New Fluorescent Porphyrins with High Two-Photon Absorption Cross-Sections Designed for Oxygen-Sensitization: Impact of Changing the Connectors in the Peripheral Arms
Abstract
1. Introduction
2. Results
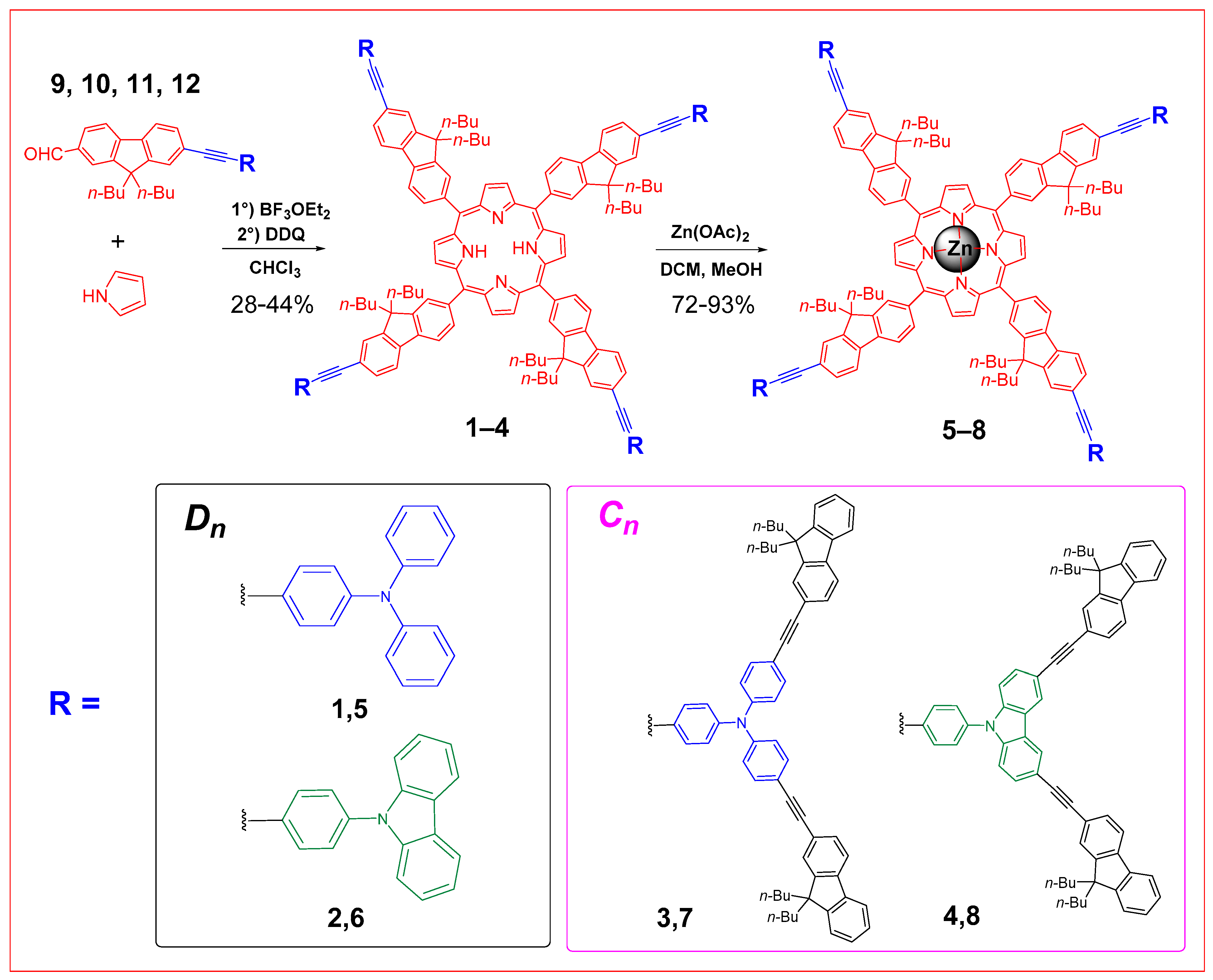
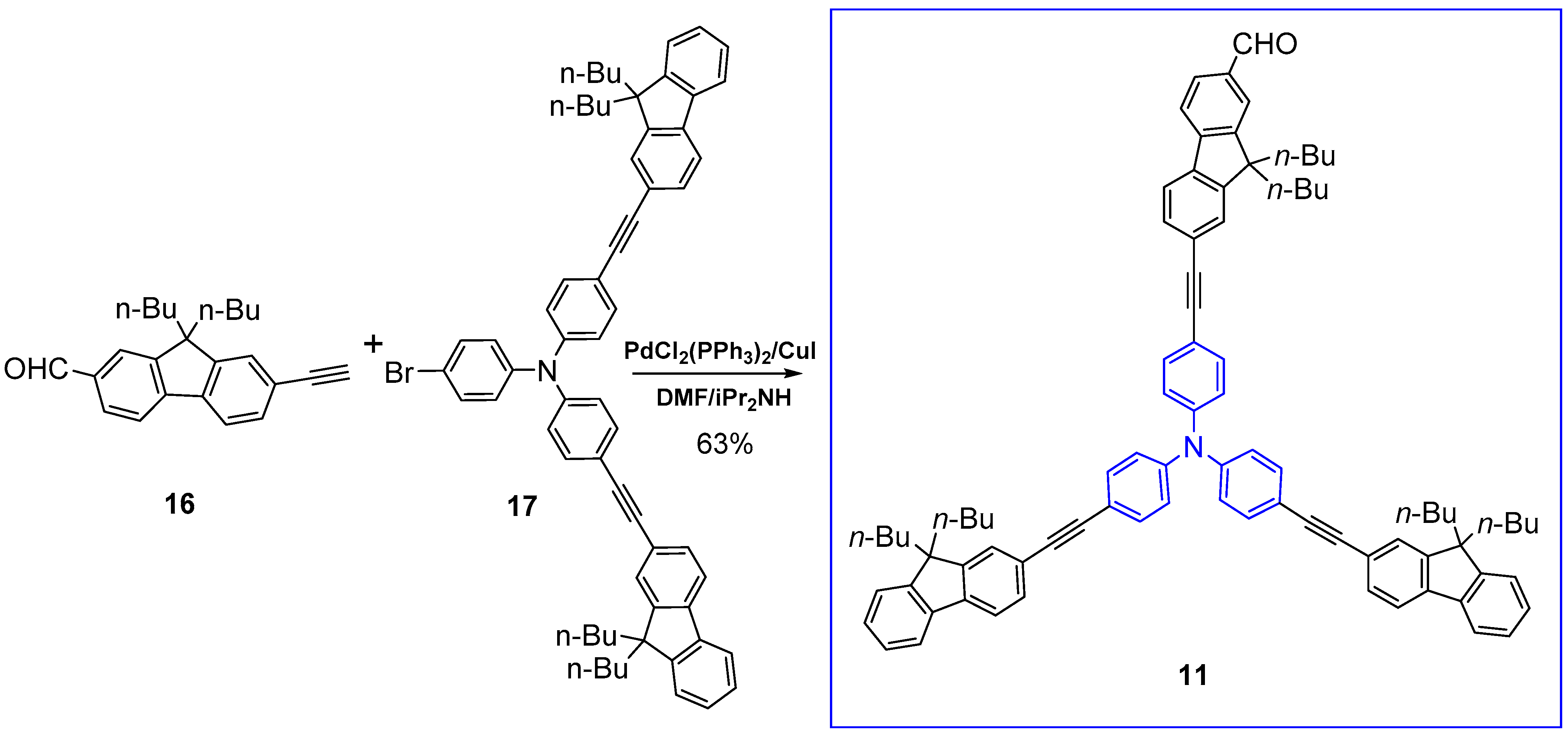
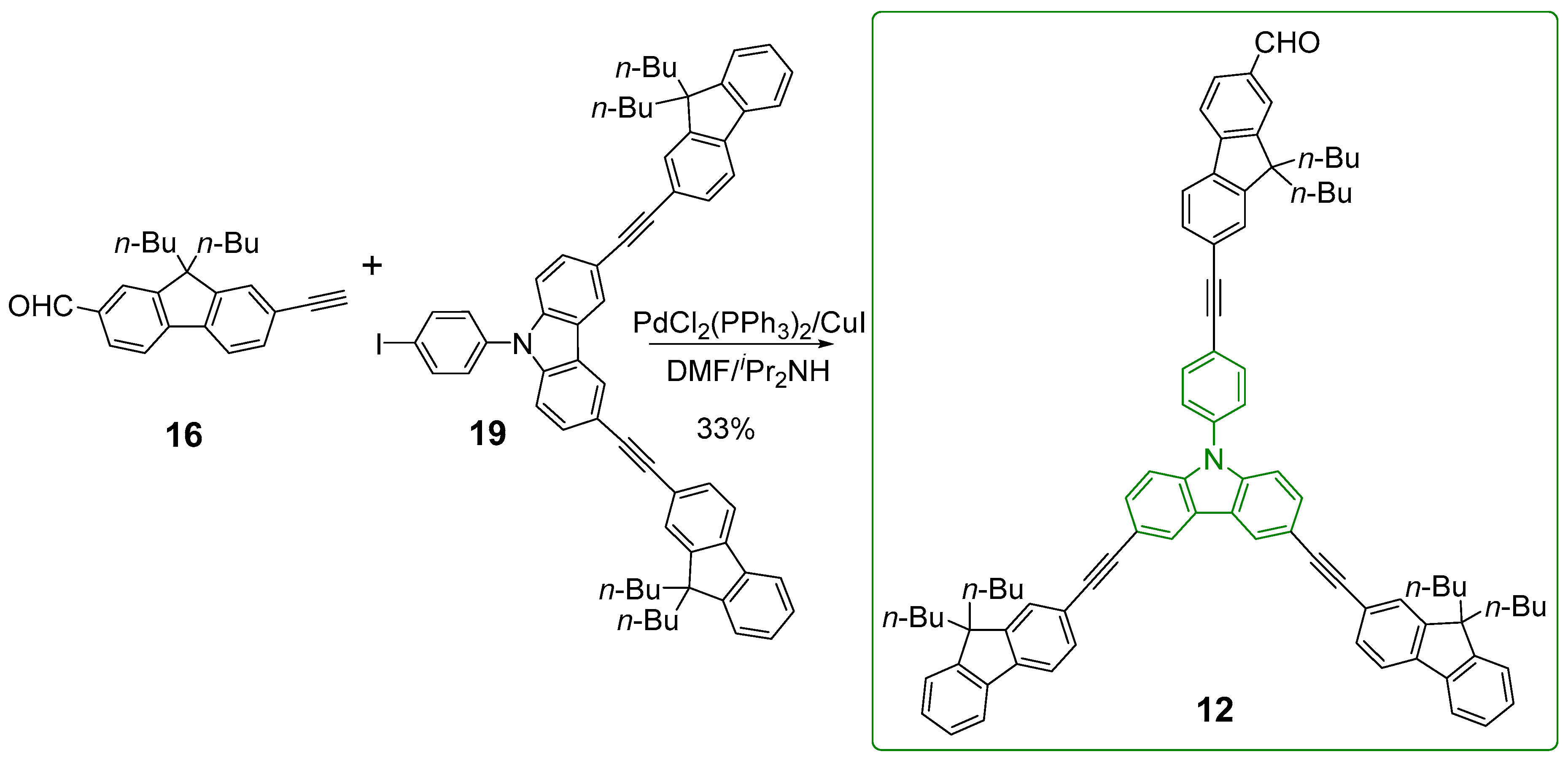
2.1. Syntheses of the Targeted Dendrimers
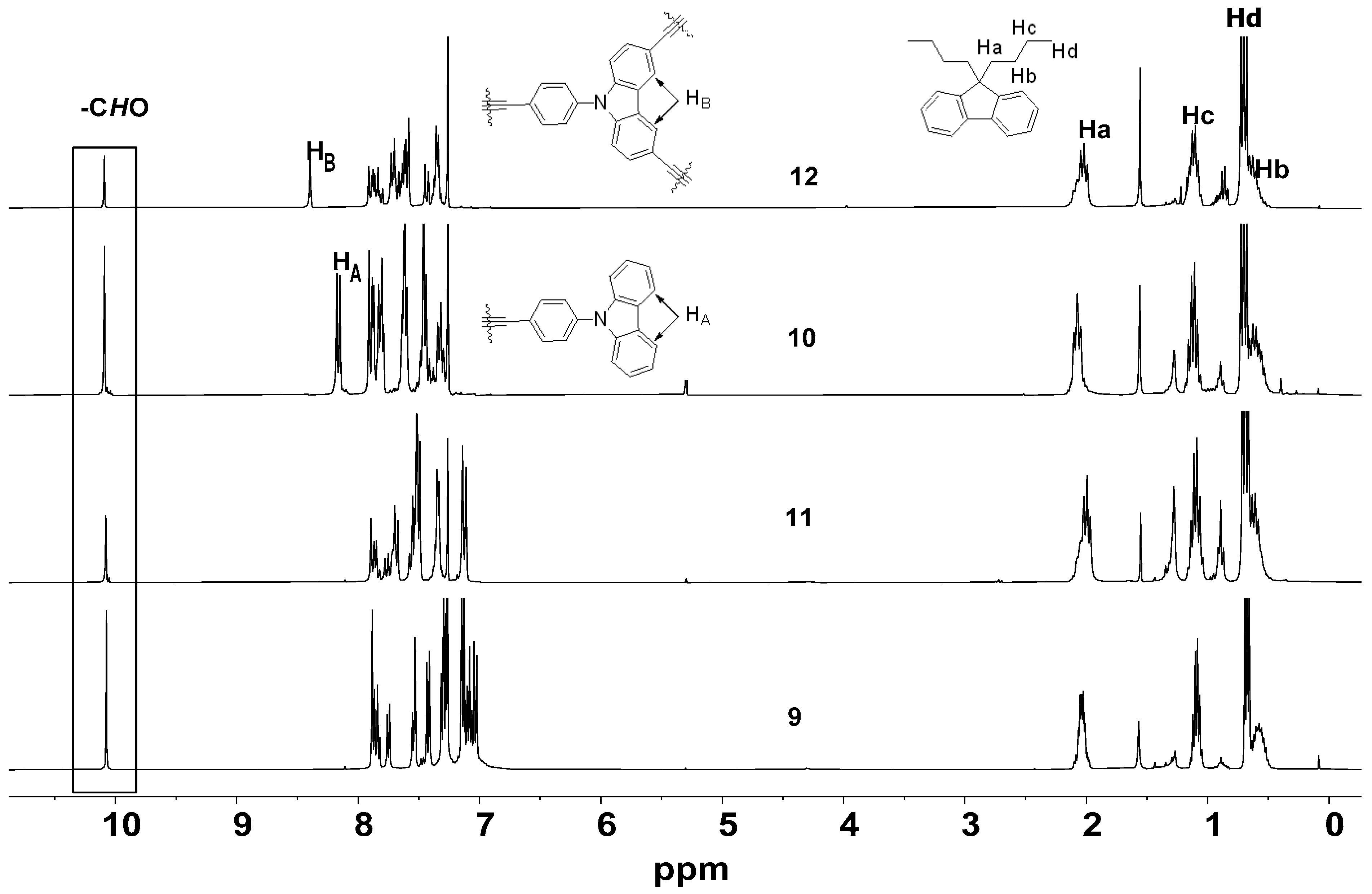
2.2. Characterization of the Dendrimers
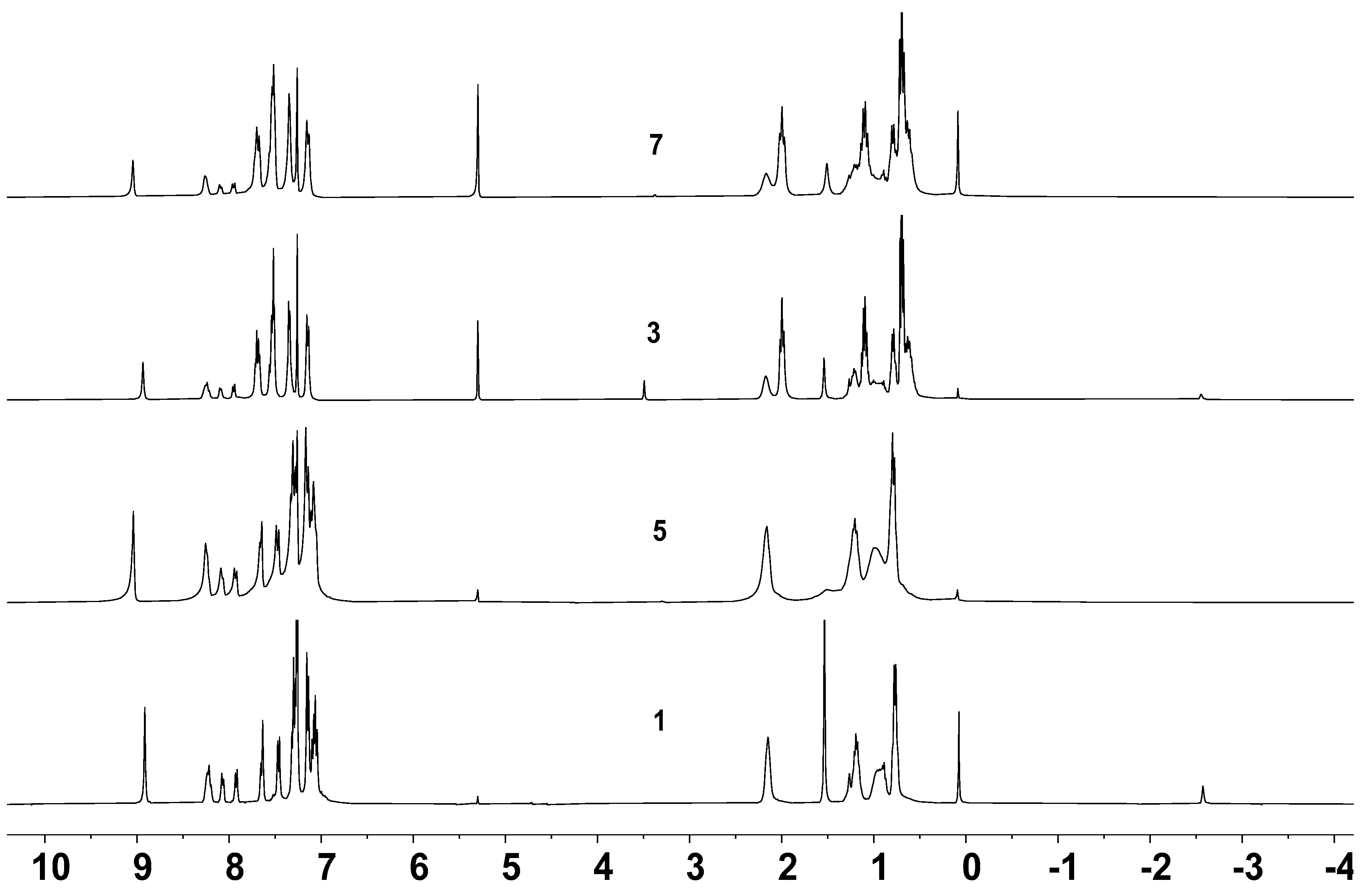
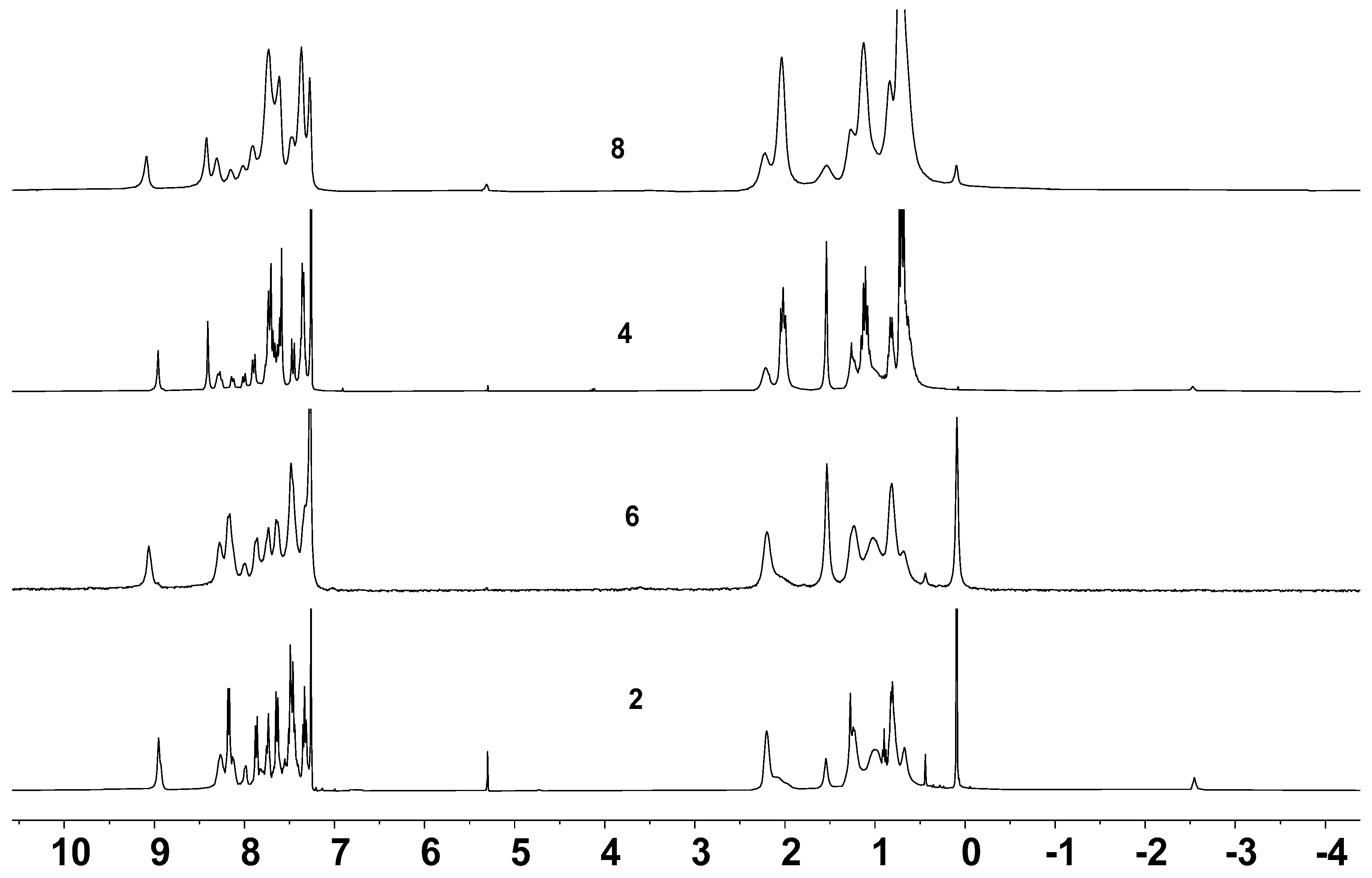
2.3. Optical Properties
3. Discussion
3.1. Detrimental Impact of Metalation by Zn(II)
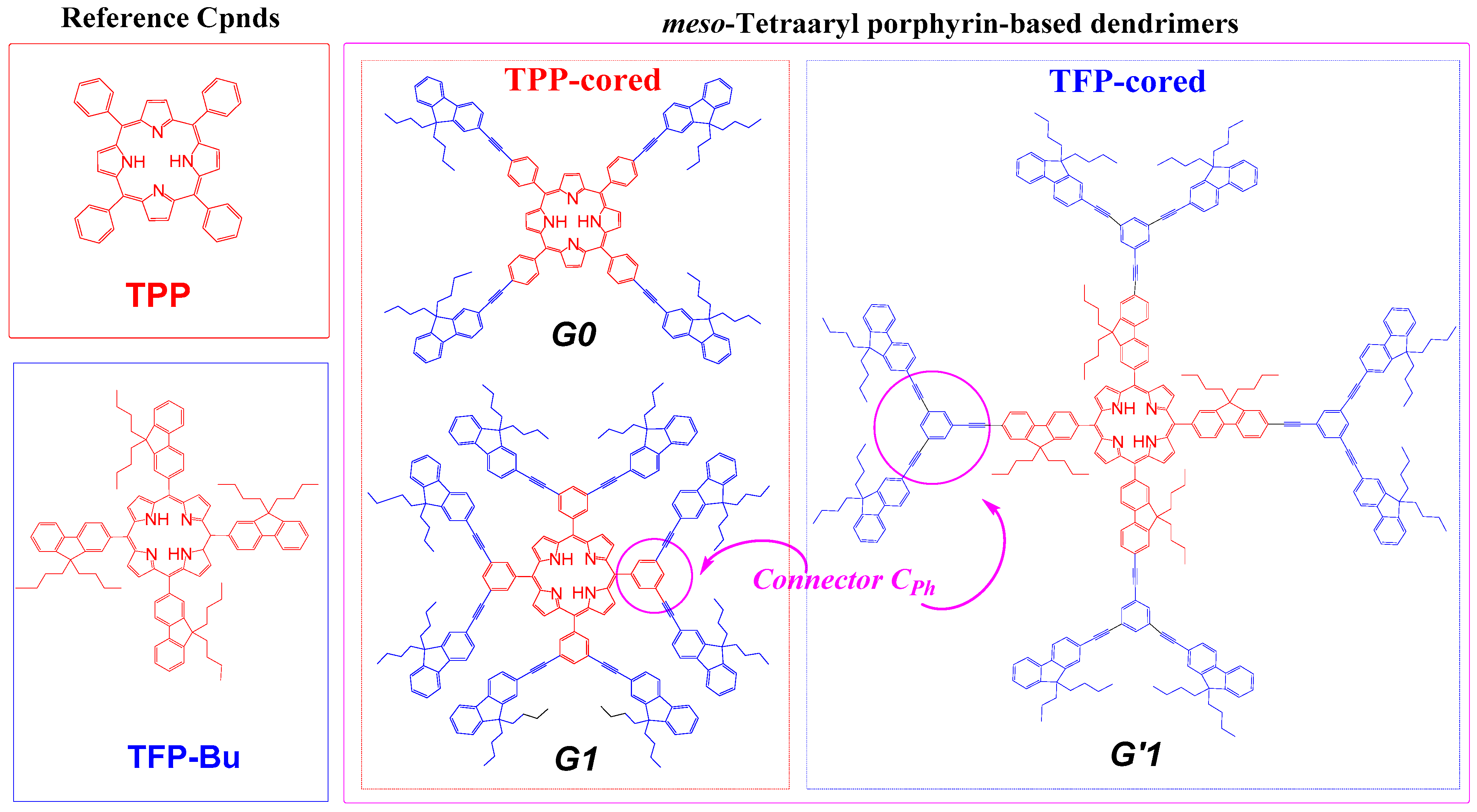
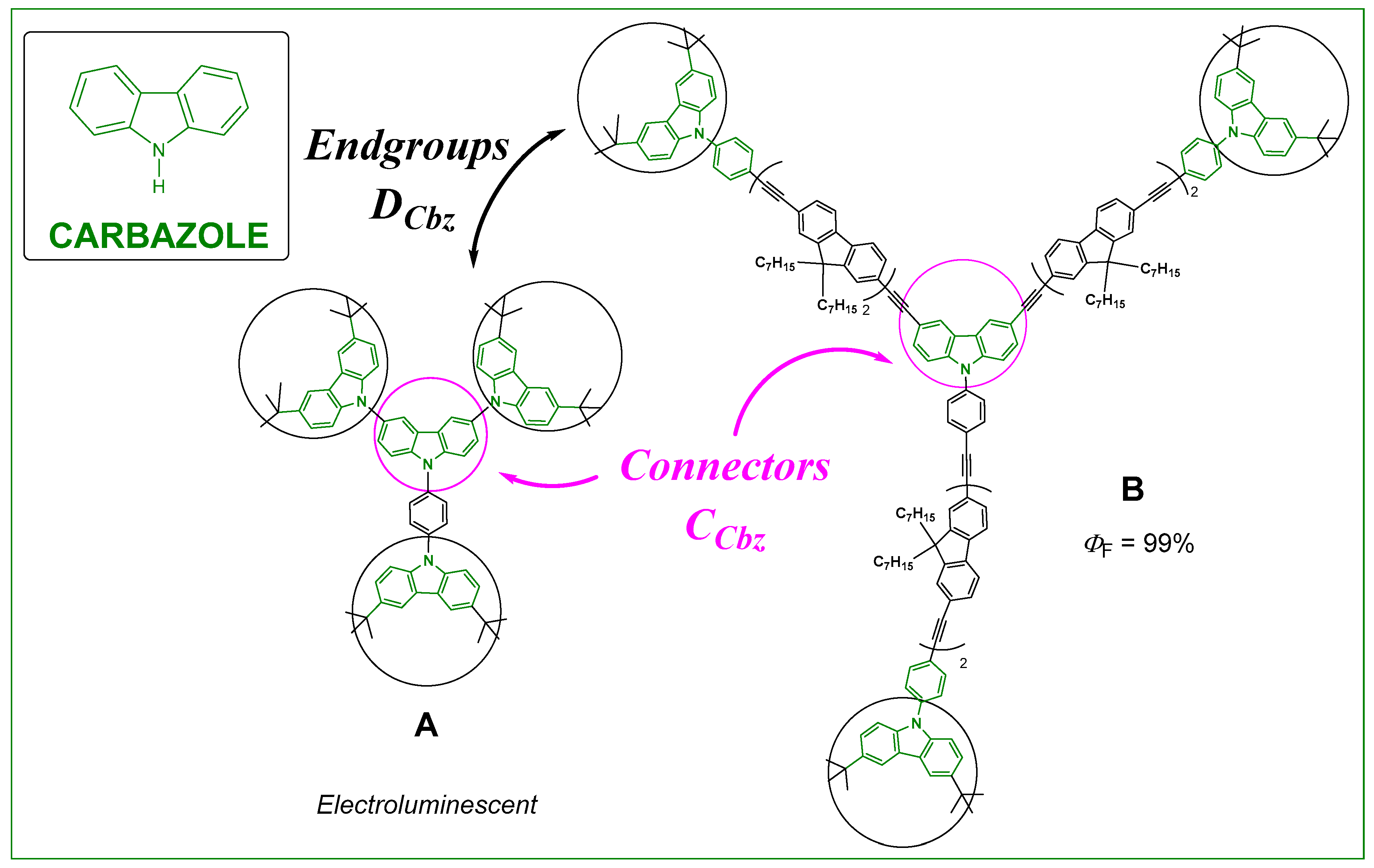
3.2. Impact of CTpa vs. CCbz Connectors on the Luminescence of These Dendrimers
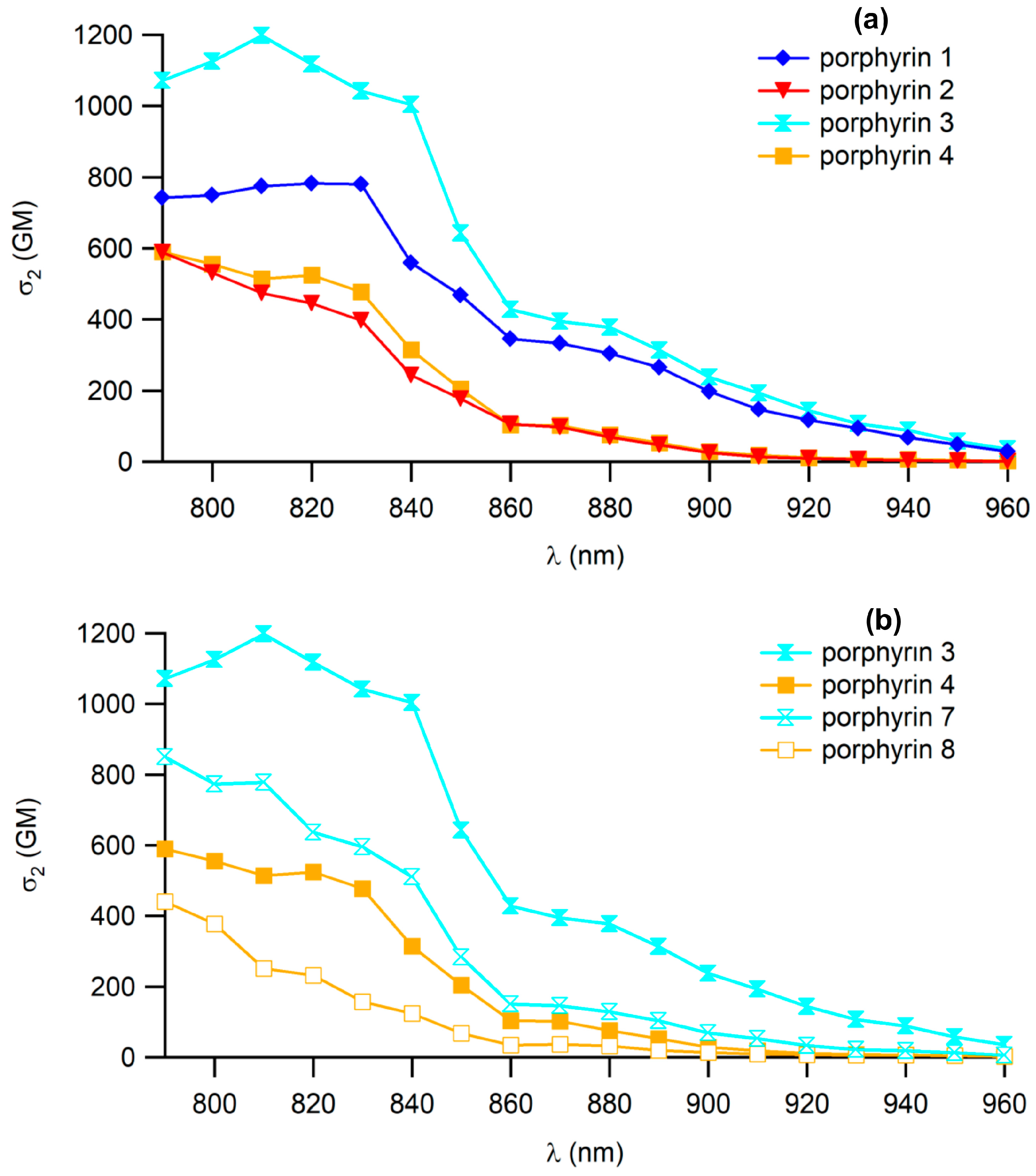
3.3. Impact of CTpa vs. CCbz Connectors on 2PA in Dendrimers
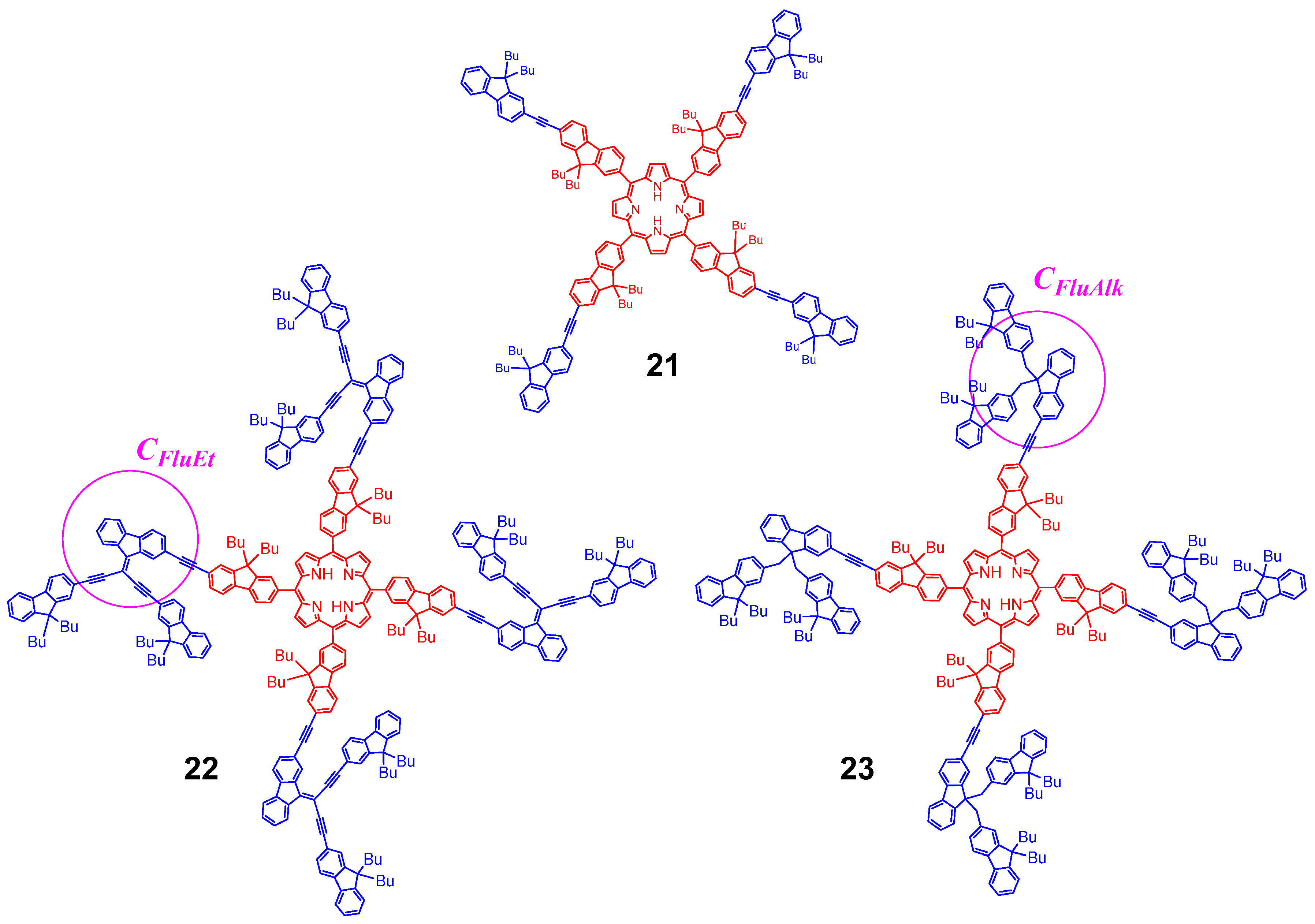
3.4. Dendrimer 3 vs. other Connectors Previously Studied
4. Conclusions
5. Experimental Section
5.1. General
5.2. Synthesis
5.3. Spectroscopic Measurements
5.4. Measurements of Singlet Oxygen Quantum Yields (ΦΔ)
5.5. Two-Photon Absorption Experiments
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Szacilowski, K. Digital Information Processing in Molecular Systems. Chem. Rev. 2008, 108, 3481–3548. [Google Scholar]
- Wasielewski, M.R. Energy, Charge, and Spin Transport in Molecules and Self-Assembled Nanostructures Inspired by Photosynthesis. J. Org. Chem. 2006, 71, 5051–5060. [Google Scholar] [CrossRef]
- Josefsen, L.B.; Boyle, R.W. Unique Diagnostic and Therapeutic Roles of Porphyrins and Phthalocyanines in Photodynamic Therapy, Imaging and Theranostics. Theranostics 2012, 2, 916–966. [Google Scholar] [PubMed]
- Pawlicki, M.; Collins, H.A.; Denning, R.G.; Anderson, H.L. Two-Photon Absorption and the Design of Two-Photon Dyes. Angew. Chem. Int. Ed. 2009, 48, 3244–3266. [Google Scholar] [CrossRef]
- Bhaumik, J.; Mittal, A.K.; Banerjee, A.; Chisti, Y.; Banerjee, U.C. Applications of phototheranostic nanoagents in photodynamic therapy. Nano Res. 2015, 8, 1373–1394. [Google Scholar] [CrossRef]
- Prabhu, P.; Patravale, V. The upcoming field of theranostic nanomedicine: An overview. J. Biomed. Nanotechnol. 2012, 8, 859–882. [Google Scholar]
- Bolze, F.; Jenni, S.; Sour, A.; Heitz, V. Molecular photosensitisers for two-photon photodynamic therapy. Chem. Commun. 2017, 53, 12857–12877. [Google Scholar] [CrossRef]
- Mongin, O.; Hugues, V.; Blanchard-Desce, M.; Merhi, A.; Drouet, S.; Yao, D.; Paul-Roth, C. Fluorenyl porphyrins for combined two-photon excited fluorescence and photosensitization. Chem. Phys. Lett. 2015, 625, 151–156. [Google Scholar] [CrossRef]
- Drouet, S.; Paul-Roth, C.O.; Simonneaux, G. Synthesis and photophysical properties of porphyrins with fluorenyl pendant arms. Tetrahedron 2009, 65, 2975–2981. [Google Scholar]
- Drouet, S.; Paul-Roth, C.O. Fluorenyl Dendrimer Porphyrins: Synthesis and Photophysical Properties. Tetrahedron 2009, 65, 10693–10700. [Google Scholar] [CrossRef]
- Yao, D.; Zhang, X.; Mongin, O.; Paul, F.; Paul-Roth, C.O. Synthesis and Characterization of New Conjugated Fluorenyl-Porphyrin Dendrimers for Optics. Chem. Eur. J. 2016, 22, 5583–5597. [Google Scholar] [CrossRef] [PubMed]
- Yao, D.; Zhang, X.; Triadon, A.; Richy, N.; Mongin, O.; Blanchard-Desce, M.; Paul, F.; Paul-Roth, C.O. New Conjugated meso-Tetrafluorenylporphyrin-cored Derivatives as Fluorescent Two-photon Photosensitizers for Singlet Oxygen Generation. Chem. Eur. J. 2017, 23, 2635–2647. [Google Scholar] [CrossRef]
- Zhang, X.; Ben Hassine, S.; Richy, N.; Mongin, O.; Blanchard-Desce, M.; Paul, F.; Paul-Roth, C.O. New Porphyrin Dendrimers with Fluorenyl-based Connectors: A Simple Way to improving the Optical Properties over Dendrimers featuring 1,3,5-Phenylene Connectors. New J. Chem. 2020, 44, 4144–4157. [Google Scholar] [CrossRef]
- Paul-Roth, C.O.; Williams, J.A.G.; Letessier, J.; Simonneaux, G. New tetra-aryl and bi-aryl porphyrins bearing 5,15-related fluorenyl pendants: The influence of arylation on fluorescence. Tetrahedron Lett. 2007, 48, 4317–4322. [Google Scholar] [CrossRef]
- Varnavski, O.; Yan, X.; Mongin, O.; Blanchard-Desce, M.; Goodson, T.G. III, Strongly interacting organic conjugated dendrimers with enhanced two-photon absorption. J. Phys. Chem. C 2007, 111, 149–162. [Google Scholar] [CrossRef]
- Terenziani, F.; Katan, C.; Badaeva, E.; Tretiak, S.; Blanchard-Desce, M. Enhanced Two-Photon Absorption of Organic Chromophores: Theoretical and Experimental Assessments. Adv. Mater. 2008, 20, 4641–4678. [Google Scholar] [CrossRef]
- Wan, Y.; Yan, L.; Zhao, Z.; Ma, X.; Guo, Q.; Jia, M.; Lu, P.; Ramos-Ortiz, G.; Maldonado, J.L.; Rodríguez, M.; et al. Gigantic two-photon absorption cross sections and strong two-photon excited fluorescence in pyrene core dendrimers with fluorene/carbazole as dendrons and acetylene as linkages. J. Phys. Chem. B 2010, 114, 11737–11745. [Google Scholar] [CrossRef]
- Ding, J.; Zhang, B.; Lü, J.; Xie, Z.; Wang, L.; Jing, X.; Wang, F. Solution-processable carbazole-based conjugated dendritic hosts for power-efficient blue-electrophosphorescent devices. Adv. Mater. 2009, 21, 4983–4986. [Google Scholar] [CrossRef]
- Zhao, Z.; Xu, X.; Chen, X.; Wang, X.; Lu, P.; Yu, G.; Liu, Y. Synthesis and Characterization of Deep Blue Emitters from Starburst Carbazole/Fluorene Compounds. Tetrahedron 2008, 64, 2658–2668. [Google Scholar] [CrossRef]
- Kannan, R.; He, G.S.; Yuan, L.; Xu, F.; Prasad, P.N.; Dombroskie, A.G.; Reinhardt, B.A.; Baur, J.W.; Vaia, R.A.; Tan, L.S. Diphenylaminofluorene-based two-photon-absorbing chromophores with various π-electron acceptors. Chem. Mater. 2001, 13, 1896–1904. [Google Scholar] [CrossRef]
- Gautier, Y.; Argouarch, G.; Malvolti, F.; Blondeau, B.; Richy, N.; Amar, A.; Boucekkine, A.; Nawara, K.; Chlebowicz, K.; Orzanowska, G.; et al. Triarylisocyanurate-Based Fluorescent Two-Photon Absorbers. ChemPlusChem 2020, 85, 411–425. [Google Scholar] [CrossRef]
- He, G.S.; Tan, L.-S.; Zheng, Q.; Prasad, P.N. Multiphoton Absorbing Materials: Molecular Designs, Characterizations, and Applications. Chem. Rev. 2008, 108, 1245–1330. [Google Scholar] [PubMed]
- Joon Lee, G.; Kim, K.; Jin, J.-I. Mechanism of one- and two-photon absorption induced photoluminescence in PPV type, electroluminescent polymer. Opt. Commun. 2002, 203, 151–157. [Google Scholar] [CrossRef]
- Tamura, K.; Fujii, T.; Shiotsuki, M.; Sanda, F.; Masuda, T. Synthesis and properties of polyacetylenes having pendent phenylethynylcarbazolyl groups. Polymer 2008, 49, 4494–4501. [Google Scholar] [CrossRef]
- Lindsey, J.S.; Hsu, H.C.; Schreiman, I.C. Synthesis of tetraphenylporphyrins under very mild conditions. Tetrahedron Lett. 1986, 27, 4969–4970. [Google Scholar] [CrossRef]
- Lindsey, J.S.; Schreiman, I.C.; Hsu, H.C.; Kearney, P.C.; Marguerettaz, A.M. Rothemund and Adler-Longo reactions revisited: Synthesis of tetraphenylporphyrins under equilibrium conditions. J. Org. Chem. 1987, 52, 827–836. [Google Scholar] [CrossRef]
- Paul-Roth, C.O.; Simonneaux, G. Porphyrins with fluorenyl and fluorenone pendant arms as red-light-emitting devices. C. R. Chim. 2006, 9, 1277–1286. [Google Scholar] [CrossRef]
- Shi, L.; He, C.; Zhu, D.; He, Q.; Li, Y.; Chen, Y.; Sun, Y.; Fu, D.; Wen, H.; Cao, J.; et al. High performance aniline vapor detection based on multi-branched fluorescent triphenylamine-benzothiadiazole derivatives: Branch effect and aggregation control of the sensing performance. J. Mater. Chem. 2012, 22, 11629–11635. [Google Scholar]
- Li, Q.; Guo, H.; Ma, L.; Wu, W.; Liu, Y.; Zhao, J. Tuning the photophysical properties of N^ N Pt (II) bisacetylide complexes with fluorene moiety and its applications for triplet–triplet-annihilation based upconversion. J. Mater. Chem. 2012, 22, 5319–5329. [Google Scholar] [CrossRef]
- Sonogashira, K.; Tohda, Y.; Hagihara, N. A convenient synthesis of acetylenes: Catalytic substitutions of acetylenic hydrogen with bromoalkenes, iodoarenes and bromopyridines. Tetrahedron Lett. 1975, 16, 4467–4470. [Google Scholar] [CrossRef]
- Pfoertner, K.H. “Photochemistry” in Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH: Weinheim, Germany, 2002. [Google Scholar]
- Wu, M.S.; Tang, W.C. Dye Compound and Photoelectric Component Using the Same. U.S. Patent US2010122729 (A1), 20 May 2010. [Google Scholar]
- Tian, Y.; Wu, W.; Chen, C.; Strovas, T.; Li, Y.; Jin, Y.; Su, F.; Meldrum, D.R.; Jen, A.K.Y. 2,1,3-Benzothiadiazole (BTD)-moiety-containing red emitter conjugated amphiphilic poly(ethylene glycol)-block-poly(3-caprolactone) copolymers for bioimaging. J. Mater. Chem. 2010, 20, 1728–1736. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.E.; Dong, X.Z.; Chen, W.Q.; Zhao, Z.S.; Duan, X.M. Novel photoinitiator with a radical quenching moiety for confining radical diffusion in two-photon induced photopolymerization. J. Mater. Chem. 2011, 21, 5650–5659. [Google Scholar] [CrossRef]
- Cao, D.; Zhu, L.; Liu, Z.; Lin, W. Through bond energy transfer (TBET)-based fluorescent chemosensors. J. Photochem. Photobiol. C 2020, 44, 100371. [Google Scholar] [CrossRef]
- Kim, H.M.; Cho, B.R. Two-photon materials with large two-photon cross sections. Structure-property relationship. Chem. Commun. 2009, 45, 153–164. [Google Scholar] [CrossRef]
- Wilkinson, F.; Helman, W.P.; Ross, A.B. Quantum Yields for the Photosensitized Formation of the Lowest Electronically Excited Singlet State of molecular Oxygen in Solution. J. Phys. Chem. Ref. Data 1993, 22, 113–262. [Google Scholar] [CrossRef]
- Yuan Chiu, K.; Xiang Su, T.; Hong Li, J.; Lin, T.-H.; Liou, G.-S.; Cheng, S.-H. Novel trends of electrochemical oxidation of amino-substituted triphenylamine derivatives. J. Electroanal. Chem. 2005, 575, 95–101. [Google Scholar] [CrossRef]
- Karon, K.; Lapkowski, M. Carbazole electrochemistry: A short review. J. Solid State Electrochem. 2015, 19, 2601–2610. [Google Scholar] [CrossRef]
- Cao, D.-X.; Fang, Q.; Wang, D.; Liu, Z.-Q.; Xue, G.; Xu, G.-B.; Yu, W.-T. Synthesis and Two-Photon-Excited Fluorescence of Benzothiazole-Based Compounds with Various π-Electron Donors. Eur. J. Org. Chem. 2003, 2003, 3628–3636. [Google Scholar] [CrossRef]
- Shi, L.; Nguyen, C.; Daurat, M.; Richy, N.; Gary-Bobo, M.; Cammas-Marion, S.; Mongin, O.; Paul-Roth, C.O.; Paul, F. Encapsulation of hydrophobic porphyrins into biocompatible nanoparticles: An easy way to benefit of their two-photon photo-therapeutic effect without hydrophilic functionalization. Cancers 2022, 14, 2358. [Google Scholar] [CrossRef]
- Demas, N.; Crosby, G.A. Measurement of photoluminescence quantum yields. J. Phys. Chem. 1971, 75, 991–1024. [Google Scholar]
- Eaton, G.R.; Eaton, S.S. EPR studies of long-range intramolecular electron-electron exchange interaction. Acc. Chem. Res. 1988, 21, 107–113. [Google Scholar] [CrossRef]
- Xu, C.; Webb, W.W. Measurement of two-photon excitation cross sections of molecular fluorophores with data from 690 to 1050 nm. J. Opt. Soc. Am. B 1996, 13, 481–491. [Google Scholar] [CrossRef]
- Werts, M.H.V.; Nerambourg, N.; Pélégry, D.; Le Grand, Y.; Blanchard-Desce, M. Action cross sections of two-photon excited luminescence of some Eu(III) and Tb(III) complexes. Photochem. Photobiol. Sci. 2005, 4, 531–538. [Google Scholar] [CrossRef] [PubMed]


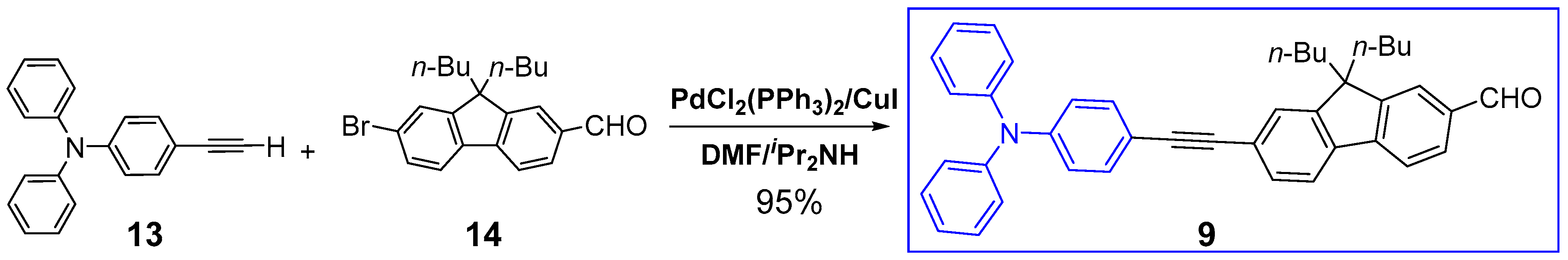
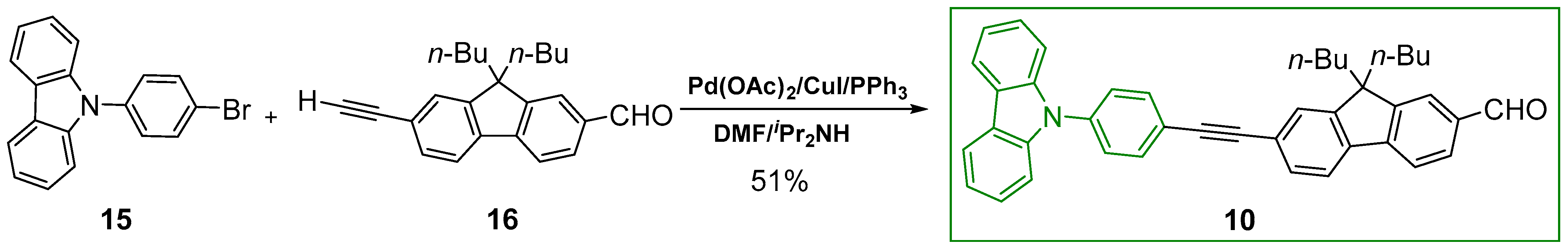
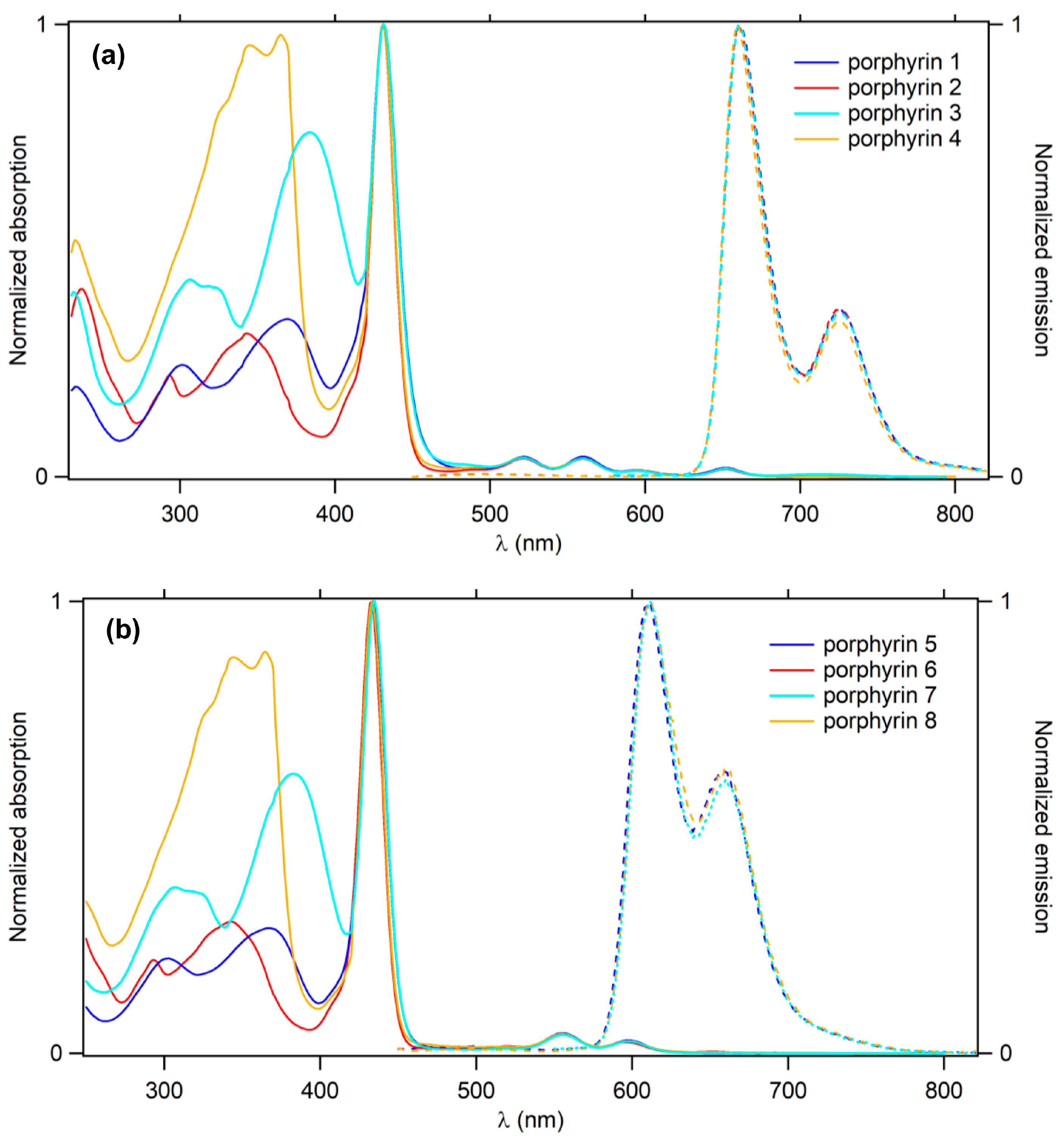
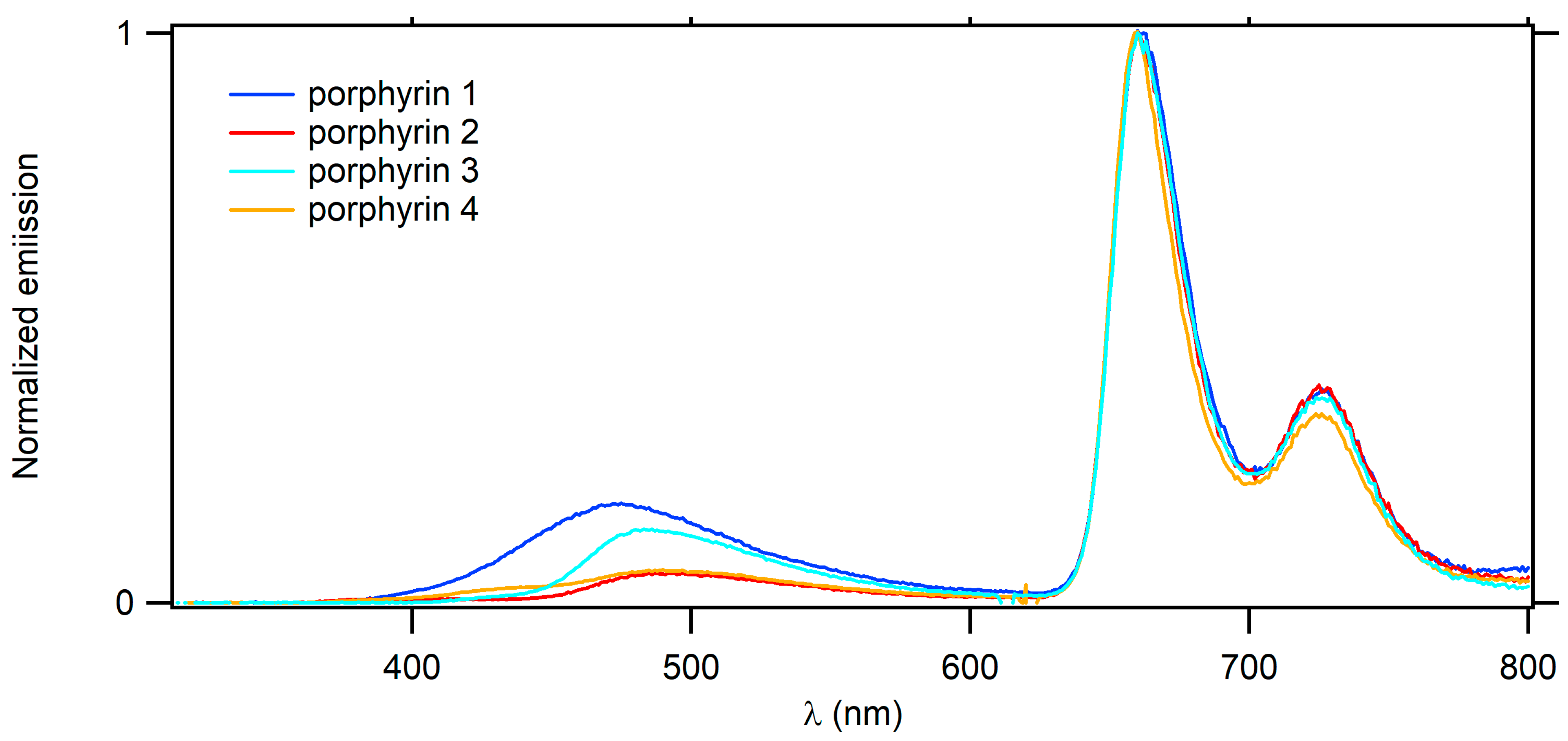

| Cmpd | λabs Dendron (nm) | λabs Soret (nm) | ε Soret (103 M−1 cm−1) | λabs Q Bands (nm) | λem (nm) | ΦF a | |
|---|---|---|---|---|---|---|---|
| Q(0,0) | Q(0,1) | ||||||
| TFP-Bu | - | 427 | - | 519,555,596,652 | 659 | 725 | 0.20 |
| 1 | 370 | 431 | 479 | 521,560,595,652 | 661 | 726 | 0.21 |
| 2 | 343 | 431 | 536 | 521,559,594,652 | 660 | 726 | 0.20 |
| 3 | 306,384 | 431 | 610 | 522,561,594,632 | 661 | 726 | 0.19 |
| 4 | 365 | 431 | 518 | 521,559,595,652 | 660 | 726 | 0.20 |
| 5 | 303,366 | 434 | 569 | 556,598 | 611 | 657 | 0.09 |
| 6 | 343 | 433 | 543 | 555,596 | - | - | - |
| 7 | 307,383 | 435 | 661 | 555,598 | 612 | 660 | 0.09 |
| 8 | 345,365 | 433 | 544 | 555,598 | 611 | 660 | 0.09 |
| Cmpd | λ2PAmax (nm) | σ2 a (GM) | ΦF b | ΦΔ c | σ2.ΦFmax (GM) | σ2.ΦΔmax (GM) |
|---|---|---|---|---|---|---|
| TFP | 790 | 90 | 0.24 | 0.60 | 22 | 54 |
| G’1 | 790 | 730 | 0.24 | 0.61 | 175 | 445 |
| 21 | 790 | 770 | 0.23 | 0.62 | 177 | 477 |
| 22 | 790 | 1450 | 0.17 | 0.46 | 247 | 667 |
| 23 | 790 | 840 | 0.12 | 0.40 | 101 | 336 |
| 1 | 830 | 780 | 0.21 | 0.70 | 164 | 546 |
| 2 | 790 | 590 | 0.20 | 0.68 | 118 | 401 |
| 3 | 810 | 1200 | 0.19 | 0.64 | 228 | 768 |
| 4 | 790 | 590 | 0.20 | 0.66 | 118 | 389 |
| 5 | 790 | 590 | 0.09 | 0.57 | 53 | 336 |
| 7 | 790 | 850 | 0.09 | 0.65 | 77 | 553 |
| 8 | 790 | 440 | 0.09 | 0.62 | 40 | 273 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, L.; Sun, Z.; Richy, N.; Mongin, O.; Blanchard-Desce, M.; Paul, F.; Paul-Roth, C.O. New Fluorescent Porphyrins with High Two-Photon Absorption Cross-Sections Designed for Oxygen-Sensitization: Impact of Changing the Connectors in the Peripheral Arms. Photochem 2023, 3, 336-359. https://doi.org/10.3390/photochem3030021
Shi L, Sun Z, Richy N, Mongin O, Blanchard-Desce M, Paul F, Paul-Roth CO. New Fluorescent Porphyrins with High Two-Photon Absorption Cross-Sections Designed for Oxygen-Sensitization: Impact of Changing the Connectors in the Peripheral Arms. Photochem. 2023; 3(3):336-359. https://doi.org/10.3390/photochem3030021
Chicago/Turabian StyleShi, Limiao, Zhipeng Sun, Nicolas Richy, Olivier Mongin, Mireille Blanchard-Desce, Frédéric Paul, and Christine O. Paul-Roth. 2023. "New Fluorescent Porphyrins with High Two-Photon Absorption Cross-Sections Designed for Oxygen-Sensitization: Impact of Changing the Connectors in the Peripheral Arms" Photochem 3, no. 3: 336-359. https://doi.org/10.3390/photochem3030021
APA StyleShi, L., Sun, Z., Richy, N., Mongin, O., Blanchard-Desce, M., Paul, F., & Paul-Roth, C. O. (2023). New Fluorescent Porphyrins with High Two-Photon Absorption Cross-Sections Designed for Oxygen-Sensitization: Impact of Changing the Connectors in the Peripheral Arms. Photochem, 3(3), 336-359. https://doi.org/10.3390/photochem3030021