Machine Learning Models and Technologies for Evidence-Based Telehealth and Smart Care: A Review
Abstract
1. Introduction
2. Background and Related Work
2.1. Evidence-Based Telehealth and Smart Care
2.2. Machine Learning
- Supervised Learning (SL) Models: These models learn from labeled data to make predictions or classifications. SL is a machine learning paradigm where input objects and desired output values are used to train a model [28]. The model maps new data on expected output values, ensuring the algorithm can correctly determine output values for unseen instances. The statistical quality of an algorithm is measured through the generalization error. SL models learn from labeled training data, aiming to find patterns in the data to predict target variables for new, unseen data. Common supervised learning algorithms include linear regression, decision trees, SVMs, neural networks, random forests, logistic regression, and naive bayes.
- Unsupervised Learning (UL) Models: Unsupervised learning is the process of grouping data into clusters using automated algorithms to learn underlying relationships or features [29]. Common UL models analyze unlabeled data to discover hidden patterns or structures. Common algorithms include clustering, dimensionality reduction techniques, and association rule learning. Unlike supervised learning, UL learns patterns exclusively from unlabeled data, aiming to build a concise representation of the world through mimicry, generating imaginative content from unlabeled data.
- Reinforcement Learning (RL) Models: RL is a model that involves discrete environment states, agent actions, and scalar reinforcement signals. It differs from supervised learning by not presenting input/output pairs and requiring agents to gather experience [30]. It focuses on finding a balance between exploration and exploitation, aiming to maximize long-term rewards, and is closely related to artificial intelligence search and planning issues.
- Deep Learning (DL) Models: DL is a rapidly growing machine learning technique that has significantly impacted human life through applications like virtual personal assistants and automated number-plate recognition, but it also faces challenges and controversies [31]. Deep learning (DL) algorithms are neural networks used to learn hierarchical data representations. They can be supervised, semi-supervised, unsupervised, or reinforcement based. Common DL algorithms include CNN for image analysis, RNN for sequential data analysis, and LSTM for time series data, particularly effective in image and speech recognition.
3. Research Methodology
3.1. Objectives
3.2. Research Questions
4. Materials and Methods
4.1. Search Strategy
4.2. Study Selection Criteria
- focus on users/patients,
- include the use of telehealth and SC as a system, and
- monitor the performance of systems through clinical trials.
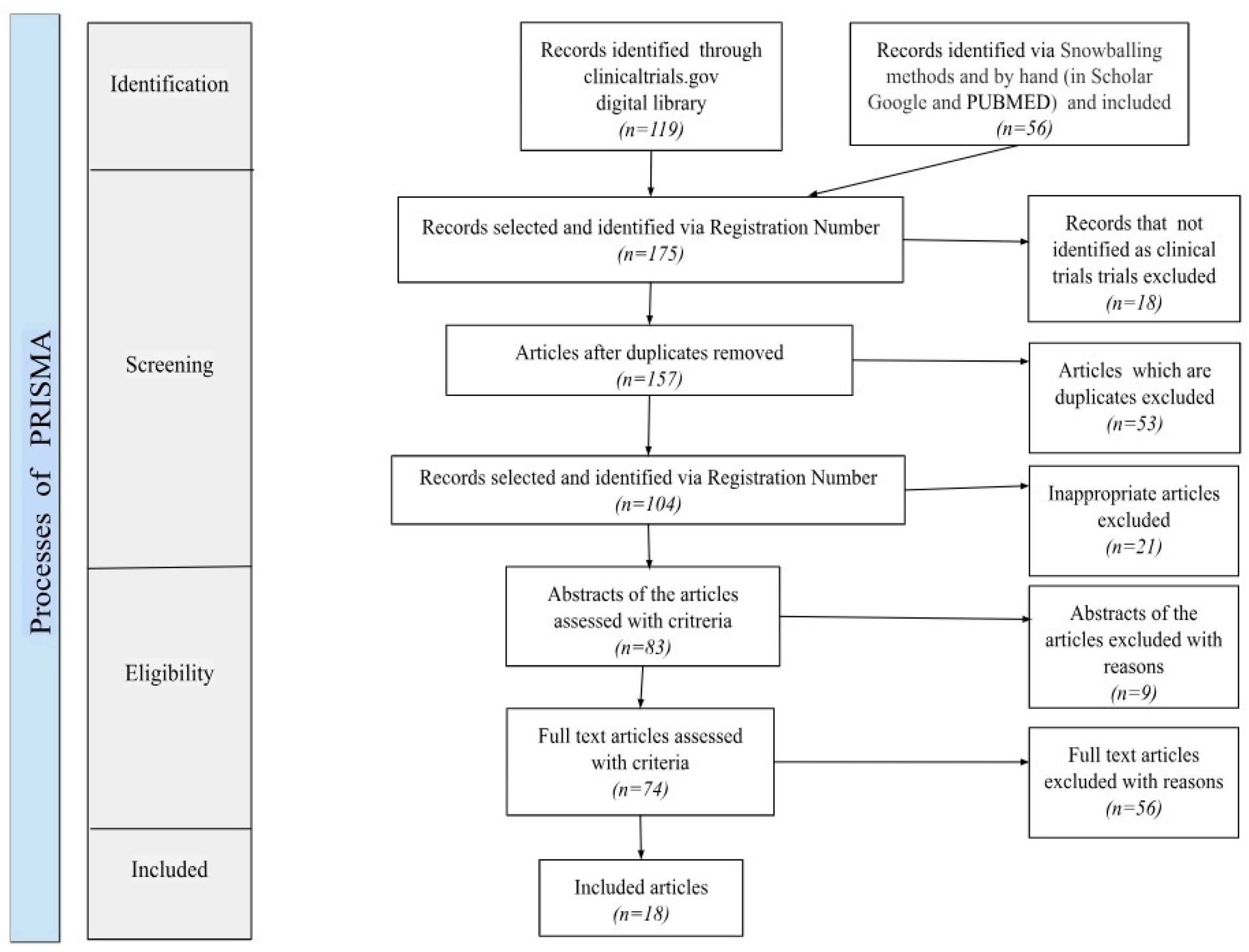
4.3. Screening Process
4.4. Data Extraction and Synthesis Strategy
- The Health Conditions (HCs) domain includes diseases, disorders, injuries, and other health problems (e.g., disease diagnosis, clustering, subtyping, and anomaly detection), with the ICD-11 [32] being the classification standard for these health conditions based on their etiology, manifestation, and location [33].
- The Classification of Digital Health Interventions (DHIs) categories include targeted client communication (e.g., health behavior modeling), healthcare provider-related functionalities (e.g., clinical decision support, telemedicine), health system management (e.g., healthcare resource allocation), and data services (e.g., predictive analytics) [34].
- The Interventions on Health Conditions (ICHI) domain includes preventive, curative, rehabilitative, and palliative (e.g., drug discovery; personalized medicine; genomics and precision medicine) actions to improve or maintain health status. These interventions are classified by the ICHI based on their target, action, means, and provider [33].
- Disease diagnosis
- Disease clustering and subtyping
- Anomaly detection
- Electronic health records
- Telemedicine
- Image analysis
- Patient risk stratification
- Natural language processing
- Clinical decision support
- Healthcare resource allocation
- Health behavior modeling
- Predictive analytics
- Drug discovery
- Personalized medicine
- Genomics and precision medicine
5. Results
5.1. Retrieved Studies
5.2. Descriptive Elements of the Studies
5.2.1. C—Diseases
5.2.2. Respiratory Tract Diseases (C08)
5.2.3. Nervous System Diseases (C10)
5.2.4. Nutritional and Metabolic Diseases (C18)
5.2.5. Neurodevelopmental Disorders (F03.625)
5.3. Classification of Machine Learning Models Based on Their Specific Applications
5.4. Machine Learning Sectors in Evidence-Based Telehealth and Smart Care
- Disease diagnosis/management healthcare sector: Approaches identified in this sector mainly belong to two studies on the supervised machine learning approach [50,53], one study on the deep learning approach [44], one study [46] on all approaches, and one more study [48] that uses statistical analysis, the backbone of ML, but does not describe an ML approach clearly.Basically, this sector uses algorithms that can be trained on labeled medical data to classify and diagnose diseases based on symptoms, medical images, or patient data. These algorithms are being used in various fields, including cancer diagnosis, cardiovascular disease diagnosis, diabetes diagnosis, respiratory disease diagnosis, and neurological disease diagnosis.The study in ref. [44] uses logistic regression analysis to compare the performance of deep learning black boxes with classical statistical approaches. It uses univariate and multivariate analyses, including logistic regression, to understand the relationship between input features and classification results. This helps in comparing the performance of these models with classical statistical approaches.
- Disease clustering and subtyping healthcare sector: Approaches identified in this sector belong two studies on the supervised machine learning approach [42,50]; one study (as described in two articles) on the unsupervised approach [39,40]; one jointly examining supervised, semi-supervised, and unsupervised machine learning approaches [43]; and one more with an unclear approach [48].Disease clustering and subtyping is a method of identifying patterns or groups within a disease population based on specific characteristics, such as clinical features or genetic markers. This helps researchers understand the disease’s heterogeneity and potentially identify different disease mechanisms or treatment approaches for each subtype, thereby improving their understanding of the disease [43].Disease clustering involves using unsupervised and etiology-independent clustering analysis to identify patient groups with similar characteristics or disease patterns. This method uses machine learning techniques to classify patients into distinct phenogroups based on clinical characteristics and treatment responses [50]. More specific ML has been applied to disease subtyping in various types of diseases. Some examples include multiple sclerosis (MS) [43], and chronic obstructive pulmonary disease (COPD) [42].
- Anomaly detection healthcare sector: One study [43] is identified in this health sector that mainly belongs to the supervised machine learning approach.
- The development of anomaly detection algorithms in health and medicine is crucial for identifying deviations from normal patterns, aiding in the early diagnosis of health conditions. More specifically, it helps in identifying outliers in physiological signals, abnormal heart rate variability, and unusual patterns in patient data, such as changes in speech or language [56].
- Electronic health records (EHR) healthcare sector: Approaches [41,54] identified in this health sector mainly belong to the supervised machine learning approach.The supervised learning approach in the EHR sector uses algorithms that can analyze large volumes of patient data from electronic health records including medical history, lab results, medications, and demographics.The results can help identify high-risk patients, predict patients’ results, support clinical decisions, monitor diseases, and more generally support in the health management of the population and the improvement of the supply of health care.
- Telemedicine healthcare sector: Approaches identified in this sector belong to the supervised machine learning approach [52,53,54], to the reinforcement machine learning approach [51], to the supervised and deep machine learning and neural networks approaches [49], and to an unclear approach [48].More specifically, supervised learning algorithms can be employed in telemedicine applications to analyze patient data collected remotely and provide diagnostic recommendations or monitor disease progression [52,53,54].Moreover, the deep learning approach can analyze data from wearable devices, detect anomalies, and provide personalized health recommendations. This approach enables remote monitoring and the early detection of health issues [49].
- Image analysis healthcare sector: One study [49] is identified in this sector that belongs to the supervised, deep machine learning and neural networks approaches. This study aims to implement machine learning and artificial intelligence in optimizing healthcare for patients with cardiac implantable electronic devices. This study is an open product, available for additional testing and improvement with supplementary functionalities: quality of life assessment, teleconsultation, video-streaming, and automated image recognition.
- Patient risk stratification healthcare sector: In this sector, two studies [47,53] are identified, the first dealing with all types of machine learning and the second with the supervised machine learning approach.In these studies, algorithms are used to analyze personal data, identify patterns, and group patients based on their profiles. This approach enables the use of personalized medicine, helps healthcare providers prioritize interventions, and allocates resources effectively. More specifically, in the second study [53] various machine learning algorithms were applied for risk stratification, with the SVM model showing the best prediction performance at 84.7%.
- Natural language processing (NLP) healthcare sector: The studies identified in this healthcare sector mainly belong to all types of machine learning methods [46] with an emphasis on the supervised learning approach [38].NLP can be used for various tasks such as text classification, sentiment analysis, named entity recognition, machine translation, text generation, and question answering. These techniques classify text, analyze sentiment, extract named entities, and develop translation models. They can also generate human-like text and answer questions based on a given text or knowledge base [46].Also, a random forest machine learning algorithm, as a supervised ML approach, that combines multiple decision trees to make predictions was used in three experiments in the study by ref. [38] to show the accuracy of pain scores in chronic cancer patients.
- Clinical decision support healthcare sector: One study [48] is identified in this sector, but it is unclear what machine learning approach it supports. Clinical decision support can provide decision support to healthcare professionals by analyzing patient data and recommending appropriate treatment options.
- Healthcare resource allocation healthcare sector: One study [53] is clearly identified as managing issues related to the management of limited resources, alongside managing medical issues. Resource allocation is the process of allocating available resources to various uses, particularly in health care. However, at-risk individuals often find it difficult to comply due to the cost of diagnostic tests and scarce medical resources. Resource constraints can affect health care by reducing access to care, compromising quality of care, and limiting treatment options, thus leading to worse health outcomes, and exacerbating existing health disparities. Resource allocation is critical to optimizing productivity, managing costs, and ensuring the strategic use of resources. Thus, beyond the medical issues they cover, many applications in the health field aim to solve the issue of limited resources. For example, BGEM™ is a cloud-based solution that uses advanced machine learning functions to monitor multiple digital biomarkers and provide targeted information to high-risk individuals who would otherwise not have access to the prevention and early treatment of their health problems.
- Predictive analytics healthcare sector: Approaches identified in this health sector mainly belong to the supervised machine learning approach [41,42,43,45,50].Supervised ML approaches are widely utilized for predictive analytics in healthcare, including predictive analytics for classification, regression, clustering, time series analysis, and recommendation systems [47].Ref. [41] studies predictive management in healthcare. ML analysis and modeling based on available data have shown promise in predicting outcomes (such as admission and length of stay) for severe COPD exacerbation. These models utilize EHR at triage assessment to make predictions.Also, the authors of ref. [43] studied supervised, semi-supervised, and unsupervised ML techniques in a multimodal machine-learning-based system for anomaly and fall detection, combining heuristics and hard rules based on acceleration magnitude features.
- Drug discovery healthcare sector: In this sector, one study [47] is identified which deals with all machine learning approaches.The development of this sector can help identify patterns and relationships in large datasets of chemical compounds, aiding in the discovery of new drugs and understanding their mechanisms of action.Supervised learning, reinforcement learning, and deep learning are algorithms used in drug discovery to predict the effectiveness and safety of new compounds. Supervised learning predicts drug efficacy based on molecular structures and biological data, reinforcement learning optimizes candidate selection and design, and deep learning analyzes large datasets to identify potential candidates.
- Personalized medicine healthcare sector: Approaches identified in this health sector mainly belong to the supervised machine learning approach [45,55]. Moreover, one study belongs to supervised, deep machine learning, and neural networks [49], and one more study [47] studies all approaches.Personalized medicine is used to develop personalized care plans based on individual patient characteristics, optimizing treatment effectiveness, and minimizing side effects.Decision trees are often used for specific cases including personalized medicine and treatment recommendations are made based on historical data to guide treatment decisions, benefit–risk judgment, and quality analysis. These tools help weigh different treatments and predict the optimal medication for individual patients, ultimately improving overall patient outcomes [47].
- Genomics and precision medicine healthcare sector: In this sector, one study is identified that belongs to the supervised machine learning approach [53].
5.5. Machine Learning Algorithms in Evidence-Based Telehealth and Smart Care
- Supervised machine learning algorithms:
- A linear classifier: This is a type of machine learning algorithm that separates data points into different classes using a linear decision boundary. Linear classifiers are used in various cases [43,53] in EBTM and SC. These studies demonstrate the use of linear SVM classifiers in different contexts, such as fall detection and blood glucose level detection.A linear classifier classifies data based on a linear combination of input features. In terms of classification, linear classifiers can be classified into two main types: binary linear classifiers and multi-class linear classifiers.Binary linear classifiers are used for binary classification tasks, where the goal is to separate data points into two classes. A binary linear classifier is a machine learning model used for binary classification tasks, dividing instances into two classes based on features. It assigns a class label to each instance based on its position on the linear boundary and makes predictions by calculating a weighted sum of feature values [43].Examples of binary linear classifiers include logistic regression and SVM with linear kernels [53].Multi-class linear classifiers are used for multi-class classification tasks, where the goal is to separate data points into more than two classes. An example of a multi-class linear classifier is the SVM with a linear kernel.Mosquera-Lopez et al. [43] discuss the use of support vector machine (SVM) models (the linear SVM classifier and SVM model with a radial basis function kernel) for fall detection.Specifically, the linear SVM classifier separates classes by finding a hyperplane that maximally separates the data points of different classes in the feature space. It assigns new instances to classes based on which side of the hyperplane they fall on. This type of classifier is commonly used in tasks such as image classification, text categorization, and sentiment analysis [43].Indeed, according to [53], the SVM can be used as both a binary linear classifier and a multi-class linear classifier.Overall, linear classifiers are effective for linearly separable data and can provide good performance in many classification tasks.
- Reinforcement machine learning algorithms.
- Contextual bandit algorithm: This algorithm is used in an in-home monitoring system to make recommendations based on the caregiver’s interaction history, current behaviors, and other observations. It helps increase the utility of the system’s recommendations and the acceptance of those recommendations by the end users [45].
- Support of many types of machine learning algorithms.
- Machine learning fall detection algorithms: These algorithms use ML techniques to detect falls. The accuracy of the detector is improved by training the algorithm using real-world fall data from the target population [43].This algorithm combines an auto-encoder, which is an unsupervised learning algorithm, and a hyper-ensemble of balanced random forests to detect fall candidates based on acceleration data. The auto-encoder re-constructs the input acceleration signal, identifying fall candidates with a root-mean-square error (RMSE) higher than a certain threshold. The hyper-ensemble of balanced random forests assigns final labels to fall candidates, reducing false positives. This two-stage classification method aims to improve fall detection accuracy in real-world scenarios by combining acceleration and movement features. By combining both acceleration and movement features, this algorithm aims to improve the accuracy of fall detection in real-world scenarios [43].
- APPRAISE-RS: This algorithm is used to develop recommender systems that provide automated, updated, participatory, and personalized treatments. It uses rule-based systems that belong to the symbolic or knowledge-based learning approach and the GRADE heuristic to form recommendations [47]. Specifically, rule-based systems, categorized as supervised, unsupervised, or reinforcement learning, use rules to make decisions or solve problems. They can also be integrated with deep learning models for enhanced performance.
- Real-time self-learning algorithm: This is an algorithm that can continuously learn from and adapt to new data in real time. Real-time self-learning algorithms can be implemented using various methods, such as neural networks, deep reinforcement learning for autonomous driving simulations, and unsupervised learning for real-time learning from unlabeled data.The study by ref. [49] is designed to update its knowledge and improve its performance as it receives new information. This type of algorithm is often used in applications where the data are dynamic and constantly changing, such as in online recommendation systems, fraud detection, or autonomous vehicles. The algorithm uses techniques such as online learning or incremental learning to update its model and make predictions or decisions. This algorithm is used in the health care optimization of patients with cardiac implantable electronic devices (CIED).
5.6. Machine Learning Tasks used in Evidence-Based Telehealth and Smart Care
5.7. Functional and Technical Features of ML Systems Technology Applied to Evidence-Based Telehealth and Smart Care
5.8. Evaluation Metrics in Evidence-Based Telecare and Smart Care
6. Discussion
7. Future Directions and Challenges
8. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 12-Item Short Form Health Survey | SF-12 |
| Artificial intelligence | AI |
| Cardiac implantable electronic devices | CIED |
| Chronic obstructive pulmonary disease | COPD |
| Classification of Digital Health Interventions | DHIs |
| Context-aware computing | CAC |
| Convolutional neural networks | CNN |
| Deep learning | DL |
| Deep q-networks | DQNs |
| Digital health technology | DHT |
| Electronic health records | EHR |
| Evidence-based telehealth | EBTH |
| EuroQol-5 Dimension | EQ-5D |
| Fuzzy K-nearest neighbors | FuzzyKNN |
| Hierarchical clustering principal components | HCPC |
| International Classification of Diseases 11th Revision | ICD-11 |
| K-nearest neighbor | KNN |
| Long short-term memory | LSTM |
| Multiple correspondence analysis | MCA |
| Mobilized ML Autism Risk Assessment | MARA |
| Multiple sclerosis | MS |
| Natural language processing | NLP |
| Non-predictable breakthrough cancer pain | NP-BTcP |
| Point-of-care | POC |
| Principal component analysis | PCA |
| Machine learning | ML |
| Photoplethysmography | PPG |
| Research question | RQ |
| Smart care | SC |
| Supervised learning | SL |
| Reinforcement deep learning | RDL |
| Reinforcement learning | RL |
| Recurrent neural networks | RNN |
| Support vector machine | SVM |
| Type 2 diabetes mellitus | T2DM |
| Unsupervised learning | UL |
References
- Quer, G.; Arnaout, R.; Henne, M.; Arnaout, R. Machine Learning and the Future of Cardiovascular Care: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2021, 77, 300–313. [Google Scholar] [CrossRef]
- Maier-Hein, L.; Eisenmann, M.; Sarikaya, D.; März, K.; Collins, T.; Malpani, A.; Fallert, J.; Feussner, H.; Giannarou, S.; Mascagni, P.; et al. Surgical data science -from concepts toward clinical translation. Med. Image Anal. 2022, 76, 102306. [Google Scholar] [CrossRef]
- Walsh, A.E.; Naughton, G.; Sharpe, T.; Zajkowska, Z.; Malys, M.; van Heerden, A.; Mondelli, V. Remote measurement technologies for depression in young people: A realist review with meaningful lived experience involvement and recommendations for future research and practice. medRxiv 2022. Available online: https://www.medrxiv.org/content/10.1101/2022.06.16.22276510v1.abstract (accessed on 18 June 2023). [CrossRef]
- Nokas, G.; Kotsilieris, T. Preventing Keratoconus through Eye Rubbing Activity Detection: A Machine Learning Approach. Electronics 2023, 12, 1028. [Google Scholar] [CrossRef]
- Gupta, R.; Srivastava, D.; Sahu, M.; Tiwari, S.; Ambasta, R.K.; Kumar, P. Artificial intelligence to deep learning: Machine intelligence approach for drug discovery. Mol. Divers. 2021, 25, 1315–1360. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, A.M.; Yousefpoor, E.; Yousefpoor, M.S.; Mehmood, Z.; Haider, A.; Hosseinzadeh, M.; Ali Naqvi, R. Machine Learning (ML) in Medicine: Review, Applications, and Challenges. Sci. China Ser. A Math. 2021, 9, 2970. [Google Scholar] [CrossRef]
- Orriols-Puig, A.; Casillas, J.; Bernadó-Mansilla, E. Fuzzy-UCS: A Michigan-Style Learning Fuzzy-Classifier System for Supervised Learning. IEEE Trans. Evol. Comput. 2009, 13, 260–283. [Google Scholar] [CrossRef]
- Pereira, T.; Lemos, L.; Cardoso, S.; Silva, D.; Rodrigues, A.; Santana, I.; de Mendonça, A.; Guerreiro, M.; Madeira, S.C. Predicting progression of mild cognitive impairment to dementia using neuropsychological data: A supervised learning approach using time windows. BMC Med. Inform. Decis. Mak. 2017, 17, 110. [Google Scholar] [CrossRef] [PubMed]
- Ramentol, E.; Caballero, Y.; Bello, R.; Herrera, F. SMOTE-RSB*: A hybrid preprocessing approach based on oversampling and undersampling for high imbalanced data-sets using SMOTE and rough sets theory. Knowl. Inf. Syst. 2021, 33, 245–265. [Google Scholar] [CrossRef]
- Janssens, T.; Antanas, L.; Derde, S.; Vanhorebeek, I.; Van den Berghe, G.; Grandas, F.G. Charisma: An integrated approach to automatic H&E-stained skeletal muscle cell segmentation using supervised learning and novel robust clump splitting. Med. Image Anal. 2013, 17, 1206–1219. [Google Scholar]
- Derrac, J.; Chiclana, F.; García, S.; Herrera, F. IFSA-EUSFLAT—An Interval Valued K-Nearest Neighbors Classifier. In Proceedings of the 2015 Conference of the International Fuzzy Systems Association and the European Society for Fuzzy Logic and Technology, Asturias, Spain, 30 June–3 July 2015. [Google Scholar] [CrossRef]
- Garg, P.; Mohanty, A.; Ramisetty, S.; Kulkarni, P.; Horne, D.; Pisick, E.; Singhal, S.S. Artificial intelligence and allied subsets in early detection and preclusion of gynecological cancers. Biochim. et Biophys. Acta (BBA)-Rev. Cancer 2023, 1878, 189026. [Google Scholar] [CrossRef]
- Carlin, C.; Taylor, A.; van Loon, I.; McDowell, G.; Burns, S.; McGinness, P.; Lowe, D.J. Role for artificial intelligence in respiratory diseases—Chronic obstructive pulmonary disease. J. Hosp. Manag. Health Policy 2021, 5, 27. [Google Scholar] [CrossRef]
- Isaksen, J.L.; Baumert, M.; Hermans, A.N.L.; Maleckar, M.; Linz, D. Artificial intelligence for the detection, prediction, and management of atrial fibrillation. Herzschrittmacherther. Elektrophysiol. 2022, 33, 34–41. [Google Scholar] [CrossRef]
- Masanneck, L.; Gieseler, P.; Gordon, W.J.; Meuth, S.G.; Stern, A.D. Evidence from ClinicalTrials.gov on the growth of Digital Health Technologies in neurology trials. NPJ Digit. Med. 2023, 6, 23. [Google Scholar] [CrossRef]
- Christopoulou, S.C. Impacts on Context Aware Systems in Evidence-Based Health Informatics: A Review. Healthcare 2022, 10, 685. [Google Scholar] [CrossRef]
- Gautam, N.; Ghanta, S.N.; Mueller, J.; Mansour, M.; Chen, Z.; Puente, C.; Ha, Y.M.; Tarun, T.; Dhar, G.; Sivakumar, K.; et al. Artificial Intelligence, Wearables and Remote Monitoring for Heart Failure: Current and Future Applications. Diagnostics 2022, 12, 2964. [Google Scholar] [CrossRef]
- Shamshad, F.; Khan, S.; Zamir, S.W.; Khan, M.H.; Hayat, M.; Khan, F.S.; Fu, H. Transformers in Medical Imaging: A Survey. Available online: http://arxiv.org/abs/2201.09873 (accessed on 10 February 2024).
- Telemedicine-Mesh-NCBI. National Center for Biotechnology Information. Telemedicine-Mesh-NCBI. Available online: https://www.ncbi.nlm.nih.gov/mesh/?term=telehealth (accessed on 1 March 2024).
- Tian, S.; Yang, W.; Grange, J.M.L.; Wang, P.; Huang, W.; Ye, Z. Smart healthcare: Making medical care more intelligent. Glob. Health J. 2019, 3, 62–65. [Google Scholar] [CrossRef]
- Evidence-Based Practice-MeSH-NCBI. Available online: https://www.ncbi.nlm.nih.gov/mesh/?term=Evidence-based+health (accessed on 10 February 2024).
- The Association of Faculties of Medicine of Canada. AFMC Primer on Population Health—An AFMC Public Health Educators’ Network Resource. 2014. Available online: http://www.ubccpd.ca/sites/default/files/documents/AFMC_Primer_on_Population_Health_2014-12-23.pdf (accessed on 10 February 2024).
- Sackett, D.L.; Rosenberg, W.M.C.; Gray, J.A.M.; Haynes, R.B.; Richardson, W.S. Evidence based medicine: What it is and what it isn’t. BMJ 1996, 312, 71–72. [Google Scholar] [CrossRef] [PubMed]
- Ammenwerth, E.; Schnell-Inderst, P.; Siebert, U. Vision and challenges of Evidence-Based Health Informatics: A case study of a CPOE meta-analysis. Int. J. Med. Inform. 2010, 79, e83–e88. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.ama-assn.org/about/research/ama-digital-health-care-2022-study-findings (accessed on 10 February 2024).
- Available online: https://www.ama-assn.org/system/files/telehealth-survey-report.pdf (accessed on 10 February 2024).
- Christopoulou, S.C.; Kotsilieris, T.; Anagnostopoulos, I. Evidence-based health and clinical informatics: A systematic review on randomized controlled trials. Health Technol. 2018, 8, 137–150. [Google Scholar] [CrossRef]
- Mohri, M.; Rostamizadeh, A.; Talwalkar, A. Foundations of Machine Learning; MIT Press: Cambridge, MA, USA, 2012. [Google Scholar]
- Berry, M.W.; Mohamed, A.; Yap, B.W. Supervised and Unsupervised Learning for Data Science; Springer Nature: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Kaelbling, L.P.; Littman, M.L.; Moore, A.W. Reinforcement Learning: A Survey. J. Artif. Intell. Res. 1996, 68, 5103. [Google Scholar] [CrossRef]
- Egger, J.; Pepe, A.; Gsaxner, C.; Jin, Y.; Li, J.; Kern, R. Deep learning-a first meta-survey of selected reviews across scientific disciplines, their commonalities, challenges and research impact. PeerJ Comput Sci. 2021, 7, e773. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://icd.who.int/en (accessed on 1 March 2024).
- Available online: https://www.who.int/standards/classifications (accessed on 10 February 2024).
- Available online: https://www.isfteh.org/files/media/WHO-RHR-18.06-eng1.pdf (accessed on 1 March 2024).
- Arksey, H.; O’Malley, L. Scoping studies: Towards a methodological framework. Int. J. Soc. Res. Methodol. 2005, 8, 19–32. [Google Scholar] [CrossRef]
- Yepes-Nuñez, J.J.; Urrútia, G.; Romero-García, M.; Alonso-Fernández, S. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Rev. Esp. Cardiol. 2021, 74, 790–799. [Google Scholar]
- Dalkey, N.; Helmer, O. An Experimental Application of the DELPHI Method to the Use of Experts. Manag. Sci. 1963, 9, 458–467. [Google Scholar] [CrossRef]
- Ordun, C.; Cha, A.N.; Raff, E.; Gaskin, B.; Hanson, A.; Rule, M.; Purushotham, S.; Gulley, J.L. Intelligent Sight and Sound: A Chronic Cancer Pain Dataset. 2022. Available online: http://arxiv.org/abs/2204.04214 (accessed on 18 June 2023).
- Cuomo, A.; Cascella, M.; Vittori, A.; Baciarello, M.; Badino, M.; Bignami, E. Comments on Telemedicine for Managing Cancer Pain. A Great Opportunity to be Exploited for CLinical and Research Purposes. Pain Physician 2023, 26, E108–E110. [Google Scholar]
- Cascella, M.; Crispo, A.; Esposito, G.; Forte, C.A.; Coluccia, S.; Porciello, G.; Amore, A.; Bimonte, S.; Mercadante, S.; Caraceni, A.; et al. Multidimensional Statistical Technique for Interpreting the Spontaneous Breakthrough Cancer Pain Phenomenon. A Secondary Analysis from the IOPS-MS Study. Cancers 2021, 13, 4018. [Google Scholar] [CrossRef]
- Taylor, A.; Lowe, D.J.; McDowell, G.; Lua, S.; Burns, S.; McGinness, P.; Carlin, C.M. Remote-Management of COPD: Evaluating the Implementation of Digital Innovation to Enable Routine Care (RECEIVER): The protocol for a feasibility and service adoption observational cohort study. BMJ Open Respir. Res. 2021, 8, e000905. [Google Scholar] [CrossRef]
- Secher, P.H.; Hangaard, S.; Kronborg, T.; Hæsum, L.K.E.; Udsen, F.W.; Hejlesen, O.; Bender, C. Clinical implementation of an algorithm for predicting exacerbations in patients with COPD in telemonitoring: A study protocol for a single-blinded randomized controlled trial. Trials 2022, 23, 356. [Google Scholar] [CrossRef]
- Mosquera-Lopez, C.; Wan, E.; Shastry, M.; Folsom, J.; Leitschuh, J.; Condon, J.; Rajhbeharrysingh, U.; Hildebrand, A.; Cameron, M.H.; Jacobs, P.G. Automated Detection of Real-World Falls: Modeled from People with Multiple Sclerosis. IEEE J. Biomed. Health Inform. 2021, 25, 1975–1984. [Google Scholar] [CrossRef]
- Varghese, J.; Niewöhner, S.; Soto-Rey, I.; Schipmann-Miletić, S.; Warneke, N.; Warnecke, T.; Dugas, M. A Smart Device System to Identify New Phenotypical Characteristics in Movement Disorders. Front. Neurol. 2019, 10, 48. [Google Scholar] [CrossRef] [PubMed]
- Rose, K.M.; Gordon, K.C.; Schlegel, E.C.; Mccall, M.; Gao, Y.; Ma, M.; Lenger, K.A.; Ko, E.; Wright, K.D.; Wang, H.; et al. Smarthealth technology study protocol to improve relationships between older adults with dementia and family caregivers. J. Adv. Nurs. 2021, 77, 2519–2529. [Google Scholar] [CrossRef] [PubMed]
- Hampsey, E.; Meszaros, M.; Skirrow, C.; Strawbridge, R.; Taylor, R.H.; Chok, L.; Aarsland, D.; Al-Chalabi, A.; Chaudhuri, R.; Weston, J.; et al. Protocol for Rhapsody: A longitudinal observational study examining the feasibility of speech phenotyping for remote assessment of neurodegenerative and psychiatric disorders. BMJ Open 2022, 12, e061193. [Google Scholar] [CrossRef] [PubMed]
- López, B.; Raya, O.; Baykova, E.; Saez, M.; Rigau, D.; Cunill, R.; Mayoral, S.; Carrion, C.; Serrano, D.; Castells, X. APPRAISE-RS: Automated, updated, participatory, and personalized treatment recommender systems based on GRADE methodology. Heliyon 2023, 9, e13074. [Google Scholar] [CrossRef] [PubMed]
- Redfern, J.; Coorey, G.; Mulley, J.; Scaria, A.; Neubeck, L.; Hafiz, N.; Pitt, C.; Weir, K.; Forbes, J.; Parker, S.; et al. A digital health intervention for cardiovascular disease management in primary care (CONNECT) randomized controlled trial. NPJ Digit. Med. 2020, 3, 117. [Google Scholar] [CrossRef] [PubMed]
- Michalik, J.; Cacko, A.; Polinski, J.; Pawlik, K.; Tataj, E.; Gawalko, M.; Grabowski, M. An interactive assistant for patients with cardiac implantable electronic devices: A study protocol of the LUCY trial. Medicine 2018, 97, e12556. [Google Scholar] [CrossRef] [PubMed]
- Fayol, A.; Wack, M.; Livrozet, M.; Carves, J.; Domengé, O.; Vermersch, E.; Mirabel, M.; Karras, A.; Le Guen, J.; Blanchard, A.; et al. Aetiological classification and prognosis in patients with heart failure with preserved ejection fraction. ESC Heart Fail. 2022, 9, 519–530. [Google Scholar] [CrossRef] [PubMed]
- Ash, G.I.; Griggs, S.; Nally, L.M.; Stults-Kolehmainen, M.; Jeon, S.; Brandt, C.; Gulanski, B.I.; Spanakis, E.K.; Baker, J.S.; Whittemore, R.; et al. Evaluation of Web-Based and In-Person Methods to Recruit Adults with Type 1 Diabetes for a Mobile Exercise Intervention: Prospective Observational Study. JMIR Diabetes 2021, 6, e28309. [Google Scholar] [CrossRef]
- Klarskov, C.K.; Windum, N.A.; Olsen, M.T.; Dungu, A.M.; Jensen, A.K.; Lindegaard, B.; Pedersen-Bjergaard, U.; Kristensen, P.L. Telemetric Continuous Glucose Monitoring During the COVID-19 Pandemic in Isolated Hospitalized Patients in Denmark: A Randomized Controlled Exploratory Trial. Diabetes Technol. Ther. 2022, 24, 102–112. [Google Scholar] [CrossRef]
- Shi, B.; Dhaliwa, S.S.; Wong, J.; Lam, N.W.; Zhou, E.; Paitimusa, V.; Ang, S.B. BGEMTM: Assessing Elevated Blood Glucose Levels Using Machine Learning and Wearable Photo plethysmography Sensors. TechRxiv 2023. [Google Scholar] [CrossRef]
- Fritz, B.; King, C.; Chen, Y.; Kronzer, A.; Abraham, J.; Abdallah, A.B.; Avidan, M. Protocol for the perioperative outcome risk assessment with computer learning enhancement (Periop ORACLE) randomized study. F1000Research 2022, 11, 653. [Google Scholar] [CrossRef] [PubMed]
- Voss, C.; Schwartz, J.; Daniels, J.; Kline, A.; Haber, N.; Washington, P.; Wall, D.P. Effect of Wearable Digital Intervention for Improving Socialization in Children with Autism Spectrum Disorder: A Randomized Clinical Trial. JAMA Pediatr. 2019, 173, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Shastry, K.A.; Shastry, A. An integrated deep learning and natural language processing approach for continuous remote monitoring in digital health. Decis. Anal. J. 2023, 8, 100301. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, L.; Wang, C. Trends in using deep learning algorithms in biomedical prediction systems. Front. Neurosci. 2023, 17, 1256351. [Google Scholar] [CrossRef] [PubMed]
| Rank | NCT Number | Articles | Authors | Title |
|---|---|---|---|---|
| 1. C—Diseases 1.1. Neoplasms—C04 | ||||
| 1 | NCT04442425 | [38] | (Ordun et al., 2022) | Intelligent Sight and Sound: A Chronic Cancer Pain Dataset |
| 2 | NCT04726228 | [39,40] | (Cuomo et al., 2023); (Cascella et al., 2021) | Comments on ‘Telemedicine for Managing Cancer Pain. A Great Opportunity to be Exploited for CLinical and Research Purposes; Multidimensional Statistical Technique for Interpreting the Spontaneous Breakthrough Cancer Pain Phenomenon. A Secondary Analysis from the IOPS-MS Study |
| 1.2 Respiratory Tract Diseases C08 | ||||
| 1.2.1 Pulmonary Disease C08.381 | ||||
| 3 | NCT04240353 | [41] | (Taylor et al., 2021) | RECEIVER: Digital Service Model for Chronic Obstructive Pulmonary Disease (COPD) |
| 4 | NCT05218525 | [42] | (Secher et al., 2022) | Clinical implementation of an algorithm for predicting exacerbations in patients with COPD in telemonitoring: a study protocol for a single-blinded randomized controlled trial |
| 1.2 Nervous System Diseases C10 1.2.1 Multiple Sclerosis C10.114.375.500 | ||||
| 5 | NCT02583386 | [43] | (Mosquera-Lopez et al., 2021) | Automated Detection of Real-World Falls: Modeled From People With Multiple Sclerosis |
| 6 | NCT03638479 | [44] | (Varghese et al., 2019) | A Smart Device System to Identify New Phenotypical Characteristics in Movement Disorders |
| 7 | NCT04536701 | [45] | (Rose et al., 2021) | Smarthealth technology study protocol to improve relationships between older adults with dementia and family caregivers |
| 8 | NCT04939818 | [46] | (Hampsey et al., 2022) | Protocol for Rhapsody: a longitudinal observational study examining the feasibility of speech phenotyping for remote assessment of neurodegenerative and psychiatric disorders |
| 1.2.2 Dyskinesias C10.597.350 | ||||
| 9 | NCT04228094 | [47] | (López et al., 2023) | APPRAISE-RS: Automated, updated, participatory, and personalized treatment recommender systems based on GRADE methodology |
| 1.3 Cardiovascular Diseases C14 | ||||
| 10 | ACTRN12613000715774 | [48] | (Redfern et al., 2020) | A digital health intervention for cardiovascular disease management in primary care (CONNECT) randomized controlled trial |
| 11 | NCT03474315 | [49] | (Michalik et al., 2018) | An interactive assistant for patients with cardiac implantable electronic devices: A study protocol of the LUCY trial |
| 12 | NCT04189029 | [50] | (Fayol et al., 2022) | Aetiological classification and prognosis in patients with heart failure with preserved ejection fraction |
| 1.4 Nutritional and Metabolic Diseases C18 1.4.1 Glucose Metabolism Disorders C18.452.394 | ||||
| 13 | NCT04204733 | [51] | (Ash et al., 2021) | Evaluation of Web-Based and In-Person Methods to Recruit Adults with Type 1 Diabetes for a Mobile Exercise Intervention: Prospective Observational Study |
| 14 | NCT04430608 | [52] | (Klarskov et al., 2022) | Telemetric Continuous Glucose Monitoring During the COVID-19 Pandemic in Isolated Hospitalized Patients in Denmark: A Randomized Controlled Exploratory Trial |
| 15 | NCT05504096 | [53] | (Shi et al., 2023) | Assessing Elevated Blood Glucose Levels Using Machine Learning and Wearable Photo plethysmography Sensors |
| 1.5. Pathological Conditions, Signs and Symptoms C23 1.5.1 Intraoperative Complications C23.550.5 | ||||
| 16 | NCT03923699 | [54] | (B. Fritz et al., 2022) | Protocol for the perioperative outcome risk assessment with computer learning enhancement (Periop ORACLE) randomized study |
| 2. F- Psychiatry and Psychology 2.1 Neurodevelopmental Disorders F03.625 2.1.1 Autism Spectrum Disorder F03.625.164.113.500 | ||||
| 17 | NCT03569176 | [55] | (Voss et al., 2019) | Effect of Wearable Digital Intervention for Improving Socialization in Children with Autism Spectrum Disorder: A Randomized Clinical Trial |
| Health Do-Main Task | Disease Diagnosis/Management | Disease Clustering and Subtyping | Anomaly Detection | Electronic Health Records | Telemedicine | Image Analysis | Patient Risk Stratification | Natural Language Processing (NLP) | Clinical Decision Support | Healthcare Resource Allocation | Predictive Analytics | Drug Discovery | Personalized Medicine | Genomics and Precision Medicine | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ref. of the Task | |||||||||||||||
| Supervised Machine Learning Approach | |||||||||||||||
| [38] | ✓ | ||||||||||||||
| [41] | ✓ | ✓ | |||||||||||||
| [42] | ✓ | ✓ | |||||||||||||
| [45] | ✓ | ✓ | |||||||||||||
| [50] | ✓ | ✓ | ✓ | ||||||||||||
| [52] | ✓ | ||||||||||||||
| [53] | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||||
| [54] | ✓ | ✓ | |||||||||||||
| [55] | ✓ | ||||||||||||||
| Unsupervised Machine Learning Approach | |||||||||||||||
| [39,40] | ✓ | ||||||||||||||
| Reinforcement Machine Learning Approach | |||||||||||||||
| [51] | ✓ | ||||||||||||||
| Deep Machine learning Approach | |||||||||||||||
| [44] | ✓ | ||||||||||||||
| Supervised & Deep Machine learning & Neural Networks | |||||||||||||||
| [49] | ✓ | ✓ | ✓ | ||||||||||||
| Supervised, semi-supervised and Unsupervised Machine Learning Approach | |||||||||||||||
| [43] | ✓ | ✓ | ✓ | ||||||||||||
| All Approaches | |||||||||||||||
| [46] | ✓ | ✓ | |||||||||||||
| [47] | ✓ | ✓ | ✓ | ||||||||||||
| Unclear Approach | |||||||||||||||
| [48] | ✓ | ✓ | ✓ | ✓ | |||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Christopoulou, S.C. Machine Learning Models and Technologies for Evidence-Based Telehealth and Smart Care: A Review. BioMedInformatics 2024, 4, 754-779. https://doi.org/10.3390/biomedinformatics4010042
Christopoulou SC. Machine Learning Models and Technologies for Evidence-Based Telehealth and Smart Care: A Review. BioMedInformatics. 2024; 4(1):754-779. https://doi.org/10.3390/biomedinformatics4010042
Chicago/Turabian StyleChristopoulou, Stella C. 2024. "Machine Learning Models and Technologies for Evidence-Based Telehealth and Smart Care: A Review" BioMedInformatics 4, no. 1: 754-779. https://doi.org/10.3390/biomedinformatics4010042
APA StyleChristopoulou, S. C. (2024). Machine Learning Models and Technologies for Evidence-Based Telehealth and Smart Care: A Review. BioMedInformatics, 4(1), 754-779. https://doi.org/10.3390/biomedinformatics4010042