Vigour Index on Time Basis Calculation on Agastache mexicana Subsp. mexicana Throughout Induced Hydric Stress: SiO2 and Artificial Shade Application Effects
Abstract
1. Introduction
2. Results
2.1. Statistical Results of (General, SDL, and ASL Effects)
2.2. Agronomic Interpretation
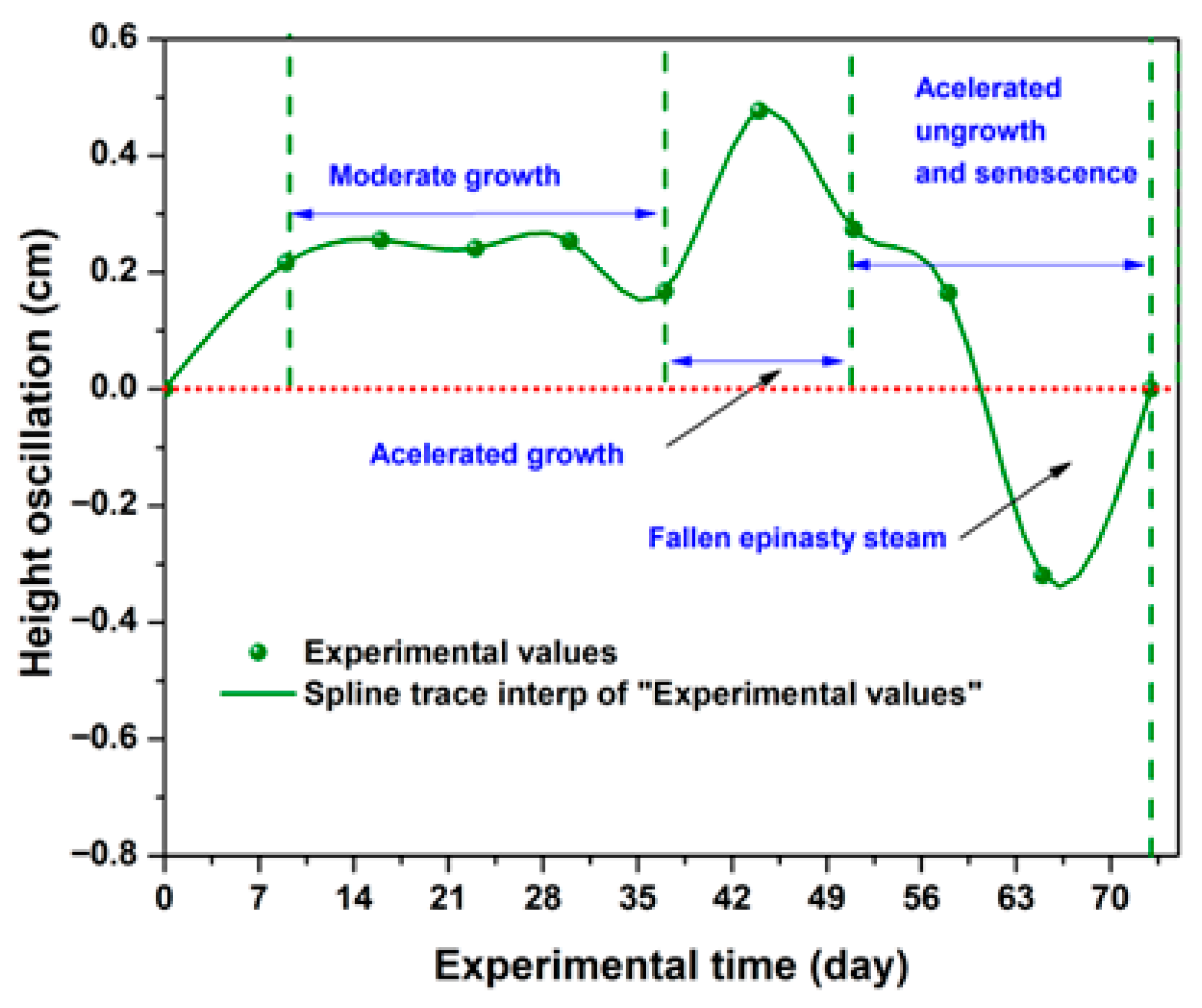
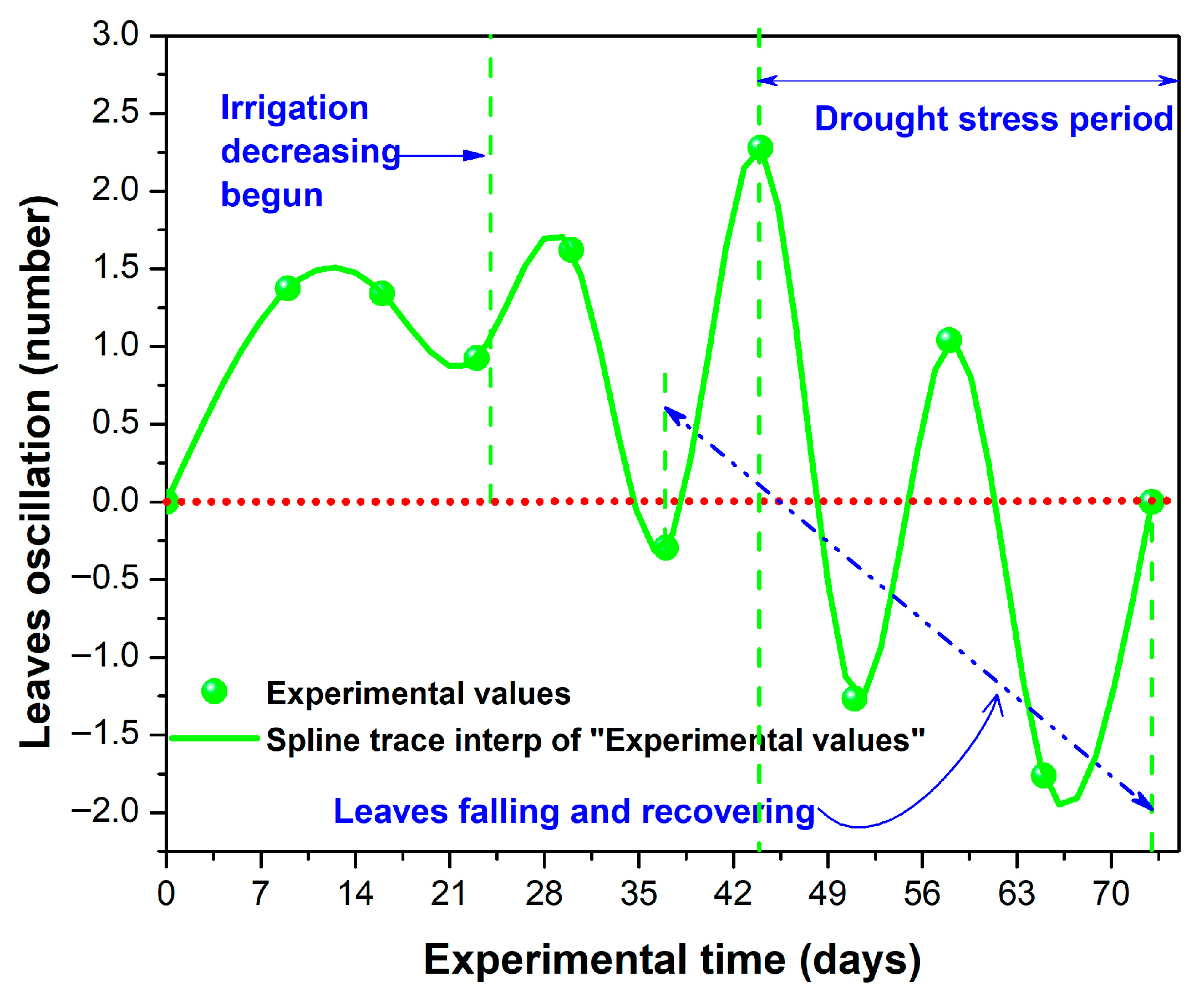
2.2.1. Generalities
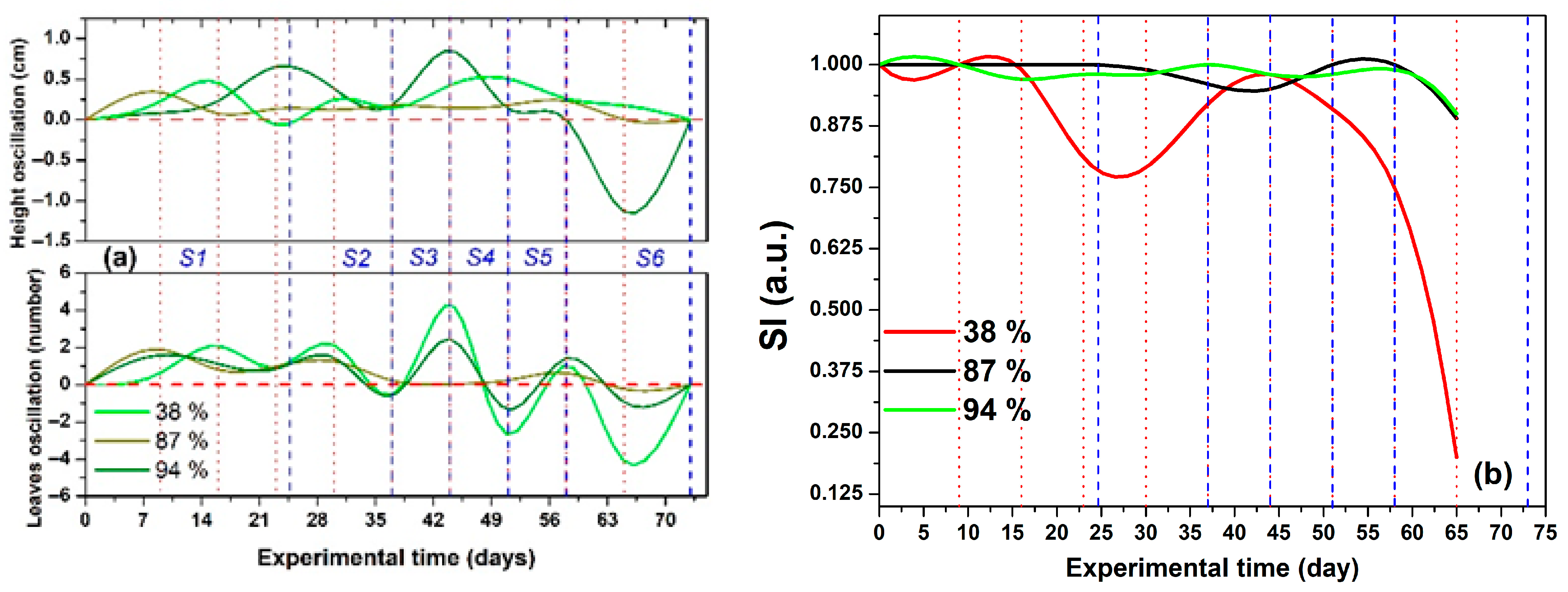
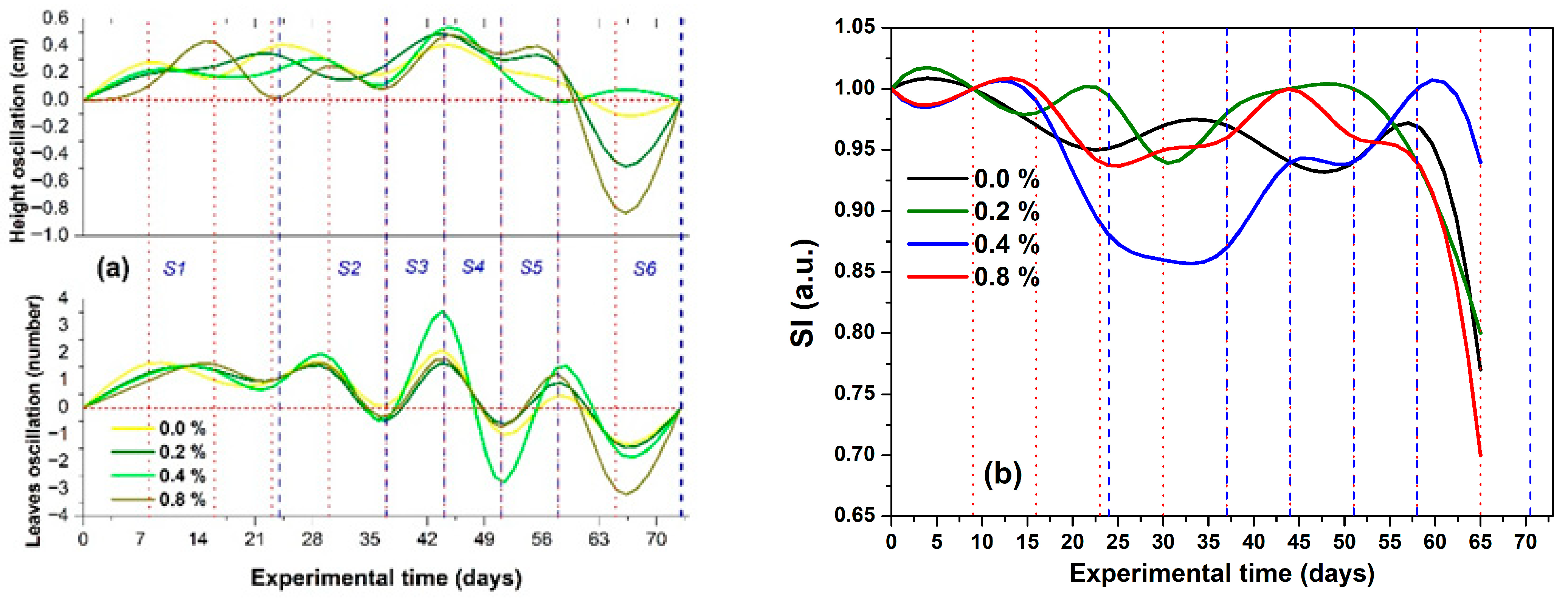
2.2.2. Irrigation, SDL, and ASL Effects on Height, Leaf Number, SI, and
Stage 1 (W1–W3)
Stage 2 (W4 and W5)
Stage 3 (W6)
Stage 4 (W7)
Stage 5 (W8)
Stage 6 (W9 and the Final Experimental Period)
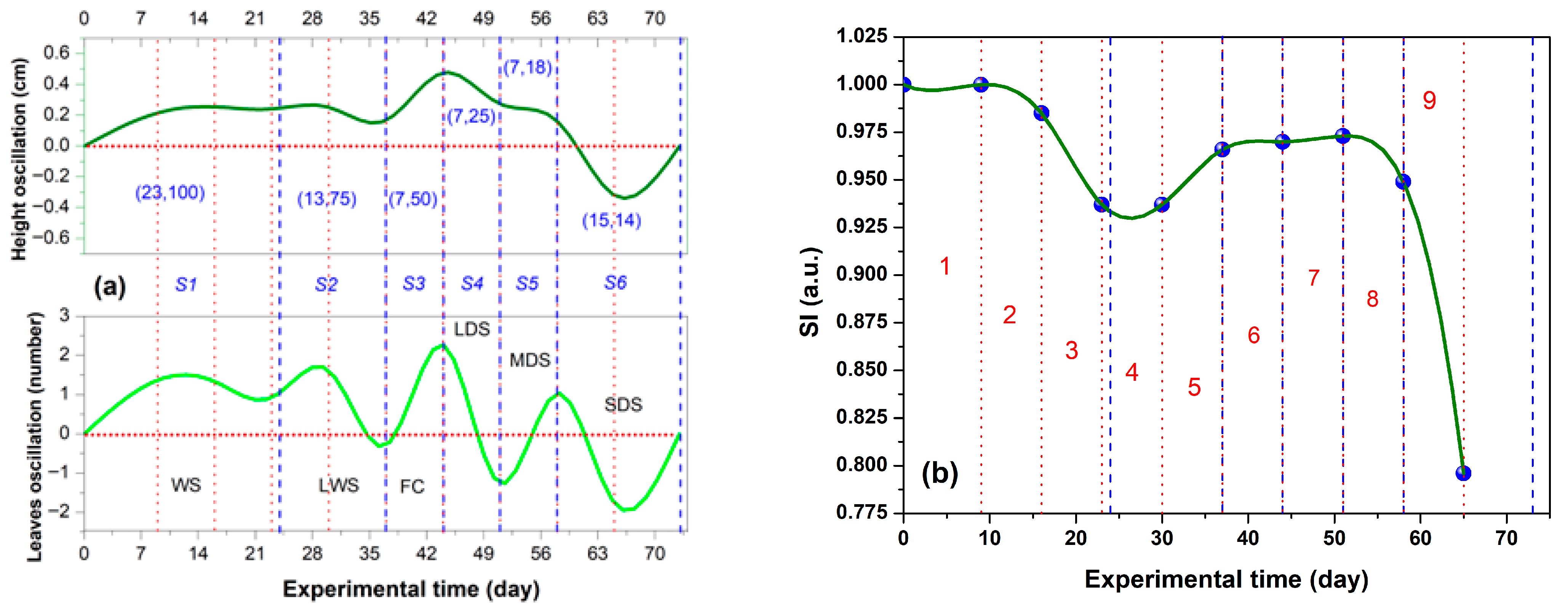
2.3. Water Regimes Effect and Identification of the Stages with Soil Water Availability and Hydric Stress
3. Discussion
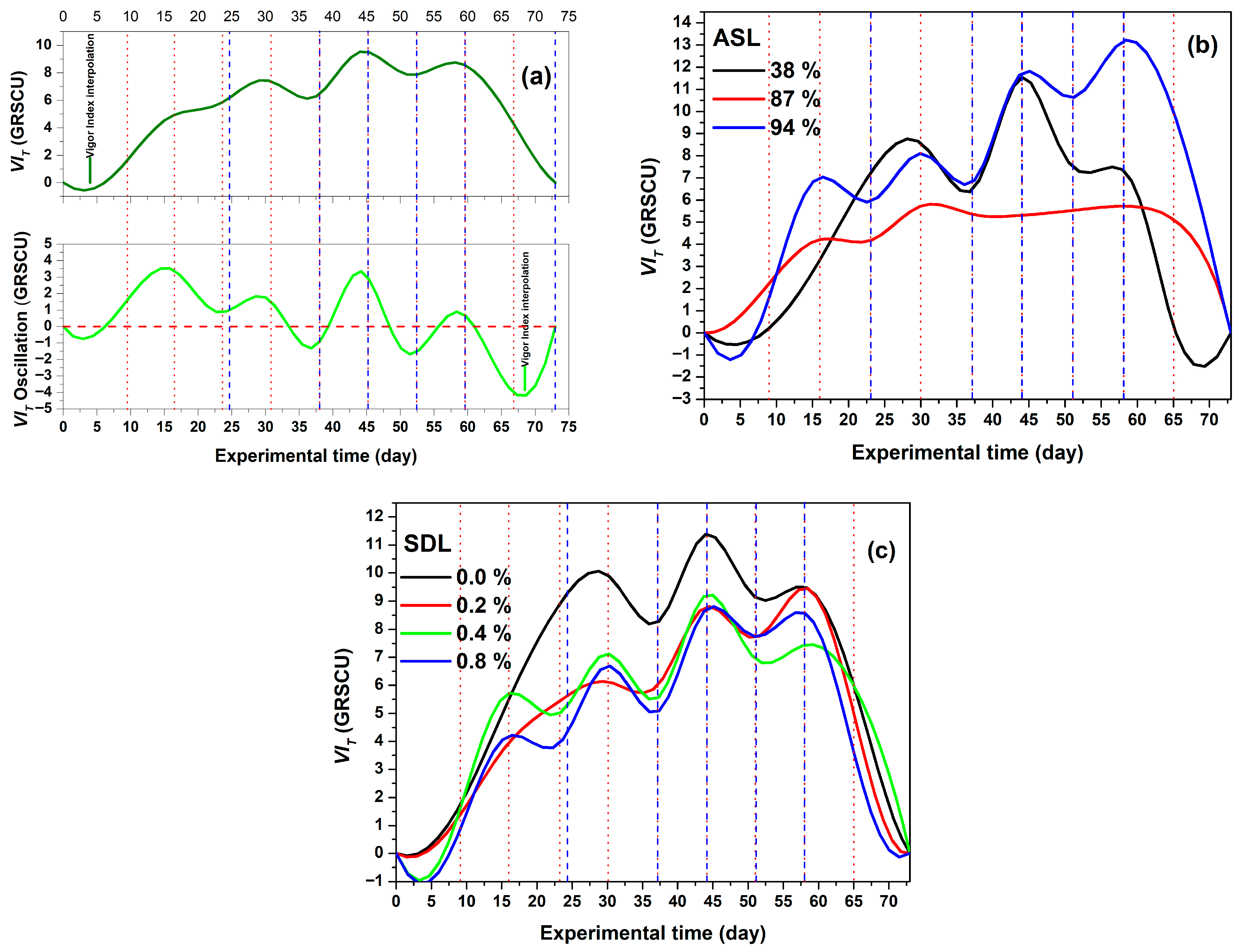
3.1. Comparison of Elements to Calculate Vigour Index and Importance of Calculation
3.2. Water Regimes Effect Linked to Water Available in the Soil and Stress Type
3.2.1. Waterlogging Stress
3.2.2. Field Capacity
3.2.3. Drought Stress
3.3. ASL and SDL Effects on , Linked to the Water Regimes
4. Material and Methods
4.1. Experimental Place and Preparation of Bio-Space
4.2. Substrate Preparation, Seed Sowing, and Experiment Design
4.3. Artificial Shade Level Measurements
4.4. Water Regimes Calculation
4.5. Measurements and Indexes
4.5.1. Measurements of Amm’s Plants
4.5.2. Calculations
Vigour Index
Survival Index
4.6. Statistical Analysis
5. Conclusions
- (1)
- WS and LWS (S1 and S2, respectively): During these early stages, the seedlings and plants were young, resulting in limited growth and a small number of leaves. Consequently, the general values were the lowest, while the general values of the showed a gradual increase, following a double sigmoidal profile.
- (2)
- S3 as a transition stage (FC): at this stage, plant growth in terms of height and leaf number peaked, leading to the highest general values for both SI and .
- (3)
- LDS, MDS, and SDS (S4, S5, and S6, respectively): During this phase, reduced irrigation levels led to drought stress. The plants that perished were those that had experienced the most significant growth but could not meet their water demands for survival. Additionally, the general values of both SI and consistently decreased until they reached zero.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Santillán-Ramírez, M.A.; López-Villafranco, M.E.; Aguilar-Rodríguez, S.; Aguilar-Contreras, A. Estudio etnobotánico, arquitectura foliar y anatomía vegetativa de Agastache mexicana ssp. mexicana y A. mexicana ssp. xolocotziana. Rev. Mex. Biodivers. 2008, 79, 513–524. [Google Scholar]
- Martínez-Gordillo, M.; Fragoso-Martínez, I.; del Rosario García-Peña, M.; Montiel, O. Géneros de Lamiaceae de México, diversidad y endemismo. Rev. Mex. Biodivers. 2013, 84, 30–86. [Google Scholar] [CrossRef]
- Ventura-Martínez, R.; Rodríguez, R.; González-Trujano, M.E.; Ángeles-López, G.E.; Déciga-Campos, M.; Gómez, C. Spasmogenic and spasmolytic activities of Agastache mexicana ssp. mexicana and A. mexicana ssp. xolocotziana methanolic extracts on the guinea pig ileum. J. Ethnopharmacol. 2017, 196, 58–65. [Google Scholar]
- Vogel, H. Cómo Producir y Procesar Plantas Medicinales y Aromáticas de Calidad; FIA: Santiago de Chile, Chile, 2003. [Google Scholar]
- Das, M.; Trivedi, A.P. Essentiality of seed standards for the cultivation of medicinal plants—A review. Curr. Hortic. 2021, 9, 9–16. [Google Scholar] [CrossRef]
- Murúa Carrizo, F. Efecto de la época y densidad de siembra de comino (Cuminum cyminum L.) sobre el rendimiento, sus componentes y la calidad de semilla. Hortic. Argent. 2011, 30, 9–17. [Google Scholar]
- Pérez-Martínez, L.V.; Rodríguez-Castillo, N.A.; Ríos, O.V.; Melgarejo, L.M. Germinación y dormancia de semillas. In Semillas de Plantas de Páramo: Ecología y Métodos de Germinación Aplicados a la Restauración Ecológica; UNAL: Bogotá, Colombia, 2014; pp. 63–113. [Google Scholar]
- Borza, J.K.; Westerman, P.R.; Liebman, M. Comparing estimates of seed viability in three foxtail (Setaria) species using the imbibed seed crush test with and without additional tetrazonlium testing. Weed Technol. 2007, 21, 518–522. [Google Scholar] [CrossRef]
- Milošević, M.; Vujaković, M.; Karagić, Đ. Vigour tests as indicators of seed viability. Genetika 2010, 42, 103–118. [Google Scholar] [CrossRef]
- Delouche, J.C.; Caldwell, W.P. Seed vigor and vigor tests. In Proceedings of the Association of Official Seed; Association of Official Seed Analysts: Wichita, KS, USA, 1960. [Google Scholar]
- Kwon, M.Y.; Woo, S.Y. Plants’ responses to drought and shade environments. Afr. J. Biotechnol. 2016, 15, 29–31. [Google Scholar] [CrossRef]
- Aroca, R. Plant responses to drought stress. In From Morphological to Molecular Features; Springer: Berlin/Heidelberg, Germany, 2012; pp. 1–5. [Google Scholar]
- Sun, Y.; Wang, C.; Chen, H.Y.; Ruan, H. Response of plants to water stress: A meta-analysis. Front. Plant Sci. 2020, 11, 978. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, M.A. Waterlogging stress in plants: A review. Afr. J. Agric. Res. 2012, 7, 1976–1981. [Google Scholar] [CrossRef]
- Khalid, M.F.; Zakir, I.; Khan, R.I.; Irum, S.; Sabir, S.; Zafar, N.; Ahmad, S.; Abbas, M.; Ahmed, T.; Hussain, S. Effect of water stress (drought and waterlogging) on medicinal plants. In Medicinal Plants: Their Response to Abiotic Stress; Springer Nature: Singapore, 2023; pp. 169–182. [Google Scholar]
- Nikam, V.; Chavan, P. Influence of water deficit and waterlogging on the mineral status of a medicinal plant Chlorophytum borivilianum. Acta Bot. Hung. 2009, 51, 105–113. [Google Scholar] [CrossRef]
- Zhou, W.; Chen, F.; Meng, Y.; Chandrasekaran, U.; Luo, X.; Yang, W.; Shu, K. Plant waterlogging/flooding stress responses: From seed germination to maturation. Plant Physiol. Biochem. 2020, 148, 228–236. [Google Scholar] [CrossRef]
- Chen, C.; Luo, X.; Jin, G.; Cheng, Z.; Pan, X.; Zhu, G.; Li, S.; Zhu, Y.; Tang, N. Shading effect on survival, growth, and contents of secondary metabolites in micropropagated Anoectochilus plantlets. Braz. J. Bot. 2017, 40, 599–607. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, L.; He, J.; Li, J.A.; Zhang, H.; Li, Y.; Gu, Y.; Luo, H.; Lu, M.; Lu, K.; et al. Effects of different shade treatments on Melaleuca seedling growth and physiological properties. BMC Plant Biol. 2025, 25, 203. [Google Scholar] [CrossRef]
- Zainal, A.; Satria, B.; Mawati, I.; Wulantika, T. The effect of shade on germination and seedling growth of two dogfruit (Pithecellobium jiringa) Genotypes. J. Appl. Agric. Sci. Technol. 2024, 8, 211–227. [Google Scholar] [CrossRef]
- Jarvis, P.G. The adaptability to light intensity of seedlings of Quercus petraea (Matt.) Liebl. J. Ecol. 1964, 52, 545–571. [Google Scholar] [CrossRef]
- Rahimi, S.; Hatami, M.; Ghorbanpour, M. Silicon-nanoparticle mediated changes in seed germination and vigor index of marigold (Calendula officinalis L.) compared to silicate under PEG-induced drought stress. Gesunde Pflanz. 2021, 73, 575–589. [Google Scholar] [CrossRef]
- Ma, J.F.; Yamaji, N. Silicon uptake and accumulation in higher plants. Trends Plant Sci. 2006, 11, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.J.; Nascimento, C.W.A.; Gouveia-Neto, A.S. Assessment of cadmium toxicities in potted garlic plants. Acta Physiol. Plant 2017, 38, 211. [Google Scholar]
- Kaya, C.; Tuna, L.; Higgs, D. Effect of silicon on plant growth and mineral nutrition of maize grown under water-stress conditions. J. Plant Nutr. 2006, 29, 1469–1480. [Google Scholar] [CrossRef]
- Cao, B.L.; Ma, Q.; Zhao, Q.; Wang, L.; Xu, K. Effects of silicon on absorbed light allocation, antioxidant enzymes, and ultrastructure of chloroplasts in tomato leaves. Sci. Hortic. 2015, 194, 53–62. [Google Scholar] [CrossRef]
- Jesus, E.G.D.; Fatima, R.T.D.; Guerrero, A.C.; Araújo, J.L.D.; Brito, M.E. Growth and gas exchanges of arugula plants under silicon fertilisation and water restriction. Rev. Bras. Eng. Agrícola Ambient. 2018, 22, 119–124. [Google Scholar] [CrossRef]
- Zia-ur-Rehman, M.; Khalid, H.; Akmal, F.; Waqar, M.; Rizwan, M.; Qayyum, F.; Nadeem, M. Silicon and antioxidant defense system against. In Silicon in Plants: Advances and Future Prospects; CRC Press: Boca Raton, FL, USA, 2016; p. 321. [Google Scholar]
- Sacala, E. Role of silicon in plant resistance to water stress. J. Elem. 2009, 14, 619–630. [Google Scholar] [CrossRef]
- Sapre, S.S.; Vakharia, D.N. Role of silicon under water deficit stress in wheat: A biochemical perspective: A review. Agric. Rev. 2016, 37, 109–116. [Google Scholar] [CrossRef]
- Coskun, D.; Britto, D.T.; Huynh, W.Q.; Kronzucker, H.J. The role of silicon in higher plants under salinity and drought stress. Front. Plant Sci. 2016, 7, 1072. [Google Scholar] [CrossRef] [PubMed]
- Gharineh, M.H.; Karmollachaab, A. Effect of silicon on physiological characteristics of wheat growth under water-deficit stress induced by PEG. Int. J. Agron. Plant Prod. 2013, 4, 1543–1548. [Google Scholar]
- Khan, I.; Awan, S.A.; Rizwan, M.; Ali, S.; Hassan, M.J.; Brestic, M.; Zhang, X.; Huang, L. Effects of silicon on heavy metal uptake at the soil-plant interface: A review. Ecotoxicol. Environ. Saf. 2021, 222, 112510. [Google Scholar] [CrossRef]
- Hussain, S.; Mumtaz, M.; Manzoor, S.; Shuxian, L.; Ahmed, I.; Skalicky, M.; Brestic, M.; Rastogi, A.; Ulhassan, Z.; Shafiq, I.; et al. Foliar application of silicon improves growth of soybean by enhancing carbon metabolism under shading conditions. Plant Physiol. Biochem. 2021, 159, 43–52. [Google Scholar] [CrossRef]
- Pan, T.; Zhang, J.; He, L.; Hafeez, A.; Ning, C.; Cai, K. Silicon enhances plant resistance of rice against submergence stress. Plants 2021, 10, 767. [Google Scholar] [CrossRef]
- Gowda, R.; Rani, K.U.; Roopashree, B.; Sowmya, K.J. Validating protocol and deciphering the nanoparticulate seed treatment in enhancing seed quality of soybean, pigeon pea, and groundnut. Int. J. Plant Soil Sci. 2023, 35, 94–103. [Google Scholar] [CrossRef]
- Singh-Sangwan, N.E.E.L.A.M.; Abad Farooqi, A.H.; Singh Sangwan, R. Effect of drought stress on growth and essential oil metabolism in lemongrasses. New Phytol. 1994, 128, 173–179. [Google Scholar] [CrossRef]
- Ibrahim, M.S.C.; Meng, T.H.; Ahmad, A.; Ghazali, M.S.M.; Abdullah, W.R.W.; Chuen, N.L. Potential of nanosilicon dioxide extraction from silicon-rich agricultural wastes as a plant growth promoter. Adv. Nat. Sci. Nanosci. Nanotechnol. 2022, 13, 033001. [Google Scholar] [CrossRef]
- Akhtar, N.; Ilyas, N. Role of nanosilica in boosting the activities of metabolites in Triticum aestivum facing drought stress. Plant Soil 2022, 477, 99–115. [Google Scholar] [CrossRef]
- Shashikanthalu, S.P.; Ramireddy, L.; Radhakrishnan, M. Stimulation of the germination and seedling growth of Cuminum cyminum L. seeds by cold plasma. J. Appl. Res. Med. Aromat. Plants 2020, 18, 100259. [Google Scholar] [CrossRef]
- Dorri, M.A.; Kamkar, B.; Aghdasi, M.; Safahani, A.R. Germination and seedling growth of Silybum Marianum as a medicinal plant under salinity stress. Plant Breed. Seed Sci. 2019, 79, 25–38. [Google Scholar] [CrossRef]
- Navarro, M.; Febles, G.; Torres, V. Conceptual bases for estimating seed vigor through growth indicators and initial development. Pastos Forrajes 2012, 35, 233–246. [Google Scholar]
- Steiner, J.J. Seedling rate of development index: Indicator of vigor and seedling growth response. Crop Sci. 1990, 30, 1264–1271. [Google Scholar] [CrossRef]
- Finch-Savage, W.E.; Bassel, G.W. Seed vigour and crop establishment: Extending performance beyond adaptation. J. Exp. Bot. 2016, 67, 567–591. [Google Scholar] [CrossRef]
- Varban, R.; Vidican, R.; Vârban, D.; Stoie, A.; Gâdea, Ș.; Vâtcă, S.; Stoian, V.; Crișan, I.; Stoian, V. Modelling plant morphometric parameters as predictors for successful cultivation of some medicinal Agastache species. Not. Bot. Horti Agrobot. Cluj-Napoca 2022, 50, 12638. [Google Scholar] [CrossRef]
- Hunt, R.; Evans, G.C. Classical data on the growth of maize: Curve fitting with statistical analysis. New Phytol. 1980, 86, 155–180. [Google Scholar] [CrossRef]
- Merma Aroni, J.L. Interpolación Chebyshev como Función Membresía en Lógica Difusa para Control de Humedad en Plantas en vivero Forestal. 2017. Available online: https://repositorio.uap.edu.pe/handle/20.500.12990/8931 (accessed on 5 June 2024).
- Phukan, U.J.; Mishra, S.; Timbre, K.; Luqman, S.; Shukla, R.K. Mentha arvensis exhibit better adaptive characters in contrast to Mentha piperita when subjugated to sustained waterlogging stress. Protoplasma 2014, 251, 603–614. [Google Scholar] [CrossRef] [PubMed]
- Walters, C.; Wheeler, L.M.; Grotenhuis, J.M. Longevity of seeds stored in a genebank: Species characteristics. Seed Sci. Res. 2005, 15, 1–20. [Google Scholar] [CrossRef]
- Meir, P.; Wood, T.E.; Galbraith, D.R.; Brando, P.M.; Da Costa, A.C.; Rowland, L.; Ferreira, L.V. Threshold responses to soil moisture deficit by trees and soil in tropical rain forests: Insights from field experiments. BioScience 2015, 65, 882–892. [Google Scholar] [CrossRef] [PubMed]
- Kirkham, M.B. Field capacity, wilting point, available water, and the nonlimiting water range. In Principles of Soil and Plant Water Relations; Elsevier: Amsterdam, The Netherlands, 2014; pp. 153–170. [Google Scholar]
- Zhang, Y.; Chen, X.; Geng, S.; Zhang, X. A review of soil waterlogging impacts, mechanisms, and adaptive strategies. Front. Plant Sci. 2025, 16, 1545912. [Google Scholar] [CrossRef] [PubMed]
- Pardos, J.A. Plant responses to soil waterlogging. For. Syst. 2004, 13, 101–107. [Google Scholar] [CrossRef]
- Baracaldo, A.; Carvajal, R.; Romero, A.P.; Prieto, A.; García, F.J.; Fischer, G.; Miranda, D. El anegamiento afecta el crecimiento y producción de biomasa en tomate chonto (Solanum lycopersicum L.), cultivado bajo sombrío. Rev. Colomb. Cienc. Hortícolas 2014, 8, 92–102. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Vegetation stress: An introduction to the stress concept in plants. J. Plant Physiol. 1996, 148, 4–14. [Google Scholar] [CrossRef]
- Quezada, R.A.P.; Ontiveros, J.L.R.; Hernández, V.A.G. Transpiración, potencial hídrico y prolina en zarzamora bajo déficit hídrico. Terra Latinoam. 1999, 17, 125–130. [Google Scholar]
- Datta, S.; Taghvaeian, S.; Stivers, J. Understanding Soil Water Content and Thresholds for Irrigation Management. 2017. Available online: https://openresearch.okstate.edu/server/api/core/bitstreams/9465ba33-0677-4818-8075-c8b4e263aa15/content (accessed on 5 June 2024).
- Oklahoma State University. Division of Agricultural Sciences and Natural Resources. Determining Feld Capacity Using Continuous Soil Water Content Data. 2025. Available online: https://extension.okstate.edu/fact-sheets/print-publications/pss/determining-field-capacity-using-continuous-soil-water-content-data.pdf (accessed on 5 June 2024).
- Zotarelli, L.; Dukes, M.D.; Morgan, K.T. Interpretation of Soil Moisture Content to Determine Soil Field Capacity and Avoid Over-Irrigating Sandy Soils Using Soil Moisture Sensors: ae460/ae460, 2/2010; EDIS: Gainesville, FL, USA, 2010; Volume 2010.
- Yan, S.; Weng, B.; Jing, L.; Bi, W. Effects of drought stress on water content and biomass distribution in summer maize (Zea mays L.). Front. Plant Sci. 2023, 14, 1118131. [Google Scholar] [CrossRef]
- Fu, Z.; Ciais, P.; Feldman, A.F.; Gentine, P.; Makowski, D.; Prentice, I.C.; Stoy, P.C.; Bastos, A.; Wigneron, J.P. Critical soil moisture thresholds of plant water stress in terrestrial ecosystems. Sci. Adv. 2022, 8, eabq7827. [Google Scholar] [CrossRef]
- Estrada-Prado, W.; Lescay-Batista, E.; Álvarez-Fonseca, A.; Maceo-Ramos, Y.C. Niveles de humedad en el suelo en la producción de bulbos de cebolla. Agron. Mesoam. 2015, 26, 112–117. [Google Scholar] [CrossRef]
- Parra Quezada, R.A.; Rodríguez Ontiveros, J.L.; González Hernández, V.A. Transpiration, water potential and proline in blackberry under hydric stress. Terra Latinoam. 1999, 17, 125–130. [Google Scholar]
- Wood, J.D.; Gu, L.; Hanson, P.J.; Frankenberg, C.; Sack, L. The ecosystem wilting point defines drought response and recovery of a Quercus-Carya forest. Glob. Change Biol. 2023, 29, 2015–2029. [Google Scholar] [CrossRef]
- Horvath, D.P.; Anderson, J.V.; Chao, W.S.; Foley, M.E. Knowing when to grow: Signals regulating bud dormancy. Trends Plant Sci. 2003, 8, 534–540, Erratum in Trends Plant Sci. 2004, 9, 12. [Google Scholar] [CrossRef] [PubMed]
- Minguez, M.P.; Casanueva, G.M.; de Dios García, J.; Alonso, F.J.G.; Sainz, R.C. Respuesta temporal al ambiente lumínico y la sequía inducida en el regenerado de una masa mixta en el entorno mediterráneo. Cuad. De La Soc. Española Cienc. For. 2019, 45, 1–18. [Google Scholar]
- Moradi, P.; Ford-Lloyd, B.; Pritchard, J. Plant-water responses of different medicinal plant thyme (Thymus spp.) species to drought stress conditions. Aust. J. Crop Sci. 2014, 8, 666–673. [Google Scholar]
- Yao, N.; Li, Y.; Xu, F.; Liu, J.; Chen, S.; Ma, H.; Chau, H.W.; Li Liu, D.; Li, M.; Feng, H.; et al. Permanent wilting point plays an important role in simulating winter wheat growth under water deficit conditions. Agric. Water Manag. 2020, 229, 105954. [Google Scholar] [CrossRef]
- Amissah, L.; Brown, W.H. Wilting, survival, growth, and physiological response of trees and arable crops to drought and shade. Ghana J. For. 2022, 38, 1–20. [Google Scholar]
- Valladares, F.; Arrieta, S.; Aranda, I.; Lorenzo, D.; Sánchez-Gómez, D.; Tena, D.; Suárez, F.; Pardos, J.A. Shade tolerance, photoinhibition sensitivity and phenotypic plasticity of Ilex aquifolium in continental Mediterranean sites. Tree Physiol. 2005, 25, 1041–1052. [Google Scholar] [CrossRef] [PubMed]
- Guarnaschelli, A.B.; Garau, A.M.; Lemcoff, J.H.; Pathauer, P. Impacto de la sombra y la sequía sobre aspectos fisiológicos y de crecimiento en diversos orígenes de E. globulus subsp. globulus. Boletín Inf. CIDEU 2007, 3, 91–98. [Google Scholar]
- Sánchez, C.L.R. Estudios Preliminares Sobre los Efectos de Altas Temperaturas en la Respuesta de Plantas de Tomate (Solanum lycopersicum L.) Sometidas a Estrés por Inundación. Master’s Thesis, Universidad Nacional del Nordeste, Corrientes, Argentina, 2023. [Google Scholar]
- Martínez-López, M.; Tinoco-Ojanguren, C.; Martorell, C. Drought tolerance increases with seed size in a semiarid grassland from southern Mexico. Plant Ecol. 2020, 221, 989–1003. [Google Scholar] [CrossRef]
- Morales-Barrera, J.; Reséndiz-Muñoz, J.; Cruz-Lagunas, B.; Fernández-Muñoz, J.L.; Godínez-Jaimes, F.; de Jesús Adame-Zambrano, T.; Vázquez-Villamar, M.; Romero-Rosales, T.; Zagaceta-Álvarez, M.T.; Aguilar-Cruz, K.A.; et al. Abiotic stress effect on Agastache mexicana subsp. mexicana Yield: Cultivated in two contrasting environments with organic nutrition and artificial shading. Plants 2024, 13, 2661. [Google Scholar] [PubMed]
- Palma-Tenango, M.; Sánchez-Fernández, R.E.; Soto-Hernández, M. A systematic approach to Agastache mexicana research: Biology, agronomy, phytochemistry, and bioactivity. Molecules 2021, 26, 3751. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.R-project.org/ (accessed on 20 September 2023).
- Fox, J.; Weisberg, S. An R Companion to Applied Regression, 3rd ed.; Sage: Thousand Oaks, CA, USA, 2019; Available online: https://cran.r-project.org/web/packages/car/index.html (accessed on 20 September 2023).
- Felipe de Mendiburu. Agricolae: Statistical Procedures for Agricultural Research. R Package Version 1.3-5. 2021. Available online: https://cran.r-project.org/web/packages/agricolae/index.html (accessed on 20 September 2023).
- Gross, J.; Ligges, U. Nortest: Tests for Normality. R Package Version 1.0-4. 2015. Available online: https://cran.r-project.org/web/packages/nortest/ (accessed on 20 September 2023).

| ASL | W1 | W2 | W3 | W4 | W5 | W6 | W7 | W8 | W9 |
|---|---|---|---|---|---|---|---|---|---|
| 38% | 0.21 b | 3.29 b | 7.20 a | 8.58 a | 6.41 a | 11.54 a | 7.49 a | 7.36 a,b | 1.96 c |
| 87% | 2.24 a | 4.21 b | 3.98 a | 5.73 a | 5.37 a | 5.31 b | 5.52 a | 5.72 b | 5.13 b |
| 94% | 1.59 a | 7.02 a | 5.92 a | 8.10 a | 6.76 a | 11.74 a | 10.62 a | 13.18 a | 10.02 a |
| SDL | W1 | W2 | W3 | W4 | W5 | W6 | W7 | W8 | W9 |
|---|---|---|---|---|---|---|---|---|---|
| 0.0% | 1.70 a | 5.50 a | 8.81 a | 9.93 a | 8.22 a | 11.38 a | 9.14 a | 9.51 a | 7.02 a |
| 0.2% | 1.35 a | 3.95 a | 5.40 a | 6.13 a | 5.96 a | 8.80 a | 7.69 a | 9.48 a | 5.38 a |
| 0.4% | 1.53 a | 5.71 a | 4.97 a | 7.13 a | 5.51 a | 9.23 a | 6.94 a | 7.44 a | 6.43 a |
| 0.8% | 0.8 a | 4.20 a | 3.63 a | 6.70 a | 5.03 a | 8.71 a | 7.74 a | 8.59 a | 3.97 a |
| Week | DF | SS | MS | F | p | R2 | |
|---|---|---|---|---|---|---|---|
| W1 | ASL | 2 | 47.51 | 23.75 | 22.03 | <0.01 1 | 0.61 |
| SDL | 3 | 5.53 | 1.84 | 1.71 | 0.18 | ||
| ASL:SDL | 6 | 8.27 | 1.38 | 1.28 | 0.29 | ||
| General | 11 | 212.50 | 19.32 | 5.93 | <0.01 2 | 0.64 | |
| W2 | ASL | 2 | 4.90 | 2.45 | 9.05 | <0.01 3 | 0.44 |
| SDL | 3 | 1.74 | 0.58 | 2.14 | 0.11 | ||
| ASL:SDL | 6 | 1.11 | 0.19 | 0.69 | 0.66 | ||
| General | 11 | 7.75 | 0.70 | 2.60 | <0.01 4 | 0.44 | |
| W3 | ASL | 2 | 2.26 | 1.13 | 1.64 | 0.21 | 0.18 |
| SDL | 3 | 1.53 | 0.51 | 0.74 | 0.53 | ||
| ASL:SDL | 6 | 1.75 | 0.29 | 0.42 | 0.86 | ||
| General | 11 | 5.54 | 0.50 | 0.73 | 0.70 | 0.18 | |
| W4 | ASL | 2 | 8.27 | 4.14 | 1.07 | 0.35 | 0.24 |
| SDL | 3 | 15.58 | 5.19 | 1.35 | 0.27 | ||
| ASL:SDL | 6 | 20.15 | 3.36 | 0.87 | 0.53 | ||
| General | 11 | 44.00 | 4.00 | 1.04 | 0.44 | 0.24 | |
| W5 | ASL | 2 | 3.05 | 1.52 | 0.40 | 0.67 | 0.18 |
| SDL | 3 | 13.71 | 4.57 | 1.21 | 0.32 | ||
| ASL:SDL | 6 | 12.98 | 2.16 | 0.57 | 0.75 | ||
| General | 11 | 29.73 | 2.70 | 0.72 | 0.72 | ||
| W6 | ASL | 2 | 47.72 | 23.86 | 5.30 | <0.01 5 | 0.33 |
| SDL | 3 | 11.81 | 3.94 | 0.87 | 0.46 | ||
| ASL:SDL | 6 | 20.39 | 3.40 | 0.75 | 0.61 | ||
| General | 11 | 79.92 | 7.27 | 1.61 | 0.14 | 0.33 | |
| W7 | ASL | 2 | 24.24 | 12.12 | 2.87 | 0.07 | 0.30 |
| SDL | 3 | 6.56 | 2.19 | 0.52 | 0.67 | ||
| ASL:SDL | 6 | 34.31 | 5.72 | 1.36 | 0.26 | ||
| General | 11 | 65.11 | 5.92 | 1.40 | 0.21 | 0.30 | |
| W8 | ASL | 2 | 54.20 | 27.10 | 4.19 | 0.02 | 0.30 |
| SDL | 3 | 4.65 | 1.55 | 0.24 | 0.87 | ||
| ASL:SDL | 6 | 43.35 | 7.23 | 1.12 | 0.37 | ||
| General | 11 | 102.21 | 9.29 | 1.44 | 0.20 | 0.30 | |
| W9 | ASL | 2 | 124.84 | 62.42 | 13.15 | <0.01 6 | 0.47 |
| SDL | 3 | 19.62 | 6.54 | 1.38 | 0.27 | ||
| ASL:SDL | 6 | 8.29 | 1.38 | 0.29 | 0.94 | ||
| General | 11 | 152.75 | 13.89 | 2.93 | <0.01 7 | 0.47 |
| Seeds by Treatment | |||||
|---|---|---|---|---|---|
| ASL (%) | SDL (%) | Total | |||
| 0.0 | 0.2 | 0.4 | 0.8 | ||
| 38 | τ1 = 80 | τ4 = 80 | τ7 = 80 | τ10 = 80 | 320 |
| 87 | τ2 = 80 | τ5 = 80 | τ8 = 80 | τ11 = 80 | 320 |
| 94 | τ3 = 80 | τ6 = 80 | τ9 = 80 | τ12 = 80 | 320 |
| Total | 240 | 240 | 240 | 240 | 960 |
| Stage/Concept | S1 | S2 | S3 | S4 | S5 | S6 |
|---|---|---|---|---|---|---|
| Duration days | 23 | 13 | 7 | 7 | 7 | 15 |
| Accumulated days | 24 | 37 | 44 | 51 | 58 | 73 |
| Water regime (mm/day) | 7.82 | 5.86 | 3.91 | 1.95 | 1.39 | 1.11 |
| Percentage decrease in water regime * | 0 | 25 | 50 | 75 | 82 | 86 |
| % increase or decrease in water regime ** | 50 | 33 | 0 | 50 | 65 | 72 |
| Parameter | W1 | W2 | W3 | W4 | W5 | W6 | W7 | W8 | W9 |
|---|---|---|---|---|---|---|---|---|---|
| 0.1964 | 0 | 0 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | |
| SW | 0.25 | 0.61 | <0.01 | 0.15 | 0.47 | 0.68 | 0.46 | 0.64 | 0.19 |
| B | <0.01 | 0.22 | <0.01 | 0.15 | 0.14 | 0.63 | 0.75 | 0.37 | 0.91 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cruz-Lagunas, B.; Delgado-Núñez, E.J.; Reséndiz-Muñoz, J.; Godínez-Jaimes, F.; Urbieta-Parrazales, R.; Zagaceta-Álvarez, M.T.; Pureco-Leyva, Y.Y.; Fernández-Muñoz, J.L.; Gruintal-Santos, M.A. Vigour Index on Time Basis Calculation on Agastache mexicana Subsp. mexicana Throughout Induced Hydric Stress: SiO2 and Artificial Shade Application Effects. Stresses 2025, 5, 63. https://doi.org/10.3390/stresses5040063
Cruz-Lagunas B, Delgado-Núñez EJ, Reséndiz-Muñoz J, Godínez-Jaimes F, Urbieta-Parrazales R, Zagaceta-Álvarez MT, Pureco-Leyva YY, Fernández-Muñoz JL, Gruintal-Santos MA. Vigour Index on Time Basis Calculation on Agastache mexicana Subsp. mexicana Throughout Induced Hydric Stress: SiO2 and Artificial Shade Application Effects. Stresses. 2025; 5(4):63. https://doi.org/10.3390/stresses5040063
Chicago/Turabian StyleCruz-Lagunas, Blas, Edgar Jesús Delgado-Núñez, Juan Reséndiz-Muñoz, Flaviano Godínez-Jaimes, Romeo Urbieta-Parrazales, María Teresa Zagaceta-Álvarez, Yeimi Yuleni Pureco-Leyva, José Luis Fernández-Muñoz, and Miguel Angel Gruintal-Santos. 2025. "Vigour Index on Time Basis Calculation on Agastache mexicana Subsp. mexicana Throughout Induced Hydric Stress: SiO2 and Artificial Shade Application Effects" Stresses 5, no. 4: 63. https://doi.org/10.3390/stresses5040063
APA StyleCruz-Lagunas, B., Delgado-Núñez, E. J., Reséndiz-Muñoz, J., Godínez-Jaimes, F., Urbieta-Parrazales, R., Zagaceta-Álvarez, M. T., Pureco-Leyva, Y. Y., Fernández-Muñoz, J. L., & Gruintal-Santos, M. A. (2025). Vigour Index on Time Basis Calculation on Agastache mexicana Subsp. mexicana Throughout Induced Hydric Stress: SiO2 and Artificial Shade Application Effects. Stresses, 5(4), 63. https://doi.org/10.3390/stresses5040063







