Synthesis and Characterization of New Tetradentate N2O2-Based Schiff’s Base Cu (II) Complexes for Dye Photodegradation
Abstract
:1. Introduction
2. Materials and Methods
Experimental Procedure for Photomineralization of Dye Pollutants
3. Results and Discussion
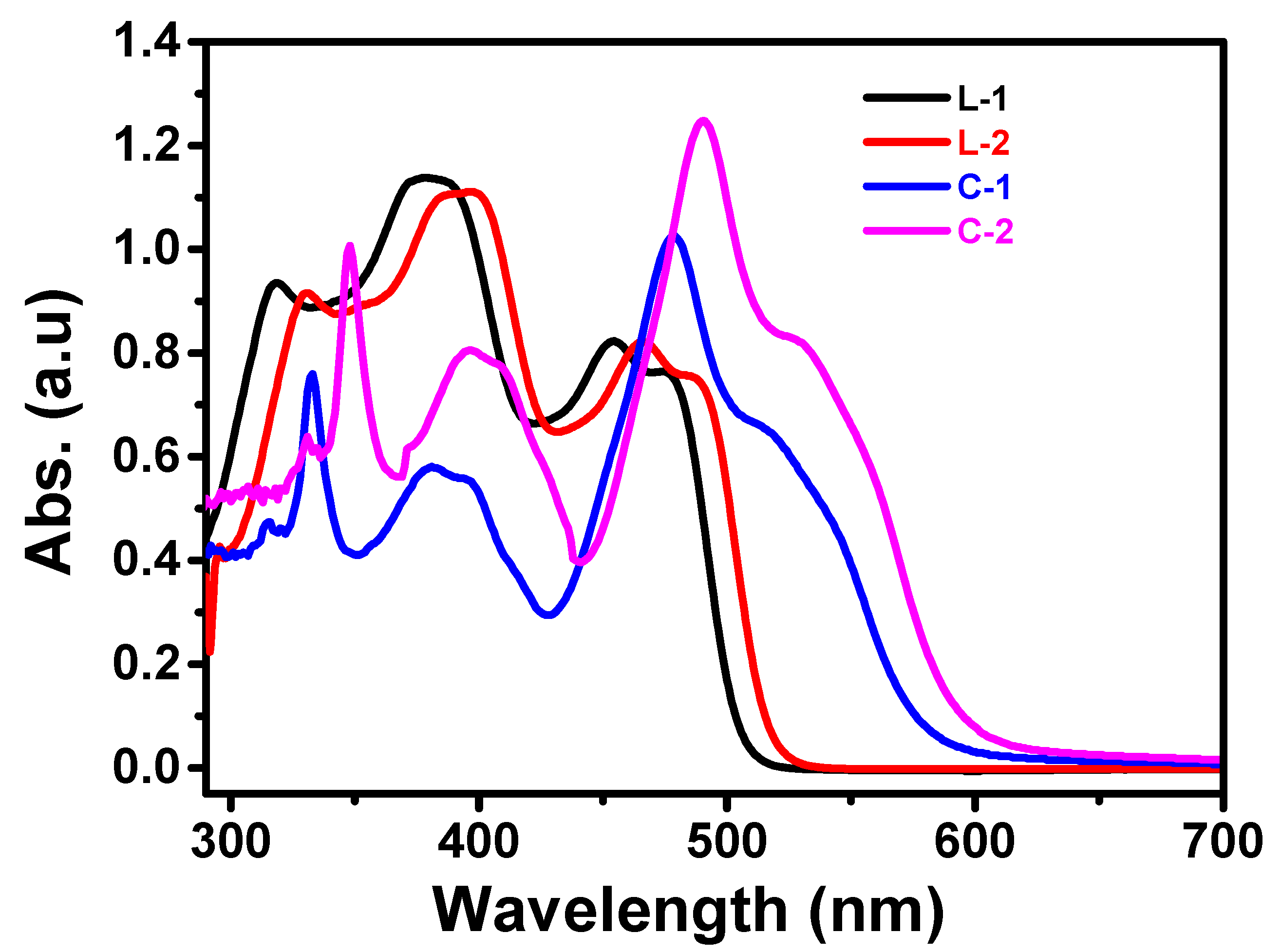
3.1. Absorption and Emission Studies
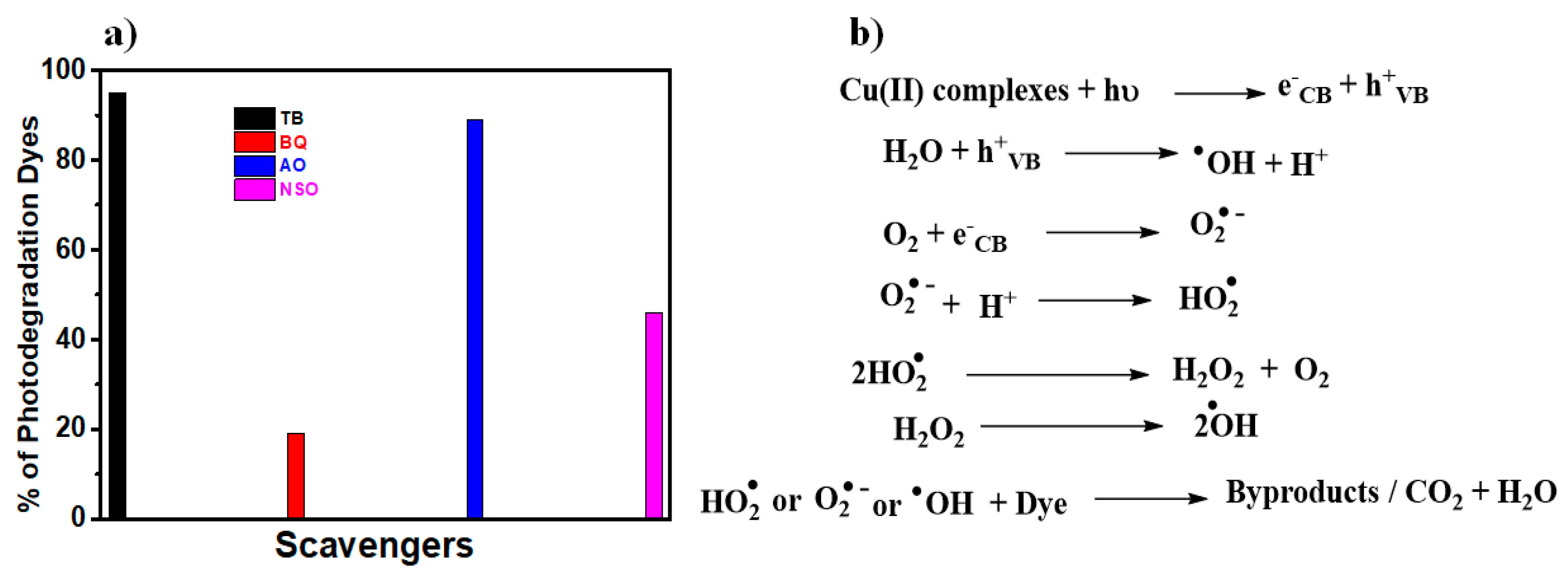
3.2. Photocatalysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mehmet, T. Polydentate Schiff-base ligands and their Cd(II) and Cu(II) metal complexes: Synthesis, characterization, biological activity and electrochemical properties. J. Coord. Chem. 2007, 60, 2051–2065. [Google Scholar]
- Tunde, L.Y.; Segun, D.O.; Sizwe, Z.; Hezekiel, M.K.; Isiaka, A.L.; Monsurat, M.L.; Nonhlangabezo, M. Design of New Schiff-Base Copper(II) Complexes: Synthesis, Crystal Structures, DFT Study, and Binding Potency toward Cytochrome P450 3A4. ACS Omega 2021, 6, 13704–13718. [Google Scholar]
- Liu, X.; Manzur, C.; Novoa, N.; Celedón, S.; Carrillo, D.; Hamon, J.-R. Multidentate unsymmetrically-substituted Schiff bases and their metal complexes: Synthesis, functional materials properties, and applications to catalysis. Coord. Chem. Rev. 2018, 357, 144–172. [Google Scholar] [CrossRef]
- Wesley, J.A.; Kalidasa, M.K.; Neelakantan, M.A. Review on Schiff bases and their metal complexes as organic photovoltaic materials. Renew. Sustain. Energy Rev. 2014, 36, 220–227. [Google Scholar] [CrossRef]
- Alberto, A.-M.; Viviana, R.-M.; Farrah, C.-B.; Jesús, R.P.-U.; Fernando, C.-C.; Dorian, P.-C.; Raúl, C.-P.; Galdina, V.S.-M.; Bethsy, A.A.-C.; David, M.-M. Pincer Complexes Derived from Tridentate Schiff Bases for Their Use as Antimicrobial Metallopharmaceuticals. Inorganics 2022, 10, 134. [Google Scholar]
- Andreas, W.; Ulrich, S.S. Metal-Terpyridine Complexes in Catalytic Application—A Spotlight on the Last Decade. ChemCatChem 2020, 12, 2890–2941. [Google Scholar]
- Takuya, S.; Ken-ichi, F. Recent Advances in Homogeneous Catalysis via Metal–Ligand Cooperation Involving Aromatization and Dearomatization. Catalysts 2020, 10, 635. [Google Scholar]
- Kazimer, L.S.; Travis, R.B.; Tehshik, P.Y. Dual Catalysis Strategies in Photochemical Synthesis. Chem. Rev. 2016, 116, 10035–10074. [Google Scholar]
- Manas, S.; Tannistha, R.B.; Armando, J.L.P.; Luísa, M.D.R.S.M. Aroylhydrazone Schiff Base Derived Cu(II) and V(V) Complexes: Efficient Catalysts towards Neat Microwave-Assisted Oxidation of Alcohols. Int. J. Mol. Sci. 2020, 21, 2832. [Google Scholar]
- Ebrahimipour, S.Y.; Maryam, M.; Masoud, T.M.; Jim, S.; Joel, T.M.; Iran, S. Synthesis and structure elucidation of novel salophen-based dioxo-uranium(VI) complexes: In-vitro and in-silico studies of their DNA/BSA-binding properties and anticancer activity. Eur. J. Med. Chem. 2017, 140, 172–186. [Google Scholar] [CrossRef]
- Atkins, R.; Brewer, G.; Kokot, E.; Mockler, G.M.; Sinn, E. Copper (II) and nickel (II) complexes of unsymmetrical tetradentate Schiff base ligands. Inorg. Chem. 1985, 24, 127–134. [Google Scholar] [CrossRef]
- Kushwah, N.P.; Pal, M.K.; Wadawale, A.P.; Jain, V.K. Diorgano-gallium and-indium complexes with salophen ligands: Synthesis, characterization, crystal structure and C–C coupling reactions. J. Organomet. Chem. 2009, 694, 2375–2379. [Google Scholar] [CrossRef]
- Santarupa, T.; Partha, R.; Ray, J.B.; Fallah, M.S.E.; Javier, T.; Eugenio, G.; Samiran, M. Ferromagnetic Coupling in a New Copper(II) Schiff Base Complex with Cubane Core: Structure, Magnetic Properties, DFT Study and Catalytic Activity. Eur. J. Inorg. Chem. 2009, 2009, 4385–4395. [Google Scholar]
- Rong, M.; Wang, J.; Shen, Y.; Han, J. Catalytic oxidation of alcohols by a novel manganese Schiff base ligand derived from salicylaldehyd and l-Phenylalanine in ionic liquids. Catal. Commun. 2012, 20, 51–53. [Google Scholar] [CrossRef]
- Teerawat, K.; Duangdao, C.; Bussaba, P.; Ratanon, C. Degradation of Methylene Blue with a Cu(II)–Quinoline Complex Immobilized on a Silica Support as a Photo-Fenton-Like Catalyst. ACS Omega 2022, 7, 33258–33265. [Google Scholar]
- Soroceanu, A.; Cazacu, M.; Shova, S.; Turta, C.; Kožíšek, J.; Gall, M.; Breza, M.; Rapta, P.; Mac Leod, T.C.; Pombeiro, A.J. Copper (II) complexes with Schiff bases containing a disiloxane unit: Synthesis, structure, bonding features and catalytic activity for aerobic oxidation of benzyl alcohol. Eur. J. Inorg. Chem. 2013, 2013, 1458–1474. [Google Scholar] [CrossRef]
- Ran, J.; Li, X.; Zhao, Q.; Qu, Z.; Li, H.; Shi, Y.; Chen, G. Synthesis, structures and photocatalytic properties of a mononuclear copper complex with pyridine-carboxylato ligands. Inorg. Chem. Commun. 2010, 13, 526–528. [Google Scholar] [CrossRef]
- Mahesh, S.; Ramesh, G.; Venkanna, G.; Prabhakar, C.; Koteshwar, R.R.; Someshwar, P. Effective photodegradation of organic pollutantsin the presence of mono and bi-metallic complexes under visible-light irradiation. J. Photochem. Photobiol. A Chem. 2021, 406, 112996. [Google Scholar]
- Venkanna, G.; Jakeer, A.; Mahesh, S.; Bhongiri, Y.; Ritu, M.; Chetti, P.; Someshwar, P. Evolution of physical and photocatalytic properties of new Zn(II) and Ru(II) complexes. Polyhedron 2019, 170, 412–423. [Google Scholar]
- Someshwar, P.; Mahesh, S.; Ravinder, G.; Vithal, M.; Yu, T.T. New photocatalyst for allylic aliphatic C–H bond activation and degradation of organic pollutants: Schiff base Ti(IV) complexes. RSC Adv. 2015, 5, 58504–58513. [Google Scholar]
- Guguloth, V.; Ahemed, J.; Subburu, M.; Guguloth, V.C.; Chetti, P.; Pola, S. A very fast photodegradation of dyes in the presence of new Schiff’s base N4-macrocyclic Ag-doped Pd (II) complexes under visible-light irradiation. J. Photochem. Photobiol. A Chem. 2019, 382, 111975. [Google Scholar] [CrossRef]
- Mahesh, S.; Ramesh, G.; Prabhakar, C.; Someshwar, P. Photooxidation of 2,2′-(Ethyne-1,2-diyl)dianilines: An Enhanced Photocatalytic Properties of New Salophen-Based Zn(II) Complexes. Photochem 2022, 2, 358–375. [Google Scholar]
- Jakeer, A.; Jakeer, P.; Venkateshwar, R.D.; Ranjith, K.; Ramesh, G.; Yadagiri, B.; Prabhakar, C.; Someshwar, P. Synthesis of new Zn (II) complexes for photo decomposition of organic dye pollutants, industrial wastewater and photo-oxidation of methyl arenes under visible-light. J. Photochem. Photobiol. A Chem. 2021, 419, 113455. [Google Scholar]
- Venkateshwar, R.D.; Mahesh, S.; Ramesh, G.; Manohar, B.; Prabhakar, C.; Babu, N.S.; Penumaka, N.; Yadagiri, B.; Someshwar, P. A new Zn(II) complex-composite material: Piezoenhanced photomineralization of organic pollutants and wastewater from the lubricant industry. Environ. Sci. Water Res. Technol. 2021, 7, 1737–1747. [Google Scholar]
- Rohini, V.; Ranjith, K.; Radhika, P.; Mahesh, S.; Ramesh, G.; Manohar, B.; Someshwar, P.; Prabhakar, C. Enhanced piezo-photocatalytic properties of new salophen based Ti (IV) complexes. Inorg. Chem. Commun. 2023, 148, 110272. [Google Scholar]
- Reddy, G.R.; Balasubramanian, S.; Chennakesavulu, K. Zeolite encapsulated Ni(ii) and Cu(ii) complexes with tetradentate N2O2 Schiff base ligand: Catalytic activity towards oxidation of benzhydrol and degradation of rhodamine-B. J. Mater. Chem. A 2014, 2, 15598–15610. [Google Scholar] [CrossRef]
- Hafsa, S.; Qureshi Fozia, M.S.; Haque, Z. Biosynthesis of Flower-Shaped CuO Nanostructures and Their Photocatalytic and Antibacterial Activities. Nano-Micro Lett. 2020, 12, 29. [Google Scholar]
- Jiang, D.; Jianbin, X.; Liqiong, W.; Wei, Z.; Yuegang, Z.; Xinheng, L. Photocatalytic performance enhancement of CuO/Cu2Oheterostructures for photodegradation of organic dyes: Effects of CuO morphology. Appl. Catal. B Environ. 2017, 211, 199–204. [Google Scholar] [CrossRef]
- Janusz, G.; Katarzyna, Ś.; Julia, K.; Maciej, W. Multinuclear Ni(ii) and Cu(ii) complexes of a meso 6 + 6 macrocyclic amine derived from trans-1,2-diaminocyclopentane and 2,6-diformylpyridine. Dalton Trans. 2022, 51, 9735–9747. [Google Scholar]
- Gülnur, K.K. Synthesis of new Schiff base and its Ni(II), Cu(II), Zn(II) and Co(II) complexes; photophysical, fluorescence quenching and thermal studies. J. Mol. Struct. 2022, 1256, 132534. [Google Scholar]
- Nooshin, K.; Alison, Z.; Sheida, E. Bioactive Ni(II), Cu(II) and Zn(II) complexes with an N3 functionalized Schiff base ligand: Synthesis, structural elucidation, thermodynamic and DFT calculation studies. Inorg. Chim. Acta 2022, 541, 121083. [Google Scholar]
- Rasha, M.K.; Ahmed, S.; Aly, H.A.; Mohamed, M.A.F.-A. Development of a novel and potential chemical sensor for colorimetric detection of Pd(II) or Cu(II) in E-wastes. Microchem. J. Part A 2022, 172, 106951. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16W, Revision B.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Minji, Y.; Youngtak, O.; Sugyeong, H.; June, S.L.; Ramireddy, B.; Sun, H.K.; Filipe, M.M.; Sang, O.K.; Dong, H.K. Synergistically enhanced photocatalytic activity of graphitic carbonnitride and WO3nanohybrids mediated by photo-Fenton reaction and H2O2. Appl. Catal. B Environ. 2017, 206, 263–270. [Google Scholar]
- Jiahui, L.; Xinghui, L.; Yan, C.; Huijun, L.; Ruochang, L.; Xiaoli, D.; Hyoyoung, L.; Hongchao, M. Highly Enhanced Photoelectrocatalytic Oxidation via Cooperative Effect of Neighboring Two Different Metal Oxides for Water Purification. J. Phys. Chem. C 2020, 124, 11525–11535. [Google Scholar]












| Complex | g|| | g⊥ | gave |
|---|---|---|---|
| C-1 | 2.36 | 2.088 | 2.224 |
| C-2 | 2.38 | 2.099 | 2.239 |
| Name | λonset (nm) | Bandgap Energy (eV) | Surface Area (m2/g) |
|---|---|---|---|
| L-1 | 504 | 2.46 | 5.8 |
| L-2 | 512 | 2.42 | 6.9 |
| C-1 | 584 | 2.12 | 45.6 |
| C-2 | 599 | 2.07 | 62.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vallavoju, R.; Kore, R.; Parikirala, R.; Subburu, M.; Gade, R.; Kumar, V.; Raghavender, M.; Chetti, P.; Pola, S. Synthesis and Characterization of New Tetradentate N2O2-Based Schiff’s Base Cu (II) Complexes for Dye Photodegradation. Photochem 2023, 3, 274-287. https://doi.org/10.3390/photochem3020016
Vallavoju R, Kore R, Parikirala R, Subburu M, Gade R, Kumar V, Raghavender M, Chetti P, Pola S. Synthesis and Characterization of New Tetradentate N2O2-Based Schiff’s Base Cu (II) Complexes for Dye Photodegradation. Photochem. 2023; 3(2):274-287. https://doi.org/10.3390/photochem3020016
Chicago/Turabian StyleVallavoju, Rohini, Ranjith Kore, Radhika Parikirala, Mahesh Subburu, Ramesh Gade, Vipin Kumar, Matta Raghavender, Prabhakar Chetti, and Someshwar Pola. 2023. "Synthesis and Characterization of New Tetradentate N2O2-Based Schiff’s Base Cu (II) Complexes for Dye Photodegradation" Photochem 3, no. 2: 274-287. https://doi.org/10.3390/photochem3020016
APA StyleVallavoju, R., Kore, R., Parikirala, R., Subburu, M., Gade, R., Kumar, V., Raghavender, M., Chetti, P., & Pola, S. (2023). Synthesis and Characterization of New Tetradentate N2O2-Based Schiff’s Base Cu (II) Complexes for Dye Photodegradation. Photochem, 3(2), 274-287. https://doi.org/10.3390/photochem3020016








