Multiplex-PCR Detection of an Atypical Leuconostoc mesenteroides subsp. jonggajibkimchii Phenotype Dominating the Terminal Spoilage Microbial Association of a Fresh Greek Whey Cheese Stored at 4 °C in Vacuum
Abstract
1. Introduction
2. Materials and Methods
2.1. Whey Cheese Spoilage Isolates and Culture Conditions
2.2. Biochemical Differentiation of the Leuconostoc spp. Isolates
2.3. Differentiation and Identification of the Leuconostoc spp. Isolates by Multiplex-PCR
3. Results
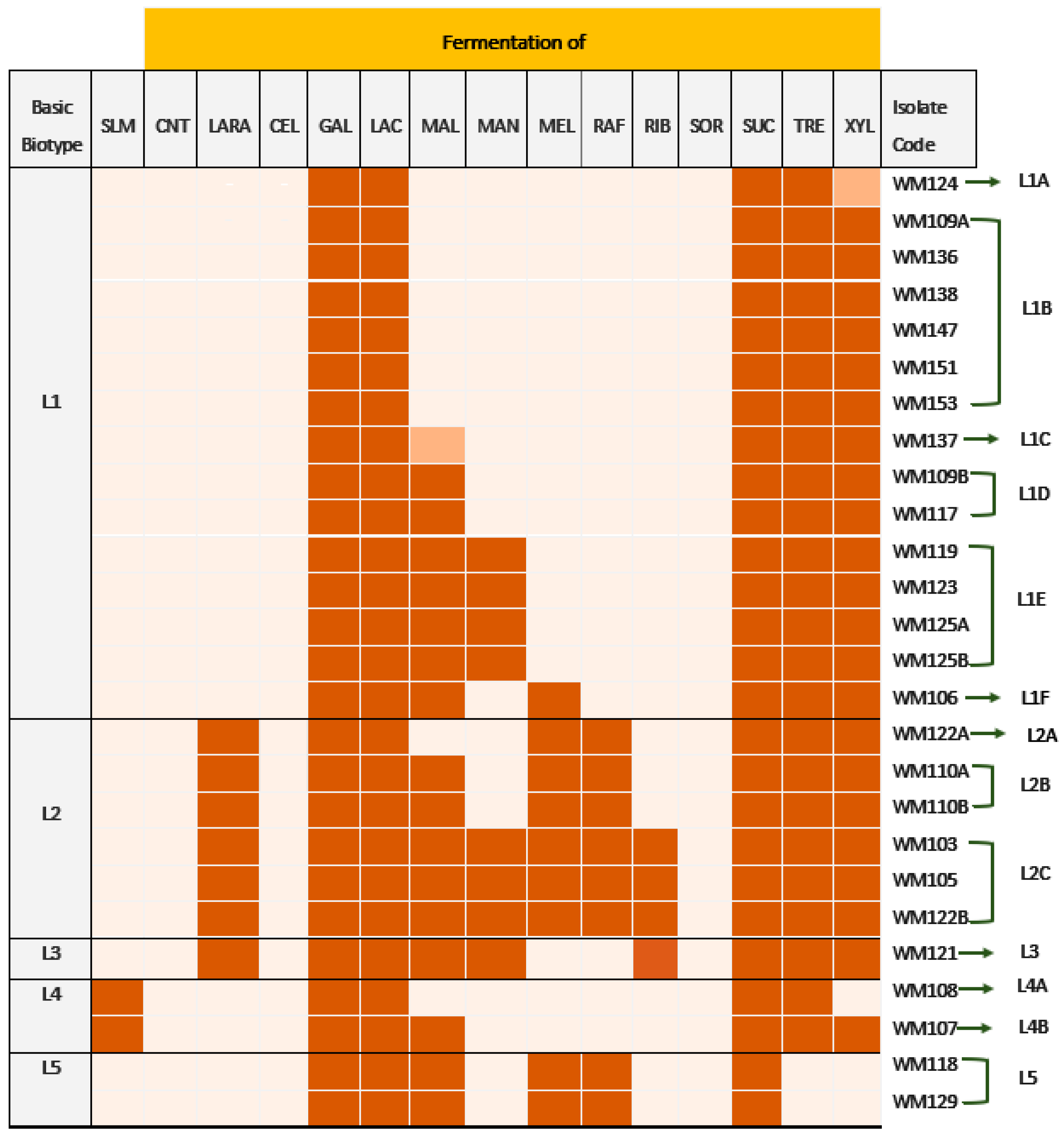
3.1. Biotyping of the Whey Cheese Spoilage Leuconostoc spp. Isolates
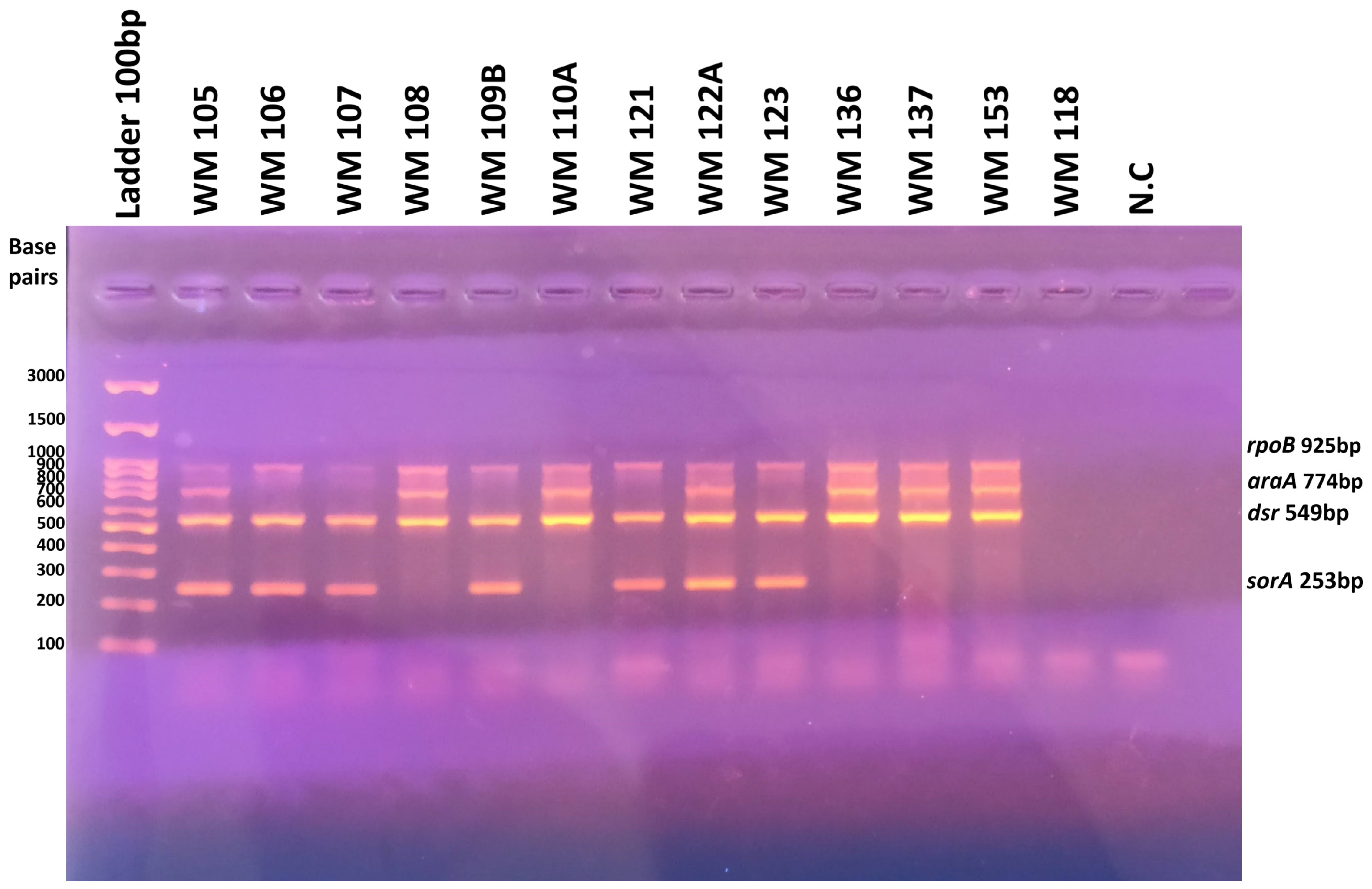
3.2. Classification of the Whey Cheese Spoilage Ln. mesenteroides Isolates by Multiplex-PCR—Prevalence of Isolates with a Gene Profile Specific to Ln. mesenteroides subsp. jonggajibkimchii
| Species Identification/ Isolate Code | Strain Biotype | LAra 4 | Rib 5 | DXyl 6 | Gal 10 | Glu 11 | Fru 12 | Mne 13 | Man 18 | MDG 21 | NAG 22 | Arb 24 | Esc 25 | Sal 26 | Mal 28 | Lac 29 | Mel 30 | Sac 31 | Tre 32 | Raf 35 | Tur 40 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ln. mesenteroides | |||||||||||||||||||||
| WM105 | L2C | + | + | + | + | + | + | + | (+)d | + | + | (+) | + | + | + | + | + | + | + | + | + |
| WM121 | L3 | + | + | + | + | + | + | + | (+)d | + | + | (+) | + | + | + | + | - | + | + | - | + |
| WM119 | L1E | - | - | + | + | + | + | + | (+)d | + | + | (+) | (+) | + | + | + | - | + | + | - | + |
| WM106 | L1F | - | - | + | + | + | + | + | - | + | + | - | (+) | + | + | + | + | + | + | - | + |
| WM107 | L4B | - | - | + | + | + | + | + | - | + | + | - | (+) | - | + | + | - | + | + | - | + |
| WM117 | L1D | - | - | + | + | + | + | + | - | + | + | - | (+) | - | + | + | - | + | + | - | + |
| WM137 | L1C | - | - | + | + | + | + | + | - | - | + | - | - | - | + | + | - | + | + | - | - |
| WM136 | L1B | - | - | + | + | + | + | + | - | - | + | - | - | - | - | + | - | + | + | - | - |
| WM138 | L1B | - | - | + | + | + | + | + | - | - | + | - | - | - | - | + | - | + | + | - | - |
| WM147 | L1B | - | - | + | + | + | + | + | - | - | + | - | - | - | - | + | - | + | + | - | - |
| WM151 | L1B | - | - | + | + | + | + | + | - | - | + | - | - | - | - | + | - | + | + | - | - |
| WM153 | L1B | - | - | + | + | + | + | + | - | - | + | - | - | - | - | + | - | + | + | - | - |
| WM108 | L4A | - | - | - | + | + | + | + | - | - | + | - | - | - | - | + | - | + | + | - | - |
| WM124 | L1A | - | - | - | + | + | + | + | - | - | + | - | - | - | - | + | - | + | + | - | - |
| Ln. lactis | |||||||||||||||||||||
| WM118 | L5 | - | - | - | + | + | + | + | - | - | + | - | - | - | + | + | + | + | - | + | - |
| WM129 | L5 | - | - | - | + | + | + | + | - | - | + | - | - | - | + | + | + | + | - | + | - |
| Species Identification | Strain Code | Target Genes Detected by Multiplex-PCR | Subspecies Identification | Reference | Multiplex Profile | Basic Biotype | Updated Biotype | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Reference strains (literature/our previous data) | rpoB | araA | dsr | sorA | ||||||
| Ln. mesenteroides | ATCC 19254T | + | - | - | - | cremoris | [46] | S1 | NA | NA |
| Ln. mesenteroides | DSM 20484T | + | - | + | - | dextranicum | [46] | S2 | NA | NA |
| Ln. mesenteroides | DRC1506T | + | + | + | - | jonggajibkimchii | [46] | S3 | NA | NA |
| Ln. mesenteroides | ATCC 8293T | + | + | + | + | mesenteroides | [46] | S4 | NA | NA |
| Ln. mesenteroides | ACA-DC 0750 | + | + | + | + | mesenteroides | [45] | S4 | NA | NA |
| Ln. mesenteroides | ACA-DC 0493 | + | - | + | - | dextranicum | [45] | S2 | NA | NA |
| Ln. mesenteroides | ACA-DC 0231 | + | - | + | + | dextranicum (atypical) | [45] | S5 | NA | NA |
| Anthotyros cheese strains | This study | |||||||||
| Ln. mesenteroides | WM124 | + | - | + | + | dextranicum (atypical) | S5 | L1 | L1A | |
| Ln. mesenteroides | WM109A | + | + | + | - | jonggajibkimchii | S3 | L1 | L1B | |
| Ln. mesenteroides | WM136 | + | + | + | - | jonggajibkimchii | S3 | L1 | L1B | |
| Ln. mesenteroides | WM138 | + | + | + | - | jonggajibkimchii | S3 | L1 | L1B | |
| Ln. mesenteroides | WM147 | + | + | + | - | jonggajibkimchii | S3 | L1 | L1B | |
| Ln. mesenteroides | WM151 | + | + | + | - | jonggajibkimchii | S3 | L1 | L1B | |
| Ln. mesenteroides | WM153 | + | + | + | - | jonggajibkimchii | S3 | L1 | L1B | |
| Ln. mesenteroides | WM137 | + | + | + | - | jonggajibkimchii | S3 | L1 | L1C | |
| Ln. mesenteroides | WM109B | + | - | + | + | dextranicum (atypical) | S5 | L1 | L1D | |
| Ln. mesenteroides | WM117 | + | - | + | + | dextranicum (atypical) | S5 | L1 | L1D | |
| Ln. mesenteroides | WM119 | + | - | + | + | dextranicum (atypical) | S5 | L1 | L1E | |
| Ln. mesenteroides | WM123 | + | - | + | + | dextranicum (atypical) | S5 | L1 | L1E | |
| Ln. mesenteroides | WM125A | + | - | + | + | dextranicum (atypical) | S5 | L1 | L1E | |
| Ln. mesenteroides | WM125B | + | - | + | + | dextranicum (atypical) | S5 | L1 | L1E | |
| Ln. mesenteroides | WM106 | + | - | + | + | dextranicum (atypical) | S5 | L1 | L1F | |
| Ln. mesenteroides | WM122A | + | + | + | + | mesenteroides | S4 | L2 | L2A | |
| Ln. mesenteroides | WM110A | + | + | + | - | jonggajibkimchii | S3 | L2 | L2B | |
| Ln. mesenteroides | WM110B | + | + | + | - | jonggajibkimchii | S3 | L2 | L2B | |
| Ln. mesenteroides | WM103 | + | + | + | + | mesenteroides | S4 | L2 | L2C | |
| Ln. mesenteroides | WM105 | + | + | + | + | mesenteroides | S4 | L2 | L2C | |
| Ln. mesenteroides | WM122B | + | + | + | + | mesenteroides | S4 | L2 | L2C | |
| Ln. mesenteroides | WM121 | + | - | + | + | dextranicum (atypical) | S5 | L3 | L3 | |
| Ln. mesenteroides | WM108 | + | + | + | - | jonggajibkimchii | S3 | L4 | L4A | |
| Ln. mesenteroides | WM107 | + | - | + | + | dextranicum (atypical) | S5 | L4 | L4B | |
| Ln. lactis | WM118 | - | - | - | - | None (N/A) | No bands | L5 | L5 | |
| Ln. lactis | WM129 | - | - | - | - | None (N/A) | No bands | L5 | L5 | |
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Montel, M.-C.; Buchin, S.; Mallet, A.; Delbés-Paus, C.; Vuitton, D.A.; Desmasures, N.; Berthier, F. Traditional cheeses: Rich and diverse microbiota with associated benefits. Int. J. Food Microbiol. 2014, 177, 136–154. [Google Scholar] [CrossRef] [PubMed]
- Aryana, K.J.; Olson, D.W. A 100-year review: Yogurt and other cultured dairy products. J. Dairy Sci. 2017, 100, 9987–10013. [Google Scholar] [CrossRef] [PubMed]
- Coelho, M.C.; Malcata, F.X.; Silva, C.C.G. Lactic acid bacteria in raw-milk cheeses: From starter cultures to probiotic functions. Foods 2022, 11, 2276. [Google Scholar] [CrossRef] [PubMed]
- Schillinger, U.; Holzapfel, W.H.; Björkroth, K.J. Lactic acid bacteria. In Food Spoilage Microorganisms; Blackburn, C.W., Ed.; Woodhead Publishing Limited: Cambridge, UK, 2006; pp. 541–578. [Google Scholar]
- Hassan, A.N. Possibilities and challenges of exopolysaccharide-producing lactic cultures in dairy foods. J. Dairy Sci. 2008, 91, 1282–1298. [Google Scholar] [CrossRef] [PubMed]
- Quiberoni, A.; Guglielmotti, D.; Reinheimer, J. New and classical spoilage bacteria causing widespread blowing in Argentinean soft and semihard cheeses. Int. J. Dairy Technol. 2008, 61, 358–363. [Google Scholar] [CrossRef]
- Gobbetti, M.; De Angelis, M.; Di Cagno, R.; Mancini, L.; Fox, P.F. Pros and cons for using non-starter lactic acid bacteria (NSLAB) as secondary/adjunct starters for cheese ripening. Trends Food Sci. Technol. 2015, 45, 167–178. [Google Scholar] [CrossRef]
- Machado, S.G.; Bagliniére, F.; Marchsand, S.; Van Coillie, E.; Vanetti, M.C.D.; De Block, J.; Heyndrickx, M. The biodiversity of the microbiota producing heat-resistant enzymes responsible for spoilage in processed bovine milk and dairy products. Front. Microbiol. 2017, 8, 302. [Google Scholar] [CrossRef] [PubMed]
- Quigley, L.; O’Sullivan, O.; Stanton, C.; Beresford, T.P.; Ross, R.P.; Fitzgerald, G.F.; Cotter, P.D. The complex microbiota of raw milk. FEMS Microbiol. Rev. 2013, 37, 664–698. [Google Scholar] [CrossRef] [PubMed]
- Boor, K.; Fromm, H. Managing microbial spoilage in the dairy industry. In Food Spoilage Microorganisms; Blackburn, C.W., Ed.; Woodhead Publishing Limited: Cambridge, UK, 2006; pp. 171–193. [Google Scholar]
- Doyle, C.J.; Gleeson, D.; Jordan, K.; Beresford, T.P.; Ross, R.P.; Fitzgerald, G.F.; Cotter, P.D. Anaerobic sporeformers and their significance with respect to milk and dairy products. Int. J. Food Microbiol. 2015, 197, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Sadiq, F.A.; Burmølle, M.; Wang, N.I.; He, G.Q. Insights into psychrotrophic bacteria in raw milk: A review. J. Food Prot. 2019, 82, 1148–1159. [Google Scholar] [CrossRef] [PubMed]
- Odeyemi, O.A.; Alegbeleye, O.O.; Strateva, M.; Stratev, D. Understanding spoilage microbial community and spoilage mechanisms in foods of animal origin. Comp. Rev. Food Sci. Food Saf. 2020, 19, 311–331. [Google Scholar] [CrossRef] [PubMed]
- Pintado, M.E.; Macedo, A.C.; Malcata, F.X. Review: Technology, chemistry and microbiology of whey cheeses. Food Sci. Technol. Int. 2001, 7, 105–116. [Google Scholar] [CrossRef]
- Hough, G.; Puglieso, M.L.; Sanchez, R.; Da Silva, O.M. Sensory and microbiological shelf-life of a commercial Ricotta cheese. J. Dairy Sci. 1999, 82, 454–459. [Google Scholar] [CrossRef]
- Pala, C.; Scarano, C.; Venusti, M.; Sardo, D.; Casti, D.; Cossu, F.; Lamon, S.; Spanu, V.; Ibba, M.; Marras, M. Shelf life evaluation of Ricotta Fresca sheep cheese in modified atmosphere packaging. Ital. J. Food Saf. 2016, 5, 5502. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sattin, E.; Andreani, N.A.; Carraro, L.; Fasolato, L.; Balzan, S.; Novelli, E.; Squartini, A.; Telatin, A.; Simionati, B.; Cardazzo, B. Microbial dynamics during shelf-life of industrial Ricotta cheese and identification of a Bacillus strain as a cause of a pink discolouration. Food Microbiol. 2016, 57, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Spanu, C.; Scarano, C.; Spanu, V.; Pala, C.; Casti, D.; Lamon, S.; Cossu, F.; Ibba, M.; Nieddu, G.; De Santis, E.P.L. Occurrence and behavior of Bacillus cereus in naturally contaminated Ricotta Salata cheese during refrigerated storage. Food Microbiol. 2016, 58, 135–138. [Google Scholar] [CrossRef]
- Di Pierro, P.; Sorrentino, A.; Mariniello, L.; Giosafatto, C.V.L.; Porta, R. Chitosan/whey protein film as active coating to extend Ricotta cheese shelf-life. LWT-Food Sci. Technol. 2011, 44, 2324–2327. [Google Scholar] [CrossRef]
- Spanu, C.; Piras, F.; Mocci, A.M.; Nieddu, G.; De Santis, E.P.L.; Scarano, C. Use of Carnobacterium spp. protective culture in MAP packed Ricotta Fresca cheese to control Pseudomonas spp. Food Microbiol. 2018, 74, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Sameli, N.; Sioziou, E.; Bosnea, L.; Kakouri, A.; Samelis, J. Assessment of the spoilage microbiota during refrigerated (4 °C) vacuum-packed storage of fresh Greek Anthotyros whey cheese without or with a crude enterocin A-B-P-containing extract. Foods 2021, 10, 2946. [Google Scholar] [CrossRef] [PubMed]
- Gu, C.T.; Wang, F.; Li, C.Y.; Liu, F.; Huo, G.C. Leuconostoc mesenteroides subsp. suionicum subsp. nov. Int. J. Syst. Evol. Microbiol. 2012, 62, 1548–1551. [Google Scholar] [CrossRef] [PubMed]
- Jeon, H.H.; Kim, K.H.; Chun, B.H.; Ryu, B.H.; Han, N.S.; Jeon, C.O. A proposal of Leuconostoc mesenteroides subsp. jonggajibkimchii subsp. nov. and reclassification of Leuconostoc mesenteroides subsp. suionicum (Gu et al., 2012) as Leuconostoc suionicum sp. nov. based on complete genome sequences. Int. J. Syst. Evol. Microbiol. 2017, 67, 2225–2230. [Google Scholar] [PubMed]
- Hemme, D.; Foucaud-Scheunemann, C. Leuconostoc, characteristics, use in dairy technology and prospects in functional foods. Int. Dairy J. 2004, 14, 467–494. [Google Scholar] [CrossRef]
- Pogačić, T.; Chuat, V.; Madec, M.-N.; Samaržija, D.; Lortal, S.; Valence, F. Phenotypic traits of genetically closely related Leuconostoc spp. Int. Dairy J. 2014, 39, 96–101. [Google Scholar] [CrossRef]
- Terzić-Vidojević, A.; Mihajlovic, S.; Uzelac, G.; Veljović, K.; Tolinački, M.; Nikolic, M.; Topisirovic, L.; Kojic, M. Characterization of lactic acid bacteria isolated from artisanal Travnik young cheeses, sweet creams and sweet kajmaks over four seasons. Food Microbiol. 2014, 39, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Juanes, F.; Teixeira-Martín, V.; González-Buitrago, J.M.; Velázquez, E.; Flores-Félix, J.D. Identification of species and subspecies of lactic acid bacteria present in Spanish cheeses type “Torta” by MALDI-TOF MS and pheS gene analyses. Microorganisms 2020, 8, 301. [Google Scholar] [CrossRef] [PubMed]
- Terzić-Vidojević, A.; Veljović, K.; Tolinački, M.; Živković, M.; Lukić, J.; Lozo, J.; Fira, Đ.; Jovčić, B.; Strahinić, I.; Begović, J.; et al. Diversity of non-starter lactic acid bacteria in autochthonous dairy products from Western Balkan countries—technological and probiotic properties. Food Res. Int. 2020, 136, 109494. [Google Scholar] [CrossRef] [PubMed]
- Ruppitsch, W.; Nisic, A.; Hyden, P.; Cabal, A.; Sucher, J.; Stöger, A.; Allerberger, F.; Martinović, A. Genetic diversity of Leuconostoc mesenteroides isolates from traditional Montenegrin brine cheese. Microorganisms 2021, 9, 1612. [Google Scholar] [CrossRef]
- Samelis, J.; Kakouri, A.; Pappa, E.C.; Matijašic, B.B.; Georgalaki, M.D.; Tsakalidou, E.; Rogelj, I. Microbial stability and safety of traditional Greek Graviera cheese: Characterization of the lactic acid bacterial flora and culture-independent detection of bacteriocin genes in the ripened cheeses and their microbial consortia. J. Food Prot. 2010, 73, 1294–1303. [Google Scholar] [CrossRef] [PubMed]
- Litopoulou-Tzanetaki, E.; Tzanetakis, N. The microfloras of traditional Greek cheeses. Microbiol. Spectr. 2014, 2, CM-0009-2012. [Google Scholar] [CrossRef]
- Vandera, E.; Kakouri, A.; Koukkou, A.-I.; Samelis, J. Major ecological shifts within the dominant non starter lactic acid bacteria in mature Greek Graviera cheese as affected by the starter culture type. Int. J. Food Microbiol. 2019, 290, 15–26. [Google Scholar] [CrossRef]
- Gantzias, C.; Lappa, I.K.; Aerts, M.; Georgalaki, M.; Manolopoulou, E.; Papadimitriou, K.; De Brandt, E.; Tsakalidou, E.; Vandamme, P. MALDI-TOF MS profiling of non-starter lactic acid bacteria from artisanal cheeses of the Greek island of Naxos. Int. J. Food Microbiol. 2020, 323, 108586. [Google Scholar] [CrossRef] [PubMed]
- Zoumpopoulou, G.; Papadimitriou, K.; Alexandraki, V.; Mavrogonatou, E.; Alexopoulou, K.; Anastasiou, R.; Georgalaki, M.; Kletsas, D.; Tsakalidou, E.; Giaouris, E. The microbiota of Kalathaki and Melichloro Greek artisanal cheeses comprises functional lactic acid bacteria. LWT 2020, 130, 109570. [Google Scholar] [CrossRef]
- Apostolakos, I.; Paramithiotis, S.; Mataragas, M. Comparative genomic analysis reveals the functional traits and safety status of lactic acid bacteria retrieved from artisanal cheeses and raw sheep milk. Foods 2023, 12, 599. [Google Scholar] [CrossRef] [PubMed]
- Frantzen, C.A.; Kot, W.; Pedersen, T.B.; Ardö, Y.M.; Broadbent, J.R.; Neve, H.; Hansen, L.H.; Dal Bello, F.; Østlie, H.M.; Kleppen, H.P. Genomic characterization of dairy associated Leuconostoc species and diversity of leuconostocs in undefined mixed mesophilic starter cultures. Front. Microbiol. 2017, 8, 132. [Google Scholar] [CrossRef] [PubMed]
- Paramithiotis, S.; Kouretas, K.; Drosinos, E.H. Effect of ripening stage on the development of the microbial community during spontaneous fermentation of green tomatoes. J. Sci. Food Agric. 2014, 94, 1600–1606. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Liu, S.; Ma, J.; Xu, X.; Wang, H. Evaluation of the spoilage heterogeneity of meat-borne Leuconostoc mesenteroides by metabonomics and in-situ analysis. Food Res. Int. 2022, 156, 111365. [Google Scholar] [CrossRef] [PubMed]
- Holzapfel, W.H.; Björkroth, J.A.; Dicks, L.M.T. Genus I Leuconostoc van Tieghem 1878, 198 AL. In Bergey’s Manual of Systematic Bacteriology, The Firmicutes, 2nd ed.; Whitman, W.B., Ed.; Springer: New York, NY, USA, 2009; Volume 3, pp. 624–635. [Google Scholar]
- De Paula, A.T.; Jeronymo-Ceneviva, A.B.; Todorov, S.D.; Penna, A.L.B. The two faces of Leuconostoc mesenteroides in food systems. Food Rev. Int. 2015, 31, 147–171. [Google Scholar] [CrossRef]
- Samelis, J. Managing microbial spoilage in the meat industry. In Food Spoilage Microorganisms; Blackburn, C.W., Ed.; Woodhead Publishing Limited: Cambridge, UK, 2006; pp. 213–286. [Google Scholar]
- Iulietto, M.F.; Sechi, P.; Borgogni, E.; Cenci-Goga, B.T. Meat spoilage: A critical review of a neglected alteration due to ropy slime producing bacteria. Ital. J. Anim. Sci. 2015, 14, 4011. [Google Scholar] [CrossRef]
- Chun, B.H.; Kim, K.H.; Jeon, H.H.; Lee, S.H.; Jeon, C.O. Pan-genomic and transcriptomic analyses of Leuconostoc mesenteroides provide insights into its genomic and metabolic features and roles in kimchi fermentation. Sci. Rep. 2017, 7, 11504. [Google Scholar] [CrossRef] [PubMed]
- Rai, R.; Tamang, J.P. In vitro and genetic screening of probiotic properties of lactic acid bacteria isolated from naturally fermented cow-milk and yak-milk products of Sikkim, India. World J. Microbiol. Biotechnol. 2022, 38, 25. [Google Scholar] [CrossRef] [PubMed]
- Sioziou, E.; Kakouri, A.; Bosnea, L.; Samelis, J. Antilisterial activity of raw sheep milk from two native Epirus breeds: Culture-dependent identification, bacteriocin gene detection and primary safety evaluation of the antagonistic LAB biota. Curr. Res. Microb. Sci. 2024, 6, 100209. [Google Scholar] [CrossRef] [PubMed]
- Ricciardi, A.; Storti, L.V.; Zotta, T.; Felis, G.E.; Parente, E. Analysis of rpoB polymorphism and PCR-based approaches for the identification of Leuconostoc mesenteroides at the species and subspecies level. Int. J. Food Microbiol. 2020, 318, 108474. [Google Scholar] [CrossRef] [PubMed]
- Querol, A.; Barrio, E.; Huerta, T.; Ramon, D. Molecular monitoring of wine fermentations conducted by active dry yeast strains. Appl. Environ. Microbiol. 1992, 58, 2948–2953. [Google Scholar] [CrossRef] [PubMed]
- Tsafrakidou, P.; Sameli, N.; Bosnea, L.; Chorianopoulos, N.; Samelis, J. Assessment of the spoilage microbiota in minced free-range chicken meat during storage at 4 °C in retail modified atmosphere packages. Food Microbiol. 2021, 99, 103822. [Google Scholar] [CrossRef] [PubMed]
- Garvie, E.I. Proposal of neotype strains for Leuconostoc mesenteroides (Tsenkovskii) van Tieghem, Leuconostoc dextranicum (Beijerinck) Hucker and Pederson, and Leuconostoc cremoris (Knudsen and Sørensen) Garvie. Int. J. Syst. Bacteriol. 1979, 29, 149–151. [Google Scholar] [CrossRef]
- Garvie, E.I. Leuconostoc mesenteroides subsp. cremoris (Knudsen and Sørensen) comb. nov. and Leuconostoc mesenteroides subsp. dextranicum (Beijerinck) comb. nov. Int. J. Syst. Bacteriol. 1983, 33, 118–119. [Google Scholar]
- Raimondi, S.; Candeliere, F.; Amaretti, A.; Costa, S.; Vertuani, S.; Spampinato, G.; Rossi, M. Phylogenomic analysis of the genus Leuconostoc. Front. Microbiol. 2022, 13, 897656. [Google Scholar] [CrossRef] [PubMed]
- Kalogridou-Vassiliadou, D.; Tzanetakis, N.; Litopoulou-Tzanetaki, E. Microbiological and physico-chemical characteristics of “Anthotyro”, a Greek traditional whey cheese. Food Microbiol. 1994, 11, 15–19. [Google Scholar] [CrossRef]
- Cibik, R.; Lepage, E.; Tailliez, P. Molecular diversity of Leuconostoc mesenteroides and Leuconostoc citreum isolated from traditional French cheeses as revealed by RAPD fingerprinting, 16S rDNA sequencing and 16S rDNA fragment amplification. Syst. Appl. Microbiol. 2000, 23, 267–278. [Google Scholar] [CrossRef] [PubMed]
- Samelis, J.; Kakouri, A. Growth inhibitory and selective pressure effects of sodium diacetate on the spoilage microbiota of frankfurters stored at 4 °C and 12 °C in vacuum. Foods 2021, 10, 74. [Google Scholar] [CrossRef]
- Villani, F.; Moschetti, G.; Blaiotta, G.; Coppola, S. Characterization of strains of Leuconostoc mesenteroides by analysis of soluble whole-cell protein pattern, DNA fingerprinting and restriction of ribosomal DNA. J. Appl. Microbiol. 1997, 82, 578–588. [Google Scholar] [CrossRef] [PubMed]
- Moschetti, G.; Blaiotta, G.; Villani, F.; Coppola, S. Specific detection of Leuconostoc mesenteroides subsp. mesenteroides with DNA primers identified by randomly amplified polymorphic DNA analysis. Appl. Environ. Microbiol. 2000, 66, 422–424. [Google Scholar] [PubMed]
- Papatsaroucha, E.; Pavlidou, S.; Hatzikamari, M.; Lazaridou, A.; Torriani, S.; Gerasopoulos, D.; Litopoulou-Tzanetaki, E. Preservation of pears in water in the presence of Sinapis arvensis seeds: A Greek tradition. Int. J. Food Microbiol. 2012, 159, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Kaur, J.; Lee, S.; Park, Y.S. Genetic diversity analysis of Leuconostoc mesenteroides from Korean vegetables and food products by multilocus sequence typing. Appl. Microbiol. Biotechnol. 2018, 102, 4853–4861. [Google Scholar] [CrossRef] [PubMed]
- Biruk, A.M.; Furik, N.N.; Tarashkevich, Y.S.; Savelyeva, T.A. Construction of specific primers for identification of Leuconostoc mesenteroides subspecies. Proc. Nat. Acad. Sci. USA 2020, 58, 244–256. [Google Scholar] [CrossRef]
- Zeller-Peronnet, V.; Brockmann, E.; Pavlovic, M.; Timke, M.; Busch, U.; Huber, I. Potential and limitations of MALDI-TOF MS for discrimination within the species Leuconostoc mesenteroides and Leuconostoc pseudomesenteroides. J. Consum. Prot. Food Saf. 2013, 8, 205–214. [Google Scholar] [CrossRef]
- Hantsis-Zacharov, E.; Halpern, M. Culturable psychrotrophic bacterial communities in raw milk and their proteolytic and lipolytic traits. Appl. Environ. Microbiol. 2007, 73, 7162–7168. [Google Scholar] [CrossRef] [PubMed]
- Sameli, N.; Samelis, J. Growth and biocontrol of Listeria monocytogenes in Greek Anthotyros whey cheese without or with a crude enterocin A-B-P extract: Interactive effects of the native spoilage microbiota during vacuum-packed storage at 4 °C. Foods 2022, 11, 334. [Google Scholar] [CrossRef] [PubMed]
- Bassi, D.; Gazzola, S.; Sattin, E.; Dal Bello, F.; Simionati, B.; Cocconcelli, P.S. Lactic acid bacteria adjunct cultures exert a mitigation effect against spoilage microbiota in fresh cheese. Microorganisms 2020, 8, 1199. [Google Scholar] [CrossRef] [PubMed]
- Madureira, A.R.; Pintado, M.E.; Gomes, A.M.P.; Malcata, F.X. Incorporation of probiotic bacteria in whey cheese: Decreasing the risk of microbial contamination. J. Food Prot. 2011, 74, 1194–1199. [Google Scholar] [CrossRef] [PubMed]
- Silva, C.C.G.; Silva, S.P.M.; Ribeiro, S.C. Application of bacteriocins and protective cultures in dairy food preservation. Front. Microbiol. 2018, 9, 594. [Google Scholar] [CrossRef]
- Yi, Y.; Li, P.; Zhao, F.; Zhang, T.; Shan, Y.; Wang, X.; Liu, B.; Chen, Y.; Zhao, X.; Lu, X. Current status and potentiality of class II bacteriocins from lactic acid bacteria: Structure, mode of action and applications in the food industry. Trends Food Sci. Technol. 2022, 120, 387–401. [Google Scholar] [CrossRef]
- Dimov, S. The controversial nature of some non-starter lactic acid bacteria actively participating in cheese ripening. BioTech 2023, 12, 63. [Google Scholar] [CrossRef] [PubMed]
- Campedelli, I.; Flórez, A.B.; Salvetti, E.; Delgado, S.; Orrù, L.; Cattivelli, L.; Alegria, A.; Fellis, G.E.; Torriani, S.; Mayo, B. Draft genome sequence of three antibiotic-resistant Leuconostoc mesenteroides strains of dairy origin. Gen. Announc. 2015, 3, e01018-15. [Google Scholar] [CrossRef] [PubMed]
| Group/ Isolate Biotype | Cheese Batch/ Treatment | Isolate Code 2 | Species Identification | Closest Ref. Strain in BLAST | 16S rRNA Gene Seq Similarity |
|---|---|---|---|---|---|
| L1 | C/CN | WM106 | Ln. mesenteroides | MT545072.1 | 100 |
| C/CN | WM109A | Ln. mesenteroides | NT | - | |
| C/CN | WM109B | Ln. mesenteroides | MT545072.1 | 100 | |
| C/Ent+ | WM117 | Ln. mesenteroides | NT | - | |
| C/Ent+ | WM119 | Ln. mesenteroides | NT | - | |
| C/Ent+ | WM123 | Ln. mesenteroides | MT545101.1 | 100 | |
| C/Ent+ | WM124 | Ln. mesenteroides | NT | - | |
| C/Ent+ | WM125A | Ln. mesenteroides | NT | - | |
| C/Ent+ | WM125B | Ln. mesenteroides | NT | - | |
| D/CN | WM136 | Ln. mesenteroides | MT545072.1 | 100 | |
| D/CN | WM137 | Ln. mesenteroides | MT545072.1 | 100 | |
| D/CN | WM138 | Ln. mesenteroides | NT | - | |
| D/Ent+ | WM147 | Ln. mesenteroides | NT | - | |
| D/Ent+ | WM151 | Ln. mesenteroides | NT | - | |
| D/Ent+ | WM153 | Ln. mesenteroides | MT545072.1 | 100 | |
| L2 | C/CN | WM103 | Ln. mesenteroides | NT | - |
| C/CN | WM105 | Ln. mesenteroides | MT545072.1 | 100 | |
| C/CN | WM110A | Ln. mesenteroides | MT545113.1 | 100 | |
| C/CN | WM110B | Ln. mesenteroides | NT | - | |
| C/Ent+ | WM122A | Ln. mesenteroides | MT545072.1 | 100 | |
| C/Ent+ | WM122B | Ln. mesenteroides | NT | - | |
| L3 | C/Ent+ | WM121 | Ln. mesenteroides | MT545113.1 | 100 |
| L4 | C/CN | WM107 | Ln. mesenteroides | MT545113.1 | 100 |
| C/CN | WM108 | Ln. mesenteroides | MT545072.1 | 100 | |
| L5 | C/Ent+ | WM118 | Ln. lactis | MF354765.1 | 100 |
| C/Ent+ | WM129 | Ln. lactis | NT | - |
| Gene | Primer | Sequence (5′-3′) | Amplicon Size (bp) | Annealing Temperature (°C) | Reference |
|---|---|---|---|---|---|
| rpoB | rpob-F | GTCCGCATTGATCGCACGC | 952 | 60 | Ricciardi et al. [46] |
| rpob-R | CACCCGGTCCAAGAGCTGAC | ||||
| araA | L-ara-F | TTTGGCTGGACGGTTGACT | 744 | ||
| L-ara-R | TGTTGTGTGATGTCCGCCAC | ||||
| dsr | dextran-F | TGGCACCATTACCATAACGAACT | 549 | ||
| dextran-R | TGCCAGCAGTCGATCAATATGG | ||||
| sorA | PTS-sorb-F | GTGCCTTACTCCCCTGTGTAG | 253 | ||
| PTS-sorb-R | TCCTCGTCTTCCTCATCATCGT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sameli, N.; Sioziou, E.; Bosnea, L.; Paramithiotis, S.; Samelis, J. Multiplex-PCR Detection of an Atypical Leuconostoc mesenteroides subsp. jonggajibkimchii Phenotype Dominating the Terminal Spoilage Microbial Association of a Fresh Greek Whey Cheese Stored at 4 °C in Vacuum. Appl. Microbiol. 2024, 4, 1124-1141. https://doi.org/10.3390/applmicrobiol4030076
Sameli N, Sioziou E, Bosnea L, Paramithiotis S, Samelis J. Multiplex-PCR Detection of an Atypical Leuconostoc mesenteroides subsp. jonggajibkimchii Phenotype Dominating the Terminal Spoilage Microbial Association of a Fresh Greek Whey Cheese Stored at 4 °C in Vacuum. Applied Microbiology. 2024; 4(3):1124-1141. https://doi.org/10.3390/applmicrobiol4030076
Chicago/Turabian StyleSameli, Nikoletta, Eleni Sioziou, Loulouda Bosnea, Spiros Paramithiotis, and John Samelis. 2024. "Multiplex-PCR Detection of an Atypical Leuconostoc mesenteroides subsp. jonggajibkimchii Phenotype Dominating the Terminal Spoilage Microbial Association of a Fresh Greek Whey Cheese Stored at 4 °C in Vacuum" Applied Microbiology 4, no. 3: 1124-1141. https://doi.org/10.3390/applmicrobiol4030076
APA StyleSameli, N., Sioziou, E., Bosnea, L., Paramithiotis, S., & Samelis, J. (2024). Multiplex-PCR Detection of an Atypical Leuconostoc mesenteroides subsp. jonggajibkimchii Phenotype Dominating the Terminal Spoilage Microbial Association of a Fresh Greek Whey Cheese Stored at 4 °C in Vacuum. Applied Microbiology, 4(3), 1124-1141. https://doi.org/10.3390/applmicrobiol4030076








