Aflatoxin B1-Induced Neurobehavioral Alterations in Chickens: Inhibition of Brain Acetylcholinesterase Activity, Induction of Oxidative Stress, and Promotion of Inflammatory Gene Expression
Abstract
1. Introduction
2. Results
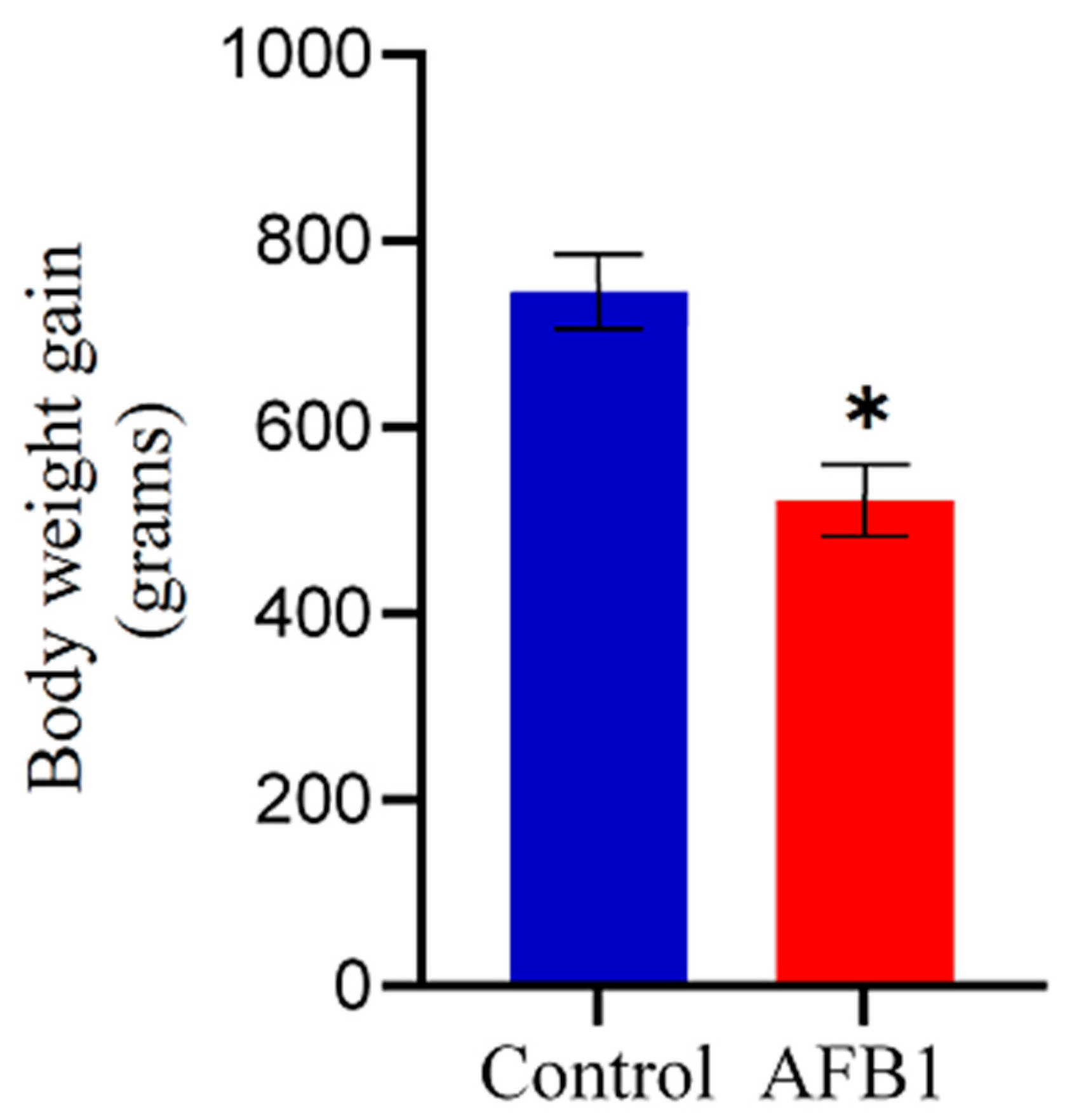
2.1. Clinical Observation of Intoxicated Chickens
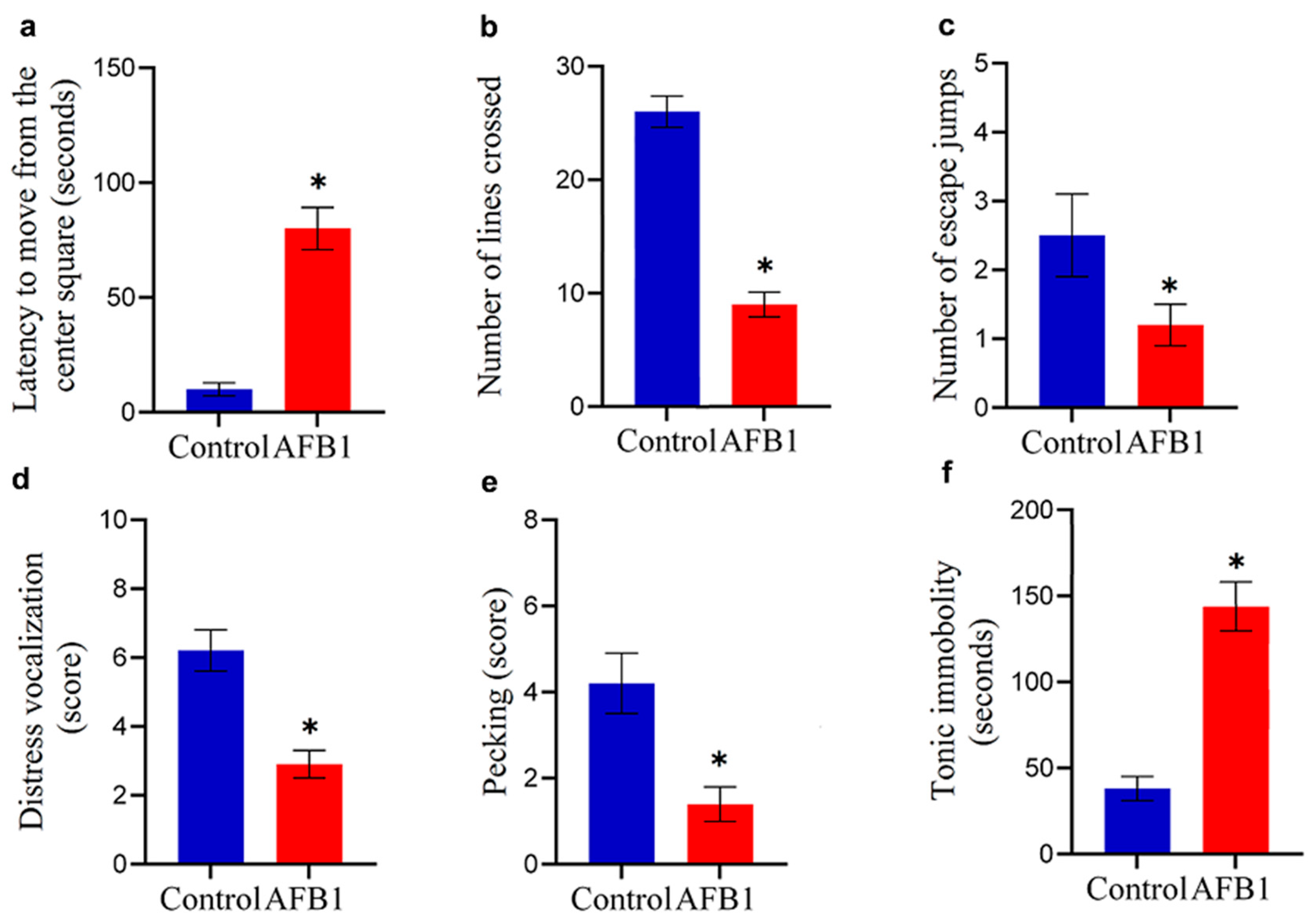
2.2. Neurobehavioral Effects of AFB1 Based on Open-Field Activity and Tonic Immobility
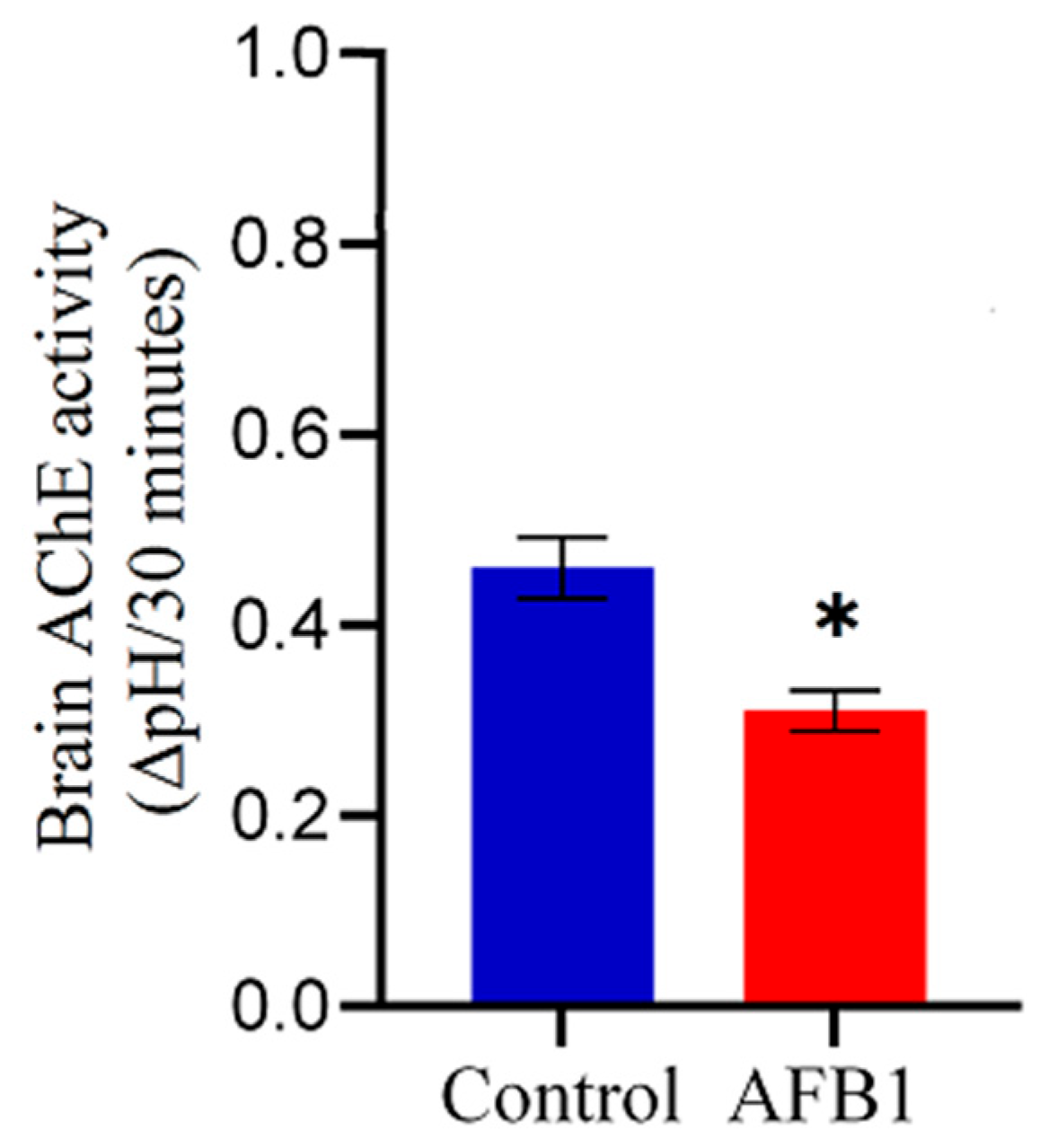
2.3. Effect of AFB1 on Brain AChE Activity
2.4. Antioxidant Imbalance in the Brain of AFB1-Treated Chickens
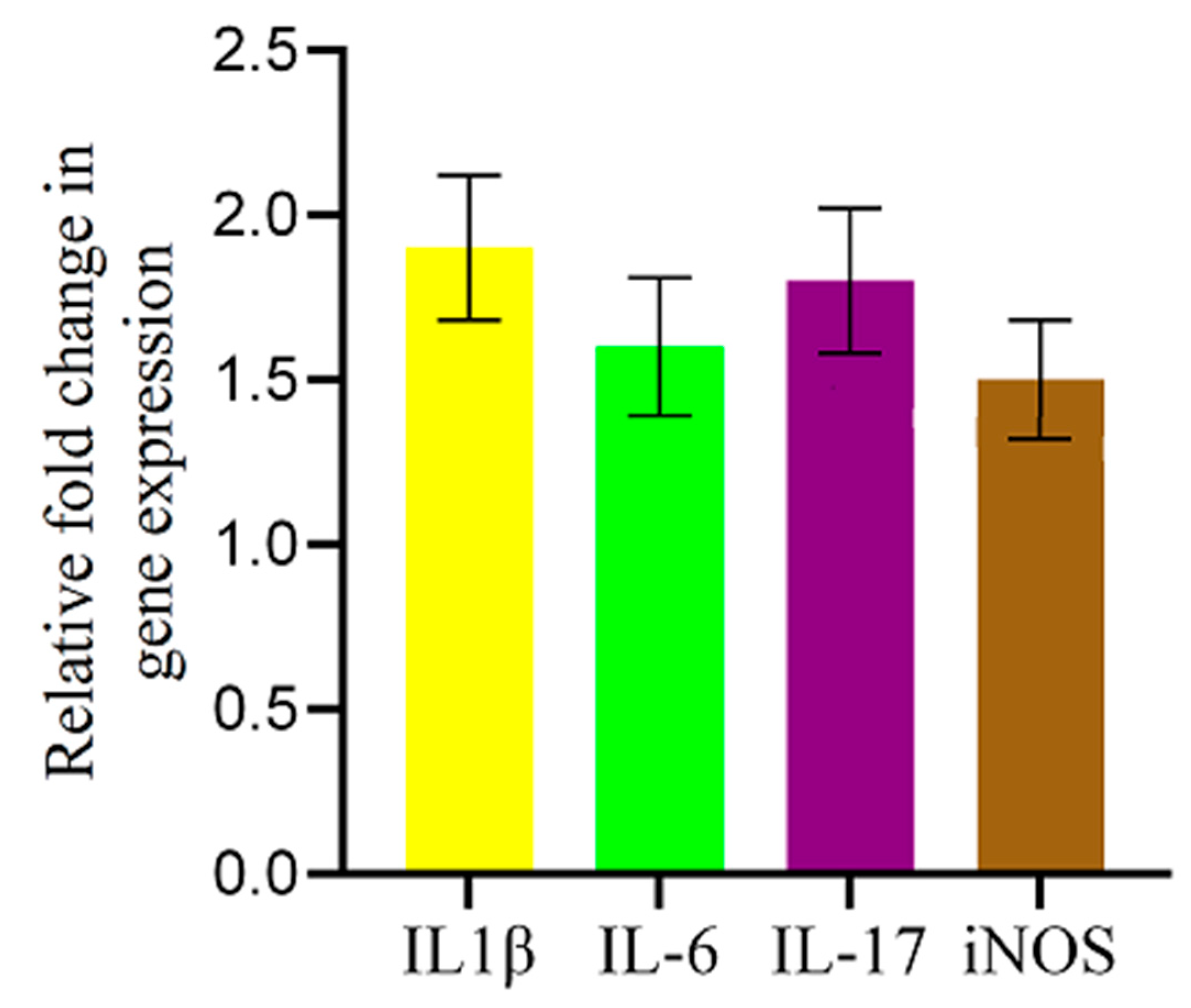
2.5. AFB1-Induced Alterations in Inflammation-Related Gene Expression in the Chicken Brain
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Chicken Husbandry and Treatment
4.3. Preparation of AFB1-Contaminated Diet
4.4. Experimental Design
4.5. Open Field Test
4.6. Tonic Immobility Test
4.7. Determination of Brain AChE Activity
4.8. Assessment of Oxidative Stress Indicators in the Brain
4.9. Gene Expression Related to Pro-Inflammatory Cytokines
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fouché, T.; Claassens, S.; Maboeta, M. Aflatoxins in the Soil Ecosystem: An Overview of Its Occurrence, Fate, Effects and Future Perspectives. Mycotoxin Res. 2020, 36, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Fouad, A.M.; Ruan, D.; El Senousey, H.A.K.; Chen, W.; Jiang, S.; Zheng, C. Harmful Effects and Control Strategies of Aflatoxin B1 Produced by Aspergillus Flavus and Aspergillus Parasiticus Strains on Poultry: Review. Toxins 2019, 11, 176. [Google Scholar] [CrossRef]
- Abrehame, S.; Manoj, V.R.; Hailu, M.; Chen, Y.Y.; Lin, Y.C.; Chen, Y.P. Aflatoxins: Source, Detection, Clinical Features and Prevention. Processes 2023, 11, 204. [Google Scholar] [CrossRef]
- Lewis, L.; Onsongo, M.; Njapau, H.; Schurz-Rogers, H.; Luber, G.; Kieszak, S.; Nyamongo, J.; Backer, L.; Dahiye, A.M.; Misore, A.; et al. Aflatoxin Contamination of Commercial Maize Products during an Outbreak of Acute Aflatoxicosis in Eastern and Central Kenya. Environ. Health Perspect. 2005, 113, 1763–1767. [Google Scholar] [CrossRef] [PubMed]
- Yunus, A.W.; Razzazi-Fazeli, E.; Bohm, J. Aflatoxin B1 in Affecting Broiler’s Performance, Immunity, and Gastrointestinal Tract: A Review of History and Contemporary Issues. Toxins 2011, 3, 566–590. [Google Scholar] [CrossRef]
- Alharthi, A.S.; Al Sulaiman, A.R.; Aljumaah, R.S.; Alabdullatif, A.A.; Elolimy, A.A.; Alqhtani, A.H.; Al-Garadi, M.A.; Abudabos, A.M. Protective Effect of Date Pits on Growth Performance, Carcass Traits, Blood Indices, Intestinal Morphology, Nutrient Digestibility, and Hepatic Aflatoxin Residues of Aflatoxin B1-Exposed Broilers. Agriculture 2022, 12, 476. [Google Scholar] [CrossRef]
- Altyar, A.E.; Kensara, O.A.; Sayed, A.A.; Aleya, L.; Almutairi, M.H.; Zaazouee, M.S.; Elshanbary, A.A.; El-Demerdash, F.M.; Abdel-Daim, M.M. Acute Aflatoxin B1-Induced Hepatic and Cardiac Oxidative Damage in Rats: Ameliorative Effects of Morin. Heliyon 2023, 9, e21837. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, F.; Zhou, X.; Liu, M.; Zang, H.; Liu, X.; Shan, A.; Feng, X. Alleviation of Oral Exposure to Aflatoxin B1-Induced Renal Dysfunction, Oxidative Stress, and Cell Apoptosis in Mice Kidney by Curcumin. Antioxidants 2022, 11, 1082. [Google Scholar] [CrossRef]
- Subramaniam, S.; Sabran, M.R.; Stanslas, J.; Kirby, B.P. Effect of Aflatoxin B1 Exposure on the Progression of Depressive-like Behavior in Rats. Front. Nutr. 2022, 9, 1032810. [Google Scholar] [CrossRef]
- Dai, C.; Tian, E.; Li, H.; Gupta, S.D.; Hao, Z.; Wang, Z.; Velkov, T.; Shen, J. Molecular Mechanisms of Aflatoxin Neurotoxicity and Potential Neuroprotective Agents. Food Sci. Hum. Wellness 2023, 13, 2445–2455. [Google Scholar] [CrossRef]
- Cao, W.; Yu, P.; Yang, K.P.; Cao, D. Aflatoxin B1: Metabolism, Toxicology, and Its Involvement in Oxidative Stress and Cancer Development. Toxicol. Mech. Methods 2022, 32, 395–419. [Google Scholar] [CrossRef] [PubMed]
- Eaton, D.L.; Williams, D.E.; Coulombe, R.A. Species Differences in the Biotransformation of Aflatoxin B1: Primary Determinants of Relative Carcinogenic Potency in Different Animal Species. Toxins 2025, 17, 30. [Google Scholar] [CrossRef]
- Park, S.; Lee, J.Y.; You, S.; Song, G.; Lim, W. Neurotoxic Effects of Aflatoxin B1 on Human Astrocytes in Vitro and on Glial Cell Development in Zebrafish in Vivo. J. Hazard. Mater. 2020, 386, 121639. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Chen, Q.; Wang, L.; Gao, X.; Zhu, W.; Mu, P.; Deng, Y. Aflatoxin B1 Induces Neurotoxicity through Reactive Oxygen Species Generation, Dna Damage, Apoptosis, and s-Phase Cell Cycle Arrest. Int. J. Mol. Sci. 2020, 21, 6517. [Google Scholar] [CrossRef]
- Song, C.; Wang, Z.; Cao, J.; Dong, Y.; Chen, Y. Neurotoxic Mechanisms of Mycotoxins: Focus on Aflatoxin B1 and T-2 Toxin. Environ. Pollut. 2024, 356, 124359. [Google Scholar] [CrossRef]
- Pohanka, M. Cholinesterases, a Target of Pharmacology and Toxicology. Biomed. Pap. 2011, 155, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Liu, S.B.; Zhang, Q.; Tan, H.Z. Effects of Aflatoxin B1 on Growth Performance, Carcass Traits, Organ Index, Blood Biochemistry and Oxidative Status in Chinese Yellow Chickens. J. Vet. Med. Sci. 2023, 85, 1015–1022. [Google Scholar] [CrossRef]
- Aytekin Sahin, G.; Karabulut, D.; Unal, G.; Sayan, M.; Sahin, H. Effects of Probiotic Supplementation on Very Low Dose AFB1-Induced Neurotoxicity in Adult Male Rats. Life Sci. 2022, 306, 120798. [Google Scholar] [CrossRef]
- Braga, A.C.M.; Souto, N.S.; Cabral, F.L.; Dassi, M.; Rosa, É.V.F.; Guarda, N.D.S.; Royes, L.F.F.; Fighera, M.R.; Moresco, R.N.; Oliveira, M.S. Intermittent Exposure to Aflatoxin B1 Did Not Affect Neurobehavioral Parameters and Biochemical Markers of Oxidative Stress. Brain Sci. 2023, 13, 386. [Google Scholar] [CrossRef]
- Coulombe Jr, R.A.; Sharma, R.P. Effect of Repeated Dietary Exposure of Aflatoxin B1 on Brain Biogenic Amines and Metabolites in the Rat. Toxicol. Appl. Pharmacol. 1985, 80, 496–501. [Google Scholar] [CrossRef]
- Linardaki, Z.I.; Lamari, F.N.; Margarity, M. Saffron (Crocus sativus L.) Tea Intake Prevents Learning/Memory Defects and Neurobiochemical Alterations Induced by Aflatoxin B1 Exposure in Adult Mice. Neurochem. Res. 2017, 42, 2743–2754. [Google Scholar] [CrossRef] [PubMed]
- Cometa, M.F.; Lorenzini, P.; Fortuna, S.; Volpe, M.T.; Meneguz, A.; Palmery, M. In Vitro Inhibitory Effect of Aflatoxin B 1 on Acetylcholinesterase Activity in Mouse Brain. Toxicology 2005, 206, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.C.R.; Hawkins, E.; Jahr, F.M.; McClay, J.L.; Deshpande, L.S. Repeated Exposure to Chlorpyrifos Is Associated with a Dose-Dependent Chronic Neurobehavioral Deficit in Adult Rats. Neurotoxicology 2022, 90, 172–183. [Google Scholar] [CrossRef]
- Guo, Y.; Balasubramanian, B.; Zhao, Z.-H.; Liu, W.-C. Marine Algal Polysaccharides Alleviate Aflatoxin B1-Induced Bursa of Fabricius Injury by Regulating Redox and Apoptotic Signaling Pathway in Broilers. Poult. Sci. 2021, 100, 844–857. [Google Scholar] [CrossRef]
- Gugliandolo, E.; Peritore, A.F.; D’Amico, R.; Licata, P.; Crupi, R. Evaluation of Neuroprotective Effects of Quercetin against Aflatoxin B1-Intoxicated Mice. Animals 2020, 10, 898. [Google Scholar] [CrossRef]
- Mehrzad, J.; Malvandi, A.M.; Alipour, M.; Hosseinkhani, S. Environmentally Relevant Level of Aflatoxin B1 Elicits Toxic Pro-Inflammatory Response in Murine CNS-Derived Cells. Toxicol. Lett. 2017, 279, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, I.; Sun, X.; Wang, H.; Li, W.; Wang, X.; Cheng, P.; Li, S.; Zhang, X.; Hamid, S. Curcumin Successfully Inhibited the Computationally Identified CYP2A6 Enzyme-Mediated Bioactivation of Aflatoxin B1 in Arbor Acres Broiler. Front. Pharmacol. 2017, 8, 143. [Google Scholar] [CrossRef]
- Li, S.; Han, M.; Zhang, Y.; Ishfaq, M.; Liu, R.; Wei, G.; Zhang, X.; Zhang, X. Effect of Curcumin as Feed Supplement on Immune Response and Pathological Changes of Broilers Exposed to Aflatoxin B1. Biomolecules 2022, 12, 1188. [Google Scholar] [CrossRef]
- Fernandez, A.; Verde, M.T.; Gascon, M.; Ramos, J.; Gomez, J.; Luco, D.F.; Chavez, G. Variations of Clinical Biochemical Parameters of Laying Hens and Broiler Chickens Fed Aflatoxin-Containing Feed. Avian Pathol. 1994, 23, 37–47. [Google Scholar] [CrossRef]
- Hernandez, E.; James, F.; Torrey, S.; Widowski, T.; Schwean-Lardner, K.; Monteith, G.; Turner, P.V. Evaluation of Brain Death in Laying Hens During On-Farm Killing by Cervical Dislocation Methods or Pentobarbital Sodium Injection. Front. Vet. Sci. 2019, 6, 297. [Google Scholar] [CrossRef]
- Mohammad, F.K.; Faris, G.A.M. Behavioral Effects of Acute Manganese Chloride Administration in Chickens. Biol. Trace Elem. Res. 2006, 110, 265–273. [Google Scholar] [CrossRef]
- Alnuaimi, S.I.; Al-Abdaly, Y.Z. Neurobehavioral Toxicity of Copper Sulfate Accompanied by Oxidative Stress and Histopathological Alterations in Chicks’ Brain. Iraqi J. Vet. Sci. 2023, 37, 53–60. [Google Scholar] [CrossRef]
- Michel, H.O. An Electrometric Method for the Determination of Red Blood Cell and Plasma Cholinesterase Activity. J. Lab. Clin. Med. 1949, 34, 1564–1568. [Google Scholar]
- Mohammad, F.K.; Faris, G.A.; Al-Kassim, N.A. A Modified Electrometric Method for Measurement of Erythrocyte Acetylcholinesterase Activity in Sheep. Vet. Hum. Toxicol. 1997, 39, 337–339. [Google Scholar] [PubMed]
- Mohammad, F.K.; St Omer, V.E. Modifications of Michel’s Electrometric Method for Rapid Measurement of Blood Cholinesterase Activity in Animals: A Minireview. Vet. Hum. Toxicol. 1982, 24, 119–121. [Google Scholar] [PubMed]
- Alias, A.S. The Use of an Electrometric Method for Measurement of Cholinesterase Activity in Plasma and Tissues of Local Doves. In Proceedings of the 11th Scientific Congress, Faculty of Veterinary Medicine, Assiut University, Assiut, Egypt, 5–7 December 2004; Volume 1, pp. 241–259. [Google Scholar]
- Abass, K.S.; Mohammad, F.K. Validation of an Electrometric Method for Cholinesterase Measurement in the Plasma and Tissues of the Chicken. In Proceedings of the 11th Scientific Congress, Faculty of Veterinary Medicine, Assiut University, Assiut, Egypt, 5–7 December 2004; Volume 1, pp. 241–259. [Google Scholar]
- Al-Badrany, Y.M.A.; Mohammad, F.K. Effects of Acute and Repeated Oral Exposure to the Organophosphate Insecticide Chlorpyrifos on Open-Field Activity in Chicks. Toxicol. Lett. 2007, 174, 110–116. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing Real-Time PCR Data by the Comparative CT Method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Salvador, C.; Cortes, A.L.; Pandiri, A.R.; Gimeno, I.M. Cytokine Expression in the Eye and Brain of Chickens Following Infection with a Very Virulent plus Marek’s Disease Virus Strain. Vet. Immunol. Immunopathol. 2021, 237, 110277. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liang, J.; Jiang, C.; Cui, J.; Hong, L.; Hao, Z.; Tang, Y.; Liu, Y.; Cui, X.; Teng, X. Se Alleviated Pb-Caused Neurotoxicity in Chickens: SPS2-GPx1-GSH-IL-2/IL-17-NO Pathway, Selenoprotein Suppression, Oxidative Stress, and Inflammatory Injury. Antioxidants 2024, 13, 370. [Google Scholar] [CrossRef]

| Gene | Sense Primer | Antisense Primer | Reference |
|---|---|---|---|
| IL1β | 5′-GCTCTACATGTCGTGTGTGATGAG-3′ | 5′-TGTCGATGTCCCGCATGA-3′ | [40] |
| IL-6 | 5′-AAATCCCTCCTCGCCAATCT-3′ | 5′-CCCTCACGGTCTTCTCCATAAA-3′ | [41] |
| IL-17 | 5′-CATGTTGTCAGCCAGCATTTCT-3′ | 5′-CATCTTTTTGGGTTAGGCATCC-3′ | [41] |
| iNOS | 5′-AGGCCAAACATCCCTGGAGGTC-3′ | 5′-TCATAGAGACGGTGCTGCCAG-3′ | [40] |
| 28S | 5′-GGCGAAGCCAGAGGAAACT-3′ | 5′-GACGACCGATTTGCACGTC–3′ | [40] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Selman, W.H.; Alyasari, N.K.H.; Al-Karagoly, H. Aflatoxin B1-Induced Neurobehavioral Alterations in Chickens: Inhibition of Brain Acetylcholinesterase Activity, Induction of Oxidative Stress, and Promotion of Inflammatory Gene Expression. Stresses 2025, 5, 34. https://doi.org/10.3390/stresses5020034
Selman WH, Alyasari NKH, Al-Karagoly H. Aflatoxin B1-Induced Neurobehavioral Alterations in Chickens: Inhibition of Brain Acetylcholinesterase Activity, Induction of Oxidative Stress, and Promotion of Inflammatory Gene Expression. Stresses. 2025; 5(2):34. https://doi.org/10.3390/stresses5020034
Chicago/Turabian StyleSelman, Wisam Hussein, Noora Kadhim Hadi Alyasari, and Hassan Al-Karagoly. 2025. "Aflatoxin B1-Induced Neurobehavioral Alterations in Chickens: Inhibition of Brain Acetylcholinesterase Activity, Induction of Oxidative Stress, and Promotion of Inflammatory Gene Expression" Stresses 5, no. 2: 34. https://doi.org/10.3390/stresses5020034
APA StyleSelman, W. H., Alyasari, N. K. H., & Al-Karagoly, H. (2025). Aflatoxin B1-Induced Neurobehavioral Alterations in Chickens: Inhibition of Brain Acetylcholinesterase Activity, Induction of Oxidative Stress, and Promotion of Inflammatory Gene Expression. Stresses, 5(2), 34. https://doi.org/10.3390/stresses5020034







