Increasing the Photocatalytic Activity of BiVO4 by Naked Co(OH)2 Nanoparticle Cocatalysts
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Synthesis of BVO Nanoflakes
2.3. Cobalt Cocatalyst Deposition
2.4. Photoelectrodes Preparation
2.5. Characterizations
2.6. PEC Water Oxidation Measurements
2.7. Evaluation of Photocatalytic Activity
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Queiroz, B.D.; Fernandes, J.A.; Martins, C.A.; Wender, H. Photocatalytic Fuel Cells: From Batch to Microfluidics. J. Environ. Chem. Eng. 2022, 10, 107611. [Google Scholar] [CrossRef]
- Khan, N.; Stelo, F.; Santos, G.H.C.; Rossi, L.M.; Gonçalves, R.V.; Wender, H. Recent Advances on Z-Scheme Engineered BiVO4-Based Semiconductor Photocatalysts for CO2 Reduction: A Review. Appl. Surf. Sci. Adv. 2022, 11, 100289. [Google Scholar] [CrossRef]
- Ding, T.; Zhou, Y.; Ong, W.L.; Ho, G.W. Hybrid Solar-Driven Interfacial Evaporation Systems: Beyond Water Production towards High Solar Energy Utilization. Mater. Today 2021, 42, 178–191. [Google Scholar] [CrossRef]
- Ullah, S.; Fayeza; Khan, A.A.; Jan, A.; Aain, S.Q.; Neto, E.P.F.; Serge-Correales, Y.E.; Parveen, R.; Wender, H.; Rodrigues-Filho, U.P.; et al. Enhanced Photoactivity of BiVO4/Ag/Ag2O Z-Scheme Photocatalyst for Efficient Environmental Remediation under Natural Sunlight and Low-Cost LED Illumination. Colloids Surf. A Physicochem. Eng. Asp. 2020, 600, 124946. [Google Scholar] [CrossRef]
- Keane, D.A.; McGuigan, K.G.; Ibanez, P.F.; Polo-Lopez, M.I.; Byrne, J.A.; Dunlop, P.S.M.; O’Shea, K.; Dionysiou, D.D.; Pillai, S.C. Solar Photocatalysis for Water Disinfection: Materials and Reactor Design. Catal. Sci. Technol. 2014, 4, 1211–1226. [Google Scholar] [CrossRef]
- Mills, A.; le Hunte, S. An Overview of Semiconductor Photocatalysis. J. Photochem. Photobiol. A Chem. 1997, 108, 1–35. [Google Scholar] [CrossRef]
- Gonçalves, R.V.; Wender, H.; Khan, S.; Melo, M.A. Photocatalytic Water Splitting by Suspended Semiconductor Particles. In Nanoenergy; Springer International Publishing: Cham, Switzerland, 2018; pp. 107–140. [Google Scholar]
- Wender, H.; Gonçalves, R.V.; Dias, C.S.B.; Zapata, M.J.M.; Zagonel, L.F.; Mendonça, E.C.; Teixeira, S.R.; Garcia, F. Photocatalytic Hydrogen Production of Co(OH)2 Nanoparticle-Coated α-Fe2O3 Nanorings. Nanoscale 2013, 5, 9310–9316. [Google Scholar] [CrossRef] [PubMed]
- Reddy, C.V.; Koutavarapu, R.; Reddy, K.R.; Shetti, N.P.; Aminabhavi, T.M.; Shim, J. Z-Scheme Binary 1D ZnWO4 Nanorods Decorated 2D NiFe2O4 Nanoplates as Photocatalysts for High Efficiency Photocatalytic Degradation of Toxic Organic Pollutants from Wastewater. J. Environ. Manag. 2020, 268, 110677. [Google Scholar] [CrossRef] [PubMed]
- Peter, L. Fundamental Aspects of Photoelectrochemical Water Splitting at Semiconductor Electrodes. Curr. Opin. Green Sustain. Chem. 2021, 31, 100505. [Google Scholar] [CrossRef]
- Kumar, S.; Rodene, D.D.; Gupta, R.B. Recent Advancements in Semiconductor Materials for Photoelectrochemical Water Splitting for Hydrogen Production Using Visible Light. Renew. Sustain. Energy Rev. 2018, 89, 228–248. [Google Scholar] [CrossRef]
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO2 Photocatalysis: Mechanisms and Materials. Chem. Rev. 2014, 114, 9919–9986. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Han, X.; Liu, S.; Lv, Y.; Zhao, Y.; Li, Y.; Wang, L. Nitrogen-Doped Cobalt-Iron Oxide Cocatalyst Boosting Photoelectrochemical Water Splitting of BiVO4 Photoanodes. Appl. Catal. B 2022, 320, 121947. [Google Scholar] [CrossRef]
- Tian, T.; Jiang, G.; Li, Y.; Xiang, W.; Fu, W. Unveiling the Activity and Stability of BiVO 4 Photoanodes with Cocatalyst for Water Oxidation. Renew. Energy 2022, 199, 132–139. [Google Scholar] [CrossRef]
- Cao, H.; Wang, T.; Li, J.; Wu, J.; Du, P. A Molecular Cobaloxime Cocatalyst and Ultrathin FeOOH Nanolayers Co-Modified BiVO4 Photoanode for Efficient Photoelectrochemical Water Oxidation. J. Energy Chem. 2022, 69, 497–505. [Google Scholar] [CrossRef]
- Ullah, S.; Ferreira-Neto, E.P.; Pasa, A.A.; Alcântara, C.C.J.; Acuña, J.J.S.; Bilmes, S.A.; Martínez Ricci, M.L.; Landers, R.; Fermino, T.Z.; Rodrigues-Filho, U.P. Enhanced Photocatalytic Properties of Core@shell SiO2@TiO2 Nanoparticles. Appl. Catal. B 2015, 179, 333–343. [Google Scholar] [CrossRef]
- Ferreira-Neto, E.P.; Ullah, S.; da Silva, T.C.A.; Domeneguetti, R.R.; Perissinotto, A.P.; de Vicente, F.S.; Rodrigues-Filho, U.P.; Ribeiro, S.J.L. Bacterial Nanocellulose/MoS2 Hybrid Aerogels as Bifunctional Adsorbent/Photocatalyst Membranes for in-Flow Water Decontamination. ACS Appl. Mater. Interfaces 2020, 12, 41627–41643. [Google Scholar] [CrossRef]
- Jiang, R.; Li, W.; Zhu, K.; Ye, W.; Zhu, G.; Jia, G.; Xu, F.; Wang, J.; Tao, T.; Wang, Y.; et al. TiO2/β-C3N4 for Sunlight-Driven Overall Water Splitting. J. Alloy. Compd. 2022, 920, 166045. [Google Scholar] [CrossRef]
- Singla, S.; Sharma, S.; Basu, S.; Shetti, N.P.; Reddy, K.R. Graphene/Graphitic Carbon Nitride-Based Ternary Nanohybrids: Synthesis Methods, Properties, and Applications for Photocatalytic Hydrogen Production. FlatChem 2020, 24, 100200. [Google Scholar] [CrossRef]
- You, J.; Guo, Y.; Guo, R.; Liu, X. A Review of Visible Light-Active Photocatalysts for Water Disinfection: Features and Prospects. Chem. Eng. J. 2019, 373, 624–641. [Google Scholar] [CrossRef]
- Ullah, S.; Ferreira-Neto, E.P.; Hazra, C.; Parveen, R.; Rojas-Mantilla, H.D.; Calegaro, M.L.; Serge-Correales, Y.E.; Rodrigues-Filho, U.P.; Ribeiro, S.J.L. Broad Spectrum Photocatalytic System Based on BiVO4 and NaYbF4:Tm3+ Upconversion Particles for Environmental Remediation under UV-Vis-NIR Illumination. Appl. Catal. B 2019, 243, 121–135. [Google Scholar] [CrossRef]
- Gomes, L.E.; Nogueira, A.C.; da Silva, M.F.; Plaça, L.F.; Maia, L.J.Q.; Gonçalves, R.V.; Ullah, S.; Khan, S.; Wender, H. Enhanced Photocatalytic Activity of BiVO4/Pt/PtOx Photocatalyst: The Role of Pt Oxidation State. Appl. Surf. Sci. 2021, 567, 150773. [Google Scholar] [CrossRef]
- Song, M.; Wu, Y.; Zheng, G.; Du, C.; Su, Y. Junction of Porous G-C3N4 with BiVO4 Using Au as Electron Shuttle for Cocatalyst-Free Robust Photocatalytic Hydrogen Evolution. Appl. Surf. Sci. 2019, 498, 143808. [Google Scholar] [CrossRef]
- Kumar, N.; Krishnarao, N.; Singh, S.A. Environmental Cocatalyst Free Z-Schematic Enhanced H 2 Evolution over LaVO4/BiVO4 Composite Photocatalyst Using Ag as an Electron Mediator. Appl. Catal. B 2018, 220, 512–523. [Google Scholar] [CrossRef]
- Ju, P.; Hao, L.; Zhang, Y.; Sun, J.; Dou, K.; Lu, Z.; Liao, D.; Zhai, X.; Sun, C. Facile Fabrication of a Novel Spindlelike MoS2/BiVO4 Z-Scheme Heterostructure with Superior Visible-Light-Driven Photocatalytic Disinfection Performance. Sep. Purif. Technol. 2022, 299, 121706. [Google Scholar] [CrossRef]
- Zhou, B.; Zhao, X.; Liu, H.; Qu, J.; Huang, C.P. Synthesis of Visible-Light Sensitive M-BiVO4 (M = Ag, Co, and Ni) for the Photocatalytic Degradation of Organic Pollutants. Sep. Purif. Technol. 2011, 77, 275–282. [Google Scholar] [CrossRef]
- Shi, H.; Li, Y.; Wang, K.; Li, S.; Wang, X.; Wang, P.; Chen, F.; Yu, H. Mass-Transfer Control for Selective Deposition of Well-Dispersed AuPd Cocatalysts to Boost Photocatalytic H2O2 Production of BiVO4. Chem. Eng. J. 2022, 443, 136429. [Google Scholar] [CrossRef]
- Huang, J.; Tian, Y.; Wang, Y.; Liu, T. Journal of Solid State Chemistry Load CoOx Cocatalyst on Photoanode by Spin Coating and Calcination for Enhanced Photoelectrochemical Water Oxidation: A Case Study on BiVO4. J. Solid State Chem. 2021, 299, 122154. [Google Scholar] [CrossRef]
- Ma, N.; Xu, J.; Bian, Z.; Yang, Y.; Zhang, L.; Wang, H. BiVO4 Plate with Fe and Ni Oxyhydroxide Cocatalysts for the Photodegradation of Sulfadimethoxine Antibiotics under Visible-Light Irradiation. Chem. Eng. J. 2020, 389, 123426. [Google Scholar] [CrossRef]
- Vishlaghi, M.B.; Kahraman, A.; Apaydin, S.; Usman, E.; Aksoy, D.; Balkan, T.; Munir, S.; Harfouche, M.; Ogasawara, H.; Kaya, S. The Significance of the Local Structure of Cobalt-Based Catalysts on the Photoelectrochemical Water Oxidation Activity of BiVO4. Electrochim. Acta 2021, 366, 137467. [Google Scholar] [CrossRef]
- Li, X.; Jia, M.; Lu, Y.; Li, N.; Zheng, Y.Z.; Tao, X.; Huang, M. Co(OH)2/BiVO4 Photoanode in Tandem with a Carbon-Based Perovskite Solar Cell for Solar-Driven Overall Water Splitting. Electrochim. Acta 2020, 330, 135183. [Google Scholar] [CrossRef]
- Adenle, A.; Ma, D.K.; Qu, D.P.; Chen, W.; Huang, S. BiVO4 Hollow Microplates: Controlled Synthesis and Enhanced Photocatalytic Activity Achieved through One-Step Boron Doping and Co(OH)2 Loading. CrystEngComm 2017, 19, 6305–6313. [Google Scholar] [CrossRef]
- Guima, K.; Gomes, L.E.; Fernandes, J.A.; Wender, H.; Martins, C.A. Harvesting Energy from an Organic Pollutant Model Using a New 3D-Printed Microfluidic Photo Fuel Cell. ACS Appl. Mater. Interfaces 2020, 12, 54563–54572. [Google Scholar] [CrossRef] [PubMed]
- Rosa, W.S.; Rabelo, L.G.; Zampaulo, L.G.T.; Gonçalves, R.V. Ternary Oxide CuWO4/BiVO4/FeCoOx Films for Photoelectrochemical Water Oxidation: Insights into the Electronic Structure and Interfacial Band Alignment. ACS Appl. Mater. Interfaces 2022, 14, 22858–22869. [Google Scholar] [CrossRef] [PubMed]
- Melo, M.A.; Centurion, H.A.; Machado, G.; Souza, F.L.; Gonçalves, R.V. Binary Transition Metal NiFeOx and CoFeOx Cocatalysts Boost the Photodriven Water Oxidation over Fe2TiO5 Nanoparticles. ChemNanoMat 2022, 8, e202100510. [Google Scholar] [CrossRef]
- Malathi, A.; Madhavan, J.; Ashokkumar, M.; Arunachalam, P. A Review on BiVO4 Photocatalyst: Activity Enhancement Methods for Solar Photocatalytic Applications. Appl. Catal. A Gen. 2018, 555, 47–74. [Google Scholar] [CrossRef]
- Nikam, S.; Joshi, S. Irreversible Phase Transition in BiVO4 Nanostructures Synthesized by a Polyol Method and Enhancement in Photo Degradation of Methylene Blue. RSC Adv. 2016, 6, 107463–107474. [Google Scholar] [CrossRef]
- Merupo, V.I.; Velumani, S.; Oza, G.; Makowska-Janusik, M.; Kassiba, A. Structural, Electronic and Optical Features of Molybdenum-Doped Bismuth Vanadium Oxide. Mater. Sci. Semicond. Process. 2015, 31, 618–623. [Google Scholar] [CrossRef]
- Zhou, D.; Li, W.-B.; Pang, L.-X.; Guo, J.; Qi, Z.-M.; Shao, T.; Yao, X.; Randall, C.A. Phase Evolution and Microwave Dielectric Properties of XBi2/3MoO4–(1 − x)BiVO4(0.0 ≤ x ≤ 1.0) Low Temperature Firing Ceramics. Dalton Trans. 2014, 43, 7290–7297. [Google Scholar] [CrossRef]
- Hardcastle, F.D.; Wachs, I.E. Determination of Vanadium-Oxygen Bond Distances and Bond Orders by Raman Spectroscopy. J. Phys. Chem. 1991, 95, 5031–5041. [Google Scholar] [CrossRef]
- Malashchonak, M.V.; Streltsov, E.A.; Kuliomin, D.A.; Kulak, A.I.; Mazanik, A.V. Monoclinic Bismuth Vanadate Band Gap Determination by Photoelectrochemical Spectroscopy. Mater. Chem. Phys. 2017, 201, 189–193. [Google Scholar] [CrossRef]
- Yan, M.; Yan, Y.; Wu, Y.; Shi, W.; Hua, Y. Microwave-Assisted Synthesis of Monoclinic–Tetragonal BiVO4 Heterojunctions with Enhanced Visible-Light-Driven Photocatalytic Degradation of Tetracycline. RSC Adv. 2015, 5, 90255–90264. [Google Scholar] [CrossRef]
- Dong, P.; Xi, X.; Zhang, X.; Hou, G.; Guan, R. Template-Free Synthesis of Monoclinic BiVO4 with Porous Structure and Its High Photocatalytic Activity. Materials 2016, 9, 685. [Google Scholar] [CrossRef] [PubMed]
- Cooper, J.K.; Gul, S.; Toma, F.M.; Chen, L.; Liu, Y.S.; Guo, J.; Ager, J.W.; Yano, J.; Sharp, I.D. Indirect Bandgap and Optical Properties of Monoclinic Bismuth Vanadate. J. Phys. Chem. C 2015, 119, 2969–2974. [Google Scholar] [CrossRef]
- Wang, Z.; Huang, X.; Wang, X. Recent Progresses in the Design of BiVO4-Based Photocatalysts for Efficient Solar Water Splitting. Catal. Today 2019, 335, 31–38. [Google Scholar] [CrossRef]
- Tayebi, M.; Lee, B.K. Recent Advances in BiVO4 Semiconductor Materials for Hydrogen Production Using Photoelectrochemical Water Splitting. Renew. Sustain. Energy Rev. 2019, 111, 332–343. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, T.; Xu, T.; Li, Y.; Wang, C. Size Effect of Pt Co-Catalyst on Photocatalytic Efficiency of g-C3N4 for Hydrogen Evolution. Appl. Surf. Sci. 2019, 464, 36–42. [Google Scholar] [CrossRef]
- Zhang, W.; Li, R.; Zhao, X.; Chen, Z.; Law, A.W.K.; Zhou, K. A Cobalt-Based Metal–Organic Framework as Cocatalyst on BiVO4 Photoanode for Enhanced Photoelectrochemical Water Oxidation. ChemSusChem 2018, 11, 2710–2716. [Google Scholar] [CrossRef]
- Liu, C.; Luo, H.; Xu, Y.; Wang, W.; Liang, Q.; Mitsuzaki, N.; Chen, Z. Cobalt–Phosphate-Modified Mo:BiVO4 Mesoporous Photoelectrodes for Enhanced Photoelectrochemical Water Splitting. J. Mater. Sci. 2019, 54, 10670–10683. [Google Scholar] [CrossRef]
- Tan, H.L.; Wen, X.; Amal, R.; Ng, Y.H. BiVO4 {010} and {110} Relative Exposure Extent: Governing Factor of Surface Charge Population and Photocatalytic Activity. J. Phys. Chem. Lett. 2016, 7, 1400–1405. [Google Scholar] [CrossRef]
- Lin, X.; Hou, J.; Jiang, S.; Lin, Z.; Wang, M.; Che, G. A Z-Scheme Visible-Light-Driven Ag/Ag3PO4/Bi2MoO6 Photocatalyst: Synthesis and Enhanced Photocatalytic Activity. RSC Adv. 2015, 5, 104815–104821. [Google Scholar] [CrossRef]
- Geng, Y.; Zhang, P.; Li, N.; Sun, Z. Synthesis of Co Doped BiVO4 with Enhanced Visible-Light Photocatalytic Activities. J. Alloy. Compd. 2015, 651, 744–748. [Google Scholar] [CrossRef]
- Zhang, L.; Dai, Z.; Zheng, G.; Yao, Z.; Mu, J. Superior Visible Light Photocatalytic Performance of Reticular BiVO4 Synthesized via a Modified Sol–Gel Method. RSC Adv. 2018, 8, 10654–10664. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, A.C.; Gomes, L.E.; Ferencz, J.A.P.; Rodrigues, J.E.F.S.; Gonçalves, R.V.; Wender, H. Improved Visible Light Photoactivity of CuBi2O4/CuO Heterojunctions for Photodegradation of Methylene Blue and Metronidazole. J. Phys. Chem. C 2019, 123, 25680–25690. [Google Scholar] [CrossRef]
- Ye, K.H.; Li, H.; Huang, D.; Xiao, S.; Qiu, W.; Li, M.; Hu, Y.; Mai, W.; Ji, H.; Yang, S. Enhancing Photoelectrochemical Water Splitting by Combining Work Function Tuning and Heterojunction Engineering. Nat. Commun. 2019, 10, 3687. [Google Scholar] [CrossRef]
- Patil, S.S.; Mali, M.G.; Hassan, M.A.; Patil, D.R.; Kolekar, S.S.; Ryu, S.-W. One-Pot in Situ Hydrothermal Growth of BiVO4/Ag/RGO Hybrid Architectures for Solar Water Splitting and Environmental Remediation. Sci. Rep. 2017, 7, 8404. [Google Scholar] [CrossRef]
- Yuan, Q.; Chen, L.; Xiong, M.; He, J.; Luo, S.; Au, C.; Yin, S. Cu2O/BiVO4 Heterostructures: Synthesis and Application in Simultaneous Photocatalytic Oxidation of Organic Dyes and Reduction of Cr(VI) under Visible Light. Chem. Eng. J. 2014, 255, 394–402. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, M.; Cui, W.; Sui, H. Synthesis and Characterization of a Core-Shell BiVO4@g-C3N4 Photo-Catalyst with Enhanced Photocatalytic Activity under Visible Light Irradiation. RSC Adv. 2017, 7, 8167–8177. [Google Scholar] [CrossRef]
- Zhou, J.; Li, J.; Liu, K.; Lan, L.; Song, H.; Chen, X. Free-Standing Cobalt Hydroxide Nanoplatelet Array Formed by Growth of Preferential-Orientation on Graphene Nanosheets as Anode Material for Lithium-Ion Batteries. J. Mater. Chem. A 2014, 2, 20706–20713. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, Z.; Chen, D.; Song, Y.; Wang, R. Synthesis and Electrochemical Properties of Porous α-Co(OH)2 and Co3O4 Microspheres. Prog. Nat. Sci. Mater. Int. 2017, 27, 197–202. [Google Scholar] [CrossRef]
- Bazylewski, P.; Boukhvalov, D.W.; Kukharenko, A.I.; Kurmaev, E.Z.; Hunt, A.; Moewes, A.; Lee, Y.H.; Cholakh, S.O.; Chang, G.S. The Characterization of Co-Nanoparticles Supported on Graphene. RSC Adv. 2015, 5, 75600–75606. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Payne, B.P.; Grosvenor, A.P.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving Surface Chemical States in XPS Analysis of First Row Transition Metals, Oxides and Hydroxides: Cr, Mn, Fe, Co and Ni. Appl. Surf. Sci. 2011, 257, 2717–2730. [Google Scholar] [CrossRef]
- Yang, J.; Liu, H.; Martens, W.N.; Frost, R.L. Synthesis and Characterization of Cobalt Hydroxide, Cobalt Oxyhydroxide, and Cobalt Oxide Nanodiscs. J. Phys. Chem. C 2010, 114, 111–119. [Google Scholar] [CrossRef]
- Cole, K.M.; Kirk, D.W.; Thorpe, S.J. Co(OH)2 Powder Characterized by x-Ray Photoelectron Spectroscopy (XPS) and Ultraviolet Photoelectron Spectroscopy (UPS). Surf. Sci. Spectra 2020, 27, 024013. [Google Scholar] [CrossRef]
- Hermans, Y.; Klein, A.; Ellmer, K.; van de Krol, R.; Toupance, T.; Jaegermann, W. Energy-Band Alignment of BiVO4 from Photoelectron Spectroscopy of Solid-State Interfaces. J. Phys. Chem. C 2018, 122, 20861–20870. [Google Scholar] [CrossRef]
- Kalasina, S.; Phattharasupakun, N.; Maihom, T.; Promarak, V.; Sudyoadsuk, T.; Limtrakul, J.; Sawangphruk, M. Novel Hybrid Energy Conversion and Storage Cell with Photovoltaic and Supercapacitor Effects in Ionic Liquid Electrolyte. Sci. Rep. 2018, 8, 12192. [Google Scholar] [CrossRef]
- Suksomboon, M.; Kongsawatvoragul, K.; Duangdangchote, S.; Sawangphruk, M. Reducing the Energy Band Gap of Cobalt Hydroxide Nanosheets with Silver Atoms and Enhancing Their Electrical Conductivity with Silver Nanoparticles. ACS Omega 2021, 6, 20804–20811. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Liu, L.; Oguzie, E.E.; Li, Y.; Wang, F. CoPi/Co(OH)2 Modified Ta3N5 as New Photocatalyst for Photoelectrochemical Cathodic Protection of 304 Stainless Steel. Materials 2019, 12, 134. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wang, D.; Han, H.; Li, C. Roles of Cocatalysts in Photocatalysis and Photoelectrocatalysis. Acc. Chem. Res. 2013, 46, 1900–1909. [Google Scholar] [CrossRef]
- Stelo, F.; Kublik, N.; Ullah, S.; Wender, H. Recent Advances in Bi2MoO6 Based Z-Scheme Heterojunctions for Photocatalytic Degradation of Pollutants. J. Alloy. Compd. 2020, 829, 154591. [Google Scholar] [CrossRef]
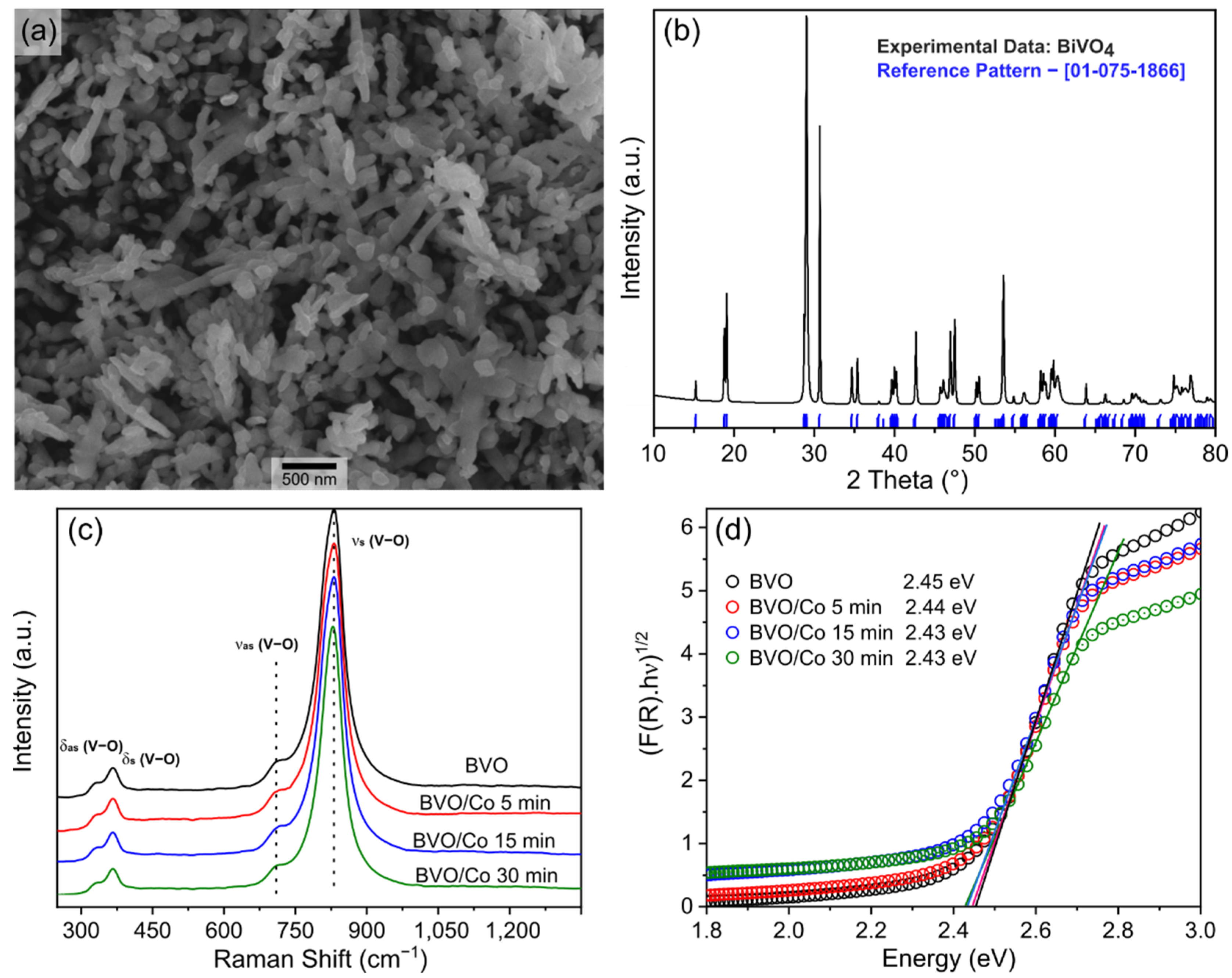


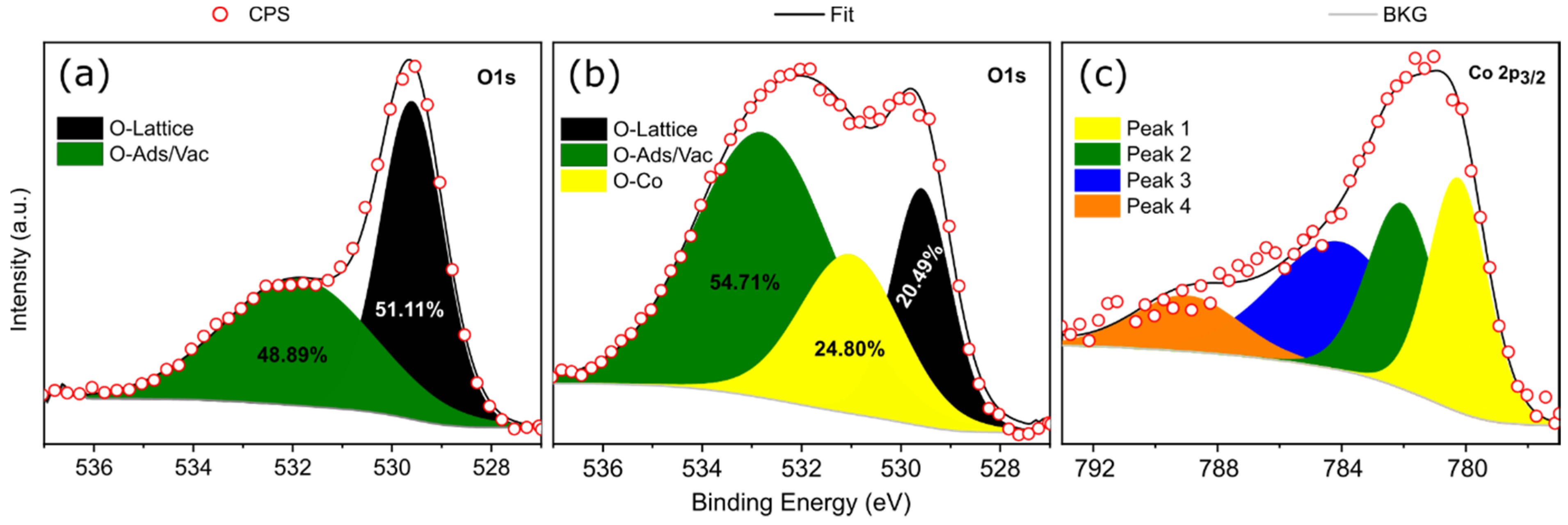
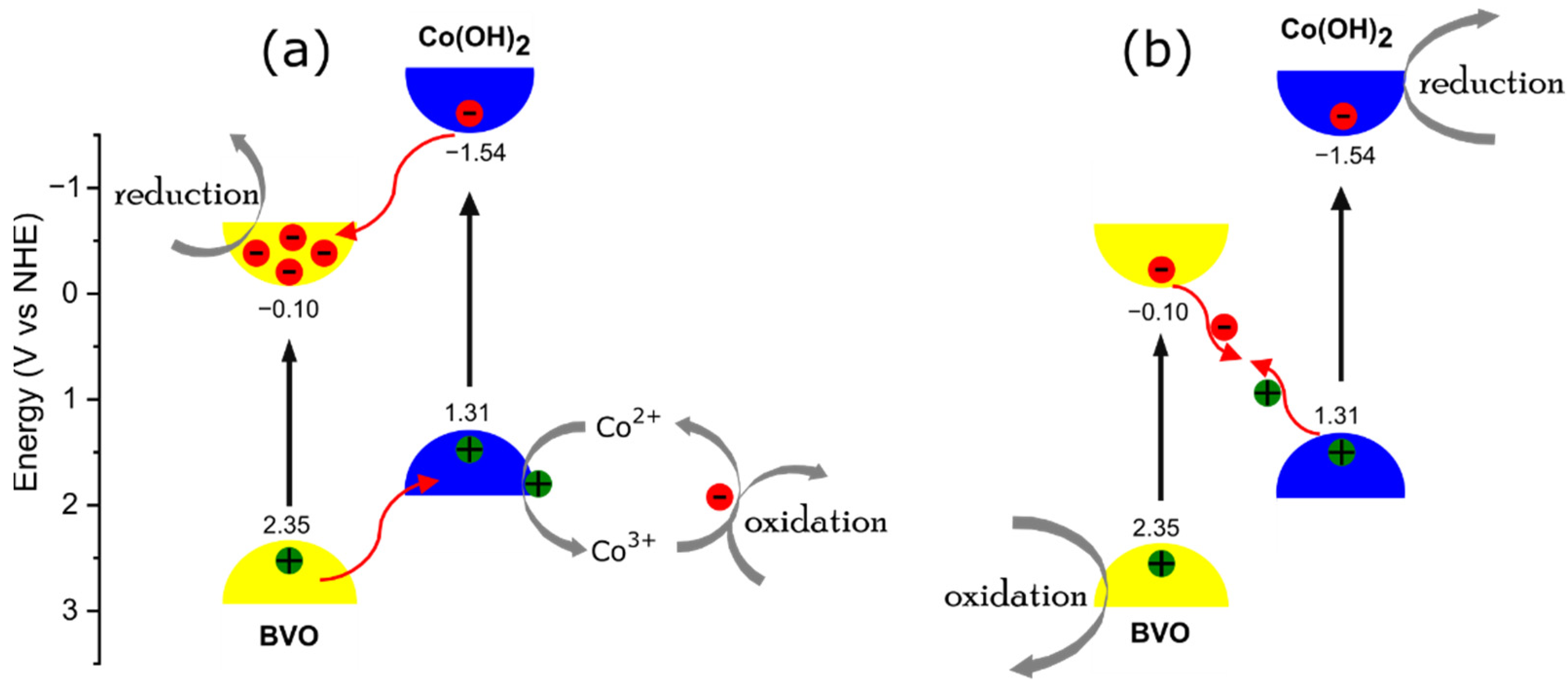
| SAMPLES | BVO | BVO/Co(5 min) | BVO/Co(15 min) | BVO/Co(30 min) |
|---|---|---|---|---|
| Rct (KΩ) | 91.9 | 17.3 | 76.4 | 101.8 |
| Rs (Ω) | 22 | 22 | 22 | 22 |
| CPE-P | 0.90 | 0.85 | 0.91 | 0.92 |
| CPE-T (µF) | 25.3 | 32.7 | 17.2 | 14.1 |
| Samples | O 1s | Co 2p3/2 | |||||
|---|---|---|---|---|---|---|---|
| Peak | Binding Energy (eV) | FWHM (eV) | Area (%) | Peak | Binding Energy (eV) | FWHM (eV) | |
| BVO | O_Lattice | 529.60 | 1.48 | 51.11 | - | - | - |
| O_Ads/Vac | 531.90 | 3.55 | 48.89 | - | - | - | |
| BVO/Co (45 min) | O_Lattice | 529.58 | 1.41 | 20.49 | Peak 1 | 780.23 | 2.2 |
| O-Co | 531.02 | 2.44 | 24.80 | Peak 2 | 782.10 | 2.6 | |
| O_Ads/Vac | 532.78 | 3.31 | 54.71 | Peak 3 | 785.89 | 5.0 | |
| - | - | - | - | Peak 4 | 790.57 | 4.0 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gomes, L.E.; Plaça, L.F.; Rosa, W.S.; Gonçalves, R.V.; Ullah, S.; Wender, H. Increasing the Photocatalytic Activity of BiVO4 by Naked Co(OH)2 Nanoparticle Cocatalysts. Photochem 2022, 2, 866-879. https://doi.org/10.3390/photochem2040055
Gomes LE, Plaça LF, Rosa WS, Gonçalves RV, Ullah S, Wender H. Increasing the Photocatalytic Activity of BiVO4 by Naked Co(OH)2 Nanoparticle Cocatalysts. Photochem. 2022; 2(4):866-879. https://doi.org/10.3390/photochem2040055
Chicago/Turabian StyleGomes, Luiz E., Luiz F. Plaça, Washington S. Rosa, Renato V. Gonçalves, Sajjad Ullah, and Heberton Wender. 2022. "Increasing the Photocatalytic Activity of BiVO4 by Naked Co(OH)2 Nanoparticle Cocatalysts" Photochem 2, no. 4: 866-879. https://doi.org/10.3390/photochem2040055
APA StyleGomes, L. E., Plaça, L. F., Rosa, W. S., Gonçalves, R. V., Ullah, S., & Wender, H. (2022). Increasing the Photocatalytic Activity of BiVO4 by Naked Co(OH)2 Nanoparticle Cocatalysts. Photochem, 2(4), 866-879. https://doi.org/10.3390/photochem2040055







