-Skłodowska 2013, 31, 35–41. [Google Scholar]
- Czech, A.; Merska, M.; Ognik, K. Blood Immunological and Biochemical Indicators in Turkey Hens Fed Diets With a Different Content of the Yeast Yarrowia Lipolytica. Ann. Anim. Sci. 2014, 14, 935–946. [Google Scholar] [CrossRef]
- Czech, A.; Smolczyk, A.; Ognik, K.; Wlazło, Ł.; Nowakowicz-Dębek, B.; Kiesz, M. Effect of dietary supplementation with Yarrowia lipolytica or Saccharomyces cerevisiae yeast and probiotic additives on haematological parameters and the gut microbiota in piglets. Res. Vet. Sci. 2018, 119, 221–227. [Google Scholar] [CrossRef]
- Czech, A.; Smolczyk, A.; Ognik, K.; Kiesz, M. Nutritional Value of Yarrowia Lipolytica Yeast and its Effect on Growth Performance Indicators in Piglets. Ann. Anim. Sci. 2016, 16, 1091–1100. [Google Scholar] [CrossRef]
- Hatlen, B.; Berge, G.M.; Odom, J.M.; Mundheim, H.; Ruyter, B. Growth performance, feed utilisation and fatty acid deposition in Atlantic salmon, Salmo salar L., fed graded levels of high-lipid/high-EPA Yarrowia lipolytica biomass. Aquaculture 2012, 364–365, 39–47. [Google Scholar] [CrossRef]
- Volff, V.; Kurosaki, J.K.d.A.R.; Retcheski, M.C.; Maximovski, L.V.; Escorsin, K.J.S.; Tormen, L.; Romão, S.; Pinto, V.Z.; Bitencourt, T.B.; Cazarolli, L.H. In Vivo Yarrowia lipolytica Fermented Biomass Feed Supplementation for Shrimp and Its Effects Upon Frozen Meat Storage. J. Aquat. Food Prod. Technol. 2022, 31, 785–800. [Google Scholar] [CrossRef]
- Licona-Jain, A.; Campa-Córdova, Á.; Luna-González, A.; Racotta, I.S.; Tello, M.; Angulo, C. Dietary supplementation of marine yeast Yarrowia lipolytica modulates immune response in Litopenaeus vannamei. Fish Shellfish Immunol. 2020, 105, 469–476. [Google Scholar] [CrossRef]
- Alvarez Sánchez, A.; Nolasco, H.; Peña-Rodríguez, A.; Mejia, H. In vitro digestibility of Yarrowia lipolytica yeast and growth performance in whiteleg shrimp Litopenaeus vannamei. J. Fish. Aquat. Sci. 2018, 18, 395–404. [Google Scholar] [CrossRef]
- Stefańska, B.; Komisarek, J.; Stanisławski, D.; Gąsiorek, M.; Kasprowicz-Potocka, M.; Frankiewicz, A.; Nowak, W. The effect of Yarrowia lipolytica culture on growth performance, ruminal fermentation and blood parameters of dairy calves. Anim. Feed. Sci. Technol. 2018, 243, 72–79. [Google Scholar] [CrossRef]
- Tsirigka, A.; Theodosiou, E.; Patsios, S.I.; Tsoureki, A.; Andreadelli, A.; Papa, E.; Aggeli, A.; Karabelas, A.J.; Makris, A.M. Novel evolved Yarrowia lipolytica strains for enhanced growth and lipid content under high concentrations of crude glycerol. Microb. Cell Factories 2023, 22, 62. [Google Scholar] [CrossRef]
- Drzymała, K.; Mirończuk, A.M.; Pietrzak, W.; Dobrowolski, A. Rye and Oat Agricultural Wastes as Substrate Candidates for Biomass Production of the Non-Conventional Yeast Yarrowia lipolytica. Sustainability 2020, 12, 7704. [Google Scholar] [CrossRef]
- Juszczyk, P.; Rywińska, A.; Kosicka, J.; Tomaszewska-Hetman, L.; Rymowicz, W. Sugar Alcohol Sweetener Production by Yarrowia lipolytica Grown in Media Containing Glycerol. Molecules 2023, 28, 6594. [Google Scholar] [CrossRef]
- Workman, M.; Holt, P.; Thykaer, J. Comparing cellular performance of Yarrowia lipolytica during growth on glucose and glycerol in submerged cultivations. AMB Express 2013, 3, 58. [Google Scholar] [CrossRef]
- Quispe, C.A.G.; Coronado, C.J.R.; Carvalho, J.A., Jr. Glycerol: Production, consumption, prices, characterization and new trends in combustion. Renew. Sustain. Energy Rev. 2013, 27, 475–493. [Google Scholar] [CrossRef]
- Kamzolova, S.V.; Lunina, J.N.; Samoilenko, V.A.; Morgunov, I.G. Effect of Nitrogen Concentration on the Biosynthesis of Citric Acid, Protein, and Lipids in the Yeast Yarrowia lipolytica. Biomolecules 2022, 12, 1421. [Google Scholar] [CrossRef] [PubMed]
- Juszczyk, P.; Tomaszewska, L.; Kita, A.; Rymowicz, W. Biomass production by novel strains of Yarrowia lipolytica using raw glycerol, derived from biodiesel production. Bioresour. Technol. 2013, 137, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Peterson, E.C.; Siao, R.; Chua, G.G.; Busran, C.T.; Pavlovic, R.; Thong, A.; Hermansen, C.; Sofeo, N.; Kanagasundaram, Y.; Weingarten, M.; et al. Single cell protein and oil production from solid cocoa fatty acid distillates co-fed ethanol. Bioresour. Technol. 2023, 387, 129630. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Chen, Z.; Hu, P.; Zhang, S.; Luo, G. Two-stage fermentation enhanced single-cell protein production by Yarrowia lipolytica from food waste. Bioresour. Technol. 2022, 361, 127677. [Google Scholar] [CrossRef]
- Alves, S.J.F.; Pires, E.B.E.; Alexandre, M.A.d.S.; Santos, C.C.A.d.A.; Martin, J.G.P.; Campelo, P.H.; Martins, E.; Eller, M.R. Single-cell proteins as alternative sources of proteins and nutrients. Food Res. Int. 2025, 214, 116631. [Google Scholar] [CrossRef]
- Gao, L.; Khoo, S.C.; Zhang, Z.; Wu, X. Trends in sustainable single-cell protein from non-grain feedstocks. Trends Biotechnol. 2025. [Google Scholar] [CrossRef] [PubMed]
- Soh, B.X.P.; Vignes, M.; Smith, N.W.; von Hurst, P.R.; McNabb, W.C. Evaluation of protein intake and protein quality in New Zealand vegans. PLoS ONE 2025, 20, e0314889. [Google Scholar] [CrossRef] [PubMed]
- Wanders, A.J.; Heerschop, S.N.; Biesbroek, S.; Dötsch-Klerk, M. Replacing Animal Meat with Plant-Based Meat Alternatives: The Impact of Protein Quality on Protein Adequacy in the Dutch Diet. Curr. Dev. Nutr. 2025, 9, 104562. [Google Scholar] [CrossRef] [PubMed]
- Jach, M.E.; Malm, A. Yarrowia lipolytica as an Alternative and Valuable Source of Nutritional and Bioactive Compounds for Humans. Molecules 2022, 27, 2300. [Google Scholar] [CrossRef]
- Machuca, C.; Méndez-Martínez, Y.; Reyes-Becerril, M.; Angulo, C. Yeast β-Glucans as Fish Immunomodulators: A Review. Animals 2022, 12, 2154. [Google Scholar] [CrossRef]
- Velazquez-Carriles, C.; Macias-Rodríguez, M.E.; Carbajal-Arizaga, G.G.; Silva-Jara, J.; Angulo, C.; Reyes-Becerril, M. Immobilizing yeast β-glucan on zinc-layered hydroxide nanoparticle improves innate immune response in fish leukocytes. Fish Shellfish Immunol. 2018, 82, 504–513. [Google Scholar] [CrossRef]
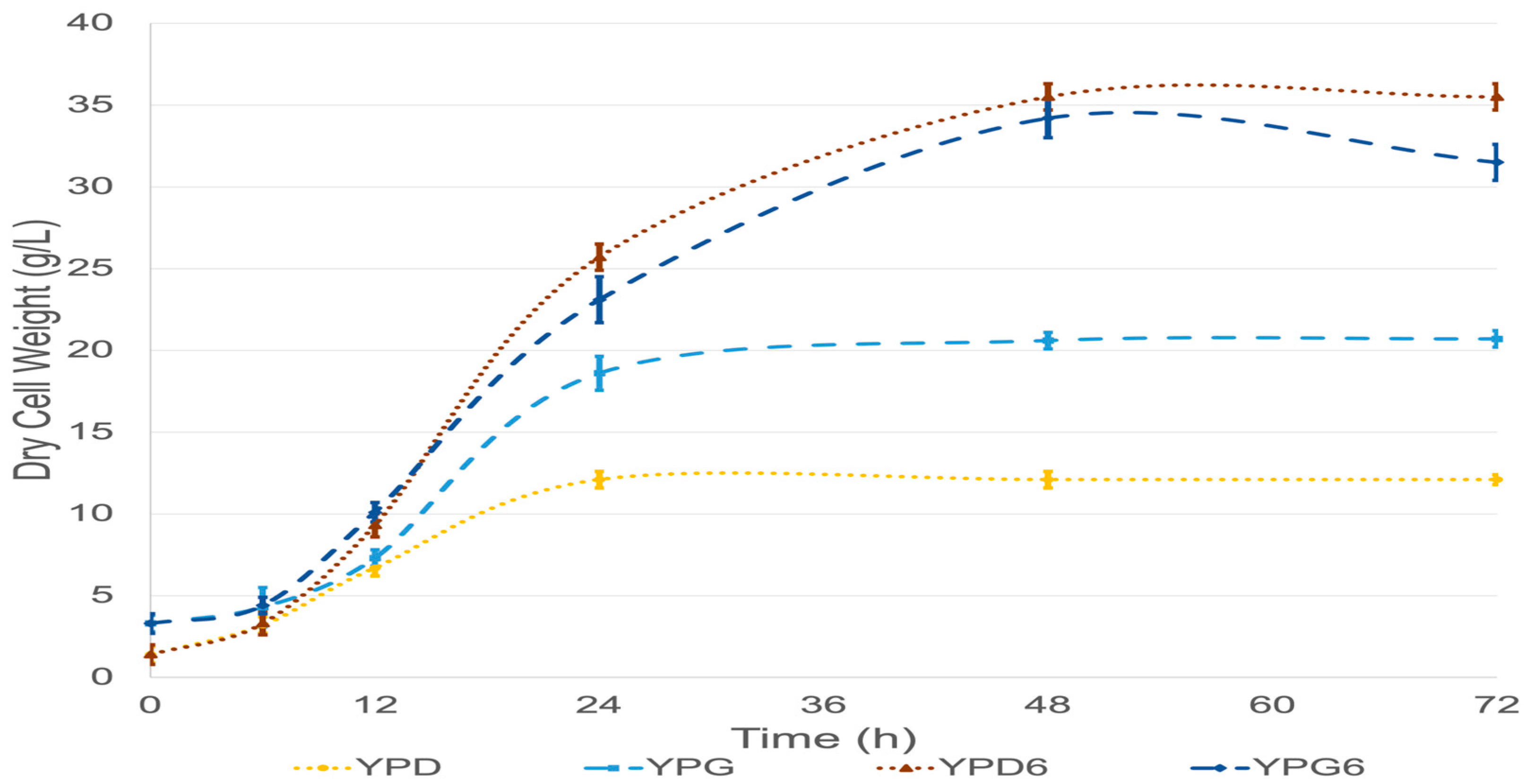
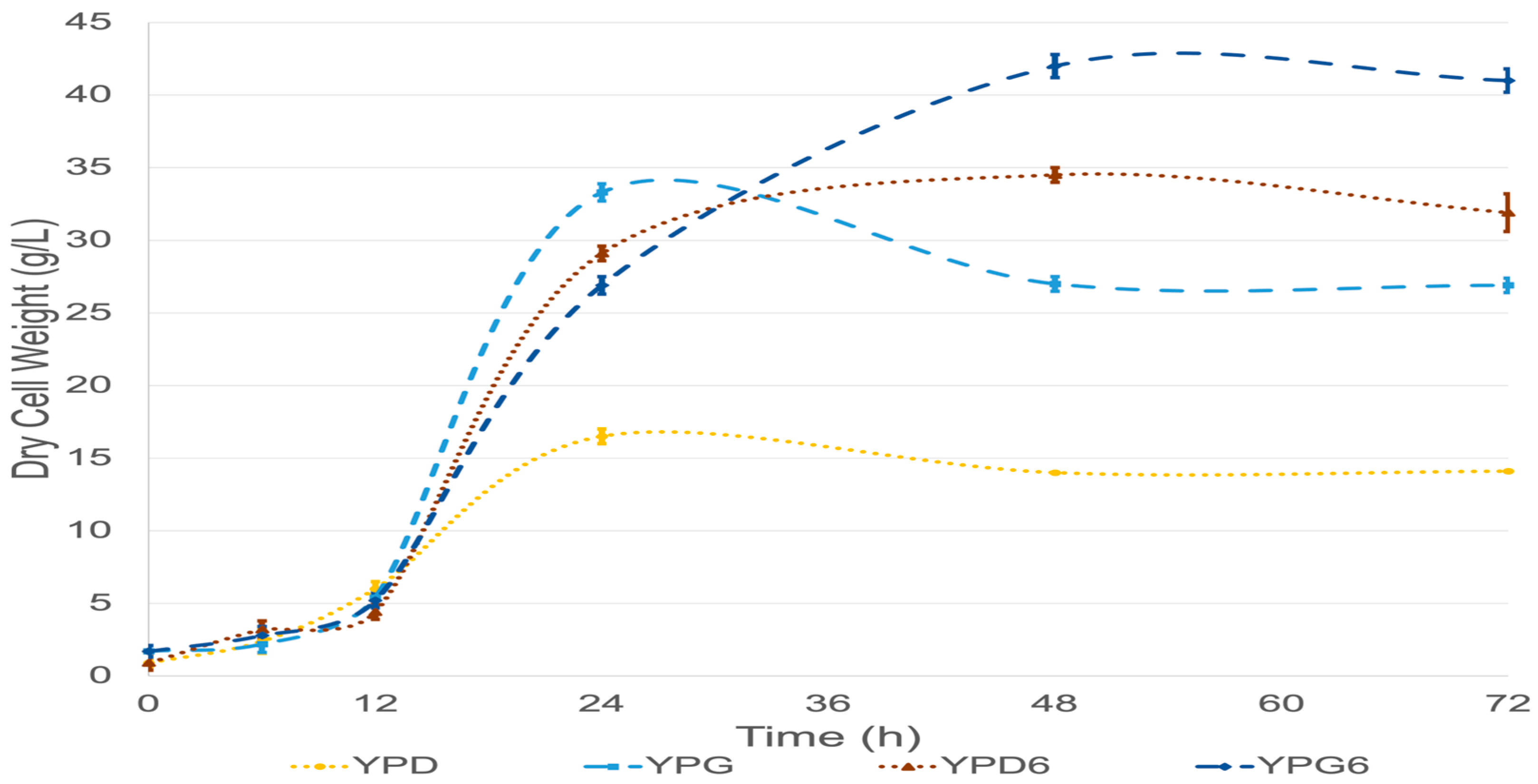
| Parameter | ATCC 9773 | NRRL Y-50997 | ||||||
|---|---|---|---|---|---|---|---|---|
| YPD | YPD6 | YPG | YPG6 | YPD | YPD6 | YPG | YPG6 | |
| Biomass (DCW, g/L) | 12.1 ± 0.5 a | 35.5 ± 0.8 | 20.6 ± 0.5 b | 34.2 ± 1.2 c | 16.5 ± 0.5 a | 34.5 ± 0.5 | 33.3 ± 0.6 b | 42 ± 0.8 c |
| YX/S (g biomass/g carbon source) | 0.61 ± 0.025 d | 0.59 ± 0.013 | 1.03 ± 0.025 e | 0.57 ± 0.02 f | 0.83 ± 0.025 d | 0.58 ± 0.008 | 1.67 ± 0.03 e | 0.7 ± 0.013 f |
| μ (h−1) | 0.07 ± 006 | 0.06 ± 0.006 | 0.08 ± 0.01 g | 0.05 ± 0.003 | 0.1 ± 0.02 | 0.06 ± 0.007 | 0.15 ± 0.02 g | 0.06 ± 0.007 |
| Parameter | ATCC 9773 * | NRRL Y-50997 * |
|---|---|---|
| Protein (%) | 58.8 | 58.2 |
| Essential amino acids (%) | 62.6 a | 41.5 a |
| Lipids (%) | 3.04 | 6.35 |
| Carbohydrates (%) | 32.32 | 28.57 |
| Ashes (%) | 5.80 | 6.88 |
| Amino Acid | FAO Requirements | ATCC 9773 | NRRL Y-50997 | Y. lipolytica | S. cerevisiae | C. utilis | Egg | Cow Milk |
|---|---|---|---|---|---|---|---|---|
| Arg | - | 17.6 ± 0.19 | 40.5 ± 1.50 | 48 | 46.5 | 32 | 11.5 | 33 |
| His * | 15 | 72.7 ± 0.94 | 19.3 ± 0.37 | 26 | 23.5 | 16 | 4 | 37 |
| Ile * | 30 | 41.1 ± 1.87 | 22.5 ± 0.19 | 44 | 37 | 48 | 68 | 40 |
| Leu * | 59 | 97.8 ± 1.87 | 45.0 ± 1.50 | 68 | 63 | 71 | 90 | 88 |
| Lys * | 45 | 125.1 ± 2.62 a | 106.9 ± 2.62 b | 70 | 65 | 51 | 63 | 78 |
| Cys | - | <1.2 ± 0.00 | <1.2 ± 0.00 | 11 | 9 | 24 | 24 | 9 |
| Met * | - | 19.1 ± 0.19 | 14.6 ± 0.00 | 12 | 14 | 15.5 | 32 | 29 |
| SAA | 22 | 20.3 ± 0.19 | 15.8 ± 0.00 | 23 | 23 | 39.5 | 56 | 38 |
| Phe * | - | 72.7 ± 0.19 | 64.8 ± 0.37 | 40 | 33 | 41 | 63 | 47 |
| Trp * | - | 47.9 ± 0.56 | 41.7 ± 0.37 | 47 | 9 | 39 | 16 | ND |
| Tyr | - | 17.6 ± 0.37 | 13.2 ± 0.00 | 66 | 26 | 20 | 195 | 16 |
| AAA | 38 | 138.2 ± 1.12 | 119.7 ± 0.74 | 153 | 68 | 100 | 98.5 | 63 |
| Thr * | 23 | 35.3 ± 0.75 | 30.4 ± 0.94 | 48 | 48 | 41 | 50 | 48.7 |
| Val * | 39 | 58.8 ± 0.56 | 38.2 ± 0.94 | 53 | 53 | 55 | 74 | 47.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torres-Añorve, D.; Sandoval, G. Comparative Analysis of the Biomass Production and Nutritional Profiles of Two Wild-Type Strains of Yarrowia lipolytica. Appl. Microbiol. 2025, 5, 77. https://doi.org/10.3390/applmicrobiol5030077
Torres-Añorve D, Sandoval G. Comparative Analysis of the Biomass Production and Nutritional Profiles of Two Wild-Type Strains of Yarrowia lipolytica. Applied Microbiology. 2025; 5(3):77. https://doi.org/10.3390/applmicrobiol5030077
Chicago/Turabian StyleTorres-Añorve, David, and Georgina Sandoval. 2025. "Comparative Analysis of the Biomass Production and Nutritional Profiles of Two Wild-Type Strains of Yarrowia lipolytica" Applied Microbiology 5, no. 3: 77. https://doi.org/10.3390/applmicrobiol5030077
APA StyleTorres-Añorve, D., & Sandoval, G. (2025). Comparative Analysis of the Biomass Production and Nutritional Profiles of Two Wild-Type Strains of Yarrowia lipolytica. Applied Microbiology, 5(3), 77. https://doi.org/10.3390/applmicrobiol5030077








