Influence of Isolation Source on the Probiotic Properties and Health Benefits of Yeasts: Insights from Metabarcoding and Cultivation Approaches
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and Preparation
2.2. Metabarcoding Analysis
2.3. Isolation and Characterization of Yeasts
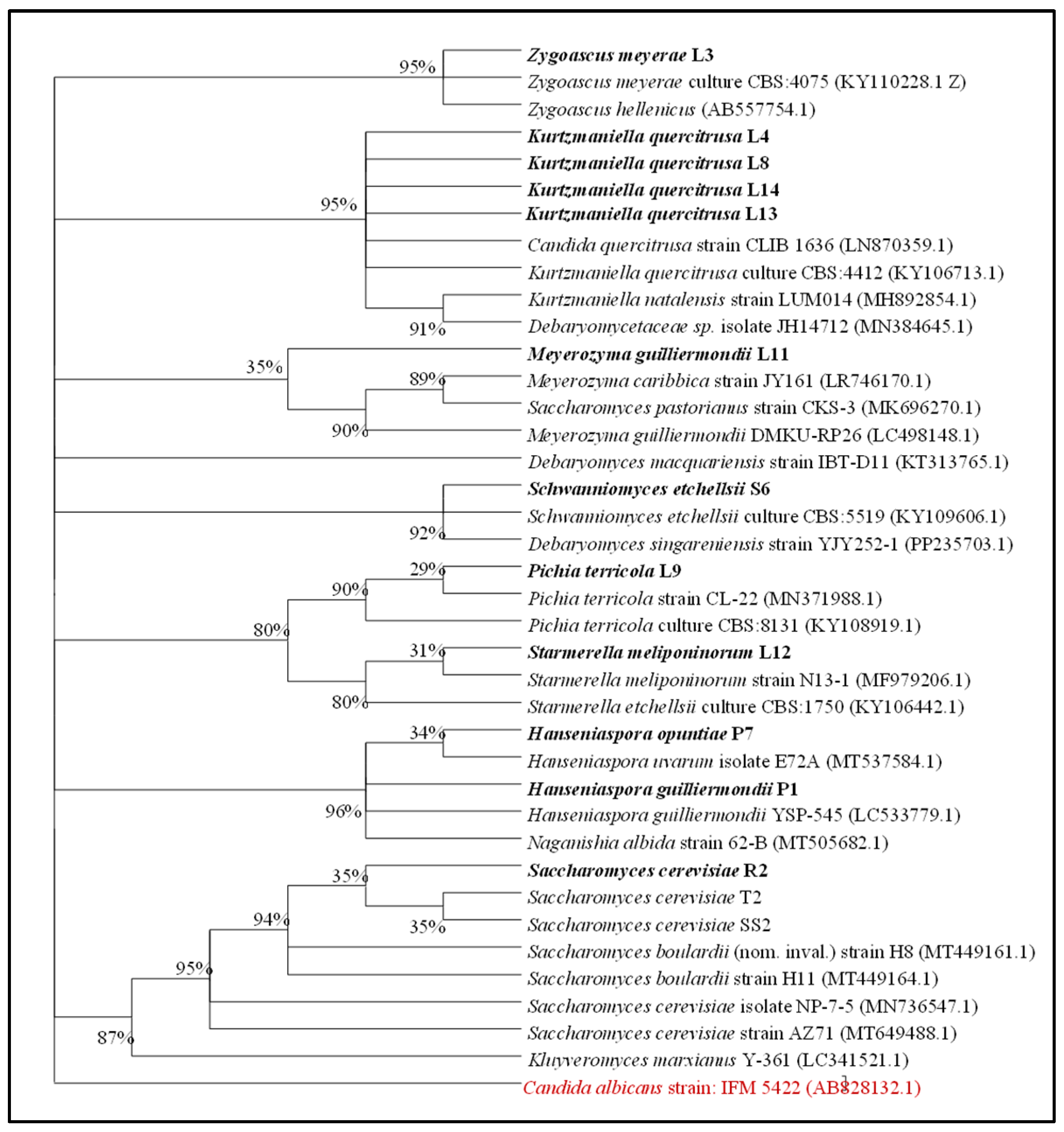
2.4. Identification and Phylogenetic Analysis of the Potential Probiotic Yeasts
2.5. Evaluation of Probiotic Properties of the Yeast Isolates
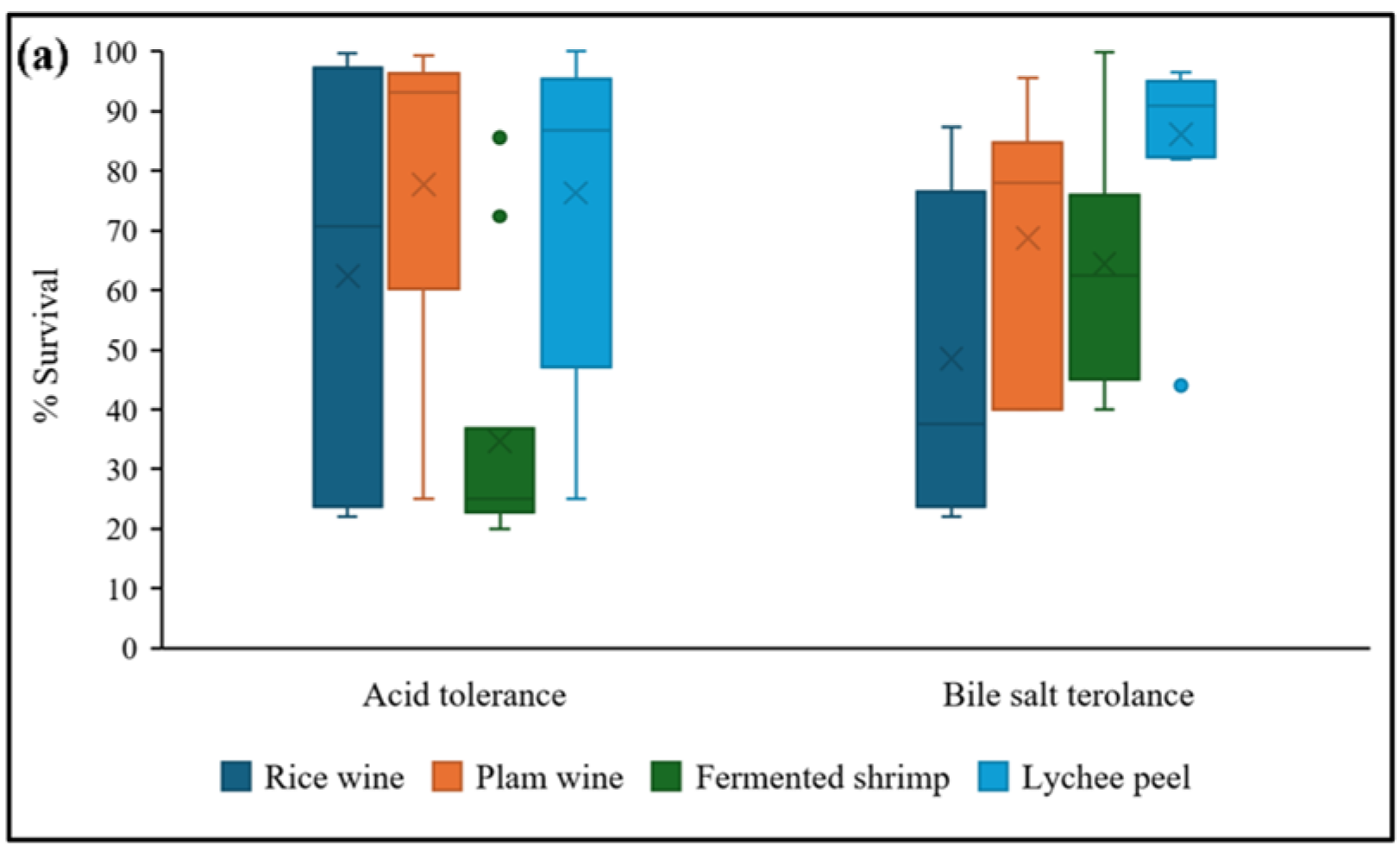
2.5.1. Acid and Bile Salt Tolerance
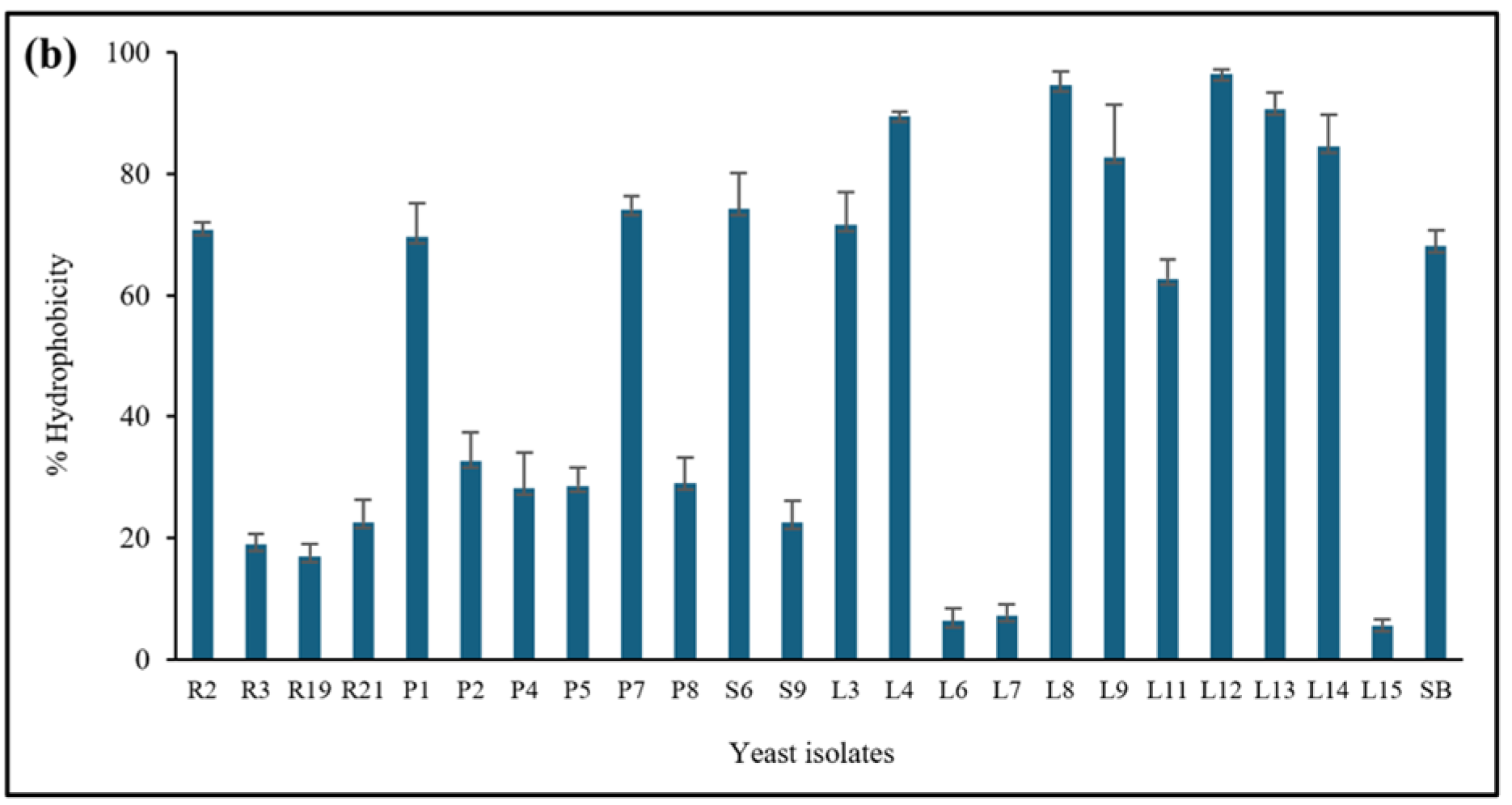
2.5.2. Cell Surface Hydrophobicity
2.6. Evaluation of Health Benefit Properties of the Potential Probiotic Yeasts
2.6.1. Test of Antioxidant Activity
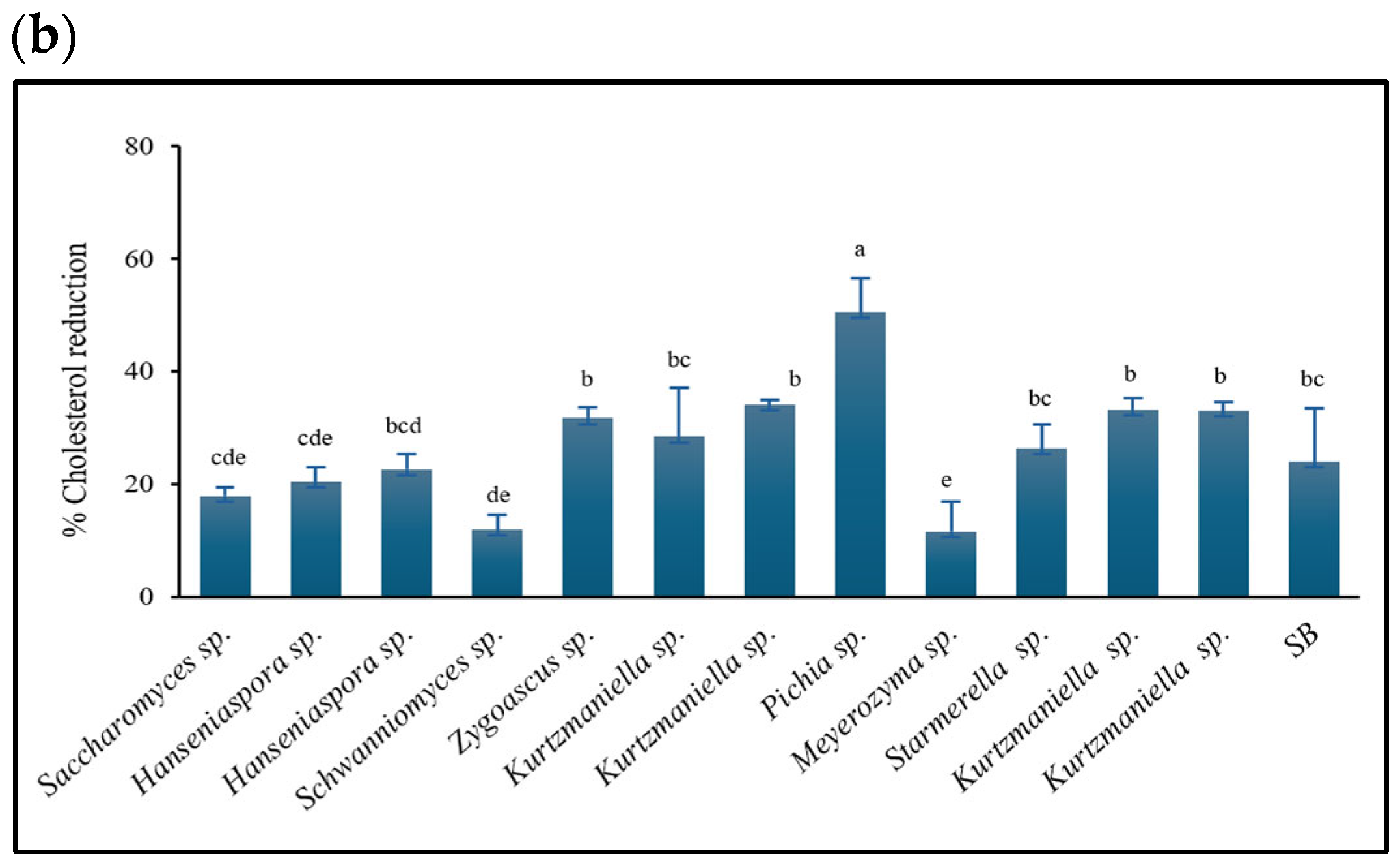
2.6.2. Test of Cholesterol Reduction
2.7. Statistical Analysis
3. Results
3.1. Yeast Composition in Each Source of Isolate Through Metabarcoding and Cultural Analyses
3.2. Identification and Phylogenetic Analysis
3.3. Probiotic Properties
3.3.1. Acid and Bile Salt Tolerance
3.3.2. Cell Surface Hydrophobicity
3.4. Potential Health Benefit Properties
3.4.1. Antioxidant Activity
3.4.2. Cholesterol-Lowering Capacity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO; FAO. Guidelines for the Evaluation of Probiotics in Food; Food and Agriculture Organization of the United Nations and World Health Organization Working Group: Geneva, Switzerland, 2002. [Google Scholar]
- de Melo Pereira, G.V.; de Oliveira Coelho, B.; Júnior, A.I.M.; Thomaz-Soccol, V.; Soccol, C.R. How to select a probiotic? A review and update of methods and criteria. Biotechnol. Adv. 2018, 36, 2060–2076. [Google Scholar] [CrossRef] [PubMed]
- Rima, H.; Steve, L.; Ismail, F. Antimicrobial and Probiotic Properties of Yeasts: From Fundamental to Novel Applications. Front. Microbiol. 2012, 3, 421. [Google Scholar] [CrossRef] [PubMed]
- McFarland, L.V. Systematic review and meta-analysis of Saccharomyces boulardii in adult patients. World J. Gastroenterol. WJG 2010, 16, 2202. [Google Scholar] [CrossRef] [PubMed]
- Gürkan Özlü, B.; Terzi, Y.; Uyar, E.; Shatila, F.; Yalçın, H.T. Characterization and determination of the potential probiotic yeasts isolated from dairy products. Biologia 2022, 77, 1471–1480. [Google Scholar] [CrossRef]
- Zhao, M.; Huang, X.; Zhang, H.; Zhang, Y.; Gänzle, M.; Yang, N.; Nishinari, K.; Fang, Y. Probiotic encapsulation in water-in-water emulsion via heteroprotein complex coacervation of type-A gelatin/sodium caseinate. Food Hydrocoll. 2020, 105, 105790. [Google Scholar] [CrossRef]
- Xiang, H.; Sun-Waterhouse, D.; Waterhouse, G.I.; Cui, C.; Ruan, Z. Fermentation-enabled wellness foods: A fresh perspective. Food Sci. Hum. Wellness 2019, 8, 203–243. [Google Scholar] [CrossRef]
- Heo, S.; Kim, T.; Na, H.-E.; Lee, G.; Park, J.-H.; Park, H.-J.; Jeong, D.-W. Safety assessment systems for microbial starters derived from fermented foods. J. Microbiol. Biotechnol. 2022, 32, 1219. [Google Scholar] [CrossRef]
- Huang, H.; Wang, L.; Xiang, X.; Bi, F.; Zhang, Z. Morphological, chemical, and biosynthetic changes in pericarp waxes in response to the browning of litchi fruit during storage. Postharvest Biol. Technol. 2022, 191, 111968. [Google Scholar] [CrossRef]
- Ribeiro, R.A.; Bourbon-Melo, N.; Sá-Correia, I. The cell wall and the response and tolerance to stresses of biotechnological relevance in yeasts. Front. Microbiol. 2022, 13, 953479. [Google Scholar] [CrossRef]
- Savitha, T.; Sankaranarayanan, A. Role of probiotic microbes exerting nutritional properties. In Advances in Probiotics; Academic Press: Cambridge, MA, USA, 2021; pp. 163–184. [Google Scholar] [CrossRef]
- Kechagia, M.; Basoulis, D.; Konstantopoulou, S.; Dimitriadi, D.; Gyftopoulou, K.; Skarmoutsou, N.; Fakiri, E.M. Health benefits of probiotics: A review. Int. Sch. Res. Not. 2013, 2013, 481651. [Google Scholar] [CrossRef]
- Amaretti, A.; Di Nunzio, M.; Pompei, A.; Raimondi, S.; Rossi, M.; Bordoni, A. Antioxidant properties of potentially probiotic bacteria: In vitro and in vivo activities. Appl. Microbiol. Biotechnol. 2013, 97, 809–817. [Google Scholar] [CrossRef]
- Tofalo, R.; Fusco, V.; Böhnlein, C.; Kabisch, J.; Logrieco, A.F.; Habermann, D.; Cho, G.-S.; Benomar, N.; Abriouel, H.; Schmidt-Heydt, M. The life and times of yeasts in traditional food fermentations. Crit. Rev. Food Sci. Nutr. 2020, 60, 3103–3132. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, Y.; Kim, Y.J.; Park, Y. Conjugated linoleic acid: Potential health benefits as a functional food ingredient. Annu. Rev. Food Sci. Technol. 2016, 7, 221–244. [Google Scholar] [CrossRef]
- Palla, M.; Conte, G.; Grassi, A.; Esin, S.; Serra, A.; Mele, M.; Giovannetti, M.; Agnolucci, M. Novel yeasts producing high levels of conjugated linoleic acid and organic acids in fermented doughs. Foods 2021, 10, 2087. [Google Scholar] [CrossRef] [PubMed]
- Kunyeit, L.; KA, A.-A.; Rao, R.P. Application of probiotic yeasts on candida species associated infection. J. Fungi 2020, 6, 189. [Google Scholar] [CrossRef] [PubMed]
- Tedersoo, L.; Bahram, M.; Zinger, L.; Nilsson, R.H.; Kennedy, P.G.; Yang, T.; Anslan, S.; Mikryukov, V. Best practices in metabarcoding of fungi: From experimental design to results. Mol. Ecol. 2022, 31, 2769–2795. [Google Scholar] [CrossRef] [PubMed]
- Prakitchaiwattana, C.J.; Fleet, G.H.; Heard, G.M. Application and evaluation of denaturing gradient gel electrophoresis to analyse the yeast ecology of wine grapes. FEMS Yeast Res. 2004, 4, 865–877. [Google Scholar] [CrossRef]
- Tsuji, A.; Kozawa, M.; Tokuda, K.; Enomoto, T.; Koyanagi, T. Robust domination of Lactobacillus sakei in microbiota during traditional Japanese sake starter yamahai-moto fermentation and the accompanying changes in metabolites. Curr. Microbiol. 2018, 75, 1498–1505. [Google Scholar] [CrossRef]
- Qian, M.; Ruan, F.; Zhao, W.; Dong, H.; Bai, W.; Li, X.; Huang, X.; Li, Y. The dynamics of physicochemical properties, microbial community, and flavor metabolites during the fermentation of semi-dry Hakka rice wine and traditional sweet rice wine. Food Chem. 2023, 416, 135844. [Google Scholar] [CrossRef]
- Harrigan, W. Laboratory Methods in Food and Dairy Microbiology; Academic Press: Cambridge, MA, USA, 1976; Volume 18, pp. 226–227. [Google Scholar] [CrossRef]
- Silva, G.A.D.; Bernardi, T.L.; Schaker, P.D.C.; Menegotto, M.; Valente, P. Rapid yeast DNA extraction by boiling and freeze-thawing without using chemical reagents and DNA purification. Braz. Arch. Biol. Technol. 2012, 55, 319–327. [Google Scholar] [CrossRef]
- Nutaratat, P.; Boontham, W.; Khunnamwong, P. A Novel Yeast Genus and Two Novel Species Isolated from Pineapple Leaves in Thailand: Savitreella phatthalungensis gen. nov., sp. nov. and Goffeauzyma siamensis sp. nov. J. Fungi 2022, 8, 118. [Google Scholar] [CrossRef] [PubMed]
- Khullar, G.; Det-udom, R.; Prombutar, P.; Prakitchaiwattana, C. Probiogenomic analysis and safety assessment of Bacillus isolates using Omics approach in combination with In-vitro. LWT 2022, 159, 113216. [Google Scholar] [CrossRef]
- Amorim, J.C.; Piccoli, R.H.; Duarte, W.F. Probiotic potential of yeasts isolated from pineapple and their use in the elaboration of potentially functional fermented beverages. Food Res. Int. 2018, 107, 518–527. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Xing, Z.; Li, C.; Wang, J.; Wang, Y. Molecular mechanisms and in vitro antioxidant effects of Lactobacillus plantarum MA2. Food Chem. 2017, 221, 1642–1649. [Google Scholar] [CrossRef]
- Ngongang, E.F.T.; Tiencheu, B.; Fossi, B.T.; Nchanji, G.T.; Shiynyuy, D.M.; Mbiapo, F.T.; François, Z.N. Isolation and identification of cholesterol lowering probiotic yeast from palm raffia (Raffia mambillensis) wine. J. Adv. Biol. Biotechnol. (JABB) 2017, 11, 1–17. [Google Scholar] [CrossRef]
- Agarbati, A.; Canonico, L.; Marini, E.; Zannini, E.; Ciani, M.; Comitini, F. Potential probiotic yeasts sourced from natural environmental and spontaneous processed foods. Foods 2020, 9, 287. [Google Scholar] [CrossRef]
- Chanprasartsuk, O.; Prakitchaiwattana, C.; Sanguandeekul, R. Comparison of Methods for Identification of Yeasts Isolated during Spontaneous Fermentation of Freshly Crushed Pineapple Juices. J. Agric. Sci. Technol. 2013, 15, 1779–1790. [Google Scholar]
- Rosenberg, M.; Gutnick, D.; Rosenberg, E. Adherence of bacteria to hydrocarbons: A simple method for measuring cell-surface hydrophobicity. FEMS Microbiol. Lett. 1980, 9, 29–33. [Google Scholar] [CrossRef]
- Punyauppa-Path, S.; Kiatprasert, P.; Sawaengkaew, J.; Mahakhan, P.; Phumkhachorn, P.; Rattanachaikunsopon, P.; Khunnamwong, P.; Srisuk, N. Diversity of fermentative yeasts with probiotic potential isolated from Thai fermented food products. AIMS Microbiol. 2022, 8, 575. [Google Scholar] [CrossRef]
- Tamang, J.P.; Lama, S. Probiotic properties of yeasts in traditional fermented foods and beverages. J. Appl. Microbiol. 2022, 132, 3533–3542. [Google Scholar] [CrossRef]
- Prakitchaiwattana, C.; Fleet, G.H.; Heard, G.M. Nutrients for yeast growth on grape berry exudates. Food Agric. Sci. Technol. 2020, 6, 1–11. [Google Scholar]
- Al Daccache, M.; Salameh, D.; Chamy, L.E.; Koubaa, M.; Maroun, R.G.; Vorobiev, E.; Louka, N. Evaluation of the fermentative capacity of an indigenous Hanseniaspora sp. strain isolated from Lebanese apples for cider production. FEMS Microbiol. Lett. 2020, 367, fnaa093. [Google Scholar] [CrossRef]
- Bora, S.S.; Keot, J.; Das, S.; Sarma, K.; Barooah, M. Metagenomics analysis of microbial communities associated with a traditional rice wine starter culture (Xaj-pitha) of Assam, India. 3 Biotech 2016, 6, 153. [Google Scholar] [CrossRef]
- Anupma, A.; Tamang, J.P. Diversity of filamentous fungi isolated from some amylase and alcohol-producing starters of India. Front. Microbiol. 2020, 11, 905. [Google Scholar] [CrossRef]
- Mu, Z.; Yang, Y.; Xia, Y.; Zhang, H.; Ni, B.; Ni, L.; Wang, G.; Xiong, Z.; Song, X.; Ai, L. Enhancement of the aromatic alcohols and health properties of Chinese rice wine by using a potentially probiotic Saccharomyces cerevisiae BR14. LWT 2023, 181, 114748. [Google Scholar] [CrossRef]
- Huang, Z.-R.; Hong, J.-L.; Xu, J.-X.; Li, L.; Guo, W.-L.; Pan, Y.-Y.; Chen, S.-J.; Bai, W.-D.; Rao, P.-F.; Ni, L. Exploring core functional microbiota responsible for the production of volatile flavour during the traditional brewing of Wuyi Hong Qu glutinous rice wine. Food Microbiol. 2018, 76, 487–496. [Google Scholar] [CrossRef]
- Das, S.; Tamang, J.P. Changes in microbial communities and their predictive functionalities during fermentation of toddy, an alcoholic beverage of India. Microbiol. Res. 2021, 248, 126769. [Google Scholar] [CrossRef] [PubMed]
- Canonico, L.; Galli, E.; Agarbati, A.; Comitini, F.; Ciani, M. Starmerella bombicola and Saccharomyces cerevisiae in wine sequential fermentation in aeration condition: Evaluation of ethanol reduction and analytical profile. Foods 2021, 10, 1047. [Google Scholar] [CrossRef] [PubMed]
- Martin, V.; Valera, M.J.; Medina, K.; Dellacassa, E.; Schneider, R.; Boido, E.; Carrau, F. Application of Hanseniaspora vineae to improve white wine quality. In White Wine Technology; Academic Press: Cambridge, MA, USA, 2022; pp. 99–115. [Google Scholar] [CrossRef]
- Paludan-Müller, C.; Madsen, M.; Sophanodora, P.; Gram, L.; Møller, P.L. Fermentation and microflora of plaa-som, a Thai fermented fish product prepared with different salt concentrations. Int. J. Food Microbiol. 2002, 73, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Pang, H.; Xin, X.; He, J.; Cui, B.; Guo, D.; Liu, S.; Yan, Z.; Liu, C.; Wang, X.; Nan, J. Effect of NaCl Concentration on Microbiological Properties in NaCl Assistant Anaerobic Fermentation: Hydrolase Activity and Microbial Community Distribution. Front. Microbiol. 2020, 11, 589222. [Google Scholar] [CrossRef]
- Ribeiro, R.A.; Godinho, C.P.; Vitorino, M.V.; Robalo, T.T.; Fernandes, F.; Rodrigues, M.S.; Sá-Correia, I. Crosstalk Between Yeast Cell Plasma Membrane Ergosterol Content and Cell Wall Stiffness Under Acetic Acid Stress Involving Pdr18. J. Fungi 2022, 8, 103. [Google Scholar] [CrossRef]
- Skinner, F.A.; Passmore, S.M.; Davenport, R. Biology and Activities of Yeasts; Academic Press London: London, UK, 1980; Volume 9. [Google Scholar]
- Fernández-Pacheco, P.; Rosa, I.Z.; Arévalo-Villena, M.; Gomes, E.; Pérez, A.B. Study of potential probiotic and biotechnological properties of non-Saccharomyces yeasts from fruit Brazilian ecosystems. Braz. J. Microbiol. 2021, 52, 2129–2144. [Google Scholar] [CrossRef]
- Stewart, G.G. The Structure and Function of the Yeast Cell Wall, Plasma Membrane and Periplasm. In Brewing and Distilling Yeasts; Stewart, G.G., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 55–75. [Google Scholar] [CrossRef]
- Pillet, F.; Lemonier, S.; Schiavone, M.; Formosa, C.; Martin-Yken, H.; Francois, J.M.; Dague, E. Uncovering by atomic force microscopy of an original circular structure at the yeast cell surface in response to heat shock. BMC Biol. 2014, 12, 6. [Google Scholar] [CrossRef] [PubMed]
- Pandey, P.; Zaman, K.; Prokai, L.; Shulaev, V. Comparative proteomics analysis reveals unique early signaling response of Saccharomyces cerevisiae to oxidants with different mechanism of action. Int. J. Mol. Sci. 2020, 22, 167. [Google Scholar] [CrossRef] [PubMed]
- Sousa-Lopes, A.; Antunes, F.; Cyrne, L.; Marinho, H. Decreased cellular permeability to H2O2 protects Saccharomyces cerevisiae cells in stationary phase against oxidative stress. FEBS Lett. 2004, 578, 152–156. [Google Scholar] [CrossRef] [PubMed]
- Yeats, T.H.; Rose, J.K. The formation and function of plant cuticles. Plant Physiol. 2013, 163, 5–20. [Google Scholar] [CrossRef]
- de Souza, B.M.S.; Borgonovi, T.F.; Casarotti, S.N.; Todorov, S.D.; Penna, A.L.B. Lactobacillus casei and Lactobacillus fermentum strains isolated from mozzarella cheese: Probiotic potential, safety, acidifying kinetic parameters and viability under gastrointestinal tract conditions. Probiotics Antimicrob. Proteins 2019, 11, 382–396. [Google Scholar] [CrossRef]
- Lara-Hidalgo, C.; Dorantes-Álvarez, L.; Hernández-Sánchez, H.; Santoyo-Tepole, F.; Martínez-Torres, A.; Villa-Tanaca, L.; Hernández-Rodríguez, C. Isolation of yeasts from guajillo pepper (Capsicum annuum L.) fermentation and study of some probiotic characteristics. Probiotics Antimicrob. Proteins 2019, 11, 748–764. [Google Scholar] [CrossRef]
- Lugea, A.; Salas, A.; Casalot, J.; Guarner, F.; Malagelada, J. Surface hydrophobicity of the rat colonic mucosa is a defensive barrier against macromolecules and toxins. Gut 2000, 46, 515–521. [Google Scholar] [CrossRef]
- Jaehrig, S.C.; Rohn, S.; Kroh, L.W.; Fleischer, L.-G.; Kurz, T. In vitro potential antioxidant activity of (1→3), (1→6)-β-d-glucan and protein fractions from Saccharomyces cerevisiae cell walls. J. Agric. Food Chem. 2007, 55, 4710–4716. [Google Scholar] [CrossRef]
- Doolam, B.; Mishra, B.; Surabhi, D.; Mandal, S.K.; Sada, S.; Reddy, N.R.; Panda, J.; Rustagi, S.; Mishra, A.K.; Mohanta, Y.K. A systematic review of potential bioactive compounds from Saccharomyces cerevisiae: Exploring their applications in health promotion and food development. Environ. Dev. Sustain. 2024, 27, 2945–2982. [Google Scholar] [CrossRef]
- Hathout, A.S.; Ghareeb, M.A.; Abdel-Nasser, A.; Abu-Sree, Y. Saccharomyces cerevisiae bioactive metabolites: Characterization and biological activities. ChemistrySelect 2024, 9, e202304878. [Google Scholar] [CrossRef]
- Siesto, G.; Pietrafesa, R.; Infantino, V.; Thanh, C.; Pappalardo, I.; Romano, P.; Capece, A. In vitro study of probiotic, antioxidant and anti-inflammatory activities among indigenous Saccharomyces cerevisiae strains. Foods 2022, 11, 1342. [Google Scholar] [CrossRef] [PubMed]
- Tomaro-Duchesneau, C.; Jones, M.L.; Shah, D.; Jain, P.; Saha, S.; Prakash, S. Cholesterol assimilation by Lactobacillus probiotic bacteria: An in vitro investigation. BioMed Res. Int. 2014, 2014, 380316. [Google Scholar] [CrossRef]
- Widodo, W.; Fanani, T.H.; Fahreza, M.I.; Sukarno, A.S. Cholesterol assimilation of two probiotic strains of Lactobacillus casei used as dairy starter cultures. Appl. Food Biotechnol. 2021, 8, 103–112. [Google Scholar] [CrossRef]
- Thakkar, P.N.; Modi, H.A.; Prajapati, J. Therapeutic Impacts of Probiotics—As Magic Bullet. Am. J. Biomed. Sci. 2016, 8, 97–113. [Google Scholar] [CrossRef]
- Rezaei, M.; Sanagoo, A.; Jouybari, L.; Behnampoo, N.; Kavosi, A. The effect of probiotic yogurt on blood glucose and cardiovascular biomarkers in patients with type II diabetes: A randomized controlled trial. Evid. Based Care 2017, 6, 26–35. [Google Scholar]
- Shrivastava, A.; Pal, M.; Sharma, R.K. Pichia as yeast cell factory for production of industrially important bio-products: Current trends, challenges, and future prospects. J. Bioresour. Bioprod. 2023, 8, 108–124. [Google Scholar] [CrossRef]
- Gao, J.; He, X.; Huang, W.; You, Y.; Zhan, J. Enhancing Ethanol Tolerance via the Mutational Breeding of Pichia terricola H5 to Improve the Flavor Profiles of Wine. Fermentation 2022, 8, 149. [Google Scholar] [CrossRef]
- Ivashov, V.A.; Zellnig, G.; Grillitsch, K.; Daum, G. Identification of triacylglycerol and steryl ester synthases of the methylotrophic yeast Pichia pastoris. Biochim. Biophys. Acta (BBA)—Mol. Cell Biol. Lipids 2013, 1831, 1158–1166. [Google Scholar] [CrossRef]
- Fleet, G.H. 10-Yeasts in fruit and fruit products. In Yeasts in Food; Boekhout, T., Robert, V., Eds.; Woodhead Publishing: Cambridge, UK, 2003; pp. 267–287. [Google Scholar]
- García, J.L.; Uhía, I.; Galán, B. Catabolism and biotechnological applications of cholesterol degrading bacteria. Microb. Biotechnol. 2012, 5, 679–699. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kanyakam, K.; Prakitchaiwattana, C. Influence of Isolation Source on the Probiotic Properties and Health Benefits of Yeasts: Insights from Metabarcoding and Cultivation Approaches. Appl. Microbiol. 2025, 5, 76. https://doi.org/10.3390/applmicrobiol5030076
Kanyakam K, Prakitchaiwattana C. Influence of Isolation Source on the Probiotic Properties and Health Benefits of Yeasts: Insights from Metabarcoding and Cultivation Approaches. Applied Microbiology. 2025; 5(3):76. https://doi.org/10.3390/applmicrobiol5030076
Chicago/Turabian StyleKanyakam, Kanyarat, and Cheunjit Prakitchaiwattana. 2025. "Influence of Isolation Source on the Probiotic Properties and Health Benefits of Yeasts: Insights from Metabarcoding and Cultivation Approaches" Applied Microbiology 5, no. 3: 76. https://doi.org/10.3390/applmicrobiol5030076
APA StyleKanyakam, K., & Prakitchaiwattana, C. (2025). Influence of Isolation Source on the Probiotic Properties and Health Benefits of Yeasts: Insights from Metabarcoding and Cultivation Approaches. Applied Microbiology, 5(3), 76. https://doi.org/10.3390/applmicrobiol5030076





