Predicting Childhood Obesity Using Machine Learning: Practical Considerations
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Source
- The Child Health Improvement through Computer Automation (CHICA) system; a computer-based pediatric primary care clinical decision support system that operated in eight pediatric primary care practices in Indianapolis between 2004–2019 [36]. The CHICA system includes data for over 47,000 patients on factors such as measured height and weight, demographics (e.g., child sex, age, race/ethnicity, Medicaid insurance status), and social determinants of health (e.g., parent health literacy, food and housing insecurity, parental depression, and infant feeding practices);
- The IN Standard Certificate of Live Birth (i.e., ‘birth certificate’), which consists of 235 variables covering parental sociodemographic information as well as information on prenatal care, labor/delivery, and neonatal conditions and procedures. Birth certificate data were made available from the Marion County Public Health Department (MCPHD); and
- The Social Assets and Vulnerabilities Indicators (SAVI) Project, which collects geocodes, organizes, and presents integrated data on communities in the 11-county Indianapolis metropolitan statistical area drawn from more than 30 federal, state, and local providers. All are linked to the lowest available geographic level [37]. SAVI is the nation’s largest community information system, with more than 10,000 time-series variables from 1980 to the present, including welfare, education, health, public safety, housing, demographics, locations of health facilities, health and human services, community facilities, and associated service areas.
2.2. Data Preprocessing
2.3. Model Development
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Name | Description | Data Source |
| weight | child’s weight at visit | CHICA |
| wtcentile | child’s weight percentile | CHICA |
| height | child’s height at visit | CHICA |
| htcentile | child’s height percentile | CHICA |
| insurance | What kind of insurance, if any, the patient has at time of visit | CHICA |
| any_household_members_smoke | Do any of the people that live with the child smoke? | CHICA |
| car_seat_position_01 | Does the child use a car seat, and if so, which way is it facing? | CHICA |
| fluoride_supplemented | Does the child have fluoride supplemented somehow through consumption? | CHICA |
| has_smoke_detector | Does the child’s living area have a smoke detector? | CHICA |
| hc | child’s head circumference in centimeters | CHICA |
| hccentile | child’s head circumference percentile | CHICA |
| know_how_to_save_choking_child | Do the child’s caregivers know how to perform the Heimlich maneuver on a choking child? | CHICA |
| left_alone_in_water | Is the child left alone in water? | CHICA |
| lg_failed | What question of the language developmental test did the child fail on? | CHICA |
| maternal_depression_concern | Based on a questionnaire, is there a concern that the mom might be depressed? | CHICA |
| medicationallergies | Does the child have any medication allergies and have the allergies been confirmed by a doctor or only reported by the family? | CHICA |
| painqualitative | Is the child in pain, yes, no or NA? | CHICA |
| ps_passed | What is the highest passed question for the psychosocial developmental test? | CHICA |
| sleeps_on_side_or_back | Does the child sleep on their side or back? | CHICA |
| slept_on_stomach_ever | Does the child ever sleep on their stomach? | CHICA |
| uses_walker | Does the child use a walker? | CHICA |
| baby_left_alone_could_fall | Is the baby ever left alone where they could fall? | CHICA |
| sleeps_unsafe_soft_surface | Does the child sleep on an unsafe soft surface such as a mattress that they can suffocate on if they sleep facedown? | CHICA |
| tested_smoke_detector | If the child’s living place has a smoke detector, has it been tested as working? | CHICA |
| abdomen_exam | If the child’s abdomen is examined, is it abnormal or normal? | CHICA |
| back_exam | If the child’s back is examined, is it abnormal or normal? | CHICA |
| chestlungs_exam | If the child’s chest or lungs are examined, is it abnormal or normal? | CHICA |
| extgenitalia_exam | If the child’s external genitalia is examined, is it normal or abnormal? | CHICA |
| extremities_exam | If the child’s extremities (hands, feet, nose, ears) are examined, are they normal or abnormal? | CHICA |
| fm_passed | For the fine motor skills developmental test, what is the highest question passed? | CHICA |
| general_exam | If the child had a general exam, was it normal or abnormal? | CHICA |
| gm_passed | For the gross motor skills developmental test, what is the highest passed question? | CHICA |
| head_exam | If the child’s head is examined, is it normal or abnormal? | CHICA |
| heartpulses_exam | If the child’s heart and pulse are examined, is it normal or abnormal? | CHICA |
| lg_passed | For the language developmental test, what is the highest scoring passed question? | CHICA |
| neuro_exam | If a neurological battery is done, was it normal or abnormal? | CHICA |
| nodes_exam | If the lymph nodes are checked, were they normal or abnormal? | CHICA |
| nosethroat_exam | If the nose and throat are examined, are they normal or abnormal? | CHICA |
| skin_exam | If the child’s skin is examined, was it normal or abnormal? | CHICA |
| teethgums_exam | If the child’s teeth and gums are examined, were they normal or abnormal? | CHICA |
| preferred_language | Does the child have a preferred language and if so, is it English or Spanish? | CHICA |
| burns_knowledge | Does the caregiver have knowledge of how to take care of burns? | CHICA |
| firearms_at_home | Are there any firearms in the home? | CHICA |
| firearms_where_visits | Are there any firearms where the visit is taking place? | CHICA |
| has_stairway_gates | Are there child safety gates over the stairways? | CHICA |
| household_products_out_of_reach | Are household cleaning products such as bleach out of the reach of children? | CHICA |
| matches_lighters_safe | Are matches and lighters kept in a safe manner? childproof wheel, out of reach, etc. | CHICA |
| play_area_fenced | Is the child’s play area fenced in? | CHICA |
| pool_at_house | Is there a pool the child can access? | CHICA |
| chica_devscreen_status | This is a developmental screening that states whether the child is developing normally or if they are developmentally delayed and indicate which developmental screenings have been done. | CHICA |
| seen_dentist | Has the child ever been seen by a dentist? This is unlikely to be true until after the child has teeth. | CHICA |
| taking_medications | Is the child on any medications and if so, has this list of medications been confirmed to be accurate? | CHICA |
| tv_in_room | Is there a TV in the child’s bedroom? | CHICA |
| tv_over_2hrs | Does the child watch TV for more than two hours every day? | CHICA |
| uses_bottle | Does the child use a bottle to eat? | CHICA |
| asthmastatus | Does the child have any asthma symptoms and if so, are they persistent, intermittent, uncontrolled/controlled? | CHICA |
| chica_devscreen_sx | Are there any developmental concerns? | CHICA |
| lye_drain_cleaners_in_house | Are there any lye, drain, or other more dangerous cleaners in the house? | CHICA |
| ps_failed | What question of the psychosocial test did the child fail on? | CHICA |
| stop_at_curb | Does the child stop at curbs or run straight without stopping? | CHICA |
| wears_bike_helmet | Does the child wear a bike helmet for activities where one is recommended? | CHICA |
| insurancename | What kind of insurance does the child have? | CHICA |
| parents_confident_filling_out_ | Do the parents appear confident filling out forms? | CHICA |
| parents_need_help_reading | Do the parents need help reading forms? | CHICA |
| ten_childrens_books_in_home | Are there at least 10 children’s books in the home available to the child? | CHICA |
| visittype | Is this a visit because the child is sick? | CHICA |
| chica_adhd_sx | Is the child having symptoms of ADHD? | CHICA |
| constipation_sx | Is the child having symptoms of constipation? | CHICA |
| firearms_kept_unloaded | Are any firearms kept unloaded in the household? | CHICA |
| look_both_ways | Does the child look both ways before crossing the street? | CHICA |
| unsupervised_near_water | Is the child left unsupervised near water? | CHICA |
| firearms_discussed | Has firearm safety been discussed with the child? | CHICA |
| grades_dropped_lately | Has the child’s school grades dropped recently? | CHICA |
| knows_how_to_swim | Does the child know how to swim? | CHICA |
| rides_bike_in_street | Does the child ride their bike in the street? | CHICA |
| school_suspension_this_year | Has the child been suspended from school this year? | CHICA |
| snoring | Have parents noticed that the child snores? | CHICA |
| special_education_classes | Does the child attend special education classes? | CHICA |
| escape_plan_for_fire | Has the family discussed a house fire escape plan with their child? Older children version of smoke_alarm_knows_what_to_do | CHICA |
| informant | What household member is answering the questions? | CHICA |
| smoke_alarm_knows_what_to_do | Does the child know what to do when the smoke/fire alarm is triggered? Younger children version of escape_plan_for_fire | CHICA |
| specialneeds | Does the child have special needs or accomodations? Such as ear defenders, speech therapist, etc... | CHICA |
| visit_attendee | What household member is attending the visit but not necessarily the informant? | CHICA |
| hot_water_heater_adjusted | Has the water heater been adjusted so the water can only be heated to 120 degrees farenheit? This is a scalding concern. | CHICA |
| plastic_wrappers_secured | Are plastic wrappers in the environment secured or left in an accessible area? This is a suffocation hazard. | CHICA |
| taking_solid_food | Is the child eating solid food yet? | CHICA |
| cutting_food_bite_size | Are the child’s solid foods being cut into bite size pieces before being given to the child? If no, this is a choking/suffocation hazard. | CHICA |
| carries_hot_liquids | Is the child allowed to carry hot liquids? This is a burn hazard. | CHICA |
| play_area_cooking | Does the child have an area to play and be safely in away from cooking area while caregiver is cooking? This is a burn risk if not. | CHICA |
| safety_latches_installed | Have safety latches been installed in the house? | CHICA |
| car_seat_inspection | Has the child’s car seat been inspected and if so, is it forward or rear facing? Rear facing is the safer option. | CHICA |
| developmental_referral | Has the child been referred to developmental testing and if so, have only the first steps been taken or has the appointment been made? | CHICA |
| fm_failed | What difficulty of the fine motor skills test did the child fail on? | CHICA |
| correctedvision | Does the child wear glasses or contact lenses? | CHICA |
| firearms_friends | Does the child go to friend’s houses which have firearms? | CHICA |
| plays_dangerous_items | Does the child play with dangerous items? | CHICA |
| wears_sports_protective_gear | Does the child wear protective gear while playing sports? | CHICA |
| safety_caps_on_bottles | Are there child safety caps on pill bottles around the child? | CHICA |
| wears_life_jacket | Does the child wear a life jacket in situations where that is recommended? | CHICA |
| bedtime_media | Does the child use media products at bedtime? | CHICA |
| daytime_sleepiness | Is the child sleepy during the day? | CHICA |
| questionnaireinformants | Which caregiver filled out the questionnaire? | CHICA |
| sleep_quantity | Does the child get sufficient or insufficient sleep? | CHICA |
| chica_t2dm_fh | Does the child’s medical records include family history? | CHICA |
| chica_t2dm_gdm | Did the child’s mother have gestational diabetes? | CHICA |
| chica_t2dm_lga | Was the child large for their gestational age during pregnancy? | CHICA |
| epilepsy_history | Is there a family-reported family history of epilepsy? | CHICA |
| breast_feeding_help_needed | Does the mother need help breastfeeding? | CHICA |
| oral_exam | Has the child’s mouth been examined and if so, was it normal or abnormal? | CHICA |
| bp_eval | Has the child’s blood pressure been evaluated and if so, was it elevated once or repeatedly elevated? There was no option for hypotensive in this variable. | CHICA |
| empty_container_after_use | Do caregivers empty bathwater container immediately after use? This is a drowning risk if no. | CHICA |
| well_water | Does the child’s household run off well-water? Well-water is a contamination concern. | CHICA |
| lowliteracyrisk | Is the child at risk of low literacy and if so, have they gone to a clinic to help? | CHICA |
| morning_headaches | Does the child have headaches in the morning or wake up with a headache? | CHICA |
| nocturnal_enuresis | Does the child wet the bed/pee during sleep? This question is for kids who are out of diapers. | CHICA |
| stops_breathing_at_night | Does the child’s caregiver know if the child stops breathing during the night? | CHICA |
| trouble_breathing_at_night | Does the child’s caregiver know if the child has trouble breathing during the night? | CHICA |
| wakes_with_snort | Does the child caregiver know if the child wakes up with a snort? | CHICA |
| rides_after_dark | Does the child ride the bike after sunset? | CHICA |
| knows_rules_of_road | Does the child know traffic rules? | CHICA |
| swims_fast_moving_water | Does the child swim in fast-moving water such as a river? | CHICA |
| chica_adhd_dx | Does the child have an ADHD diagnosis? | CHICA |
| doors_secure | Are the doors in the child’s home secure? | CHICA |
| sharp_edged_furniture | Are there sharp-edged furniture in the child’s home? | CHICA |
| pulseox | What was the child’s pulse oxygenation percentage at visit? | CHICA |
| has_window_guards | Does the child home have window guards? | CHICA |
| play_equipment_protected | Does the child play on safe playground equipment? | CHICA |
| asthmasymptoms | Does the child have symptoms of asthma? | CHICA |
| gm_failed | What gross motor test did the child fail on? | CHICA |
| chica_adhd_side_effects | Does the child experience side effects from their ADHD medication? | CHICA |
| irondeficiencyscreenqualitativ | Has the child been checked for iron deficiency and if so, what were the results? | CHICA |
| chica_devscreen_management | Is the child part of activities specifically made for children? | CHICA |
| normal_newborn_screen | Did the child have the normal newborn screen and if so, what were the results? | CHICA |
| vaccine_given | Has the child had the HPV, Tdap, or meningococcal vaccine given? | CHICA |
| anhedonia_past_few_weeks | Has the child been anhedonic/apathetic the last few weeks? | CHICA |
| cigarettes_snuff_friend | Does the child’s friend or friend’s household use cigarettes or snuff? | CHICA |
| cigarettes_snuff_live_with | Does someone the child lives with use snuff? | CHICA |
| ever_use_tobacco | Has the child ever used tobacco? | CHICA |
| has_drunk_alcohol | Has the child drunk alcohol at all? | CHICA |
| has_gotten_high | Has the child used an illicit substance? | CHICA |
| has_had_forced_sex_act | Has the child experienced a forced sex act? | CHICA |
| has_had_intercourse | Has the child had intercourse? | CHICA |
| sad_past_few_weeks | Has the child been sad in the past few weeks? | CHICA |
| suicide_concerns | Is there a concern of suicidality for the child? | CHICA |
| used_marijuana | Has the child used marijuana? | CHICA |
| interested_birth_control | Is the child interested in contraception? | CHICA |
| ready_to_quit | Is the child ready to quit smoking cigarettes? | CHICA |
| watches_tv | Does the child watch TV? | CHICA |
| sleep_problems | Does the child have problems sleeping? | CHICA |
| nobp | Child did not cooperate in visit; Could not check blood pressure. | CHICA |
| nohearing | Child did not cooperate in visit; Could not perform hearing exam. | CHICA |
| risk_based_hearing_screen | Has the child undergone a hearing screen that was ordered based on high risk? | CHICA |
| chica_devscreen_treatment | Does the child have a written care plan or access to family support services? | CHICA |
| anxiety_status | Does the child have an anxiety diagnosis, or has this questionnaire been deferred? | CHICA |
| phq9_score | What was the mother’s depression score on the phq9? | CHICA |
| driven_with_drunk | Has the child driven while drunk? | CHICA |
| drunk_and_activity | Has the child been drunk while doing an activity? | CHICA |
| drunk_last_month | Has the child been drunk in the last month? | CHICA |
| family_substance_abuse | Does the child’s family abuse any substances? | CHICA |
| happy_how_things_going | Is the child happy with life? | CHICA |
| uses_drugs | Does the child use drugs? | CHICA |
| sudep_risk_counseling | Is the child at risk for sudden unexpected death from epilepsy? If so, is the risk high or low? | CHICA |
| surgical_hx | Has the child had their tonsils and adenoids removed? | CHICA |
| feed_at_night | Does the child eat at night? | CHICA |
| contraceptive_method_discussed | Has birth control been discussed with the child such as condoms and hormonal birth control? | CHICA |
| abuse_otc | Does the child abuse over the counter medication? | CHICA |
| abuse_steroids | Does the child abuse steroids drugs? | CHICA |
| criticized_for_drinking | Has the child been criticized for drinking? | CHICA |
| friends_use_drugs | Has the child’s friends used drugs (other than alcohol/caffeine) in the last month? | CHICA |
| friend_drunk_last_month | Has the child’s friends been drunk in the last month? | CHICA |
| fun_in_past_two_weeks | Does the child think they’ve had fun in the last two weeks? | CHICA |
| bike_has_coaster_brakes | Does the child’s bike have coaster brakes? Coaster brakes allow you to pedal backwards to brake. | CHICA |
| past_depression_or_suicide | Has the child had any previous history of depression or suicidality? | CHICA |
| immune_compromise | Is the child immuno-compromised? | CHICA |
| prescription_for_cessation | Is the child on a prescribed nicotine replacement drug? | CHICA |
| intercourse_past_year | Has the child had intercourse in the last year? | CHICA |
| might_be_pregnant | Could the child be pregnant? | CHICA |
| medication | Does the child have a Ritalin prescription? | CHICA |
| depression_workup | Is there a developed safety plan for the child’s depression? | CHICA |
| chica_autism_risk | Is the child at a higher risk of autism due to family history? | CHICA |
| tooth_erupted | Has the child had a tooth erupt from beneath the gums yet? | CHICA |
| autism_behavior_problems | Does the child have autism related behavior problems? | CHICA |
| autism_cam | Does the child use complementary alternative medicine for autism? | CHICA |
| autism_financial_concerns | Are there financial concerns related to the child’s autism such as paying for therapy? | CHICA |
| autism_parent_needs_respite | Is the child’s caregiver in need of a break? i.e., showing symptoms of caregiver burnout | CHICA |
| patient_in_mental_health | Is the child undergoing mental health care? | CHICA |
| food_insecurity | Is the child’s caregiver worried about getting enough food and if so, has this been MD confirmed or resolved? | CHICA |
| rental_status | Is the child’s rental home clean & safe vs having issues, and has this been confirmed by an MD? | CHICA |
| snapdeniedlast30days | Has the child’s SNAP(food stamps) been denied in the last 30 days? | CHICA |
| utility_status | Has the child’s household had one of their utilities (water, power, heat, gas) shut off? Yes, no, or yes but not heat. | CHICA |
| mlp_condition_type | Is the child’s family going through an eviction, on the SNAP program, or renting? | CHICA |
| wakes_up_one_or_more_times_a_n | Does the child wake up at least once during the night? | CHICA |
| wakes_up_and_needs_help_to_sleep | Does the child wake up at night and need help getting back to sleep? | CHICA |
| sleeps_on_back | Does the child sleep on their back? | CHICA |
| slept_on_stomach_side_ever | Does the child ever sleep on their stomach or side? | CHICA |
| abuse_concern | Is there a concern that the child is being abused? | CHICA |
| constipation_dx | Has the child been diagnosed with constipation? | CHICA |
| parent_thinks_child_has_sleep_pr | Do the caregivers think that the child has problems with their sleep? | CHICA |
| eyesvision_exam | Did the child have a normal or abnormal vision exam? | CHICA |
| breastfed | Is the child being breastfed at this time? | CHICA |
| psfsicklecell | Result of pre-screening form on tablet for sickle cell anemia. | CHICA |
| negativeenvironmentalhistory | Was the child potentially exposed to something negative in their environment such as tuberculosis or lead? | CHICA |
| negativenutritionhistory | Did the child have nutrition problems such as early introduction to cow milk or needing low iron formula? | CHICA |
| negativepastmedicalhistory | Did the child have a low birth weight? | CHICA |
| cholesterol_screen | Is the child at risk of high cholesterol based on parental history? | CHICA |
| earshearing_exam | Did the child have a normal or abnormal hearing exam? | CHICA |
| hearingleft | Does the child have full or partial hearing in their left ear? | CHICA |
| hearingright | Does the child have full or partial hearing in their right ear? | CHICA |
| ppd_result | What was the result of the mother’s post-partum depression assessment? | CHICA |
| venousbloodleadqualitative | How much lead was in the child’s blood, if tested? | CHICA |
| mother_bmi | Maternal body mass index | MCPHD |
| PNC_Clinic_Type | Type of prenatal care clinic | MCPHD |
| Sex | Child’s sex | MCPHD |
| FATHER_OCCUP_DSCRP | Is child’s father employed at time of birth? | MCPHD |
| MomNativeAm | Is child’s mother Native American? | MCPHD |
| Mother_Weight_Gain_P | How many pounds the mother has gained during pregnancy. | MCPHD |
| MARRIED_NOW | Are child’s parents married at time of birth? | MCPHD |
| APGAR5 | Appearance, Pulse, Grimace, Activity, and Respiration at five minutes post birth. Score of 10 is good; one is bad. | MCPHD |
| BIRTH_WEIGHT_GRAM | Birth weight in grams from modern birth certificate | MCPHD |
| finalroute | How was the child delivered? | MCPHD |
| HEP_B_TEST | Was hepatitis B vaccine given at birth? | MCPHD |
| Apgar1 | Appearance, Pulse, Grimace, Activity, and Respiration at 1 min post birth. Score of 10 is good; one is bad. | MCPHD |
| Dad_Race9Eth | race of child’s father | MCPHD |
| Mom_Race9Eth | race of child’s mother | MCPHD |
| PREN_VISIT_NBR | number of prenatal care visits | MCPHD |
| EST_GEST | estimated gestation in weeks | MCPHD |
| MOTHER_AGE | age of the mother at birth in years | MCPHD |
| FATHER_AGE | age of the father at birth in years | MCPHD |
| PREVIOUS_LIVE_NBR | How many living babies has the mother giving birth to before? | MCPHD |
| plurality | Is this a plural or singleton birth? (twins) | MCPHD |
| BREAST_FED | Was the child breast-fed at hospital release? | MCPHD |
| MOTHER_ED | mother’s education level in years | MCPHD |
| FATHER_ED | father’s education level in years | MCPHD |
| LD_MECONIUM | delivery complication: was there meconium present at delivery? | MCPHD |
| LD_NONE | no delivery complications | MCPHD |
| LD_NON_VERTEX | delivery complication: child in non- vertex position | MCPHD |
| firstpnc | prenatal care initiated in first trimester | MCPHD |
| wtgrams | child’s birth weight in grams | MCPHD |
| PREV_BIRTH_TOTAL | number of previous live births—all birth certificates | MCPHD |
| Kotelchuck | adequacy of prenatal care index | MCPHD |
| mdpsmoke | Did the mother smoke during pregnancy? | MCPHD |
| abcond | Were abnormal conditions present at birth? | MCPHD |
| anomaly | Was a congenital anomaly found? | MCPHD |
| infect | maternal infections | MCPHD |
| labdel | labor and delivery | MCPHD |
| mmorb | maternal morbidity | MCPHD |
| methdel | method of delivery | MCPHD |
| oblab | obstetrical labor | MCPHD |
| obproc | obstetrical procedures | MCPHD |
| risk | maternal risk factor | MCPHD |
| RACE | race of the child | CHICA |
| ETHN | ethnicity of the child | CHICA |
| wic_ever | Has the child ever been in the WIC program? | CHICA/MCPHD |
| PERINPOVN1 | persons living in poverty as percentage of population | SAVI |
| VIOLENTN2 | violent crime (including simple assaults) per 1000 people | SAVI |
| VIOLNSTN2 | violent crime (not including simple assaults) per 1000 people | SAVI |
| AGGVASLTN2 | aggravated assaults per 1000 people | SAVI |
| ROBBERYN2 | robberies per 1000 people | SAVI |
| PROPERTYN2 | property crime per 1000 people | SAVI |
| THFTVHN2 | vehicle thefts per 1000 people | SAVI |
| BURGLARYN2 | burglaries per 1000 people | SAVI |
| WALKSCORE | walkability score | SAVI |
| FRRDTRAN1 | free and reduced lunch program participants as percentage of enrollment | SAVI |
| POVB185N1 | population below 185% poverty (proxy for reduced lunch) | SAVI |
| POVB125N1 | population below 125% poverty (proxy for free lunch) | SAVI |
| RESNEWPEN1 | total residential building permits per 100 housing units | SAVI |
| COMMALLPN1 | total commercial building permits per 100 housing units | SAVI |
| TREE_CANOPY | tree canopy as percentage of land area | SAVI |
| PCT_POP_FOOD_DESERT | percentage of population far from grocery stores | SAVI |
References
- Friedrich, M. Global obesity epidemic worsening. JAMA 2017, 318, 603. [Google Scholar] [CrossRef] [PubMed]
- GBD 2015 Obesity Collaborators. Health effects of overweight and obesity in 195 countries over 25 years. N. Engl. J. Med. 2017, 377, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Freedman, D.S.; Khan, L.K.; Dietz, W.H.; Srinivasan, S.R.; Berenson, G.S. Relationship of childhood obesity to coronary heart disease risk factors in adulthood: The Bogalusa Heart Study. Pediatrics 2001, 108, 712–718. [Google Scholar] [CrossRef] [PubMed]
- Must, A.; Strauss, R.S. Risks and consequences of childhood and adolescent obesity. Int. J. Obes. Relat. Metab. Disord. 1999, 23 (Suppl. 2), S2–S11. [Google Scholar] [CrossRef] [PubMed]
- Dietz, W.H. Overweight and precursors of type 2 diabetes mellitus in children and adolescents. J. Pediatr. 2001, 138, 453–454. [Google Scholar] [CrossRef]
- Taveras, E.M.; Rifas-Shiman, S.L.; Camargo, C.A., Jr.; Gold, D.R.; Litonjua, A.A.; Oken, E.; Weiss, S.T.; Gillman, M.W. Higher adiposity in infancy associated with recurrent wheeze in a prospective cohort of children. J. Allergy Clin. Immunol. 2008, 121, 1161–1166.e3. [Google Scholar] [CrossRef] [Green Version]
- Dietz, W.H. Childhood weight affects adult morbidity and mortality. J. Nutr. 1998, 128 (Suppl. 2), 411S–414S. [Google Scholar] [CrossRef]
- World Health Organization. Commission on the Social Determinants of Health; WHO: Geneva, Switzerland, 2008. [Google Scholar]
- General Assembly of the United Nations. High-Level Meeting on Non-Communicable Diseases. 2011. Available online: http://www.un.org/en/ga/president/65/issues/ncdiseases.shtml (accessed on 1 June 2021).
- Li, H.; Stein, A.D.; Barnhart, H.X.; Ramakrishnan, U.; Martorell, R. Associations between prenatal and postnatal growth and adult body size and composition. Am. J. Clin. Nutr. 2003, 77, 1498–1505. [Google Scholar] [CrossRef] [Green Version]
- Rogers, I. The influence of birthweight and intrauterine environment on adiposity and fat distribution in later life. Int. J. Obes. 2003, 27, 755–777. [Google Scholar] [CrossRef] [Green Version]
- Barlow, S.E.; Expert, C. Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: Summary report. Pediatrics 2007, 120 (Suppl. 4), S164–S192. [Google Scholar] [CrossRef] [Green Version]
- Baidal, J.A.W.; Locks, L.M.; Cheng, E.R.; Blake-Lamb, T.L.; Perkins, M.E.; Taveras, E.M. Risk factors for childhood obesity in the first 1000 days: A systematic review. Am. J. Prev. Med. 2016, 50, 761–779. [Google Scholar]
- LeCroy, M.N.; Kim, R.S.; Stevens, J.; Hanna, D.B.; Isasi, C.R. Identifying Key Determinants of Childhood Obesity: A Narrative Review of Machine Learning Studies. Child. Obes. 2021, 17, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Wiemken, T.L.; Kelley, R.R. Machine Learning in Epidemiology and Health Outcomes Research. Annu. Rev. Public Health 2019, 41, 21–36. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Tjortjis, C.; Zeng, X.; Qiao, H.; Buchan, I.; Keane, J. Comparing data mining methods with logistic regression in childhood obesity prediction. Inf. Syst. Front. 2009, 11, 449–460. [Google Scholar] [CrossRef]
- Beam, A.L.; Kohane, I.S. Big data and machine learning in health care. JAMA 2018, 319, 1317–1318. [Google Scholar] [CrossRef] [PubMed]
- Simon, G.R.; Baker, C.; Barden, G.A., 3rd; Brown, O.W.; Hardin, A.; Lessin, H.R.; Meade, K.; Moore, S.; Rodgers, C.T.; Hammer, L.D.; et al. 2014 Recommendations for Pediatric Preventive Health Care. Pediatrics 2014, 133, 568–570. [Google Scholar]
- Wolf, E.R.; Hochheimer, C.J.; Sabo, R.T.; DeVoe, J.; Wasserman, R.; Geissal, E.; Opel, D.J.; Warren, N.; Puro, J.; O’Neil, J.; et al. Gaps in well-child care attendance among primary care clinics serving low-income families. Pediatrics 2018, 142, e20174019. [Google Scholar] [CrossRef] [Green Version]
- Siddiqui, H.; Rattani, A.; Woods, N.K.; Cure, L.; Lewis, R.; Twomey, J.; Smith-Campbell, B.; Hill, T.J. A Survey on Machine and Deep Learning Models for Childhood and Adolescent Obesity. IEEE Access 2021, 9, 157337–157360. [Google Scholar] [CrossRef]
- Grow, H.M.; Cook, A.J.; Arterburn, D.E.; Saelens, B.E.; Drewnowski, A.; Lozano, P. Child obesity associated with social disadvantage of children’s neighborhoods. Soc. Sci. Med. 2010, 71, 584–591. [Google Scholar] [CrossRef] [Green Version]
- Fiechtner, L.; Block, J.; Duncan, D.T.; Gillman, M.W.; Gortmaker, S.L.; Melly, S.J.; Rifas-Shiman, S.L.; Taveras, E.M. Proximity to supermarkets associated with higher body mass index among overweight and obese preschool-age children. Prev. Med. 2013, 56, 218–221. [Google Scholar] [CrossRef] [Green Version]
- Lovasi, G.S.; Schwartz-Soicher, O.; Quinn, J.W.; Berger, D.K.; Neckerman, K.M.; Jaslow, R.; Lee, K.K.; Rundle, A. Neighborhood safety and green space as predictors of obesity among preschool children from low-income families in New York City. Prev. Med. 2013, 57, 189–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carroll-Scott, A.; Gilstad-Hayden, K.; Rosenthal, L.; Peters, S.M.; McCaslin, C.; Joyce, R.; Ickovics, J.R. Disentangling neighborhood contextual associations with child body mass index, diet, and physical activity: The role of built, socioeconomic, and social environments. Soc. Sci. Med. 2013, 95, 106–114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papas, M.A.; Alberg, A.J.; Ewing, R.; Helzlsouer, K.J.; Gary, T.L.; Klassen, A.C. The built environment and obesity. Epidemiol. Rev. 2007, 29, 129–143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dunton, G.F.; Kaplan, J.; Wolch, J.; Jerrett, M.; Reynolds, K.D. Physical environmental correlates of childhood obesity: A systematic review. Obes. Rev. Off. J. Int. Assoc. Study Obes. 2009, 10, 393–402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lovasi, G.S.; Hutson, M.A.; Guerra, M.; Neckerman, K.M. Built environments and obesity in disadvantaged populations. Epidemiol. Rev. 2009, 31, 7–20. [Google Scholar] [CrossRef] [Green Version]
- Butler, É.M.; Derraik, J.G.; Taylor, R.W.; Cutfield, W.S. Prediction models for early childhood obesity: Applicability and existing issues. Horm. Res. Paediatr. 2018, 90, 358–367. [Google Scholar] [CrossRef]
- Ziauddeen, N.; Roderick, P.J.; Macklon, N.S.; Alwan, N.A. Predicting childhood overweight and obesity using maternal and early life risk factors: A systematic review. Obes. Rev. 2018, 19, 302–312. [Google Scholar] [CrossRef] [Green Version]
- Hawkins, S.S.; Oken, E.; Gillman, M.W. Early in the life course: Time for obesity prevention. In Handbook of Life Course Health Development; Springer: Berlin/Heidelberg, Germany, 2018; pp. 169–196. [Google Scholar]
- Blake-Lamb, T.L.; Locks, L.M.; Perkins, M.E.; Woo Baidal, J.A.; Cheng, E.R.; Taveras, E.M. Interventions for Childhood Obesity in the First 1000 Days A Systematic Review. Am. J. Prev. Med. 2016, 50, 780–789. [Google Scholar] [CrossRef] [Green Version]
- St. George, S.M.; Agosto, Y.; Rojas, L.M.; Soares, M.; Bahamon, M.; Prado, G.; Smith, J.D. A developmental cascade perspective of paediatric obesity: A systematic review of preventive interventions from infancy through late adolescence. Obes. Rev. 2020, 21, e12939. [Google Scholar] [CrossRef]
- Hochreiter, S.; Schmidhuber, J. Long short-term memory. Neural Comput. 1997, 9, 1735–1780. [Google Scholar] [CrossRef]
- Wang, Y.; Canahuate, G.M.; Van Dijk, L.V.; Mohamed, A.S.; Fuller, C.D.; Zhang, X.; Marai, G.-E. Predicting late symptoms of head and neck cancer treatment using LSTM and patient reported outcomes. In Proceedings of the 25th International Database Engineering & Applications Symposium, Montreal, QC, Canada, 14–16 July 2021. [Google Scholar]
- Deng, Y.; Dolog, P.; Gass, J.-M.; Denecke, K. Obesity entity extraction from real outpatient records: When learning-based methods meet small imbalanced medical data sets. In Proceedings of the 2019 IEEE 32nd International Symposium on Computer-Based Medical Systems (CBMS), Cordoba, Spain, 5–7 June 2019. [Google Scholar]
- Anand, V.; Biondich, P.G.; Liu, G.; Rosenman, M.; Downs, S.M. Child Health Improvement through Computer Automation: The CHICA system. Stud. Health Technol. Inform. 2004, 107, 187–191. [Google Scholar] [PubMed]
- Bodenhamer, D.J.; Colbert, J.T.; Comer, K.F.; Kandris, S.M. Developing and sustaining a community information system for central Indiana: SAVI as a case study. In Community Quality-of-Life Indicators: Best Cases V; Springer: Berlin/Heidelberg, Germany, 2011; pp. 21–46. [Google Scholar]
- Kuczmarski, R.J.; Ogden, C.L.; Grummer-Strawn, L.M.; Flegal, K.M.; Guo, S.S.; Wei, R.; Mei, Z.; Curtin, L.R.; Roche, A.F.; Johnson, C.L. CDC growth charts: United States. Adv. Data 2000, 314, 1–27. [Google Scholar]
- Hammond, R.; Athanasiadou, R.; Curado, S.; Aphinyanaphongs, Y.; Abrams, C.; Messito, M.J.; Gross, R.; Katzow, M.; Jay, M.; Razavian, N.; et al. Predicting childhood obesity using electronic health records and publicly available data. PLoS ONE 2019, 14, e0215571. [Google Scholar] [CrossRef] [PubMed]
- Lachenbruch, P.A.; Mickey, M.R. Estimation of error rates in discriminant analysis. Technometrics 1968, 10, 1–11. [Google Scholar] [CrossRef]
- Fatima, Y.; Doi, S.; Mamun, A. Sleep quality and obesity in young subjects: A meta-analysis. Obes. Rev. 2016, 17, 1154–1166. [Google Scholar] [CrossRef] [PubMed]
- Matricciani, L.; Paquet, C.; Galland, B.; Short, M.; Olds, T. Children’s sleep and health: A meta-review. Sleep Med. Rev. 2019, 46, 136–150. [Google Scholar] [CrossRef]
- Harrington, J.W.; Nguyen, V.Q.; Paulson, J.F.; Garland, R.; Pasquinelli, L.; Lewis, D. Identifying the “tipping point” age for overweight pediatric patients. Clin. Pediatr. 2010, 49, 638–643. [Google Scholar] [CrossRef]
- Sim, L.A.; Lebow, J.; Wang, Z.; Koball, A.; Murad, M.H. Brief primary care obesity interventions: A meta-analysis. Pediatrics 2016, 138, e20160149. [Google Scholar] [CrossRef] [Green Version]
- Gupta, M.; Phan, T.-L.T.; Bunnell, T.; Beheshti, R. Obesity Prediction with EHR Data: A deep learning approach with interpretable elements. arXiv 2019, arXiv:191202655. [Google Scholar]
- Thamrin, S.A.; Arsyad, D.S.; Kuswanto, H.; Lawi, A.; Nasir, S. Predicting Obesity in Adults Using Machine Learning Techniques: An analysis of Indonesian Basic Health Research 2018. Front. Nutr. 2021, 8, 252. [Google Scholar] [CrossRef]
- Dugan, T.M.; Mukhopadhyay, S.; Carroll, A.; Downs, S. Machine Learning Techniques for Prediction of Early Childhood Obesity. Appl. Clin. Inform. 2015, 6, 506–520. [Google Scholar] [PubMed] [Green Version]
- Chatterjee, A.; Gerdes, M.W.; Martinez, S.G. Identification of risk factors associated with obesity and overweight—A machine learning overview. Sensors 2020, 20, 2734. [Google Scholar] [CrossRef] [PubMed]
- DeGregory, K.W.; Kuiper, P.; DeSilvio, T.; Pleuss, J.D.; Miller, R.; Roginski, J.W.; Fisher, C.B.; Harness, D.; Viswanath, S.; Heymsfield, S.B.; et al. A review of machine learning in obesity. Obes. Rev. 2018, 19, 668–685. [Google Scholar] [CrossRef] [PubMed]
- Colmenarejo, G. Machine Learning Models to Predict Childhood and Adolescent Obesity: A Review. Nutrients 2020, 12, 2466. [Google Scholar] [CrossRef]
- Montañez, C.A.C.; Fergus, P.; Hussain, A.; Al-Jumeily, D.; Abdulaimma, B.; Hind, J.; Radi, N. Machine learning approaches for the prediction of obesity using publicly available genetic profiles. In Proceedings of the 2017 International Joint Conference on Neural Networks (IJCNN), Anchorage, AK, USA, 14–19 May 2017. [Google Scholar]
- Santorelli, G.; Petherick, E.S.; Wright, J.; Wilson, B.; Samiei, H.; Cameron, N.; Johnson, W. Developing prediction equations and a mobile phone application to identify infants at risk of obesity. PLoS ONE 2013, 8, e71183. [Google Scholar] [CrossRef]
- Weng, S.F.; Redsell, S.A.; Nathan, D.; Swift, J.A.; Yang, M.; Glazebrook, C. Estimating overweight risk in childhood from predictors during infancy. Pediatrics 2013, 132, e414–e421. [Google Scholar] [CrossRef] [Green Version]
- Vrijkotte, T.G.; Oostvogels, A.J.; Stronks, K.; Roseboom, T.J.; Hof, M.H. Growth patterns from birth to overweight at age 5–6 years of children with various backgrounds in socioeconomic status and country of origin: The ABCD study. Pediatric Obes. 2020, 15, e12635. [Google Scholar] [CrossRef] [Green Version]
| Category | Source | Description |
|---|---|---|
| Prenatal | MCPHD | Maternal risk factor during pregnancy |
| MCPHD | Method of delivery: vaginal versus cesarean section | |
| MCPHD | Child’s birthweight (in grams) | |
| Demographic | MCPHD | Child sex |
| CHICA | Child’s ethnicity | |
| CHICA | Child’s age at the clinical encounter | |
| CHICA | Preferred language of the child | |
| MCPHD | Biological mother’s age at delivery | |
| MCPHD | Biological mother’s race and ethnicity | |
| MCPHD | Father’s race and ethnicity | |
| Environmental | CHICA | Blood lead level |
| CHICA/MCPHD | Flag for if the child has ever been enrolled in the WIC program | |
| SAVI | Percentage of the local population living in a food desert, based on child’s address at birth | |
| CHICA | Parent is confident filling out health forms | |
| CHICA | Who attended the visit (e.g., mother, father, grandparent, etc.) | |
| CHICA | Flag for low health literacy risk, as determined by a validated screener | |
| CHICA | Parent response to “Are all the doors in your house that lead outside, to stairs, or potentially dangerous areas secured against [child] opening them?” | |
| Developmental | CHICA | Flag for developmental delay |
| CHICA | Parent reports concerns about the child’s behavioral development | |
| Sleep Quality | CHICA | Parent response to “Does [child] often wake up one or more times per night, and does an adult go to him/her?” |
| CHICA | Parent response to “Do you think [child] has a sleep problem?” | |
| Clinical | CHICA | Type of clinic visit (routine versus sick visit) |
| CHICA | Prior BMI measurements | |
| CHICA | Time between clinical encounters |
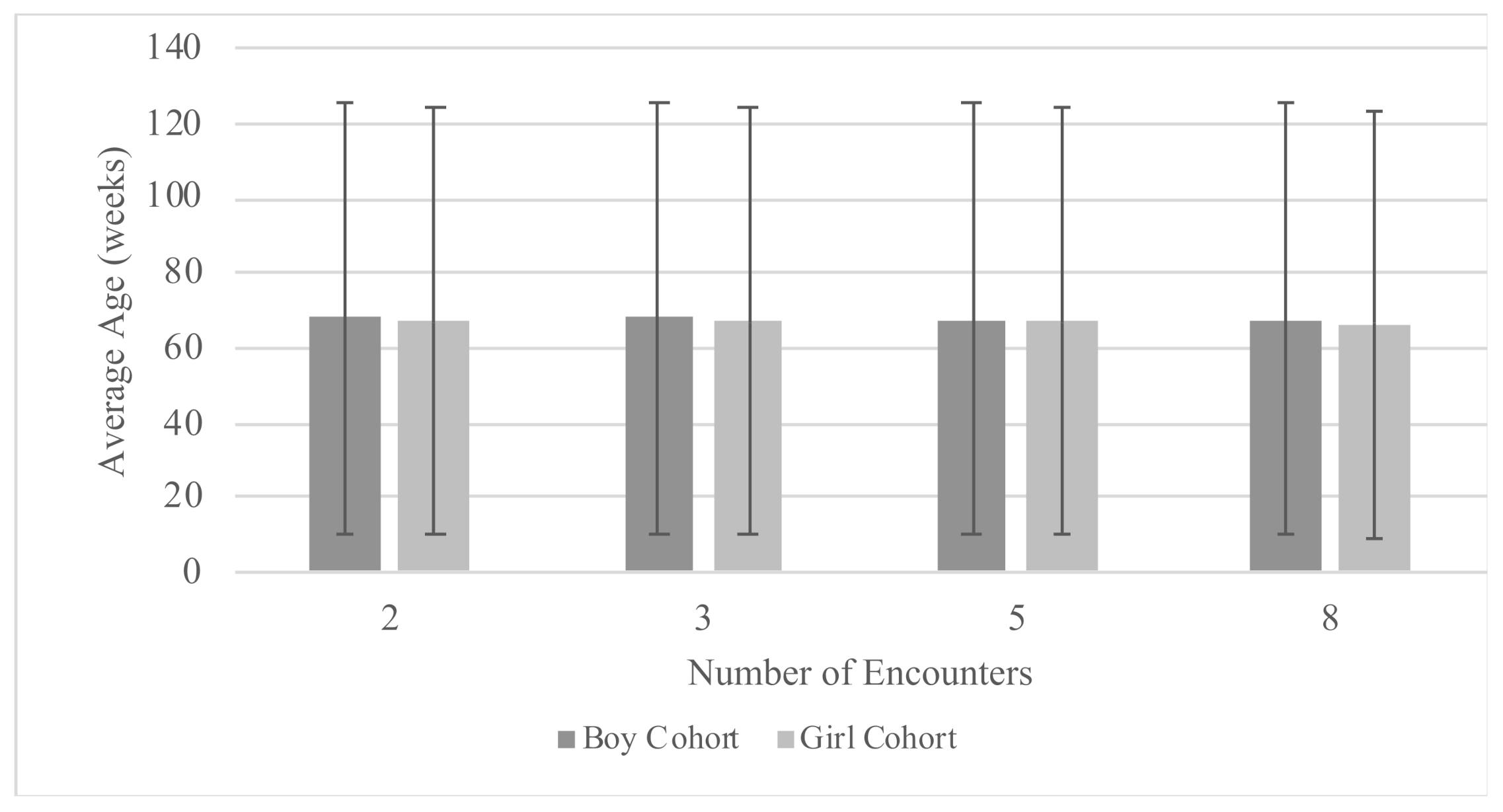
| Population | N | BMI | Age at Encounter (Weeks) | Encounters per Patient * |
|---|---|---|---|---|
| Mean (SD) | ||||
| Training Cohort | ||||
| Male | 2694 | 16.79 (2.26) | 67.54 (57.43) | 12.56 (4.44) |
| Female | 2614 | 16.39 (2.22) | 66.75 (57.22) | 12.01 (3.69) |
| Combined | 5308 | 16.59 (2.25) | 67.16 (57.33) | 12.29 (4.10) |
| Testing Cohort | ||||
| Male | 657 | 16.71 (2.20) | 69.07 (58.09) | 12.55 (4.18) |
| Female | 649 | 16.38 (2.20) | 67.28 (56.92) | 12.28 (4.17) |
| Combined | 1306 | 16.55 (2.21) | 68.19 (57.52) | 12.42 (4.18) |
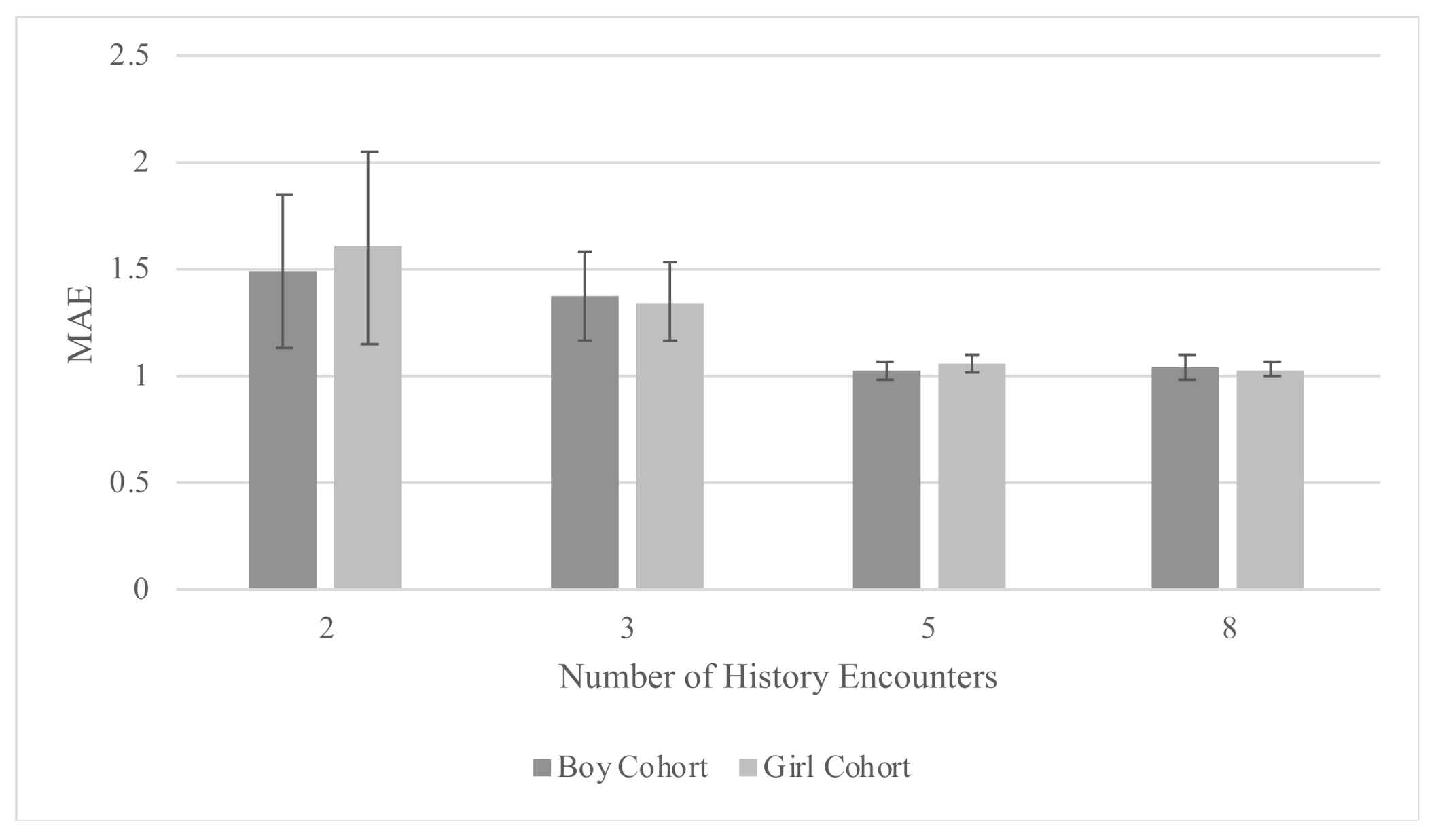
| History (Encounters) | MAE (SD) | R2 | Prediction Horizon (Weeks) |
|---|---|---|---|
| Boy Cohort | |||
| 8 | 1.04 (0.06) | 0.68 (0.02) | 21.56 (17.06) |
| 5 | 1.02 (0.04) | 0.68 (0.02) | 20.48 (16.87) |
| 3 | 1.37 (0.21) | 0.58 (0.07) | 18.83 (16.1) |
| 2 | 1.49 (0.36) | 0.55 (0.09) | 17.79 (15.73) |
| Girl Cohort | |||
| 8 | 1.03 (0.03) | 0.71 (0.01) | 22.71 (17.39) |
| 5 | 1.06 (0.04) | 0.69 (0.01) | 21.18 (17.22) |
| 3 | 1.35 (0.18) | 0.62 (0.04) | 19.36 (16.37) |
| 2 | 1.60 (0.45) | 0.49 (0.14) | 18.25 (16.02) |
| Combined Cohort | |||
| 5 | 0.98 (0.03) | 0.72 (0.01) | 20.87 (17.09) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, E.R.; Steinhardt, R.; Ben Miled, Z. Predicting Childhood Obesity Using Machine Learning: Practical Considerations. BioMedInformatics 2022, 2, 184-203. https://doi.org/10.3390/biomedinformatics2010012
Cheng ER, Steinhardt R, Ben Miled Z. Predicting Childhood Obesity Using Machine Learning: Practical Considerations. BioMedInformatics. 2022; 2(1):184-203. https://doi.org/10.3390/biomedinformatics2010012
Chicago/Turabian StyleCheng, Erika R., Rai Steinhardt, and Zina Ben Miled. 2022. "Predicting Childhood Obesity Using Machine Learning: Practical Considerations" BioMedInformatics 2, no. 1: 184-203. https://doi.org/10.3390/biomedinformatics2010012
APA StyleCheng, E. R., Steinhardt, R., & Ben Miled, Z. (2022). Predicting Childhood Obesity Using Machine Learning: Practical Considerations. BioMedInformatics, 2(1), 184-203. https://doi.org/10.3390/biomedinformatics2010012







