Plasma-Treated Water Effect on Sporulating Bacillus cereus vs. Non-Sporulating Listeria monocytogenes Biofilm Cell Vitality
Abstract
1. Introduction
2. Materials and Methods
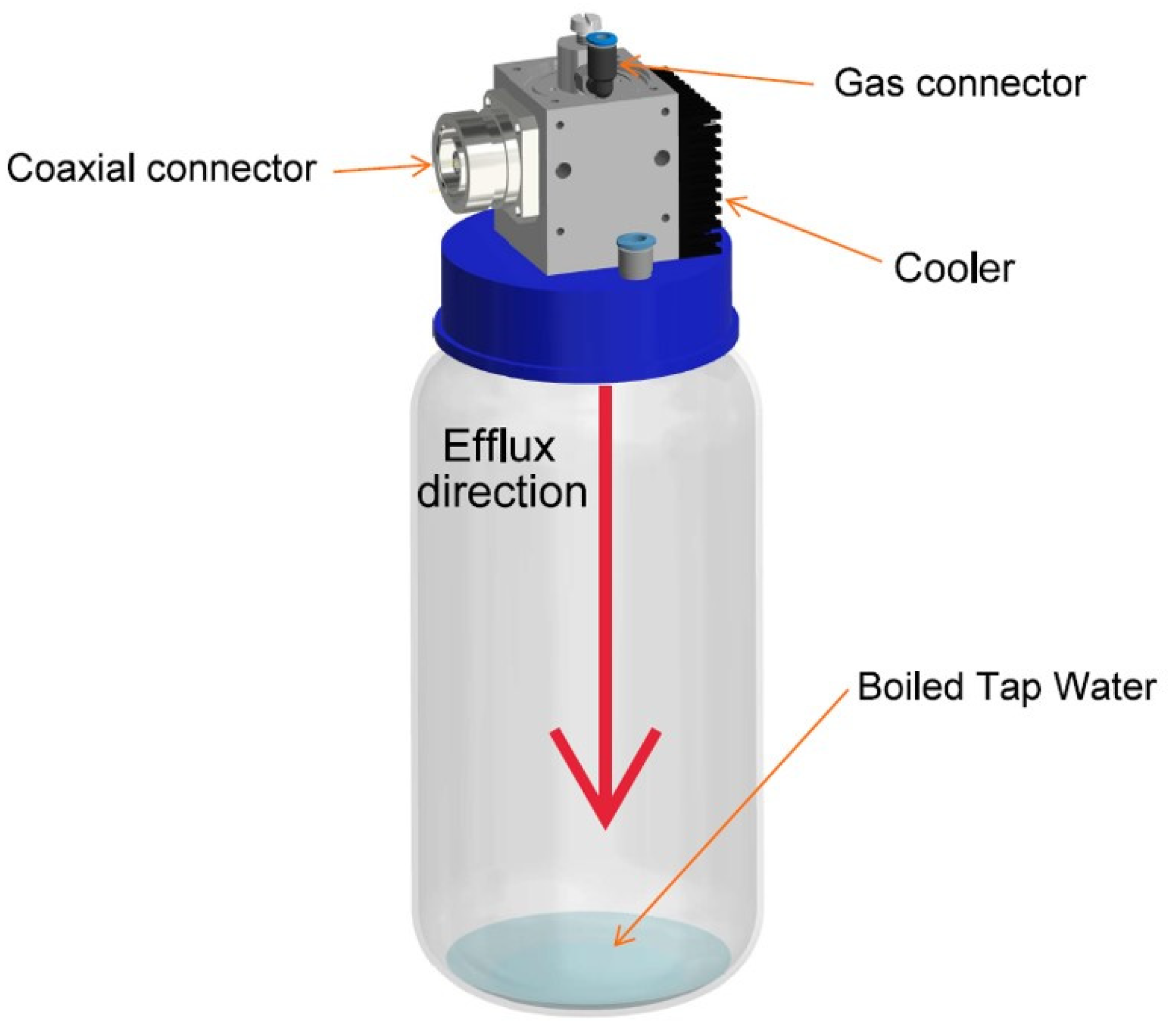
2.1. Producing PTW Using Microwave Plasma Source
2.2. Growth of Bacillus cereus and Listeria monocytogenes Biofilms
2.3. PTW Treatment on B. cereus and L. monocytogenes Biofilms
2.4. Fluorescence Live/Dead Assay of PTW-Treated Biofilms
2.5. XTT Assay
2.6. Quantification of RONS in PTW
2.7. Determining Hydrogen Peroxide Concentration Using Voltammetry
2.8. Statistics
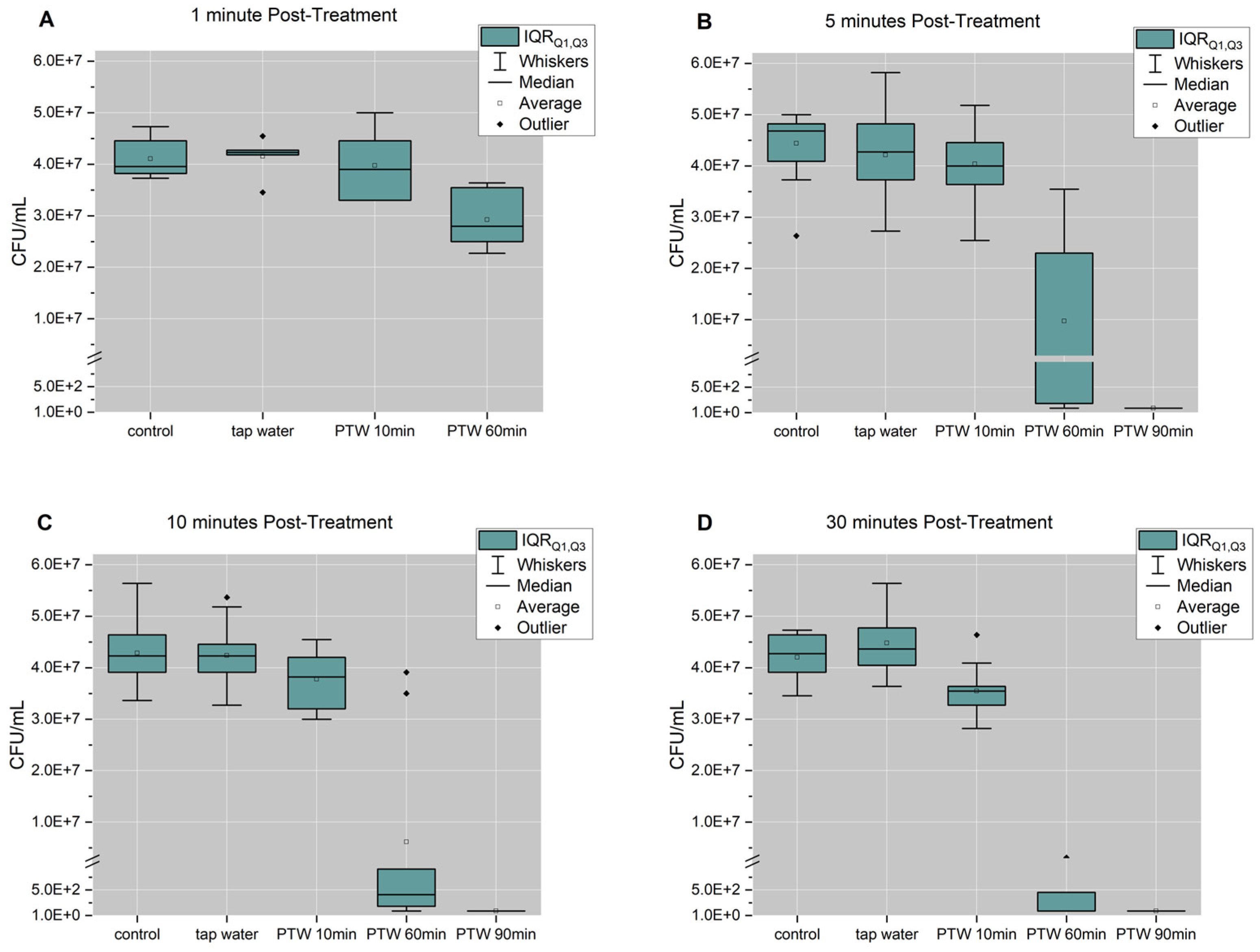
3. Results
3.1. Chemical Differences of PTW Between Pre-Treatment Times
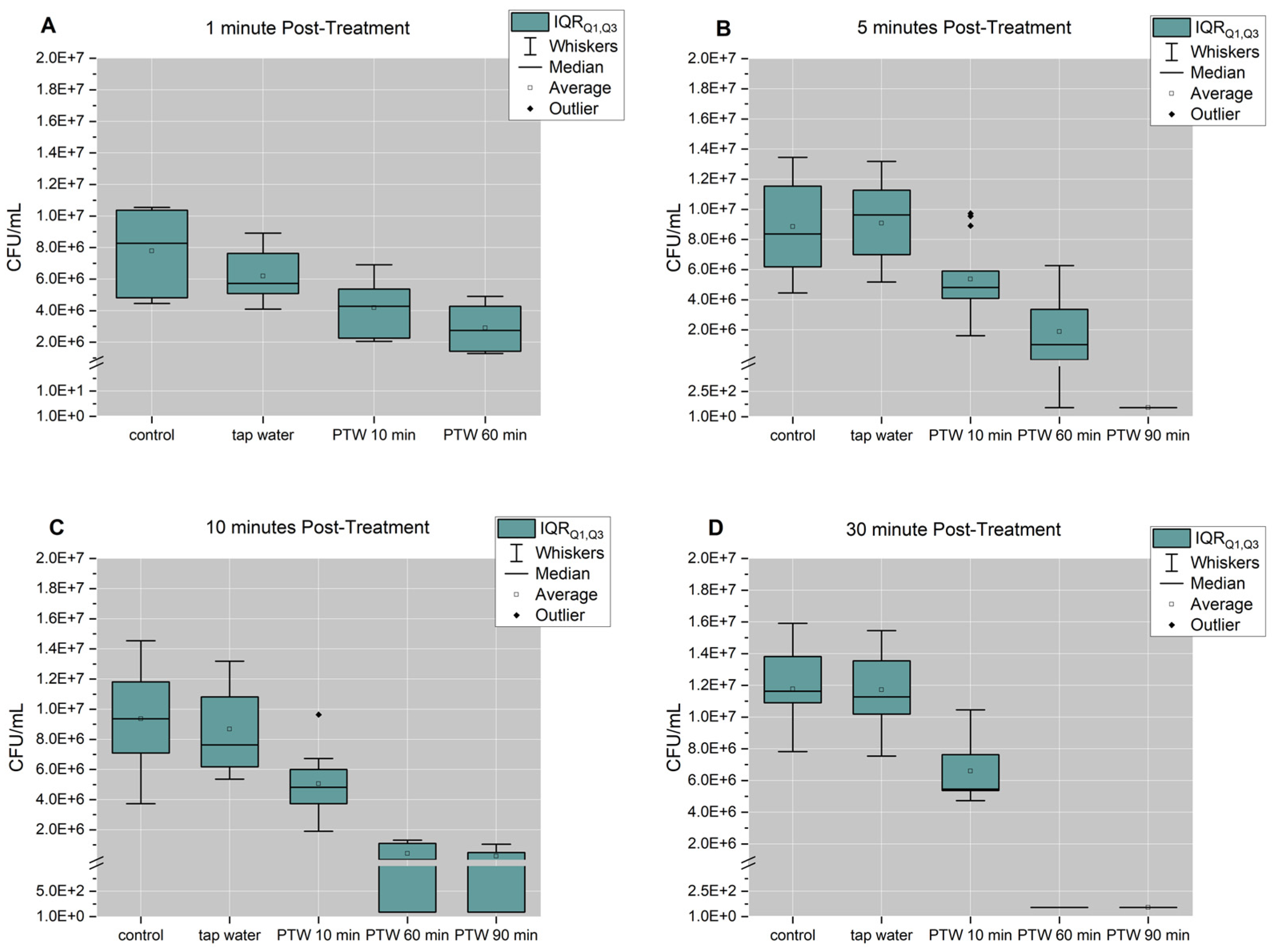
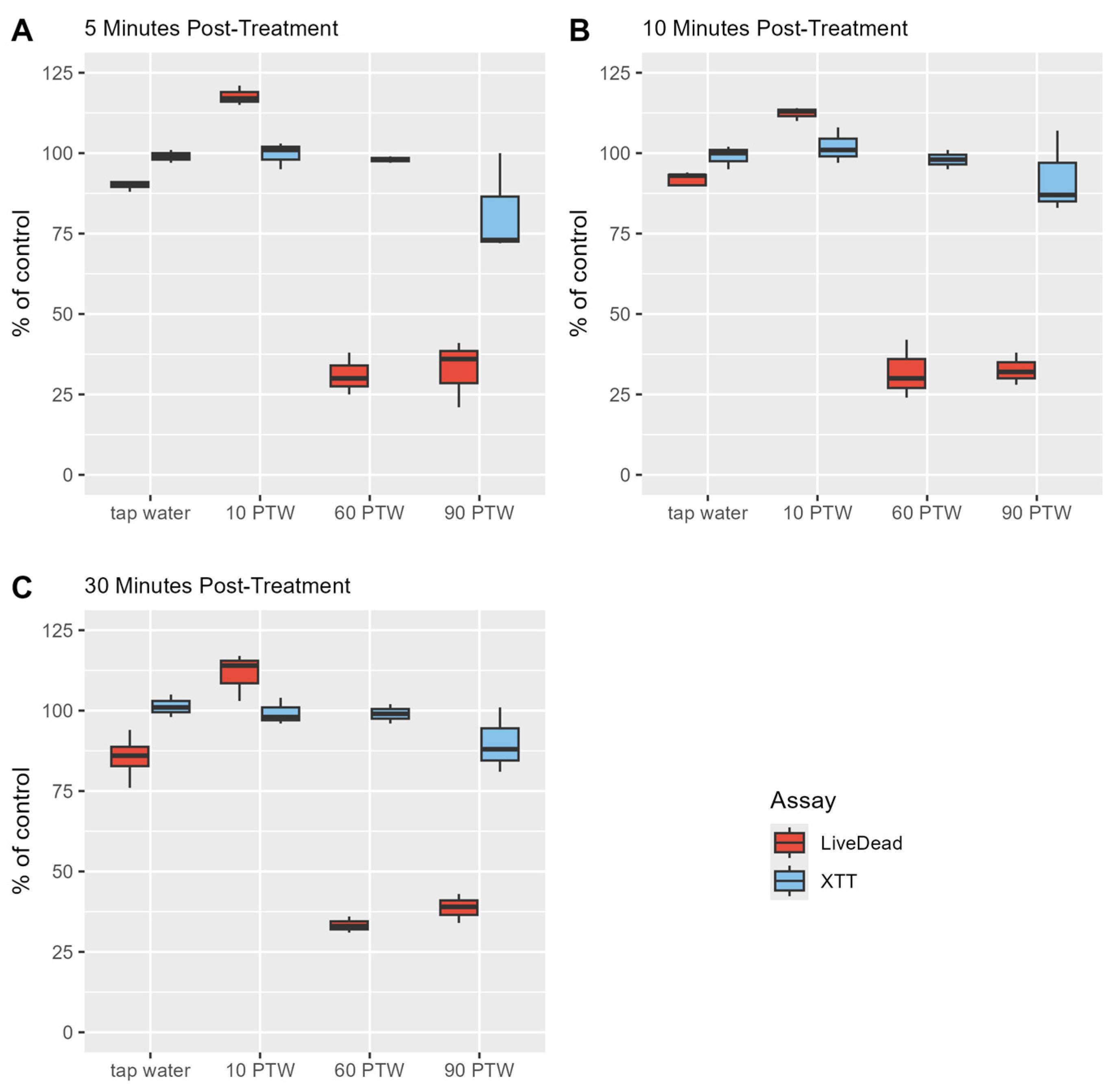
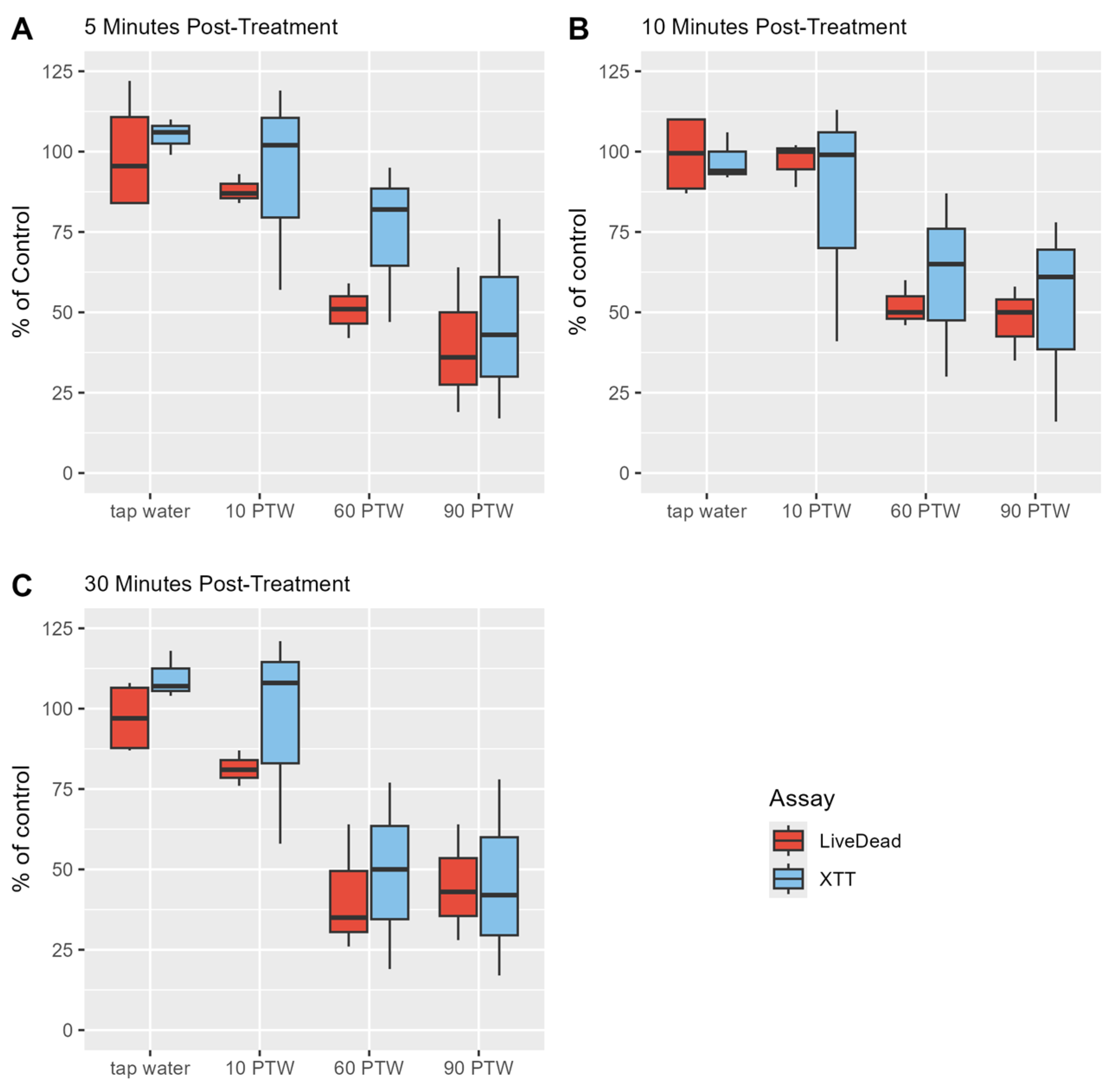
3.2. Optimal Pre-Treatment and Post-Treatment Time to Reduce Proliferation Activity of Biofilm Cells
| Scenario | Pairing | Resulting p-Value | αadapted | Tested Sample Size |
|---|---|---|---|---|
| 1 min post-treatment | Control—tap water | 0.66883 | 0.0167 | n = 6 |
| 1 min post-treatment | Control—PTW 10 min | 0.62987 | 0.0167 | n = 6 |
| 1 min post-treatment | Control—PTW 60 min | 0.00216 | 0.0167 | n = 6 |
| 5 min post-treatment | Control—tap water | 0.32580 | 0.0125 | n = 18 |
| 5 min post-treatment | Control—PTW 10 min | 0.02829 | 0.0125 | n = 15 |
| 5 min post-treatment | Control—PTW 60 min | 1.28935∙10−8 | 0.0125 | n = 15 |
| 5 min post-treatment | Control—PTW 90 min | 5.82751∙10−4 | 0.0125 | n = 7 |
| 10 min post-treatment | Control—tap water | 0.83274 | 0.0125 | n = 18 |
| 10 min post-treatment | Control—PTW 10 min | 0.02541 | 0.0125 | n = 15 |
| 10 min post-treatment | Control—PTW 60 min | 9.61483∙10−6 | 0.0125 | n = 12 |
| 10 min post-treatment | Control—PTW 90 min | 4.11353∙10−5 | 0.0125 | n = 9 |
| 30 min post-treatment | Control—tap water | 0.43611 | 0.0125 | n = 12 |
| 30 min post-treatment | Control—PTW 10 min | 0.00695 | 0.0125 | n = 9 |
| 30 min post-treatment | Control—PTW 60 min | 4.11353∙10−5 | 0.0125 | n = 9 |
| 30 min post-treatment | Control—PTW 90 min | 4.11353∙10−5 | 0.0125 | n = 9 |
3.3. 60 min and 90 min PTW Lead to a Reduction in Vitality of Biofilm Cells
3.4. PTW Treatment Had Mild Effects on the Metabolic Activity of B. cereus Biofilm Cells and Moderate Effects on L. monocytogenes
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Food Supply. Our World in Data. Available online: https://ourworldindata.org/food-supply (accessed on 30 October 2024).
- Fellows, P.J. Food Processing Technology: Principles and Practice; Woodhead Publishing: Cambridge, UK, 2022. [Google Scholar]
- How Food Gets Contaminated: The Food Production Chain. U.S. Centers for Disease Control and Prevention (CDC). 2024. Available online: https://www.cdc.gov/foodborne-outbreaks/foodproductionchain/index.html (accessed on 1 August 2025).
- Bryan, F.L. Risks of practices, procedures and processes that lead to outbreaks of foodborne diseases. J. Food Prot. 1988, 51, 663–673. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Biological Hazards (BIOHAZ); Koutsoumanis, K.; Allende, A.; Bolton, D.; Bover-Cid, S.; Chemaly, M.; De Cesare, A.; Herman, L.; Hilbert, F.; Lindqvist, R. Persistence of microbiological hazards in food and feed production and processing environments. Efsa J. 2024, 22, e8521. [Google Scholar]
- Busta, F. Thermal inactivation characteristics of bacterial spores at ultrahigh temperatures. Appl. Microbiol. 1967, 15, 640–645. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Farid, M. A review on recent development in non-conventional food sterilization technologies. J. Food Eng. 2016, 182, 33–45. [Google Scholar] [CrossRef]
- Goluch, Z.; Barbara, K.; Haraf, G.; Wołoszyn, J.; Okruszek, A.; Wereńska, M. Impact of various types of heat processing on the energy and nutritional values of goose breast meat. Poult. Sci. 2021, 100, 101473. [Google Scholar] [CrossRef]
- Zhao, X.; Guo, Y.; Zhang, Y.; Pang, X.; Wang, Y.; Lv, J.; Zhang, S. Effects of different heat treatments on Maillard reaction products and volatile substances of camel milk. Front. Nutr. 2023, 10, 1072261. [Google Scholar] [CrossRef]
- Shin, S.; Bhowmik, S.R. Thermal kinetics of color changes in pea puree. J. Food Eng. 1995, 24, 77–86. [Google Scholar] [CrossRef]
- Imaizumi, T.; Tanaka, F.; Uchino, T. Effects of mild heating treatment on texture degradation and peroxidase inactivation of carrot under pasteurization conditions. J. Food Eng. 2019, 257, 19–25. [Google Scholar] [CrossRef]
- Alkhafaji, S.R.; Farid, M.M. PEF Assisted Thermal Sterilization (PEF-ATS) Process-Inactivation of Geobacillus sterothermophilus Spores. J. Food Sci. Eng. 2012, 2, 403. [Google Scholar]
- Chandrapala, J.; Oliver, C.; Kentish, S.; Ashokkumar, M. Ultrasonics in food processing. Ultrason. Sonochem. 2012, 19, 975–983. [Google Scholar] [CrossRef]
- Gayán, E.; Álvarez, I.; Condón, S. Inactivation of bacterial spores by UV-C light. Innov. Food Sci. Emerg. Technol. 2013, 19, 140–145. [Google Scholar] [CrossRef]
- Wilson, D.R.; Dabrowski, L.; Stringer, S.; Moezelaar, R.; Brocklehurst, T.F. High pressure in combination with elevated temperature as a method for the sterilisation of food. Trends Food Sci. Technol. 2008, 19, 289–299. [Google Scholar] [CrossRef]
- Laroussi, M.; Leipold, F. Evaluation of the roles of reactive species, heat, and UV radiation in the inactivation of bacterial cells by air plasmas at atmospheric pressure. Int. J. Mass Spectrom. 2004, 233, 81–86. [Google Scholar] [CrossRef]
- Patil, S.; Moiseev, T.; Misra, N.; Cullen, P.; Mosnier, J.; Keener, K.; Bourke, P. Influence of high voltage atmospheric cold plasma process parameters and role of relative humidity on inactivation of Bacillus atrophaeus spores inside a sealed package. J. Hosp. Infect. 2014, 88, 162–169. [Google Scholar] [CrossRef]
- Moreau, M.; Orange, N.; Feuilloley, M.G.J. Non-thermal plasma technologies: New tools for bio-decontamination. Biotechnol. Adv. 2008, 26, 610–617. [Google Scholar] [CrossRef]
- Schnabel, U.; Handorf, O.; Yarova, K.; Zessin, B.; Zechlin, S.; Sydow, D.; Zellmer, E.; Stachowiak, J.; Andrasch, M.; Below, H. Plasma-treated air and water—Assessment of synergistic antimicrobial effects for sanitation of food processing surfaces and environment. Foods 2019, 8, 55. [Google Scholar] [CrossRef]
- Xu, Y.; Tian, Y.; Ma, R.; Liu, Q.; Zhang, J. Effect of plasma activated water on the postharvest quality of button mushrooms, Agaricus bisporus. Food Chem. 2016, 197, 436–444. [Google Scholar] [CrossRef]
- Schnabel, U.; Andrasch, M.; Stachowiak, J.; Weit, C.; Weihe, T.; Schmidt, C.; Muranyi, P.; Schlüter, O.; Ehlbeck, J. Sanitation of fresh-cut endive lettuce by plasma processed tap water (PPtW)–Up-scaling to industrial level. Innov. Food Sci. Emerg. Technol. 2019, 53, 45–55. [Google Scholar] [CrossRef]
- Yawut, N.; Mekwilai, T.; Vichiansan, N.; Braspaiboon, S.; Leksakul, K.; Boonyawan, D. Cold plasma technology: Transforming food processing for safety and sustainability. J. Agric. Food Res. 2024, 18, 101383. [Google Scholar] [CrossRef]
- Handorf, O.; Below, H.; Schnabel, U.; Riedel, K.; Ehlbeck, J. Investigation of the chemical composition of plasma-treated water by MidiPLexc and its antimicrobial effect on L. monocytogenes and Pseudomonas fluorescens monospecies suspension cultures. J. Phys. D Appl. Phys. 2020, 53, 305204. [Google Scholar] [CrossRef]
- Flemming, H.-C.; Wingender, J.; Szewzyk, U.; Steinberg, P.; Rice, S.A.; Kjelleberg, S. Biofilms: An emergent form of bacterial life. Nat. Rev. Microbiol. 2016, 14, 563–575. [Google Scholar] [CrossRef] [PubMed]
- Galie, S.; García-Gutiérrez, C.; Miguélez, E.M.; Villar, C.J.; Lombó, F. Biofilms in the food industry: Health aspects and control methods. Front. Microbiol. 2018, 9, 898. [Google Scholar] [CrossRef]
- Zhao, A.; Sun, J.; Liu, Y. Understanding bacterial biofilms: From definition to treatment strategies. Front. Cell. Infect. Microbiol. 2023, 13, 1137947. [Google Scholar] [CrossRef] [PubMed]
- Bottone, E.J. Bacillus cereus, a volatile human pathogen. Clin. Microbiol. Rev. 2010, 23, 382–398. [Google Scholar] [CrossRef]
- Basta, M.; Annamaraju, P. Bacterial Spores; StatPearls Publishing: St. Petersburg, FL, USA, 2020. [Google Scholar]
- Novak, J.S.; Call, J.; Tomasula, P.; Luchansky, J.B. An Assessment of Pasteurization Treatment of Water, Media, and Milk with Respect to Bacillus Spores†. J. Food Prot. 2005, 68, 751–757. [Google Scholar] [CrossRef]
- Kamat, A.S.; Nerkar, D.P.; Nair, P.M. Bacillus cereus in some Indian foods, incidence and antibiotic, heat and radiation resistance. J. Food Saf. 1989, 10, 31–41. [Google Scholar] [CrossRef]
- Spanu, C.; Scarano, C.; Spanu, V.; Pala, C.; Casti, D.; Lamon, S.; Cossu, F.; Ibba, M.; Nieddu, G.; De Santis, E.P.L. Occurrence and behavior of Bacillus cereus in naturally contaminated ricotta salata cheese during refrigerated storage. Food Microbiol. 2016, 58, 135–138. [Google Scholar] [CrossRef]
- Cossart, P. Illuminating the landscape of host–pathogen interactions with the bacterium Listeria monocytogenes. Proc. Natl. Acad. Sci. USA 2011, 108, 19484–19491. [Google Scholar] [CrossRef]
- Bonsaglia, E.; Silva, N.; Júnior, A.F.; Júnior, J.A.; Tsunemi, M.; Rall, V. Production of biofilm by Listeria monocytogenes in different materials and temperatures. Food Control 2014, 35, 386–391. [Google Scholar] [CrossRef]
- European Food Safety Authority; European Centre for Disease Prevention and Control. The European Union One Health 2023 Zoonoses report. EFSA J. 2024, 22, e9106. [Google Scholar] [PubMed]
- Scallan, E.; Hoekstra, R.M.; Angulo, F.J.; Tauxe, R.V.; Widdowson, M.A.; Roy, S.L.; Jones, J.L.; Griffin, P.M. Foodborne illness acquired in the United States–major pathogens. Emerg. Infect. Dis. 2011, 17, 7–15. [Google Scholar] [CrossRef]
- McDowell, R.H.; Sands, E.M.; Friedman, H. Bacillus cereus. In StatPearls [Internet]; StatPearls Publishing: St. Petersburg, FL, USA, 2023. [Google Scholar]
- Ingwer Gemahlen, Marke GÜLCAN, 150 Gramm. Lebensmittelwarnung, 2025. Available online: https://www.lebensmittelwarnung.de/___lebensmittelwarnung.de/Meldungen/2025/05_Mai/20250506_03_NW_Ingwer/2025-05-06_03_NW_Ingwer_Meldung.html (accessed on 30 July 2025).
- Rückruf: Bacillus cereus—HEALTHY POWERFOOD ruft Sprossenprodukte Zurück. Produkt Warnung, 2025. Available online: https://www.produktwarnung.eu/2025/02/28/rueckruf-bacillus-cereus-healthy-powerfood-ruft-sprossenprodukte-zurueck/33675 (accessed on 30 July 2025).
- Whitworth, J. Oatly Recalls Drink Because of Bacillus cereus; 2 Sick with 27 Other Complaints Filed. Food Safety News. 2022. Available online: https://www.foodsafetynews.com/2022/04/oatly-recalls-drink-because-of-bacillus-cereus-2-sick-with-27-other-complaints-filed/ (accessed on 30 July 2025).
- Smithfield Packaged Meats Corp. Dba Margherita Meats Inc. Recalls Pepperoni Products Due to Possible Bacillus Cereus Contamination. Food Safety and Inspection Service U.S. Department of Agriculture. 2021. Available online: https://www.fsis.usda.gov/recalls-alerts/smithfield-packaged-meats-corp.-dba-margherita-meats-inc.-recalls-pepperoni-products (accessed on 30 July 2025).
- How Listeria Spreads. U.S. Centers for Disease Control and Prevention (CDC). 2024. Available online: https://www.cdc.gov/listeria/causes/index.html (accessed on 30 July 2025).
- Listeria. European Food Safety Authority (EFSA). 2025. Available online: https://www.efsa.europa.eu/en/topics/topic/listeria (accessed on 30 July 2025).
- “Enoki Mushroom”, 100 Gramm, Marke “Super Fruta”. Lebensmittelwarnung. 2025. Available online: https://www.lebensmittelwarnung.de/___lebensmittelwarnung.de/Meldungen/2025/07_Juli/250728_29_BY_Enoki_Pilze/250728_29_BY_Enoki_Pilze.html (accessed on 30 July 2025).
- Gourmet Schweizer Raclette Käse, 400 Gramm. Lebensmittelwarnung. 2025. Available online: https://www.lebensmittelwarnung.de/___lebensmittelwarnung.de/Meldungen/2025/05_Mai/250508_07_NW_Raclette/250508_07_NW_Raclette_Meldung.html (accessed on 30 July 2025).
- Kräuterfilets nach Matjesart in Öl, 500 Gramm und 1000 Gramm. Lebensmittelwarnung. 2025. Available online: https://www.lebensmittelwarnung.de/___lebensmittelwarnung.de/Meldungen/2025/07_Juli/250725_26_HB_Kraeutermatjes/250725_HB_Kraeutermatjes_Meldung.html (accessed on 30 July 2025).
- Abbey Specialty Foods Recalls Wicklow Gold Cheddar Nettle & Chive 5.2 oz and Wicklow Gold Cheddar Tomato & Herb 5.2 oz Because of Possible Health Risk. U.S. Food & Drug Administration. 2025. Available online: https://www.fda.gov/safety/recalls-market-withdrawals-safety-alerts/abbey-specialty-foods-recalls-wicklow-gold-cheddar-nettle-chive-52-oz-and-wicklow-gold-cheddar (accessed on 30 July 2025).
- Duda Farm Fresh Foods, Inc. Issues Advisory for 1587 Cases of 4 in/1.6 oz Bundle Marketside Celery Sticks Because of Possible Health Risk. U.S. Food & Drug Administration. 2025. Available online: https://www.fda.gov/safety/recalls-market-withdrawals-safety-alerts/duda-farm-fresh-foods-inc-issues-advisory-1587-cases-4-in16-oz-bundle-marketside-celery-sticks (accessed on 30 July 2025).
- Albertsons Companies Stores in Arkansas, Louisiana, Oklahoma and Texas Voluntarily Recalls Select Items Containing Tuna Salad from Reser’s Fine Foods Due to an Ingredient Recall Linked to Possible Listeria Monocytogenes Contamination. U.S. Food & Drug Administration. 2025. Available online: https://www.fda.gov/safety/recalls-market-withdrawals-safety-alerts/albertsons-companies-stores-arkansas-louisiana-oklahoma-and-texas-voluntarily-recalls-select-items (accessed on 30 July 2025).
- Bast, E. Mikrobiologische Methoden: Eine Einführung in Grundlegende Arbeitstechniken; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- O’Halloran, M.P.; Pravda, M.; Guilbault, G.G. Prussian Blue bulk modified screen-printed electrodes for H2O2 detection and for biosensors. Talanta 2001, 55, 605–611. [Google Scholar] [CrossRef] [PubMed]
- Schnabel, U.; Handorf, O.; Stachowiak, J.; Boehm, D.; Weit, C.; Weihe, T.; Schäfer, J.; Below, H.; Bourke, P.; Ehlbeck, J. Plasma-functionalized water: From bench to prototype for fresh-cut lettuce. Food Eng. Rev. 2021, 13, 115–135. [Google Scholar] [CrossRef]
- Armstrong, R.A. When to use the Bonferroni correction. Ophthalmic Physiol. Opt. 2014, 34, 502–508. [Google Scholar] [CrossRef]
- Thirumdas, R.; Kothakota, A.; Annapure, U.; Siliveru, K.; Blundell, R.; Gatt, R.; Valdramidis, V.P. Plasma activated water (PAW): Chemistry, physico-chemical properties, applications in food and agriculture. Trends Food Sci. Technol. 2018, 77, 21–31. [Google Scholar] [CrossRef]
- Wong, K.S.; Chew, N.S.; Low, M.; Tan, M.K. Plasma-activated water: Physicochemical properties, generation techniques, and applications. Processes 2023, 11, 2213. [Google Scholar] [CrossRef]
- Girard, P.-M.; Arbabian, A.; Fleury, M.; Bauville, G.; Puech, V.; Dutreix, M.; Sousa, J.S. Synergistic effect of H2O2 and NO2 in cell death induced by cold atmospheric He plasma. Sci. Rep. 2016, 6, 29098. [Google Scholar] [CrossRef]
- Lu, P.; Boehm, D.; Cullen, P.; Bourke, P. Controlled cytotoxicity of plasma treated water formulated by open-air hybrid mode discharge. Appl. Phys. Lett. 2017, 110, 264102. [Google Scholar] [CrossRef]
- Senesi, S.; Ghelardi, E. Production, secretion and biological activity of Bacillus cereus enterotoxins. Toxins 2010, 2, 1690–1703. [Google Scholar] [CrossRef] [PubMed]
- Magalhães, R.; Mena, C.; Ferreira, V.; Silva, J.; Almeida, G.; Gibbs, P.; Teixeira, P. Bacteria: Listeria monocytogenes. In History, Science and Methods; Elsevier: Amsterdam, The Netherlands, 2014; pp. 450–461. [Google Scholar]
- Hariram, U.; Labbé, R. Spore prevalence and toxigenicity of Bacillus cereus and Bacillus thuringiensis isolates from US retail spices. J. Food Prot. 2015, 78, 590–596. [Google Scholar] [CrossRef]
- McKenney, P.T.; Driks, A.; Eichenberger, P. The Bacillus subtilis endospore: Assembly and functions of the multilayered coat. Nat. Rev. Microbiol. 2013, 11, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Rutala, W.A.; Weber, D.J. Infection control: The role of disinfection and sterilization. J. Hosp. Infect. 1999, 43, S43–S55. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yang, L.; Kjellerup, B.V.; Xu, Z. Viable but nonculturable (VBNC) state, an underestimated and controversial microbial survival strategy. Trends Microbiol. 2023, 31, 1013–1023. [Google Scholar] [CrossRef]
- Ayrapetyan, M.; Oliver, J.D. The viable but non-culturable state and its relevance in food safety. Curr. Opin. Food Sci. 2016, 8, 127–133. [Google Scholar] [CrossRef]
- Li, L.; Mendis, N.; Trigui, H.; Oliver, J.D.; Faucher, S.P. The importance of the viable but non-culturable state in human bacterial pathogens. Front. Microbiol. 2014, 5, 258. [Google Scholar] [CrossRef]
- Weihe, T.; Yao, Y.; Opitz, N.; Wagner, R.; Krall, J.; Schnabel, U.; Below, H.; Ehlbeck, J. Plasma-Treated Water: A Comparison with Analog Mixtures of Traceable Ingredients. Microorganisms 2023, 11, 932. [Google Scholar] [CrossRef]
- Pagán, E.; Pavli, F.; Happiette, S.; Berdejo, D.; Gatt, R.; Pagán, R.; Valdramidis, V.; García-Gonzalo, D. Isolation and characterization of resistant variants of Salmonella Typhimurium after sequential exposure to plasma activated water (PAW). Innov. Food Sci. Emerg. Technol. 2024, 93, 103633. [Google Scholar] [CrossRef]
| Mean p-Value (Wilcoxon Test, α = 0.05) | Pre-Treatment | |||
|---|---|---|---|---|
| Tap Water | 10 min PTW | 60 min PTW | 90 min PTW | |
| pH | 8.35 | 2.78 6.3 · 10−4 | 1.88 4.1 · 10−4 | 1.84 0.093 |
| Conductivity (µS/cm) | 794.6 | 1829.7 8.2 · 10−5 | 10,317.2 4.1 · 10−5 | 11,335.8 0.077 |
|
ORP
(mV) | 153 | 570 4.0 · 10−5 | 638 4.1 · 10−5 | 650 4.1 · 10−5 |
|
Nitrite
(mg/L) | ND * | 53.0 7.7 · 10−4 | 89.6 4.9 · 10−3 | 112.4 1.0 |
|
Nitrate
(mg/L) | ND | 468.8 3.1 · 10−4 | 2040.6 4.1 · 10−5 | 2428.0 0.019 |
| Hydrogen peroxide (µg/mL) | ND | 196.3 3.1 · 10−4 | 540.3 4.1 · 10−5 | 749.9 7.8 · 10−3 |
| Scenario | Pairing | Resulting p-Value | αadapted | Tested Sample Size |
|---|---|---|---|---|
| 1 min post-treatment | Control—tap water | 0.39394 | 0.0167 | n = 6 |
| 1 min post-treatment | Control—PTW 10 min | 0.04113 | 0.0167 | n = 6 |
| 1 min post-treatment | Control—PTW 60 min | 0.00866 | 0.0167 | n = 6 |
| 5 min post-treatment | Control—tap water | 0.87636 | 0.0125 | n = 21 |
| 5 min post-treatment | Control—PTW 10 min | 0.00204 | 0.0125 | n = 15 |
| 5 min post-treatment | Control—PTW 60 min | 7.60714∙10−7 | 0.0125 | n = 15 |
| 5 min post-treatment | Control—PTW 90 min | 7.39602∙10−7 | 0.0125 | n = 12 |
| 10 min post-treatment | Control—tap water | 0.48124 | 0.0125 | n = 21 |
| 10 min post-treatment | Control—PTW 10 min | 0.00239 | 0.0125 | n = 15 |
| 10 min post-treatment | Control—PTW 60 min | 1.28935∙10−8 | 0.0125 | n = 15 |
| 10 min post-treatment | Control—PTW 90 min | 7.39602∙10−7 | 0.0125 | n = 12 |
| 30 min post-treatment | Control—tap water | 0.70534 | 0.0125 | n = 15 |
| 30 min post-treatment | Control—PTW 10 min | 7.81571∙10−4 | 0.0125 | n = 9 |
| 30 min post-treatment | Control—PTW 60 min | 4.11353∙10−5 | 0.0125 | n = 9 |
| 30 min post-treatment | Control—PTW 90 min | 7.39602∙10−7 | 0.0125 | n = 12 |
| Scenario | Tested Agent | Resulting p-Value | αadapted | Tested Sample Size |
|---|---|---|---|---|
| 1 min post-treatment | Tap water | 0.03344 | 0.0167 | n = 6 |
| 1 min post-treatment | PTW 10 min | 0.00492 | 0.0167 | n = 6 |
| 1 min post-treatment | PTW 60 min | 0.00500 | 0.0167 | n = 6 |
| 5 min post-treatment | Tap water | 0.33069 | 0.0125 | n = 21 |
| 5 min post-treatment | PTW 10 min | 0.00052 | 0.0125 | n = 15 |
| 5 min post-treatment | PTW 60 min | 0.54437 | 0.0125 | n = 12 |
| 5 min post-treatment | PTW 90 min | 0.05170 | 0.0125 | n = 9 |
| 10 min post-treatment | Tap water | 0.48375 | 0.0125 | n = 21 |
| 10 min post-treatment | PTW 10 min | 0.00183 | 0.0125 | n = 15 |
| 10 min post-treatment | PTW 60 min | 0.01655 | 0.0125 | n = 12 |
| 10 min post-treatment | PTW 90 min | 0.00040 | 0.0125 | n = 9 |
| 30 min post-treatment | Tap water | 0.21016 | 0.0125 | n = 15 |
| 30 min post-treatment | PTW 10 min | 0.00092 | 0.0125 | n = 9 |
| 30 min post-treatment | PTW 60 min | 0.10199 | 0.0125 | n = 9 |
| 30 min post-treatment | PTW 90 min | 0.00037 | 0.0125 | n = 12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nestel, S.; Wagner, R.; Meister, M.; Weihe, T.; Schnabel, U. Plasma-Treated Water Effect on Sporulating Bacillus cereus vs. Non-Sporulating Listeria monocytogenes Biofilm Cell Vitality. Appl. Microbiol. 2025, 5, 80. https://doi.org/10.3390/applmicrobiol5030080
Nestel S, Wagner R, Meister M, Weihe T, Schnabel U. Plasma-Treated Water Effect on Sporulating Bacillus cereus vs. Non-Sporulating Listeria monocytogenes Biofilm Cell Vitality. Applied Microbiology. 2025; 5(3):80. https://doi.org/10.3390/applmicrobiol5030080
Chicago/Turabian StyleNestel, Samantha, Robert Wagner, Mareike Meister, Thomas Weihe, and Uta Schnabel. 2025. "Plasma-Treated Water Effect on Sporulating Bacillus cereus vs. Non-Sporulating Listeria monocytogenes Biofilm Cell Vitality" Applied Microbiology 5, no. 3: 80. https://doi.org/10.3390/applmicrobiol5030080
APA StyleNestel, S., Wagner, R., Meister, M., Weihe, T., & Schnabel, U. (2025). Plasma-Treated Water Effect on Sporulating Bacillus cereus vs. Non-Sporulating Listeria monocytogenes Biofilm Cell Vitality. Applied Microbiology, 5(3), 80. https://doi.org/10.3390/applmicrobiol5030080







