Unobtrusive Monitoring of Sleep Cycles: A Technical Review
Abstract
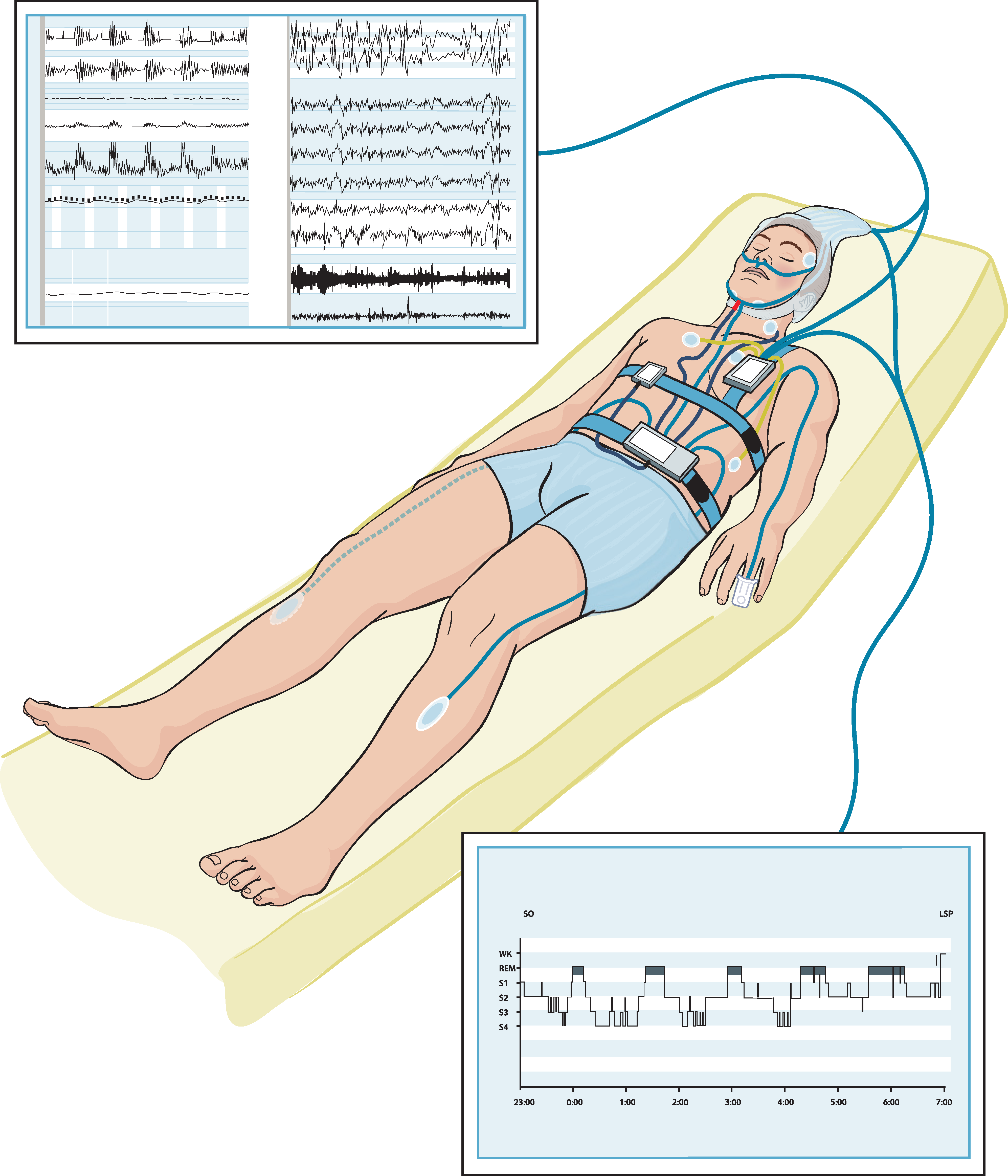
:1. Introduction
2. Methodology
3. Literature Review
4. Discussion and Viewpoints
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Keywords |
|---|
|
References
- Chattu, V.K.; Sakhamuri, S.M.; Kumar, R.; Spence, D.W.; BaHammam, A.S.; Pandi-Perumal, S.R. Insufficient Sleep Syndrome: Is It Time to Classify It as a Major Noncommunicable Disease? Sleep Sci. 2018, 11, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Schutte-Rodin, S.; Deak, M.C.; Khosla, S.; Goldstein, C.A.; Yurcheshen, M.; Chiang, A.; Gault, D.; Kern, J.; O’Hearn, D.; Ryals, S.; et al. Evaluating Consumer and Clinical Sleep Technologies: An American Academy of Sleep Medicine Update. J. Clin. Sleep Med. 2021, 17, 2275–2282. [Google Scholar] [CrossRef] [PubMed]
- Pan, Q.; Brulin, D.; Campo, E. Current Status and Future Challenges of Sleep Monitoring Systems: Systematic Review. JMIR Biomed. Eng. 2020, 5, e20921. [Google Scholar] [CrossRef]
- Worley, S.L. The Extraordinary Importance of Sleep: The Detrimental Effects of Inadequate Sleep on Health and Public Safety Drive an Explosion of Sleep Research. Pharm. Ther. 2018, 43, 758–763. [Google Scholar]
- Hussain, Z.; Sheng, Q.Z.; Zhang, W.E.; Ortiz, J.; Pouriyeh, S. A Review of the Non-Invasive Techniques for Monitoring Different Aspects of Sleep. arXiv 2021, arXiv:2104.12964. [Google Scholar] [CrossRef]
- Roomkham, S.; Lovell, D.; Cheung, J.; Perrin, D. Promises and Challenges in the Use of Consumer-Grade Devices for Sleep Monitoring. IEEE Rev. Biomed. Eng. 2018, 11, 53–67. [Google Scholar] [CrossRef]
- Tataraidze, A.B.; Anishchenko, L.N.; Korostovtseva, L.S.; Bochkarev, M.V.; Sviryaev, Y.V. Non-Contact Respiratory Monitoring of Subjects with Sleep-Disordered Breathing. In Proceedings of the 2018 International Conference “Quality Management, Transport and Information Security, Information Technologies”, St. Petersburg, Russia, 24–28 September 2018; pp. 736–738. [Google Scholar] [CrossRef]
- Liang, Z.; Chapa-Martell, M.A. A Multi-Level Classification Approach for Sleep Stage Prediction With Processed Data Derived From Consumer Wearable Activity Trackers. Front. Digit. Health 2021, 3, 665946. [Google Scholar] [CrossRef]
- Chang, F.; Patel, T.; Schulz, M.E. The “Rising Tide” of Dementia in Canada: What Does It Mean for Pharmacists and the People They Care For? Can. Pharm. J. 2015, 148, 193–199. [Google Scholar] [CrossRef] [Green Version]
- Andrew, T.L.; Rostaminia, S.; Homayounfar, S.Z.; Ganesan, D. Perspective—Longitudinal Sleep Monitoring for All: Payoffs, Challenges and Outlook. ECS Sens. Plus 2022. [Google Scholar] [CrossRef]
- Perez-Pozuelo, I.; Zhai, B.; Palotti, J.; Mall, R.; Aupetit, M.; Garcia-Gomez, J.M.; Taheri, S.; Guan, Y.; Fernandez-Luque, L. The Future of Sleep Health: A Data-Driven Revolution in Sleep Science and Medicine. Npj Digit. Med. 2020, 3, 42. [Google Scholar] [CrossRef]
- Dixon, M.; Schneider, L.D.; Yu, J.; Hsu, J.; Pathak, A.; Shin, D.; Lee, R.S.; Malhotra, M.; Mixter, K.; Mcconnell, M.V.; et al. Sleep-Wake Detection with a Contactless, Bedside Radar Sleep Sensing System; Google LLC: Seattle, WA, USA, 2021. [Google Scholar]
- Zhang, G.; Vahia, I.V.; Liu, Y.; Yang, Y.; May, R.; Cray, H.V.; McGrory, W.; Katabi, D. Contactless In-Home Monitoring of the Long-Term Respiratory and Behavioral Phenotypes in Older Adults With COVID-19: A Case Series. Front. Psychiatry 2021, 12, 754169. [Google Scholar] [CrossRef] [PubMed]
- Schütz, N.; Saner, H.; Botros, A.; Pais, B.; Santschi, V.; Buluschek, P.; Gatica-Perez, D.; Urwyler, P.; Müri, R.; Nef, T. Contactless Sleep Monitoring for Early Detection of Health Deteriorations in Community-Dwelling Older Adults: Exploratory Study. JMIR mHealth uHealth 2021, 9, e24666. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Wang, Y.; Niu, K.; Zeng, Y.; Gu, T.; Wang, L.; Guan, C.; Zhang, D. WiFi-Sleep: Sleep Stage Monitoring Using Commodity Wi-Fi Devices. IEEE Internet Things J. 2021, 8, 13900–13913. [Google Scholar] [CrossRef]
- Korhonen, I.; Iivainen, T.; Lappalainen, R.; Tuomisto, T.; Kööbi, T.; Pentikäinen, V.; Tuomisto, M.; Turjanmaa, V. TERVA: System for Long-Term Monitoring of Wellness at Home. Telemed. J. e-Health 2001, 7, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Sadek, I.; Bellmunt, J.; Kodyš, M.; Abdulrazak, B.; Mokhtari, M. Novel Unobtrusive Approach for Sleep Monitoring Using Fiber Optics in an Ambient Assisted Living Platform. In Enhanced Quality of Life and Smart Living; Mokhtari, M., Abdulrazak, B., Aloulou, H., Eds.; Springer: Cham, Switzerland, 2017; Volume 10461 LNCS, pp. 48–60. Available online: https://link.springer.com/chapter/10.1007/978-3-319-66188-9_5 (accessed on 7 March 2022). [CrossRef]
- Lima, F.; Albukhari, A.; Zhu, R.; Mescheder, U. Contactless Sleep Monitoring Measurement Setup. Proceedings 2018, 2, 1083. [Google Scholar] [CrossRef] [Green Version]
- Koyama, Y.; Nishiyama, M.; Watanabe, K. Smart Textile Using Hetero-Core Optical Fiber for Heartbeat and Respiration Monitoring. IEEE Sens. J. 2018, 18, 6175–6180. [Google Scholar] [CrossRef]
- Zhou, Z.; Padgett, S.; Cai, Z.; Conta, G.; Wu, Y.; He, Q.; Zhang, S.; Sun, C.; Liu, J.; Fan, E.; et al. Single-Layered Ultra-Soft Washable Smart Textiles for All-around Ballistocardiograph, Respiration, and Posture Monitoring during Sleep. Biosens. Bioelectron. 2020, 155, 112064. [Google Scholar] [CrossRef]
- Nam, Y.; Kim, Y.; Lee, J. Sleep Monitoring Based on a Tri-Axial Accelerometer and a Pressure Sensor. Sensors 2016, 16, 750. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, A.; Alqurashi, R.; Raghebi, Z.; Banaei-Kashani, F.; Halbower, A.C.; Vu, T. LIBS: A Bioelectrical Sensing System from Human Ears for Staging Whole-Night Sleep Study. Commun. ACM 2018, 61, 157–165. [Google Scholar] [CrossRef]
- Gu, W.; Shangguan, L.; Yang, Z.; Liu, Y. Sleep Hunter: Towards Fine Grained Sleep Stage Tracking with Smartphones. IEEE Trans. Mob. Comput. 2016, 15, 1514–1527. [Google Scholar] [CrossRef]
- Tal, A.; Shinar, Z.; Shaki, D.; Codish, S.; Goldbart, A. Validation of Contact-Free Sleep Monitoring Device with Comparison to Polysomnography. J. Clin. Sleep Med. 2017, 13, 517–522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guettari, T.; Istrate, D.; Boudy, J.; Benkelfat, B.E.; Fumel, B.; Daviet, J.C. Design and First Evaluation of a Sleep Characterization Monitoring System Using a Remote Contactless Sensor. IEEE J. Biomed. Health Inform. 2017, 21, 1511–1523. [Google Scholar] [CrossRef] [PubMed]
- Seba, A.; Istrate, D.; Guettari, T.; Ugon, A.; Pinna, A.; Garda, P. Thermal-Signature-Based Sleep Analysis Sensor. Informatics 2017, 4, 37. [Google Scholar] [CrossRef] [Green Version]
- de Zambotti, M.; Rosas, L.; Colrain, I.M.; Baker, F.C. The Sleep of the Ring: Comparison of the ŌURA Sleep Tracker Against Polysomnography. Behav. Sleep Med. 2019, 17, 124–136. [Google Scholar] [CrossRef]
- de Zambotti, M.; Goldstone, A.; Claudatos, S.; Colrain, I.M.; Baker, F.C. A Validation Study of Fitbit Charge 2TM Compared with Polysomnography in Adults. Chronobiol. Int. 2018, 35, 465–476. [Google Scholar] [CrossRef]
- Pallesen, S.; Grønli, J.; Myhre, K.; Moen, F.; Bjorvatn, B.; Hanssen, I.; Heglum, H.S.A. A Pilot Study of Impulse Radio Ultra Wideband Radar Technology as a New Tool for Sleep Assessment. J. Clin. Sleep Med. 2018, 14, 1249–1254. [Google Scholar] [CrossRef] [Green Version]
- Tuominen, J.; Peltola, K.; Saaresranta, T.; Valli, K. Sleep Parameter Assessment Accuracy of a Consumer Home Sleep Monitoring Ballistocardiograph Beddit Sleep Tracker: A Validation Study. J. Clin. Sleep Med. 2019, 15, 483–487. [Google Scholar] [CrossRef] [Green Version]
- Kalkbrenner, C.; Brucher, R.; Kesztyüs, T.; Eichenlaub, M.; Rottbauer, W.; Scharnbeck, D. Automated Sleep Stage Classification Based on Tracheal Body Sound and Actigraphy. GMS Ger. Med. Sci. 2019, 17, Doc02. [Google Scholar] [CrossRef]
- Lauteslager, T.; Kampakis, S.; Williams, A.J.; Maslik, M.; Siddiqui, F. Performance Evaluation of the Circadia Contactless Breathing Monitor and Sleep Analysis Algorithm for Sleep Stage Classification. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Montréal, QC, Canada, 20–24 July 2020; pp. 5150–5153. [Google Scholar] [CrossRef]
- Zhang, F.; Wu, C.; Wang, B.; Wu, M.; Bugos, D.; Zhang, H.; Liu, K.J.R. Smars: Sleep Monitoring via Ambient Radio Signals. IEEE Trans. Mob. Comput. 2021, 20, 217–231. [Google Scholar] [CrossRef]
- Inan, O.T.; Migeotte, P.F.; Park, K.S.; Etemadi, M.; Tavakolian, K.; Casanella, R.; Zanetti, J.; Tank, J.; Funtova, I.; Prisk, G.K.; et al. Ballistocardiography and Seismocardiography: A Review of Recent Advances. IEEE J. Biomed. Health Inform. 2015, 19, 1414–1427. [Google Scholar] [CrossRef] [Green Version]
- Imtiaz, S.A. A Systematic Review of Sensing Technologies for Wearable Sleep Staging. Sensors 2021, 21, 1562. [Google Scholar] [CrossRef] [PubMed]
- Baron, K.G.; Abbott, S.; Jao, N.; Manalo, N.; Mullen, R. Orthosomnia: Are Some Patients Taking the Quantified Self Too Far? J. Clin. Sleep Med. 2017, 13, 351–354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sadek, I.; Biswas, J.; Abdulrazak, B. Ballistocardiogram Signal Processing: A Review. Health Inf. Sci. Syst. 2019, 7, 10. [Google Scholar] [CrossRef]
- Chinoy, E.D.; Cuellar, J.A.; Huwa, K.E.; Jameson, J.T.; Watson, C.H.; Bessman, S.C.; Hirsch, D.A.; Cooper, A.D.; Drummond, S.P.A.; Markwald, R.R. Performance of Seven Consumer Sleep-Tracking Devices Compared with Polysomnography. Sleep 2021, 44, 291. [Google Scholar] [CrossRef]
- Roomkham, S.; Hittle, M.; Cheung, J.; Lovell, D.; Mignot, E.; Perrin, D. Sleep Monitoring with the Apple Watch: Comparison to a Clinically Validated Actigraph [Version 1; Peer Review: 2 Approved with Reservations, 1 Not Approved]. F1000Research 2019, 8, 754. [Google Scholar] [CrossRef] [Green Version]
- Kholghi, M.; Szollosi, I.; Hollamby, M.; Bradford, D.; Zhang, Q. A Validation Study of a Ballistocardiograph Sleep Tracker EMFIT QS against Polysomnography. J. Clin. Sleep Med. 2021; online ahead of print. [Google Scholar] [CrossRef]
- Bhat, S.; Ferraris, A.; Gupta, D.; Mozafarian, M.; De Bari, V.A.; Gushway-Henry, N.; Gowda, S.P.; Polos, P.G.; Rubinstein, M.; Seidu, H.; et al. Is There a Clinical Role for Smartphone Sleep Apps? Comparison of Sleep Cycle Detection by a Smartphone Application to Polysomnography. J. Clin. Sleep Med. 2015, 11, 709–715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wiens, A.D.; Carek, A.M.; Inan, O.T. Sternal Vibrations during Head-out Immersion: A Preliminary Demonstration of Underwater Wearable Ballistocardiography. J. Acoust. Soc. Am. 2015, 138, 342–346. [Google Scholar] [CrossRef]
- Carlson, C.; Turpin, V.R.; Suliman, A.; Ade, C.; Warren, S.; Thompson, D.E. Bed-Based Ballistocardiography: Dataset and Ability to Track Cardiovascular Parameters. Sensors 2021, 21, 156. [Google Scholar] [CrossRef]
- Cappuccio, F.P.; Stranges, S.; Kandala, N.B.; Miller, M.A.; Taggart, F.M.; Kumari, M.; Ferrie, J.E.; Shipley, M.J.; Brunner, E.J.; Marmot, M.G. Gender-Specific Associations of Short Sleep Duration with Prevalent and Incident Hypertension: The Whitehall II Study. Hypertension 2007, 50, 693–700. [Google Scholar] [CrossRef]
- Sadek, I.; Abdulrazak, B.; Mokhtari, M. Evaluating an IoT Under-Mattress Sensor Mat for Detecting Anomalies in Sleep Parameters: A Pilot Study. In Proceedings of the 2021 IEEE Canadian Conference on Electrical and Computer Engineering (CCECE), Ottawa, ON, Canada, 12–17 September 2021; pp. 1–5. [Google Scholar] [CrossRef]
- Sadek, I.; Abdulrazak, B. A Comparison of Three Heart Rate Detection Algorithms over Ballistocardiogram Signals. Biomed. Signal Process. Control 2021, 70, 103017. [Google Scholar] [CrossRef]
- Ye, B.; Khan, S.S.; Chikhaoui, B.; Iaboni, A.; Martin, L.S.; Newman, K.; Wang, A.; Mihailidis, A. Challenges in Collecting Big Data in A Clinical Environment with Vulnerable Population: Lessons Learned from A Study Using A Multi-Modal Sensors Platform. Sci. Eng. Ethics 2019, 25, 1447–1466. [Google Scholar] [CrossRef] [PubMed]

| Study | Objective | Mode of Monitoring | Subjects | Validation Method | Evaluation/Result |
|---|---|---|---|---|---|
| Nam et al. (2016) [21] | Monitoring sleep quality using vital signs. | Tri-Axial Accelerometer and Pressure Sensor | 10 | Validated with PSG and video camera | ⇨ The results showed that the proposed method can measure vital signs affecting sleep quality. ⇨ The estimators of the sleep quality equation were consistent with reference signals. |
| Nguyen et al. (2016) [22] | Presenting a lightweight and inexpensive wearable sensing system. | LIBS | 8 | Validated with PSG | ⇨ The system produced comparable accuracy with PSG for sleep stages classification. |
| Gu et al. (2016) [23] | Detecting transition between sleep stages for sleep quality monitoring and intelligent wake-up call. | Mobile service–Sleep Hunter | 15 | Validated with existing actigraphy-based products, Zeo and Jawbone Up | ⇨ Testing the data over one month provided that the detection accuracy of Sleep Hunter was 64.55%. |
| Tal et al. (2017) [24] | Validating the efficacy of the system for detecting sleep/wake state and sleep parameters against PSG. Testing if the system can detect sleep architecture in various sleeping conditions. | EarlySense system made up of piezoelectric sensor and a mobile application | 63 | Validated with PSG | ⇨ Relative to PSG, the system showed sleep detection sensitivity, specificity, and accuracy of 92.5%, 80.4%, and 90.5%, respectively. |
| Guettari et al. (2017) [25] | Detecting the presence of a person in bed and producing an estimation of the sleep quality. | Thermopile sensor | 13 | Validated with PSG | ⇨ The obtained evaluation results have shown 87% of good classifications with 95% confidence intervals for recognition of the three deducted stages. |
| Seba et al. (2017) [26] | Validating the use of a thermal radiation sensor as a sleep analysis sensor. Analyzing physical activity and thermal radiation during sleep. | Thermopile sensor, thermal camera, accelerometer, iButton | 1 | Connected to an acetimeter consisting of an inertial unit fixed on the wrist of the patient | ⇨ The study validated the efficacy of using temperature sensors for the extraction of skin temperature, actimetry, and the presence, absence, and position of a patient in a bed. |
| Zambotti et al. (2017) [27] | Comparing the output of a multi-sensor sleep tracker (ŌURA ring) to PSG for measuring sleep and sleep phases. | ŌURA ring | 41 | Validated with PSG | ⇨ The ŌURA ring showed good agreement with the PSG measurements of total sleep time, sleep onset latency, and wake after sleep onset. |
| Zambotti et al. (2018) [28] | Comparing the performance of a consumer multi-sensory wristband (Fitbit Charge 2) in measuring sleep stage classification versus PSG. | Fitbit Charge 2 | 44 | Validated with PSG | ⇨ Fitbit achieved 82% accuracy in sleep cycle classification. It overestimated total sleep time and “light sleep” but it underestimated sleep onset latency and “deep sleep”. |
| Pallesen et al. (2018) [29] | Validating the impulse radio ultra-wideband pulse-doppler radar technology against PSG for sleep assessment. | Novelda XeThru radar | 12 | Validated with PSG | ⇨ The mean values obtained for accuracy, sensitivity, specificity, and Cohen kappa were 0.931, 0.961, 0.695, and 0.670, respectively. |
| Tuominen et al. (2019) [30] | Validating the accuracy of a BCG Beddit Sleep Tracker (BST) for sleep monitoring. | Beddit Sleep Tracker | 10 | Validated through comparison with PSG | ⇨ BST was able to identify sleep onset latency with some accuracy. However, it underestimated wake after sleep onset and overestimated total sleep time and sleep efficiency. ⇨ BST did not distinguish between NREM stages and did not detect the REM stage. |
| Kalkbrenner et al. (2019) [31] | Assessing a novel type-4 monitoring system for automated sleep staging. | Type-4 monitoring system | 53 | Validated with PSG | ⇨ The system provided satisfactory results for three-stage sleep classification with an accuracy of 76.3% and Cohen’s kappa of 0.42. |
| Lauteslager et al. (2020) [32] | Assessing the performance of a radar-based system for sleep staging performance. | Circadia Contactless Breathing Monitor (model C100) | 9 | Validated with PSG | ⇨ The system produced an overall accuracy of 66.7%. |
| Zhang et al. (2021) [33] | Present the model, design, and implementation of SMARS, a sleep monitoring system based on ambient radio signals. | Ambient radio signals | 6 | Validated with PSG and Four state-of-the-art RF-based respiratory monitoring systems | ⇨ Accuracy of 88.4% for three-stage classification, coverage of up 8–10 m, and detection rate of 80%. |
| Yu et al. (2021) [15] | Presenting a Wi-Fi-Sleep, a sleep stage monitoring system to monitor and classify sleep. | Wi-Fi transceivers | 12 | Ground truth was obtained from PSG. The performance was Validated with SMARS and RF-Sleep | ⇨ Wi-Fi-Sleep showed 81.8% accuracy for four-stage sleep classification. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siyanbade, J.; Abdulrazak, B.; Sadek, I. Unobtrusive Monitoring of Sleep Cycles: A Technical Review. BioMedInformatics 2022, 2, 204-216. https://doi.org/10.3390/biomedinformatics2010013
Siyanbade J, Abdulrazak B, Sadek I. Unobtrusive Monitoring of Sleep Cycles: A Technical Review. BioMedInformatics. 2022; 2(1):204-216. https://doi.org/10.3390/biomedinformatics2010013
Chicago/Turabian StyleSiyanbade, Juwonlo, Bessam Abdulrazak, and Ibrahim Sadek. 2022. "Unobtrusive Monitoring of Sleep Cycles: A Technical Review" BioMedInformatics 2, no. 1: 204-216. https://doi.org/10.3390/biomedinformatics2010013
APA StyleSiyanbade, J., Abdulrazak, B., & Sadek, I. (2022). Unobtrusive Monitoring of Sleep Cycles: A Technical Review. BioMedInformatics, 2(1), 204-216. https://doi.org/10.3390/biomedinformatics2010013







