Decision Trees for the Analysis of Gene Expression Levels of COVID-19: An Association with Alzheimer’s Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Microarray Collection
2.2. Data Processing
2.3. Statistical Analysis
2.4. Decision Tree
- Accuracy: the total number of correct classifications divided by the size of the corresponding test set.
- Sensitivity: ability to correctly identify patients with asymptomatic and symptomatic COVID-19.
- Specificity: ability to correctly identify patients who do not have COVID-19.
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brown, E.E.; Kumar, S.; Rajji, T.K.; Pollock, B.G.; Mulsant, B.H. Anticipating and Mitigating the Impact of the COVID-19 Pandemic on Alzheimer’s Disease and Related Dementias. Am. J. Geriatr. Psychiatry 2020, 28, 712–721. [Google Scholar] [CrossRef] [PubMed]
- Habas, K.; Nganwuchu, C.; Shahzad, F.; Gopalan, R.; Haque, M.; Rahman, S.; Majumder, A.A.; Nasim, T. Resolution of Coronavirus Disease 2019 (COVID-19). Expert Rev. Anti Infect. Ther. 2020, 18, 1201–1211. [Google Scholar] [CrossRef]
- Simonetti, A.; Bernardi, E.; Sani, G. Novel Advancements in COVID-19 and Neuroscience. J. Pers. Med. 2024, 14, 143. [Google Scholar] [CrossRef]
- World Health Organization. WHO COVID-19 Dashboard. Available online: https://data.who.int/dashboards/covid19/cases (accessed on 12 May 2024).
- Gorbalenya, A.E.; Baric, R.S.; De Groot, R.J.; Drosten, C.; Gulyaeva, A.A.; Haagmans, B.L.; Lauber, C.; Leontovich, A.M.; Neuman, B.W.; Penzar, D.; et al. The Species Severe Acute Respiratory Syndrome-Related Coronavirus: Classifying 2019-nCoV and Naming It SARS-CoV-2. Nat. Microbiol. 2020, 5, 536–544. [Google Scholar] [CrossRef]
- Gheblawi, M.; Wang, K.; Viveiros, A.; Nguyen, Q.; Zhong, J.-C.; Turner, A.J.; Raizada, M.K.; Grant, M.B.; Oudit, G.Y. Angiotensin-Converting Enzyme 2: SARS-CoV-2 Receptor and Regulator of the Renin-Angiotensin System: Celebrating the 20th Anniversary of the Discovery of ACE2. Circ. Res. 2020, 126, 1456–1474. [Google Scholar] [CrossRef] [PubMed]
- Zanza, C.; Romenskaya, T.; Manetti, A.; Franceschi, F.; La Russa, R.; Bertozzi, G.; Maiese, A.; Savioli, G.; Volonnino, G.; Longhitano, Y. Cytokine Storm in COVID-19: Immunopathogenesis and Therapy. Medicina 2022, 58, 144. [Google Scholar] [CrossRef]
- Ramesh, P.; Veerappapillai, S.; Karuppasamy, R. Gene Expression Profiling of Corona Virus Microarray Datasets to Identify Crucial Targets in COVID-19 Patients. Gene Rep. 2021, 22, 100980. [Google Scholar] [CrossRef] [PubMed]
- Pacheco-Herrero, M.; Soto-Rojas, L.O.; Harrington, C.R.; Flores-Martinez, Y.M.; Villegas-Rojas, M.M.; León-Aguilar, A.M.; Martínez-Gómez, P.A.; Campa-Córdoba, B.B.; Apátiga-Pérez, R.; Corniel-Taveras, C.N.; et al. Elucidating the Neuropathologic Mechanisms of SARS-CoV-2 Infection. Front. Neurol. 2021, 12, 660087. [Google Scholar] [CrossRef]
- Baig, A.M. Neurological Manifestations in COVID-19 Caused by SARS-CoV-2. CNS Neurosci. Ther. 2020, 26, 499–501. [Google Scholar] [CrossRef]
- Jackson, C.B.; Farzan, M.; Chen, B.; Choe, H. Mechanisms of SARS-CoV-2 Entry into Cells. Nat. Rev. Mol. Cell Biol. 2022, 23, 3–20. [Google Scholar] [CrossRef]
- Wang, F.; Kream, R.M.; Stefano, G.B. Long-Term Respiratory and Neurological Sequelae of COVID-19. Med. Sci. Monit. 2020, 26, e928996. [Google Scholar] [CrossRef]
- Parks, J.M.; Smith, J.C. How to Discover Antiviral Drugs Quickly. N. Engl. J. Med. 2020, 382, 2261–2264. [Google Scholar] [CrossRef] [PubMed]
- Rudnicka-Drożak, E.; Drożak, P.; Mizerski, G.; Zaborowski, T.; Ślusarska, B.; Nowicki, G.; Drożak, M. Links between COVID-19 and Alzheimer’s Disease—What Do We Already Know? Int. J. Environ. Res. Public Health 2023, 20, 2146. [Google Scholar] [CrossRef] [PubMed]
- Carvajal Carvajal, C. Biología Molecular de La Enfermedad de Alzheimer. Med. Leg. Costa Rica 2016, 33, 2. Available online: https://www.scielo.sa.cr/scielo.php?script=sci_arttext&pid=S1409-00152016000200104 (accessed on 15 January 2025).
- Alzheimer’s Association. What Is Dementia? Available online: https://www.alz.org/alzheimers-dementia/what-is-dementia (accessed on 15 January 2025).
- Hernández-Contreras, K.A.; Martínez-Díaz, J.A.; Hernández-Aguilar, M.E.; Herrera-Covarrubias, D.; Rojas-Durán, F.; Chi-Castañeda, L.D.; García- Hernández, L.I.; Aranda-Abreu, G.E. Alterations of mRNAs and Non-Coding RNAs Associated with Neuroinflammation in Alzheimer’s Disease. Mol. Neurobiol. 2024, 61, 5826–5840. [Google Scholar] [CrossRef]
- Serrano-Castro, P.J.; Estivill-Torrús, G.; Cabezudo-García, P.; Reyes-Bueno, J.A.; Ciano Petersen, N.; Aguilar-Castillo, M.J.; Suárez-Pérez, J.; Jiménez-Hernández, M.D.; Moya-Molina, M.Á.; Oliver-Martos, B.; et al. Influencia de la infección SARS-CoV-2 sobre enfermedades neurodegenerativas y neuropsiquiátricas: ¿una pandemia demorada? Neurología 2020, 35, 245–251. [Google Scholar] [CrossRef]
- Alzheimer’s Association. 2023 Alzheimer’s Disease Facts and Figures. Alzheimers Dement. 2023, 19, 1598–1695. [Google Scholar] [CrossRef]
- Abasi, L.S.; Elathram, N.; Movva, M.; Deep, A.; Corbett, K.D.; Debelouchina, G.T. Phosphorylation Regulates Tau’s Phase Separation Behavior and Interactions with Chromatin. Commun. Biol. 2024, 7, 251. [Google Scholar] [CrossRef]
- Monteiro, A.R.; Barbosa, D.J.; Remião, F.; Silva, R. Alzheimer’s Disease: Insights and New Prospects in Disease Pathophysiology, Biomarkers and Disease-Modifying Drugs. Biochem. Pharmacol. 2023, 211, 115522. [Google Scholar] [CrossRef]
- Golzari-Sorkheh, M.; Weaver, D.F.; Reed, M.A. COVID-19 as a Risk Factor for Alzheimer’s Disease. J. Alzheimers Dis. 2023, 91, 1–23. [Google Scholar] [CrossRef]
- World Health Organization. Global Action Plan on the Public Health Response to Dementia 2017–2025. Available online: https://www.who.int/publications/i/item/global-action-plan-on-the-public-health-response-to-dementia-2017---2025 (accessed on 12 May 2024).
- Wei, H.-F.; Anchipolovsky, S.; Vera, R.; Liang, G.; Chuang, D.-M. Potential Mechanisms Underlying Lithium Treatment for Alzheimer’s Disease and COVID-19. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 2201–2214. [Google Scholar] [CrossRef] [PubMed]
- Ferini-Strambi, L.; Salsone, M. COVID-19 and Neurological Disorders: Are Neurodegenerative or Neuroimmunological Diseases More Vulnerable? J. Neurol. 2021, 268, 409–419. [Google Scholar] [CrossRef] [PubMed]
- Ciaccio, M.; Lo Sasso, B.; Scazzone, C.; Gambino, C.M.; Ciaccio, A.M.; Bivona, G.; Piccoli, T.; Giglio, R.V.; Agnello, L. COVID-19 and Alzheimer’s Disease. Brain Sci. 2021, 11, 305. [Google Scholar] [CrossRef] [PubMed]
- Matveeva, N.; Kiselev, I.; Baulina, N.; Semina, E.; Kakotkin, V.; Agapov, M.; Kulakova, O.; Favorova, O. Shared Genetic Architecture of COVID-19 and Alzheimer’s Disease. Front. Aging Neurosci. 2023, 15, 1287322. [Google Scholar] [CrossRef]
- Bombón-Albán, P.E.; González-Aparicio, I.I. Apoliproteina E Vinculado a Una Mayor Susceptibilidad al SARS-CoV-2. Acta Neurológica Colomb. 2021, 37, 158–160. [Google Scholar] [CrossRef]
- Vavougios, G.D.; Mavridis, T.; Doskas, T.; Papaggeli, O.; Foka, P.; Hadjigeorgiou, G. SARS-CoV-2-Induced Type I Interferon Signaling Dysregulation in Olfactory Networks Implications for Alzheimer’s Disease. Curr. Issues Mol. Biol. 2024, 46, 4565–4579. [Google Scholar] [CrossRef]
- Vavougios, G.D.; Tseriotis, V.-S.; Liampas, A.; Mavridis, T.; De Erausquin, G.A.; Hadjigeorgiou, G. Type I Interferon Signaling, Cognition and Neurodegeneration Following COVID-19: Update on a Mechanistic Pathogenetic Model with Implications for Alzheimer’s Disease. Front. Hum. Neurosci. 2024, 18, 1352118. [Google Scholar] [CrossRef]
- Lee, J.H.; Kanwar, B.; Khattak, A.; Balentine, J.; Nguyen, N.H.; Kast, R.E.; Lee, C.J.; Bourbeau, J.; Altschuler, E.L.; Sergi, C.M.; et al. COVID-19 Molecular Pathophysiology: Acetylation of Repurposing Drugs. Int. J. Mol. Sci. 2022, 23, 13260. [Google Scholar] [CrossRef]
- Twarowski, B.; Herbet, M. Inflammatory Processes in Alzheimer’s Disease—Pathomechanism, Diagnosis and Treatment: A Review. Int. J. Mol. Sci. 2023, 24, 6518. [Google Scholar] [CrossRef]
- Chagas, L.D.S.; Serfaty, C.A. The Influence of Microglia on Neuroplasticity and Long-Term Cognitive Sequelae in Long COVID: Impacts on Brain Development and Beyond. Int. J. Mol. Sci. 2024, 25, 3819. [Google Scholar] [CrossRef]
- Heller, M.J. DNA Microarray Technology: Devices, Systems, and Applications. Annu. Rev. Biomed. Eng. 2002, 4, 129–153. [Google Scholar] [CrossRef] [PubMed]
- Premkumar, T.; Sajitha Lulu, S. Molecular Crosstalk between COVID-19 and Alzheimer’s Disease Using Microarray and RNA-Seq Datasets: A System Biology Approach. Front. Med. 2023, 10, 1151046. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Tao, L.; Zhou, Y.; Zhu, Y.; Li, C.; Pan, Y.; Yao, P.; Qian, X.; Liu, J. Identification of biomarkers in Alzheimer’s disease and COVID-19 by bioinformatics combining single-cell data analysis and machine learning algorithms. PLoS ONE 2025, 20, e0317915. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.A.; Daley, M.; Van Nynatten, L.R.; Slessarev, M.; Cepinskas, G.; Fraser, D.D. A Reduced Proteomic Signature in Critically Ill Covid-19 Patients Determined with Plasma Antibody Micro-Array and Machine Learning. Clin. Proteom. 2024, 21, 33. [Google Scholar] [CrossRef]
- Edgar, R. Gene Expression Omnibus: NCBI Gene Expression and Hybridization Array Data Repository. Nucleic Acids Res. 2002, 30, 207–210. [Google Scholar] [CrossRef]
- Masood, K.I.; Yameen, M.; Ashraf, J.; Shahid, S.; Mahmood, S.F.; Nasir, A.; Nasir, N.; Jamil, B.; Ghanchi, N.K.; Khanum, I.; et al. Upregulated Type I Interferon Responses in Asymptomatic COVID-19 Infection Are Associated with Improved Clinical Outcome. Sci. Rep. 2021, 11, 22958. [Google Scholar] [CrossRef]
- Bioconductor Open Source Software for Bioinformatics. Available online: https://www.bioconductor.org/ (accessed on 7 May 2024).
- Sepulveda, J.L. Using R and Bioconductor in Clinical Genomics and Transcriptomics. J. Mol. Diagn. 2020, 22, 3–20. [Google Scholar] [CrossRef]
- Carvalho, B.S.; Irizarry, R.A. A Framework for Oligonucleotide Microarray Preprocessing. Bioinformatics 2010, 26, 2363–2367. [Google Scholar] [CrossRef]
- Aguado, M. Microarrays de ADN en Microbiología DNA Microarrays in Microbiology. RCCV 2007, 1, 125–134. [Google Scholar]
- Saeed, A.I.; Bhagabati, N.K.; Braisted, J.C.; Liang, W.; Sharov, V.; Howe, E.A.; Li, J.; Thiagarajan, M.; White, J.A.; Quackenbush, J. TM4 Microarray Software Suite. In Methods in Enzymology; 2006; Volume 411, pp. 134–193. ISBN 978-0-12-182816-5. [Google Scholar]
- De Ville, B. Decision Trees. WIREs Comput. Stat. 2013, 5, 448–455. [Google Scholar] [CrossRef]
- Quinlan, J.R. Induction of Decision Trees. Mach. Learn. 1986, 1, 81–106. [Google Scholar] [CrossRef]
- Charbuty, B.; Abdulazeez, A. Classification Based on Decision Tree Algorithm for Machine Learning. J. Appl. Sci. Technol. Trends 2021, 2, 20–28. [Google Scholar] [CrossRef]
- Marsland, S. Machine Learning: An Algorithmic Perspective, 2nd ed.; Chapman & Hall/CRC: Boca Raton, FL, USA, 2015; ISBN 978-1-4665-8333-7. [Google Scholar]
- Tharwat, A. Classification Assessment Methods. Appl. Comput. Inform. 2021, 17, 168–192. [Google Scholar] [CrossRef]
- Geurts, P.; Irrthum, A.; Wehenkel, L. Supervised learning with decision tree-based methods in computational and systems biology. Mol. Biosyst. 2009, 5, 1593–1605. [Google Scholar] [CrossRef]
- Waikato Environment for Knowledge Analysis the Weka Workbench. Available online: https://ml.cms.waikato.ac.nz/weka/index.html (accessed on 12 May 2024).
- Wong, T.-T. Performance Evaluation of Classification Algorithms by K-Fold and Leave-One-out Cross Validation. Pattern Recognit. 2015, 48, 2839–2846. [Google Scholar] [CrossRef]
- Jalota, C.; Agrawal, R. Feature Selection Algorithms and Student Academic Performance: A Study. In International Conference on Innovative Computing and Communications; Gupta, D., Khanna, A., Bhattacharyya, S., Hassanien, A.E., Anand, S., Jaiswal, A., Eds.; Advances in Intelligent Systems and Computing; Springer Singapore: Singapore, 2021; Volume 1165, pp. 317–328. ISBN 978-981-15-5112-3. [Google Scholar]
- Bansal, D.; Khanna, K.; Chhikara, R.; Dua, R.K.; Malhotra, R. Analysis of Classification & Feature Selection Techniques for Detecting Dementia. SSRN Electron. J. 2019. [Google Scholar] [CrossRef]
- Kamarudin, M.H.; Maple, C.; Watson, T. Hybrid Feature Selection Technique for Intrusion Detection System. Int. J. High Perform. Comput. Netw. 2019, 13, 232. [Google Scholar] [CrossRef]
- Robin, X.; Turck, N.; Hainard, A.; Tiberti, N.; Lisacek, F.; Sanchez, J.C.; Müller, M. pROC: An open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinform. 2011, 12, 77. [Google Scholar] [CrossRef]
- Ren, J.; He, T.; Li, Y.; Liu, S.; Du, Y.; Jiang, Y.; Wu, C. Network-Based Regularization for High Dimensional SNP Data in the Case–Control Study of Type 2 Diabetes. BMC Genet. 2017, 18, 44. [Google Scholar] [CrossRef]
- McCaughan, J.A.; McKnight, A.J.; Maxwell, A.P. Genetics of New-Onset Diabetes after Transplantation. J. Am. Soc. Nephrol. 2014, 25, 1037–1049. [Google Scholar] [CrossRef]
- Chang, Y.-S.; Lin, C.-Y.; Liu, T.-Y.; Huang, C.-M.; Chung, C.-C.; Chen, Y.-C.; Tsai, F.-J.; Chang, J.-G.; Chang, S.-J. Polygenic Risk Score Trend and New Variants on Chromosome 1 Are Associated with Male Gout in Genome-Wide Association Study. Arthritis Res. Ther. 2022, 24, 229. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Noda, T. Autophagosome Formation in Relation to the Endoplasmic Reticulum. J. Biomed. Sci. 2020, 27, 97. [Google Scholar] [CrossRef] [PubMed]
- Marrano, N.; Biondi, G.; Borrelli, A.; Rella, M.; Zambetta, T.; Di Gioia, L.; Caporusso, M.; Logroscino, G.; Perrini, S.; Giorgino, F.; et al. Type 2 Diabetes and Alzheimer’s Disease: The Emerging Role of Cellular Lipotoxicity. Biomolecules 2023, 13, 183. [Google Scholar] [CrossRef] [PubMed]
- Janoutová, J.; Machaczka, O.; Zatloukalová, A.; Janout, V. Is Alzheimer’s Disease a Type 3 Diabetes? A Review. Cent. Eur. J. Public Health 2022, 30, 139–143. [Google Scholar] [CrossRef]
- Hernández-Contreras, K.A.; Martínez-Díaz, J.A.; Hernández-Aguilar, M.E.; Herrera-Covarrubias, D.; Rojas-Durán, F.; Aranda Abreu, G.E. Mecanismos de Asociación Entre Enfermedad de Alzheimer y Diabetes Mellitus: La Paradoja de La Insulina. Arch. Neurocienc. 2021, 25, 45–54. [Google Scholar] [CrossRef]
- Olawore, O.; Turner, L.; Evans, M.; Johnson, S.; Huling, J.; Bramante, C.; Buse, J.; Stürmer, T. Risk of Post-Acute Sequelae of SARS-CoV-2 Infection (PASC) Among Patients with Type 2 Diabetes Mellitus on Anti-Hyperglycemic Medications. Clin. Epidemiol. 2024, 16, 379–393. [Google Scholar] [CrossRef]
- Hass, D.T.; Barnstable, C.J. Uncoupling Proteins in the Mitochondrial Defense against Oxidative Stress. Prog. Retin. Eye Res. 2021, 83, 100941. [Google Scholar] [CrossRef]
- Thangavel, R.; Kempuraj, D.; Zaheer, S.; Raikwar, S.; Ahmed, M.E.; Selvakumar, G.P.; Iyer, S.S.; Zaheer, A. Glia Maturation Factor and Mitochondrial Uncoupling Proteins 2 and 4 Expression in the Temporal Cortex of Alzheimer’s Disease Brain. Front. Aging Neurosci. 2017, 9, 150. [Google Scholar] [CrossRef]
- Sreedhar, A.; Zhao, Y. Uncoupling Protein 2 and Metabolic Diseases. Mitochondrion 2017, 34, 135–140. [Google Scholar] [CrossRef]
- Yan, X.; Xu, F.; Ji, J.; Song, P.; Pei, Y.; He, M.; Wang, Z.; You, S.; Hua, Z.; Cheng, J.; et al. Activation of UCP2 by Anethole Trithione Suppresses Neuroinflammation after Intracerebral Hemorrhage. Acta Pharmacol. Sin. 2022, 43, 811–828. [Google Scholar] [CrossRef]
- Guo, Q.; He, J.; Zhang, H.; Yao, L.; Li, H. Oleanolic Acid Alleviates Oxidative Stress in Alzheimer’s Disease by Regulating Stanniocalcin-1 and Uncoupling Protein-2 Signalling. Clin. Exp. Pharmacol. Physiol. 2020, 47, 1263–1271. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Zhao, Y.; Zhao, B. Superoxide Anion, Uncoupling Proteins and Alzheimer’s Disease. J. Clin. Biochem. Nutr. 2010, 46, 187–194. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shi, X.; Wei, T.; Hu, Y.; Wang, M.; Tang, Y. The Associations between Plasma Soluble Trem1 and Neurological Diseases: A Mendelian Randomization Study. J. Neuroinflamm. 2022, 19, 218. [Google Scholar] [CrossRef]
- Replogle, J.M.; Chan, G.; White, C.C.; Raj, T.; Winn, P.A.; Evans, D.A.; Sperling, R.A.; Chibnik, L.B.; Bradshaw, E.M.; Schneider, J.A.; et al. A TREM 1 Variant Alters the Accumulation of Alzheimer-related Amyloid Pathology. Ann. Neurol. 2015, 77, 469–477. [Google Scholar] [CrossRef]
- Jiang, T.; Gong, P.-Y.; Tan, M.-S.; Xue, X.; Huang, S.; Zhou, J.-S.; Tan, L.; Zhang, Y.-D. Soluble TREM1 Concentrations Are Increased and Positively Correlated with Total Tau Levels in the Plasma of Patients with Alzheimer’s Disease. Aging Clin. Exp. Res. 2019, 31, 1801–1805. [Google Scholar] [CrossRef] [PubMed]
- Gombart, A.F.; Pierre, A.; Maggini, S. A Review of Micronutrients and the Immune System–Working in Harmony to Reduce the Risk of Infection. Nutrients 2020, 12, 236. [Google Scholar] [CrossRef]
- Yazdanpanah, F.; Hamblin, M.R.; Rezaei, N. The Immune System and COVID-19: Friend or Foe? Life Sci. 2020, 256, 117900. [Google Scholar] [CrossRef]
- Kratzer, B.; Gattinger, P.; Trapin, D.; Ettel, P.; Körmöczi, U.; Rottal, A.; Stieger, R.B.; Sehgal, A.N.A.; Feichter, M.; Borochova, K.; et al. Differential Decline of SARS-CoV-2-specific Antibody Levels, Innate and Adaptive Immune Cells, and Shift of Th1/Inflammatory to Th2 Serum Cytokine Levels Long after First COVID-19. Allergy 2024, 79, 2482–2501. [Google Scholar] [CrossRef]
- Wu, K.-M.; Zhang, Y.-R.; Huang, Y.-Y.; Dong, Q.; Tan, L.; Yu, J.-T. The Role of the Immune System in Alzheimer’s Disease. Ageing Res. Rev. 2021, 70, 101409. [Google Scholar] [CrossRef]
- Hernández-Contreras-Contreras, K.A.; Hernández-Aguilar-Aguilar, M.E.; Herrera-Covarrubias, D.; Rojas-Durán, F.; Aranda-Abreu, G.E. ¿La Neuroinflamación Por La COVID 19 Es Una Estrategia Fallida Del Organismo Para Combatir al Virus? 2022, 2, 1–7. Available online: https://igbmgenetica.medium.com/sobre-los-autores-c1fc743c1610 (accessed on 15 January 2025).
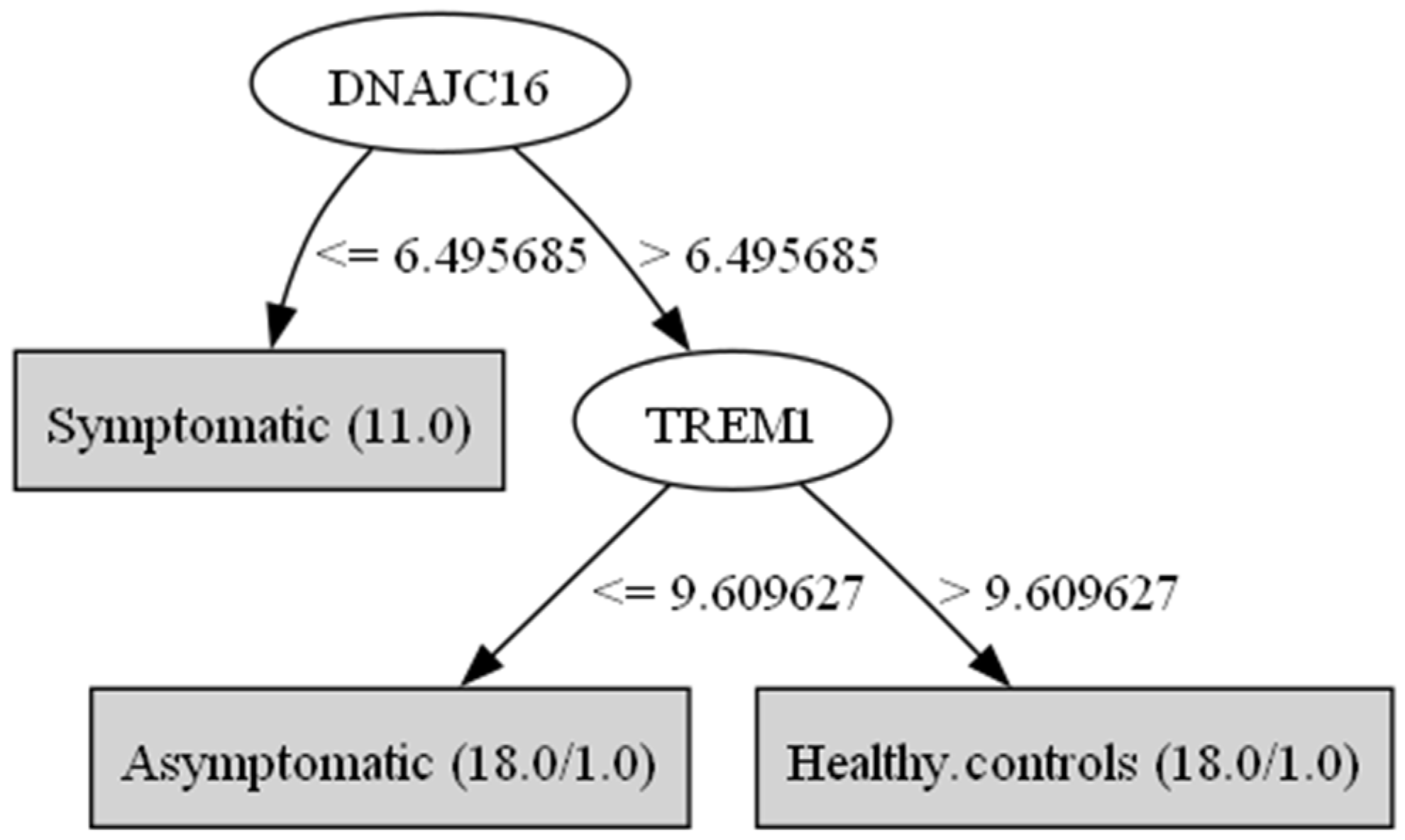
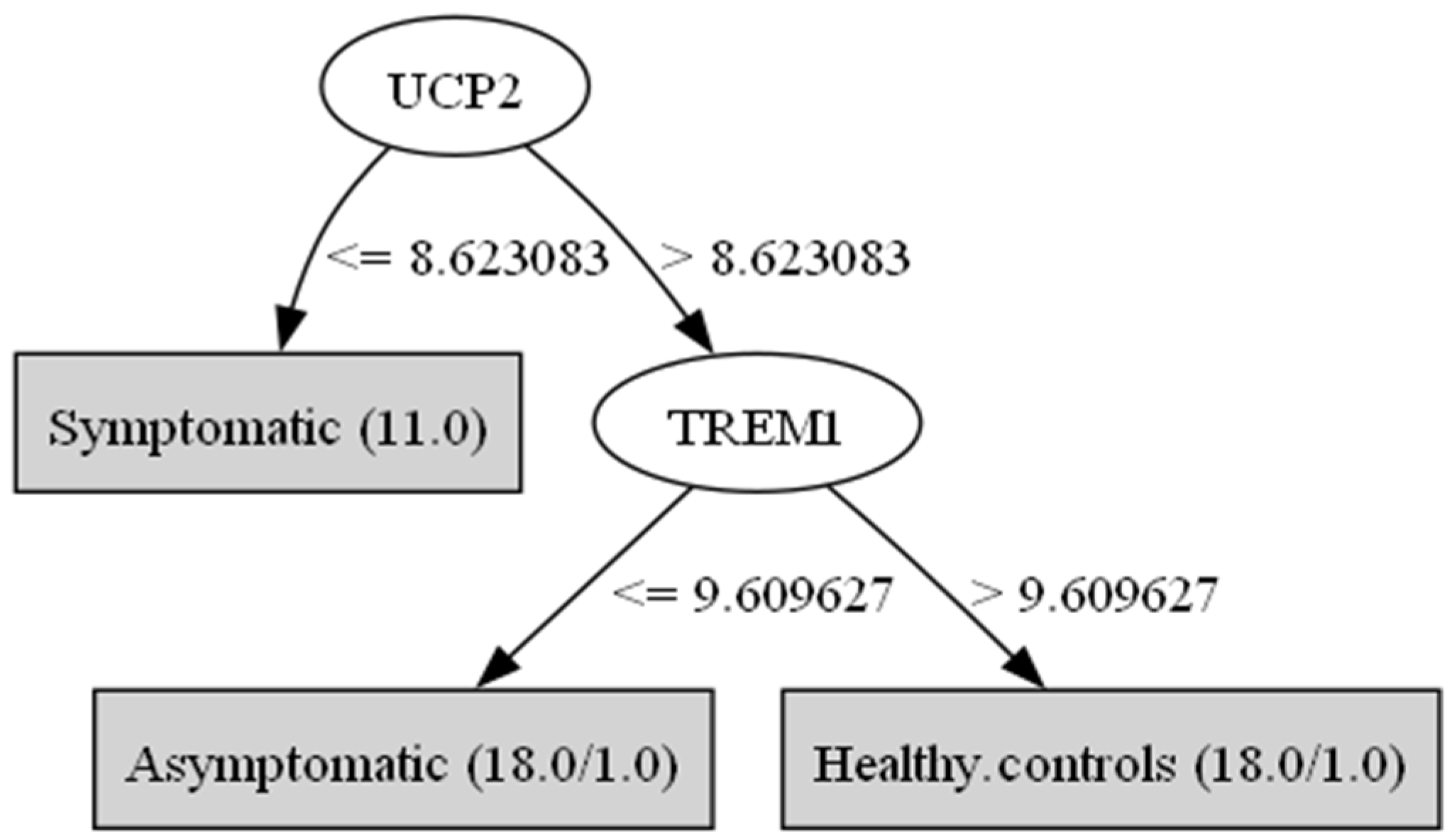
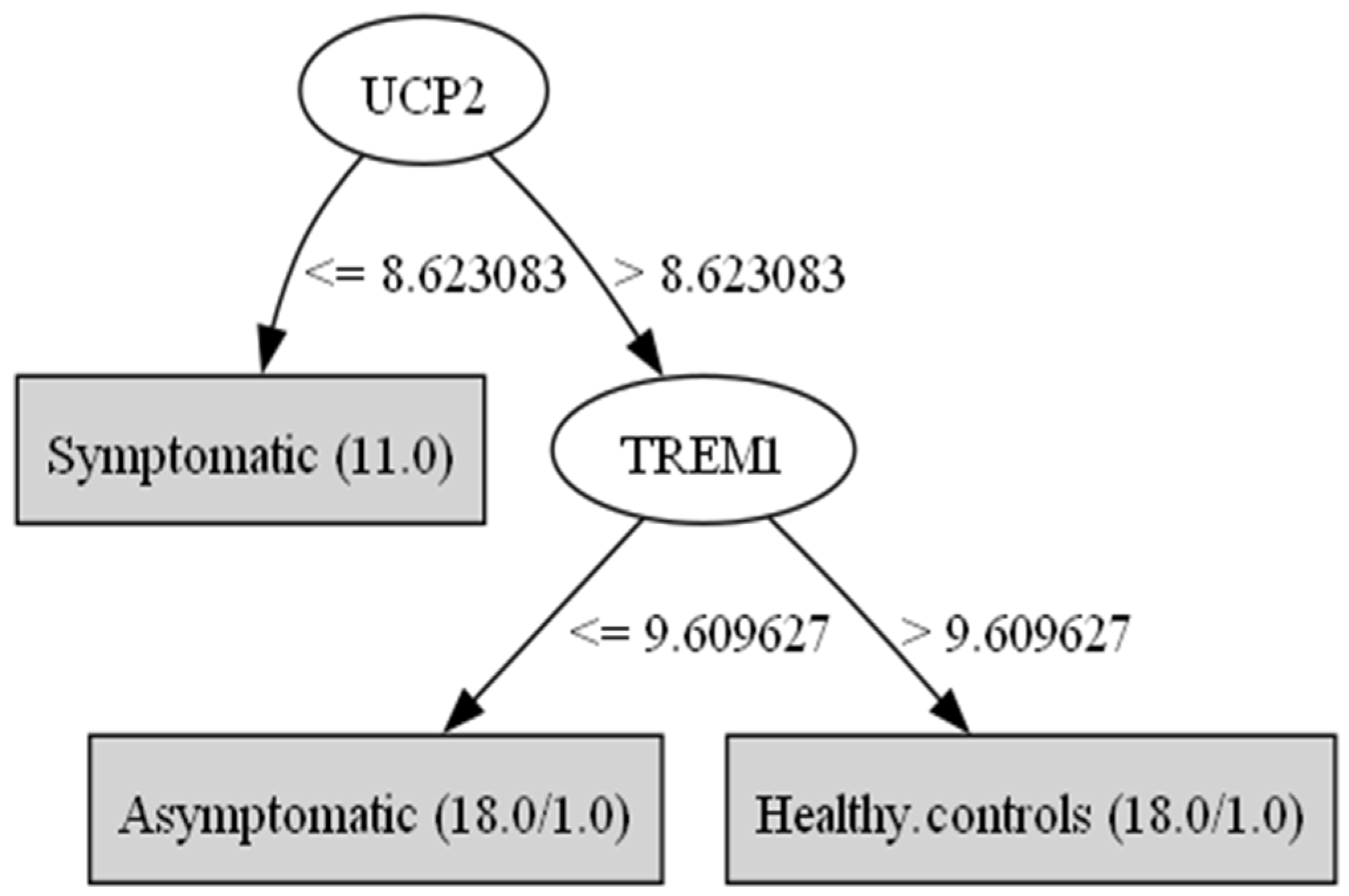
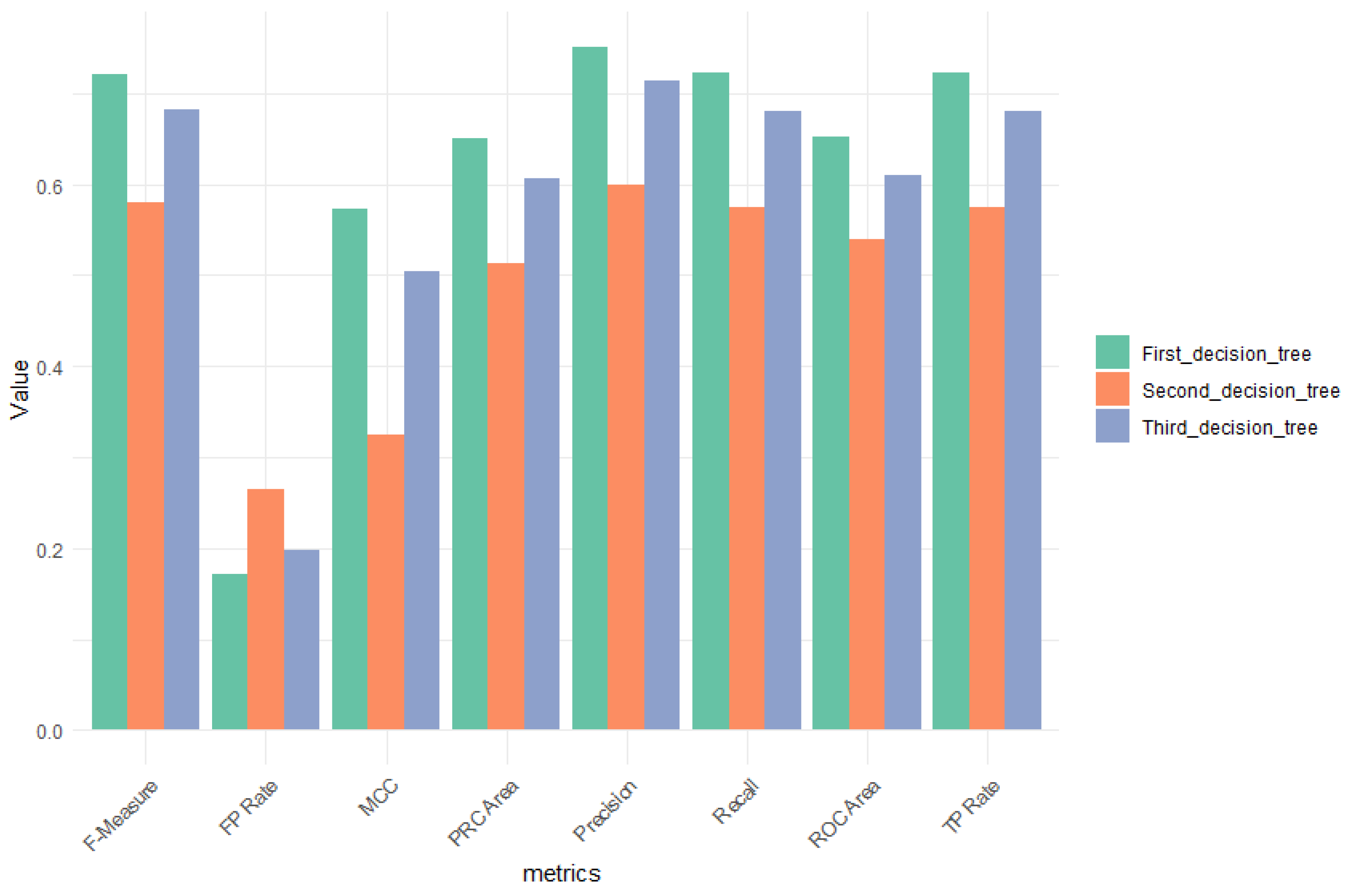
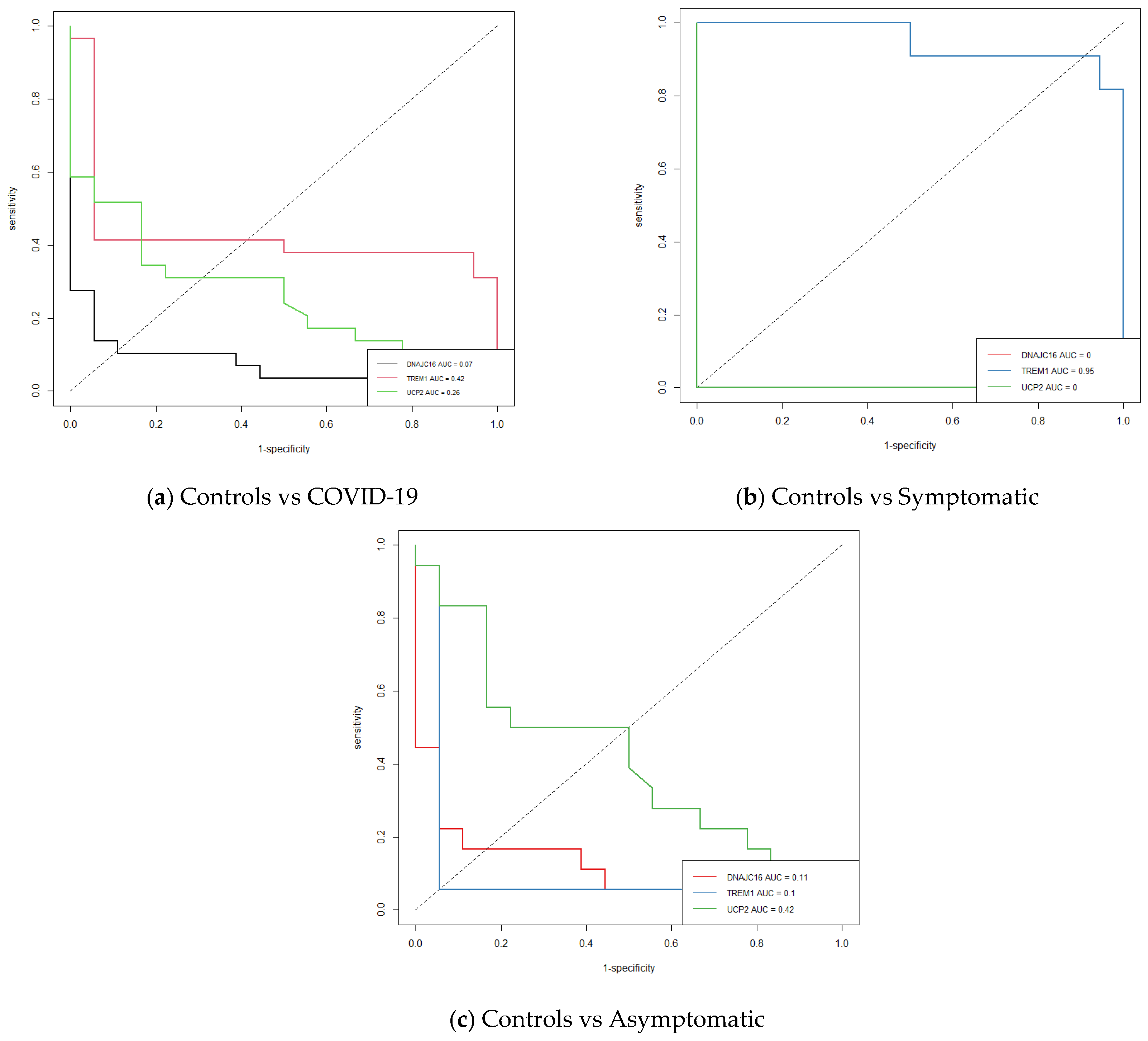
| Genes/Groups | Symptomatic Mean | Symptomatic std.dev | Asymptomatic Mean | Asymptomatic std.dev | Healthy Controls Mean | Healthy Controls std.dev | F Ratio |
|---|---|---|---|---|---|---|---|
| TMEM106A | 5.816394 | 0.3775489 | 7.4241314 | 0.23304962 | 7.463178 | 0.2478398 | 144.75156 |
| TNFAIP3 | 11.618307 | 0.4019925 | 8.287449 | 0.50382364 | 8.084149 | 0.38873255 | 257.89365 |
| IER3 | 10.167634 | 0.5320755 | 7.5864162 | 0.3664378 | 7.700342 | 0.31561947 | 173.8846 |
| ZNF394 | 7.964314 | 0.2556696 | 6.6509304 | 0.27549538 | 6.463861 | 0.2902587 | 110.72787 |
| ABCA13 | 8.67961 | 0.5993782 | 4.191325 | 1.152125 | 4.047996 | 0.5659465 | 122.08616 |
| FBXL14 | 6.397647 | 0.2234289 | 8.146227 | 0.26870775 | 8.219295 | 0.25515413 | 208.84077 |
| CD63 | 9.559783 | 0.4696372 | 7.531043 | 0.17533515 | 7.447514 | 0.3454388 | 167.34497 |
| DUSP1 | 11.526863 | 0.3501923 | 8.561377 | 0.7014648 | 8.818249 | 0.5148774 | 106.75434 |
| PLK3 | 7.3127217 | 0.4593841 | 5.3107634 | 0.29284862 | 5.438676 | 0.36669925 | 119.47468 |
| PPP1R15A | 8.71425 | 0.6500824 | 6.556414 | 0.32009986 | 6.7638645 | 0.3106922 | 103.9078 |
| a | b | c | Classification |
|---|---|---|---|
| 10 | 0 | 1 | a = symptomatic |
| 0 | 9 | 9 | b = asymptomatic |
| 0 | 3 | 15 | c = healthy controls |
| a | b | c | Classification |
|---|---|---|---|
| 9 | 0 | 2 | a = symptomatic |
| 0 | 8 | 10 | b = asymptomatic |
| 0 | 8 | 10 | c = healthy controls |
| a | b | c | Classification |
|---|---|---|---|
| 10 | 1 | 0 | a = symptomatic |
| 3 | 6 | 9 | b = asymptomatic |
| 0 | 1 | 17 | c = healthy controls |
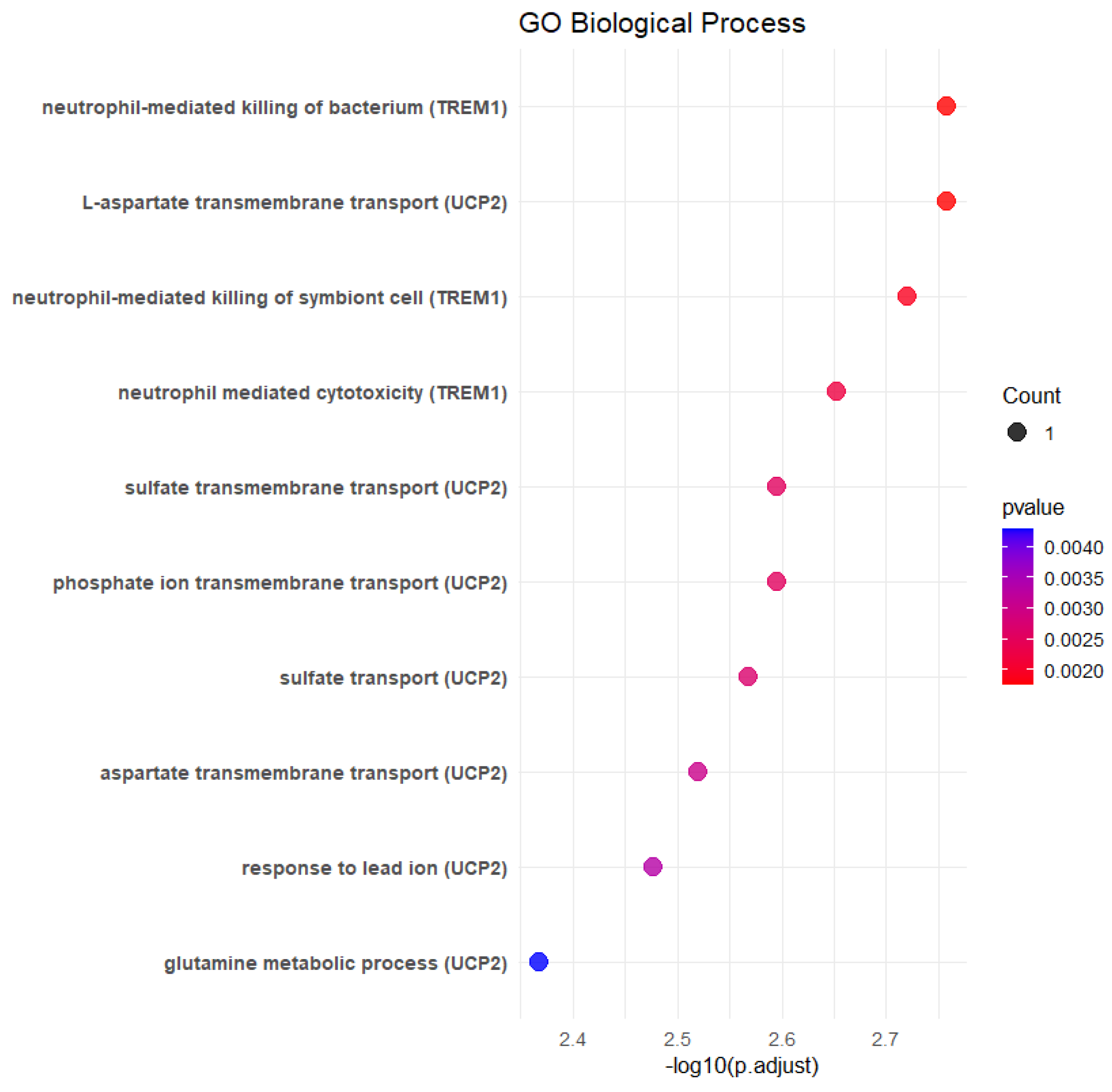
| Description | p Value | p Adjust | Gene ID | log_p Value |
|---|---|---|---|---|
| L-aspartate transmembrane transport | 0.00174788 | 0.04632427 | UCP2 | 2.75748815 |
| Neutrophil-mediated killing of bacterium | 0.00174788 | 0.04632427 | TREM1 | 2.75748815 |
| Neutrophil-mediated killing of symbiont cell | 0.00190668 | 0.04632427 | TREM1 | 2.71972261 |
| Neutrophil-mediated cytotoxicity | 0.00222422 | 0.04632427 | TREM1 | 2.65282186 |
| Phosphate ion transmembrane transport | 0.0025417 | 0.04632427 | UCP2 | 2.59487596 |
| Sulfate transmembrane transport | 0.0025417 | 0.04632427 | UCP2 | 2.59487596 |
| Sulfate transport | 0.00270041 | 0.04632427 | UCP2 | 2.56857004 |
| Aspartate transmembrane transport | 0.00301779 | 0.04632427 | UCP2 | 2.52031141 |
| Response to lead ion | 0.0033351 | 0.04632427 | UCP2 | 2.47689177 |
| Glutamine metabolic process | 0.00428662 | 0.04632427 | UCP2 | 2.36788545 |
| Phosphate ion transport | 0.00428662 | 0.04632427 | UCP2 | 2.36788545 |
| C4-dicarboxylate transport | 0.00428662 | 0.04632427 | UCP2 | 2.36788545 |
| Response to superoxide | 0.00444514 | 0.04632427 | UCP2 | 2.35211421 |
| Killing by host of symbiont cells | 0.00444514 | 0.04632427 | TREM1 | 2.35211421 |
| Response to oxygen radical | 0.00460365 | 0.04632427 | UCP2 | 2.33689727 |
| Liver regeneration | 0.00460365 | 0.04632427 | UCP2 | 2.33689727 |
| Neutrophil mediated immunity | 0.00555437 | 0.05188886 | TREM1 | 2.2553654 |
| Mitochondrial fission | 0.00634617 | 0.05188886 | UCP2 | 2.19748861 |
| Negative regulation of insulin secretion | 0.00634617 | 0.05188886 | UCP2 | 2.19748861 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torres-Sosa, J.A.; Aranda-Abreu, G.E.; Cruz-Ramírez, N.; Mestizo-Gutiérrez, S.L. Decision Trees for the Analysis of Gene Expression Levels of COVID-19: An Association with Alzheimer’s Disease. BioMedInformatics 2025, 5, 26. https://doi.org/10.3390/biomedinformatics5020026
Torres-Sosa JA, Aranda-Abreu GE, Cruz-Ramírez N, Mestizo-Gutiérrez SL. Decision Trees for the Analysis of Gene Expression Levels of COVID-19: An Association with Alzheimer’s Disease. BioMedInformatics. 2025; 5(2):26. https://doi.org/10.3390/biomedinformatics5020026
Chicago/Turabian StyleTorres-Sosa, Jesús Alberto, Gonzalo Emiliano Aranda-Abreu, Nicandro Cruz-Ramírez, and Sonia Lilia Mestizo-Gutiérrez. 2025. "Decision Trees for the Analysis of Gene Expression Levels of COVID-19: An Association with Alzheimer’s Disease" BioMedInformatics 5, no. 2: 26. https://doi.org/10.3390/biomedinformatics5020026
APA StyleTorres-Sosa, J. A., Aranda-Abreu, G. E., Cruz-Ramírez, N., & Mestizo-Gutiérrez, S. L. (2025). Decision Trees for the Analysis of Gene Expression Levels of COVID-19: An Association with Alzheimer’s Disease. BioMedInformatics, 5(2), 26. https://doi.org/10.3390/biomedinformatics5020026






