Can ‘On-Farm’ Seed Priming and Chitosan Seed Treatments Induce Host Defences in Winter Barley (Hordeum vulgare L.) under Field Conditions?
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Preparation of Seed Treatments
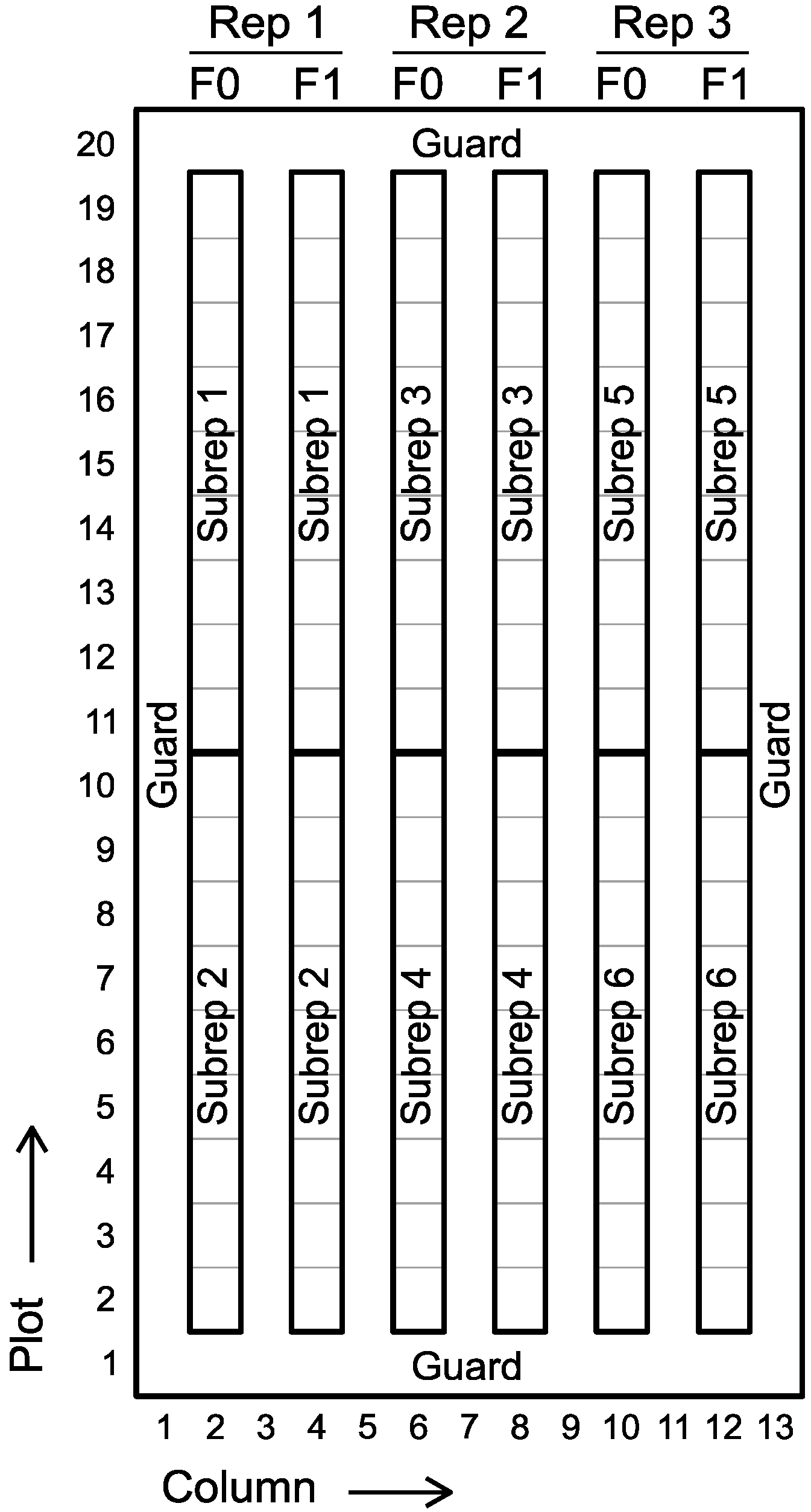
2.2. Field Sites, Experimental Design, and Crop Husbandry
2.3. In-Field Imaging
2.3.1. Image Collection
2.3.2. Image Processing for Emergence Counts
2.3.3. Image Processing for Leaf Area Index and Percentage of Senescent Tissue Estimation at Advanced Tillering
2.3.4. Image Processing for Canopy Green Cover
2.4. In-Field Measurements
2.4.1. Disease Severity
2.4.2. Height and Maturity
2.5. Yield and Grain Quality
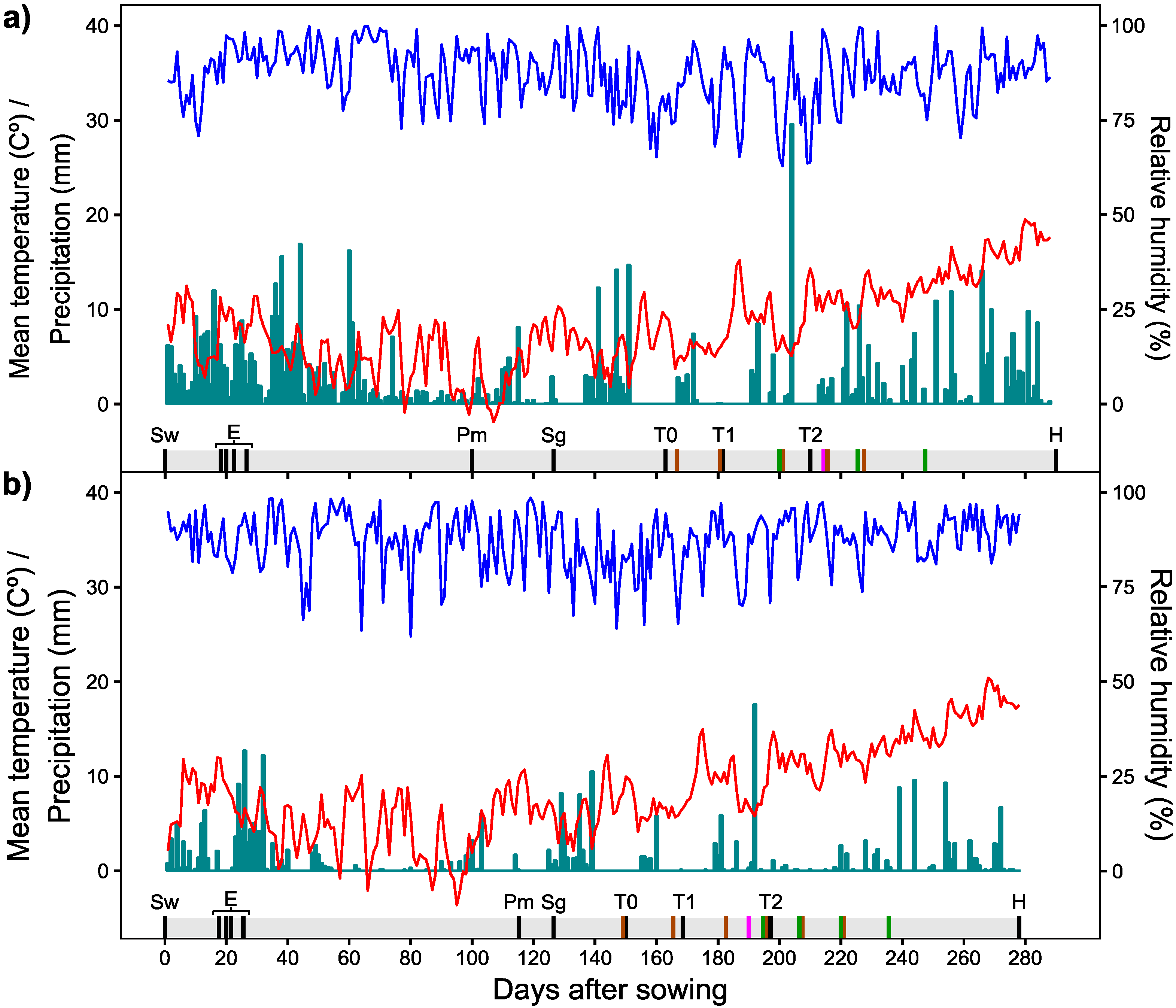
2.6. Meteorological Conditions
2.7. Data Analysis
3. Results
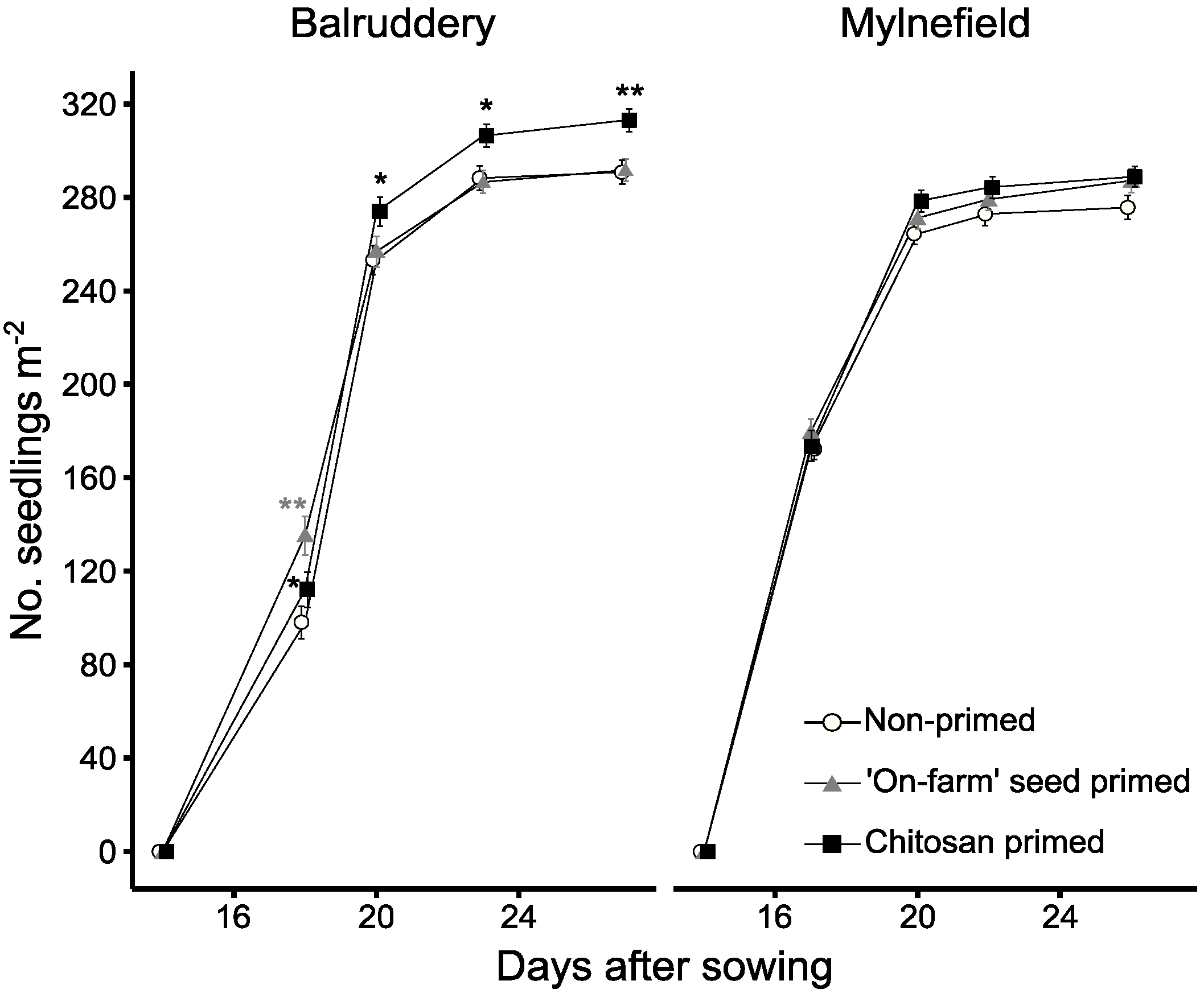
3.1. Emergency and Early Growth
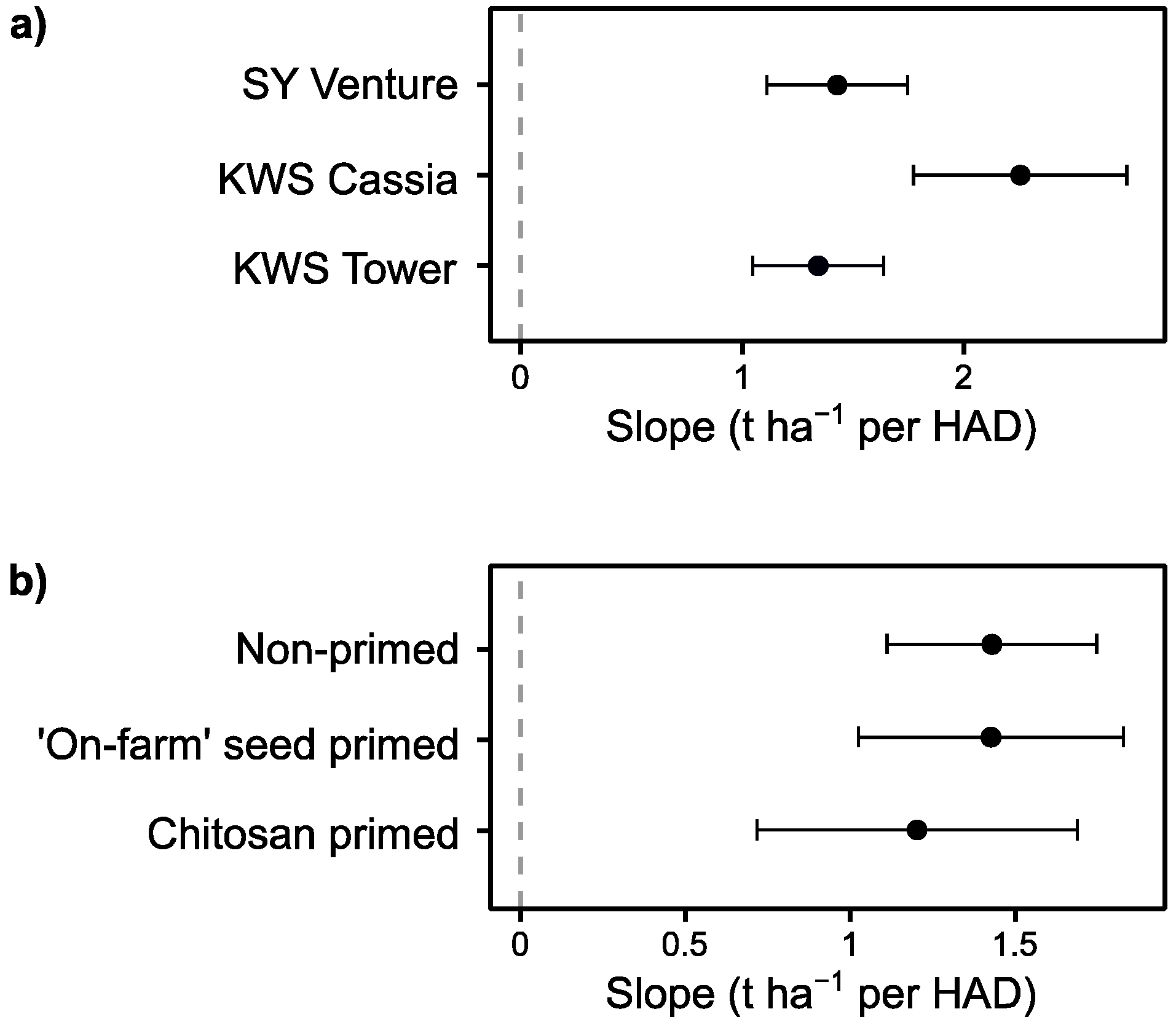
3.2. Effect of Vigour as a Candidate Trait for Tolerance in Early Epidemics
3.3. Disease Severity and Resistance
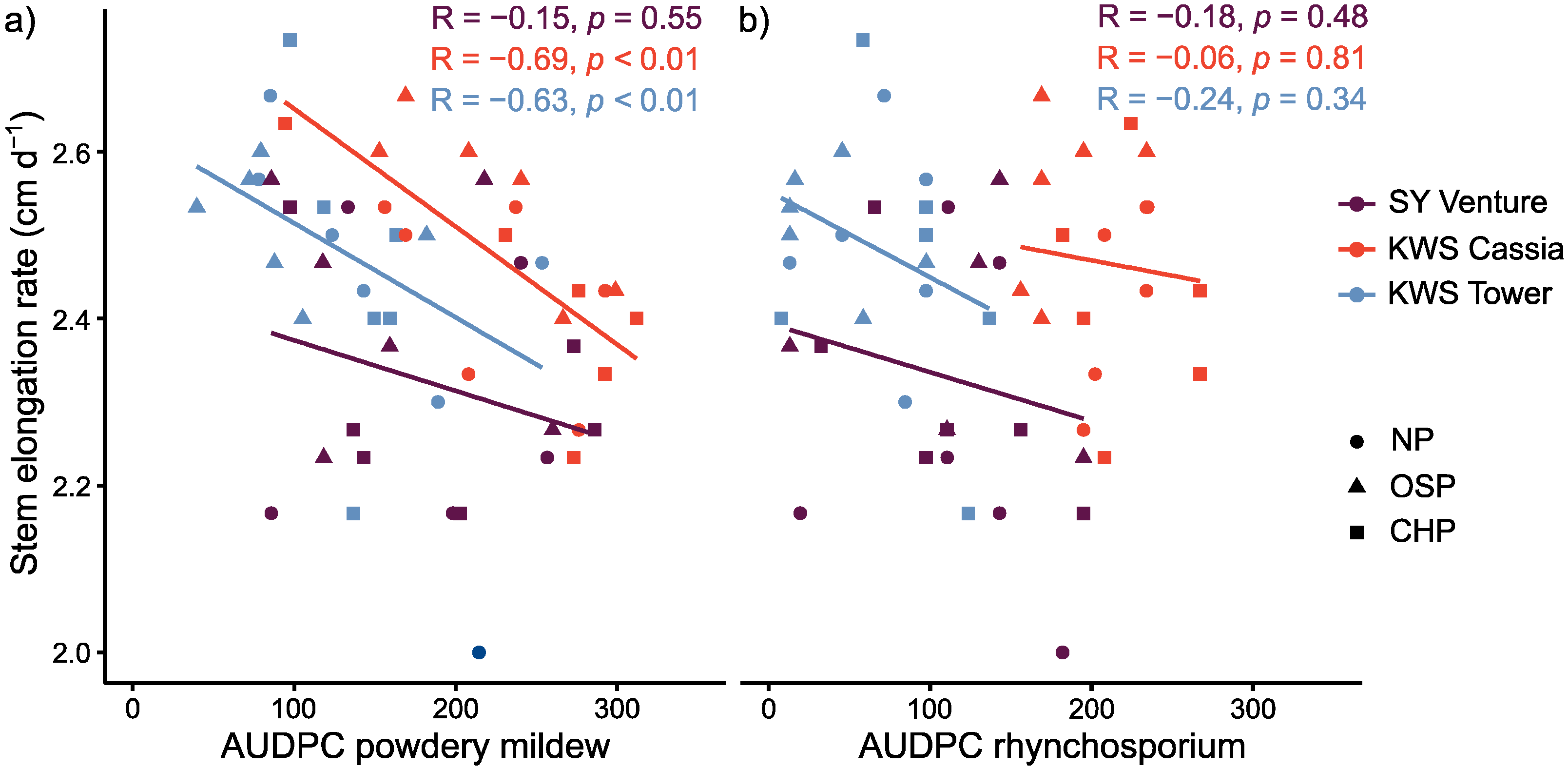
3.4. Effect of Stem Elongation Rate as a Candidate Trait for Disease ‘Escape’
3.5. Effects on Yield and Yield Components
3.6. Effects on Tolerance in Late Epidemics
4. Discussion
4.1. Induced Resistance Is Hampered under Field Conditions
4.2. Disease Tolerance and Escape in Relation to Crop Traits
4.3. Overall Tolerance in Relation to Yield as Fitness
4.4. From Emergence to Yield
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Walters, D.R.; Avrova, A.; Bingham, I.J.; Burnett, F.J.; Fountaine, J.; Havis, N.D.; Hoad, S.P.; Hughes, G.; Looseley, M.; Oxley, S.J.P.; et al. Control of foliar diseases in barley: Towards an integrated approach. Eur. J. Plant Pathol. 2012, 133, 33–73. [Google Scholar] [CrossRef]
- Ney, B.; Bancal, M.O.; Bancal, P.; Bingham, I.J.; Foulkes, J.; Gouache, D.; Paveley, N.; Smith, J. Crop architecture and crop tolerance to fungal diseases and insect herbivory. Mechanisms to limit crop losses. Eur. J. Plant Pathol. 2013, 135, 561–580. [Google Scholar] [CrossRef]
- Iriti, M.; Varoni, E.M. Moving to the Field: Plant Innate Immunity in Crop Protection. Int. J. Mol. Sci. 2017, 18, 640. [Google Scholar] [CrossRef] [PubMed]
- Alexandersson, E.; Mulugeta, T.; Lankinen, Å.; Liljeroth, E.; Andreasson, E. Plant resistance inducers against pathogens in Solanaceae species-from molecular mechanisms to field application. Int. J. Mol. Sci. 2016, 17, 1673. [Google Scholar] [CrossRef] [PubMed]
- Benhamou, N. Induction of Systemic Resistance to Fusarium Crown and Root Rot in Tomato Plants by Seed Treatment with Chitosan. Phytopathology 1994, 84, 1432. [Google Scholar] [CrossRef]
- Sharathchandra, R.G.; Raj, S.N.; Shetty, N.P.; Amruthesh, K.N.; Shetty, H.S. A Chitosan formulation ElexaTM induces downy mildew disease resistance and growth promotion in pearl millet. Crop Prot. 2004, 23, 881–888. [Google Scholar] [CrossRef]
- Reddy, M.V.B.; Arul, J.; Angers, P.; Couture, L. Chitosan treatment of wheat seeds induces resistance to Fusarium graminearum and improves seed quality. J. Agric. Food Chem. 1999, 47, 1208–1216. [Google Scholar] [CrossRef]
- Carrillo-Reche, J. ‘On-Farm’ Seed Priming: A Strategy for Ecological & Sustainable Agriculture. Ph.D. Thesis, University of Stirling, Stirling, UK, 29 April 2020. [Google Scholar]
- Li, K.; Xing, R.; Liu, S.; Li, P. Chitin and Chitosan Fragments Responsible for Plant Elicitor and Growth Stimulator. J. Agric. Food Chem. 2020, 68, 12203–12211. [Google Scholar] [CrossRef]
- Bingham, I.J.; Walters, D.R.; Foulkes, M.J.; Paveley, N.D. Crop traits and the tolerance of wheat and barley to foliar disease. Ann. Appl. Biol. 2009, 154, 159–173. [Google Scholar] [CrossRef]
- Wang, M.; Chen, Y.; Zhang, R.; Wang, W.; Zhao, X.; Du, Y.; Yin, H. Effects of chitosan oligosaccharides on the yield components and production quality of different wheat cultivars (Triticum aestivum L.) in Northwest China. Field Crop. Res. 2015, 172, 11–20. [Google Scholar] [CrossRef]
- Carrillo-Reche, J.; Vallejo-Marín, M.; Quilliam, R.S. Quantifying the potential of ‘on-farm’ seed priming to increase crop performance in developing countries. A meta-analysis. Agron. Sustain. Dev. 2018, 38, 64. [Google Scholar] [CrossRef]
- Harris, D.; Breese, W.A.; Rao, J.V.D.K.K. The improvement of crop yield in marginal environments using “on-farm” seed priming: Nodulation, nitrogen fixation and disease resisitance. Aust. J. Agric. Res. 2005, 56, 1211–1218. [Google Scholar] [CrossRef]
- Harris, D. Development and Testing of “On-Farm” Seed Priming. Adv. Agron. 2006, 90, 129–178. [Google Scholar] [CrossRef]
- Rashid, A.; Harris, D.; Hollington, P.; Ali, S. On-farm seed priming reduces yield losses of mungbean (Vigna radiata) associated with mungbean yellow mosaic virus in the North West Frontier Province of Pakistan. Crop Prot. 2004, 23, 1119–1124. [Google Scholar] [CrossRef]
- Musa, A.M.; Harris, D.; Johansen, C.; Kumar, J.V.D.K. Short duration chickpea to replace fallow after aman rice: The role of on-farm seed priming in the High Barind Tract of Bangladesh. Exp. Agric. 2001, 37, 509–521. [Google Scholar] [CrossRef][Green Version]
- Harris, D.; Joshi, A.; Khan, P.A.; Gothkar, P.; Sodhi, P.S. On-Farm Seed Priming in Semi-Arid Agriculture: Development and Evaluation in Maize, Rice and Chickpea in India Using Participatory Methods. Exp. Agric. 1999, 35, 15–29. [Google Scholar] [CrossRef]
- Lamichhane, J.R.; You, M.P.; Laudinot, V.; Barbetti, M.J.; Aubertot, J.-N. Revisiting Sustainability of Fungicide Seed Treatments for Field Crops. Plant Dis. 2020, 104, 610–623. [Google Scholar] [CrossRef]
- Kennedy, S.P.; Bingham, I.J.; Spink, J.H. Determinants of spring barley yield in a high-yield potential environment. J. Agric. Sci. 2017, 155, 60–80. [Google Scholar] [CrossRef]
- Bingham, I.J.; Topp, C.F.E. Potential contribution of selected canopy traits to the tolerance of foliar disease by spring barley. Plant Pathol. 2009, 58, 1010–1020. [Google Scholar] [CrossRef]
- Bingham, I.J.; Hoad, S.P.; Newton, A.C.; Thomas, W.T.B. Avoidance and tolerance of foliar disease in barley: Opportunities for improvement. In In Proceedings of the Dundee Conference. Crop Protection in Northern Britain, Dundee, UK, 26–27 February 2008; pp. 139–144. [Google Scholar]
- Zhan, J.; Fitt, B.D.L.; Pinnschmidt, H.O.; Oxley, S.J.P.; Newton, A.C. Resistance, epidemiology and sustainable management of Rhynchosporium secalis populations on barley. Plant Pathol. 2008, 57, 1–14. [Google Scholar] [CrossRef]
- Bingham, I.J.; Blake, J.; Foulkes, M.J.; Spink, J. Is barley yield in the UK sink limited? I. Post-anthesis radiation interception, radiation-use efficiency and source-sink balance. Field Crop. Res. 2007, 101, 198–211. [Google Scholar] [CrossRef]
- Collin, F.; Bancal, P.; Spink, J.; Appelgren, P.K.; Smith, J.; Paveley, N.D.; Bancal, M.O.; Foulkes, M.J. Wheat lines exhibiting variation in tolerance of Septoria tritici blotch differentiated by grain source limitation. Field Crop. Res. 2018, 217, 1–10. [Google Scholar] [CrossRef]
- AHDB. AHDB Recommended Lists for Cereals and Oilseeds 2018/19. Available online: https://ahdb.org.uk/rlarchive (accessed on 20 September 2018).
- Carrillo-Reche, J.; Newton, A.C.; Quilliam, R.S. Using seed respiration as a tool for calculating optimal soaking times for ‘on-farm’ seed priming of barley (Hordeum vulgare). Seed Sci. Res. 2021, 31, 116–124. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Ramirez-Garcia, J.; Almendros, P.; Quemada, M. Ground cover and leaf area index relationship in a grass, legume and crucifer crop. Plant Soil Environ. 2012, 58, 385–390. [Google Scholar] [CrossRef]
- Kefauver, S.C.; Kerfal, S.; Fernandez-Gallego, J.A.; El-Haddad, G.; Araus, J.L. CerealScanner. Available online: https://integrativecropecophysiology.com/software-development/cerealscanner/ (accessed on 28 May 2020).
- Fernandez-Gallego, J.A.; Kefauver, S.C.; Vatter, T.; Aparicio Gutiérrez, N.; Nieto-Taladriz, M.T.; Araus, J.L. Low-cost assessment of grain yield in durum wheat using RGB images. Eur. J. Agron. 2019, 105, 146–156. [Google Scholar] [CrossRef]
- AHDB. AHDB Recommended Lists (RL): Cereal Trials Protocol 2017–21; AHDB: Stoneleigh, UK, 2018; p. 31. [Google Scholar]
- Waggoner, P.E.; Berger, R.D. Defoliation, Disease, and Growth. Phytopathology 1987, 77, 393–398. [Google Scholar]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing. Available online: https://www.R-project.org/ (accessed on 15 May 2021).
- Luo, D.; Ganesh, S.; Koolaard, J. Predictmeans: Calculate Predicted Means for Linear Model. Available online: https://CRAN.R-project.org/package=predictmeans (accessed on 11 April 2020).
- Waite, T.A.; Campbell, L.G. Controlling the false discovery rate and increasing statistical power in ecological studies. Ecoscience 2006, 13, 439–442. [Google Scholar] [CrossRef]
- Foulkes, M.J.; Paveley, N.D.; Worland, A.; Welham, S.J.; Thomas, J.; Snape, J.W. Major Genetic Changes in Wheat with Potential. Phytopathology 2006, 96, 680–688. [Google Scholar] [CrossRef] [PubMed]
- Parker, S.R.; Welham, S.; Paveley, N.D.; Foulkes, J.; Scott, R.K. Tolerance of septoria leaf blotch in winter wheat. Plant Pathol. 2004, 53, 1–10. [Google Scholar] [CrossRef]
- Walters, D.R.; Ratsep, J.; Havis, N.D. Controlling crop diseases using induced resistance: Challenges for the future. J. Exp. Bot. 2013, 64, 1263–1280. [Google Scholar] [CrossRef] [PubMed]
- Walters, D.R.; Havis, N.D.; Paterson, L.; Taylor, J.; Walsh, D.J. Cultivar Effects on the Expression of Induced Resistance in Spring Barley. Plant Dis. 2011, 95, 595–600. [Google Scholar] [CrossRef]
- Kuwabara, C.; Imai, R. Molecular basis of disease resistance acquired through cold acclimation in overwintering plants. J. Plant Biol. 2009, 52, 19–26. [Google Scholar] [CrossRef]
- Wiese, J.; Bagy, M.M.K.; Schubert, S. Soil properties, but not plant nutrients (N, P, K) interact with chemically induced resistance against powdery mildew in barley. J. Plant Nutr. Soil Sci. 2003, 166, 379–384. [Google Scholar] [CrossRef]
- Khan, M.R.; Fischer, S.; Egan, D.; Doohan, F.M. Biological control of fusarium seedling blight disease of wheat and barley. Phytopathology 2006, 96, 386–394. [Google Scholar] [CrossRef] [PubMed]
- Orzali, L.; Forni, C.; Riccioni, L. Effect of chitosan seed treatment as elicitor of resistance to Fusarium graminearum in wheat. Seed Sci. Technol. 2014, 42, 132–149. [Google Scholar] [CrossRef]
- Lamichhane, J.R.; Debaeke, P.; Steinberg, C.; You, M.P.; Barbetti, M.J.; Aubertot, J.-N. Abiotic and biotic factors affecting crop seed germination and seedling emergence: A conceptual framework. Plant Soil 2018, 432, 1–28. [Google Scholar] [CrossRef]
- Newton, A.C.; Guy, D.C. Assessing Effects of Crop History and Soil Amendments on Yields of Subsequent Crops. Agric. Sci. 2020, 11, 514–527. [Google Scholar] [CrossRef]
- Newton, A.C.; Dashwood, E.P. The Interaction of Humidity and Resistance Elicitors on Expression of Polygenic Resistance of Barley to Mildew. J. Phytopathol. 1998, 146, 123–130. [Google Scholar] [CrossRef]
- Lovell, D.J.; Parker, S.R.; Hunter, T.; Royle, D.J.; Coker, R.R. Influence of crop growth and structure on the risk of epidemics by Mycosphaerella graminicola (Septoria tritici) in winter wheat. Plant Pathol. 1997, 46, 126–138. [Google Scholar] [CrossRef]
- Thirugnanasambandam, A.; Wright, K.M.; Atkins, S.D.; Whisson, S.C.; Newton, A.C. Infection of Rrs1 barley by an incompatible race of the fungus Rhynchosporium secalis expressing the green fluorescent protein. Plant Pathol. 2011, 60, 513–521. [Google Scholar] [CrossRef]
- Harris, D.; Rashid, A.; Miraj, G.; Arif, M.; Shah, H. “On-farm” seed priming with zinc sulphate solution-A cost-effective way to increase the maize yields of resource-poor farmers. Field Crop. Res. 2007, 102, 119–127. [Google Scholar] [CrossRef]
- Murungu, F.S.; Chiduza, C.; Nyamugafata, P.; Clark, L.J.; Whalley, W.R.; Finch-Savage, W.E. Effects of “on-farm seed priming” on consecutive daily sowing occasions on the emergence and growth of maize in semi-arid Zimbabwe. Field Crop. Res. 2004, 89, 49–57. [Google Scholar] [CrossRef]
- Farooq, M.; Basra, S.M.A.; Rehman, H.; Saleem, B.A. Seed Priming Enhances the Performance of Late Sown Wheat (Triticum aestivum L.) by Improving Chilling Tolerance. J. Agron. Crop Sci. 2008, 194, 55–60. [Google Scholar] [CrossRef]
- Aune, J.B.; Ousman, A. Effect of Seed Priming and Micro-Dosing of Fertilizer on Sorghum and Pearl Millet in Western Sudan. Exp. Agric. 2011, 47, 419–430. [Google Scholar] [CrossRef]
- Arisnabarreta, S.; Miralles, D.J. Critical period for grain number establishment of near isogenic lines of two- and six-rowed barley. Field Crop. Res. 2008, 107, 196–202. [Google Scholar] [CrossRef]
- Bingham, I.J.; Hoad, S.P.; Thomas, W.T.B.; Newton, A.C. Yield response to fungicide of spring barley genotypes differing in disease susceptibility and canopy structure. Field Crop. Res. 2012, 139, 9–19. [Google Scholar] [CrossRef]
- Bingham, I.J.; Young, C.; Bounds, P.; Gravouil, C.; Paveley, N.D. Mechanisms by which fungicides increase grain sink capacity and yield of spring barley when visible disease severity is low or absent. Field Crop. Res. 2021, 261, 108011. [Google Scholar] [CrossRef]
- Ruske, R.E.; Gooding, M.J.; Jones, S.A. The effects of triazole and strobilurin fungicide programmes on nitrogen uptake, partitioning, remobilization and grain N accumulation in winter wheat cultivars. J. Agric. Sci. 2003, 140, 395–407. [Google Scholar] [CrossRef]
- García del Moral, M.B.; García del Moral, L.F. Tiller production and survival in relation to grain yield in winter and spring barley. Field Crop. Res. 1995, 44, 85–93. [Google Scholar] [CrossRef]
- Subedi, K.D.; Ma, B.L. Seed priming does not improve corn yield in a humid temperate environment. Agron. J. 2005, 97, 211–218. [Google Scholar] [CrossRef]
- Giri, G.S.; Schillinger, W.F. Seed Priming Winter Wheat for Germination, Emergence, and Yield. Crop Sci. 2003, 43, 2135–2141. [Google Scholar] [CrossRef]
- Rashid, A.; Hollington, P.A.; Harris, D.; Khan, P. On-farm seed priming for barley on normal, saline and saline-sodic soils in North West Frontier Province, Pakistan. Eur. J. Agron. 2006, 24, 276–281. [Google Scholar] [CrossRef]
- Wojtyla, Ł.; Lechowska, K.; Kubala, S.; Garnczarska, M. Molecular processes induced in primed seeds—increasing the potential to stabilize crop yields under drought conditions. J. Plant Physiol. 2016, 203, 116–126. [Google Scholar] [CrossRef] [PubMed]



| Cultivar | Date Listed | Type | Resistance Mildew 1 | Resistance Rhynchosporium 1 |
|---|---|---|---|---|
| SY Venture | 2012 | Two-row malting | 6 | 4 |
| KWS Cassia | 2010 | Two-row feed | 4 | 4 |
| KWS Tower | 2014 | Two-row feed | 5 | 5 |
| Site | Sowing Date | Latitude, Longitude | Elevation (m) | Soil Texture | Previous Crops | Harvest Date |
|---|---|---|---|---|---|---|
| Balruddery-Hutchens | 17 October | 56°29′03.5″ N 3°06′34.4″ W | 118 | Sandy loam | Barley (2017), Peas (2018) | 31 July |
| Mylnefield-East Loan | 29 October | 56°27′21.4″ N 3°04′25.2″ W | 13 | Sandy loam | Barley since 1986 | 2 August |
| Treatment | Commercial Product | Active Ingredient | Rate (L ha−1) | GS Applied 1 |
|---|---|---|---|---|
| T0 | Proline Corbel | Prothioconazole Fenpropimorph | 0.5 0.5 | GS 30 |
| T1 | Siltra Xpro Rover 500 Vegas | Bixafen and prothioconazole Chlorothalonil Cyflufenamid | 0.6 1 0.3 | GS 31–32 |
| T2 | Tucana Imprex Joules Proline | Pyraclostrobin Fluxapyroxad Chlorothalonil Prothioconazole | 1 2 1 0.3 | GS 49 |
| Site | Term | AUDPC Powdery Mildew | AUDPC Rhynchosporium |
|---|---|---|---|
| Balruddery | Fun | 0.069 | <0.001 |
| Cv | <0.001 | <0.001 | |
| Tr | 0.954 | 0.136 | |
| Fun × Cv | 0.212 | <0.001 | |
| Fun × Tr | 0.563 | 0.165 | |
| Cv × Tr | 0.870 | 0.243 | |
| Fun × Cv × Tr | 0.701 | 0.285 | |
| Mylnefield | Fun | 0.003 | <0.001 |
| Cv | <0.001 | <0.001 | |
| Tr | 0.040 | 0.189 | |
| Fun × Cv | <0.001 | <0.001 | |
| Fun × Tr | 0.079 | 0.190 | |
| Cv × Tr | 0.981 | 0.458 | |
| Fun × Cv × Tr | 0.943 | 0.457 |
| Tr | |||
|---|---|---|---|
| NP | OSP | CHP | |
| AUDPC powdery mildew | |||
| Balruddery | 723 a | 724 a | 725 a |
| Mylnefield | 453 ab | 427 b | 462 a |
| AUDPC rhynchosporium * | |||
| Balruddery | 156 a | 189 a | 176 a |
| Mylnefield | 135 a | 115 a | 141 a |
| Site | Term | GY (t ha−1) | G no. (m−2) | TGW (g) |
|---|---|---|---|---|
| Balruddery | Fun | 0.010 | 0.009 | 0.060 |
| Cv | <0.001 | <0.001 | <0.001 | |
| Tr | 0.028 | 0.005 | 0.264 | |
| Fun × Cv | 0.003 | 0.041 | 0.022 | |
| Fun × Tr | 0.193 | 0.281 | 0.237 | |
| Cv × Tr | 0.864 | 0.819 | 0.609 | |
| Fun × Cv × Tr | 0.762 | 0.829 | 0.436 | |
| Mylnefield | Fun | 0.015 | 0.045 | <0.001 |
| Cv | 0.047 | <0.001 | <0.001 | |
| Tr | 0.072 | 0.076 | 0.983 | |
| Fun × Cv | 0.630 | 0.738 | 0.023 | |
| Fun × Tr | 0.103 | 0.243 | 0.103 | |
| Cv × Tr | 0.793 | 0.817 | 0.969 | |
| Fun × Cv × Tr | 0.082 | 0.111 | 0.460 |
| Tr | |||
|---|---|---|---|
| NP | OSP | CHP | |
| GY (t ha−1) | |||
| Balruddery | 7.89 a | 7.54 b | 7.77 ab |
| Mylnefield | 6.75 a | 6.77 a | 6.51 a |
| G no. (m−2) | |||
| Balruddery | 13,929 a | 13,211 b | 13,736 a |
| Mylnefield | 11,692 a | 11,723 a | 11,281 a |
| TGW (g) | |||
| Balruddery | 56.5 a | 57.0 a | 56.5 a |
| Mylnefield | 57.8 a | 57.8 a | 57.8 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carrillo-Reche, J.; Newton, A.C.; Ferrando-Molina, F.; Quilliam, R.S. Can ‘On-Farm’ Seed Priming and Chitosan Seed Treatments Induce Host Defences in Winter Barley (Hordeum vulgare L.) under Field Conditions? Crops 2021, 1, 68-87. https://doi.org/10.3390/crops1020008
Carrillo-Reche J, Newton AC, Ferrando-Molina F, Quilliam RS. Can ‘On-Farm’ Seed Priming and Chitosan Seed Treatments Induce Host Defences in Winter Barley (Hordeum vulgare L.) under Field Conditions? Crops. 2021; 1(2):68-87. https://doi.org/10.3390/crops1020008
Chicago/Turabian StyleCarrillo-Reche, Javier, Adrian C. Newton, Francesc Ferrando-Molina, and Richard S. Quilliam. 2021. "Can ‘On-Farm’ Seed Priming and Chitosan Seed Treatments Induce Host Defences in Winter Barley (Hordeum vulgare L.) under Field Conditions?" Crops 1, no. 2: 68-87. https://doi.org/10.3390/crops1020008
APA StyleCarrillo-Reche, J., Newton, A. C., Ferrando-Molina, F., & Quilliam, R. S. (2021). Can ‘On-Farm’ Seed Priming and Chitosan Seed Treatments Induce Host Defences in Winter Barley (Hordeum vulgare L.) under Field Conditions? Crops, 1(2), 68-87. https://doi.org/10.3390/crops1020008







