Effect of Enterocins A and B on the Viability and Virulence Gene Expression of Listeria monocytogenes in Sliced Dry-Cured Ham
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microorganisms and Culture Conditions
2.2. Enterocins Extract
2.3. Dry-Cured Ham Samples
2.4. L. monocytogenes Enumeration
2.5. RNA Extraction and Retrotranscription
2.6. L. monocytogenes Relative Gene Expression
2.7. Data and Statistical Analysis
3. Results and Discussion
3.1. Effect of Enterocins on L. monocytogenes Population
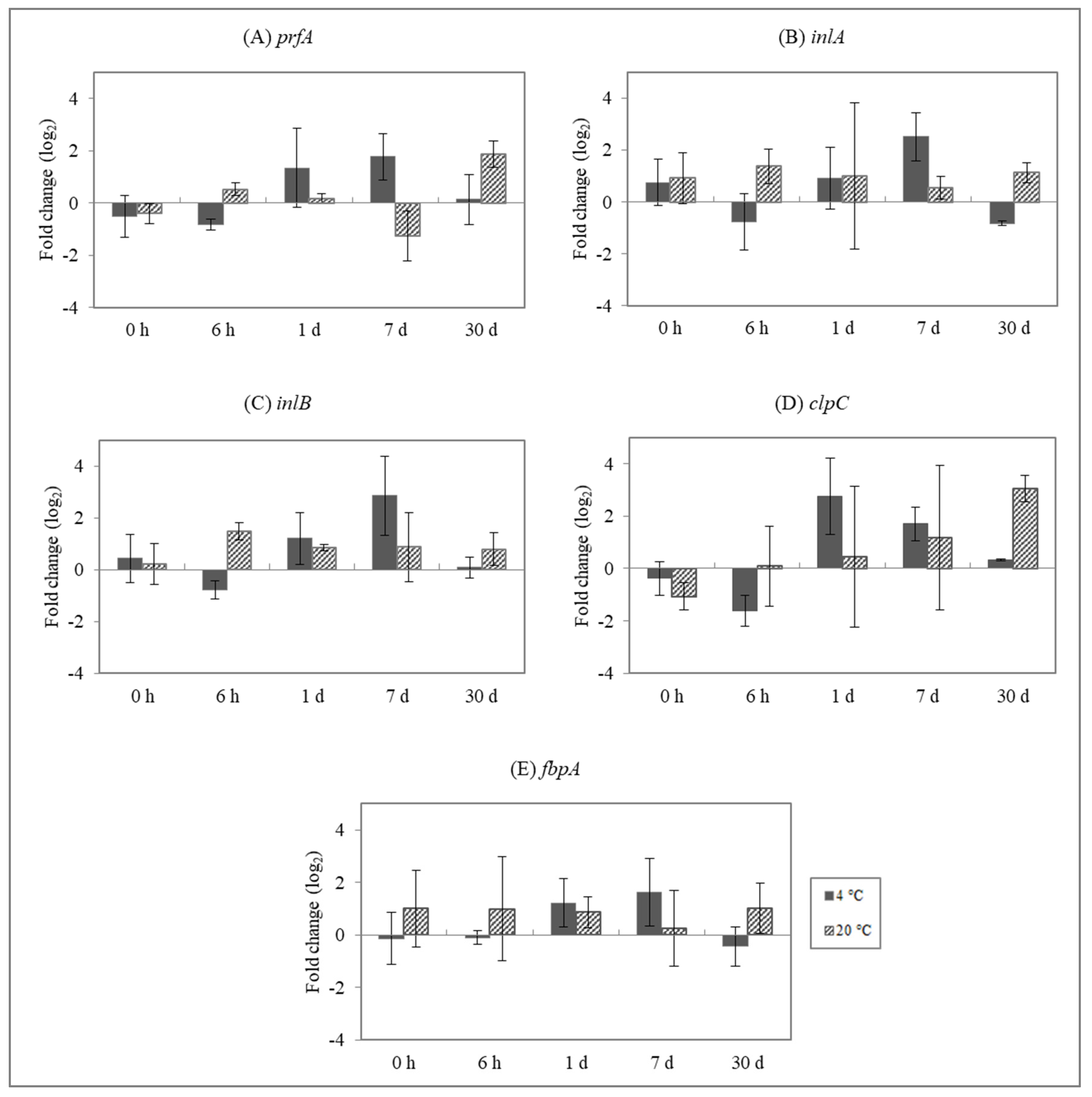
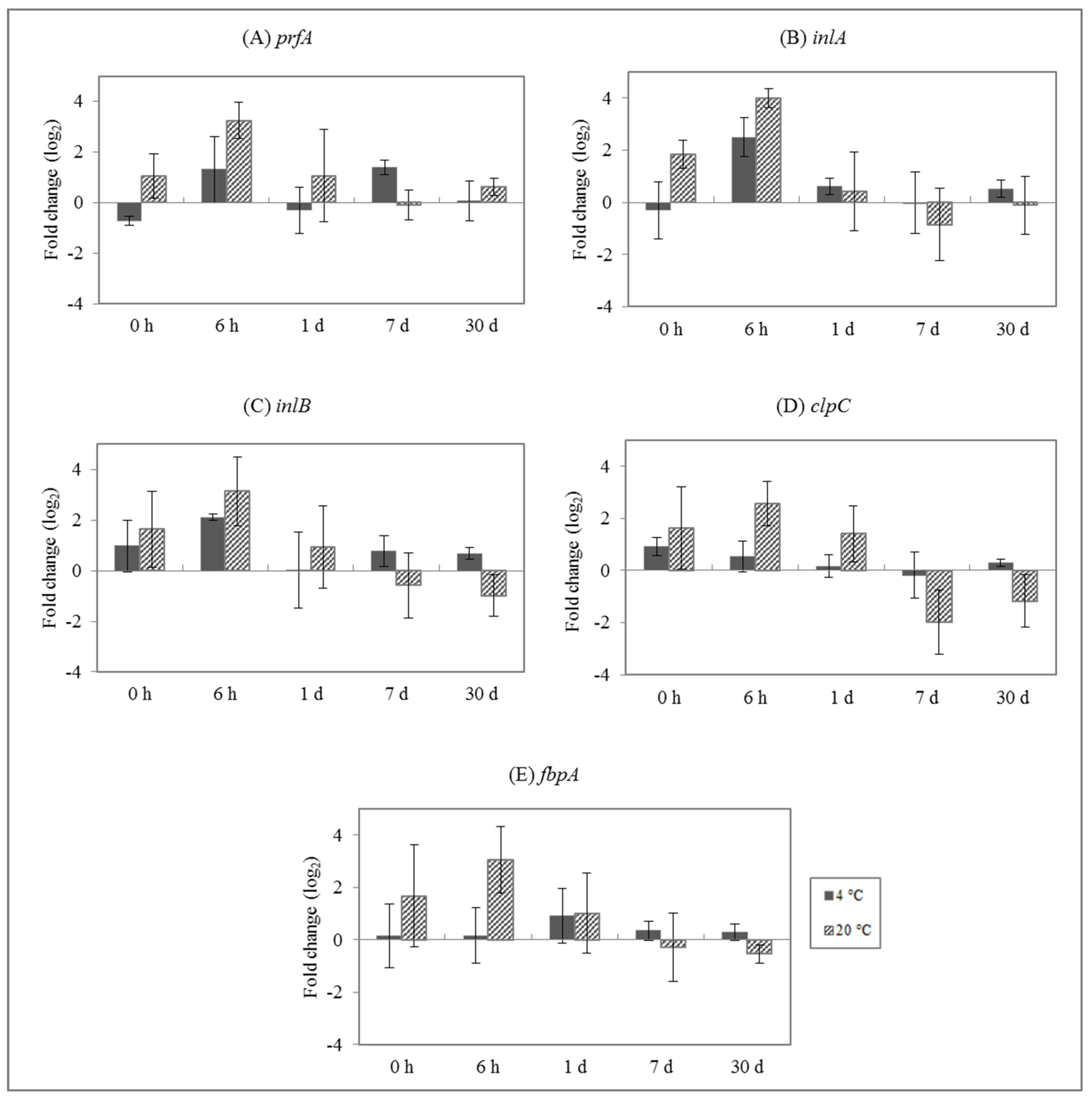
3.2. Effect of Enterocins on L. monocytogenes Gene Expression
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- EFSA (European Food Safety Authority); ECDC (European Centre for Disease Prevention and Control). The European Union One Health 2019 Zoonoses Report. EFSA J. 2021, 19, e06406. [Google Scholar]
- Vázquez-Boland, J.A.; Domínguez-Bernal, G.; González-Zorn, B.; Kreft, J.; Goebel, W. Pathogenicity islands and virulence evolution in Listeria. Microbes Infect. 2001, 3, 571–584. [Google Scholar] [CrossRef]
- Camejo, A.; Carvalho, F.; Reis, O.; Leitão, E.; Sousa, S.; Cabanes, D. The arsenal of virulence factors deployed by Listeria monocytogenes to promote its cell infection cycle. Virulence 2011, 2, 379–394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nair, S.; Milohanic, E.; Berche, P. ClpC ATPase Is Required for Cell Adhesion and Invasion of Listeria monocytogenes. Infect. Immun. 2000, 68, 7061–7068. [Google Scholar] [CrossRef] [Green Version]
- Pizarro-Cerdá, J.; Kühbacher, A.; Cossart, P. Entry of Listeria monocytogenes in Mammalian Epithelial Cells: An Updated View. Cold Spring Harb. Perspect. Med. 2012, 2, a010009. [Google Scholar] [CrossRef] [PubMed]
- De las Heras, A.; Cain, R.J.; Bielecka, M.K.; Vazquez-Boland, J.A. Regulation of Listeria virulence: PrfA master and commander. Curr. Opin. Microbiol. 2011, 14, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Ricci, A.; Allende, A.; Bolton, D.; Chemaly, M.; Davies, R.; Fernández Escámez, P.S.; Girones, R.; Herman, L.; Koutsoumanis, K.; Nørrung, B.; et al. Listeria monocytogenes contamination of ready-to-eat foods and the risk for human health in the EU. EFSA J. 2018, 16, 5134. [Google Scholar]
- Reynolds, A.; Harrison, M.; Rose-Morrow, R.; Lyon, C. Validation of Dry Cured Ham Process for Control of Pathogens. J. Food Sci. 2001, 66, 1373–1379. [Google Scholar] [CrossRef]
- Serra-Castelló, C.; Jofré, A.; Garriga, M.; Bover-Cid, S. Modeling and designing a Listeria monocytogenes control strategy for dry-cured ham taking advantage of water activity and storage temperature. Meat Sci. 2020, 165, 108131. [Google Scholar] [CrossRef]
- Lin, C.-M.; Takeuchi, K.; Zhang, L.; Dohm, C.B.; Meyer, J.D.; Hall, P.A.; Doyle, M.P. Cross-Contamination between Processing Equipment and Deli Meats by Listeria monocytogenes. J. Food Prot. 2006, 69, 71–79. [Google Scholar] [CrossRef]
- Hadjicharalambous, C.; Grispoldi, L.; Cenci-Goga, B. Quantitative risk assessment of Listeria monocytogenes in a traditional RTE product. EFSA J. 2019, 17, e170906. [Google Scholar] [CrossRef] [Green Version]
- Alía, A.; Andrade, M.J.; Rodríguez, A.; Martín, I.; Pérez-Baltar, A.; Medina, M.; Córdoba, J.J. Prevalence and characterization of Listeria monocytogenes in deboning and slicing areas of Spanish dry-cured ham processing. LWT-Food Sci. Technol. 2020, 128, 109498. [Google Scholar] [CrossRef]
- D’Arrigo, M.; Mateo-Vivaracho, L.; Guillamón, E.; Fernández-León, M.F.; Bravo, D.; Peirotén, Á.; Medina, M.; García-Lafuente, A. Characterization of persistent Listeria monocytogenes strains from ten dry-cured ham processing facilities. Food Microbiol. 2020, 92, 103581. [Google Scholar] [CrossRef] [PubMed]
- EC (European Commission). Commission regulation (EC) No 2073/2005 of 15 November 2005 on microbiological criteria for foodstuffs. Off. J. Eur. Union 2005, L338, 1–29. [Google Scholar]
- USDA-FSIS (Food Safety and Inspection Service). FSIS Compliance Guideline: Controlling Listeria Monocytogenes in Post-Lethality Exposed Ready-to-Eat Meat and Poultry Products. Guideline ID: FSIS-GD-2014-0001; 2014. Available online: https://www.fsis.usda.gov/wps/wcm/connect/d3373299-50e6-47d6-a577-e74a1e549fde/Controlling-Lm-RTE-Guideline.pdf?MOD=AJPERES (accessed on 16 June 2021).
- Du, L.; Liu, F.; Zhao, P.; Zhao, T.; Doyle, M.P. Characterization of Enterococcus durans 152 bacteriocins and their inhibition of Listeria monocytogenes in ham. Food Microbiol. 2017, 68, 97–103. [Google Scholar] [CrossRef]
- Marcos, B.; Jofré, A.; Aymerich, T.; Monfort, J.M.; Garriga, M. Combined effect of natural antimicrobials and high pressure processing to prevent Listeria monocytogenes growth after a cold chain break during storage of cooked ham. Food Control 2008, 19, 76–81. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Baltar, A.; Serrano, A.; Bravo, D.; Montiel, R.; Medina, M. Combined Effect of High Pressure Processing with Enterocins or Thymol on the Inactivation of Listeria monocytogenes and the Characteristics of Sliced Dry-cured Ham. Food Bioprocess Technol. 2019, 12, 288–297. [Google Scholar] [CrossRef]
- Montiel, R.; Quesille-Villalobos, A.; Alessandria, V.; Medina, M.; Cocolin, L.S.; Rantsiou, K. Antilisterial Effect and Influence on Listeria monocytogenes Gene Expression of Enterocin or Enterococcus faecalis in Sliced Dry-Cured Ham Stored at 7 °C. J. Food Prot. 2019, 82, 1598–1606. [Google Scholar] [CrossRef]
- Ortiz, S.; López, V.; Villatoro, D.; López, P.; Dávila, J.C.; Martínez-Suárez, J.V. A 3-year surveillance of the genetic diversity and persistence of Listeria monocytogenes in an Iberian pig slaughterhouse and processing plant. Foodborne Pathog. Dis. 2010, 7, 1177–1184. [Google Scholar] [CrossRef]
- Rodríguez, E.; Gonzalez, B.; Gaya, P.; Nuñez, M.; Medina, M. Diversity of bacteriocins produced by lactic acid bacteria isolated from raw milk. Int. Dairy J. 2000, 10, 7–15. [Google Scholar] [CrossRef]
- Garriga, M.; Aymerich, T.; Costa, S.; Monfort, J.; Hugas, M. Bactericidal synergism through bacteriocins and high pressure in a meat model system during storage. Food Microbiol. 2002, 19, 509–518. [Google Scholar] [CrossRef]
- Barefoot, S.F.; Klaenhammer, T.R. Detection and activity of lactacin B, a bacteriocin produced by Lactobacillus acidophilus. Appl. Environ. Microbiol. 1983, 45, 1808–1815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rantsiou, K.; Mataragas, M.; Alessandria, V.; Cocolin, L. Expression of virulence genes of Listeria monocytogenes in food. J. Food Saf. 2012, 32, 161–168. [Google Scholar] [CrossRef]
- Pérez-Baltar, A.; Serrano, A.; Medina, M.; Montiel, R. Effect of high pressure processing on the inactivation and the relative gene transcription patterns of Listeria monocytogenes in dry-cured ham. LWT-Food Sci. Technol. 2020, 139, 110555. [Google Scholar] [CrossRef]
- Sue, D.; Fink, D.; Wiedmann, M.; Boor, K.J. σB-dependent gene induction and expression in Listeria monocytogenes during osmotic and acid stress conditions simulating the intestinal environment. Microbiology 2004, 150, 3843–3855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olesen, I.; Thorsen, L.; Jespersen, L. Relative transcription of Listeria monocytogenes virulence genes in liver pâtés with varying NaCl content. Int. J. Food Microbiol. 2010, 141, S60–S68. [Google Scholar] [CrossRef]
- Kazmierczak, M.J.; Wiedmann, M.; Boor, K.J. Contributions of Listeria monocytogenes σB and PrfA to expression of virulence and stress reponse genes during extra- and intracellular growth. Microbiology 2006, 152, 1827–1838. [Google Scholar] [CrossRef] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Hereu, A.; Bover-Cid, S.; Garriga, M.; Aymerich, T. High hydrostatic pressure and biopreservation of dry-cured ham to meet the Food Safety Objectives for Listeria monocytogenes. Int. J. Food Microbiol. 2011, 154, 107–112. [Google Scholar] [CrossRef]
- Jofré, A.; Aymerich, T.; Monfort, J.M.; Garriga, M. Application of enterocins A and B, sakacin K and nisin to extend the safe shelf-life of pressurized ready-to-eat meat products. Eur. Food Res. Technol. 2008, 228, 159–162. [Google Scholar] [CrossRef]
- Buncic, S.; Avery, S.M.; Rocourt, J.; Dimitrijevic, M. Can food-related environmental factors induce different behaviour in two key serovars, 4b and 1/2a, of Listeria monocytogenes? Int. J. Food Microbiol. 2001, 65, 201–212. [Google Scholar] [CrossRef]
- Moorhead, S.M.; Dykes, G.A. The Role of the sigB gene in the general stress response of Listeria monocytogenes varies between a strain of serotype 1/2a and a strain of serotype 4c. Curr. Microbiol. 2003, 46, 461–466. [Google Scholar] [CrossRef]
- Pérez-Baltar, A.; Alía, A.; Rodríguez, A.; Córdoba, J.J.; Medina, M.; Montiel, R. Impact of Water Activity on the Inactivation and Gene Expression of Listeria monocytogenes during Refrigerated Storage of Pressurized Dry-Cured Ham. Foods 2020, 9, 1092. [Google Scholar] [CrossRef] [PubMed]
- Wałecka-Zacharska, E.; Gmyrek, R.; Skowron, K.; Kosek-Paszkowska, K.; Bania, J. Duration of heat stress effect on invasiveness of Listeria monocytogenes strains. BioMed. Res. Int. 2018, 2018, 1457480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schrama, D.; Helliwell, N.; Neto, L.; Faleiro, M.L. Adaptation of Listeria monocytogenes in a simulated cheese medium: Effects on virulence using the Galleria mellonella infection model. Lett. Appl. Microbiol. 2013, 56, 421–427. [Google Scholar] [CrossRef]
- Alía, A.; Rodríguez, A.; Andrade, M.J.; Gómez, F.M.; Córdoba, J.J. Combined effect of temperature, water activity and salt content on the growth and gene expression of Listeria monocytogenes in a dry-cured ham model system. Meat Sci. 2019, 155, 16–19. [Google Scholar] [CrossRef]
- Lucas, J.; Alía, A.; Velasco, R.; Selgas, M.; Cabeza, M. Effect of E-beam treatment on expression of virulence and stress-response genes of Listeria monocytogenes in dry-cured ham. Int. J. Food Microbiol. 2021, 340, 109057. [Google Scholar] [CrossRef]
- Duodu, S.; Holst-Jensen, A.; Skjerdal, T.; Cappelier, J.-M.; Pilet, M.-F.; Loncarevic, S. Influence of storage temperature on gene expression and virulence potential of Listeria monocytogenes strains grown in a salmon matrix. Food Microbiol. 2010, 27, 795–801. [Google Scholar] [CrossRef]
- Miranda, R.O.; Campos-Galvão, M.E.M.; Nero, L.A. Expression of genes associated with stress conditions by Listeria monocytogenes in interaction with nisin producer Lactococcus lactis. Food Res. Int. 2018, 105, 897–904. [Google Scholar] [CrossRef]
- Ye, K.; Zhang, X.; Huang, Y.; Liu, J.; Liu, M.; Zhou, G. Bacteriocinogenic Enterococcus faecium inhibits the virulence property of Listeria monocytogenes. LWT-Food Sci. Technol. 2018, 89, 87–92. [Google Scholar] [CrossRef]
- Bowman, J.; Bittencourt, C.R.; Ross, T. Differential gene expression of Listeria monocytogenes during high hydrostatic pressure processing. Microbiology 2008, 154, 462–475. [Google Scholar] [CrossRef] [Green Version]
- Laursen, M.F.; Bahl, M.I.; Licht, T.R.; Gram, L.; Knudsen, G.M. A single exposure to a sublethal pediocin concentration initiates a resistance-associated temporal cell envelope and general stress response in Listeria monocytogenes. Environ. Microbiol. 2014, 17, 1134–1151. [Google Scholar] [CrossRef] [PubMed]
- Osanai, A.; Li, S.-J.; Asano, K.; Sashinami, H.; Hu, D.-L.; Nakane, A. Fibronectin binding protein, FbpA, is an adhesin responsible for pathogenesis of Listeria monocytogenes infection. Microbiol. Immunol. 2013, 57, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Ross, W.H.; Whiting, R.C.; Van Stelten, A.; Nightingale, K.K.; Wiedmann, M.; Scott, V.N. Variation in Listeria monocytogenes Dose Responses in Relation to Subtypes Encoding a Full-Length or Truncated Internalin A. Appl. Environ. Microbiol. 2011, 77, 1171–1180. [Google Scholar] [CrossRef] [Green Version]
- Ortiz, S.; López-Alonso, V.; Rodríguez, P.; Martínez-Suárez, J.V. The Connection between Persistent, Disinfectant-Resistant Listeria monocytogenes Strains from Two Geographically Separate Iberian Pork Processing Plants: Evidence from Comparative Genome Analysis. Appl. Environ. Microbiol. 2016, 82, 308–317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Gene Name | Function and Scope of Use | Sequence (5′→3′) | Reference |
|---|---|---|---|
| IGS | Reference gene | IGS1: GGCCTATAGCTCAGCTGGTTA | [24] |
| IGS2: GCTGAGCTAAGGCCCCATAAA | |||
| P: HEX-CCATCGACCTCACGCTTATCAGGC-TAMRA | [25] | ||
| inlA | Internalization in the host cell | F: GGTCTCACAAACAGATCTAGACCAAGT | [26] |
| R: TCAAGTATTCCACTCCATCGATAGATT | |||
| P: HEX-TCCCTAATCTATCCGCCTGAAGCGTTG-TAMRA | |||
| inlB | Internalization in the host cell | F: AAGCAAGATTTCATGGGAGAGT | [27] |
| R: TTACCGTTCCATCAACATCATAACTT | |||
| P: HEX-CCACTGAAAGAGGTTTACACA-TAMRA | |||
| clpC | ATPase involved in cell adhesion and invasion | F: GCGGCTGTTCAAGGTCAAG | [27] |
| R: TTGCCAATTCGCTTTAGTTTCTT | |||
| P: HEX-AAAGCAGCGTCATTACG-TAMRA | |||
| fbpA | Involved in efficient colonization of host tissues | F: AAATCAATGAACTATTTCCGGAAAG | [27] |
| R: CATGGAGCTTGCTAAAC | |||
| P: HEX-CTAGAGGAGCATAAGGAA-TAMRA | |||
| prfA | Transcriptional regulator, virulence | F: CAATGGGATCCACAAGAATATTGTAT | [28] |
| R: AATAAAGCCAGACATTATAACGAAAGC | |||
| P: HEX-TGTAAATTCATGATGGTCCCGTTCTCGCT-TAMRA |
| Time (d) | |||||||
|---|---|---|---|---|---|---|---|
| Strain | Temperature (°C) | Treatment | 0 | 1 | 7 | 14 | 30 |
| S2 | 4 | Control | 6.29 ± 0.18aD | 5.61 ± 0.16aC | 5.18 ± 0.14aB | 5.26 ± 0.20aB | 4.84 ± 0.32aA |
| ENT | 5.80 ± 0.15bE | 5.20 ± 0.17bD | 4.90 ± 0.09bC | 4.59 ± 0.15bB | 4.16 ± 0.13bA | ||
| 20 | Control | 6.18 ± 0.09aD | 5.63 ± 0.20aC | 5.07 ± 0.09aB | 4.80 ± 0.27aB | 4.21 ± 0.25aA | |
| ENT | 5.67 ± 0.27bC | 4.84 ± 0.12bB | 4.63 ± 0.29bB | 4.08 ± 0.42bA | 3.81 ± 0.29bA | ||
| S7-2 | 4 | Control | 6.25 ± 0.11aD | 5.84 ± 0.08aC | 5.46 ± 0.17aAB | 5.51 ± 0.13aB | 5.26 ± 0.13aA |
| ENT | 5.63 ± 0.18bB | 5.26 ± 0.09bB | 4.84 ± 0.37bA | 4.86 ± 0.33bA | 4.69 ± 0.13bA | ||
| 20 | Control | 6.25 ± 0.20aC | 5.72 ± 0.28aBC | 5.38 ± 0.33aB | 5.18 ± 0.33aAB | 4.72 ± 0.53aA | |
| ENT | 5.67 ± 0.12bB | 5.27 ± 0.10bB | 4.35 ± 0.32bA | 4.27 ± 0.83bA | 4.49 ± 0.18aA | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-Baltar, A.; Medina, M.; Montiel, R. Effect of Enterocins A and B on the Viability and Virulence Gene Expression of Listeria monocytogenes in Sliced Dry-Cured Ham. Appl. Microbiol. 2022, 2, 1-11. https://doi.org/10.3390/applmicrobiol2010001
Pérez-Baltar A, Medina M, Montiel R. Effect of Enterocins A and B on the Viability and Virulence Gene Expression of Listeria monocytogenes in Sliced Dry-Cured Ham. Applied Microbiology. 2022; 2(1):1-11. https://doi.org/10.3390/applmicrobiol2010001
Chicago/Turabian StylePérez-Baltar, Aida, Margarita Medina, and Raquel Montiel. 2022. "Effect of Enterocins A and B on the Viability and Virulence Gene Expression of Listeria monocytogenes in Sliced Dry-Cured Ham" Applied Microbiology 2, no. 1: 1-11. https://doi.org/10.3390/applmicrobiol2010001
APA StylePérez-Baltar, A., Medina, M., & Montiel, R. (2022). Effect of Enterocins A and B on the Viability and Virulence Gene Expression of Listeria monocytogenes in Sliced Dry-Cured Ham. Applied Microbiology, 2(1), 1-11. https://doi.org/10.3390/applmicrobiol2010001






