Interaction between Trichoderma sp., Pseudomonas putida, and Two Organic Amendments on the Yield and Quality of Strawberries (Fragaria x annanasa cv. San Andreas) in the Huaral Region, Peru
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Sowing
2.2. Design and Treatments
2.3. Inoculant Preparation
2.4. Biometric Parameters
2.5. Performance and Quality Parameters
2.6. Statistical Analysis
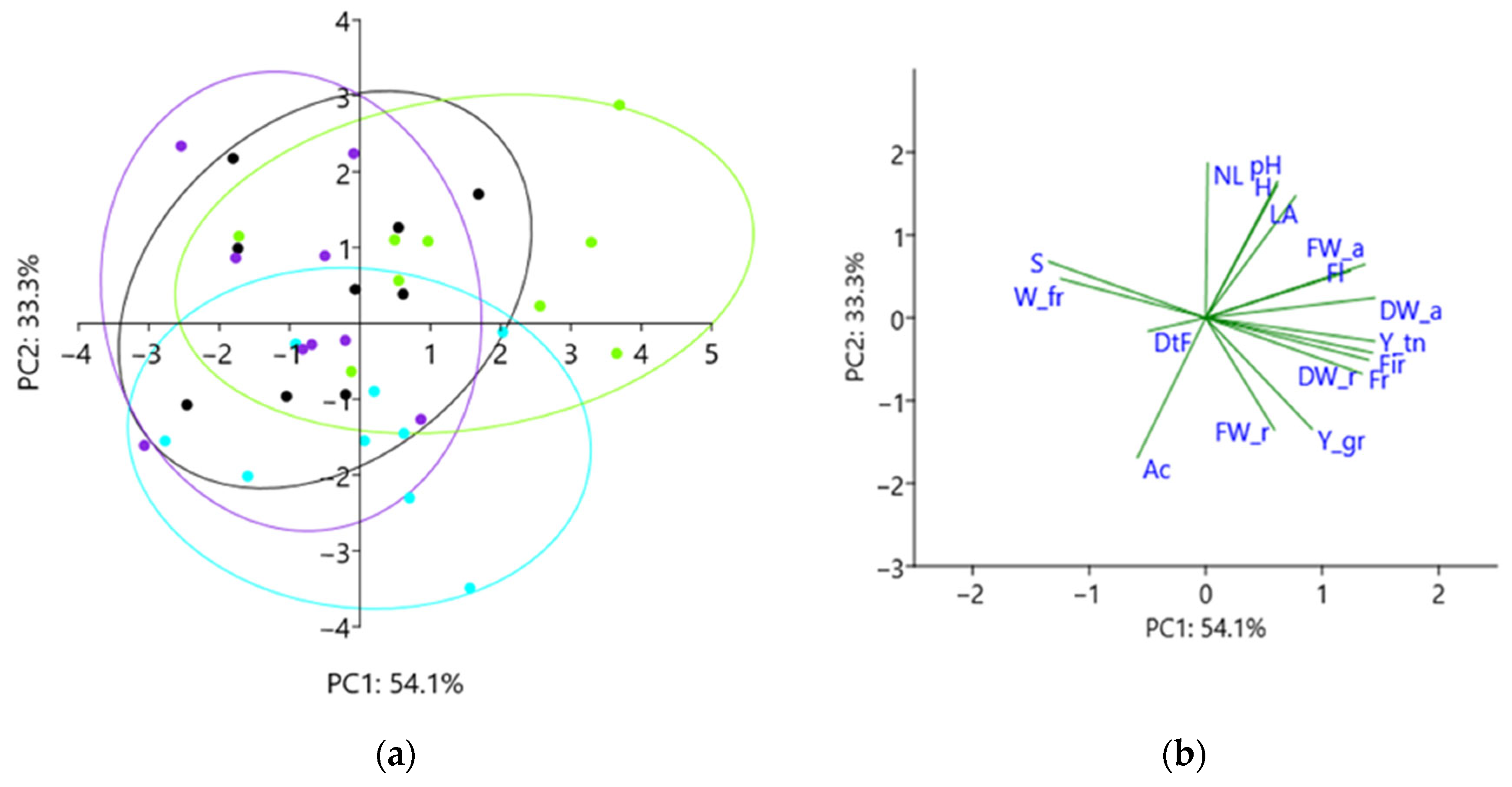
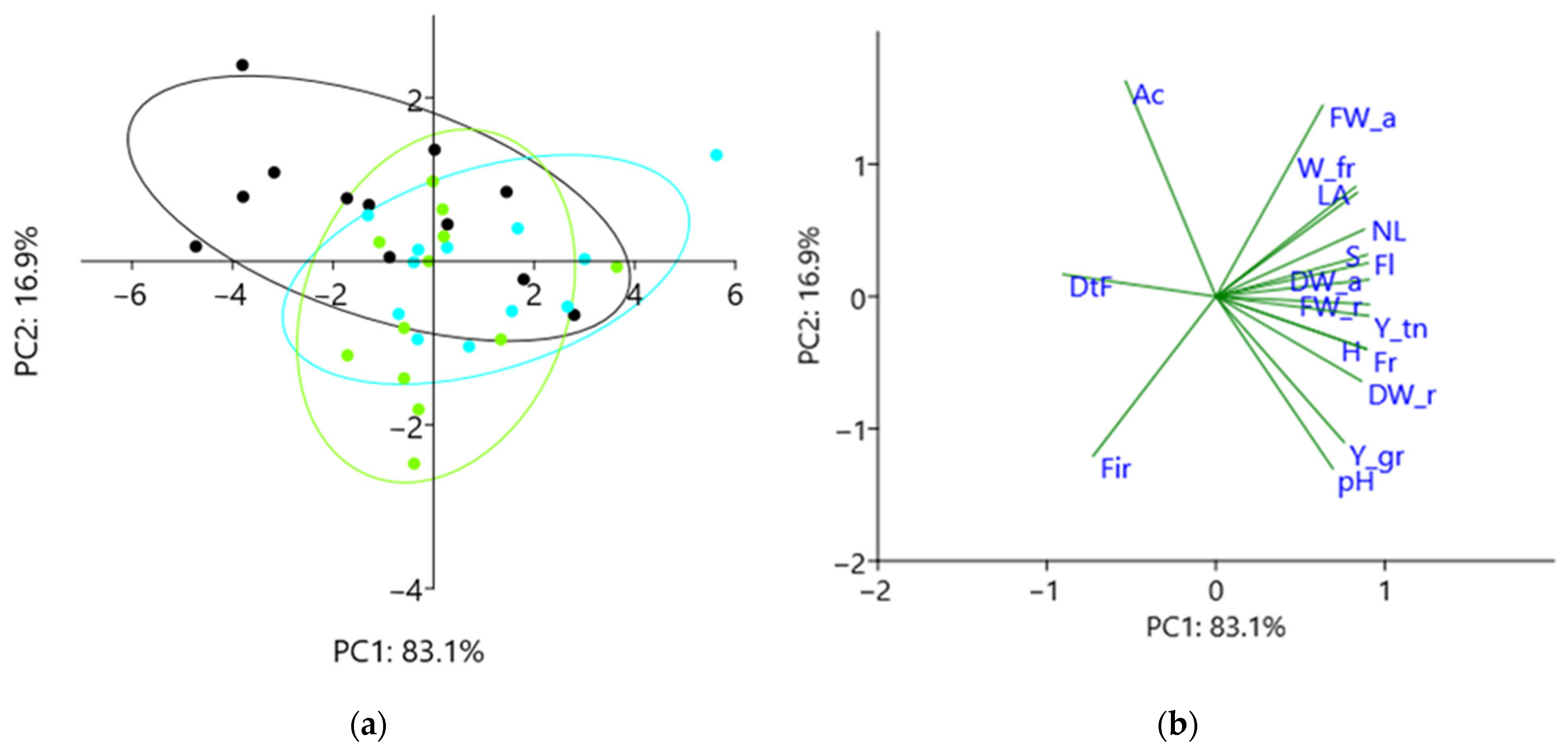
2.7. Principal Component Analysis
3. Results
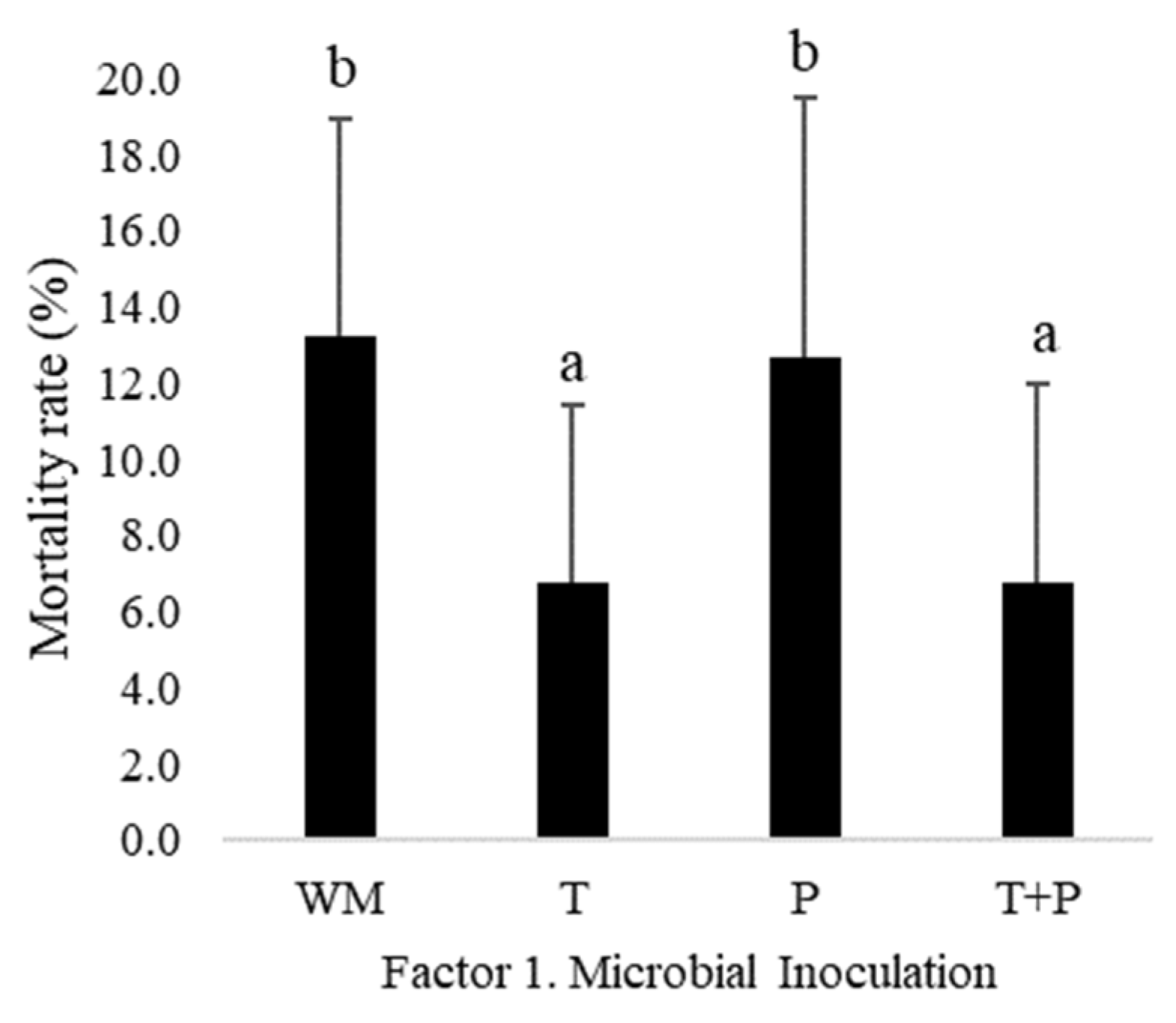
3.1. Strawberry Plant Rooting Percentage (Fragaria sp. var. San Andreas)
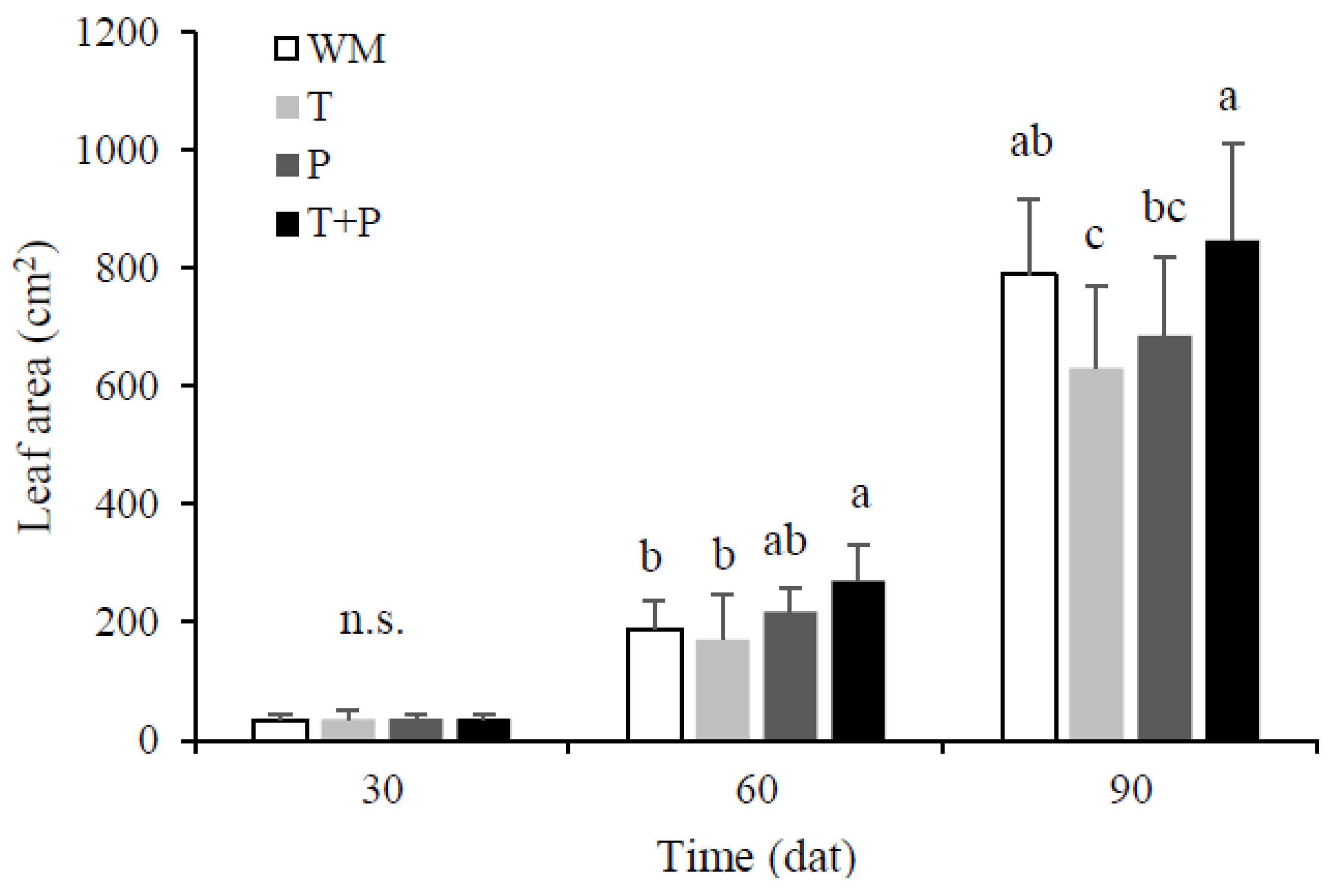
3.2. Number of Leaves, Plant Height, and Leaf Area
3.3. Fresh and Dry Biomass
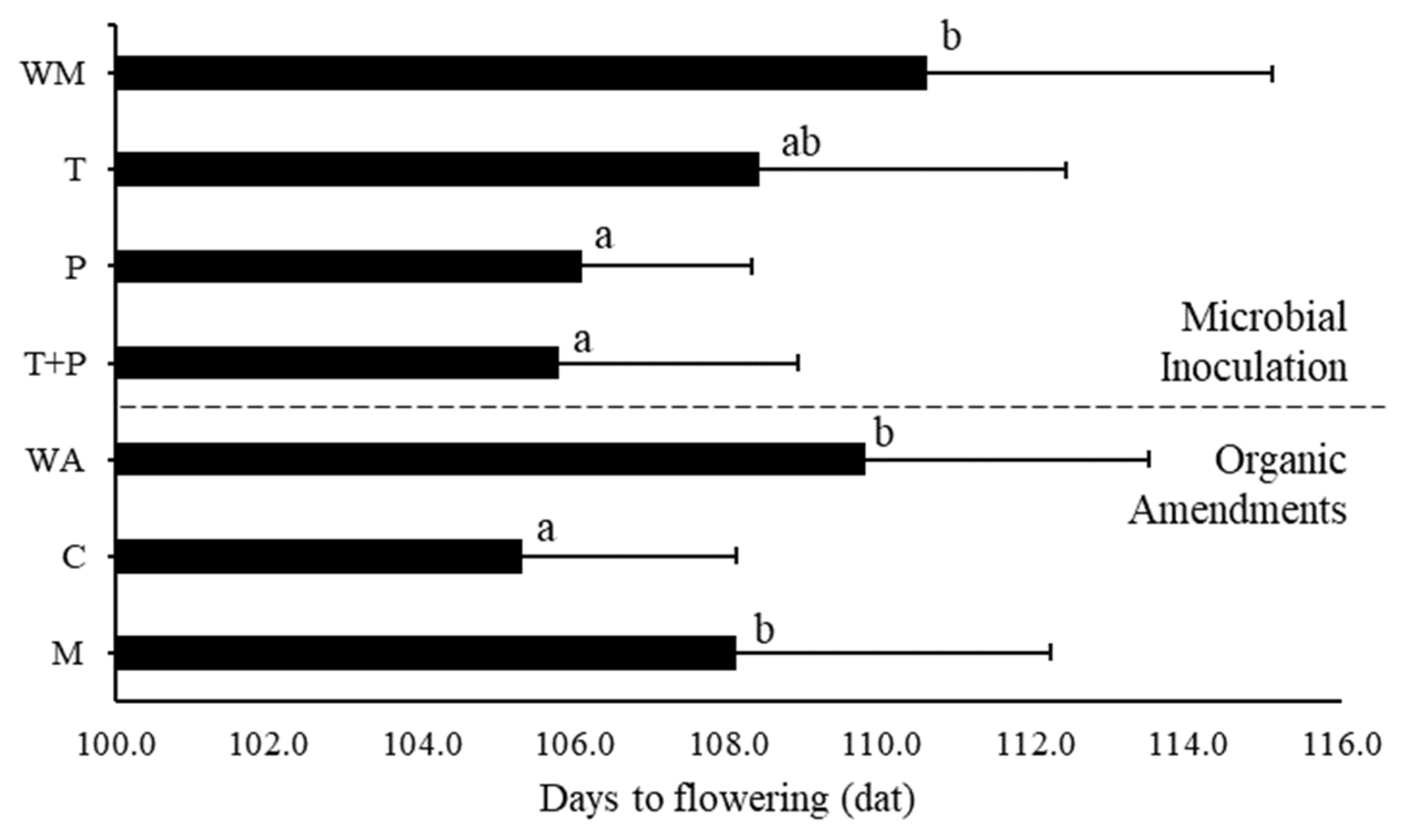
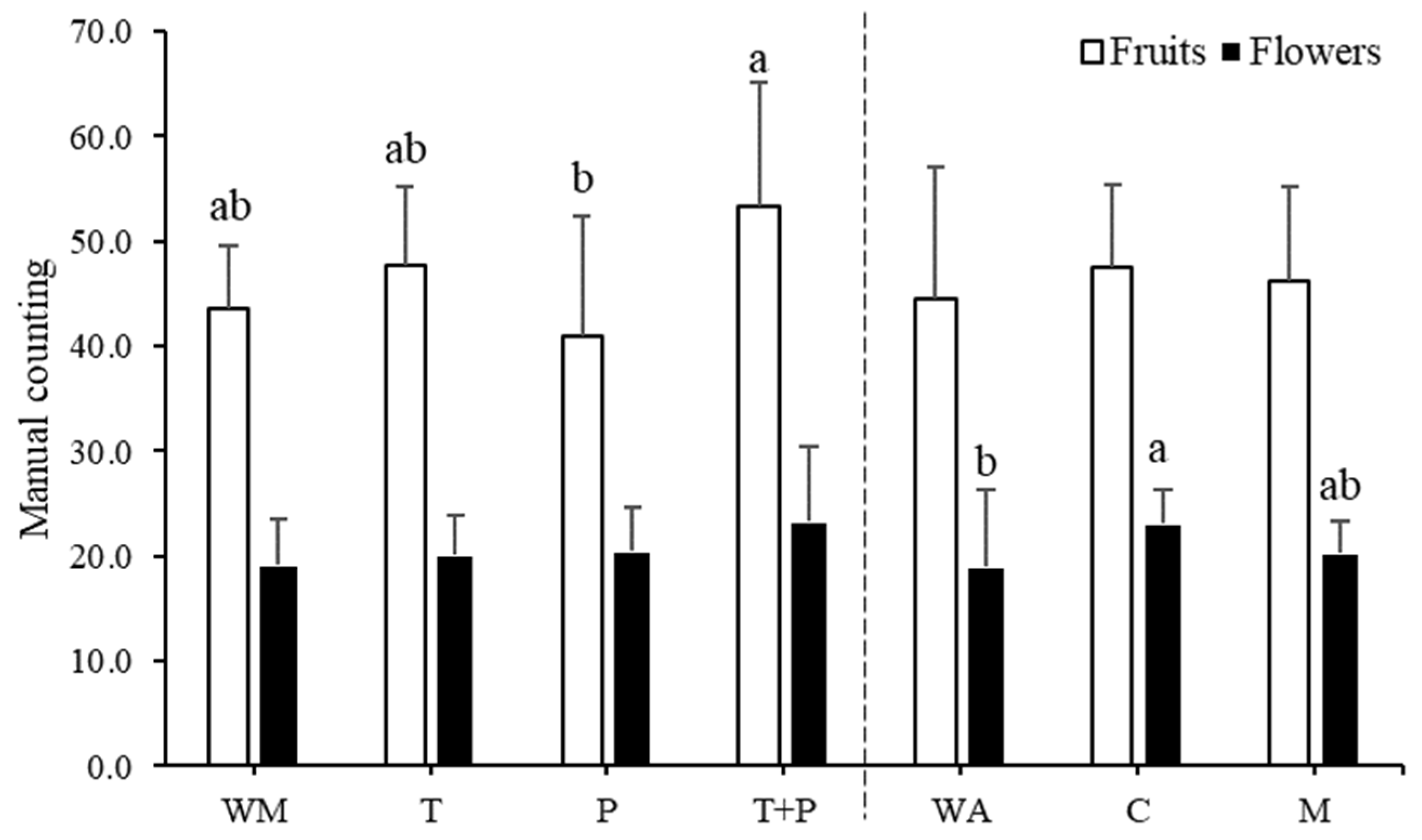
3.4. Flowering, Fruit, and Yield Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fernández-Lara, R.; Gordillo, B.; Rodríguez-Pulido, F.J.; Lourdes González-Miret, M.; del Villar-Martínez, A.A.; Dávila-Ortiz, G.; Heredia, F.J. Assessment of the Differences in the Phenolic Composition and Color Characteristics of New Strawberry (Fragaria x Ananassa Duch.) Cultivars by HPLC–MS and Imaging Tristimulus Colorimetry. Food Res. Int. 2015, 76, 645–653. [Google Scholar] [CrossRef] [PubMed]
- FAO. FAOSTAT Database. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 1 March 2024).
- Giampieri, F.; Forbes-Hernandez, T.Y.; Gasparrini, M.; Alvarez-Suarez, J.M.; Afrin, S.; Bompadre, S.; Quiles, J.L.; Mezzetti, B.; Battino, M. Strawberry as a Health Promoter: An Evidence Based Review. Food Funct. 2015, 6, 1386–1398. [Google Scholar] [CrossRef] [PubMed]
- Parodi, G.; Benavides, M. Desarrollo Del Cultivo de Berries En Perú. In Avances en el Cultivo de las Berries en el Trópico; Sociedad Colombiana de Ciencias Hortícolas: Bogotá, Colombia, 2021; pp. 91–101. ISBN 978-958-59886-1-3. [Google Scholar]
- Flores-Bernedo, J.; Vásquez-Castro, J. Susceptibility to Spirodiclofen in field populations of Tetranychus urticae (Acari: Tetranychidae) from strawberry plantations in Lima region, Peru. Peruv. J. Agron. 2020, 4, 61–67. [Google Scholar] [CrossRef]
- Beyer Arteaga, A.A.; Rodríguez Quispe, P.; Collantes González, R.D.; Joyo Coronado, G. Factores Socioeconómicos, Productivos y Fuentes de Información Sobre Plaguicidas Para Productores de Fragaria x Ananassa En Cañete, Lima, Perú. Idesia Arica 2017, 35, 31–37. [Google Scholar] [CrossRef][Green Version]
- FAO. Emissions Due to Agriculture Global, Regional and Country Trends 2000–2018. In FAOSTAT Analytical Brief Series N° 18; FAO: Rome, Italy, 2020; ISSN 2709-0078. [Google Scholar]
- FAO. World Agricultural towards 2015/2030. An FAO Perspective; FAO: Rome, Italy, 2003; ISBN 92-5-104835-5. [Google Scholar]
- Alexandratos, N.; Bruinsma, J. World Agriculture towards 2030/2050: The 2012 Revision. ESA Working Paper No. 12-03. 2012. Available online: https://www.fao.org/4/ap106e/ap106e.pdf (accessed on 13 June 2024).
- Anuradha; Goyal, R.; Sindhu, S. Response of Strawberry (Fragaria × Ananassa Duch.) to PGPR Inoculation. Bangladesh J. Bot. 2020, 49, 1071–1076. [Google Scholar] [CrossRef]
- Pulido-Blanco, V.C.; Insuasty-Burbano, O.I.; Sarmiento-Naizaque, Z.X.; Ramírez-Durán, J. Carmenta theobromae (Busck, 1910), Pest of Guava in Colombia: Biology, Life Cycle and Natural Enemies. Heliyon 2020, 6, e05489. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Pataczek, L.; Hilger, T.H.; Zahir, Z.A.; Hussain, A.; Rasche, F.; Schafleitner, R.; Solberg, S.Ø. Perspectives of Microbial Inoculation for Sustainable Development and Environmental Management. Front. Microbiol. 2018, 9, 02992. [Google Scholar] [CrossRef] [PubMed]
- Hermosa, R.; Viterbo, A.; Chet, I.; Monte, E. Plant-beneficial effects of Trichoderma and of its genes. Microbiology 2012, 158, 17–25. [Google Scholar] [CrossRef]
- Cano, M.A. Interacción de microorganismos benéficos en plantas: Micorrizas, Trichoderma spp. y Pseudomonas spp. Una revision. Rev. UDCA Actual. Divulg. Científica 2011, 14, 15–31. [Google Scholar]
- Sah, S.; Krishnani, S.; Singh, R. Pseudomonas mediated nutritional and growth promotional activities for sustainable food security. Curr. Res. Microb. Sci. 2021, 2, 100084. [Google Scholar] [CrossRef]
- Murillo Montoya, S.A.; Mendoza Mora, A.; Fadul Vásquez, C.J. La importancia de las enmiendas orgánicas en la conservación del suelo y la producción agrícola. Rev. Colomb. Investig. Agroindustriales 2020, 7, 58–68. [Google Scholar] [CrossRef]
- Ajema, W. Effect of compost in Improving Soil Properties an Its Consequent Effect on crop production—A review. J. Nat. Sci. Res. 2021, 12, 15–25. [Google Scholar]
- Memenza, A.; Cauty, N.; Sotelo, F.; Ramos, E. Standardize Strawberry Crops by Applying the Quality Function Deployment in Huaura, Perú. In Proceedings of the 18th LACCEI International Multi-Conference for Engineering, Education, and Technology: Engineering, Integration, and Alliances for a Sustainable Development “Hemispheric Cooperation for Competitiveness and Prosperity on A Knowledge-Based Economy”, Virtual, 27–31 July 2020; Latin American and Caribbean Consortium of Engineering Institutions: Buenos Aires, Argentina, 2020. [Google Scholar]
- Lombardi, N.; Caira, S.; Troise, A.D.; Scaloni, A.; Vitaglione, P.; Vinale, F.; Marra, R.; Salzano, A.M.; Lorito, M.; Woo, S.L. Trichoderma Applications on Strawberry Plants Modulate the Physiological Processes Positively Affecting Fruit Production and Quality. Front. Microbiol. 2020, 11, 01364. [Google Scholar] [CrossRef] [PubMed]
- Martín, L.; Velázquez, E.; Rivas, R.; Mateos, P.F.; Martínez-Molina, E.; Rodríguez-Barrueco, C.; Peix, A. Effect of Inoculation with a Strain of Pseudomonas fragi in the Growth and Phosphorous Content of Strawberry Plants. In Proceedings of the First International Meeting on Microbial Phosphate Solubilization, Salamanca, Spain, 16–19 July 2002; Velázquez, E., Rodríguez-Barrueco, C., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 309–315. [Google Scholar]
- Serret-López, M.; Espinosa-Victoria, D.; Gómez-Rodríguez, O.; Delgadillo-Martínez, J.; Serret-López, M.; Espinosa-Victoria, D.; Gómez-Rodríguez, O.; Delgadillo-Martínez, J. Tolerancia de plantas de fresa (Fragaria × ananassa Duch.) premicorrizadas con Rhizophagus intraradices e inoculadas con PGPR’s a Phytophthora capsici. Agrociencia 2016, 50, 1107–1121. [Google Scholar]
- Sarmiento, G.; Amézquita, M.; Mena, L. Use of bocashi and effective microorganisms as an ecological alternative in strawberry crops in arid zones. Sci. Agropecu. 2019, 10, 55–61. [Google Scholar] [CrossRef]
- Rosero Cisneros, N.G. Efectos de la Aplicación de Trichoderma Harzianum Sobre la Incidencia de “Damping off” en el Cultivo de Fresa (Fragaria vesca L.) en la Zona de el Quinche Provincia de Pichincha. Bachelor Thesis, UTB, Babahoyo, Ecuador, 2011. [Google Scholar]
- Kumari, N.; Thakur, A. Black Root Rot of Strawberry: A Disease Complex. Int. J. Econ. Plants 2022, 9, 158–163. [Google Scholar] [CrossRef]
- Admassie, M.; Alemu, T.; Mulatu, A. Microbial biocontrol by Trichoderma, its biological interactions and mode of actions. Pak. J. Phytopathol. 2019, 31, 123–146. [Google Scholar] [CrossRef]
- Jiang, H.; Zhang, L.; Zhang, J.; Ojaghian, M.R.; Hyde, K.D. Antagonistic Interaction between Trichoderma asperellum and Phytophthora capsici in Vitro. J. Zhejiang Univ. Sci. B 2016, 17, 271–281. [Google Scholar] [CrossRef]
- Faghih, S.; Zarei, A.; Ghobadi, C. Positive Effects of Plant Growth Regulators on Physiology Responses of Fragaria × Ananassa Cv. ‘Camarosa’ under Salt Stress. Int. J. Fruit Sci. 2019, 19, 104–114. [Google Scholar] [CrossRef]
- Demiral, M.A. Effect of Salt Stress on Concentration of Nitrogen and Phosphorus in Root and Leaf of Strawberry Plant. Eurasian J. Soil Sci. EJSS 2017, 6, 357–364. [Google Scholar] [CrossRef]
- Wang, Y.-F.; Hou, X.-Y.; Deng, J.-J.; Yao, Z.-H.; Lyu, M.-M.; Zhang, R.-S. Auxin Response Factor 1 Acts as a Positive Regulator in the Response of Poplar to Trichoderma asperellum Inoculation in Overexpressing Plants. Plants 2020, 9, 272. [Google Scholar] [CrossRef]
- Garnica-Vergara, A.; Barrera-Ortiz, S.; Muñoz-Parra, E.; Raya-González, J.; Méndez-Bravo, A.; Macías-Rodríguez, L.; Ruiz-Herrera, L.F.; López-Bucio, J. The Volatile 6-Pentyl-2H-Pyran-2-One from Trichoderma atroviride Regulates Arabidopsis thaliana Root Morphogenesis via Auxin Signaling and ETHYLENE INSENSITIVE 2 Functioning. New Phytol. 2016, 209, 1496–1512. [Google Scholar] [CrossRef]
- Vico, G.; Way, D.A.; Hurry, V.; Manzoni, S. Can Leaf Net Photosynthesis Acclimate to Rising and More Variable Temperatures? Plant Cell Environ. 2019, 42, 1913–1928. [Google Scholar] [CrossRef]
- Esparza-Reynoso, S.; Ruíz-Herrera, L.F.; Pelagio-Flores, R.; Macías-Rodríguez, L.I.; Martínez-Trujillo, M.; López-Coria, M.; Sánchez-Nieto, S.; Herrera-Estrella, A.; López-Bucio, J. Trichoderma atroviride-Emitted Volatiles Improve Growth of Arabidopsis Seedlings through Modulation of Sucrose Transport and Metabolism. Plant Cell Environ. 2021, 44, 1961–1976. [Google Scholar] [CrossRef] [PubMed]
- Harman, G.E.; Doni, F.; Khadka, R.B.; Uphoff, N. Endophytic Strains of Trichoderma Increase Plants’ Photosynthetic Capability. J. Appl. Microbiol. 2021, 130, 529–546. [Google Scholar] [CrossRef]
- Bononi, L.; Chiaramonte, J.B.; Pansa, C.C.; Moitinho, M.A.; Melo, I.S. Phosphorus-Solubilizing Trichoderma spp. from Amazon Soils Improve Soybean Plant Growth. Sci. Rep. 2020, 10, 2858. [Google Scholar] [CrossRef]
- Singh, R.; Ryu, J.; Kim, S.W. Microbial Consortia Including Methanotrophs: Some Benefits of Living Together. J. Microbiol. Seoul Korea 2019, 57, 939–952. [Google Scholar] [CrossRef] [PubMed]
- Youssef, S.; Tartoura, K.; Abdelraouf, G. Evaluation of Trichoderma harzianum and Serratia proteamaculans Effect on Disease Suppression, Stimulation of ROS-Scavenging Enzymes and Improving Tomato Growth Infected by Rhizoctonia solani. Biol. Control 2016, 100, 79–86. [Google Scholar] [CrossRef]
- Hossain, B.; Rashid, M.H.A. Efficiency of Using Different Organic Manures on Growth, Yield and Quality of Strawberry through Various Planting Media. Trends Sci. 2022, 19, 4484. [Google Scholar] [CrossRef]
- Poveda, J.; Eugui, D. Combined Use of Trichoderma and Beneficial Bacteria (Mainly Bacillus and Pseudomonas): Development of Microbial Synergistic Bio-Inoculants in Sustainable Agriculture. Biol. Control 2022, 176, 105100. [Google Scholar] [CrossRef]
- de Andrade, F.M.; de Assis Pereira, T.; Souza, T.P.; Guimarães, P.H.S.; Martins, A.D.; Schwan, R.F.; Pasqual, M.; Dória, J. Beneficial Effects of Inoculation of Growth-Promoting Bacteria in Strawberry. Microbiol. Res. 2019, 223–225, 120–128. [Google Scholar] [CrossRef]
- Rueda, D.; Valencia, G.; Soria, N.; Rueda Benítez, B.; Bangeppagari, M.; Kundapur, R.; Selvanayagam, M. Effect of Azospirillum spp. and Azotobacter spp. on the Growth and Yield of Strawberry (Fragaria vesca) in Hydroponic System under Different Nitrogen Levels. J. Appl. Pharm. Sci. 2016, 6, 48–54. [Google Scholar] [CrossRef]
- Asghar, W.; Kataoka, R. Effect of Co-Application of Trichoderma spp. with Organic Composts on Plant Growth Enhancement, Soil Enzymes and Fungal Community in Soil. Arch. Microbiol. 2021, 203, 4281–4291. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Shi, Y.; Kong, L.; Tong, L.; Cao, H.; Zhou, H.; Lv, Y. Long-term application of bio-compost increased soil microbial community diversity and altered its composition and network. Microorganisms 2022, 10, 462. [Google Scholar] [CrossRef] [PubMed]
- Leao, L.F.S.; Guzman, T.; Ferguson, L.M.; Fernandez, G.E.; Louws, F.J. Population Dynamics of Trichoderma in Fumigated and Compost-Amended Soil and on Strawberry Roots. Appl. Soil Ecol. 2007, 35, 237–246. [Google Scholar] [CrossRef]
- Kumari, A.; Goyal, R.K.; Choudhary, M.; Sindhu, S.S. Effects of some plant growth promoting rhizobacteria (PGPR) strains on growth and flowering of chrysanthemum. J. Crop Weed 2016, 12, 7–15. [Google Scholar]
- Cipriano, M.A.P.; Freitas, S.S. Effect of Pseudomonas putida on chrysanthemum growth under greenhouse and field conditions. Afr. J. Agric. Res. 2017, 13, 302–310. [Google Scholar] [CrossRef]
- Scotti, R.; Bonanomi, G.; Scelza, R.; Zoina, A.; Rao, M.A. Organic Amendments as Sustainable Tool to Recovery Fertility in Intensive Agricultural Systems. J. Soil Sci. Plant Nutr. 2015, 15, 333–352. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, H.; Dai, Y.; Tian, H.; Zhou, W.; Lv, J. Soil organic carbon transformation and dynamics of microorganisms under different organic amendments. Sci. Total Environ. 2021, 750, 141719. [Google Scholar] [CrossRef]
- Rayne, N.; Aula, L. Livestock Manure and the Impacts on Soil Health: A Review. Soil Syst. 2020, 4, 64. [Google Scholar] [CrossRef]
- Mehraj, H.; Ahsan, M.; Hussain, M.; Rahman, M.; Jamal ddin, A.U. Response of Different Organic Matters in Strawberry. Bangladesh Res. Publ. J. 2014, 10, 151–161. [Google Scholar]
- Apu, S.; Biswas, M.; Bhuiyan, M.; Gomasta, J.; Easmin, S.; Kayesh, E. Effect of Organic Amendments and Arbuscular Mycorrhizal Fungi on Plant Growth, Yield and Quality of Strawberry. Ann. Bangladesh Agric. 2023, 26, 71–82. [Google Scholar] [CrossRef]
- Sayğı, H. Effects of Organic Fertilizer Application on Strawberry (Fragaria vesca L.) Cultivation. Agronomy 2022, 12, 1233. [Google Scholar] [CrossRef]

| Treat. | Factor 1. Microbial Inoculation | Factor 2. Organic Amendments Application |
|---|---|---|
| T1 | Without microbial inoculation (WM) | Without amendments (WA) |
| T2 | Compost (C) | |
| T3 | Manure + Leaf litter (M) | |
| T4 | Trichoderma viride (T) | Without amendments (WA) |
| T5 | Compost (C) | |
| T6 | Manure + Leaf litter (M) | |
| T7 | Pseudomonas putida (P) | Without amendments (WA) |
| T8 | Compost (C) | |
| T9 | Manure + Leaf litter (M) | |
| T10 | Trichoderma viride + Pseudomonas putida (T + P) | Without amendments (WA) |
| T11 | Compost (C) | |
| T12 | Manure + Leaf litter (M) |
| Treatment | Number of Leaves | Plant Height | Leaf Area |
|---|---|---|---|
| per Plant | (cm) | (cm2) | |
| Interaction | n.s. | n.s. | n.s. |
| Factor 1. Microbial inoculation | |||
| WM | 13.06 ± 1.6 a | 11.89 ± 1.2 n.s. | 788 ± 128 ab |
| T | 10.44 ± 2.3 b | 10.73 ± 1.3 | 630 ± 140 c |
| P | 13.24 ± 2.2 a | 11.26 ± 1.4 | 686 ± 133 bc |
| T + P | 13.45 ± 3.4 a | 12.17 ± 1.2 | 847 ± 164 a |
| Factor 2. Organic amendment application | |||
| WA | 11.53 ± 1.4 b | 11.16 ± 1.2 n.s. | 732 ± 158 n.s. |
| C | 14.12 ± 2.7 a | 11.82 ± 1.4 | 780 ± 161 |
| M | 12.00 ± 3.0 ab | 11.55 ± 1.4 | 701 ± 167 |
| Treatment | Fresh Biomass | Dry Biomass | ||
|---|---|---|---|---|
| 90 dat | Harvest | 90 dat | Harvest | |
| Factor 1. Microbial inoculation | ||||
| WM | 37.8 ± 9.7 ab | 90.3 ± 13.2 n.s. | 7.6 ± 2.3 b | 25.2 ± 5.8 n.s. |
| T | 34.3 ± 6.0 b | 92.2 ± 26.5 | 8.3 ± 1.4 b | 25.8 ± 5.3 |
| P | 33.3 ± 6.2 b | 85.3 ± 26.6 | 8.1 ± 1.5 b | 23.9 ± 5.2 |
| T + P | 42.7 ± 8.5 a | 109.4 ± 26.4 | 9.7 ± 1.5 a | 29 ± 6.1 |
| Factor 2. Organic amendment application | ||||
| WA | 34.5 ± 5.8 n.s. | 95.2 ± 16.9 n.s. | 7.9 ± 1.4 b | 25.0 ± 4.4 n.s |
| C | 40.7 ± 8.9 | 93.4 ± 24.1 | 9.5 ± 1.7 a | 27.2 ± 6.2 |
| M | 35.5 ± 8.9 | 93.3 ± 34.6 | 7.6 ± 2.0 b | 25.8 ± 6.6 |
| Treatment | pH | Acidity | Sugars | Firmness | Size |
|---|---|---|---|---|---|
| (g Citric Acid∙100 mL−1) | (Brix°) | (kg) | (cm2) | ||
| Interaction | n.s. | n.s. | n.s. | n.s. | n.s. |
| Factor 1. Microbial inoculation | |||||
| WM | 3.37 ± 0.06 n.s. | 0.71 ± 0.06 n.s. | 13.33 ± 1.80 n.s. | 0.14 ± 0.03 n.s. | 1471 ± 107 n.s. |
| T | 3.33 ± 0.10 | 0.76 ± 0.08 | 12.84 ± 2.22 | 0.15 ± 0.03 | 1451 ± 55 |
| P | 3.38 ± 0.12 | 0.73 ± 0.09 | 13.65 ± 1.83 | 0.13 ± 0.03 | 1541 ± 110 |
| T + P | 3.40 ± 0.04 | 0.69 ± 0.04 | 12.7 ± 2.22 | 0.16 ± 0.09 | 1435 ± 179 |
| Factor 2. Organic amendments application | |||||
| WA | 3.33 ± 0.09 n.s. | 0.76 ± 0.07 a | 12.99 ± 1.77 n.s. | 0.14 ± 0.03 n.s. | 1492 ± 169 n.s. |
| C | 3.39 ± 0.06 | 0.71 ± 0.06 ab | 13.33 ± 2.12 | 0.14 ± 0.04 | 1485 ± 88 |
| M | 3.39 ± 0.09 | 0.69 ± 0.07 b | 13.08 ± 2.17 | 0.14 ± 0.08 | 1447 ± 102 |
| Treatment | Fruit Weight | Yield | |
|---|---|---|---|
| (g Fruit−1) | (g Fruit Plant−1) | (t ha−1) | |
| Interaction | n.s. | n.s. | n.s. |
| Factor 1. Microbial inoculation | |||
| WM | 21.29 ± 1.15 n.s. | 173.6 ± 65.3 n.s. | 9.3 ± 1.1 n.s. |
| T | 20.61 ± 1.23 | 212.9 ± 45.7 | 9.8 ± 1.5 |
| P | 20.96 ± 1.89 | 183.7 ± 73.7 | 8.9 ± 3.0 |
| T + P | 20.36 ± 1.32 | 201.2 ± 84.3 | 10.3 ± 2.0 |
| Factor 2. Organic amendment application | |||
| WA | 20.91 ± 1.60 n.s. | 152.7 ± 78.0 b | 9.3 ± 2.7 n.s. |
| C | 21.10 ± 1.52 | 214.4 ± 48.8 a | 9.9 ± 1.6 |
| M | 20.41 ± 1.09 | 211.4 ± 58.4 a | 9.6 ± 1.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huasasquiche, L.; Ccori, T.; Alejandro, L.; Cántaro-Segura, H.; Samaniego, T.; Solórzano, R. Interaction between Trichoderma sp., Pseudomonas putida, and Two Organic Amendments on the Yield and Quality of Strawberries (Fragaria x annanasa cv. San Andreas) in the Huaral Region, Peru. Appl. Microbiol. 2024, 4, 1110-1123. https://doi.org/10.3390/applmicrobiol4030075
Huasasquiche L, Ccori T, Alejandro L, Cántaro-Segura H, Samaniego T, Solórzano R. Interaction between Trichoderma sp., Pseudomonas putida, and Two Organic Amendments on the Yield and Quality of Strawberries (Fragaria x annanasa cv. San Andreas) in the Huaral Region, Peru. Applied Microbiology. 2024; 4(3):1110-1123. https://doi.org/10.3390/applmicrobiol4030075
Chicago/Turabian StyleHuasasquiche, Lucero, Thania Ccori, Leonela Alejandro, Héctor Cántaro-Segura, Tomás Samaniego, and Richard Solórzano. 2024. "Interaction between Trichoderma sp., Pseudomonas putida, and Two Organic Amendments on the Yield and Quality of Strawberries (Fragaria x annanasa cv. San Andreas) in the Huaral Region, Peru" Applied Microbiology 4, no. 3: 1110-1123. https://doi.org/10.3390/applmicrobiol4030075
APA StyleHuasasquiche, L., Ccori, T., Alejandro, L., Cántaro-Segura, H., Samaniego, T., & Solórzano, R. (2024). Interaction between Trichoderma sp., Pseudomonas putida, and Two Organic Amendments on the Yield and Quality of Strawberries (Fragaria x annanasa cv. San Andreas) in the Huaral Region, Peru. Applied Microbiology, 4(3), 1110-1123. https://doi.org/10.3390/applmicrobiol4030075







