Identifying Winter Light Stress in Conifers Using Proximal Hyperspectral Imaging and Machine Learning
Abstract
1. Introduction
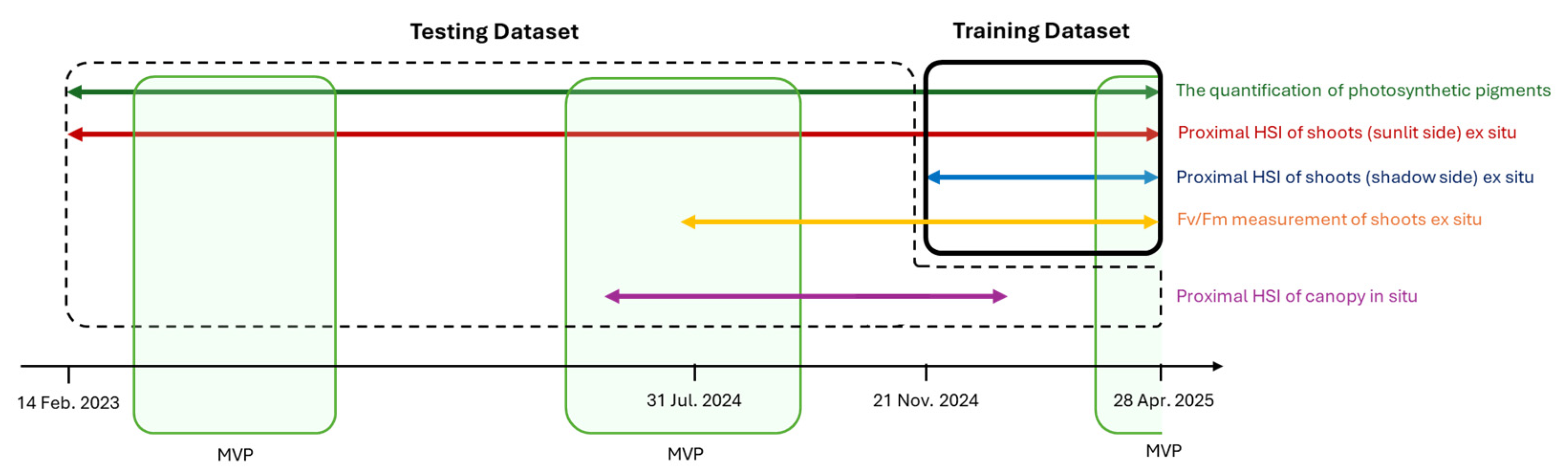
- Time series of proximal HSI data of the sunlit side of conifers shoots at intervals of 7–10 days for the period from 14 February 2023 to 28 April 2025.
- Time series of proximal HSI data of the shaded side of conifers shoots at intervals of 7–10 days for the period from 21 November 2024 to 28 April 2025.
- Time series of photosynthetic pigment content in sunlit shoots for the period from 14 February 2023 to 28 April 2025.
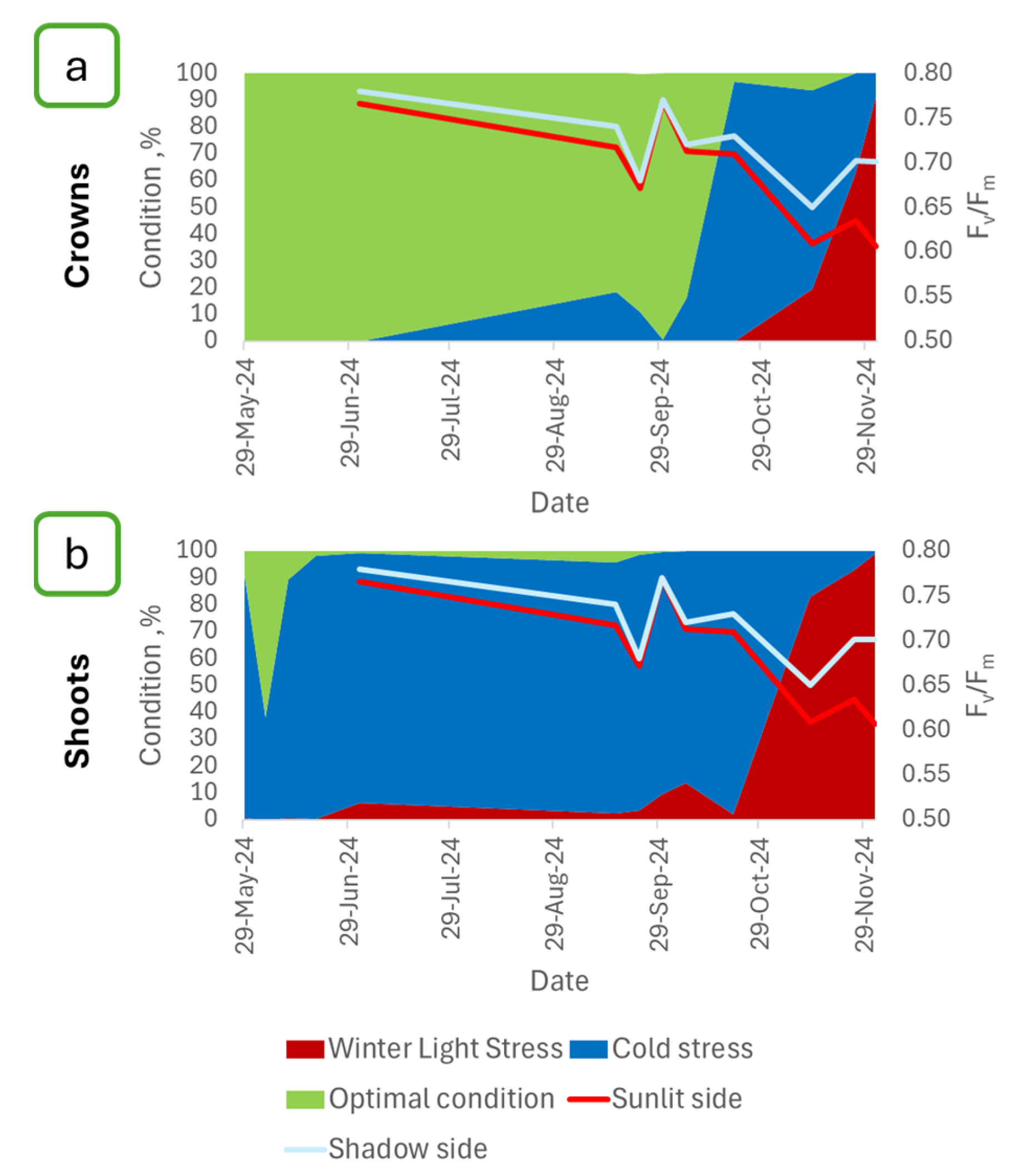
- Time series of maximum quantum yield of PSII (Fv/Fm) of photosynthesis of sunlit and shaded sides of shoots for the period from 31 July 2024 to 28 April 2025.
2. Results
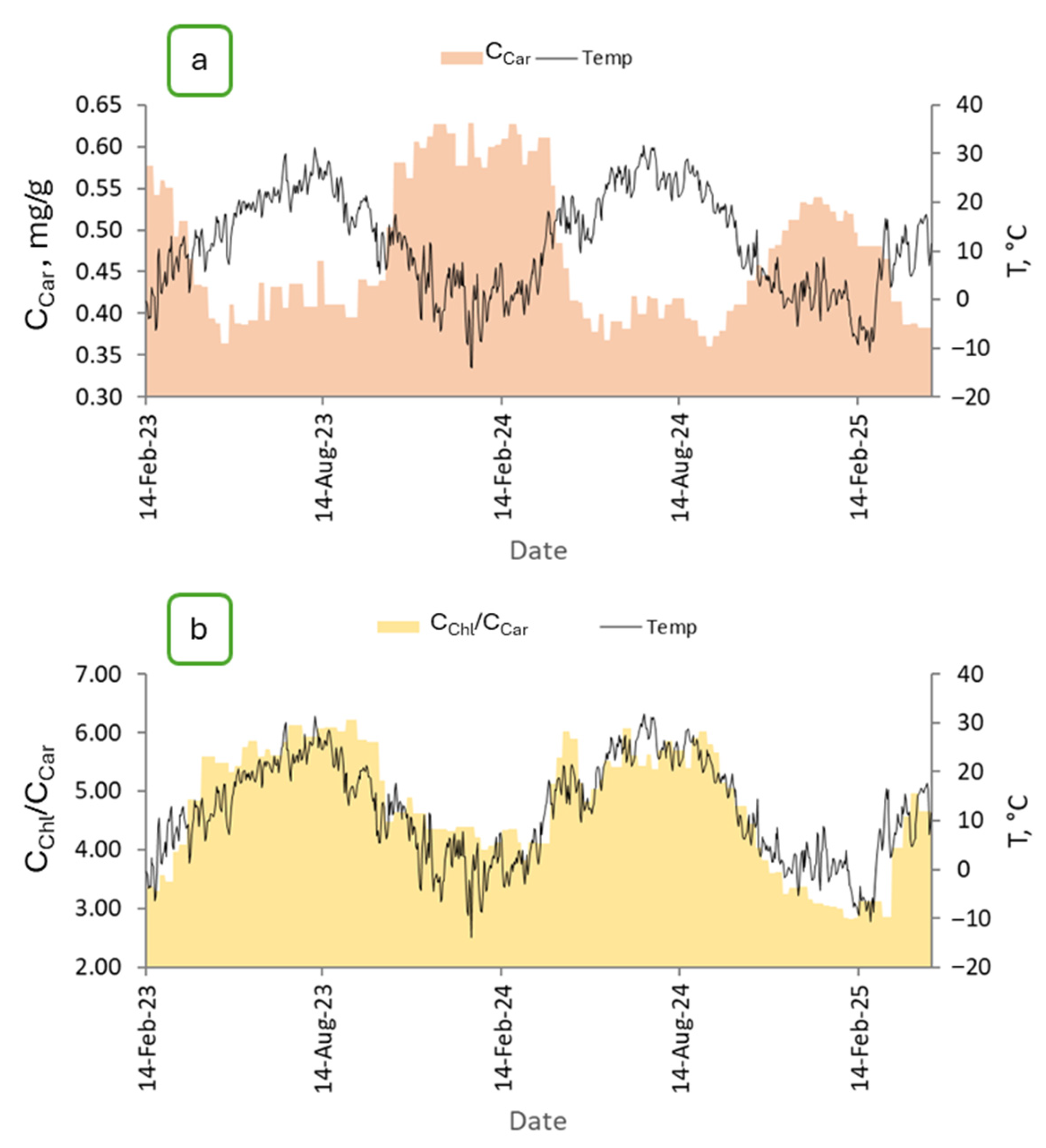
2.1. The Nature of the Annual Cycle of Carotenoids and the Ratio of Chlorophylls to Carotenoids of P. orientalis Shoots
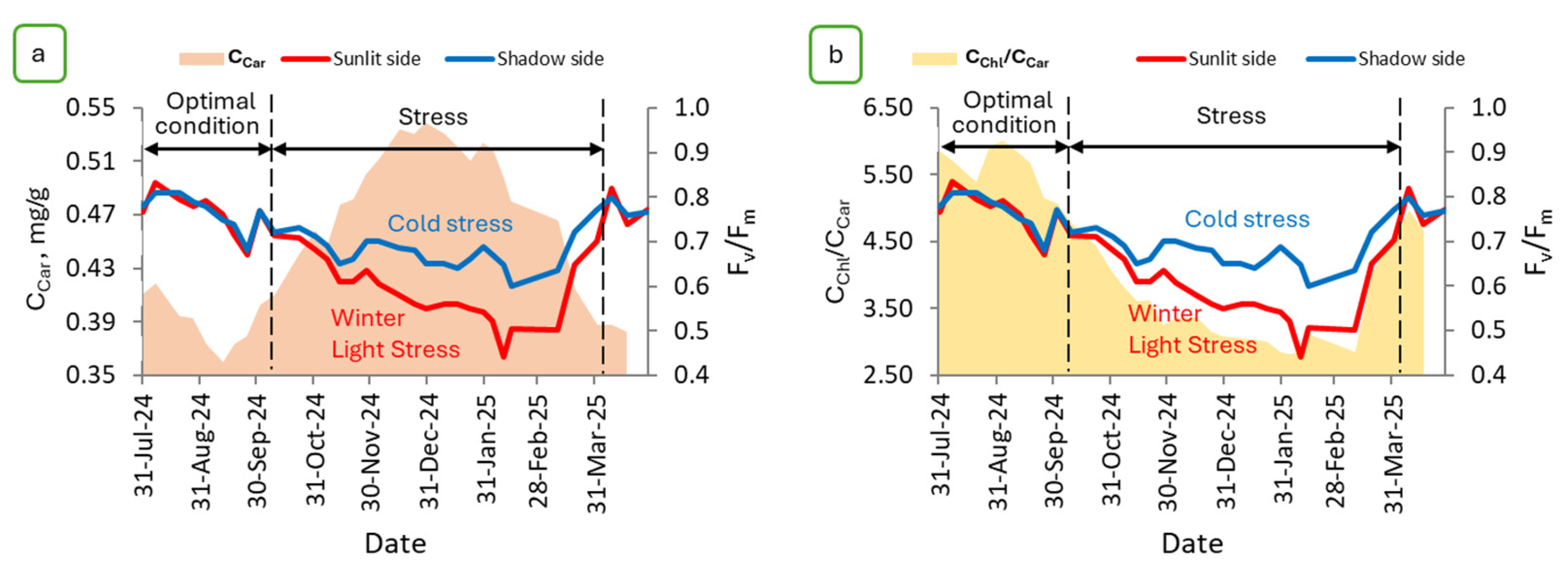
2.2. Estimation of the Maximum Quantum Yield of PSII Shoots of P. orientalis
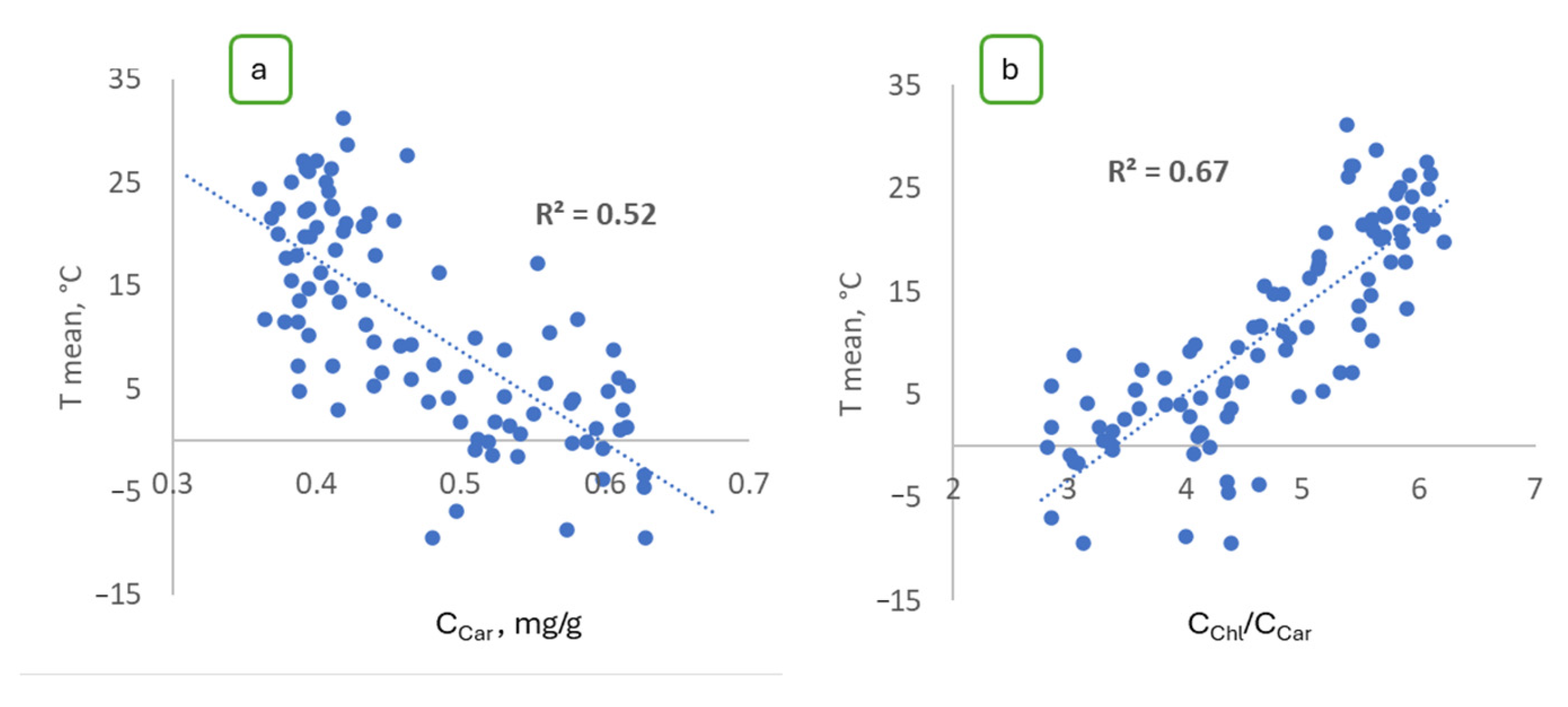
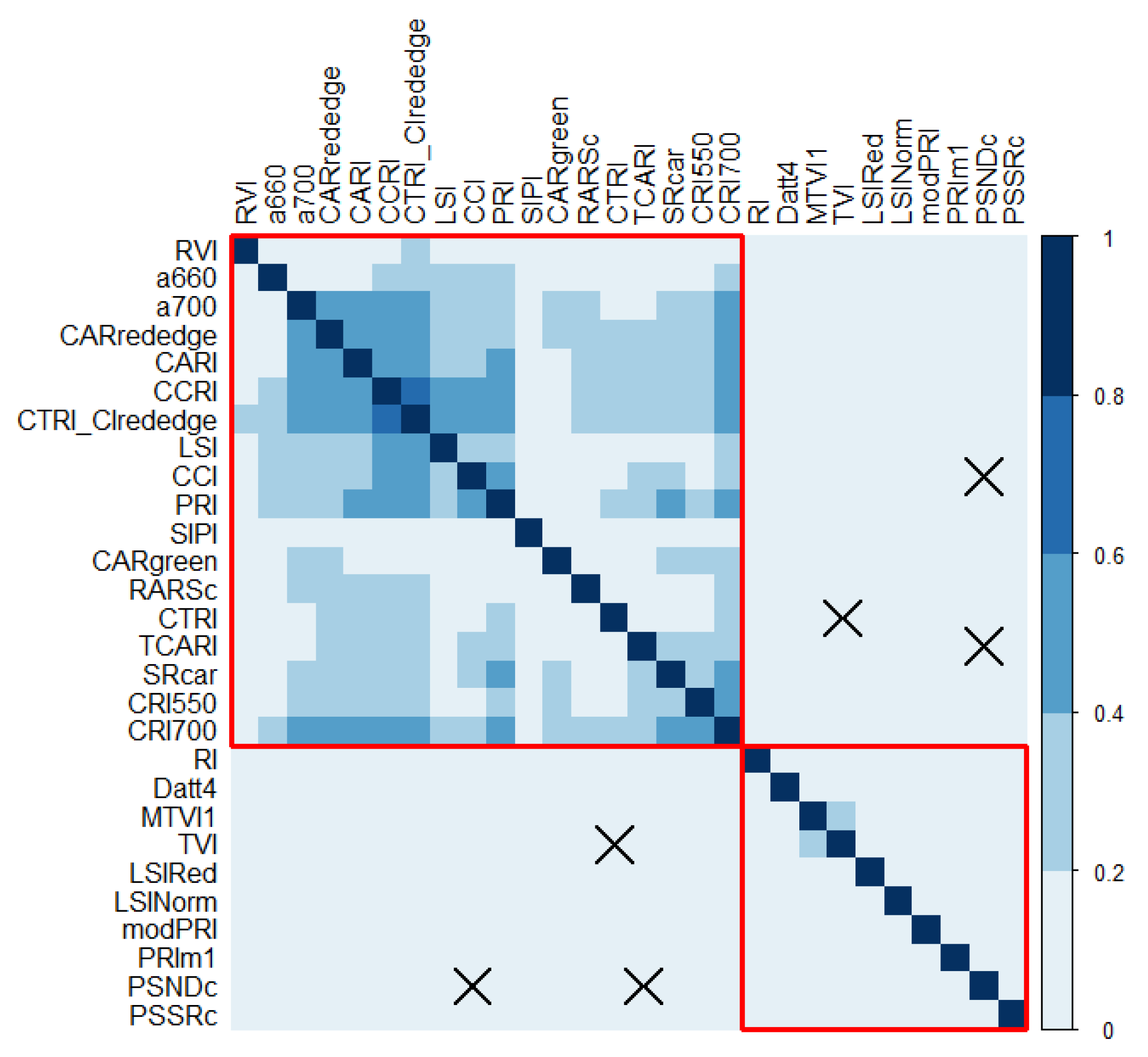
2.3. The Results of the Exploratory Data Analysis for Vegetation Indices
2.4. Results of Modeling the Light Stress of P. orientalis Shoots
2.5. Results of Testing the RF Model on Platycladus orientalis Crowns
3. Discussion
4. Materials and Methods
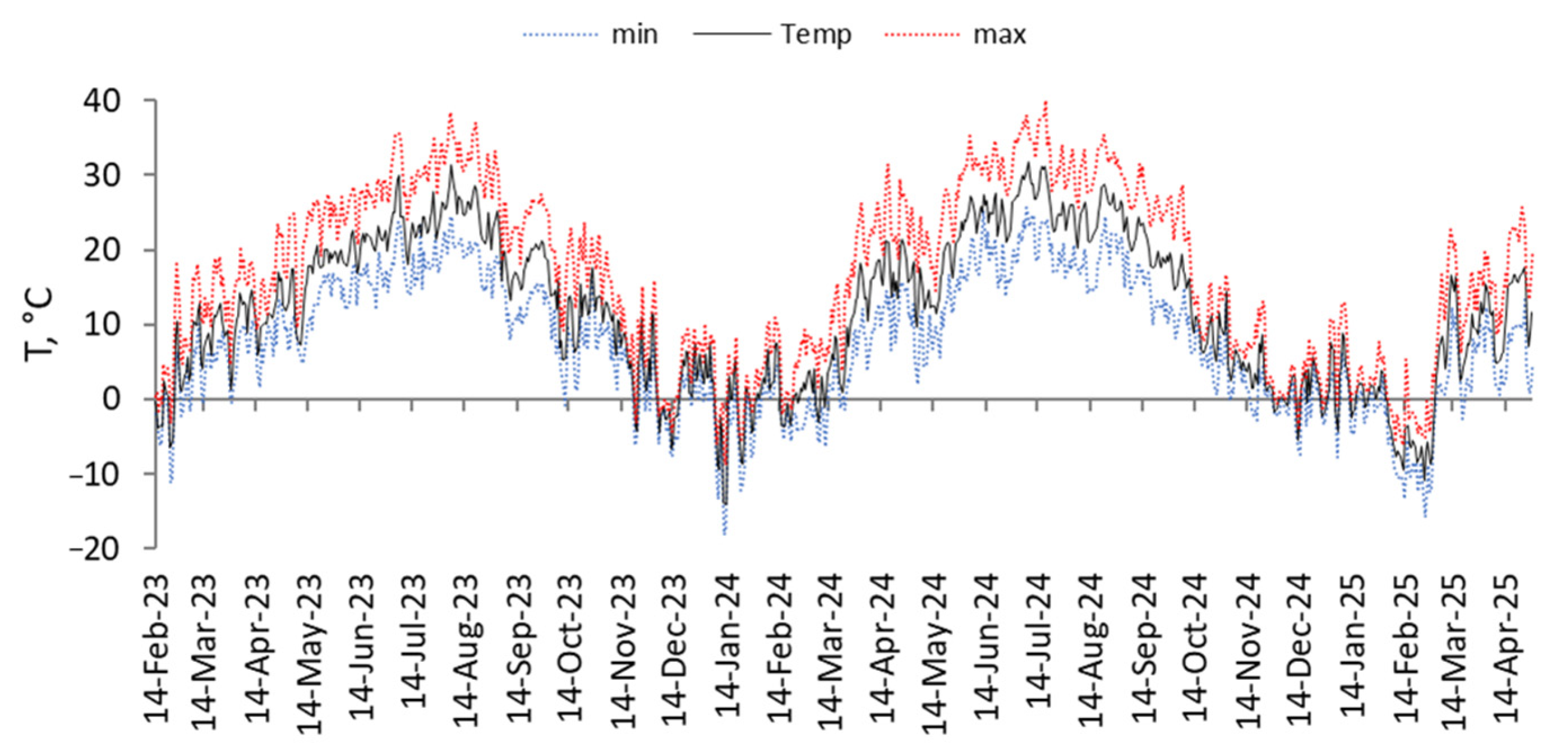
4.1. Experiment Timing
4.2. Study Area
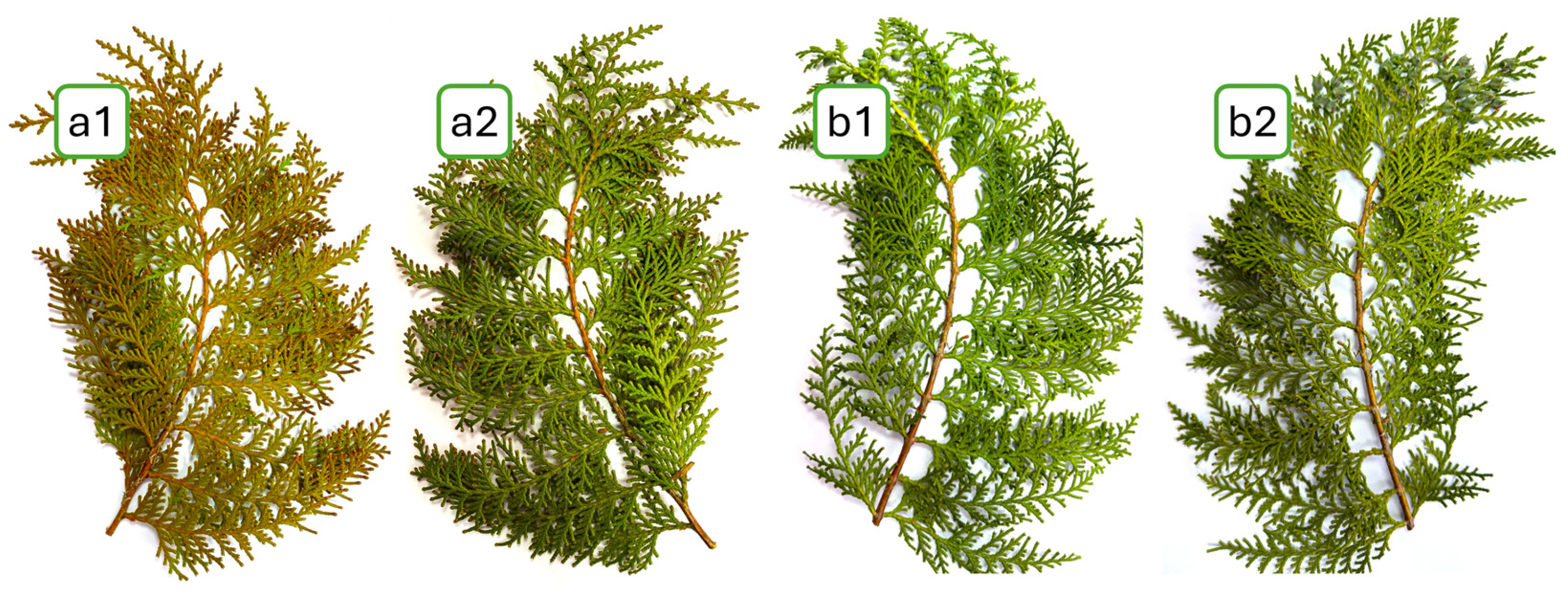
4.3. Object of Study
4.4. Sampling of P. orientalis Shoots for Laboratory Research
4.5. Hyperspectral Imaging Methodology
4.6. Preprocessing of Hyperspectral Imagery Data
4.7. Determination of Photosynthetic Pigment Content
4.8. Measurement of Maximum Quantum Yields of Photosystem II (Fv/Fm)
4.9. Data Analytics
5. Limitations of the Study
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chakhvashvili, E.; Machwitz, M.; Antala, M.; Rozenstein, O.; Prikaziuk, E.; Schlerf, M.; Naethe, P.; Wan, Q.; Komárek, J.; Klouek, T.; et al. Crop stress detection from UAVs: Best practices and lessons learned for exploiting sensor synergies. Precis. Agric. 2024, 25, 2614–2642. [Google Scholar] [CrossRef]
- Kashyap, B.; Kumar, R. Sensing Methodologies in Agriculture for Monitoring Biotic Stress in Plants Due to Pathogens and Pests. Inventions 2021, 6, 29. [Google Scholar] [CrossRef]
- Kramer, K.; Bouriaud, L.; Feindt, P.H.; van Wassenaer, L.; Glanemann, N.; Hanewinkel, M.; van der Heide, M.; Hengeveld, G.M.; Hoogstra, M.; Ingram, V.; et al. Roadmap to develop a stress test for forest ecosystem services supply. One Earth 2022, 5, 25–34. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, Y.; Zhu, J.-K. Thriving under Stress: How Plants Balance Growth and the Stress Response. Dev. Cell 2020, 55, 529–543. [Google Scholar] [CrossRef]
- Yadav, S.; Modi, P.; Dave, A.; Vijapura, A.; Patel, D.; Patel, M. Effect of Abiotic Stress on Crops. In Sustainable Crop Production; IntechOpen: London, UK, 2020. [Google Scholar]
- Dey, S.; Raichaudhuri, A. Abiotic Stress in Plants; Intech Open: London, UK, 2022. [Google Scholar] [CrossRef]
- Ghosh, P.; Nandi, R.; Das, S.; Roy, A.; Parvin, S.; Dalal, D.; Dalal, D. Plants Response to Abiotic Stress: A Review. Sarcouncil J. Plant Agron. 2023, 1, 1–6. [Google Scholar] [CrossRef]
- Liu, M.; Wang, Y.; Zhang, H.; Hao, Y.; Wu, H.; Shen, H.; Zhang, P. Mechanisms of photoprotection in overwintering evergreen conifers: Sustained quenching of chlorophyll fluorescence. Plant Physiol. Biochem. 2024, 210, 108638. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.Y.; Bräutigam, K.; Hüner, N.P.A.; Ensminger, I. Champions of winter survival: Cold acclimation and molecular regulation of cold hardiness in evergreen conifers. New Phytol. 2020, 229, 675–691. [Google Scholar] [CrossRef] [PubMed]
- Ensminger, I.; Berninger, F.; Streb, P. Response of photosynthesis to low temperature. In Terrestrial Photosynthesis in Achanging Environment: A Molecular, Physiological, and Ecological Approach; Flexas, J., Loreto, F., Medrano, H., Eds.; Cambridge University Press: Cambridge, UK, 2012; pp. 276–293. [Google Scholar]
- Öquist, G.; Hüner, N.P.A. Photosynthesis of overwintering evergreen plants. Annu. Rev. Plant Biol. 2003, 54, 329–355. [Google Scholar] [CrossRef] [PubMed]
- Crosatti, C.; Rizza, F.; Badeck, F.W.; Mazzucotelli, E.; Cattivelli, L. Harden the chloroplast to protect the plant. Physiol. Plant. 2013, 147, 55–63. [Google Scholar] [CrossRef]
- Shavnin, S.A.; Yusupov, I.A.; Marina, N.V.; Montile, A.A.; Golikov, D.Y. Seasonal changes in chlorophyll and carotenoid content in needles of scots pines (Pinus sylvestris L.) Exposed to the thermal field of a gas flare. Russ. J. Plant Physiol. 2021, 68, 526–535. [Google Scholar] [CrossRef]
- William, W.A.; Zarter, C.R.; Ebbert, V.; Demmig-Adams, B. Photoprotective Strategies of Overwintering Evergreens. BioScience 2004, 54, 41–49. [Google Scholar] [CrossRef]
- Shi, Y.; Ke, X.; Yang, X.; Liu, Y.; Hou, X. Plants response to light stress. J. Genet. Genom. 2022, 49, 735–747. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Ming, Y.; Wang, H.-B.; Jin, H.-L. Strategies for adaptation to high light in plants. Abiotech 2024, 5, 381–393. [Google Scholar] [CrossRef] [PubMed]
- Szymanska, R.; Slesak, I.; Orzechowska, A.; Kruk, J. Physiological and biochemical responses to high light and temperature stress in plants. Environ. Exp. Bot. 2017, 139, 165–177. [Google Scholar] [CrossRef]
- Baránková, B.; Lazár, D.; Nauš, J. Analysis of the effect of chloroplast arrangement on optical properties of green tobacco leaves. Remote Sens. Environ. 2016, 174, 181–196. [Google Scholar] [CrossRef]
- Araguirang, G.E.; Richter, A.S. Activation of anthocyanin biosynthesis in high light–what is the initial signal? New Phytol. 2022, 236, 2037–2043. [Google Scholar] [CrossRef]
- Ottander, C.; Campbell, D.; Oquist, G. Seasonal changes in photosystem II organisation and pigment composition in Pinus sylvestris. Planta 1995, 197, 176–183. [Google Scholar] [CrossRef]
- Zhu, H.; Li, X.; Zhai, W.; Liu, Y.; Gao, Q.; Liu, J.; Ren, L.; Chen, H.; Zhu, Y. Effects of low light on photosynthetic properties, antioxidant enzyme activity, and anthocyanin accumulation in purple pak-choi (Brassica campestris ssp. Chinensis Makino). PLoS ONE 2017, 12, e0179305. [Google Scholar] [CrossRef]
- Ruban, A.V. Nonphotochemical chlorophyll fluorescence quenching: Mechanism and effectiveness in protecting plants from photodamage. Plant Physiol. 2016, 170, 1903–1916. [Google Scholar] [CrossRef]
- Frechette, E.; Chang, C.Y.; Ensminger, I. Photoperiod and temperature constraints on the relationship between the photochemical reflectance index (PRI) and the light-use efficiency of photosynthesis in Pinus strobus. Tree Physiol. 2016, 36, 311–324. [Google Scholar] [CrossRef]
- Latowski, D.; Kuczyґnska, P.; Strzałka, K. Xanthophyll cycle—A mechanism protecting plants against oxidative stress. Redox Rep. 2011, 16, 78–90. [Google Scholar] [CrossRef]
- Adams, R.W.W.; Demmig-Adams, B.; Rosenstiel, T.N.; Ebbert, V. Dependence of photosynthesis and energy dissipation activity upon growth form and light environment during the winter. Photosynth. Res. 2001, 67, 51–62. [Google Scholar] [CrossRef]
- Sveshnikov, D.; Ensminger, I.; Ivanov, A.G.; Campbell, D.; Lloyd, J.; Funk, C.; Hüner, N.P.A.; Öquist, G. Excitation energy partitioning and quenching during cold acclimation in Scots pine. Tree Physiol. 2006, 26, 325–336. [Google Scholar] [CrossRef]
- Busch, F.A. Photorespiration in the context of Rubisco biochemistry, CO2 diffusion and metabolism. Plant J. 2020, 101, 919–939. [Google Scholar] [CrossRef] [PubMed]
- Murata, N.; Nishiyama, Y. ATP is a driving force in the repair of photosystem II during photoinhibition. Plant Cell Environ. 2018, 41, 285–299. [Google Scholar] [CrossRef] [PubMed]
- Kothari, S.; Schweiger, A.K. Plant spectra as integrative measures of plant phenotypes. J. Ecol. 2022, 110, 2536–2554. [Google Scholar] [CrossRef]
- Solovchenko, A.; Shurygin, B.; Nesterov, D.A.; Sorokin, D.V. Towards the synthesis of spectral imaging and machine learning-based approaches for non-invasive phenotyping of plants. Biophys. Rev. 2023, 15, 939–946. [Google Scholar] [CrossRef] [PubMed]
- Okyere, F.G.; Cudjoe, D.K.; Virlet, N.; Castle, M.; Riche, A.B.; Greche, L.; Mohareb, F.; Simms, D.; Mhada, M.; Hawkesford, M.J. Hyperspectral Imaging for Phenotyping Plant Drought Stress and Nitrogen Interactions Using Multivariate Modeling and Machine Learning Techniques in Wheat. Remote Sens. 2024, 16, 3446. [Google Scholar] [CrossRef]
- Dmitriev, P.A.; Kozlovsky, B.L.; Dmitrieva, A.A.; Varduni, T.V.; Lysenko, V.S. Light Stress Detection in Ficus elastica with Hyperspectral Indices. AgriEngineering 2024, 6, 3297–3311. [Google Scholar] [CrossRef]
- Bhargava, A.; Sachdeva, A.; Sharma, K.; Alsharif, M.H.; Uthansakul, P.; Uthansakul, M. Hyperspectral imaging and its applications: A review. Heliyon 2024, 10, 33208. [Google Scholar] [CrossRef]
- Akewar, M.; Chandak, M. Hyperspectral Imaging Algorithms and Applications: A Review. TechRxiv. 2024. [Google Scholar] [CrossRef]
- Montero, D.; Aybar, C.; Mahecha, M.D.; Martinuzzi, F.; Söchting, M.; Wieneke, S. A standardized catalogue of spectral indices to advance the use of remote sensing in Earth system research. Sci. Data 2023, 10, 197. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Su, B. Significant Remote Sensing Vegetation Indices: A Review of Developments and Applications. J. Sens. 2017, 2017, 1353691. [Google Scholar] [CrossRef]
- César, H.-H.; Goulden, M.L. Plant Traits Help Explain the Tight Relationship between Vegetation Indices and Gross Primary Production. Remote Sens. 2020, 12, 1405. [Google Scholar] [CrossRef]
- Farella, M.M.; Barnes, M.L.; Breshears, D.D.; Mitchell, J.; van Leeuwen, W.J.D.; Gallery, R.E. Evaluation of Vegetation Indices and Imaging Spectroscopy to Estimate Foliar Nitrogen across Disparate Biomes. Ecosphere 2022, 13, e3992. [Google Scholar] [CrossRef]
- Shumaila, K.; Chandni, Z.; Tayyaba, R.; Sara, A.; Aimen, A.; Bushra, A.; Mujahid, A. The significance of chlorophylls and carotenoids in enhancing seed tolerance to abiotic stress. Biol. Clin. Sci. Res. J. 2024, 5, 1081. [Google Scholar] [CrossRef]
- Zhou, X.; Huang, W.; Zhang, J.; Kong, W.; Casa, R.; Huang, Y. A novel combined spectral index for estimating the ratio of carotenoid to chlorophyll content to monitor crop physiological and phenological status. Int. J. Appl. Earth Obs. Geoinf. 2019, 76, 128–142. [Google Scholar] [CrossRef]
- He, C.; Sun, J.; Chen, Y.; Wang, L.; Shi, S.; Qiu, F.; Tagesson, T. A new vegetation index combination for leaf carotenoid-to-chlorophyll ratio: Minimizing the effect of their correlation. Int. J. Digit. Earth 2023, 16, 272–288. [Google Scholar] [CrossRef]
- Wang, H.; Shi, R.; Liu, P.D.; Gao, W. Dual NDVI Ratio Vegetation Index: A Kind of Vegetation Index Assessing Leaf Carotenoid Content Based on Leaf Optical Properties Model. Guang Pu Xue Yu Guang Pu Fen Xi 2016, 36, 2189–2194. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Zygielbaum, A.I.; Arkebauer, T.J.; Walter-Shea, E.A.; Solovchenko, A. Stress detection in vegetation based on remotely sensed light absorption coefficient. Int. J. Remote Sens. 2024, 45, 259–277. [Google Scholar]
- Chappelle, E.W.; Kim, M.S.; McMurtrey, J.E., III. Ratio analysis of reflectance spectra (RARS): An algorithm for the remote estimation of the concentrations of chlorophyll a, chlorophyll b, and carotenoids in soybean leaves. Remote Sens. Environ. 1992, 39, 239–247. [Google Scholar] [CrossRef]
- Blackburn, G.A. Spectral indices for estimating photosynthetic pigment concentrations: A test using senescent tree leaves. Int. J. Remote Sens. 1998, 19, 657–675. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Zur, Y.; Chivkunova, O.B.; Merzlyak, M.N. Assessing Carotenoid Content in Plant Leaves with Reflectance Spectroscopy. Photochem. Photobiol. 2002, 75, 272–281. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Keydan, G.P.; Merzlyak, M.N. Three-band model for noninvasive estimation of chlorophyll, carotenoids, and anthocyanin contents in higher plant leaves. Geophys. Res. Lett. 2006, 33, L11402. [Google Scholar] [CrossRef]
- Zhou, X.; Wenjiang, H.; Weiping, K.; Huichun, Y.; Yingying, D.; Raffaele, C. Assessment of leaf carotenoids content with a new carotenoid index: Development and validation on experimental and model data. Int. J. Appl. Earth Obs. Geoinf. 2017, 57, 24–35. [Google Scholar] [CrossRef]
- Weiping, K.; Huang, W.; Zhou, X.; Song, X.; Casa, R. Estimation of carotenoid content at the canopy scale using the carotenoid triangle ratio index fromin situand simulated hyperspectral data. J. Appl. Remote Sens. 2016, 10, 026035. [Google Scholar] [CrossRef]
- Gamon, J.A.; Penuelas, J.; Field, C.B. A narrow-waveband spectral index that tracks diurnal changes in photosynthetic efficiency. Remote Sens. Environ. 1992, 41, 35–44. [Google Scholar] [CrossRef]
- Broge, N.H.; Leblanc, E. Comparing prediction power and stability of broadband and hyperspectral vegetation indices for estimation of green leaf area index and canopy chlorophyll density. Remote Sens. Environ. 2001, 76, 156–172. [Google Scholar] [CrossRef]
- Haboudane, D.; Miller, J.R.; Tremblay, N.; Zarco-Tejada, P.J.; Dextraze, L. Integrated narrow-band vegetation indices for prediction of crop chlorophyll content for application to precision agriculture. Remote Sens. Environ. 2002, 81, 416–426. [Google Scholar] [CrossRef]
- Hernandez-Clemente, R.; Navarro-Cerrillo, R.M.; Suбrez, L.; Morales, F.; Zarco-Tejada, P.J. Assessing structural effects on PRI for stress detection in conifer forests. Remote Sens. Environ. 2011, 115, 2360–2375. [Google Scholar] [CrossRef]
- Hernandez-Clemente, R.; Navarro-Cerrillo, R.M.; Zarco-Tejada, P.J. Carotenoid content estimation in a heterogeneous conifer forest using narrowband indices and PROSPECT + DART simulations. Remote Sens. Environ. 2012, 127, 298–315. [Google Scholar] [CrossRef]
- Haboudane, D. Hyperspectral vegetation indices and novel algorithms for predicting green LAI of crop canopies: Modeling and validation in the context of precision agriculture. Remote Sens. Environ. 2004, 90, 337–352. [Google Scholar] [CrossRef]
- Datt, B. Remote Sensing of Chlorophyll a, Chlorophyll b, Chlorophyll a+b, and Total Carotenoid Content in Eucalyptus Leaves. Remote Sens. Environ. 1998, 66, 111–121. [Google Scholar] [CrossRef]
- Chen, S.; Kosugi, Y.; Jiao, L.; Sakabe, A.; Epron, D.; Nakaji, T.; Noda, H.; Hikosaka, K.; Nasahara, K.N. Winter leaf reddening and photoprotection accessed by vegetation indices and its influence on canopy light-use efficiency of a Japanese cypress (Chamaecyparis obtusa) forest. Agric. For. Meteorol. 2025, 363, 110427. [Google Scholar] [CrossRef]
- Escadafal, R.; Huete, A. Etude des propriétés spectrales des sols arides appliquée à l′amélioration des indices de végétation obtenus par télédétection. Comptes Rendus L′Académie Sci. 1991, 312, 1385–1391. [Google Scholar]
- Gitelson, A. Towards a generic approach to remote non-invasive estimation of foliar carotenoid-tochlorophyll ratio. J. Plant Physiol. 2020, 252, 153227. [Google Scholar] [CrossRef]
- Yang, F.; Li, J.; Gan, X.; Qian, Y.; Wu, X.; Yang, Q. Assessing nutritional status of Festuca arundinacea by monitoring photosynthetic pigments from hyperspectral data. Comput. Electron. Agric. 2010, 70, 52–59. [Google Scholar] [CrossRef]
- Gamon, J.A.; Huemmrich, K.F.; Wong, C.Y.; Ensminger, I.; Garrity, S.; Hollinger, D.Y.; Noormets, A.; Penuelas, J. A remotely sensed pigment index reveals photosynthetic phenology in evergreen conifers. Proc. Natl. Acad. Sci. USA 2016, 113, 13087–13092. [Google Scholar] [CrossRef]
- Penuelas, J.; Baret, F.; Filella, I. Semi-empirical indices to assess carotenoids/chlorophyll a ratio from leaf spectral reflectance. Photosynthetica 1995, 31, 221–230. [Google Scholar]
- Dmitriev, P.A.; Kozlovsky, B.L.; Dmitrieva, A.A.; Lysenko, V.S.; Chokheli, V.A.; Varduni, T.V. Indication of Light Stress in Ficus elastica Using Hyperspectral Imaging. AgriEngineering 2023, 5, 2253–2265. [Google Scholar] [CrossRef]
- Dmitriev, P.A.; Kozlovsky, B.L.; Dmitrieva, A.A. Assessing the phenological state of evergreen conifers using hyperspectral imaging time series. Remote Sens. Appl. Soc. Environ. 2024, 36, 101342. [Google Scholar] [CrossRef]
- Guo, J.; Liu, X.; Ge, W.; Zhao, L.; Fan, W.; Zhang, X.; Lu, Q.; Xing, X.; Zhou, Z. Tracking photosynthetic phenology using spectral indices at the leaf and canopy scales in temperate evergreen and deciduous trees. Agric. For. Meteorol. 2024, 344, 109809. [Google Scholar] [CrossRef]
- Sasagawa, T.; Akitsu, T.K.; Ide, R.; Takagi, K.; Takanashi, S.; Nakaji, T.; Nasahara, K.N. Accuracy Assessment of Photochemical Reflectance Index (PRI) and Chlorophyll Carotenoid Index (CCI) Derived from GCOM-C/SGLI with In Situ Data. Remote Sens. 2022, 14, 5352. [Google Scholar] [CrossRef]
- Gamon, J.; Serrano, L.; Surfus, J. The photochemical reflectance index: An optical indicator of photosynthetic radiation use efficiency across species, functional types, and nutrient levels. Oecologia 1997, 112, 492–501. [Google Scholar] [CrossRef] [PubMed]
- Rakgoale, P.B.; Ngetar, S.N. Detecting Invasive Alien Plant Species Using Remote Sensing, Machine Learning and Deep Learning. J. Sens. 2024, 23, 8854675. [Google Scholar] [CrossRef]
- Grewal, R.; Singh Kasana, S.; Kasana, G. Machine Learning and Deep Learning Techniques for Spectral Spatial Classification of Hyperspectral Images: A Comprehensive Survey. Electronics 2023, 12, 488. [Google Scholar] [CrossRef]
- Swe, K.N.; Noguchi, N. Comparison of machine learning and deep learning models for the assessment of rondo wine grape quality with a hyperspectral camera. Smart Agric. Technol. 2024, 8, 100474. [Google Scholar] [CrossRef]
- Saha, D.; Manickavasagan, A. Machine learning techniques for analysis of hyperspectral images to determine quality of food products: A review. Curr. Res. Food Sci. 2021, 4, 28–44. [Google Scholar] [CrossRef]
- Dmitriev, P.A.; Kozlovsky, B.L.; Dmitrieva, A.A. Vegetation and Dormancy States Identification in Coniferous Plants Based on Hyperspectral Imaging Data. Horticulturae 2024, 10, 241. [Google Scholar] [CrossRef]
- Ma, J.; Chen, X.; Han, F.; Song, Y.; Zhou, B.; Nie, Y.; Li, Y.; Niu, S. The long road to bloom in conifers. For. Res. 2022, 2, 16. [Google Scholar] [CrossRef]
- Röhrig, E.; Ulrich, B. Temperate deciduous forests. Ecosyst. World 1991, 7, 557–558. [Google Scholar]
- Thomas, S.C.; MacLellan, J. Boreal and Temperate Forests. For. For. Plants 2009, 1, 152. [Google Scholar]
- Vankat, J. Boreal and Temperate Forests. Encycl. Life Sci. 2002. [Google Scholar] [CrossRef]
- Venäläinen, A.; Lehtonen, I.; Laapas, M.; Ruosteenoja, K.; Tikkanen, O.P.; Viiri, H.; Ikonen, V.P.; Peltola, H. Climate change induces multiple risks to boreal forests and forestry in Finland: A literature review. Glob. Change Biol. 2020, 26, 4178–4196. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Manzanedo, R.; Xu, G.; Lapenis, A.G. Editorial: Achieving sustainable development goal 13: Resilience and adaptive capacity of temperate and boreal forests under climate change. Front. For. Glob. Change 2024, 7, 1356686. [Google Scholar] [CrossRef]
- Sheikh, M.A.; Tiwari, A.; Anjum, J.; Sharma, S. Dynamics of carbon storage and status of standing vegetation in temperate coniferous forest ecosystem of north western Himalaya India. Vegetos 2021, 34, 822–833. [Google Scholar] [CrossRef]
- Zhang, Q.; Luan, R.; Wang, M.; Zhang, J.; Yu, F.; Ping, Y.; Qiu, L. Research Progress of Spectral Imaging Techniques in Plant Phenotype Studies. Plants 2024, 13, 3088. [Google Scholar] [CrossRef]
- Cotrozzi, L. Spectroscopic detection of forest diseases: A review (1970–2020). J. For. Res. 2022, 33, 21–38. [Google Scholar] [CrossRef]
- Paul, N.; Sunil, G.C.; Horvath, D.; Sun, X. Deep learning for plant stress detection: A comprehensive review of technologies, challenges, and future directions. Comput. Electron. Agric. 2025, 229, 109734. [Google Scholar] [CrossRef]
- Ye, D.; Wu, L.; Li, X.; Atoba, T.O.; Wu, W.; Weng, H. A Synthetic Review of Various Dimensions of Non-Destructive Plant Stress Phenotyping. Plants 2023, 12, 1698. [Google Scholar] [CrossRef]
- Williams, D.; Karley, A.; Britten, A.; McCallum, S.; Graham, J. Raspberry plant stress detection using hyperspectral imaging. Plant Direct 2023, 7, e490. [Google Scholar] [CrossRef] [PubMed]
- Blackburn, G.A. Hyperspectral remote sensing of plant pigments. J. Exp. Bot. 2007, 58, 855–867. [Google Scholar] [CrossRef] [PubMed]
- Yan, K.; Gao, S.; Yan, G.; Ma, X.; Chen, X.; Zhu, P.; Li, J.; Gao, S.; Gastellu-Etchegorry, J.-P.; Myneni, R.B.; et al. A global systematic review of the remote sensing vegetation indices. Int. J. Appl. Earth Obs. Geoinf. 2025, 139, 104560. [Google Scholar] [CrossRef]
- Wong, C.Y.S. Plant optics: Underlying mechanisms in remotely sensed signals for phenotyping applications. AoB Plants 2023, 15, plad039. [Google Scholar] [CrossRef]
- Ashraf, M.; Harris, P.J.C. Photosynthesis under stressful environments: An overview. Photosynthetica 2013, 51, 163–190. [Google Scholar] [CrossRef]
- Sun, Y.; Qin, Q.; Zhang, Y.; Ren, H.; Han, G.; Zhang, Z.; Zhang, T.; Wang, B. A leaf chlorophyll vegetation index with reduced LAI effect based on Sentinel-2 multispectral red-edge information. Comput. Electron. Agric. 2025, 236, 110500. [Google Scholar] [CrossRef]
- Cheshkova, A.F. A review of hyperspectral image analysis techniques for plant disease detection and identification. Vavilovskii Zhurnal Genet. I Sel. Vavilov J. Genet. Breed. 2022, 26, 202–213. [Google Scholar] [CrossRef]
- Wan, L.; Li, H.; Li, C.; Wang, A.; Yang, Y.; Wang, P. Hyperspectral Sensing of Plant Diseases: Principle and Methods. Agronomy 2022, 12, 1451. [Google Scholar] [CrossRef]
- Poblete, T.; Watt, M.S.; Buddenbaum, H.; Zarco-Tejada, P.J. Chlorophyll content estimation in radiata pine using hyperspectral imagery: A comparison between empirical models, scaling-up algorithms, and radiative transfer inversions. Agric. For. Meteorol. 2025, 362, 110402. [Google Scholar] [CrossRef]
- Mertens, S.; Verbraeken, L.; Sprenger, H.; Demuynck, K.; Maleux, K.; Cannoot, B.; De Block, J.; Maere, S.; Nelissen, H.; Bonaventure, G.; et al. Proximal Hyperspectral Imaging Detects Diurnal and Drought-Induced Changes in Maize Physiology. Front. Plant Sci. 2021, 12, 640914, Erratum in Front. Plant Sci. 2024, 15, 1379654. [Google Scholar] [CrossRef]
- Misra, G.; Cawkwell, F.; Wingler, A. Status of Phenological Research Using Sentinel-2 Data: A Review. Remote Sens. 2020, 12, 2760. [Google Scholar] [CrossRef]
- Zeng, L.; Wardlow, B.D.; Xiang, D.; Hu, S.; Li, D. A Review of Vegetation Phenological Metrics Extraction Using Time-Series, Multispectral Satellite Data. Remote Sens. Environ. 2020, 237, 111511. [Google Scholar] [CrossRef]
- Berra, E.F.; Gaulton, R. Remote sensing of temperate and boreal forest phenology: A review of progress, challenges and opportunities in the intercomparison of in-situ and satellite phenological metrics. For. Ecol. Manag. 2021, 480, 118663. [Google Scholar] [CrossRef]
- Dronova, I.; Taddeo, S. Remote sensing of phenology: Towards the comprehensive indicators of plant community dynamics from species to regional scales. J. Ecol. 2022, 110, 1460–1484. [Google Scholar] [CrossRef]
- Lu, B.; Dao, P.D.; Liu, J.; He, Y.; Shang, J. Recent Advances of Hyperspectral Imaging Technology and Applications in Agriculture. Remote Sens. 2020, 12, 2659. [Google Scholar] [CrossRef]
- Mishra, P.; Lohumi, S.; Haris, A.K.; Nordon, A. Close-range hyperspectral imaging of whole plants for digital phenotyping: Recent applications and illumination correction approaches. Comput. Electron. Agric. 2020, 178, 105780. [Google Scholar] [CrossRef]
- Han, Q.; Wang, T.; Jiang, Y.; Fischer, R.; Li, C. Phenological variation decreased carbon uptake in European forests during 1999–2013. For. Ecol. Manag. 2018, 427, 45–51. [Google Scholar] [CrossRef]
- Schrodt, F.; de la Barreda Bautista, B.; Williams, C.; Boyd, D.S.; Schaepman-Strub, G.; Santos, M.J. Integrating biodiversity, remote sensing, and auxiliary information for the study of ecosystem functioning and conservation at large spatial scales. In Remote Sensing of Plant Biodiversity; Springer International Publishing: Berlin/Heidelberg, Germany, 2020; pp. 449–484. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, Y.; Wen, R.; Zhou, C.; Xu, L.; Xi, X. Mapping Spatiotemporal Changes in Vegetation Growth Peak and the Response to Climate and Spring Phenology over Northeast China. Remote Sens. 2020, 12, 3977. [Google Scholar] [CrossRef]
- Lebrini, Y.; Boudhar, A.; Laamrani, A.; Htitiou, A.; Lionboui, H.; Salhi, A.; Chehbouni, A.; Benabdelouahab, T. Mapping and Characterization of Phenological Changes over Various Farming Systems in an Arid and Semi-Arid Region Using Multitemporal Moderate Spatial Resolution Data. Remote Sens. 2021, 13, 578. [Google Scholar] [CrossRef]
- Denny, E.G.; Gerst, K.L.; Miller-Rushing, A.J.; Tierney, G.L.; Crimmins, T.M.; Enquist, C.A.F.; Guertin, P.; Rosemartin, A.H.; Schwartz, M.D.; Thomas, K.A.; et al. Standardized phenology monitoring methods to track plant and animal activity for science and resource management applications. Int. J. Biometeorol. 2014, 58, 591–601. [Google Scholar] [CrossRef]
- Vyse, K.; Pagter, M.; Zuther, E.; Hincha, D.K. Deacclimation after cold acclimation—A crucial, but widely neglected part of plant winter survival. J. Exp. Bot. 2019, 70, 4595–4604. [Google Scholar] [CrossRef]
- Weiser, C.J. Cold Resistance and Injury in Woody Plants. Science 1970, 169, 1269–1278. [Google Scholar] [CrossRef] [PubMed]
- Beck, E.H.; Heim, R.; Hansen, J. Plant resistance to cold stress: Mechanisms and environmental signals triggering frost hardening and dehardening. J. Biosci. 2004, 29, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Seyednasrollah, B.; Bowling, D.R.; Cheng, R.; Logan, B.A.; Magney, T.S.; Frankenberg, C.; Yang, J.C.; Young, A.M.; Hufkens, K.; Arain, M.A.; et al. Seasonal variation in the canopy color of temperate evergreen conifer forests. New Phytol. 2021, 229, 2586–2600. [Google Scholar] [CrossRef]
- Ida, K. Eco-physiological studies on the response of taxodiaceous conifers to shading, with special reference to the behaviour of leaf pigments—I. Distribution of carotenoids in green and autumnal reddish brown leaves of gymnosperms. Bot. Mag. 1981, 94, 41–54. [Google Scholar] [CrossRef]
- Körner, C.; Möhl, P.; Hiltbrunner, E. Four ways to define the growing season. Ecol. Lett. 2023, 26, 1277–1292. [Google Scholar] [CrossRef]
- Dmitriev, P.A.; Dmitrieva, A.A.; Kozlovsky, B.L. Random Reflectance: A New Hyperspectral Data Preprocessing Method for Improving the Accuracy of Machine Learning Algorithms. AgriEngineering 2025, 7, 90. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solutionts. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef]
- Verhoeven, A. Recovery kinetics of winter stressed conifers: The effects of growth light environment, extent of the season, and species. Nat Prec. 2010. [Google Scholar] [CrossRef]
- Nippert, J.B.; Duursma, R.A.; Marshall, J.D. Seasonal variation in photosynthetic capacity of montane conifers. Funct. Ecol. 2004, 18, 876–886. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2025; Available online: https://www.R-project.org/ (accessed on 9 August 2025).
- Liaw, A.; Wiener, M. Classification and Regression by Random Forest. R News 2002, 2, 18–22. [Google Scholar]
- Venables, W.N.; Ripley, B.D. Modern Applied Statistics with S, 4th ed.; Springer: New York, NY, USA, 2002. [Google Scholar]
- Zuur, A.F.; Ieno, E.N.; Elphick, C.S. A protocol for data exploration to avoid common statistical problems. Methods Ecol. Evol. 2010, 1, 3–14. [Google Scholar] [CrossRef]




| Vegetation Index | Formula | Reference |
|---|---|---|
| Ccar VIs | ||
| RARSc | R760/R500 | [44] |
| PSSRc | R800/R470 | [45] |
| PSNDc | (R800 − R470)/(R800 + R470) | [45] |
| CRI550 | R510–1 − R550−1 | [46] |
| CRI700 | R510–1 − R700−1 | [46] |
| CARred-edge | (R510−1 − R700−1) × R770 | [47] |
| CARgreen | (R510−1 − R550−1) × R770 | [47] |
| CARI | (R720 − R521)/R521 | [48] |
| CTRI | [1.2 × [1.2 × (R800 − R550) − 2.5 × (R670 − R550)]]/R531 | [49] |
| PRI | (R531 − R570)/(R531 + R570) | [50] |
| TVI | 0.5 × [120 × (R750 − R550) − 200 × (R670 − R480)] | [51] |
| TCARI | 3 × [(R700 − R670) − 0.2 × (R700 − R550) × (R700/R670)] | [52] |
| PRIm1 | (R512 − R531)/(R512 + R531) | [53] |
| SRcar | R515/R570 | [54] |
| MTVI1 | 1.2 × [1.2 × (R800 − R550) − 2.5 × (R670 − R550)] | [55] |
| Datt4 | R672/(R550 × R708) | [56] |
| RI | (R470 − R540)/(R470 + R540) | [57,58] |
| Cchl/Ccar VIs | ||
| CCRI = CARI/CIred-edge | [(R720 − R521)/R521]/(R750 − R705)/R705 | [40] |
| (α500/α700) − 1 | [(R800/R500)/(R800/R700)] − 1 | [59] |
| α500 − α660 | (R800/R500) − (R800/R660) | [59] |
| RVI | R761/R738 | [60] |
| CCI | (R528 − R645)/(R528 + R645) | [61] |
| CTRI/CIred-edge | [[1.2 × [1.2 × (R800 − R550) − 2.5 × (R670 − R550)]]/R531]/[(R750 − R705)/R705] | [41] |
| SIPI | (R800 − R445)/(R800 + R680) | [62] |
| LS VIs | ||
| modPRI515/550 | (R515 − R550)/(R515 + R550) | [43] |
| LSI | mean(R666:682)/mean(R552:594) | [63] |
| LSIRed | R674/R654 | [32] |
| LSINorm | (R674 − R654)/(R674 + R654) | [32] |
| VI | VIF | VI | VIF | VI | VIF |
|---|---|---|---|---|---|
| CCRI | 4.10 | LSI | 2.16 | Datt4 | 1.51 |
| CRI700 | 3.88 | TCARI | 1.96 | PRIm1 | 1.45 |
| PRI | 3.52 | CTRI | 1.73 | LSINorm | 1.45 |
| CARI | 3.16 | RARSc | 1.66 | LSIRed | 1.43 |
| CCI | 3.13 | CARgreen | 1.66 | RVI | 1.42 |
| a700 | 2.87 | MTVI1 | 1.64 | SIPI | 1.40 |
| SRcar | 2.53 | a660 | 1.57 | RI | 1.39 |
| CARrededge | 2.51 | modPRI | 1.56 | PSNDc | 1.13 |
| CRI550 | 2.48 | TVI | 1.56 | PSSRc | 1.10 |
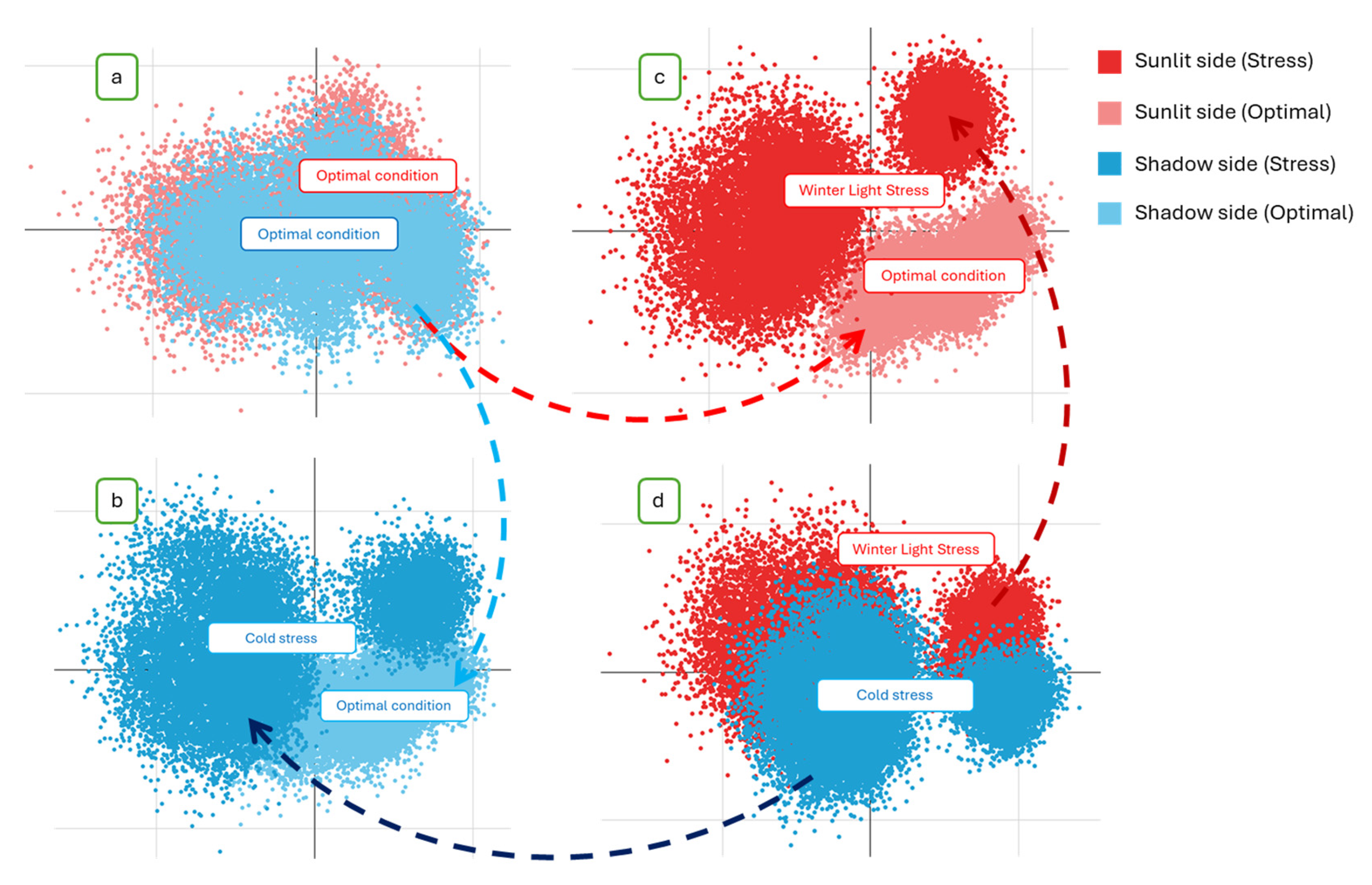
| Group | State | Winter Light Stress | Optimal Condition | Cold Stress | Class. Error, % | OOB Estimate of Error Rate, % |
|---|---|---|---|---|---|---|
| ‘Winter Light Stress’ & ‘Optimal condition’ | Winter Light Stress | 17,938 | 62 | - | 0.3 | 0.35 |
| Optimal condition | 64 | 17,936 | - | 0.4 | ||
| ‘Cold stress’ & ‘Optimal condition’ | Cold stress | - | 563 | 17,437 | 3.1 | 3.19 |
| Optimal condition | - | 17,415 | 585 | 3.3 | ||
| ‘Winter Light Stress’ & ‘Cold stress’ | Cold stress | 3346 | - | 14,654 | 18.6 | 15.94 |
| Winter Light Stress | 15,606 | - | 2394 | 13.3 | ||
| ‘Winter Light Stress’ & ‘Cold stress’ & ‘Optimal condition’ | Cold stress | 14,147 | 3239 | 614 | 21.4 | 12.8 |
| Winter Light Stress | 2477 | 15,512 | 11 | 13.8 | ||
| Optimal condition | 562 | 7 | 17,431 | 3.2 |
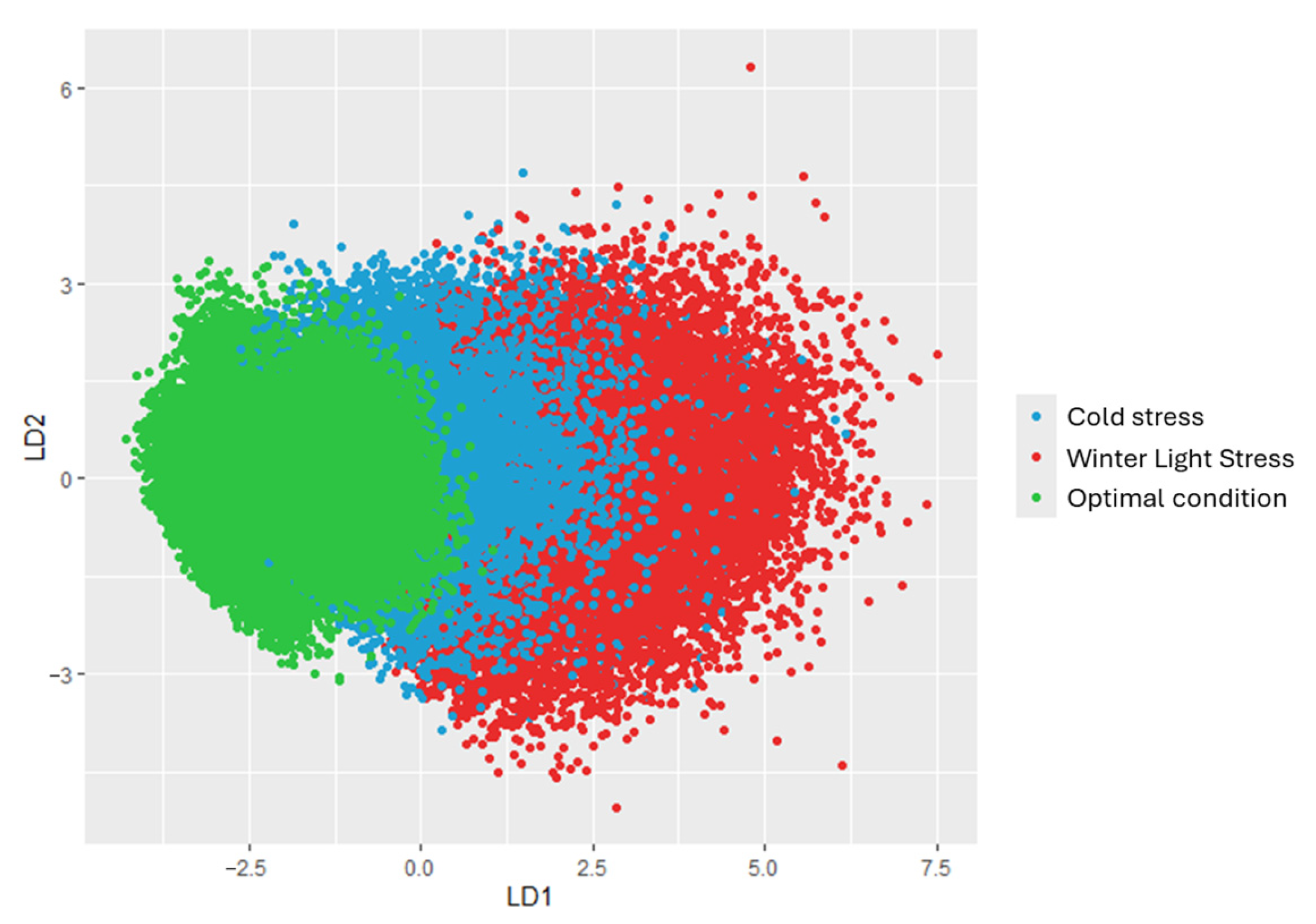
| Combination of States | Discrimination Equations | Correctness Rate, % |
|---|---|---|
| «Winter Light Stress» and «Optimal condition» | LD1 = 0.722 × CCI − 0.749 × CCRI + 0.567 × PRI − 0.670 × PRIm1 | 96.7 |
| «Cold stress» and «Optimal condition» | LD1 = 0.349 × CRI550 − 0.470 × CARI − 0.704 × CCRI − 0.395 × modPRI − 0.369 × PRIm1 − 0.384 × TVI | 90.4 |
| «Winter Light Stress» and «Cold stress» | LD1 = − 0.667 × CCI + 0.460 × CCRI − 0.359 × LSIRed − 0.449 × PRI + 0.320 × PRIm1 | 79.9 |
| «Winter Light Stress» and «Cold stress» and «Optimal condition» | LD1 = 0.486 × CCI − 0.748 × CCRI − 0.421 × modPRI + 0.443 × PRI − 0.459 × PRIm1 − 0.220 × TVI LD2 = 0.818 × CCI − 0.312 × CCRI − 0.265 × modPRI + 0.300 × PRI − 0.106 × PRIm1 − 0.834 × TVI | 78.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dmitriev, P.A.; Kozlovsky, B.L.; Dmitrieva, A.A.; Sereda, M.M.; Varduni, T.V.; Lysenko, V.S. Identifying Winter Light Stress in Conifers Using Proximal Hyperspectral Imaging and Machine Learning. Stresses 2025, 5, 62. https://doi.org/10.3390/stresses5040062
Dmitriev PA, Kozlovsky BL, Dmitrieva AA, Sereda MM, Varduni TV, Lysenko VS. Identifying Winter Light Stress in Conifers Using Proximal Hyperspectral Imaging and Machine Learning. Stresses. 2025; 5(4):62. https://doi.org/10.3390/stresses5040062
Chicago/Turabian StyleDmitriev, Pavel A., Boris L. Kozlovsky, Anastasiya A. Dmitrieva, Mikhail M. Sereda, Tatyana V. Varduni, and Vladimir S. Lysenko. 2025. "Identifying Winter Light Stress in Conifers Using Proximal Hyperspectral Imaging and Machine Learning" Stresses 5, no. 4: 62. https://doi.org/10.3390/stresses5040062
APA StyleDmitriev, P. A., Kozlovsky, B. L., Dmitrieva, A. A., Sereda, M. M., Varduni, T. V., & Lysenko, V. S. (2025). Identifying Winter Light Stress in Conifers Using Proximal Hyperspectral Imaging and Machine Learning. Stresses, 5(4), 62. https://doi.org/10.3390/stresses5040062







