Salicylic Acid Pretreatment Modulates Wheat Responses to Glyphosate
Abstract
:1. Introduction
2. Materials and Methods
3. Results
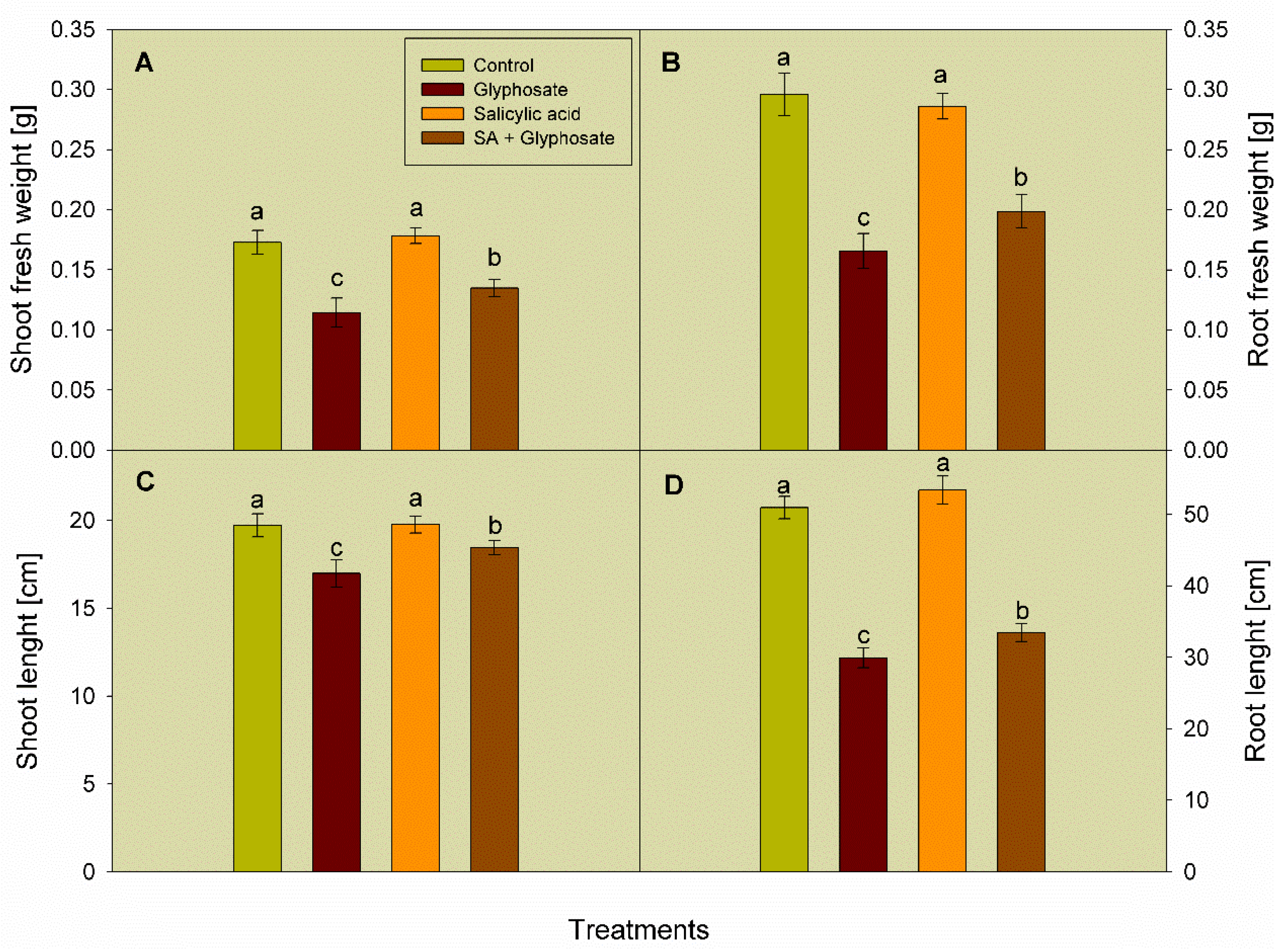
3.1. Effect of SA and Glyphosate Treatments on Growth Traits
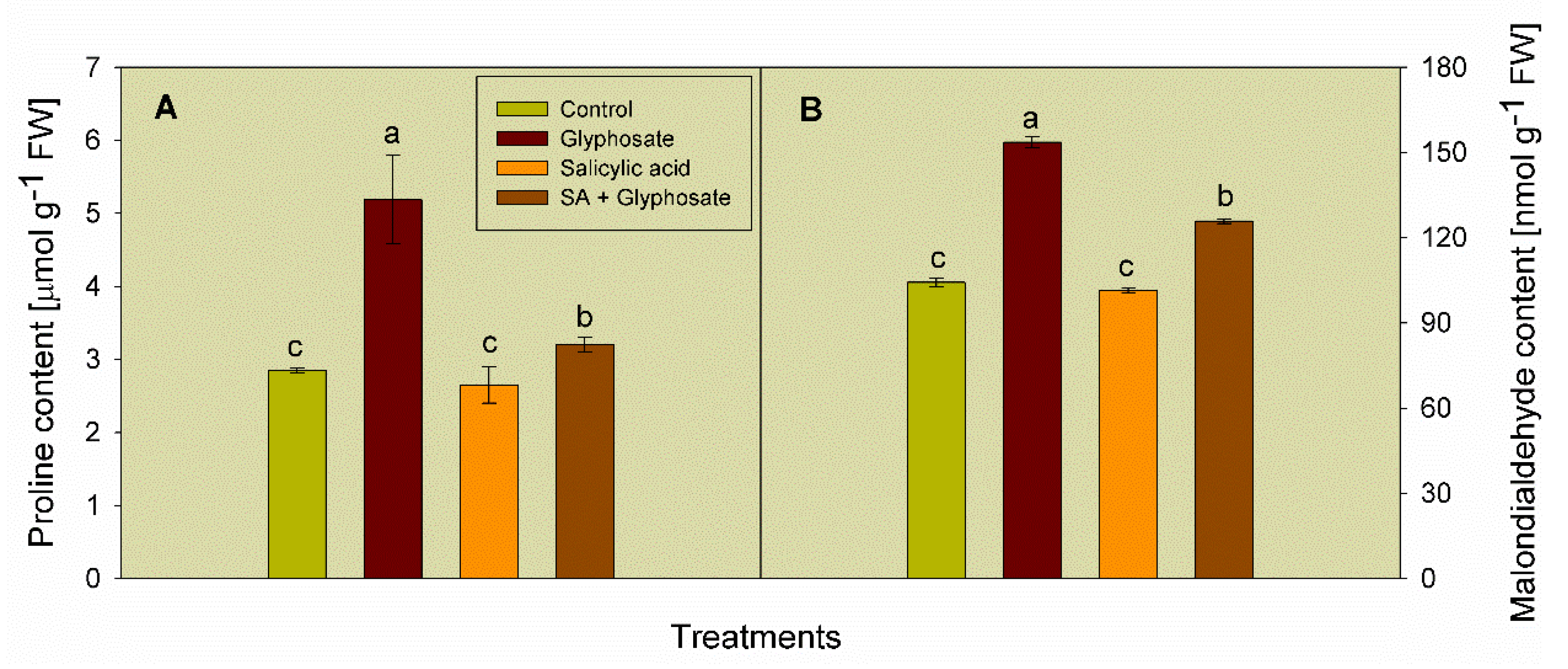
3.2. Effect of SA and Glyphosate Treatments on Stress Biomarkers Content
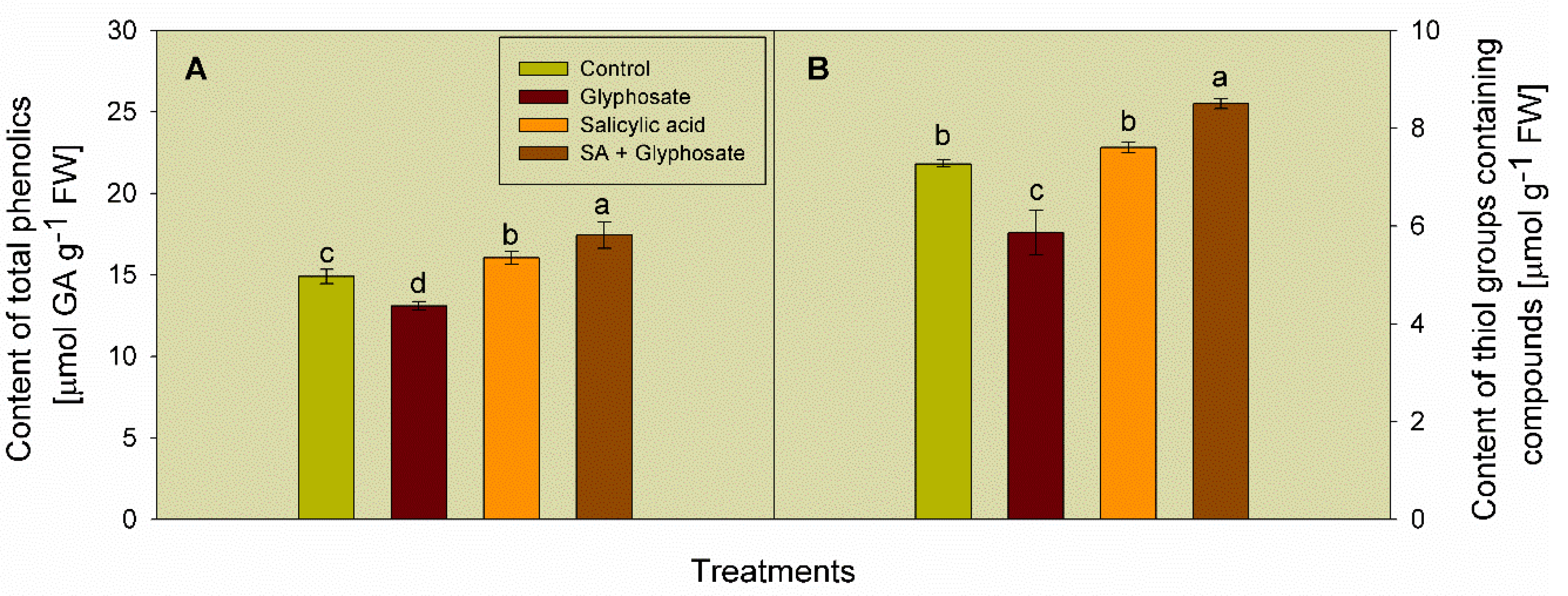
3.3. Effect of SA and Glyphosate Treatments on Non-Enzymatic Antioxidant Content
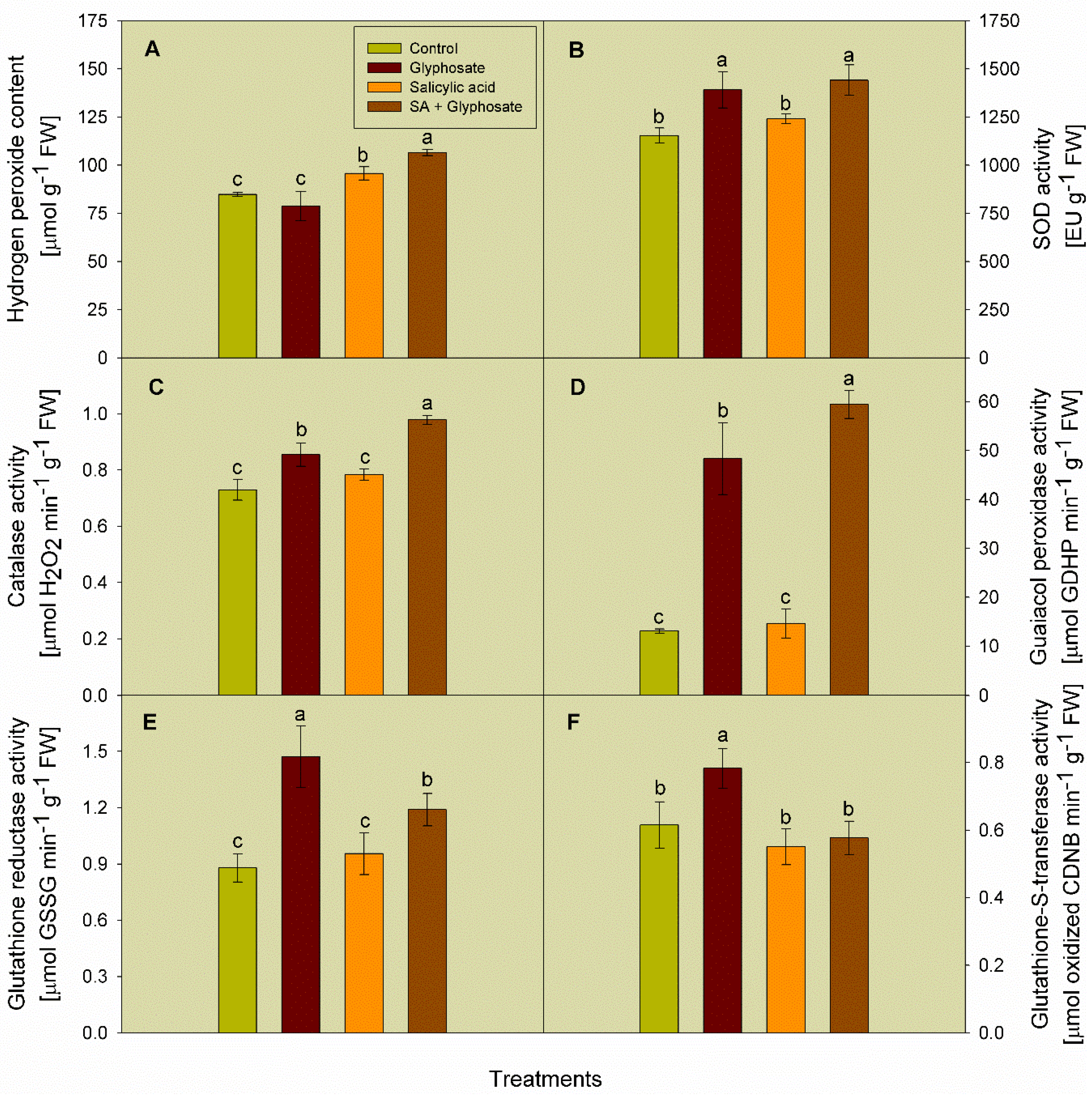
3.4. Effect of SA and Glyphosate Treatments on the Activity of Some Antioxidant Enzymes, and the Content of Hydrogen Peroxide
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gomes, M.; Smedbol, É.; Chalifour, A.; Hénault-Ethier, L.; Labrecque, M.; Lepage, L.; Lucotte, M.; Juneau, P. Alteration of plant physiology by glyphosate and its by-product aminomethylphosphonic acid: An overview. J. Exp. Bot. 2014, 65, 4691–4703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Freitas-Silva, L.; Rodríguez-Ruiz, M.; Houmani, H.; da Silva, L.C.; Palma, J.M.; Corpas, F.J. Glyphosate-induced oxidative stress in Arabidopsis thaliana affecting peroxisomal metabolism and triggers activity in the oxidative phase of the pentose phosphate pathway (OxPPP) involved in NADPH generation. J. Plant Physiol. 2017, 218, 196–205. [Google Scholar] [CrossRef]
- Ferreira, M.F.; Torres, C.; Bracamonte, E.; Galetto, L. Effects of the herbicide glyphosate on non-target plant native species from Chaco forest (Argentina). Ecotoxicol. Environ. Saf. 2017, 144, 360–368. [Google Scholar]
- Sergiev, I.; Todorova, D.; Shopova, E.; Brankova, L.; Jankauskienė, J.; Jurkonienė, S.; Gavelienė, V.; Mockevičiūtė, R. Assessment of synthetic auxin type compounds as potential modulators of herbicide action in Pisum sativum L. Biologia 2020, 75, 1845–1853. [Google Scholar] [CrossRef]
- Cobb, A.H.; Reade, J.P. Herbicides and Plant Physiology; John Wiley & Sons, Inc.: West Sussex, UK, 2010; p. 296. [Google Scholar]
- Miteva, L.; Ivanov, S.; Alexieva, V.; Karanov, E. Effect of herbicide glyphosate on glutathione levels, glutathi-one-S-transferase and glutathione reductase activities in two plant species. Compt. Rend. Acad. Bulg. Sci. 2003, 56, 79–84. [Google Scholar]
- Miteva, L.; Tsoneva, J.; Ivanov, S.; Alexieva, V. Alterations of the content of hydrogen peroxide and malondialdehyde and the activity of some antioxidant enzymes in the roots and leaves of pea and wheat plants exposed to glyphosate. Compt. Rend. Acad. Bulg. Sci. 2005, 58, 733–738. [Google Scholar]
- Miteva, L.P.-E.; Ivanov, S.V.; Alexieva, V.S. Alterations in glutathione pool and some related enzymes in leaves and roots of pea plants treated with the herbicide glyphosate. Russ. J. Plant Physiol. 2010, 57, 131–136. [Google Scholar] [CrossRef]
- Mondal, S.; Kumar, M.; Haque, S.; Kundu, D. Phytotoxicity of glyphosate in the germination of Pisum sativum and its effect on germinated seedlings. Environ. Health Toxicol. 2017, 32, e2017011. [Google Scholar] [CrossRef] [Green Version]
- Maldani, M.; Aliyat, F.Z.; Cappello, S.; Morabito, M.; Giarratana, F.; Nassiri, L.; Ibijbijen, J. Effect of glyphosate and paraquat on seed germination, amino acids, photosynthetic pigments and plant morphology of Vicia faba, Phaseolus vulgaris and Sorghum bicolor. Environ. Sustain. 2021, 1–11. [Google Scholar] [CrossRef]
- Jiang, L.-X.; Jin, L.-G.; Guo, Y.; Tao, B.; Qiu, L.-J. Glyphosate effects on the gene expression of the apical bud in soybean (Glycine max). Biochem. Biophys. Res. Commun. 2013, 437, 544–549. [Google Scholar] [CrossRef]
- Singh, H.; Singh, N.B.; Singh, A.; Hussain, I. Exogenous application of salicylic acid to alleviate glyphosate stress in So-lanum lycopersicum. Int. J. Veg. Sci. 2017, 23, 552–566. [Google Scholar] [CrossRef]
- Khan, S.; Zhou, J.L.; Ren, L.; Mojiri, A. Effects of glyphosate on germination, photosynthesis and chloroplast morphology in tomato. Chemosphere 2020, 258, 127350. [Google Scholar] [CrossRef] [PubMed]
- Sergiev, I.G.; Alexieva, V.S.; Ivanov, S.V.; Moskova, I.I.; Karanov, E.N. The phenylurea cytokinin 4PU-30 protects maize plants against glyphosate action. Pestic. Biochem. Physiol. 2006, 85, 139–146. [Google Scholar] [CrossRef]
- Gomes, M.P.; Richardi, V.S.; Bicalho, E.M.; da Rocha, D.C.; da Silva, M.A.N.; Soffiatti, P.; Garcia, Q.S.; Sant’Anna-Santos, B.F. Effects of Ciprofloxacin and Roundup on seed germination and root development of maize. Sci. Total Environ. 2019, 651, 2671–2678. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Lu, G.-H.; Yang, Y.-H. Salicylic Acid Signaling and its Role in Responses to Stresses in Plants. In Mechanism of Plant Hormone Signaling under Stress; Wiley: New York, NY, USA, 2017; pp. 413–441. [Google Scholar]
- Khan, M.I.R.; Fatma, M.; Per, T.S.; Anjum, N.A.; Khan, N.A. Salicylic acid-induced abiotic stress tolerance and underlying mechanisms in plants. Front. Plant Sci. 2015, 6, 462. [Google Scholar] [CrossRef] [Green Version]
- Hayat, Q.; Hayat, S.; Irfan, M.; Ahmad, A. Effect of exogenous salicylic acid under changing environment: A review. Environ. Exp. Bot. 2010, 68, 14–25. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Nahar, K.; Bhuiyan, T.I.A.T.F.; Anee, T.I.; Inafuku, M.; Oku, H.; Fujita, M. Salicylic Acid: An All-Rounder in Regulating Abiotic Stress Responses in Plants. In Phytohormones-Signaling Mechanisms and Crosstalk in Plant Development and Stress Responses; IntechOpen: London, UK, 2017; pp. 31–75. [Google Scholar]
- Wang, J.; Lv, M.; Islam, F.; Gill, R.A.; Yang, C.; Ali, B.; Yan, G.; Zhou, W. Salicylic acid mediates antioxidant defense system and ABA pathway related gene expression in Oryza sativa against quinclorac toxicity. Ecotoxicol. Environ. Saf. 2016, 133, 146–156. [Google Scholar] [CrossRef]
- Radwan, D.E. Salicylic acid induced alleviation of oxidative stress caused by clethodim in maize (Zea mays L.) leaves. Pestic. Biochem. Physiol. 2012, 102, 182–188. [Google Scholar] [CrossRef]
- Cui, J.; Zhang, R.; Wu, G.L.; Zhu, H.M.; Yang, H. Salicylic acid reduces napropamide toxicity by preventing its accumu-lation in rapeseed (Brassica napus L.). Arch. Environ. Contam. Toxicol. 2010, 59, 100–108. [Google Scholar] [CrossRef]
- Liang, L.; Lu, Y.L.; Yang, H. Toxicology of isoproturon to the food crop wheat as affected by salicylic acid. Environ. Sci. Pollut. Res. 2012, 19, 2044–2054. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, Q. Exogenous salicylic acid alleviates the toxicity of chlorpyrifos in wheat plants (Triticum aestivum). Ecotoxicol. Environ. Saf. 2017, 137, 218–224. [Google Scholar] [CrossRef]
- Liu, T.; Li, T.; Zhang, L.; Li, H.; Liu, S.; Yang, S.; An, Q.; Pan, C.; Zou, N. Exogenous salicylic acid alleviates the accumula-tion of pesticides and mitigates pesticide-induced oxidative stress in cucumber plants (Cucumis sativus L.). Ecotoxicol. Environ. Saf. 2021, 208, 111654. [Google Scholar] [CrossRef]
- Shiferaw, B.; Smale, M.; Braun, H.-J.; Duveiller, E.; Reynolds, M.; Muricho, G. Crops that feed the world 10. Past successes and future challenges to the role played by wheat in global food security. Food Secur. 2013, 5, 291–317. [Google Scholar] [CrossRef] [Green Version]
- Fayez, K.A.; Ali, E.F. Impact of Glyphosate Herbicide and Salicylic Acid on Seed Germination, Cell Structure and Physiological Activities of Faba Bean (Vicia faba L.) Plant. Ann. Res. Rev. Biol. 2017, 17, 1–15. [Google Scholar] [CrossRef]
- Spormann, S.; Soares, C.; Fidalgo, F. Salicylic acid alleviates glyphosate-induced oxidative stress in Hordeum vulgare L. J. Environ. Manag. 2019, 241, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Akbulut, G.B.; Yigit, E.; Bayram, D. Investigation of the Effects of Salicylic Acid on Some Biochemical Parameters in Zea mays to Glyphosate Herbicide. J. Environ. Anal. Toxicol. 2015, 5, 271. [Google Scholar] [CrossRef] [Green Version]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Kramer, G.F.; Norman, H.A.; Krizek, D.T.; Mirecki, R.M. influence of UV-B radiation on polyamines, lipid peroxidation and membrane lipids in cucumber. Phytochemistry 1991, 30, 2101–2108. [Google Scholar] [CrossRef]
- Alexieva, V.; Sergiev, I.; Mapelli, S.; Karanov, E. The effect of drought and ultraviolet radiation on growth and stress markers in pea and wheat. Plant Cell Environ. 2001, 24, 1337–1344. [Google Scholar] [CrossRef]
- Swain, T.; Goldstein, L. Methods in Polyphenol Chemistry; Pridham, J.B., Ed.; Pergamon Press: Oxford, UK, 1964; pp. 131–146. [Google Scholar]
- Ellman, G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef]
- Beauchamp, C.; Fridovich, I. Superoxide dismutase. Improved assay and an assay applicable to acrylamide gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- Aebi, M. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar]
- Dias, M.A.; Costa, M.M. Effect of Low Salt Concentrations on Nitrate Reductase and Peroxidase of Sugar Beet Leaves. J. Exp. Bot. 1983, 34, 537–543. [Google Scholar] [CrossRef]
- Smith, I.K.; Vierheller, T.L.; Thorne, C.A. Assay of glutathione reductase in crude tissue homogenates using 5,5′-dithiobis(2-nitrobenzoic acid). Anal. Biochem. 1988, 175, 408–413. [Google Scholar] [CrossRef]
- Gronwald, J.W.; Fuerst, E.P.; Eberlein, C.V.; Egli, M.A. Effect of herbicide antidotes on glutathione content and gluta-thione S-transferase activity of sorghum shoots. Pestic. Biochem. Physiol. 1987, 29, 66–76. [Google Scholar] [CrossRef]
- Varshney, S.; Khan, M.I.R.; Masood, A.; Per, T.S.; Rasheed, F.; A Khan, N. Contribution of Plant Growth Regulators in Mitigation of Herbicidal Stress. J. Plant Biochem. Physiol. 2015, 3, 160. [Google Scholar] [CrossRef] [Green Version]
- Langaro, A.C.; Agostinetto, D.; Ruchel, Q.; Garcia, J.R.; Perboni, L.T. Oxidative stress caused by the use of preemergent herbicides in rice crops. Rev. Ciência Agronômica 2017, 48, 358–364. [Google Scholar] [CrossRef]
- Savvides, A.; Ali, S.; Tester, M.; Fotopoulos, V. Chemical Priming of Plants Against Multiple Abiotic Stresses: Mission Possible? Trends Plant Sci. 2016, 21, 329–340. [Google Scholar] [CrossRef] [Green Version]
- Soares, C.; Pereira, R.; Spormann, S.; Fidalgo, F. Is soil contamination by a glyphosate commercial formulation truly harmless to non-target plants?—Evaluation of oxidative damage and antioxidant responses in tomato. Environ. Pollut. 2019, 247, 256–265. [Google Scholar] [CrossRef]
- Hayat, S.; Hayat, Q.; Alyemeni, M.N.; Wani, A.S.; Pichtel, J.; Ahmad, A. Role of proline under changing environments: A review. Plant Signal Behav. 2012, 7, 1456–1466. [Google Scholar] [CrossRef] [Green Version]
- Hossain, M.A.; Li, Z.-G.; Hoque, T.S.; Burritt, D.J.; Fujita, M.; Munné-Bosch, S. Heat or cold priming-induced cross-tolerance to abiotic stresses in plants: Key regulators and possible mechanisms. Protoplasma 2018, 255, 399–412. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, F.E.L.; Silveira, J.A.G. H2O2-retrograde signaling as a pivotal mechanism to understand priming and cross stress tolerance in plants. In Priming-Mediated Stress and Cross-Stress Tolerance in Crop Plants; Hossain, M.A., Liu, F., Bur-ritt, D., Fujita, M., Huang, B., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 57–78. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shopova, E.; Brankova, L.; Katerova, Z.; Dimitrova, L.; Todorova, D.; Sergiev, I.; Talaat, N.B. Salicylic Acid Pretreatment Modulates Wheat Responses to Glyphosate. Crops 2021, 1, 88-96. https://doi.org/10.3390/crops1020009
Shopova E, Brankova L, Katerova Z, Dimitrova L, Todorova D, Sergiev I, Talaat NB. Salicylic Acid Pretreatment Modulates Wheat Responses to Glyphosate. Crops. 2021; 1(2):88-96. https://doi.org/10.3390/crops1020009
Chicago/Turabian StyleShopova, Elena, Liliana Brankova, Zornitsa Katerova, Ljudmila Dimitrova, Dessislava Todorova, Iskren Sergiev, and Neveen B. Talaat. 2021. "Salicylic Acid Pretreatment Modulates Wheat Responses to Glyphosate" Crops 1, no. 2: 88-96. https://doi.org/10.3390/crops1020009
APA StyleShopova, E., Brankova, L., Katerova, Z., Dimitrova, L., Todorova, D., Sergiev, I., & Talaat, N. B. (2021). Salicylic Acid Pretreatment Modulates Wheat Responses to Glyphosate. Crops, 1(2), 88-96. https://doi.org/10.3390/crops1020009






