Bacterial Resistance to Mercury: A Mini-Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Focus Questions
2.2. Information Sources
2.3. Risk of Bias Assessment
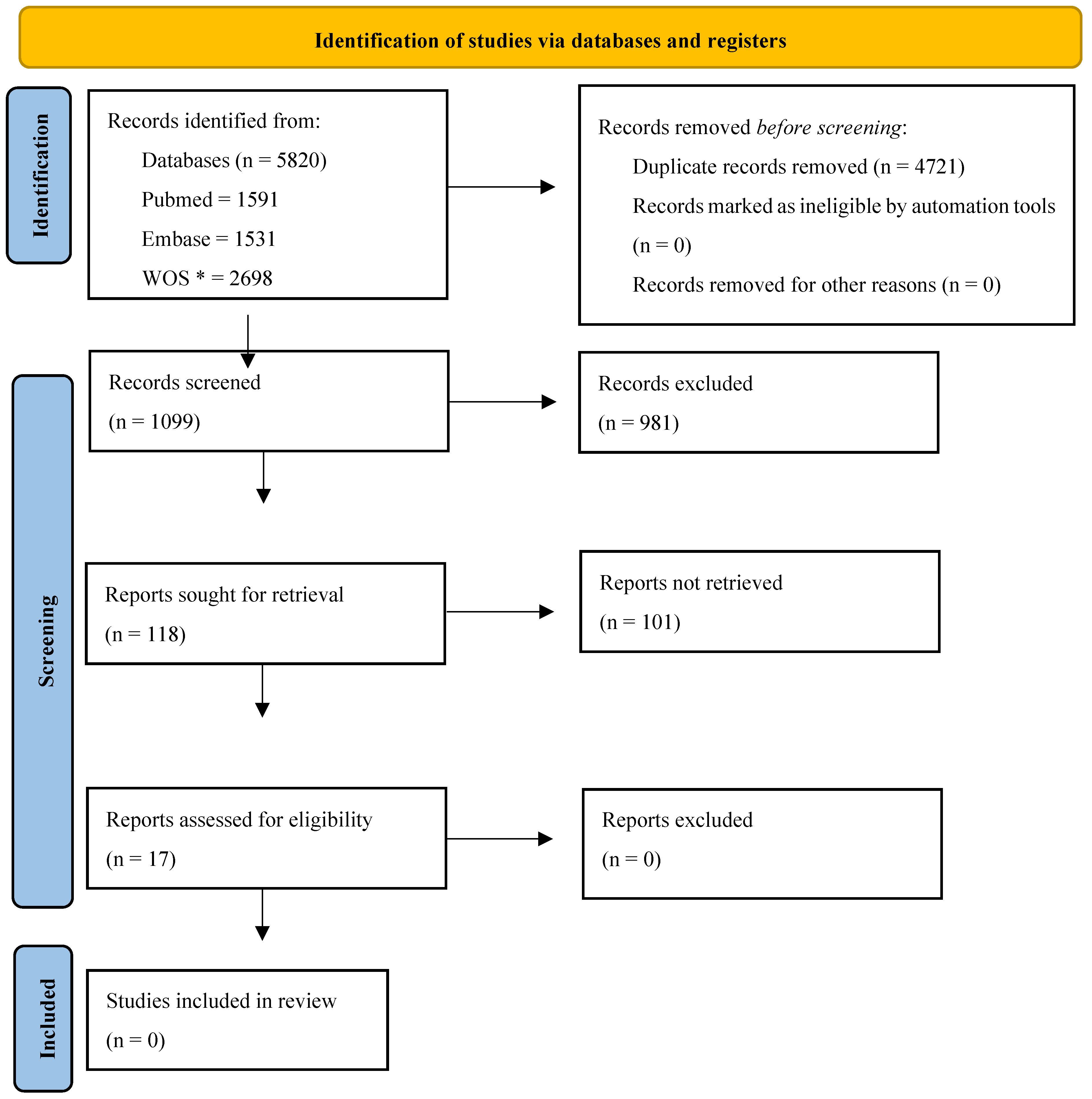
3. Results
3.1. Literature Search
3.2. Bacteria and Mercury Concentration
| Source | Bacteria | Genes | Experimental Design | [Hg] Tested | [Hg] Max 1 | References |
|---|---|---|---|---|---|---|
| Water | Staphylococcus epidermidis | merA, merT, merC and merR | PCR | 40–100 µM | 100 µM | [29] |
| Laboratory | Synechocystis sp. | merA and merR | PCR | 0–500 µM | 500 µM | [34] |
| Wellwater | Achromobacter xylosoxidans | merR, merT, merP, merC, merA, merD, merE and merR | WGS | - | - | [48] |
| Laboratory | Escherichia coli | merR | Laboratory manipulation | 5 nmol L−1 | 5 nmol L−1 | [49] |
| Soil | Enterobacter cloacae, Enterobacter ludwigii and Klebsiella pneumoniae | mer operon (merA) | Laboratory manipulation | 10–250 µM and 25–500 µM | 200 µM and 500 µM | [50] |
| Laboratory | Salmonella enterica I 4,[5],12:i:- | merR and merT | In silico | - | - | [51] |
| Laboratory | anammox bacteria of the genus Candidatus Kuenenia | merA, merB, merD and merR | PCR | 0–50 mg L−1 | 50 mg L−1 | [52] |
| Slaughterhouses | Escherichia coli | merA and merC | PCR | 25 μg/mL and 50 μg/mL | 25 μg/mL and 50 μg/mL | [24] |
| River water | Klebsiella sp., Escherichia coli, Serratia marcescens, Proteus sp., Citrobacter sp., Pseudomonas sp., Acinetobacter sp. and Enterobacter sp. | merA, merD, merR, merP, merT and merB | Isolation | 10 mg L−1 | 10 mg L−1 | [53] |
| Water of aquaculture system | Aeromonas sp., Salmonella sp., Shewanella sp., Pseudomonas aeruginosa, Myroides odoratus, Serratia liquefaciens, Vibrio fluvialis and Chryseobacterium sp. | merA | PCR | 0.005–2.5 mM | 2 mM | [54] |
| Laboratory | Escherichia coli K12 | merR | - | - | [55] | |
| Laboratory | Escherichia coli | merR | Laboratory manipulation | mmol L−1 | 10 μmol L−1 | [56] |
| Laboratory | Bacillus cereus, Bacillus sp. and Brevundimonas diminuta | merA, merP, merT and merB | Isolation | 50–500 ppm | 500 ppm | [57] |
| Wastewater | Rheinheimera tangshanensis | merT, merR, merC, and merA | PCR | 0–120 mg L−1 | solid medium: 120 mg L−1 and liquid medium: 60 mg L−1 | [58] |
| Seawater | Pseudomonas, Bacillus and Pseudoalteromonas | merA | PCR | 25–100 mg L−1 | 100 mg L−1 | [59] |
| Wastewater | Pseudomonas | merR, merA and merT | WGS | - | 60 ppm | [60] |
| Soil | Pseudoxanthomonas sp. | merA | PCR | 1–6 mg L−1 and 5–80 mg L−1 | 3 mg L−1 and 40 mg L−1 | [61] |
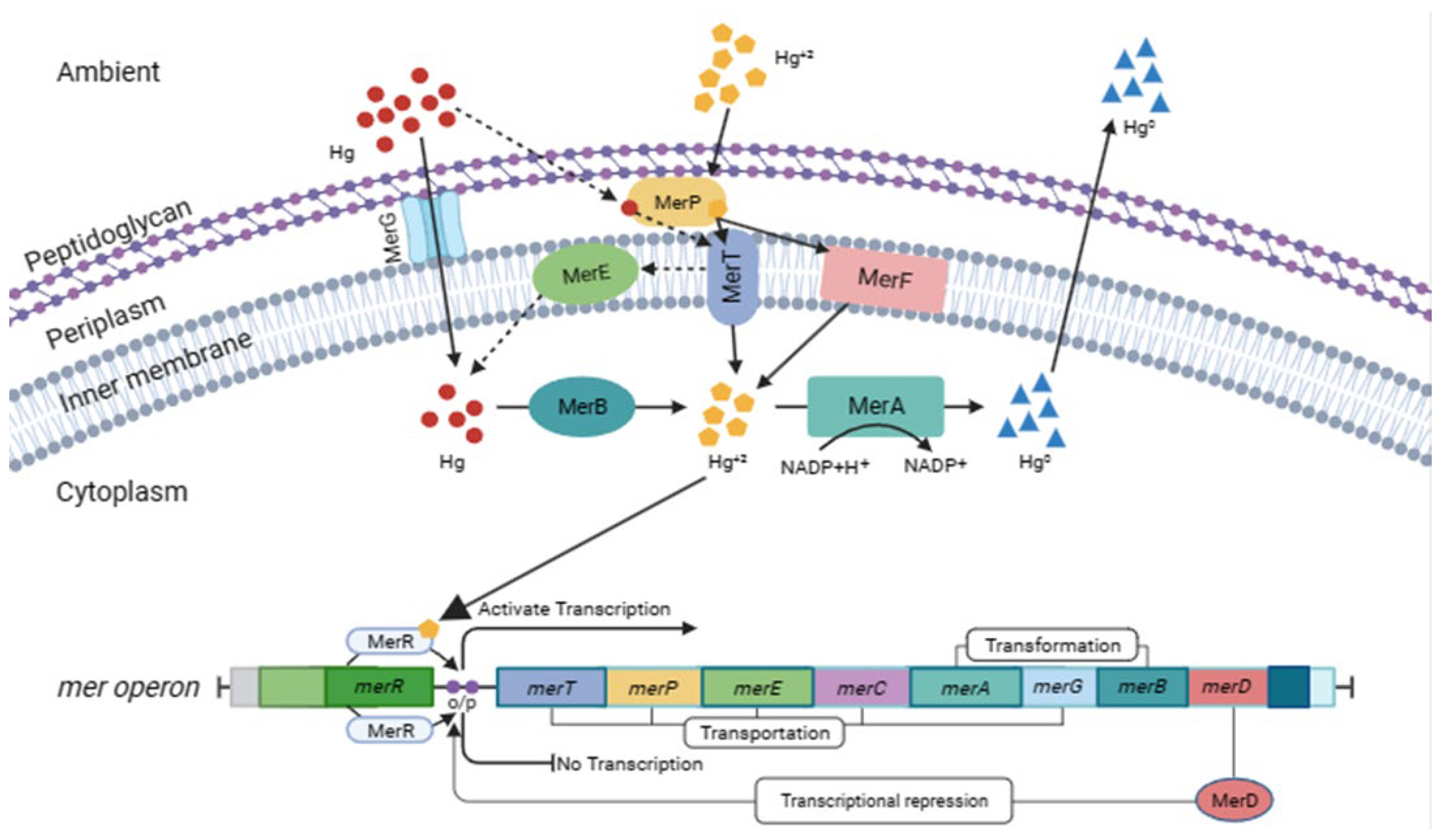
3.3. Genes Involved
3.4. Environments in Which the Gene Was Found
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Li, L.; Flora, J.R.V.; Caicedo, J.M.; Berge, N.D. Investigating the role of feedstock properties and process conditions on products formed during the hydrothermal carbonization of organics using regression techniques. Bioresour. Technol. 2015, 187, 263–274. [Google Scholar] [CrossRef]
- Mahbub, K.R.; Krishnan, K.; Naidu, R.; Andrews, S.; Megharaj, M. Mercury toxicity to terrestrial biota. Ecol. Indic. 2017, 74, 451–462. [Google Scholar] [CrossRef]
- UN Environment. Global Mercury Assessment 2018 [WWW Document]. 2019. Available online: https://www.unep.org/globalmercurypartnership/resources/report/global-mercury-assessment-2018 (accessed on 6 February 2023).
- Azevedo, J.S.; Braga, E.S.; Favaro, D.T.; Perretti, A.R.; Rezende, C.E.; Souza, C.M.M. Total mercury in sediments and in Brazilian Ariidae catfish from two estuaries under different anthropogenic influence. Mar. Pollut. Bull. 2011, 62, 2724–2731. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, J.D.S.; Sarkis, J.E.D.S.; Oliveira, T.A.; Ulrich, J.C. Tissue-specific mercury concentrations in two catfish species from the Brazilian coast. Braz. J. Oceanogr. 2012, 60, 209–217. [Google Scholar] [CrossRef]
- Condini, M.V.; Hoeinghaus, D.J.; Roberts, A.P.; Soulen, B.K.; Garcia, A.M. Mercury concentrations in dusky grouper Epinephelus marginatus in littoral and neritic habitats along the Southern Brazilian coast. Mar. Pollut. Bull. 2017, 115, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Alvarez, C.G.; Ruelas-Inzunza, J.; Osuna-López, J.I.; Voltolina, D.; Frías-Espericueta, M.G. Mercury content and their risk assessment in farmed shrimp Litopenaeus vannamei from NW Mexico. Chemosphere 2015, 119, 1015–1020. [Google Scholar] [CrossRef]
- Harayashiki, C.A.Y.; Reichelt-Brushett, A.; Cowden, K.; Benkendorff, K. Effects of oral exposure to inorganic mercury on the feeding behaviour and biochemical markers in yellowfin bream (Acanthopagrus australis). Mar. Environ. Res. 2018, 134, 1–15. [Google Scholar] [CrossRef]
- Hutcheson, M.S.; Smith, C.M.; Rose, J.; Batdorf, C.; Pancorbo, O.; West, C.R.; Strube, J.; Francis, C. Temporal and Spatial Trends in Freshwater Fish Tissue Mercury Concentrations Associated with Mercury Emissions Reductions. Environ. Sci. Technol. 2014, 48, 2193–2202. [Google Scholar] [CrossRef]
- Ruus, A.; Øverjordet, I.B.; Braaten, H.F.V.; Evenset, A.; Christensen, G.; Heimstad, E.S.; Gabrielsen, G.W.; Borgå, K. Methylmercury biomagnification in an Arctic pelagic food web. Environ. Toxicol. Chem. 2015, 34, 2636–2643. [Google Scholar] [CrossRef]
- Sadhu, A.K.; Kim, J.P.; Furrell, H.; Bostock, B. Methyl mercury concentrations in edible fish and shellfish from Dunedin, and other regions around the South Island, New Zealand. Mar. Pollut. Bull. 2015, 101, 386–390. [Google Scholar] [CrossRef]
- Araújo, L.C.A.; da Purificação-Júnior, A.F.; da Silva, S.M.; Lopes, A.C.S.; Veras, D.L.; Alves, L.C.; dos Santos, F.B.; Napoleão, T.H.; dos Santos Correia, M.T.; da Silva, M.V.; et al. In vitro evaluation of mercury (Hg2+) effects on biofilm formation by clinical and environmental isolates of Klebsiella pneumoniae. Ecotoxicol. Environ. Saf. 2019, 169, 669–677. [Google Scholar] [CrossRef] [PubMed]
- Rahman, Z.; Singh, V.P. Assessment of heavy metal contamination and Hg-resistant bacteria in surface water from different regions of Delhi, India. Saudi J. Biol. Sci. 2018, 25, 1687–1695. [Google Scholar] [CrossRef] [PubMed]
- Dash, H.R.; Das, S. Bioremediation of mercury and the importance of bacterial mer genes. Int. Biodeterior. Biodegrad. 2012, 75, 207–213. [Google Scholar] [CrossRef]
- Priyadarshanee, M.; Chatterjee, S.; Rath, S.; Dash, H.R.; Das, S. Cellular and genetic mechanism of bacterial mercury resistance and their role in biogeochemistry and bioremediation. J. Hazard. Mater. 2022, 423, 126985. [Google Scholar] [CrossRef] [PubMed]
- Hennebel, T.; Boon, N.; Maes, S.; Lenz, M. Biotechnologies for critical raw material recovery from primary and secondary sources: R&D priorities and future perspectives. New Biotechnol. 2015, 32, 121–127. [Google Scholar] [CrossRef]
- Ayangbenro, A.S.; Babalola, O.O. A New Strategy for Heavy Metal Polluted Environments: A Review of Microbial Biosorbents. Int. J. Environ. Res. Public. Health 2017, 14, 94. [Google Scholar] [CrossRef]
- Azubuike, C.C.; Chikere, C.B.; Okpokwasili, G.C. Bioremediation techniques-classification based on site of application: Principles, advantages, limitations and prospects. World J. Microbiol. Biotechnol. 2016, 32, 180. [Google Scholar] [CrossRef]
- Tiralerdpanich, P.; Sonthiphand, P.; Luepromchai, E.; Pinyakong, O.; Pokethitiyook, P. Potential microbial consortium involved in the biodegradation of diesel, hexadecane and phenanthrene in mangrove sediment explored by metagenomics analysis. Mar. Pollut. Bull. 2018, 133, 595–605. [Google Scholar] [CrossRef]
- Behera, B.K.; Chakraborty, H.J.; Patra, B.; Rout, A.K.; Dehury, B.; Das, B.K.; Sarkar, D.J.; Parida, P.K.; Raman, R.K.; Rao, A.R.; et al. Metagenomic Analysis Reveals Bacterial and Fungal Diversity and Their Bioremediation Potential From Sediments of River Ganga and Yamuna in India. Front. Microbiol. 2020, 11, 556136. [Google Scholar] [CrossRef]
- Taj, M.K.; Samreen, Z.; Ling, J.X.; Taj, I.; Hassani, T.M.; Yunlin, W. Escherichia coli as a model organism. Int. J. Eng. Res. Sci. Technol. 2014, 3, 1–8. [Google Scholar]
- Kane, A.L.; Al-Shayeb, B.; Holec, P.V.; Rajan, S.; Mieux, N.E.L.; Heinsch, S.C.; Psarska, S.; Aukema, K.G.; Sarkar, C.A.; Nater, E.A.; et al. Toward Bioremediation of Methylmercury Using Silica Encapsulated Escherichia coli Harboring the mer Operon. PLoS ONE 2016, 11, e0147036. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Wilson, D.B. Construction and characterization of Escherichia coli genetically engineered for bioremediation of Hg(2+)-contaminated environments. Appl. Environ. Microbiol. 1997, 63, 2442–2445. [Google Scholar] [CrossRef]
- Yang, H.; Wei, S.; Hobman, J.; Dodd, C. Antibiotic and Metal Resistance in Escherichia coli Isolated from Pig Slaughterhouses in the United Kingdom. Antibiotics 2020, 9, 746. [Google Scholar] [CrossRef] [PubMed]
- Leopold, K.; Foulkes, M.; Worsfold, P. Methods for the determination and speciation of mercury in natural waters—A review. Anal. Chim. Acta 2010, 663, 127–138. [Google Scholar] [CrossRef]
- Guedron, S.; Cossa, D.; Grimaldi, M.; Charlet, L. Methylmercury in tailings ponds of Amazonian gold mines (French Guiana): Field observations and an experimental flocculation method for in situ remediation. Appl. Geochem. Mercury Biogeochem. Cycl. Mercury Contam. Environ. 2011, 26, 222–229. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, L.; Liu, D. Characterization of a marine-isolated mercury-resistant Pseudomonas putida strain SP1 and its potential application in marine mercury reduction. Appl. Microbiol. Biotechnol. 2012, 93, 1305–1314. [Google Scholar] [CrossRef] [PubMed]
- von Canstein, H.; Li, Y.; Timmis, N.; Deckwer, W.-D.; Wagner-Döbler, I. Removal of Mercury from Chloralkali Electrolysis Wastewater by a Mercury-Resistant Pseudomonas putida Strain. Appl. Environ. Microbiol. 1999, 65, 5279–5284. [Google Scholar] [CrossRef]
- Yu, Z.; Gunn, L.; Wall, P.; Fanning, S. Antimicrobial resistance and its association with tolerance to heavy metals in agriculture production. Food Microbiol. 2017, 64, 23–32. [Google Scholar] [CrossRef]
- Mata, M.T.; Baquero, F.; Pérez-Díaz, J.C. A multidrug efflux transporter in Listeria monocytogenes. FEMS Microbiol. Lett. 2000, 187, 185–188. [Google Scholar] [CrossRef]
- Caille, O.; Rossier, C.; Perron, K. A Copper-Activated Two-Component System Interacts with Zinc and Imipenem Resistance in Pseudomonas aeruginosa. J. Bacteriol. 2007, 189, 4561–4568. [Google Scholar] [CrossRef] [PubMed]
- Dismukes, G.C.; Carrieri, D.; Bennette, N.; Ananyev, G.M.; Posewitz, M.C. Aquatic phototrophs: Efficient alternatives to land-based crops for biofuels. Curr. Opin. Biotechnol. Energy Biotechnol./Environ. Biotechnol. 2008, 19, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Heimann, K.; Cirés, S. Chapter 33—N2-Fixing Cyanobacteria: Ecology and Biotechnological Applications. In Handbook of Marine Microalgae; Kim, S.-K., Ed.; Academic Press: Boston, MA, USA, 2015; pp. 501–515. [Google Scholar] [CrossRef]
- Singh, J.S.; Kumar, A.; Rai, A.N.; Singh, D.P. Cyanobacteria: A Precious Bio-resource in Agriculture, Ecosystem, and Environmental Sustainability. Front. Microbiol. 2016, 7, 529. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Zhang, N.; Yang, X.; Song, L.; Yang, S. Toxic metal biosorption by macrocolonies of cyanobacterium Nostoc sphaeroides Kützing. J. Appl. Phycol. 2016, 28, 2265–2277. [Google Scholar] [CrossRef]
- Shen, L.; Li, Z.; Wang, J.; Liu, A.; Li, Z.; Yu, R.; Wu, X.; Liu, Y.; Li, J.; Zeng, W. Characterization of extracellular polysaccharide/protein contents during the adsorption of Cd(II) by Synechocystis sp. PCC6803. Environ. Sci. Pollut. Res. 2018, 25, 20713–20722. [Google Scholar] [CrossRef] [PubMed]
- Santos-Merino, M.; Singh, A.K.; Ducat, D.C. New applications of synthetic biology tools for cyanobacterial metabolic engineering. Front. Bioeng. Biotechnol. 2019, 7, 33. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Li, S.; Song, X.; Diao, J.; Chen, L.; Zhang, W. Toolboxes for cyanobacteria: Recent advances and future direction. Biotechnol. Adv. 2018, 36, 1293–1307. [Google Scholar] [CrossRef] [PubMed]
- Carmichael, W. A world overview—One-hundred-twenty-seven years of research on toxic cyanobacteria—Where do we go from here? In Cyanobacterial Harmful Algal Blooms: State of the Science and Research Needs, Advances in Experimental Medicine and Biology; Hudnell, H.K., Ed.; Springer: New York, NY, USA, 2008; pp. 105–125. [Google Scholar] [CrossRef]
- Catherine, Q.; Susanna, W.; Isidora, E.-S.; Mark, H.; Aurélie, V.; Jean-François, H. A review of current knowledge on toxic benthic freshwater cyanobacteria—Ecology, toxin production and risk management. Water Res. 2013, 47, 5464–5479. [Google Scholar] [CrossRef]
- Lee, J.; Lee, S.; Jiang, X. Cyanobacterial Toxins in Freshwater and Food: Important Sources of Exposure to Humans. Annu. Rev. Food Sci. Technol. 2017, 8, 281–304. [Google Scholar] [CrossRef]
- Sierra, M.A.; Martínez-Álvarez, R. Ricin and Saxitoxin: Two Natural Products That Became Chemical Weapons. J. Chem. Educ. 2020, 97, 1707–1714. [Google Scholar] [CrossRef]
- van der Merwe, D. Chapter 31—Cyanobacterial (Blue-Green Algae) Toxins. In Handbook of Toxicology of Chemical Warfare Agents, 2nd ed.; Gupta, R.C., Ed.; Academic Press: Boston, MA, USA, 2015; pp. 421–429. [Google Scholar] [CrossRef]
- Lefebvre, D.D.; Kelly, D.; Budd, K. Biotransformation of Hg(II) by Cyanobacteria. Appl. Environ. Microbiol. 2007, 73, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.; Yu, M.; Jia, L. Synergistic effects of proteasome inhibitor on TRAIL-induced apoptosis in malignant lymphoma cells. Chin. J. Clin. Oncol. 2007, 34, 9–11. [Google Scholar]
- Ren, T.; Xu, S.; Zhao, W.-B.; Zhu, J.-J. A surfactant-assisted photochemical route to single crystalline HgS nanotubes. J. Photochem. Photobiol. Chem. 2005, 173, 93–98. [Google Scholar] [CrossRef]
- Yu, D.; Zhai, J.; Yong, D.; Dong, S. A rapid and sensitive p-benzoquinone-mediated bioassay for determination of heavy metal toxicity in water. Analyst 2013, 138, 3297–3302. [Google Scholar] [CrossRef] [PubMed]
- Istiaq, A.; Shuvo, M.S.R.; Rahman, K.M.J.; Siddique, M.A.; Hossain, M.A.; Sultana, M. Adaptation of metal and antibiotic resistant traits in novel β-Proteobacterium Achromobacter xylosoxidans BHW-15. PeerJ 2019, 7, e6537. [Google Scholar] [CrossRef]
- Mangal, V.; Stenzler, B.R.; Poulain, A.J.; Guéguen, C. Aerobic and Anaerobic Bacterial Mercury Uptake is Driven by Algal Organic Matter Composition and Molecular Weight. Environ. Sci. Technol. 2019, 53, 157–165. [Google Scholar] [CrossRef]
- Gontia-Mishra, I.; Sapre, S.; Sharma, A.; Tiwari, S. Alleviation of Mercury Toxicity in Wheat by the Interaction of Mercury-Tolerant Plant Growth-Promoting Rhizobacteria. J. Plant Growth Regul. 2016, 35, 1000–1012. [Google Scholar] [CrossRef]
- Fenske, G.J.; Scaria, J. Analysis of 56,348 Genomes Identifies the Relationship between Antibiotic and Metal Resistance and the Spread of Multidrug-Resistant Non-Typhoidal Salmonella. Microorganisms 2021, 9, 1468. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, X.; Ma, Y.; Song, Y.; Li, Y.; Geng, G.; Huang, Y. Anammox biofilm system under the stress of Hg(II): Nitrogen removal performance, microbial community dynamic and resistance genes expression. J. Hazard. Mater. 2020, 395, 122665. [Google Scholar] [CrossRef]
- Mirzaei, N.; Rastegari, H.; Kargar, M. Antibiotic resistance pattern among gram negative mercury resistant bacteria isolated from contaminated environments. Jundishapur J. Microbiol. 2013, 6, e8085. [Google Scholar] [CrossRef]
- Chenia, H.; Jacobs, A. Antimicrobial resistance, heavy metal resistance and integron content in bacteria isolated from a South African tilapia aquaculture system. Dis. Aquat. Organ. 2017, 126, 199–209. [Google Scholar] [CrossRef]
- Zavilgelsky, G.B.; Kotova, V.Y.; Melkina, O.E.; Pustovoit, K.S. Antirestriction activity of the mercury resistance nonconjugative transposon Tn5053 is controlled by the protease ClpXP. Russ. J. Genet. 2014, 50, 910–915. [Google Scholar] [CrossRef]
- Wang, Y.-Z.; Li, D.; He, M. Application of internal standard method in recombinant luminescent bacteria test. J. Environ. Sci. China 2015, 35, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Kardena, E.; Panha, Y.; Helmy, Q.; Hidayat, S. Application of mercury resistant bacteria isolated from artisanal small-scale gold tailings in biotransformation of mercury (II)—contaminated. Int. J. GEOMATE 2020, 19, 106–114. [Google Scholar] [CrossRef]
- Zhao, M.; Zheng, G.; Kang, X.; Zhang, X.; Guo, J.; Wang, S.; Chen, Y.; Xue, L. Aquatic Bacteria Rheinheimera tangshanensis New Ability for Mercury Pollution Removal. Int. J. Mol. Sci. 2023, 24, 5009. [Google Scholar] [CrossRef] [PubMed]
- Joshi, G.; Meena, B.; Verma, P.; Nayak, J.; Vinithkumar, N.V.; Dharani, G. Deep-sea mercury resistant bacteria from the Central Indian Ocean: A potential candidate for mercury bioremediation. Mar. Pollut. Bull. 2021, 169, 112549. [Google Scholar] [CrossRef] [PubMed]
- Lo, K.; Lu, C.; Liu, F.; Kao, C.; Chen, S. Draft genome sequence of Pseudomonas sp. A46 isolated from mercury-contaminated wastewater. J. Basic Microbiol. 2022, 62, 1193–1201. [Google Scholar] [CrossRef] [PubMed]
- Mahbub, K.R.; Krishnan, K.; Naidu, R.; Megharaj, M. Mercury resistance and volatilization by Pseudoxanthomonas sp. SE1 isolated from soil. Environ. Technol. Innov. 2016, 6, 94–104. [Google Scholar] [CrossRef]
- Singh, D.K.; Lingaswamy, B.; Koduru, T.N.; Nagu, P.P.; Jogadhenu, P.S.S. A putative merR family transcription factor Slr0701 regulates mercury inducible expression of MerA in the cyanobacterium Synechocystis sp. PCC6803. MicrobiologyOpen 2019, 8, e00838. [Google Scholar] [CrossRef]
- Tirkey, J.; Adhikary, S.P. Cyanobacteria in biological soil crusts of India. Curr. Sci. 2005, 89, 515–521. [Google Scholar]
- Aminov, R.I.; Mackie, R.I. Evolution and ecology of antibiotic resistance genes. FEMS Microbiol. Lett. 2007, 271, 147–161. [Google Scholar] [CrossRef]
- Baquero, F.; Martínez, J.-L.; Cantón, R. Antibiotics and antibiotic resistance in water environments. Curr. Opin. Biotechnol. 2008, 19, 260–265. [Google Scholar] [CrossRef] [PubMed]
- van Elsas, J.D.; Bailey, M.J. The ecology of transfer of mobile genetic elements. FEMS Microbiol. Ecol. 2002, 42, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Roulet, M.; Lucotte, M.; Canuel, R.; Farella, N.; Courcelles, M.; Guimarães, J.-R.D.; Mergler, D.; Amorim, M. Increase in mercury contamination recorded in lacustrine sediments following deforestation in the central Amazon 1 The present investigation is part of an ongoing study, the CARUSO project (CRDI-UFPa-UQAM), initiated to determine the sources, fate and health effects of the presence of MeHg in the area of the Lower Tapajós. 1. Chem. Geol. 2000, 165, 243–266. [Google Scholar] [CrossRef]
- Aula, I.; Braunschweiler, H.; Malin, I. The watershed flux of mercury examined with indicators in the Tucuruí reservoir in Pará, Brazil. Sci. Total Environ. Mercury Pollut. Gold Min. Braz. 1995, 175, 97–107. [Google Scholar] [CrossRef]
- Lacerda, L.D. Evolution of Mercury Contamination in Brazil. Water. Air. Soil Pollut. 1997, 97, 247–255. [Google Scholar] [CrossRef]
- Nriagu, J.O. Mercury pollution from silver mining in colonial South America. In Proceedings of the International Symposium Perspectives for Environmental Geochemistry in Tropical Countries; Abrao, J.J., Wasserman, J.C., Silva Filho, E.V., Eds.; Lewis: London, UK, 1993; pp. 365–368. [Google Scholar]
- Kolka, R.K.; Nater, E.A.; Grigal, D.F.; Verry, E.S. Atmospheric inputs of mercury and organic carbon into a forested upland/bog watershed. Water. Air. Soil Pollut. 1999, 113, 273–294. [Google Scholar] [CrossRef]
- Pacyna, E.G.; Pacyna, J.M. Global Emission of Mercury from Anthropogenic Sources in 1995. Water. Air. Soil Pollut. 2002, 137, 149–165. [Google Scholar] [CrossRef]
- Beim, A.M.; Grosheva, E.I. Ecological chemistry of mercury contained in bleached kraft pulp mill effluents. Water. Air. Soil Pollut. 1992, 65, 135–141. [Google Scholar] [CrossRef]
- Moreira, J.C.; Pivetta, F. Human and environmental contamination by mercury from industrial uses in Brazil. Water. Air. Soil Pollut. 1997, 97, 241–246. [Google Scholar] [CrossRef]
- USEPA. Mercury Study Report to Congress EPA-452/R-97-004. 1997. Available online: https://archive.epa.gov/mercury/archive/web/pdf/volume1.pdf (accessed on 6 February 2023).
- Gworek, B.; Dmuchowski, W.; Baczewska-Dąbrowska, A.H. Mercury in the terrestrial environment: A review. Environ. Sci. Eur. 2020, 32, 128. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Mattos D’Avila, D.G.; Ferrari, R.G.; de Almeida Rodrigues, P.; Neves, G.L.; Ramos Filho, A.M.; Baptista Mano, R.F.; Conte Junior, C.A. Bacterial Resistance to Mercury: A Mini-Review. Appl. Microbiol. 2024, 4, 1630-1641. https://doi.org/10.3390/applmicrobiol4040111
de Mattos D’Avila DG, Ferrari RG, de Almeida Rodrigues P, Neves GL, Ramos Filho AM, Baptista Mano RF, Conte Junior CA. Bacterial Resistance to Mercury: A Mini-Review. Applied Microbiology. 2024; 4(4):1630-1641. https://doi.org/10.3390/applmicrobiol4040111
Chicago/Turabian Stylede Mattos D’Avila, Daniel Gonçalves, Rafaela Gomes Ferrari, Paloma de Almeida Rodrigues, Gabriel Lata Neves, Alexandre Mendes Ramos Filho, Rami Fanticelli Baptista Mano, and Carlos Adam Conte Junior. 2024. "Bacterial Resistance to Mercury: A Mini-Review" Applied Microbiology 4, no. 4: 1630-1641. https://doi.org/10.3390/applmicrobiol4040111
APA Stylede Mattos D’Avila, D. G., Ferrari, R. G., de Almeida Rodrigues, P., Neves, G. L., Ramos Filho, A. M., Baptista Mano, R. F., & Conte Junior, C. A. (2024). Bacterial Resistance to Mercury: A Mini-Review. Applied Microbiology, 4(4), 1630-1641. https://doi.org/10.3390/applmicrobiol4040111








