Modeling the Unimolecular Decay Dynamics of the Fluorinated Criegee Intermediate, CF3CHOO
Abstract
:1. Introduction
2. Methodology
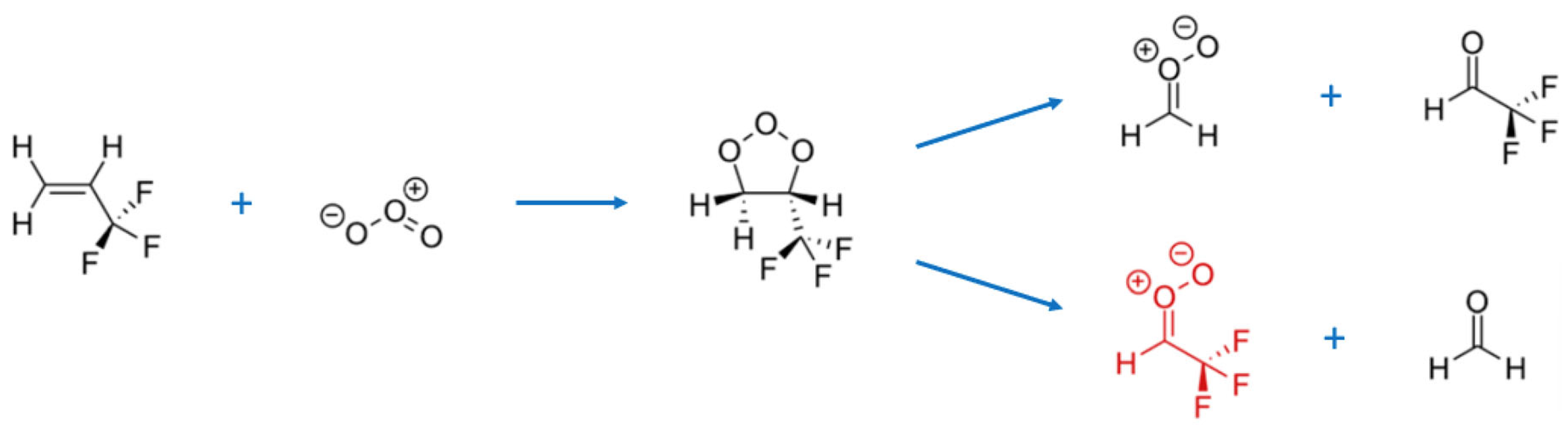
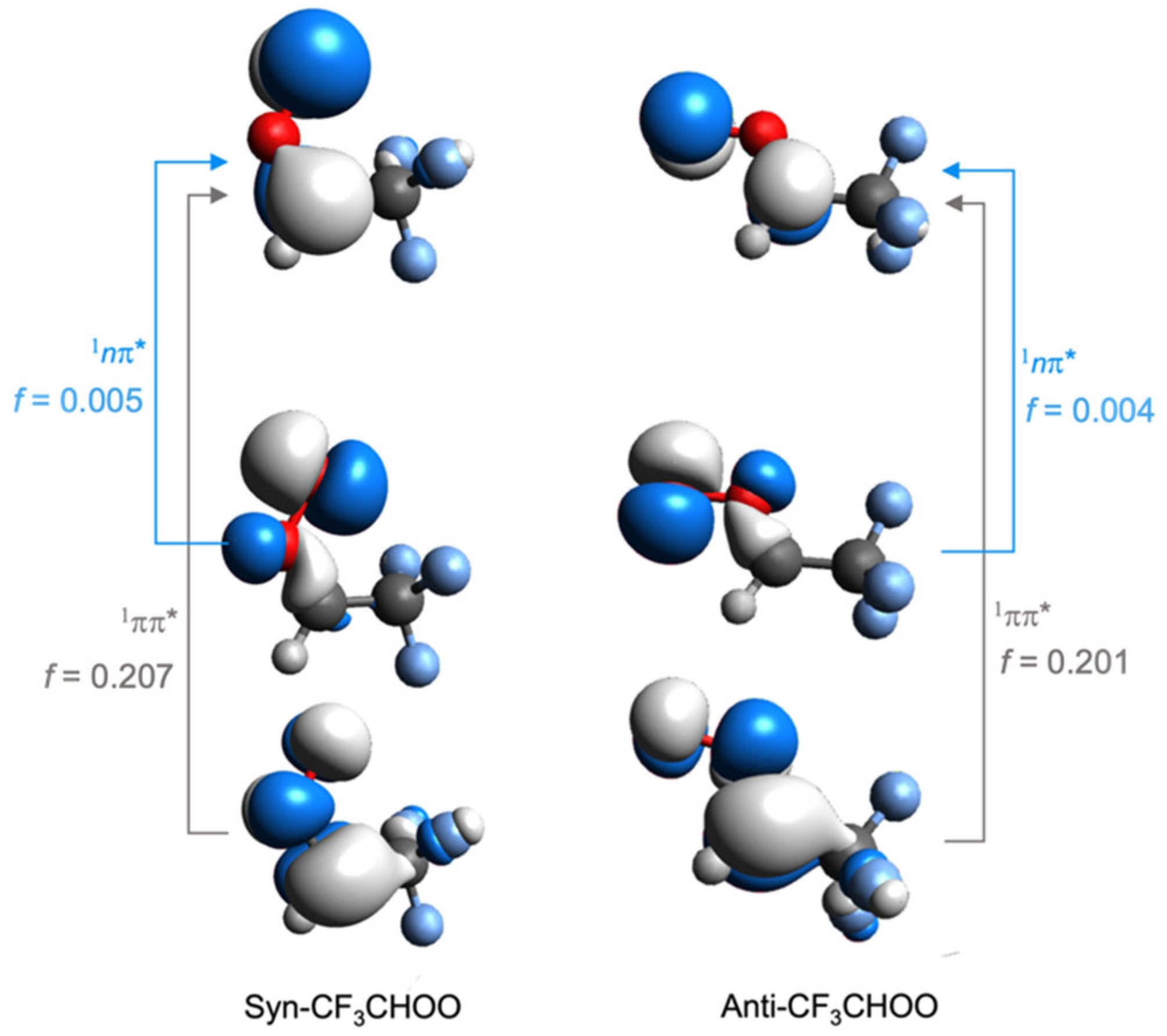
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mateu-Royo, C.; Navarro-Esbrí, J.; Mota-Babiloni, A.; Amat-Albuixech, M.; Molés, F. Thermodynamic analysis of low GWP alternatives to HFC-245fa in high-temperature heat pumps: HCFO-1224yd(Z), HCFO-1233zd(E) and HFO-1336mzz(Z). Appl. Therm. Eng. 2019, 152, 762–777. [Google Scholar] [CrossRef]
- Molés, F.; Navarro-Esbrí, J.; Peris, B.; Mota-Babiloni, A.; Barragán-Cervera, Á.; Kontomaris, K. Thermo-economic evaluation of low global warming potential alternatives to HFC-245fa in Organic Rankine Cycles. Energy Procedia 2017, 142, 1199–1205. [Google Scholar] [CrossRef]
- Navarro-Esbrí, J.; Molés, F.; Peris, B.; Mota-Babiloni, A.; Kontomaris, K. Experimental study of an Organic Rankine Cycle with HFO-1336mzz-Z as a low global warming potential working fluid for micro-scale low temperature applications. Energy 2017, 133, 79–89. [Google Scholar] [CrossRef]
- Molés, F.; Navarro-Esbrí, J.; Peris, B.; Mota-Babiloni, A.; Barragán-Cervera, Á.; Kontomaris, K. Low GWP alternatives to HFC-245fa in Organic Rankine Cycles for low temperature heat recovery: HCFO-1233zd-E and HFO-1336mzz-Z. Appl. Therm. Eng. 2014, 71, 204–212. [Google Scholar] [CrossRef]
- Fouad, W.A.; Vega, L.F. Next generation of low global warming potential refrigerants: Thermodynamic properties molecular modeling. AIChE J. 2018, 64, 250–262. [Google Scholar] [CrossRef]
- Rivela, C.B.; Tovar, C.M.; Teruel, M.A.; Barnes, I.; Wiesen, P.; Blanco, M.B. CFCs replacements: Reactivity and atmospheric lifetimes of a series of Hydrofluoroolefins towards OH radicals and Cl atoms. Chem. Phys. Lett. 2019, 714, 190–196. [Google Scholar] [CrossRef]
- Rao, P.K.; Gejji, S.P. Atmospheric degradation of HCFO-1233zd(E) initiated by OH radical, Cl atom and O3 molecule: Kinetics, reaction mechanisms and implications. J. Fluor. Chem. 2018, 211, 180–193. [Google Scholar] [CrossRef]
- Donahue, N.M.; Drozd, G.T.; Epstein, S.A.; Presto, A.A.; Kroll, J.H. Adventures in ozoneland: Down the rabbit-hole. Phys. Chem. Chem. Phys. 2011, 13, 10848–10857. [Google Scholar] [CrossRef]
- Osborn, D.L.; Taatjes, C.A. The physical chemistry of Criegee intermediates in the Gas Phase. Int. Rev. Phys. Chem. 2015, 34, 309–360. [Google Scholar] [CrossRef]
- Lester, M.I.; Klippenstein, S.J. Unimolecular Decay of Criegee Intermediates to OH Radical Products: Prompt and Thermal Decay Processes. Acc. Chem. Res. 2018, 51, 978–985. [Google Scholar] [CrossRef]
- Chhantyal-Pun, R.; Khan, M.A.H.; Taatjes, C.A.; Percival, C.J.; Orr-Ewing, A.J.; Shallcross, D.E. Criegee intermediates: Production, detection and reactivity. Int. Rev. Phys. Chem. 2020, 39, 383–422. [Google Scholar] [CrossRef]
- Liu, F.; Beames, J.M.; Petit, A.S.; McCoy, A.B.; Lester, M.I. Infrared-driven unimolecular reaction of CH3CHOO Criegee intermediates to OH radical products. Science 2014, 345, 1596–1598. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Liu, F.; Barber, V.P.; Klippenstein, S.J.; McCoy, A.B.; Lester, M.I. Deep tunneling in the unimolecular decay of CH3CHOO Criegee intermediates to OH radical products. J. Chem. Phys. 2016, 145, 234308. [Google Scholar] [CrossRef] [PubMed]
- Barber, V.P.; Pandit, S.; Esposito, V.J.; McCoy, A.B.; Lester, M.I. CH Stretch Activation of CH3CHOO: Deep Tunneling to Hydroxyl Radical Products. J. Phys. Chem. A 2019, 123, 2559–2569. [Google Scholar] [CrossRef]
- Newland, M.J.; Rickard, A.R.; Sherwen, T.; Evans, M.J.; Vereecken, L.; Muñoz, A.; Ródenas, M.; Bloss, W.J. The atmospheric impacts of monoterpene ozonolysis on global stabilised Criegee intermediate budgets and SO2 oxidation: Experiment, theory and modelling. Atmos. Chem. Phys. 2018, 18, 6095–6120. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Fang, Y.; Kidwell, N.M.; Beames, J.M.; Lester, M.I. UV Photodissociation Dynamics of the CH3CHOO Criegee Intermediate: Action Spectroscopy and Velocity Map Imaging of O-Atom Products. J. Phys. Chem. A 2015, 119, 8328–8337. [Google Scholar] [CrossRef] [PubMed]
- Karsili, T.N.V.; Marchetti, B.; Lester, M.I.; Ashfold, M.N.R. Electronic Absorption Spectroscopy and Photochemistry of Criegee Intermediates. Photochem. Photobiol. 2022, 99, 4–18. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision C.01; Gaussian Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Werner, H.-J.; Knowles, P.J.; Knizia, G.; Manby, F.R.; Schütz, M. Molpro: A general-purpose quantum chemistry program package. WIREs Comput. Mol. Sci. 2012, 2, 242–253. [Google Scholar] [CrossRef]
- Werner, H.-J.; Knowles, P.J.; Knizia, G.; Manby, F.R.; Schütz, M.; Celani, P.; Györffy, W.; Kats, D.; Korona, T.; Lindh, R.; et al. MOLPRO, Version 2018.1, a Package of Ab Initio Programs. Available online: https://www.molpro.net/ (accessed on 31 May 2023).
- Vereecken, L.; Novelli, A.; Taraborrelli, D. Unimolecular decay strongly limits the atmospheric impact of Criegee intermediates. Phys. Chem. Chem. Phys. 2017, 19, 31599–31612. [Google Scholar] [CrossRef] [Green Version]
- Vereecken, L.; Novelli, A.; Kiendler-Scharr, A.; Wahner, A. Unimolecular and water reactions of oxygenated and unsaturated Criegee intermediates under atmospheric conditions. Phys. Chem. Chem. Phys. 2022, 24, 6428–6443. [Google Scholar] [CrossRef]
- Barbatti, M.; Granucci, G.; Persico, M.; Ruckenbauer, M.; Vazdar, M.; Eckert-Maksić, M.; Lischka, H. The on-the-fly surface-hopping program system Newton-X: Application to ab initio simulation of the nonadiabatic photodynamics of benchmark systems. J. Photochem. Photobiol. A Chem. 2007, 190, 228–240. [Google Scholar] [CrossRef]
- Barbatti, M.; Ruckenbauer, M.; Plasser, F.; Pittner, J.; Granucci, G.; Persico, M.; Lischka, H. Newton-X: A surface-hopping program for nonadiabatic molecular dynamics. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2013, 4, 26–33. [Google Scholar] [CrossRef]
- Barber, V.P.; Pandit, S.; Green, A.M.; Trongsiriwat, N.; Walsh, P.J.; Klippenstein, S.J.; Lester, M.I. Four-Carbon Criegee Intermediate from Isoprene Ozonolysis: Methyl Vinyl Ketone Oxide Synthesis, Infrared Spectrum, and OH Production. J. Am. Chem. Soc. 2018, 140, 10866–10880. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Liu, T.; Caracciolo, A.; Vansco, M.F.; Trongsiriwat, N.; Walsh, P.J.; Marchetti, B.; Karsili, T.N.V.; Lester, M.I. Photodissociation dynamics of methyl vinyl ketone oxide: A four-carbon unsaturated Criegee intermediate from isoprene ozonolysis. J. Chem. Phys. 2021, 155, 174305. [Google Scholar] [CrossRef]
- McCoy, J.C.; Marchetti, B.; Thodika, M.; Karsili, T.N.V. A Simple and Efficient Method for Simulating the Electronic Absorption Spectra of Criegee Intermediates: Benchmarking on CH2OO and CH3CHOO. J. Phys. Chem. A 2021, 125, 4089–4097. [Google Scholar] [CrossRef]
- Esposito, V.J.; Liu, T.; Wang, G.; Caracciolo, A.; Vansco, M.F.; Marchetti, B.; Karsili, T.N.V.; Lester, M.I. Photodissociation Dynamics of CH2OO on Multiple Potential Energy Surfaces: Experiment and Theory. J. Phys. Chem. A 2021, 125, 6571–6579. [Google Scholar] [CrossRef]
- Antwi, E.; Bush, R.; Marchetti, B.; Karsili, T. A Direct Dynamics Study of the Exotic Photochemistry of the Simplest Criegee Intermediate, CH2OO. Phys. Chem. Chem. Phys. 2022, 24, 16724–16731. [Google Scholar] [CrossRef]
- Antwi, E.; Ratliff, J.M.; Ashfold, M.N.R.; Karsili, T.N.V. Comparing the Excited State Dynamics of CH2OO, the Simplest Criegee Intermediate, Following Vertical versus Adiabatic Excitation. J. Phys. Chem. A 2022, 126, 6236–6243. [Google Scholar] [CrossRef]
- Antwi, E.; Packer, N.A.; Ratliff, J.M.; Marchetti, B.; Karsili, T.N.V. Insights into the Ultrafast Photodissociation Dynamics of Isoprene Derived Criegee Intermediates. Photochem. Photobiol. 2022, 99, 1072–1079. [Google Scholar] [CrossRef]
- Wang, G.; Liu, T.; Zou, M.; Sojdak, C.A.; Kozlowski, M.C.; Karsili, T.N.V.; Lester, M.I. Electronic Spectroscopy and Dissociation Dynamics of Vinyl-Substituted Criegee Intermediates: 2-Butenal Oxide and Comparison with Methyl Vinyl Ketone Oxide and Methacrolein Oxide Isomers. J. Phys. Chem. A 2023, 127, 203–215. [Google Scholar] [CrossRef]
- Butcher, J.C. A Modified Multistep Method for the Numerical Integration of Ordinary Differential Equations. J. ACM 1965, 12, 124–135. [Google Scholar] [CrossRef]
- Park, J.W.; Shiozaki, T. Analytical Derivative Coupling for Multistate CASPT2 Theory. J. Chem. Theory Comput. 2017, 13, 2561–2570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shiozaki, T. BAGEL: Brilliantly Advanced General Electronic-structure Library. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2018, 8, e1331. [Google Scholar] [CrossRef] [Green Version]
- Fang, Y.; Liu, F.; Barber, V.P.; Klippenstein, S.J.; McCoy, A.B.; Lester, M.I. Communication: Real time observation of unimolecular decay of Criegee intermediates to OH radical products. J. Chem. Phys. 2016, 144, 61102. [Google Scholar] [CrossRef] [Green Version]
- Esposito, V.J.; Werba, O.; Bush, S.A.; Marchetti, B.; Karsili, T.N.V. Insights into the Ultrafast Dynamics of CH2OO and CH3CHOO Following Excitation to the Bright 1ππ* State: The Role of Singlet and Triplet States. Photochem. Photobiol. 2021, 98, 763–772. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guidry, L.M.; Poirier, C.A.; Ratliff, J.M.; Antwi, E.; Marchetti, B.; Karsili, T.N.V. Modeling the Unimolecular Decay Dynamics of the Fluorinated Criegee Intermediate, CF3CHOO. Photochem 2023, 3, 327-335. https://doi.org/10.3390/photochem3030020
Guidry LM, Poirier CA, Ratliff JM, Antwi E, Marchetti B, Karsili TNV. Modeling the Unimolecular Decay Dynamics of the Fluorinated Criegee Intermediate, CF3CHOO. Photochem. 2023; 3(3):327-335. https://doi.org/10.3390/photochem3030020
Chicago/Turabian StyleGuidry, Lily M., Courtney A. Poirier, Jordyn M. Ratliff, Ernest Antwi, Barbara Marchetti, and Tolga N. V. Karsili. 2023. "Modeling the Unimolecular Decay Dynamics of the Fluorinated Criegee Intermediate, CF3CHOO" Photochem 3, no. 3: 327-335. https://doi.org/10.3390/photochem3030020
APA StyleGuidry, L. M., Poirier, C. A., Ratliff, J. M., Antwi, E., Marchetti, B., & Karsili, T. N. V. (2023). Modeling the Unimolecular Decay Dynamics of the Fluorinated Criegee Intermediate, CF3CHOO. Photochem, 3(3), 327-335. https://doi.org/10.3390/photochem3030020






