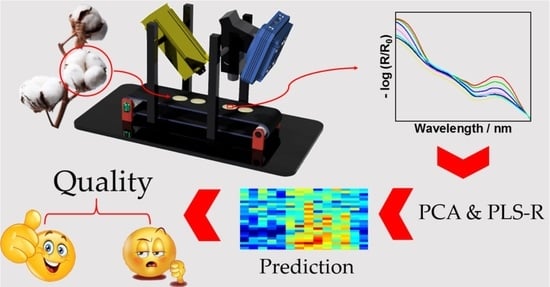
Applying UV Hyperspectral Imaging for the Quantification of Honeydew Content on Raw Cotton via PCA and PLS-R Models
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Preparation of Solutions and Samples
2.2. UV Hyperspectral Imaging Setup and Data Processing
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, H.; Memon, H. Cotton Science and Processing Technology; Springer Nature: Singapore, 2021; Volume 5, p. 565. [Google Scholar]
- EN 14278-2:2004; Textiles-Determination of Cotton Fibre Stickiness-Part 2: Method Using an Automatic Thermodetection Plate Device. DIN Deutsches Institut für Normung: Berlin, Germany, 2004.
- Al Ktash, M.; Hauler, O.; Ostertag, E.; Brecht, M. Ultraviolet-visible/near infrared spectroscopy and hyperspectral imaging to study the different types of raw cotton. J. Spectr. Imaging 2020, 9, a18. [Google Scholar] [CrossRef]
- Mansuri, A.; Somani, S.; Pathak, R. Effect of Waste Control on Yarn Parameters and Yield Improvement in Spinning Mill. J. Crit. Rev. 2020, 7, 2314–2322. [Google Scholar]
- Jiang, Y.; Li, C. Detection and discrimination of cotton foreign matter using push-broom based hyperspectral imaging: System design and capability. PLoS ONE 2015, 10, e0121969. [Google Scholar] [CrossRef]
- Barotova, A.; Xurramov, A.; Raxmatullayev, S.; Ismoilova, A. Evaluation of fiber quality indexes in different varieties of cotton plants. J. Agric. Hortic. 2023, 3, 41–46. [Google Scholar] [CrossRef]
- Bradow, J.M.; Davidonis, G.H. Quantitation of fiber quality and the cotton production-processing interface: A physiologist’s perspective. Cotton Sci. 2000, 4, 34–64. [Google Scholar]
- Campbell, B.T.; Hinze, L. Cotton production, processing and uses of cotton raw material. In Industrial Crops and Uses; CABI: Wallingford, UK, 2010; pp. 259–276. [Google Scholar]
- Chand, N.; Fahim, M. (Eds.) Cotton Reinforced Polymer Composites; Springer: Berlin/Heidelberg, Germany, 2008. [Google Scholar] [CrossRef]
- Afzal, M.I. Cotton stickiness–A marketing and processing problem: Improvement of the Marketability of Cotton Produced in Zones affected by Stickiness. In Proceedings of the Final Seminar, Lille, France, 2–4 July 2001; pp. 105–111. [Google Scholar]
- Bi, J.; Ballmer, G.; Hendrix, D.; Henneberry, T.; Toscano, N. Effect of cotton nitrogen fertilization on Bemisia argentifolii populations and honeydew production. Entomol. Exp. Appl. 2001, 99, 25–36. [Google Scholar] [CrossRef]
- Ghule, A.V.; Chen, R.K.; Tzing, S.H.; Lo, J.; Ling, Y.C. Simple and rapid method for evaluating stickiness of cotton using thermogravimetric analysis. Anal. Chim. Acta 2004, 502, 251–256. [Google Scholar] [CrossRef]
- Was-Gubala, J.; Starczak, R. Nondestructive identification of dye mixtures in polyester and cotton fibers using Raman spectroscopy and ultraviolet-visible (UV-Vis) microspectrophotometry. Appl. Spectrosc. 2015, 69, 296–303. [Google Scholar] [CrossRef]
- Abidi, N.; Hequet, E. Fourier transform infrared analysis of cotton contamination. Text. Res. J. 2007, 77, 77–84. [Google Scholar] [CrossRef]
- Chung, C.; Lee, M.; Choe, E. Characterization of cotton fabric scouring by FT-IR ATR spectroscopy. Carbohydr. Polym. 2004, 58, 417–420. [Google Scholar] [CrossRef]
- Fuhrer, L. Mapping of In-Field Cotton Fiber Quality Utilizing John Deere’s Harvest Identification System (Hid). Doctoral Dissertation, University of Georgia, Athens, GA, USA, 2022. [Google Scholar]
- Liu, Y. Recent Progress in Fourier Transform Infrared (FTIR) Spectroscopy Study of Compositional, Structural and Physical Attributes of Developmental Cotton Fibers. Materials 2013, 6, 299–313. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, J.; Beck, K. NIR characterization and measurement of the cotton content of dyed blend fabrics. Text. Res. J. 2009, 79, 675–686. [Google Scholar] [CrossRef]
- Al Ktash, M.; Stefanakis, M.; Wackenhut, F.; Jehle, V.; Ostertag, E.; Rebner, K.; Brecht, M. Prediction of Honeydew Contaminations on Cotton Samples by In-Line UV Hyperspectral Imaging. Sensors 2023, 23, 319. [Google Scholar] [CrossRef] [PubMed]
- Al Ktash, M.; Stefanakis, M.; Boldrini, B.; Ostertag, E.; Brecht, M. Characterization of Pharmaceutical Tablets Using UV Hyperspectral Imaging as a Rapid In-Line Analysis Tool. Sensors 2021, 21, 4436. [Google Scholar] [CrossRef] [PubMed]
- Al Ktash, M.; Stefanakis, M.; Englert, T.; Drechsel, M.S.; Stiedl, J.; Green, S.; Jacob, T.; Boldrini, B.; Ostertag, E.; Rebner, K.; et al. UV hyperspectral imaging as process analytical tool for the characterization of oxide layers and copper states on direct bonded copper. Sensors 2021, 21, 7332. [Google Scholar] [CrossRef]
- Boldrini, B.; Kessler, W.; Rebner, K.; Kessler, R.W. Hyperspectral imaging: A review of best practice, performance and pitfalls for in-line and on-line applications. J. Near Infrared Spectrosc. 2012, 20, 483–508. [Google Scholar] [CrossRef]
- Gowen, A.; Odonnell, C.; Cullen, P.; Downey, G.; Frias, J. Hyperspectral imaging—An emerging process analytical tool for food quality and safety control. Trends Food Sci. Technol. 2007, 18, 590–598. [Google Scholar] [CrossRef]
- Fischer, M.K.; Völkl, W.; Hoffmann, K.H. Honeydew production and honeydew sugar composition of polyphagous black bean aphid, Aphis fabae (Hemiptera: Aphididae) on various host plants and implications for ant-attendance. Eur. J. Entomol. 2005, 102, 155–160. [Google Scholar] [CrossRef]
- Hogervorst, P.A.; Wäckers, F.L.; Romeis, J. Effects of honeydew sugar composition on the longevity of Aphidius ervi. Entomol. Exp. Appl. 2007, 122, 223–232. [Google Scholar] [CrossRef]
- Victorita, B.; Marghitas, L.A.; Stanciu, O.; Laslo, L.; Dezmirean, D.; Bobis, O. High-performance liquid chromatographic analysis of sugars in Transylvanian honeydew honey. Bull. UASVM Anim. Sci. Biotechnol. 2008, 65, 229–232. [Google Scholar]
- Lottspeich, F.; Zorbas, H. Bioanalytik, 4th ed.; Spektrum, A., Ed.; Springer: Berlin/Heidelberg, Germany, 2022. [Google Scholar]
- Janshekar, H.; Brown, C.; Fiechter, A. Determination of biodegraded lignin by ultraviolet spectrophotometry. Anal. Chim. Acta 1981, 130, 81–91. [Google Scholar] [CrossRef]
- Sadeghifar, H.; Venditti, R.; Jur, J.; Gorga, R.E.; Pawlak, J.J. Cellulose-lignin biodegradable and flexible UV protection film. ACS Sustain. Chem. Eng. 2017, 5, 625–631. [Google Scholar] [CrossRef]
- Mach, H.; Volkin, D.B.; Burke, C.J.; Russell Middaugh, C. Ultraviolet absorption spectroscopy. In Protein Stability and Folding; Humana Press: Totowa, NJ, USA, 1995; pp. 91–114. [Google Scholar] [CrossRef]
- Mevik, H.; Wehrens, R. Introduction to the pls Package. J. Stat. Softw. 2023, 1–23. [Google Scholar]



| Sample Type | Sugar Concentration/wt % | msugar/mcotton |
|---|---|---|
| A | 4 | 0.4249 |
| B | 2 | 0.2413 |
| C | 1 | 0.1194 |
| D | 0.5 | 0.0609 |
| E | 0.25 | 0.02313 |
| F | 0.125 | 0.0126 |
| G | 0.0625 | 0.0143 |
| CLN | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Knoblich, M.; Al Ktash, M.; Wackenhut, F.; Jehle, V.; Ostertag, E.; Brecht, M. Applying UV Hyperspectral Imaging for the Quantification of Honeydew Content on Raw Cotton via PCA and PLS-R Models. Textiles 2023, 3, 287-293. https://doi.org/10.3390/textiles3030019
Knoblich M, Al Ktash M, Wackenhut F, Jehle V, Ostertag E, Brecht M. Applying UV Hyperspectral Imaging for the Quantification of Honeydew Content on Raw Cotton via PCA and PLS-R Models. Textiles. 2023; 3(3):287-293. https://doi.org/10.3390/textiles3030019
Chicago/Turabian StyleKnoblich, Mona, Mohammad Al Ktash, Frank Wackenhut, Volker Jehle, Edwin Ostertag, and Marc Brecht. 2023. "Applying UV Hyperspectral Imaging for the Quantification of Honeydew Content on Raw Cotton via PCA and PLS-R Models" Textiles 3, no. 3: 287-293. https://doi.org/10.3390/textiles3030019
APA StyleKnoblich, M., Al Ktash, M., Wackenhut, F., Jehle, V., Ostertag, E., & Brecht, M. (2023). Applying UV Hyperspectral Imaging for the Quantification of Honeydew Content on Raw Cotton via PCA and PLS-R Models. Textiles, 3(3), 287-293. https://doi.org/10.3390/textiles3030019