Dose-Dependent Effects of Boron on Photosynthetic and Oxidative Processes in Young Sugar Beet (Beta vulgaris L.) Plants
Abstract
1. Introduction
2. Results
2.1. Chlorophyll Content Estimation and Maximum Quantum Efficiency of PSII
2.2. Changes in Delayed Fluorescence (DF)
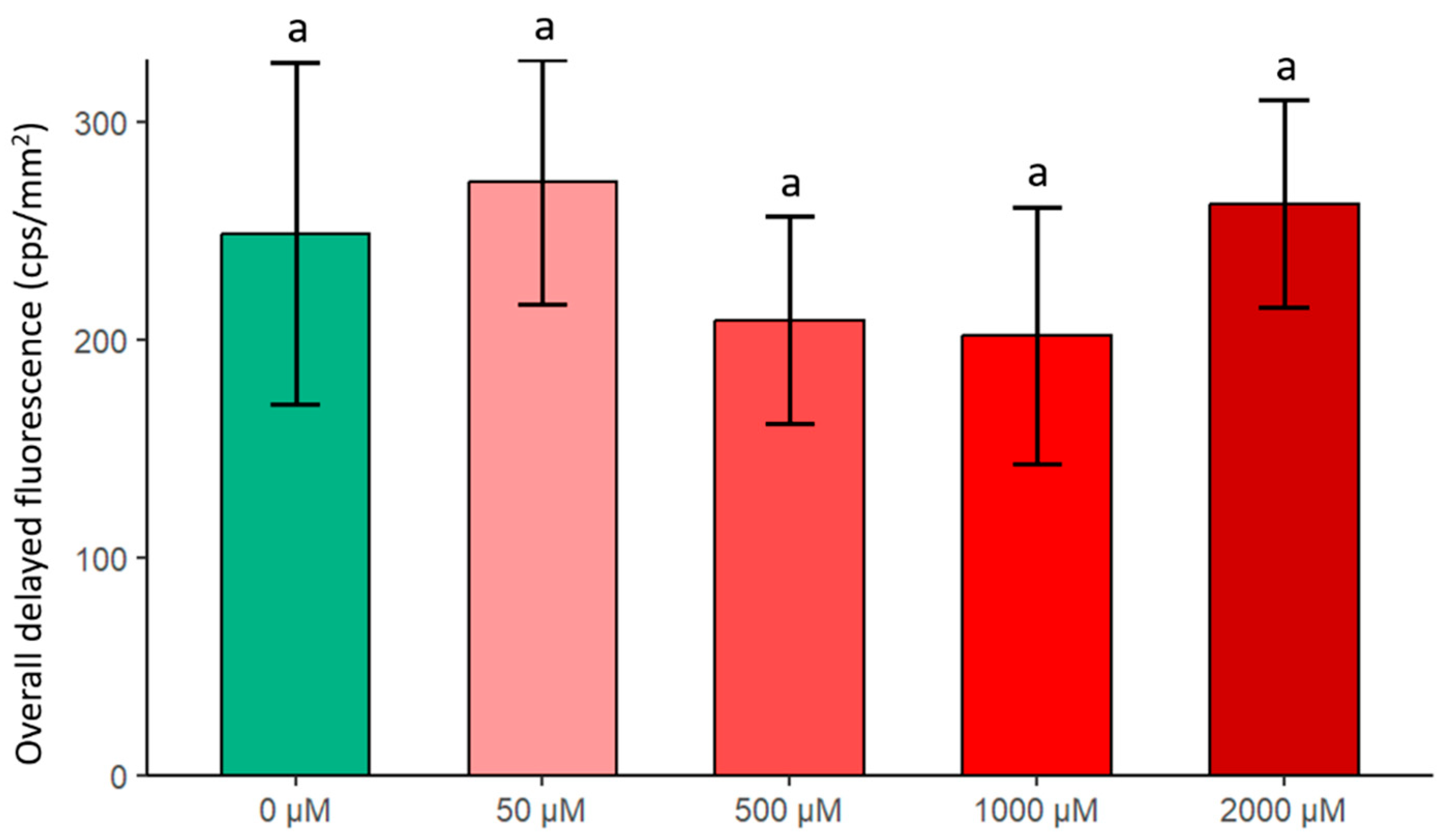
2.2.1. Changes in Overall Delayed Fluorescence (DF)
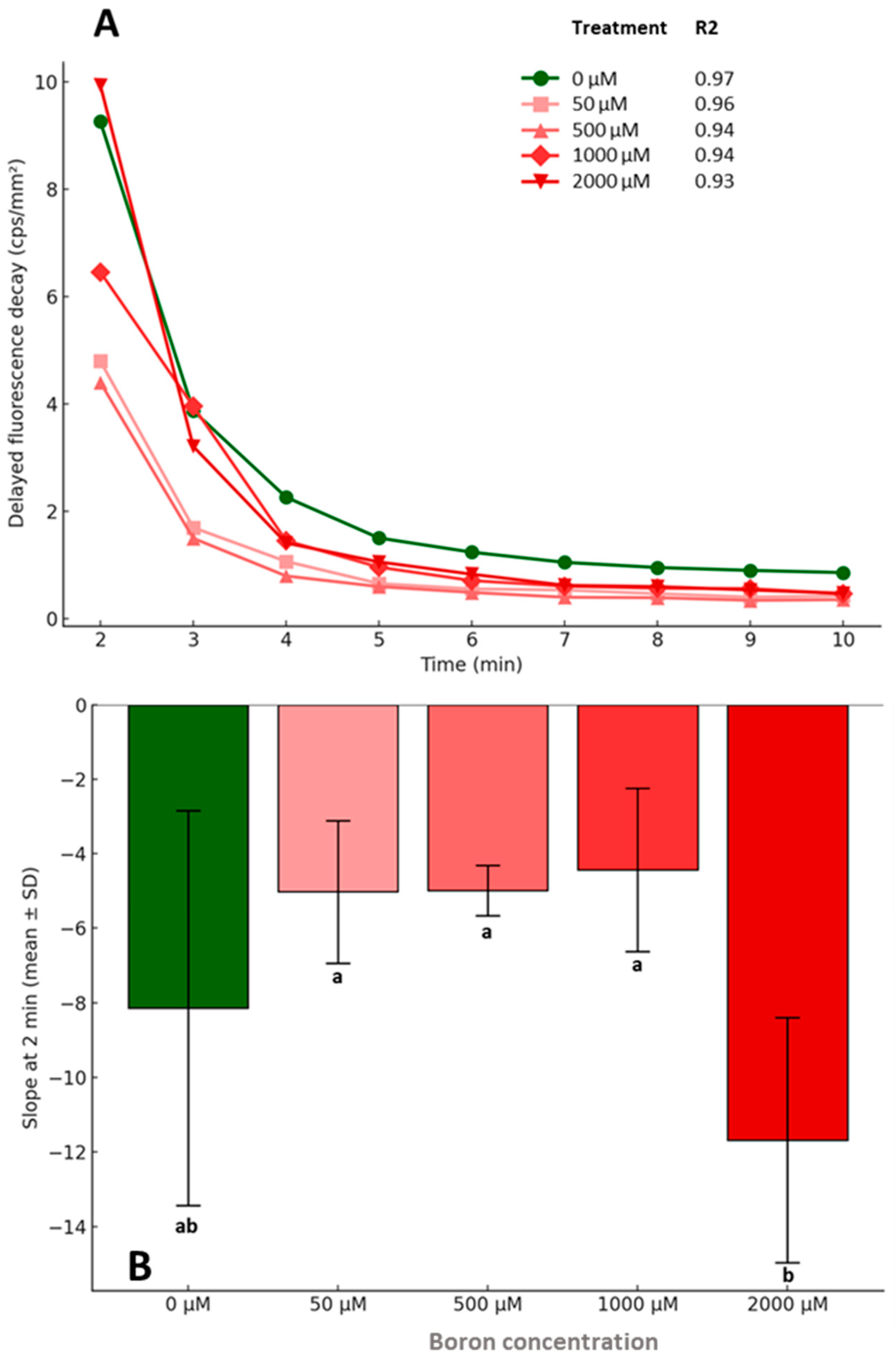
2.2.2. Changes in Delayed Fluorescence Decay
2.3. Changes in Lipidoxidation-Related Processes
2.3.1. Changes in FRAP
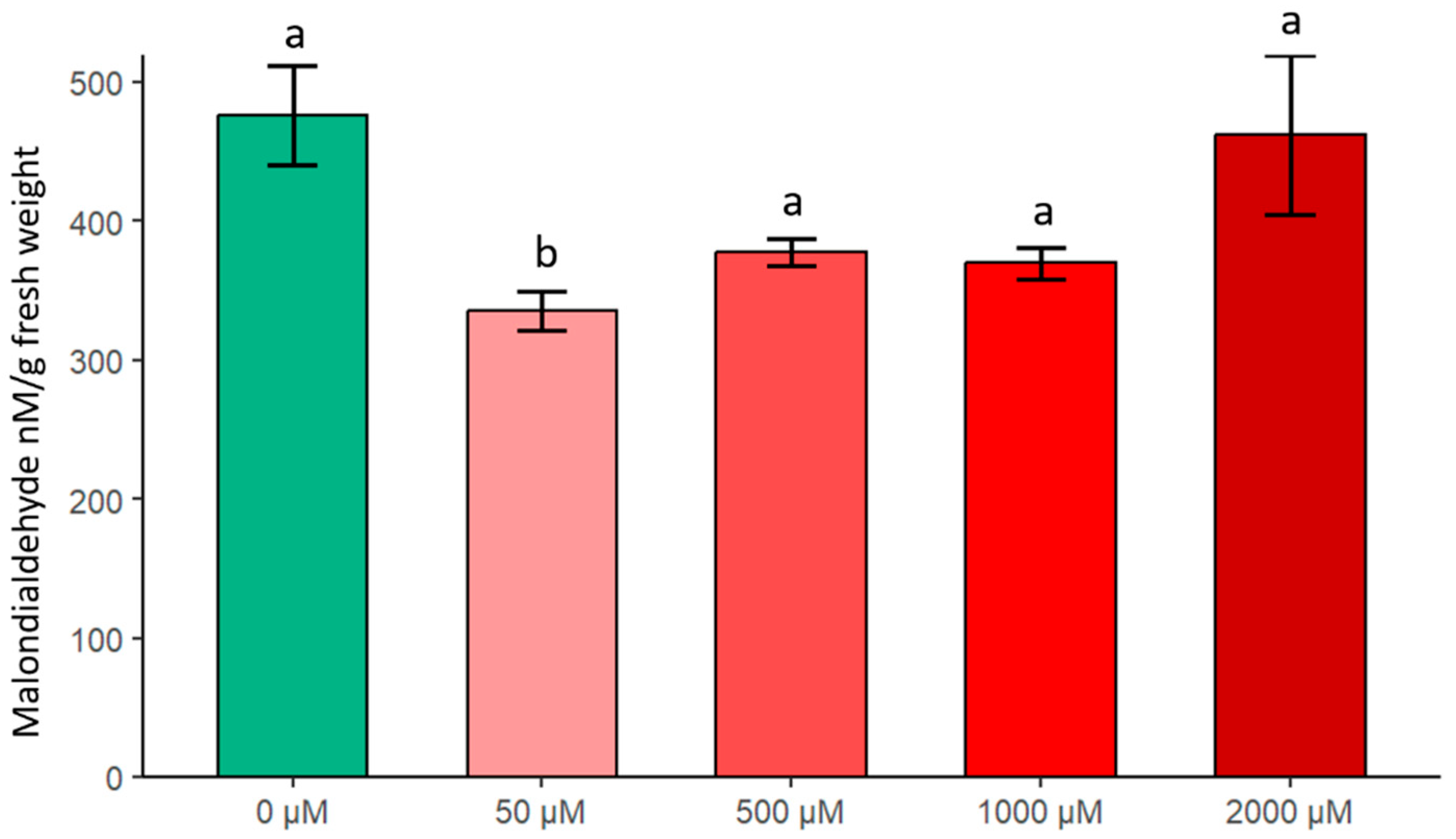
2.3.2. Changes in MDA
3. Discussion
3.1. Changes in Photosynthesis-Related Parameters: SPAD, Fv/Fm and DF
3.2. Changes in Lipidoxidation-Related Processes
4. Materials and Methods
4.1. Culture Conditions
4.2. Boron (B) Treatments
4.3. Non-Invasive Measurements
4.3.1. Estimation of Relative Chlorophyll Content
4.3.2. Measurement of Fv/Fm
4.3.3. Measurement of DF
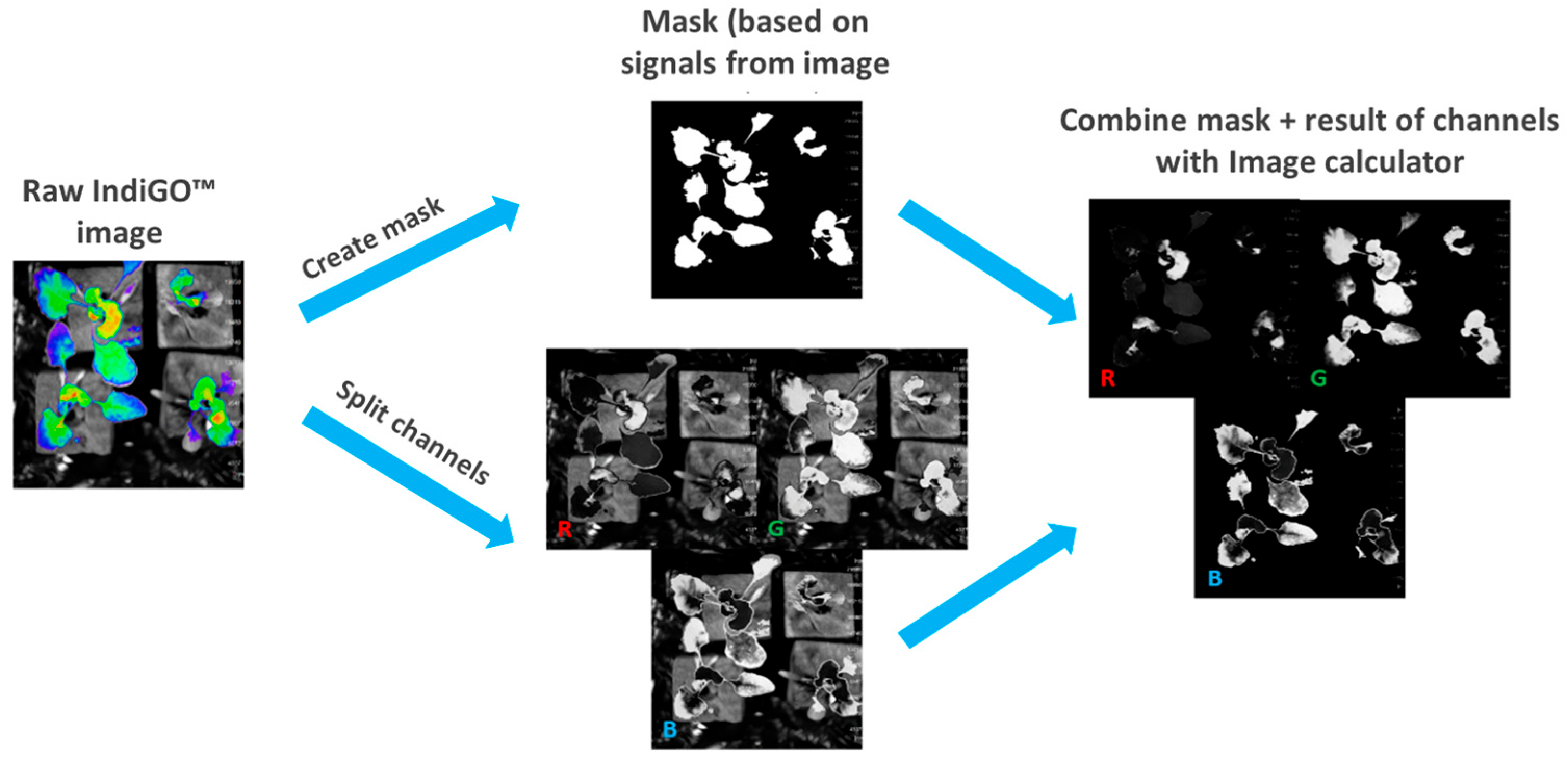
4.3.4. Image Processing and RGB Intensity Analysis
4.4. Measurement of FRAP
- Acetate buffer: 300 mM, pH 3.6
- TPTZ (2,4,6-tripyridyl-s-triazine) 10 mM in 40 mM HCl; 100 mL distilled water in 330 µL HCl
- FeCl3 × 6 H2O: 20 mM
4.5. Measurement of MDA
4.6. Statistical Analyses
- Kruskal–Wallis + Dunn post hoc with Holm correction;
- Welch ANOVA + Games–Howell post hoc;
- ANOVA + Tukey post hoc.
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AA | Ascorbic acid |
| B | Boron |
| cps | Count per second |
| DF | Delayed fluorescence |
| FRAP | Ferric reducing ability of plasma |
| Fv/Fm | Variable fluorescence over maximum fluorescence |
| HSB | Hue–Saturation–Brightness |
| MDA | Malondialdehyde |
| RGB | Red–Green–Blue |
References
- Food and Agriculture Organization of the United Nations. Available online: https://www.fao.org/faostat/en/#compare (accessed on 17 August 2025).
- Statista. Sugar Consumption Worldwide in 2023/2024, by Leading Country (in Million Metric Tons). Available online: https://www.statista.com/statistics/496002/sugar-consumption-worldwide/ (accessed on 17 August 2025).
- Hungarian Central Statistical Office. 19.1.1.12. Area Harvested of Major Arable Crops [Thousand Hectares]. Available online: https://www.ksh.hu/stadat_files/mez/hu/mez0012.html (accessed on 17 August 2025).
- Hoffmann, C.M.; Huijbregts, T.; van Swaaij, N.; Jansen, R. Impact of different environments in Europe on yield and quality of sugar beet genotypes. Eur. J. Agron. 2009, 30, 17–26. [Google Scholar] [CrossRef]
- Kiymaz, S.; Ertek, A. Yield and quality of sugar beet (Beta vulgaris L.) at different water and nitrogen levels under the climatic conditions of Kırsehir, Turkey. Agric. Water Manag. 2015, 158, 156–165. [Google Scholar] [CrossRef]
- Mekdad, A.A.A.; Rady, M.M. Response of Beta vulgaris L. to nitrogen and micronutrients in dry environment. Plant Soil Environ. 2016, 62, 23–29. [Google Scholar] [CrossRef]
- Mekdad, A.A.A.; Shaaban, A. Integrative applications of nitrogen, zinc, and boron to nutrients-deficient soil improves sugar beet productivity and technological sugar contents under semi-arid conditions. J. Plant Nutr. 2020, 43, 1935–1950. [Google Scholar] [CrossRef]
- Abdel-Motagally, F. Effect concentration and spraying time of boron on yield and quality traits of sugar beet grown in newly reclaimed soil conditions. Agric. Sci. 2015, 46, 15–26. [Google Scholar]
- Mahapatra, C.K.; Bhadra, T.; Paul, K.S. Nutrient management in sugar beet: A review. Pak. Sugar J. 2020, 35, 31–44. [Google Scholar] [CrossRef]
- Moustafa, Z.R.; Soudi, A.M.K.; Mohamed, K.E. Productivity and quality of sugar beet as influenced by nitrogen fertilizer and some micronutrients. Egypt. J. Agric. Res. 2011, 89, 1005–1017. [Google Scholar] [CrossRef]
- Rassam, G.; Dashti, M.; Dadkhah, A.; Yazdi, A.K. Root yield and quality of sugar beet in relation to foliar application of micronutrients. Ann. West Univ. Timişoara Ser. Biol. 2015, 2, 87–94. [Google Scholar]
- Zewail, R.M.Y.; El-Gmal, I.S.; Khaitov, B.; El-Desouky, H.S. Micronutrients through foliar application enhance growth, yield and quality of sugar beet (Beta vulgaris L.). J. Plant Nutr. 2020, 43, 2275–2285. [Google Scholar] [CrossRef]
- Song, X.; Wang, X.; Song, B.; Wu, Z.; Zhao, X.; Huang, W.; Riaz, M. Transcriptome Analysis Reveals the Molecular Mechanism of Boron Deficiency Tolerance in Leaves of Boron-Efficient Beta vulgaris Seedlings. Plant Physiol. Biochem. 2021, 168, 294–304. [Google Scholar] [CrossRef]
- Lewis, D.H. Boron: The essential element for vascular plants that never was. New Phytol. 2019, 221, 1685–1690. [Google Scholar] [CrossRef]
- Long, Y.; Peng, J. Interaction between Boron and Other Elements in Plants. Genes 2023, 14, 130. [Google Scholar] [CrossRef]
- Matoh, T. Boron in plant cell walls. Plant Soil 1997, 193, 59–70. [Google Scholar] [CrossRef]
- Shireen, F.; Nawaz, M.A.; Chen, C.; Zhang, Q.; Zheng, Z.; Sohail, H.; Sun, J.; Cao, H.; Huang, Y.; Bie, Z. Boron: Functions and approaches to enhance its availability in plants for sustainable agriculture. Int. J. Mol. Sci. 2018, 19, 1856. [Google Scholar] [CrossRef] [PubMed]
- Camacho-Cristóbal, J.J.; Herrera-Rodríguez, M.B.; Beato, V.M.; Rexach, J.; Navarro-Gochicoa, M.T.; Maldonado, J.M.; González-Fontes, A. The expression of several cell wall-related genes in Arabidopsis roots is down-regulated under boron deficiency. Environ. Exp. Bot. 2008, 63, 351–358. [Google Scholar] [CrossRef]
- Voxeur, A.; Fry, S.C. Glycosylinositol phosphorylceramides from Rosa cell cultures are boron-bridged in the plasma membrane and form complexes with rhamnogalacturonan II. Plant J. 2014, 79, 139–149. [Google Scholar] [CrossRef]
- Ryden, P.; Sugimoto-Shirasu, K.; Smith, A.C.; Findlay, K.; Reiter, W.D.; McCann, M.C. Tensile properties of Arabidopsis cell walls depend on both a xyloglucan cross-linked microfibrillar network and rhamnogalacturonan II-borate complexes. Plant Physiol. 2003, 132, 1033–1040. [Google Scholar] [CrossRef]
- Funakawa, H.; Miwa, K. Synthesis of borate cross-linked rhamnogalacturonan II. Front. Plant Sci. 2015, 6, 223. [Google Scholar] [CrossRef] [PubMed]
- Brdar-Jokanović, M. Boron toxicity and deficiency in agricultural plants. Int. J. Mol. Sci. 2020, 21, 1424. [Google Scholar] [CrossRef] [PubMed]
- Kohli, S.K.; Kaur, H.; Khanna, K.; Handa, N.; Bhardwaj, R.; Rinklebe, J.; Ahmad, P. Boron in plants: Uptake, deficiency and biological potential. Plant Growth Regul. 2023, 100, 267–282. [Google Scholar] [CrossRef]
- Dordas, C.; Brown, P.H. Boron deficiency affects cell viability, phenolic leakage and oxidative burst in rose cell cultures. Plant Soil 2005, 268, 293–301. [Google Scholar] [CrossRef]
- Song, B.; Hao, X.; Wang, X.; Yang, S.; Dong, Y.; Ding, Y.; Wang, Q.; Wang, X.; Zhou, J. Boron stress inhibits beet (Beta vulgaris L.) growth through influencing endogenous hormones and oxidative stress response. Soil Sci. Plant Nutr. 2019, 65, 346–352. [Google Scholar] [CrossRef]
- Han, S.; Tang, N.; Jiang, H.X.; Yang, L.T.; Li, Y.; Chen, L.S. CO2 assimilation, photosystem II photochemistry, carbohydrate metabolism and antioxidant system of citrus leaves in response to boron stress. Plant Sci. 2009, 176, 143–153. [Google Scholar] [CrossRef]
- Lu, Y.B.; Qi, Y.P.; Yang, L.T.; Lee, J.; Guo, P.; Ye, X.; Jia, M.Y.; Li, M.L.; Chen, L.S. Long-term boron-deficiency-responsive genes revealed by cDNA-AFLP differ between Citrus sinensis roots and leaves. Front. Plant Sci. 2015, 6, 585. [Google Scholar] [CrossRef] [PubMed]
- Rao, M.J.; Duan, M.; Zhou, C.; Jiao, J.; Cheng, P.; Yang, L.; Wei, W.; Shen, Q.; Ji, P.; Yang, Y.; et al. Antioxidant Defense System in Plants: Reactive Oxygen Species Production, Signaling, and Scavenging During Abiotic Stress-Induced Oxidative Damage. Horticulturae 2025, 11, 477. [Google Scholar] [CrossRef]
- Melgar, J.C.; Guidi, L.; Remorini, D.; Agati, G.; Degl’Innocenti, E.; Castelli, S.; Baratto, M.C.; Faraloni, C.; Tattini, M. Antioxidant defences and oxidative damage in salt-treated olive plants under contrasting sunlight irradiance. Tree Physiol. 2009, 29, 1187–1198. [Google Scholar] [CrossRef]
- Landi, M.; Degl’Innocenti, E.; Pardossi, A.; Guidi, L. Antioxidant and photosynthetic responses in plants under boron toxicity: A review. Am. J. Agric. Biol. Sci. 2012, 7, 255–270. [Google Scholar] [CrossRef]
- de Souza, J.P., Jr.; de Mello Prado, R.; Campos, C.N.S.; Junior, G.D.S.S.; Costa, M.G.; de Pádua Teixeira, S.; Gratão, P.L. Silicon modulate the non-enzymatic antioxidant defence system and oxidative stress in a similar way as boron in boron-deficient cotton flowers. Plant Physiol. Biochem. 2023, 197, 107594. [Google Scholar] [CrossRef]
- Pereira, G.L.; Siqueira, J.A.; Batista-Silva, W.; Cardoso, F.B.; Nunes-Nesi, A.; Araújo, W.L. Boron: More Than an Essential Element for Land Plants? Front. Plant Sci. 2021, 11, 610307. [Google Scholar] [CrossRef] [PubMed]
- Sommer, A.L.; Sorokin, H. Effects of the absence of boron and of some other essential elements on the cell and tissue structure of the root tips of Pisum sativum. Plant Physiol. 1928, 3, 237–251. [Google Scholar] [CrossRef]
- Bolaños, L.; Abreu, I.; Bonilla, I.; Camacho-Cristóbal, J.J.; Reguera, M. What Can Boron Deficiency Symptoms Tell Us about Its Function and Regulation? Plants 2023, 12, 777. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Dong, X.; Liu, L.; Wu, L.; Peng, S.; Jiang, C. Boron deficiency is correlated with changes in cell wall structure that lead to growth defects in the leaves of navel orange plants. Sci. Hortic. 2014, 176, 54–62. [Google Scholar] [CrossRef]
- Camacho-Cristóbal, J.J.; Rexach, J.; González-Fontes, A. Boron in plants: Deficiency and toxicity. J. Integr. Plant Biol. 2008, 50, 1247–1255. [Google Scholar] [CrossRef]
- Khan, M.K.; Pandey, A.; Hamurcu, M.; Avsaroglu, Z.Z.; Ozbek, M.; Omay, A.H.; Elbasan, F.; Omay, M.R.; Gokmen, F.; Topal, A.; et al. Variability in Physiological Traits Reveals Boron Toxicity Tolerance in Aegilops Species. Front. Plant Sci. 2021, 12, 736614. [Google Scholar] [CrossRef]
- Riaz, M.; Kamran, M.; El-Esawi, M.A.; Hussain, S.; Wang, X. Boron-toxicity induced changes in cell wall components, boron forms, and antioxidant defense system in rice seedlings. Ecotoxicol. Environ. Saf. 2021, 216, 112192. [Google Scholar] [CrossRef]
- Pandey, A.; Khan, M.K.; Hamurcu, M.; Brestic, M.; Topal, A.; Gezgin, S. Insight into the Root Transcriptome of a Boron-Tolerant Triticum zhukovskyi Genotype Grown under Boron Toxicity. Agronomy 2022, 12, 2421. [Google Scholar] [CrossRef]
- Huo, J.; Song, B.; Riaz, M.; Song, X.; Li, J.; Liu, H.; Huang, W.; Jia, Q.; Wu, W. High boron stress leads to sugar beet (Beta vulgaris L.) toxicity by disrupting photosystem II. Ecotoxicol. Environ. Saf. 2022, 248, 114295. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Song, B.; Huo, J.; Liu, H.; Adil, M.F.; Jia, Q.; Wu, W.; Kuerban, A.; Wang, Y.; Huang, W. Effect of boron deficiency on the photosynthetic performance of sugar beet cultivars with contrasting boron efficiencies. Front. Plant Sci. 2023, 13, 1101171. [Google Scholar] [CrossRef]
- Wu, Z.; Wang, X.; Song, B.; Zhao, X.; Du, J.; Huang, W. Responses of Photosynthetic Performance of Sugar Beet Varieties to Foliar Boron Spraying. Sugar Tech 2021, 23, 1332–1339. [Google Scholar] [CrossRef]
- Galeriani, T.M.; Neves, G.O.; Ferreira, J.H.S.; Oliveira, R.N.; Oliveira, S.L.; Calonego, J.C.; Crusciol, C.A.C. Calcium and Boron Fertilization Improves Soybean Photosynthetic Efficiency and Grain Yield. Plants 2022, 11, 2937. [Google Scholar] [CrossRef]
- Oikonomou, A.; Ladikou, E.-V.; Chatziperou, G.; Margaritopoulou, T.; Landi, M.; Sotiropoulos, T.; Araniti, F.; Papadakis, I.E. Boron excess imbalances root/shoot allometry, photosynthetic and chlorophyll fluorescence parameters and sugar metabolism in apple plants. Agronomy 2019, 9, 731. [Google Scholar] [CrossRef]
- Li, Z.; Zhou, J.; Dong, T.; Xu, Y.; Shang, Y. Application of electrochemical methods for the detection of abiotic stress biomarkers in plants. Biosens. Bioelectron. 2021, 182, 113105. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.-X.; Liu, S.-Y.; Guo, W.-Y.; Dong, J.; Nan, J.-X.; Lin, H.-Y.; Mei, L.-C.; Yang, W.-C.; Yang, G.-F. In vivo diagnostics of abiotic plant stress responses via in situ real-time fluorescence imaging. Plant Physiol. 2022, 190, 196–201. [Google Scholar] [CrossRef]
- Jócsák, I.; Malgwi, I.; Rabnecz, G.; Szegő, A.; Varga-Visi, É.; Végvári, G.; Pónya, Z. Effect of cadmium stress on certain physiological parameters, antioxidative enzyme activities and biophoton emission of leaves in barley (Hordeum vulgare L.) seedlings. PLoS ONE 2020, 15, e0240470. [Google Scholar] [CrossRef] [PubMed]
- Berden-Zrimec, M.; Drinovec, L.; Zrimec, A.; Tišler, T. Delayed fluorescence in algal growth inhibition tests. Cent. Eur. J. Biol. 2007, 2, 169–181. [Google Scholar] [CrossRef]
- Jócsák, I.; Csima, F.; Somfalvi-Tóth, K. Alterations of Photosynthetic and Oxidative Processes Influenced by the Presence of Different Zinc and Cadmium Concentrations in Maize Seedlings: Transition from Essential to Toxic Functions. Plants 2024, 13, 1150. [Google Scholar] [CrossRef]
- Song, X.; Hao, X.; Song, B.; Zhao, X.; Wu, Z.; Wang, X.; Du, J.; Huang, W.; Riaz, M.; Li, X.; et al. The Oxidative Damage and Morphological Changes of Sugar Beet (Beta vulgaris L.) Leaves at Seedlings Stage Exposed to Boron Deficiency in Hydroponics. Sugar Tech 2022, 24, 532–541. [Google Scholar] [CrossRef]
- Song, X.; Song, B.; Huo, J.; Riaz, M.; Wang, X.; Huang, W.; Zhao, S. Boron-Efficient Sugar Beet (Beta vulgaris L.) Cultivar Improves Tolerance to Boron Deficiency by Improving Leaf Traits. J. Soil Sci. Plant Nutr. 2022, 22, 4217–4227. [Google Scholar] [CrossRef]
- Porcel, R.; Bustamante, A.; Ros, R.; Serrano, R.; Mulet Salort, J.M. BvCOLD1: A novel aquaporin from sugar beet (Beta vulgaris L.) involved in boron homeostasis and abiotic stress. Plant Cell Environ. 2018, 41, 2844–2857. [Google Scholar] [CrossRef]
- Ali, M.M.; Bachik, N.A.; Muhadi, N.A.; Yusof, T.N.T.; Gomes, C. Non-destructive techniques of detecting plant diseases: A review. Physiol. Mol. Plant Pathol. 2019, 108, 101426. [Google Scholar] [CrossRef]
- Keszthelyi, S.; Pónya, Z.; Csóka, Á.; Bázár, G.; Morschhauser, T.; Donkó, T. Non-destructive imaging and spectroscopic techniques to investigate the hidden-lifestyle arthropod pests: A review. J. Plant Dis. Prot. 2020, 127, 283–295. [Google Scholar] [CrossRef]
- Ducournau, S.; Charrier, A.; Demilly, D.; Wagner, M.-H.; Trigui, G.; Dupont, A.; Hamdy, S.; Boudehri-Giresse, K.; Le Corre, L.; Landais, L.; et al. High throughput phenotyping dataset related to seed and seedling traits of sugar beet genotypes. Data Brief 2020, 29, 105201. [Google Scholar] [CrossRef]
- Yi, J.; Krusenbaum, L.; Unger, P.; Hüging, H.; Seidel, S.J.; Schaaf, G.; Gall, J. Deep learning for non-invasive diagnosis of nutrient deficiencies in sugar beet using RGB images. Sensors 2020, 20, 5893. [Google Scholar] [CrossRef]
- Lukács, H.; Jócsák, I.; Somfalvi-Tóth, K.; Keszthelyi, S. Physiological Responses Manifested by Some Conventional Stress Parameters and Biophoton Emission in Winter Wheat as a Consequence of Cereal Leaf Beetle Infestation. Front. Plant Sci. 2022, 13, 839855. [Google Scholar] [CrossRef] [PubMed]
- Jócsák, I.; Gyalog, H.; Hoffmann, R.; Somfalvi-Tóth, K. In-Vivo Biophoton Emission, Physiological and Oxidative Responses of Biostimulant-Treated Winter Wheat (Triticum aestivum L.) as Seed Priming Possibility, for Heat Stress Alleviation. Plants 2022, 11, 640. [Google Scholar] [CrossRef]
- Chaerle, L.; Hagenbeek, D.; de Bruyne, E.; van der Straeten, D. Chlorophyll fluorescence imaging for disease-resistance screening of sugar beet. Plant Cell Tissue Organ Cult. 2007, 91, 97–106. [Google Scholar] [CrossRef]
- Goltsev, V.; Zaharieva, I.; Chernev, P.; Strasser, R. Delayed Chlorophyll Fluorescence as a Monitor for Physiological State of Photosynthetic Apparatus. Biotechnol. Biotechnol. Equip. 2009, 23 (Suppl. S1), 452–457. [Google Scholar] [CrossRef]
- Zhou, R.; Kan, X.; Chen, J.; Hua, H.; Li, Y.; Ren, J.; Feng, K.; Liu, H.; Deng, D.; Yin, Z. Drought-induced changes in photosynthetic electron transport in maize probed by prompt fluorescence, delayed fluorescence, P700 and cyclic electron flow signals. Environ. Exp. Bot. 2019, 158, 51–62. [Google Scholar] [CrossRef]
- Sánchez-Moreiras, A.M.; Graña, E.; Reigosa, M.J.; Araniti, F. Imaging of Chlorophyll a Fluorescence in Natural Compound-Induced Stress Detection. Front. Plant Sci. 2020, 11, 583590. [Google Scholar] [CrossRef] [PubMed]
- Riccio, M.; Resca, E.; Bertoni, L.; Cavani, F.; Sena, P.; Ferretti, M.; Baldini, A.; Palumbo, C.; de Pol, A. RGB method in immunofluorescence investigations on stem cells. Opt. Laser Technol. 2011, 43, 317–322. [Google Scholar] [CrossRef]
- Kior, A.; Yudina, L.; Zolin, Y.; Sukhov, V.; Sukhova, E. RGB Imaging as a Tool for Remote Sensing of Characteristics of Terrestrial Plants: A Review. Plants 2024, 13, 1262. [Google Scholar] [CrossRef]
- Sánchez-Sastre, L.F.; Alte da Veiga, N.M.S.; Ruiz-Potosme, N.M.; Carrión-Prieto, P.; Marcos-Robles, J.L.; Navas-Gracia, L.M.; Martín-Ramos, P. Assessment of RGB Vegetation Indices to Estimate Chlorophyll Content in Sugar Beet Leaves in the Final Cultivation Stage. AgriEngineering 2020, 2, 128–149. [Google Scholar] [CrossRef]
- Ripoll, J.; Bertin, N.; Bidel, L.P.R.; Urban, L. A user’s view of the parameters derived from the induction curves of maximal chlorophyll a fluorescence: Perspectives for analyzing stress. Front. Plant Sci. 2016, 7, 1679. [Google Scholar] [CrossRef] [PubMed]
- Sen, A.; Alikamanoglu, S. Characterization of drought-tolerant sugar beet mutants induced with gamma radiation using biochemical analysis and isozyme variations. J. Sci. Food Agric. 2014, 94, 367–372. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Song, B.; Wu, Z.; Zhao, X.; Song, X.; Adil, M.F.; Huang, W. Insights into Physiological and Molecular Mechanisms Underlying Efficient Utilization of Boron in Different Boron Efficient Beta vulgaris L. Varieties. Plant Physiol. Biochem. 2023, 197, 107619. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- R Core Team. R Core Team R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023. [Google Scholar]
- de Mendiburu, F.; Yaseen, M. Agricolae: Statistical Procedures for Agricultural Research; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis, 2nd ed.; Springer International Publishing: Berlin/Heidelberg, Germany, 2016; pp. 187–253. [Google Scholar]
- Ogle, D. FSA: Simple Fisheries Stock Assessment Methods. 2023. Available online: https://fishr-core-team.github.io/FSA/ (accessed on 17 August 2025).





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Csima, F.; Hoffmann, R.; Kazinczi, G.; Jócsák, I. Dose-Dependent Effects of Boron on Photosynthetic and Oxidative Processes in Young Sugar Beet (Beta vulgaris L.) Plants. Stresses 2025, 5, 61. https://doi.org/10.3390/stresses5040061
Csima F, Hoffmann R, Kazinczi G, Jócsák I. Dose-Dependent Effects of Boron on Photosynthetic and Oxidative Processes in Young Sugar Beet (Beta vulgaris L.) Plants. Stresses. 2025; 5(4):61. https://doi.org/10.3390/stresses5040061
Chicago/Turabian StyleCsima, Ferenc, Richárd Hoffmann, Gabriella Kazinczi, and Ildikó Jócsák. 2025. "Dose-Dependent Effects of Boron on Photosynthetic and Oxidative Processes in Young Sugar Beet (Beta vulgaris L.) Plants" Stresses 5, no. 4: 61. https://doi.org/10.3390/stresses5040061
APA StyleCsima, F., Hoffmann, R., Kazinczi, G., & Jócsák, I. (2025). Dose-Dependent Effects of Boron on Photosynthetic and Oxidative Processes in Young Sugar Beet (Beta vulgaris L.) Plants. Stresses, 5(4), 61. https://doi.org/10.3390/stresses5040061






