Photoprotective Effects of Selected Polyphenols and Antioxidants on Naproxen Photodegradability in the Solid-State
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of a Test Sample
2.2.2. UV Irradiation Experiment
2.2.3. Evaluation of the Residual Amounts of NPX in UV-Irradiated Samples
2.2.4. Evaluation of Antioxidative Potencies
2.2.5. UV Spectral Analysis
2.3. Statistical Analysis
3. Results and Discussion
3.1. Dose Dependency of the Photoprotective Effect of Quercetin
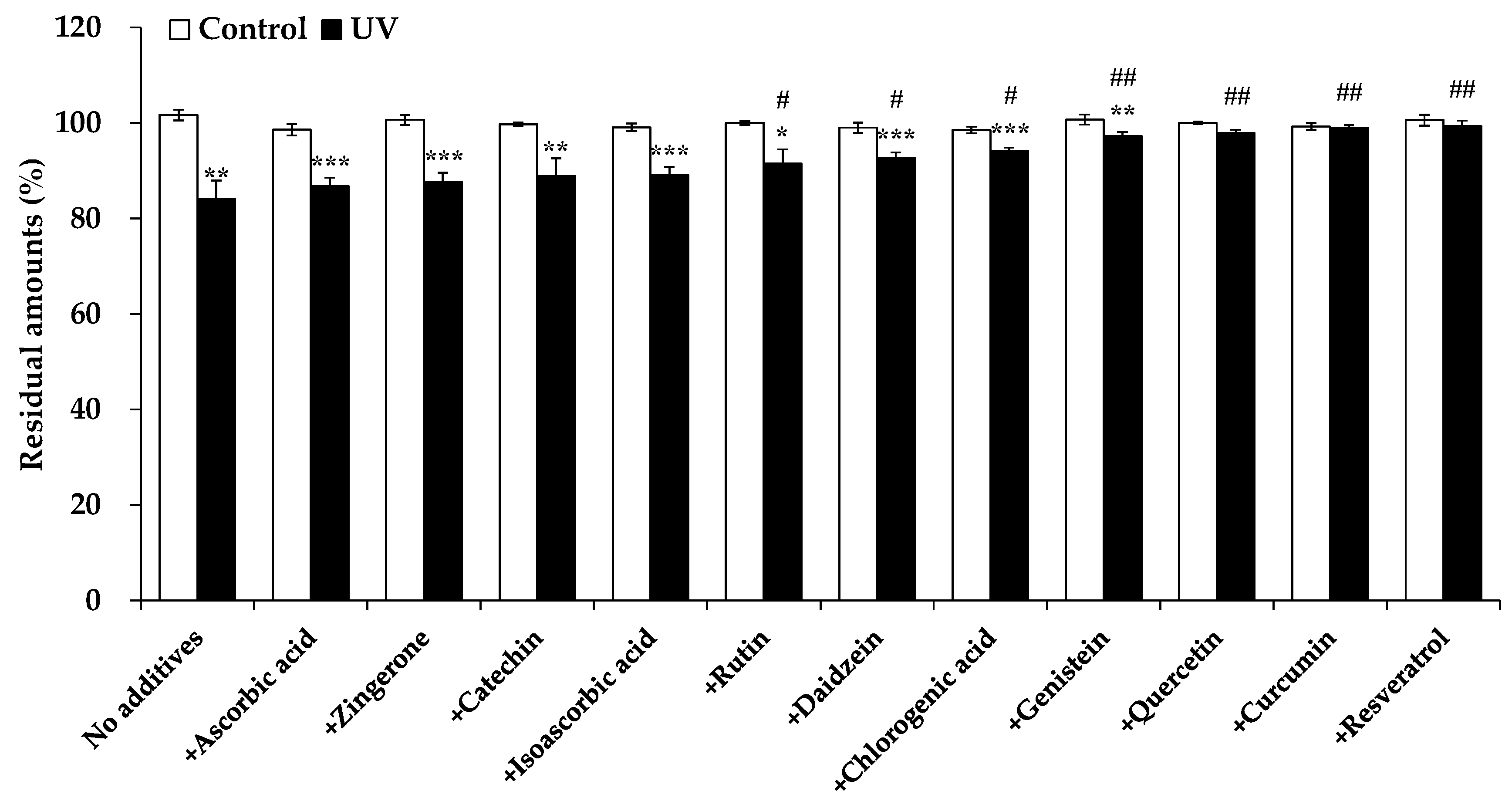
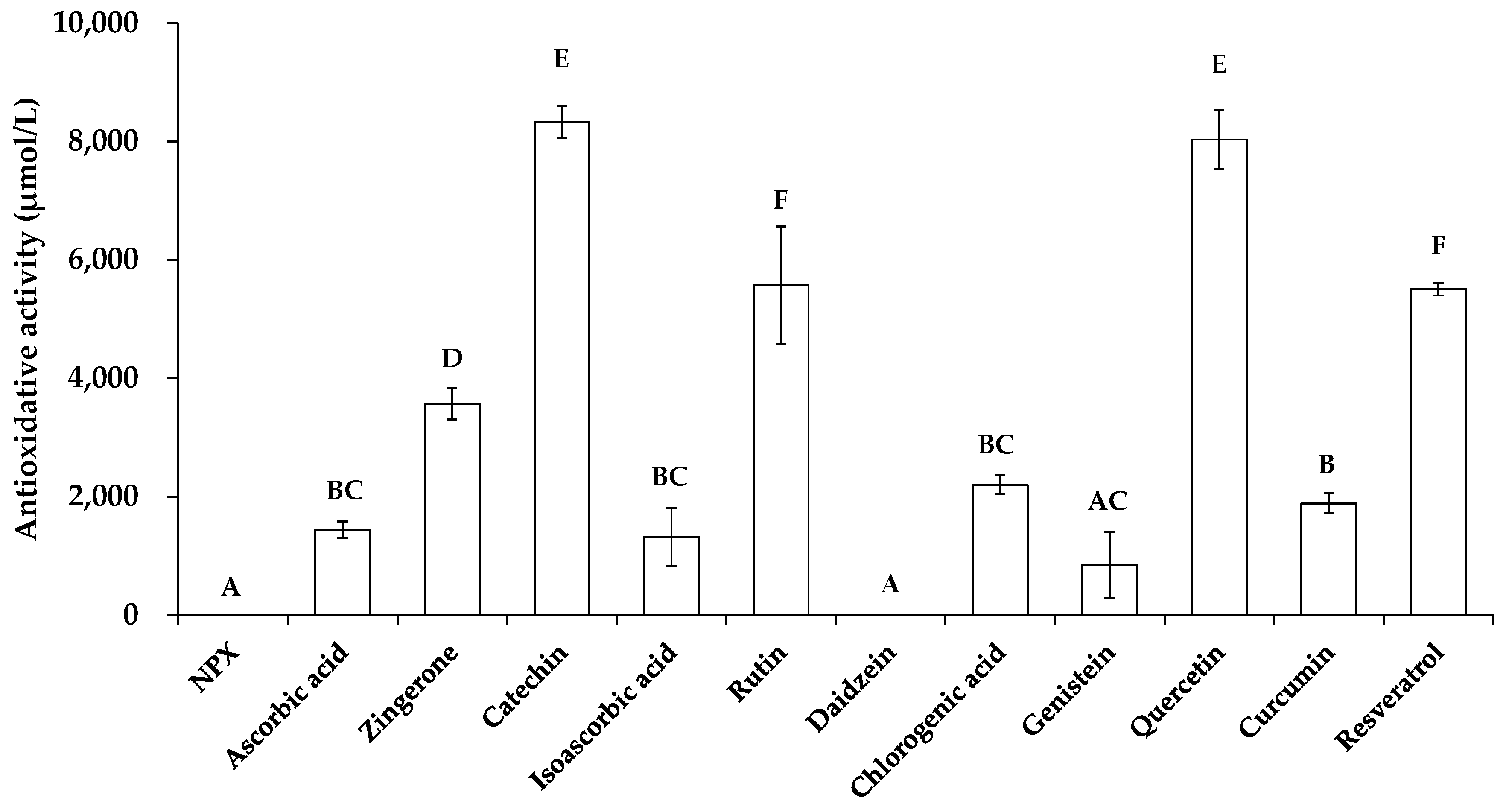
3.2. Comparison of the Photoprotective Effect of Selected Polyphenols and Antioxidants
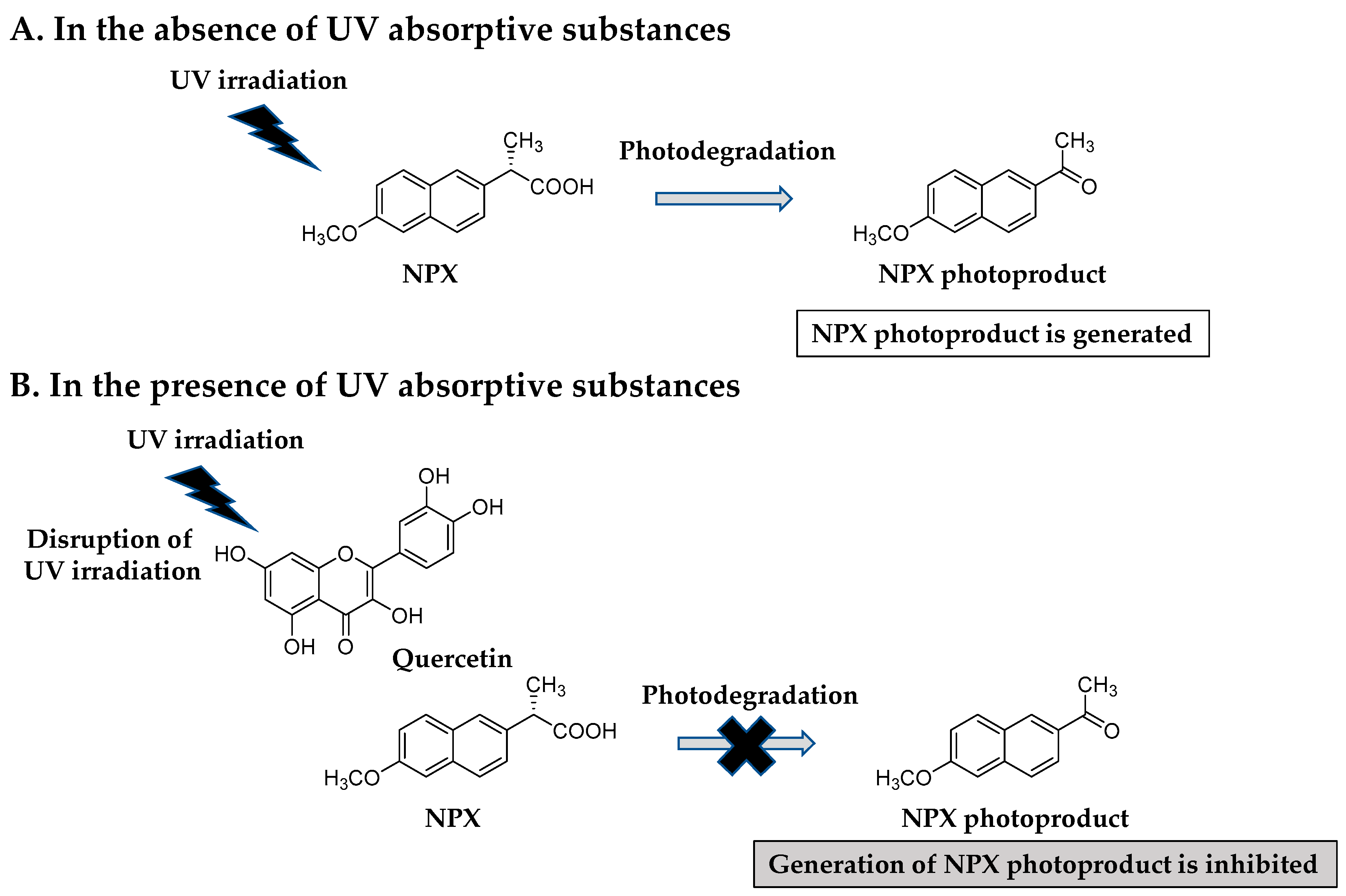
3.3. Mechanism Elucidation of the Photoprotective Effects of Selected Additives
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, N.; Li, J.M.; Liu, G.G.; Chen, X.L.; Jiang, K. Photodegradation of diclofenac in aqueous solution by simulated sunlight irradiation: Kinetics, thermodynamics and pathways. Water Sci. Technol. 2017, 75, 2163–2170. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ma, L.Y.; Li, L.S.; Xu, L. Photodegradation kinetics, transformation, and toxicity prediction of ketoprofen, carprofen, and diclofenac acid in aqueous solutions. Environ. Toxicol. Chem. 2017, 36, 3232–3239. [Google Scholar] [CrossRef] [PubMed]
- Temussi, F.; Cermola, F.; Dellagreca, M.; Iesce, M.R.; Passananti, M.; Previtera, L.; Zarrelli, A. Determination of photostability and photodegradation products of indomethacin in aqueous media. J. Pharm. Biomed. Anal. 2011, 56, 678–683. [Google Scholar] [CrossRef] [PubMed]
- Iovino, P.; Chianese, S.; Canzano, S.; Prisciandaro, M.; Musmarra, D. Ibuprofen photodegradation in aqueous solutions. Environ. Sci. Pollut. Res. Int. 2016, 23, 22993–23004. [Google Scholar] [CrossRef]
- Kawabata, K.; Sugihara, K.; Sanoh, S.; Kitamura, S.; Ohta, S. Photodegradation of pharmaceuticals in the aquatic environment by sunlight and UV-A, -B and -C irradiation. J. Toxicol. Sci. 2013, 38, 215–223. [Google Scholar] [CrossRef]
- Kawabata, K.; Akimoto, S.; Nishi, H. Cis-trans isomerization reaction of sulindac induced by UV irradiation in the aqueous media. Chromatography 2018, 39, 139–146. [Google Scholar] [CrossRef]
- Moore, D.E.; Chappuis, P.P. A comparative study of the photochemistry of the non-steroidal anti-inflammatory drugs, naproxen, benoxaprofen and indomethacin. Photochem. Photobiol. 1988, 47, 173–180. [Google Scholar] [CrossRef]
- Ishidori, M.; Lavorgna, M.; Nardelli, A.; Parrella, A.; Previtera, L.; Rubino, M. Ecotoxicity of naproxen and its phototransformation products. Sci. Total Environ. 2005, 348, 93–101. [Google Scholar] [CrossRef]
- Tu, N.; Liu, Y.; Li, R.; Lv, W.; Liu, G.; Ma, D. Experimental and theoretical investigation on photodegradation mechanisms of naproxen and its photoproducts. Chemosphere 2019, 227, 142–150. [Google Scholar] [CrossRef]
- Liang, R.; Sun, S.S.; Huang, G.; Li, M.D. Unveiling the photophysical and photochemical reaction process of naproxen via ultrafast femtosecond to nanosecond laser flash photolysis. Chem. Res. Toxicol. 2019, 32, 613–620. [Google Scholar] [CrossRef]
- Kawabata, K.; Mizuta, Y.; Ishihara, K.; Takato, A.; Oshima, S.; Akimoto, S.; Inagaki, M.; Nishi, H. Structure determination of naproxen photoproducts in the tablet generated by the UV irradiation. Chromatography 2019, 40, 157–162. [Google Scholar] [CrossRef]
- Asahi, M.; Matsubara, R.; Kawahara, M.; Ishida, T.; Emoto, C.; Suzuki, N.; Kataoka, O.; Mukai, C.; Hanaoka, M.; Ishizaki, J.; et al. Causative agent of vascular pain among photodegradation products of dacarbazine. J. Pharm. Pharmacol. 2002, 54, 1117–1122. [Google Scholar] [CrossRef] [PubMed]
- Klementová, Š.; Poncarová, M.; Kahoun, D.; Šorf, M.; Dokoupilová, E.; Fojtíková, P. Toxicity assessment of verapamil and its photodegradation products. Environ. Sci. Pollut. Res. Int. 2020, 27, 35650–35660. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Wu, W.; Zhu, Z.; Zhu, S.; Wang, H.; Zhang, L.; Fan, Z.; Chen, Y. Structure identification and toxicity evaluation of one newly-discovered dechlorinated photoproducts of chlorpyrifos. Chemosphere 2022, 301, 134822. [Google Scholar] [CrossRef]
- Klementová, Š.; Poncarová, M.; Langhansová, H.; Lieskovská, J.; Kahoun, D.; Fojtíková, P. Photodegradation of fluoroquinolones in aqueous solution under light conditions relevant to surface waters, toxicity assessment of photoproduct mixtures. Environ. Sci. Pollut. Res. Int. 2022, 29, 13941–13962. [Google Scholar] [CrossRef]
- Lassalle, Y.; Nicol, É.; Genty, C.; Bourcier, S.; Bouchonnet, S. Structural elucidation and estimation of the acute toxicity of the major UV-visible photoproduct of fludioxonil–Detection in both skin and flesh samples of grape. J. Mass Spectrom. 2015, 50, 864–869. [Google Scholar] [CrossRef]
- Nataraj, B.; Maharajan, K.; Hemalatha, D.; Rangasamy, B.; Arul, N.; Ramesh, M. Comparative toxicity of UV-filter Octyl methoxycinnamate and its photoproducts on zebrafish development. Sci. Total Environ. 2020, 718, 134546. [Google Scholar] [CrossRef]
- Coelho, L.; Almeida, I.F.; Sousa Lobo, J.M.; Sousa E Silva, J.P. Photostabilization strategies of photosensitive drugs. Int. J. Pharm. 2018, 41, 19–25. [Google Scholar] [CrossRef]
- Cagno, M.P.D. The potential of cyclodextrins as novel active pharmaceutical ingredients: A short overview. Molecules 2017, 22, 1. [Google Scholar] [CrossRef]
- Iacovino, R.; Caso, J.V.; Di Donato, C.; Malgieri, G.; Palmieri, M.; Russo, L.; Isernia, C. Cyclodextrins as complexing agents: Preparation and applications. Curr. Org. Chem. 2017, 21, 162–176. [Google Scholar] [CrossRef]
- Ioele, G.; Tavano, L.; De Luca, M. Photostability and ex-vivo permeation studies on diclofenac in topical niosomal formulations. Int. J. Pharm. 2015, 494, 490–497. [Google Scholar] [CrossRef]
- León, C.; Henríquez, C.; López, N.; Sanchez, G.; Pastén, B.; Baeza, P.; Ojeda, J. Inhibitory effect of the Ascorbic Acid on photodegradation of pharmaceuticals compounds exposed to UV-B radiation. J. Photochem. Photobiol. 2021, 7, 100035. [Google Scholar] [CrossRef]
- Kawabata, K.; Takato, A.; Oshima, S.; Akimoto, S.; Inagaki, M.; Nishi, H. Protective effect of selected antioxidants on naproxen photodegradation in aqueous media. Antioxidants 2019, 8, 424. [Google Scholar] [CrossRef] [PubMed]
- Savian, A.L.; Rodrigues, D.; Weber, J. Dithranol-loaded lipid-core nanocapsules improve the photostability and reduce the in vitro irritation potential of this drug. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 46, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Detoni, C.B.; Souto, G.D.; da Silva, A.L.M. Photostability and skin penetration of different E-resveratrol-loaded supramolecular structures. Photochem. Photobiol. 2012, 88, 913–921. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Nie, Y.; Zheng, X.Q.; Lu, J.L.; Liang, Y.R.; Ye, J.H. Ultraviolet B (UVB) photosensitivities of tea catechins and relevant chemical conversions. Molecules 2016, 21, E1345. [Google Scholar] [CrossRef]
- Sakamoto-Sasaki, S.; Sato, K.; Abe, M.; Sugimoto, N.; Maitani, T. Components of turmeric oleoresin preparations and photo-stability of curcumin. Jpn. J. Food Chem. 1998, 5, 57–63. [Google Scholar]
- Dabić, D.; Hanževački, M.; Škorić, I.; Žegura, B.; Ivanković, K.; Biošić, M.; Tolić, K.; Babić, S. Photodegradation, toxicity and density functional theory study of pharmaceutical metoclopramide and its photoproducts. Sci. Total Environ. 2022, 807, 150694. [Google Scholar] [CrossRef]
- Siciliano, A.; Guida, M.; Iesce, M.R.; Libralato, G.; Temussi, F.; Galdiero, E.; Carraturo, F.; Cermola, F.; DellaGreca, M. Ecotoxicity and photodegradation of Montelukast (a drug to treat asthma) in water. Environ Res. 2021, 202, 111680. [Google Scholar] [CrossRef]
- DellaGreca, M.; Fiorentino, A.; Isidori, M.; Lavorgna, M.; Previtera, L.; Rubino, M.; Temussi, F. Toxicity of prednisolone, dexamethasone and their photochemical derivatives on aquatic organisms. Chemosphere 2004, 54, 629–637. [Google Scholar] [CrossRef]
- Isidori, M.; Nardelli, A.; Pascarella, L.; Rubino, M.; Parrella, A. Toxic and genotoxic impact of fibrates and their photoproducts on non-target organisms. Environ. Int. 2007, 33, 635–641. [Google Scholar] [CrossRef] [PubMed]
- DellaGreca, M.; Brigante, M.; Isidori, M.; Nardelli, A.; Previtera, L.; Rubino, M.; Temussi, F. Phototransformation and ecotoxicity of the drug Naproxen-Na. Environ. Chem. Lett. 2003, 1, 237–241. [Google Scholar] [CrossRef]
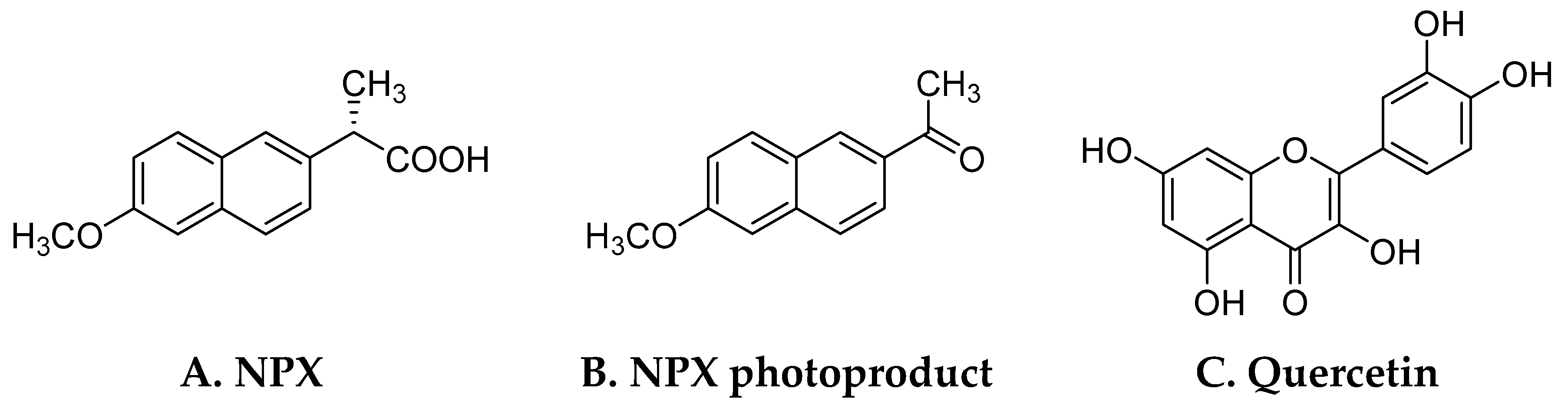
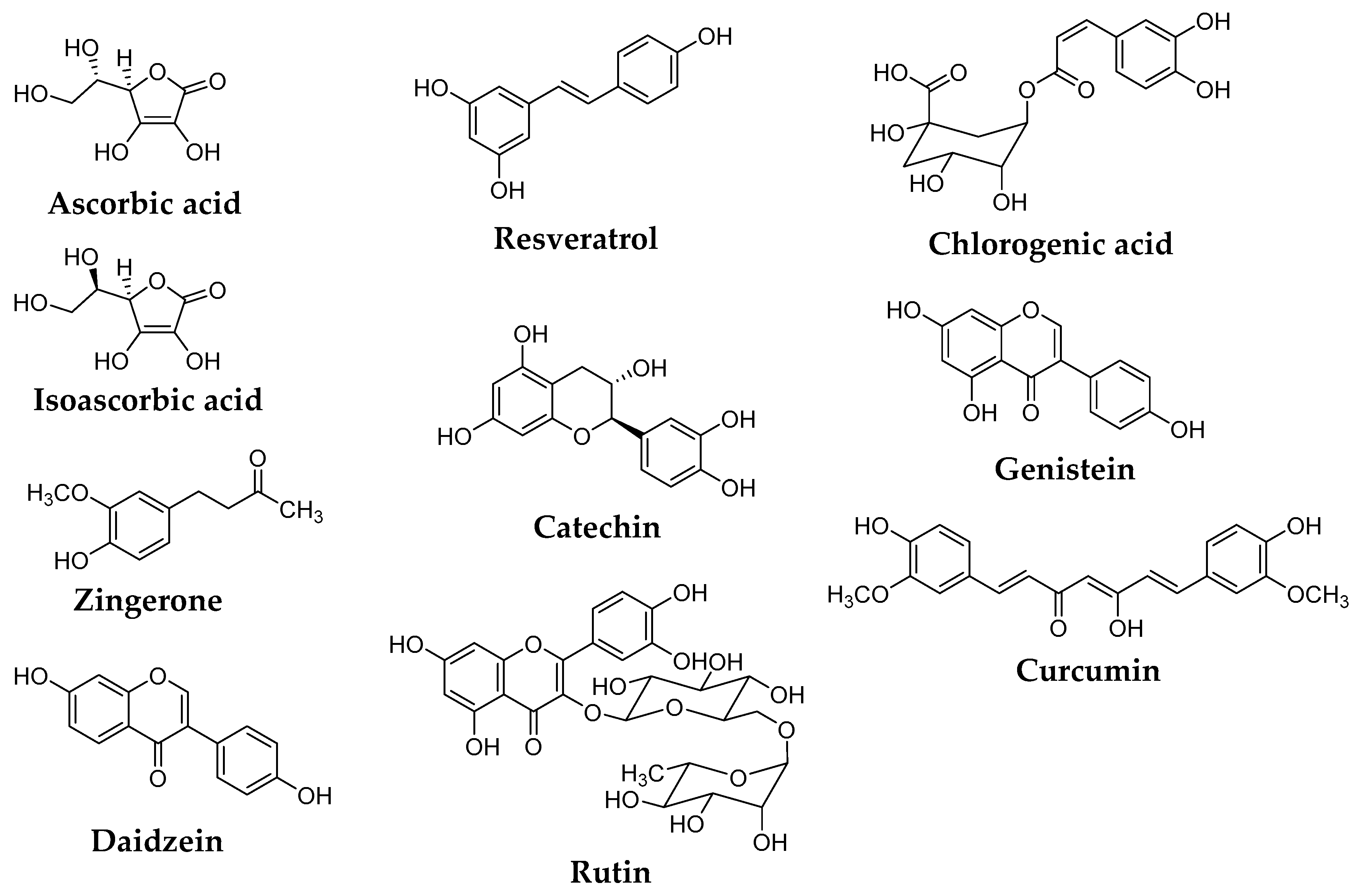


| λmax (nm) and ε (L mol−1 cm−1) in the Wavelength above 260 nm | |
|---|---|
| NPX | 332 nm (1853), 318 nm (1481), 272 nm (5178), 263 nm (5144) |
| Ascorbic acid | - |
| Zingerone | 282 nm (2984) |
| Catechin | 280 nm (4114) |
| Isoascorbic acid | - |
| Rutin | 360 nm (17,607) |
| Daidzein | 303 nm (10,279) |
| Chlorogenic acid | 329 nm (20,032), 301 nm (15,213) |
| Genistein | 262 nm (44,251) |
| Quercetin | 370 nm (23,971) |
| Curcumin | 424 nm (72,442), 263 nm (16,933) |
| Resveratrol | 308 nm (34,140) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kawabata, K.; Miyoshi, A.; Nishi, H. Photoprotective Effects of Selected Polyphenols and Antioxidants on Naproxen Photodegradability in the Solid-State. Photochem 2022, 2, 880-890. https://doi.org/10.3390/photochem2040056
Kawabata K, Miyoshi A, Nishi H. Photoprotective Effects of Selected Polyphenols and Antioxidants on Naproxen Photodegradability in the Solid-State. Photochem. 2022; 2(4):880-890. https://doi.org/10.3390/photochem2040056
Chicago/Turabian StyleKawabata, Kohei, Ayano Miyoshi, and Hiroyuki Nishi. 2022. "Photoprotective Effects of Selected Polyphenols and Antioxidants on Naproxen Photodegradability in the Solid-State" Photochem 2, no. 4: 880-890. https://doi.org/10.3390/photochem2040056
APA StyleKawabata, K., Miyoshi, A., & Nishi, H. (2022). Photoprotective Effects of Selected Polyphenols and Antioxidants on Naproxen Photodegradability in the Solid-State. Photochem, 2(4), 880-890. https://doi.org/10.3390/photochem2040056








