Development of Heat-Dry RT-LAMP Bioassay for Rapid Latent Detection of Botrytis cinerea
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical and Material
2.2. RT-LAMP Primer Design
2.3. Preparation of Conidia and Inoculation of Tomato Fruit with Fungal Pathogens
2.4. RNA Extraction
2.5. RT-LAMP Reaction and the Optimization of Heat Sources
2.6. Optimization of D- (+) Trehalose Concentrations in Heat-Dry RT-LAMP Assay
2.7. Sensitivity of Thermal-Dry Assay
2.8. Specificity of Thermal-Dry Assay
2.9. Validation with an Infected Biological Sample
2.10. Statistical Analysis
3. Results and Discussion
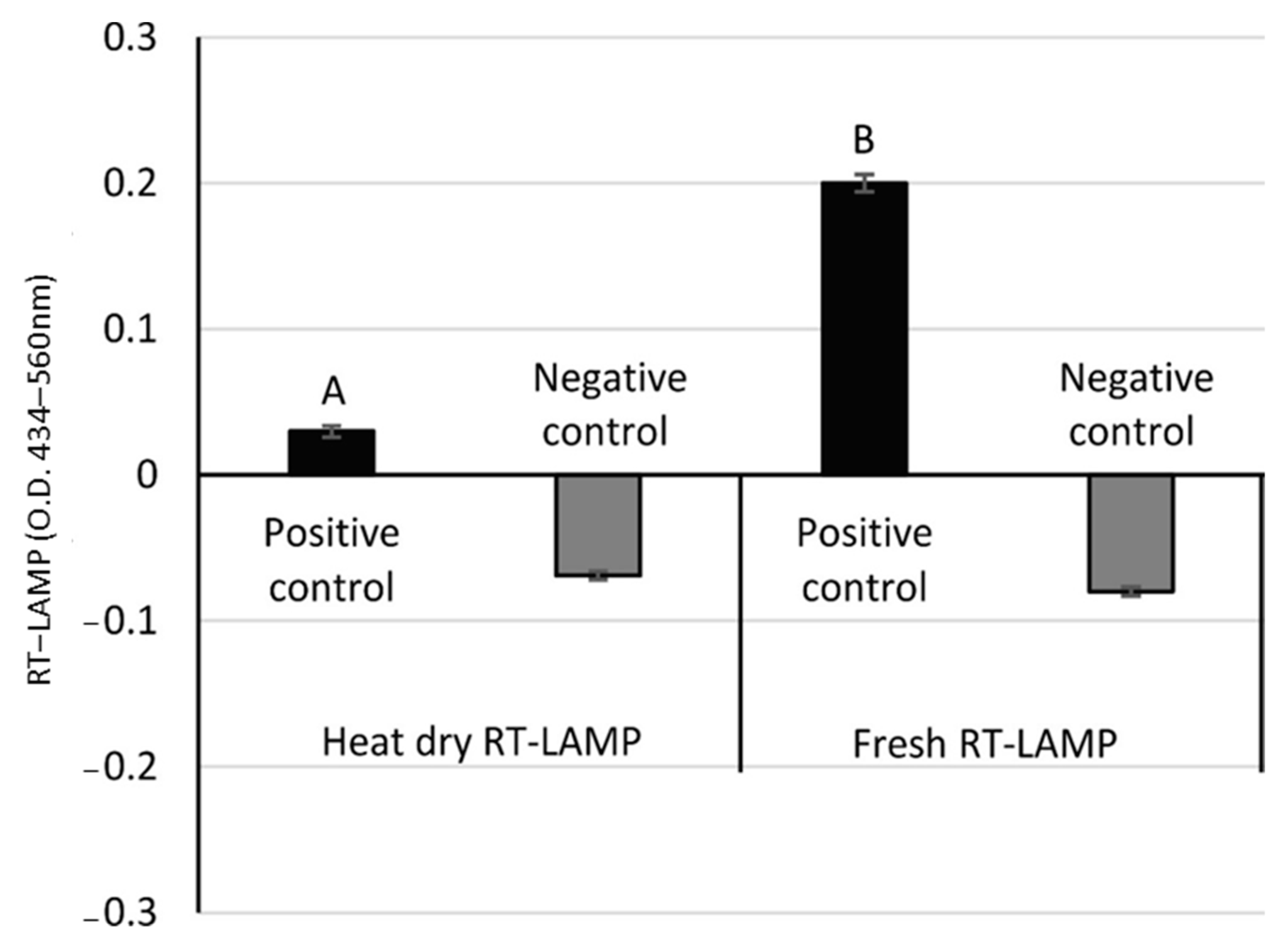
3.1. Optimization of Heat Sources
3.2. Stabilizing Dry RT-LAMP Temperature
3.3. Optimizing Trehalose Concentration and Incubation Time in Heat-Dried LAMP Assays for Enhanced Latent Botrytis cinerea Detection
3.4. Sensitivity Analysis Using Synthesized and Biological Targets
3.5. Specificity
3.6. Validation Using Infected Biological Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Alkan, N.; Fortes, A.M. Insights into molecular and metabolic events associated with fruit response to post-harvest fungal pathogens. Front. Plant Sci. 2015, 6, 889. [Google Scholar] [CrossRef] [PubMed]
- Wenneker, M.; Thomma, B.P.H.J. Latent postharvest pathogens of pome fruit and their management: From single measures to a systems intervention approach. Eur. J. Plant Pathol. 2020, 156, 663–681. [Google Scholar] [CrossRef]
- Williamson, B.; Tudzynski, B.; Tudzynski, P.; Van Kan, J.A.L. Botrytis cinerea: The cause of grey mould disease. Mol. Plant Pathol. 2007, 8, 561–580. [Google Scholar] [CrossRef] [PubMed]
- Dugan, F.M.; Lupien, S.L.; Grove, G.G. Incidence, aggressiveness and In Planta interactions of Botrytis cinerea and other filamentous fungi quiescent in grape berries and dormant buds in central Washington State. J. Phytopathol. 2002, 150, 375–381. [Google Scholar] [CrossRef]
- Edwards, S.; Seddon, B. Selective media for the specific isolation and enumeration of Botrytis cinerea conidia. Lett. Appl. Microbiol. 2001, 32, 63–66. [Google Scholar] [CrossRef]
- Wahab, H.A.; Younis, R.A.A. Early detection of gray mold in grape using conventional and molecular methods. Afr. J. Biotechnol. 2012, 11, 15251–15257. [Google Scholar] [CrossRef]
- Calvo-Garrido, C.; Usall, J.; Viñas, I.; Elmer, P.A.; Cases, E.; Teixidó, N. Potential secondary inoculum sources of Botrytis cinerea and their influence on bunch rot development in dry Mediterranean climate vineyards. Pest. Manag. Sci. 2014, 70, 922–930. [Google Scholar] [CrossRef] [PubMed]
- Jaspers, M.V.; Seyb, A.M.; Trought, M.C.T.; Balasubramaniam, R. Overwintering grapevine debris as an important source of Botrytis cinerea inoculum. Plant Pathol. 2013, 62, 130–138. [Google Scholar] [CrossRef]
- Celik, M.; Kalpulov, T.; Zutahy, Y.; Ish-Shalom, S.; Lurie, S.; Lichter, A. Quantitative and qualitative analysis of Botrytis inoculated on table grapes by qPCR and antibodies. Postharvest Biol. Technol. 2009, 52, 235–239. [Google Scholar] [CrossRef]
- Tomlinson, J.A.; Boonham, N.; Hughes, K.J.D.; Griffin, R.L.; Barker, I. On-site DNA extraction and real-time PCR for detection of Phytophthora ramorum in the field. Appl. Environ. Microbiol. 2005, 71, 6702–6710. [Google Scholar] [CrossRef]
- Lin, Y.-H.; Lin, Y.-J.; Chang, T.-D.; Hong, L.-L.; Chen, T.-Y.; Chang, P.-F.L. Development of a taqman probe-based insulated isothermal polymerase chain reaction (iiPCR) assay for detection of Fusarium oxysporum f. sp. cubense race 4. PLoS ONE 2016, 11, e0159681. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Zhou, J.; Zheng, Y.; Gamson, A.S.; Roembke, B.T.; Nakayama, S.; Sintim, H.O. Isothermal amplified detection of DNA and RNA. Mol. Biosyst. 2014, 10, 970–1003. [Google Scholar] [CrossRef] [PubMed]
- Notomi, T.; Okayama, H.; Masubuchi, H.; Yonekawa, T.; Watanabe, K.; Amino, N.; Hase, T. Loop-mediated isothermal amplification of DNA. Nucleic Acids Res. 2000, 28, e63. [Google Scholar] [CrossRef]
- Saiki, R.K.; Scharf, S.; Faloona, F.; Mullis, K.B.; Horn, G.T.; Erlich, H.A.; Arnheim, N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. 1985. Biotechnology 1992, 24, 476–480. [Google Scholar] [PubMed]
- Mohammadniaei, M.; Zhang, M.; Ashley, J.; Christensen, U.B.; Friis-Hansen, L.J.; Gregersen, R.; Lisby, J.G.; Benfield, T.L.; Nielsen, F.E.; Rasmussen, J.H.; et al. A non-enzymatic, isothermal strand displacement and amplification assay for rapid detection of SARS-CoV-2 RNA. Nat. Commun. 2021, 12, 5089. [Google Scholar] [CrossRef] [PubMed]
- Walker, G.T.; Fraiser, M.S.; Schram, J.L.; Little, M.C.; Nadeau, J.G.; Malinowski, D.P. Strand displacement amplification—An isothermal, in vitro DNA amplification technique. Nucleic Acids Res. 1992, 20, 1691–1696. [Google Scholar] [CrossRef] [PubMed]
- Fang, R.; Li, X.; Hu, L.; You, Q.; Li, J.; Wu, J.; Xu, P.; Zhong, H.; Luo, Y.; Mei, J.; et al. Cross-priming amplification for rapid detection of Mycobacterium tuberculosis in sputum specimens. J. Clin. Microbiol. 2009, 47, 845–847. [Google Scholar] [CrossRef] [PubMed]
- Woźniakowski, G.; Niczyporuk, J.S.; Samorek-Salamonowicz, E.; Gaweł, A. The development and evaluation of cross-priming amplification for the detection of avian reovirus. J. Appl. Microbiol. 2015, 118, 528–536. [Google Scholar] [CrossRef]
- Christian, A.T.; Pattee, M.S.; Attix, C.M.; Reed, B.E.; Sorensen, K.J.; Tucker, J.D. Detection of DNA point mutations and mRNA expression levels by rolling circle amplification in individual cells. Proc. Natl. Acad. Sci. USA 2001, 98, 14238–14243. [Google Scholar] [CrossRef] [PubMed]
- Kaur, M.; Ayarnah, K.; Duanis-Assaf, D.; Alkan, N.; Eltzov, E. Rapid and simple colorimetric detection of quiescent Colletotrichum in harvested fruit using reverse transcriptional loop-mediated isothermal amplification (RT-LAMP) technology. Talanta 2023, 255, 124251. [Google Scholar] [CrossRef]
- Cardoso, T.C.; Ferrari, H.F.; Bregano, L.C.; Silva-Frade, C.; Rosa, A.C.G.; Andrade, A.L. Visual detection of turkey coronavirus RNA in tissues and feces by reverse-transcription loop-mediated isothermal amplification (RT-LAMP) with hydroxynaphthol blue dye. Mol. Cell. Probes 2010, 24, 415–417. [Google Scholar] [CrossRef]
- Martineau, R.L.; Murray, S.A.; Ci, S.; Gao, W.; Chao, S.-H.; Meldrum, D.R. Improved Performance of Loop-Mediated Isothermal Amplification Assays via Swarm Priming. Anal. Chem. 2017, 89, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Tavakoli-Koopaei, R.; Javadi-Zarnaghi, F.; Aboutalebian, S.; Mirhendi, H. Malachite Green-Based Detection of SARS-CoV-2 by One-Step Reverse Transcription Loop-Mediated Isothermal Amplification. Iran. J. Sci. 2023, 47, 359–367. [Google Scholar] [CrossRef]
- Ge, A.; Liu, F.; Teng, X.; Cui, C.; Wu, F.; Liu, W.; Liu, Y.; Chen, X.; Xu, J.; Ma, B. A Palm Germ-Radar (PaGeR) for rapid and simple COVID-19 detection by reverse transcription loop-mediated isothermal amplification (RT-LAMP). Biosens. Bioelectron. 2022, 200, 113925. [Google Scholar] [CrossRef] [PubMed]
- Nzelu, C.O.; Gomez, E.A.; Cáceres, A.G.; Sakurai, T.; Martini-Robles, L.; Uezato, H.; Mimori, T.; Katakura, K.; Hashiguchi, Y.; Kato, H. Development of a loop-mediated isothermal amplification method for rapid mass-screening of sand flies for Leishmania infection. Acta Trop. 2014, 132, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Kokane, A.D.; Kokane, S.B.; Warghane, A.J.; Gubyad, M.G.; Sharma, A.K.; Reddy, M.K.; Ghosh, D.K. A Rapid and sensitive reverse transcription–loop-mediated isothermal amplification (RT-LAMP) assay for the detection of indian citrus ringspot virus. Plant Dis. 2021, 105, 1346–1355. [Google Scholar] [CrossRef]
- García-Bernalt Diego, J.; Fernández-Soto, P.; Crego-Vicente, B.; Alonso-Castrillejo, S.; Febrer-Sendra, B.; Gómez-Sánchez, A.; Vicente, B.; López-Abán, J.; Muro, A. Progress in loop-mediated isothermal amplification assay for detection of Schistosoma mansoni DNA: Towards a ready-to-use test. Sci. Rep. 2019, 9, 14744. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-W.; Weissenberger, G.; Ching, W.-M. Development of lyophilized loop-mediated isothermal amplification reagents for the detection of leptospira. Mil. Med. 2016, 181, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Coulter, F.J.; Yang, M.; Smith, J.L.; Tafesse, F.G.; Messer, W.B.; Reif, J.H. A lyophilized colorimetric RT-LAMP test kit for rapid, low-cost, at-home molecular testing of SARS-CoV-2 and other pathogens. Sci. Rep. 2022, 12, 7043. [Google Scholar] [CrossRef]
- Silva, C.J.; Adaskaveg, J.A.; Mesquida-Pesci, S.D.; Ortega-Salazar, I.B.; Pattathil, S.; Zhang, L.; Hahn, M.G.; van Kan, J.A.L.; Cantu, D.; Powell, A.L.T.; et al. Botrytis cinerea infection accelerates ripening and cell wall disassembly to promote disease in tomato fruit. Plant Physiol. 2023, 191, 575–590. [Google Scholar] [CrossRef] [PubMed]
- Francia, F.; Dezi, M.; Mallardi, A.; Palazzo, G.; Cordone, L.; Venturoli, G. Protein—Matrix Coupling/Uncoupling in ‘Dry’ Systems of Photosynthetic Reaction Center Embedded in Trehalose/Sucrose: The Origin of Trehalose Peculiarity. J. Am. Chem. Soc. 2008, 130, 10240–10246. [Google Scholar] [CrossRef]
- Cicerone, M.T.; Soles, C.L. Fast dynamics and stabilization of proteins: Binary glasses of trehalose and glycerol. Biophys. J. 2004, 86, 3836–3845. [Google Scholar] [CrossRef] [PubMed]
- Hardinge, P.; Murray, J.A.H. Reduced False Positives and Improved Reporting of Loop-Mediated Isothermal Amplification using Quenched Fluorescent Primers. Sci. Rep. 2019, 9, 7400. [Google Scholar] [CrossRef] [PubMed]
- Meagher, R.J.; Priye, A.; Light, Y.K.; Huang, C.; Wang, E. Impact of primer dimers and self-amplifying hairpins on reverse transcription loop-mediated isothermal amplification detection of viral RNA. Analyst 2018, 143, 1924–1933. [Google Scholar] [CrossRef] [PubMed]
- Oscorbin, I.; Filipenko, M. Bst polymerase—A humble relative of Taq polymerase. Comput. Struct. Biotechnol. J. 2023, 21, 4519–4535. [Google Scholar] [CrossRef] [PubMed]
- Tsai, A.M.; Udovic, T.J.; Neumann, D.A. The inverse relationship between protein dynamics and thermal stability. Biophys. J. 2001, 81, 2339–2343. [Google Scholar] [CrossRef]
- Mensink, M.A.; Frijlink, H.W.; Van Der Voort Maarschalk, K.; Hinrichs, W.L.J. How sugars protect proteins in the solid state and during drying (review): Mechanisms of stabilization in relation to stress conditions. Eur. J. Pharm. Biopharm. 2017, 114, 288–295. [Google Scholar] [CrossRef]
- Boora, S.; Khan, A.; Sharma, V.; Kaushik, S.; Mehta, P.K.; Singh, S.; Kaushik, S. RT-LAMP is a potential future molecular diagnostic tool for influenza A virus. Futur. Virol. 2023, 18, 165–175. [Google Scholar] [CrossRef]





| Species | Targeted Sequence | Primer Name | Primer Sequences 5′-3′ | Final Concentration |
|---|---|---|---|---|
| Botrytis cinerea | Latent marker (Polysaccharide Lyase family 1 protein, Table 2) | F3 | CAAATTCCACGAGATTCTCAT | 100 µM |
| B3 | ACGCTCAGCAATAGCATC | 100 µM | ||
| FIP | CGGGAGATCGATTCAAAAGATTGATTTTCACAACTCACTCAACTCAAG | 100 µM | ||
| BIP | AATGAAGCTCTCCATTCTCTCCACTTTTACCTTCAGTTGGTGTTGG | 100 µM | ||
| Loop F | AGAGCTGTCAACTTCGTTCTT | 100 µM | ||
| Loop B | CGCAGTCCTTGCGCAGTT | 100 µM |
| Description | Sequence |
|---|---|
| Latent marker of Botrytis cinerea (Polysaccharide Lyase family 1 protein) | GTGAAGATTTGCACGGCTGCGGTTGTACTGACGTTGAATGGACTCAAATCTCGTTTGACGATATGGGGGTTGAACGAGAAATCAAAAGTATATAAAGGAGAACCAAATTCCACGAGATTCTCATTCTTCACAACTCACTCAACTCAAGGCAAAGAACGAAGTTGACAGCTCTACAATCAATCTTTTGAATCGATCTCCCGCAACGAACTTTTTGAATATCCAAAAAAAAATGAAGCTCTCCATTCTCTCCACAGGACTCGCAGTCCTTGCGCAGTTCGTCTCTGCTGCTCCAACACCAACTGAAGGTGATGCTATTGCTGAGCGTGCAAACATCGCTAAGAGAGCTACTATCACCGATGTTGCCACTACCGGCTTTGCAACCCA |
| Latent marker of Alternaria alternata (Hypothetical protein partial mRNA (small ribosomal RNA unit) | ATGAAGTTCCTTGCCACCATCATCCTCCTTACCTTCCTCGCAGCCACGGTCATCGCAGGTGATTGCATGAAAGCACCTTTTCACTGCAATGGTATGTCTTTCACCTGTACCACCGCCATTCTCGTCACCCTCTTCACCACTACAGCTTCGGCGACTGAGTGTACAGTACCTGTTCTCTGCACCCGTAGCGTCGGTAAATCGCGGCTCATAGCAAACATACCCGGCTTTGAAGAATTCGGCGACTCTTACAGCAGCATCGAGTGTGCCCAGAGTCTTGTCGTGTCCTCTGTGATGGGGATGAAGACCTGCCCCGCTGTGCCATCGGTGTCTCACCCCTTCCCCCAGGTCGTCACGATATCGTCCACACCATACCCACCAACTACACGACCACCCATCTCAGCAAGAACAACACGCACCCACCCATACGGGTCGTCTGAACTGCGCGCTTACCAAGCTCCAGCGATGGTTTCTTTCTCCGCCGTCCTCATCCTAAGCTATCC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ayarnah, K.; Duanis-Assaf, D.; Alkan, N.; Eltzov, E. Development of Heat-Dry RT-LAMP Bioassay for Rapid Latent Detection of Botrytis cinerea. Appl. Microbiol. 2024, 4, 1616-1629. https://doi.org/10.3390/applmicrobiol4040110
Ayarnah K, Duanis-Assaf D, Alkan N, Eltzov E. Development of Heat-Dry RT-LAMP Bioassay for Rapid Latent Detection of Botrytis cinerea. Applied Microbiology. 2024; 4(4):1616-1629. https://doi.org/10.3390/applmicrobiol4040110
Chicago/Turabian StyleAyarnah, Khadijah, Danielle Duanis-Assaf, Noam Alkan, and Evgeni Eltzov. 2024. "Development of Heat-Dry RT-LAMP Bioassay for Rapid Latent Detection of Botrytis cinerea" Applied Microbiology 4, no. 4: 1616-1629. https://doi.org/10.3390/applmicrobiol4040110
APA StyleAyarnah, K., Duanis-Assaf, D., Alkan, N., & Eltzov, E. (2024). Development of Heat-Dry RT-LAMP Bioassay for Rapid Latent Detection of Botrytis cinerea. Applied Microbiology, 4(4), 1616-1629. https://doi.org/10.3390/applmicrobiol4040110








