Abstract
From germination to harvest, cereal crops are constantly exposed to a broad spectrum of abiotic stresses that significantly hinder their growth and productivity, posing a serious threat to global food security. Seed resilience and performance are foundational to sustainable agriculture, making the development of efficient, low-cost, and environmentally friendly strategies to enhance seed vigor and stress tolerance a critical priority. Seed priming has emerged as a promising pre-sowing technique that involves exposing seeds to specific organic or inorganic compounds under controlled conditions to improve their physiological and biochemical traits. Various priming techniques—including halopriming, chemical priming, osmopriming, hormonal priming, hydropriming, biopriming, and nanopriming—have been successfully applied in cereal crops to alleviate the adverse effects of environmental stressors. These treatments trigger a cascade of metabolic and molecular responses, including the modulation of hormonal signaling, enhancement of antioxidant defense systems, stabilization of cellular structures, and upregulation of stress-responsive genes. Together, these changes contribute to enhanced seed germination, improved growth and performance, and greater adaptability to abiotic stress conditions. This review provides a comprehensive overview of seed priming strategies in cereal crops, emphasizing their mechanisms of action and their impact on plant performance in challenging environments.
1. Introduction
Cereal crops (wheat, rice, maize, and sorghum, etc.) are the cornerstone of global agriculture, accounting for >50% of the world’s caloric supply and the primary source of nutrition for billions of people, particularly in developing countries [1]. These cereals/grains are rich in nutrients such as carbohydrates, proteins, various dietary fibers, vitamins, and minerals, and are thus essential in human diets [2]. Besides direct human consumption, cereals are a critical source of animal feed (livestock) and raw materials for an industrial sector, highlighting their versatility [3]. Their wide adaptability enables them to be grown in various agroecological zones, and they are central to the sustainable development of global food systems [4]. Europe, America, and Asia produce nearly 80% of the world’s cereal grains comprising rice, sorghum, barley, rye, oats, millet, and wheat [5]. But increased demand from population growth and changing diets pose major challenges for agricultural productivity and sustainability. Addressing these challenges requires innovative strategies to enhance cereal yield and resilience, ensuring food security for the growing global population [5]. Despite their significance, cereal crops are highly susceptible to abiotic stresses, which are intensified by climate change [6]. These stresses significantly limit crop growth, yield, and quality, posing a serious threat to global food production [6]. In cereal crops, they disrupt key physiological processes such as photosynthesis, nutrient uptake, respiration, and reproduction, ultimately reducing productivity [7]. As a result, cereals produce excessive reactive oxygen species (ROS) due to incomplete reduction in molecular oxygen [8]. These ROS inflict oxidative damage on nucleic acids, lipid membranes, and cellular proteins, leading to irreversible dysfunction of metabolic structures and, ultimately, cell death [8]. Faced with escalating climate challenges, enhancing abiotic stress tolerance in cereal crops has become a pressing priority to safeguard global food security. While traditional breeding and genetic engineering have provided valuable tools, their limitations—such as long development timelines, high costs, and regulatory constraints—highlight the need for more immediate and accessible alternatives. Among these, seed priming has gained significant attention as a simple, cost-effective, and eco-friendly strategy to boost stress resilience in plants [9]. Seed priming involves controlled hydration of seeds to initiate early metabolic processes essential for germination, followed by re-drying to halt further development [10]. This pre-conditioning enables seeds to “memorize” the priming stimulus, thereby enhancing their physiological preparedness to face environmental stress upon sowing [11]. Such stress memory is orchestrated by a suite of molecular and biochemical changes, including hormonal modulation—particularly of abscisic acid (ABA) and gibberellic acid (GA)—alongside ROS signaling and transcriptional reprogramming of stress-responsive genes [9,12]. Emerging evidence also points to a role for epigenetic modifications, such as DNA methylation and histone remodeling, in sustaining these priming effects across developmental stages [13]. A wide range of priming techniques have been developed, each tailored to specific stress contexts and crop species. These include hydropriming (water), osmopriming (using osmotic agents like PEG), halopriming (salt solutions), hormonal priming (e.g., with salicylic or gibberellic acid), biopriming (with beneficial microbes), magnetopriming, matriconditioning, and nanopriming (using nanoparticles) [14]. These methods enhance germination rates, improve seedling vigor, and prime the expression of key stress-related genes, particularly those involved in antioxidant defense, ion homeostasis, and photosynthetic efficiency [9,12,13,15]. In this context, seed priming stands out as a promising approach to enhance cereal crop performance under abiotic stress. Its multifaceted impact on molecular pathways, hormonal dynamics, and epigenetic stability positions it as a valuable tool in sustainable agriculture, especially in regions vulnerable to extreme environmental conditions. The objective of this review is to provide a comprehensive examination of the mechanisms underlying seed priming in cereal crops, with a particular focus on its role in enhancing resilience to abiotic stress. By exploring the molecular, physiological, biochemical, and transcriptomic responses to seed priming, this review aims to highlight the potential of this strategy for improving cereal crop productivity and stress tolerance in the face of climate change.
2. Overview of Seed Priming
Seed germination represents the initial phase of a plant’s life cycle, transitioning a dry, dormant seed into a metabolically active state upon water uptake [16]. This process culminates in radicle protrusion, signifying the emergence of the seedling. Germination can be divided into three distinct phases [17]: (i) imbibition, characterized by rapid water absorption; (ii) metabolic activation, involving the resumption of cellular activities; (iii) radicle emergence, marking the completion of germination. During imbibition, gibberellins (GAs), primarily GA3 stored in the embryo, are translocated to the aleurone layer of the endosperm, a protein-rich tissue [18]. This hormonal signal induces the activation of hydrolytic enzymes, including acid cysteine endopeptidases, serine carboxypeptidases, and neutral aminopeptidases, which facilitate the degradation of storage proteins into amino acids within aleurone cells [18]. Concurrently, GA3 enhances the transcription of genes encoding α-amylase, a key enzyme in starch hydrolysis [19]. This regulation is mediated by the GA-induced transcription factor GAMYB (GA-induced Myb (myeloblastosis)-like protein), which binds to the promoters of α-amylase genes, thereby promoting their expression and ensuring the mobilization of stored reserves essential for seedling growth and development [19]. Various agronomic practices, including seed priming, have been suggested to increase seed vigor [20]. Seed priming is a highly effective technique that enhances seed germination [21], promotes growth [22], and increases crop resilience to both abiotic and biotic stresses [23]. Priming is a technique that involves exposing seeds to various organic or inorganic compounds [24]. This process requires soaking seeds in specific solutions for a predetermined period under controlled conditions, followed by drying them back to their initial moisture content to prevent radicle emergence before sowing [21]. Priming activates key metabolic processes that enhance germination and seedling emergence in numerous species, particularly cereal crops [25,26,27]. Additionally, it helps mitigate the adverse effects of seed deterioration [28,29]. Primed seeds show remarkable improvements in germination rate, uniformity, and overall plant growth [30,31,32]. Multiple seed priming techniques, including halopriming, chemical priming, osmopriming, hormonal priming, hydropriming, biopriming, and nanopriming, have been applied to rice to mitigate the effects of environmental stresses [33,34,35,36,37]. Numerous scientific studies have demonstrated the activation of key biochemical and cellular mechanisms during seed priming. Antioxidant enzymes such as catalase and superoxide dismutase are synthesized, protecting cellular structures from oxidative stress [38,39]. Oxidative protection plays a crucial role in safeguarding the mitochondria [40,41], thereby supporting uninterrupted cellular respiration and maintaining ATP production. Gene expression analyses indicate that seed priming promotes cellular metabolism and facilitates the repair of DNA damage accumulated during seed aging [42]. Additionally, seed priming influences the regulation of microRNAs (miRNAs), which play a pivotal role in plant hormone biosynthesis, contributing to enhanced germination performance [43].
3. Abiotic Stresses in Cereal Crops
In recent years, the intensity of abiotic stresses has significantly increased, particularly during midday in the summer months [44]. This situation has been further aggravated by unsustainable agricultural practices [45]. Most abiotic stresses are closely linked to water availability, and their effects are more pronounced during the daytime in summer [46]. As water uptake and transport are disrupted, photosynthetic efficiency is inevitably affected [47,48,49,50,51]. Under both abiotic and biotic stress conditions, reactive oxygen species and reactive nitrogen species are generated [52]. These highly reactive molecules can interfere with various metabolic processes, and in severe cases, they may damage nuclear membranes and DNA, leading to single- or double-strand breaks [53]. Cereals, like other crops, undergo various physiological and structural modifications in response to abiotic stresses [54]. These environmental challenges include drought, extreme temperatures, waterlogging, salinity, and heavy metal toxicity. Such stress conditions significantly disrupt essential physiological processes like photosynthesis, respiration, and nutrient uptake, ultimately affecting plant growth and productivity [55]. Despite significant advances in research on abiotic stress factors in cereals, these challenges continue to intensify, posing a growing threat to crop productivity and food security. Table 1 illustrates the impact of abiotic stressors on cereals.

Table 1.
Cereal crop responses to various abiotic stresses.
4. Seed Priming Applications in Cereal Crops for Mitigating Environmental Stresses
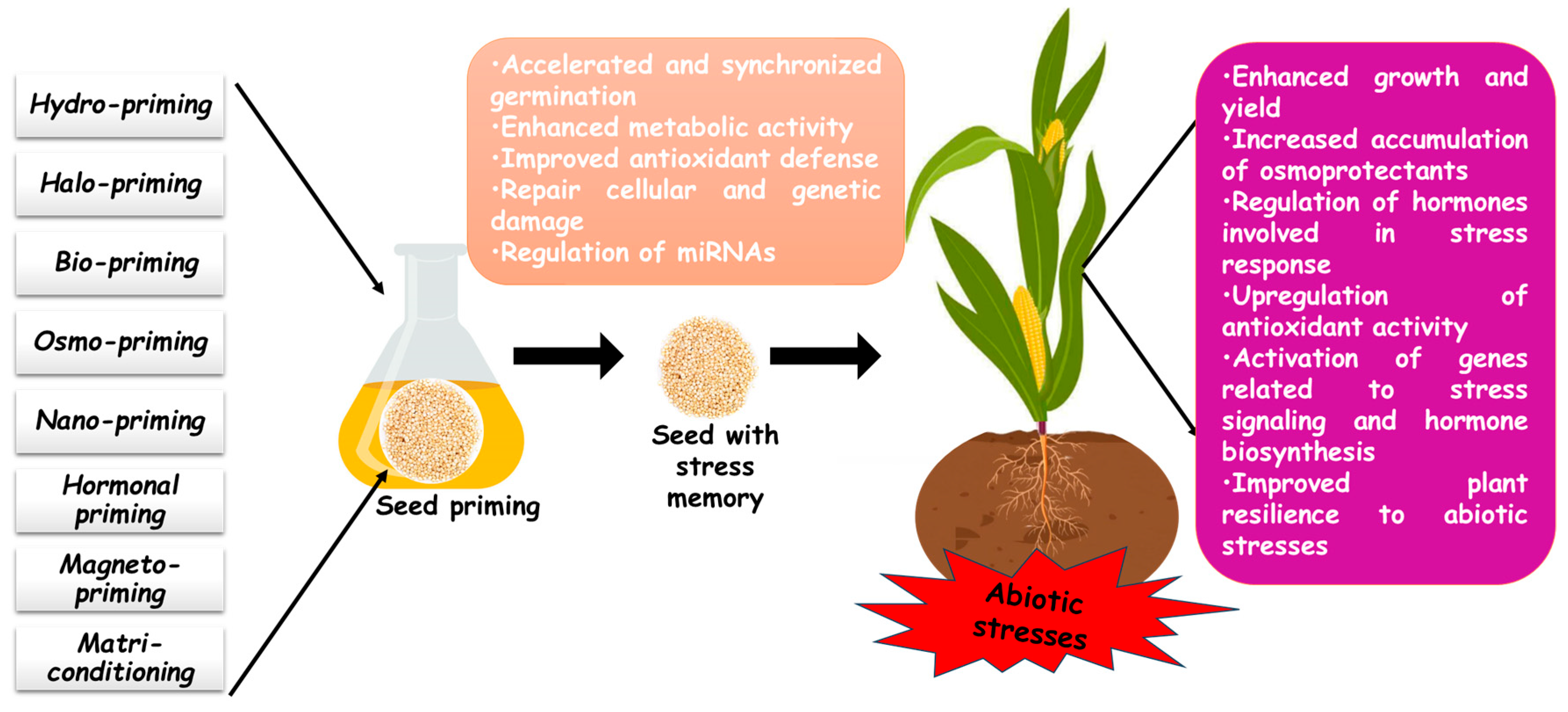
Seed priming significantly enhances cereals’ resistance to abiotic stress by boosting physiological, hormonal, and biochemical responses. The application of priming agents under adverse environmental conditions regulates various plant functions, promoting stress adaptation and improved growth. Several cereal species have demonstrated significant benefits from seed priming, including enhanced germination, seedling vigor, and stress tolerance. Therefore, this section provides an in-depth review of different seed priming techniques, highlighting their role as an eco-friendly strategy to improve plant resilience against environmental challenges such as salinity, drought, and heavy metal toxicity (Table 2 and Figure 1).
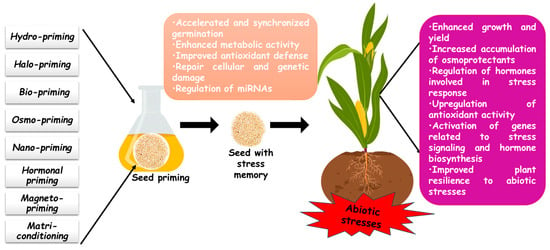
Figure 1.
Seed priming-induced modulation of abiotic stress responses in cereal crops.
4.1. Role of SP in Cereals Exposed to Salt Stress
Salt stress is known to impair seed germination by inducing osmotic imbalance and ion toxicity, particularly due to excessive accumulation of Na+ and Cl−, which hinders water uptake and metabolic activation [31]. However, seed priming has emerged as a practical and efficient approach to counteracting these effects and improving germination success in cereal crops exposed to saline environments (Table 2). For instance, pre-treatment of wheat (Triticum aestivum L.) seeds with agents such as spermidine, proline, potassium silicate, or saline water have been shown to significantly enhance germination rates and seedling vigor under salt stress [71]. Similar beneficial effects have been observed in sorghum (Sorghum bicolor L.), a species particularly vulnerable to salinity during the early stages of development. Among various priming agents tested, calcium chloride (notably at a concentration of 50 mM) demonstrated the highest efficacy, significantly enhancing germination potential, vigor index, root elongation, and biomass accumulation. These findings highlight the potential of Ca2+-based priming to improve early seedling establishment by modulating ion homeostasis and promoting cellular stability under saline stress [72]. In rice (Oryza sativa L.), nanopriming has recently emerged as an innovative strategy to improve germination and seedling establishment under salt stress. Notably, the application of chitosan-based nanoparticles (CNPs) has shown promising results in enhancing germination percentage, seedling vigor, and antioxidant activity under increasing concentrations of NaCl [73]. Seeds primed with CNPs exhibited superior morphological and physiological performance compared to hydroprimed controls, suggesting a role for nanomaterials in reinforcing the plant’s early defense mechanisms and improving stress resilience. In general, priming treatments stimulate early metabolic reactivation of cereal seeds, improving water absorption and initiating pre-germinative processes that result in better seedling establishment and growth in cereals. In primed cereal seeds, enhanced Na+/H+ antiporter activity promotes selective ion transport, reducing toxic sodium accumulation while maintaining potassium uptake, which is essential for preserving cellular homeostasis [74,75]. Furthermore, seed priming appears to induce a form of molecular “stress memory” by modulating gene expression patterns associated with hormone biosynthesis (notably gibberellins), photosynthetic capacity, and antioxidant defense pathways, thereby improving stress resilience in cereal crops [76].
4.2. Role of SP in Cereals Exposed to Drought
In cereals exposed to drought, seed priming improves the ability of seeds to absorb water, accelerating the activation of metabolic pathways crucial for seedling growth (Table 1). Recent studies demonstrate the positive effects of cereals’ priming on germination traits under water-limited conditions (Table 2). For instance, osmopriming has been shown to stimulate the expression of key enzymes involved in the germination process, including amylases, lipases, and proteases [77]. This enzymatic activation facilitates the mobilization of stored nutrients, thereby supporting embryo growth and the early stages of seedling development [78]. Moreover, priming with agents such as silicon dioxide, potassium nitrate, and salicylic acid has been reported to improve germination, early growth, vigor, and drought tolerance in cereals. These benefits are associated with increased water uptake and the enhanced activity of hydrolytic enzymes such as amylases, dehydrogenases, and xylanases [79]. Similarly, polyethylene glycol priming in sorghum has been found to increase germination efficiency by improving the mobilization of seed reserves, the percentage of reserve depletion, and total seedling dry biomass under drought stress [80]. In addition to its role in improving germination, seed priming continues to exert positive effects on cereals by enhancing subsequent plant growth and development under drought stress conditions. Recent research also points to the potential of beneficial microorganisms, such as endophytic bacteria, in supporting plant performance under water-limited environments. Notably, seed priming with certain endophytes such as Metarhizium anisopliae has been shown to improve drought tolerance in wheat by enhancing a range of physiological and biochemical parameters. These include enhanced photosynthetic efficiency, increased leaf thickness, improved root architecture, and elevated proline accumulation, which contributes to better osmotic regulation. Furthermore, plants treated with M. anisopliae exhibited reduced oxidative stress, linked to increased activity of key antioxidant enzymes including catalase, peroxidase, and superoxide dismutase. Under both moderate and severe drought conditions, these physiological improvements translated into greater biomass accumulation and significantly enhanced grain yield, with up to a 41% increase in thousand-grain weight [81]. Therefore, it can be inferred that seed priming represents an effective approach to improve the tolerance of cereal crops to abiotic stresses such as drought.
4.3. Seed Priming as a Strategy to Mitigate Heavy Metal Stress in Cereals
Seed priming has proven to be an effective technique to enhance cereal crop resilience against various heavy metals, including cadmium (Cd), lead (Pb), and chromium (Cr). Among the chemical agents, silicon nanoparticles (Si NPs) have shown particular promise in mitigating Cd toxicity in wheat. Si NP-based priming not only improved plant growth and chlorophyll content but also significantly enhanced antioxidant enzyme activities, leading to reduced oxidative stress. This treatment decreased Cd accumulation—especially in grains—while promoting silicon uptake, with reductions reaching up to 75% in grains compared to unprimed controls [82]. In parallel, the use of potassium nitrate (KNO3) as a priming agent demonstrated protective effects against Pb stress in maize. KNO3 priming improved germination, photosynthetic pigment levels, and antioxidant responses, especially in roots where Pb accumulation was highest, while limiting its translocation to aerial parts [83]. In addition to chemical approaches, biological priming using beneficial microorganisms has gained attention for its capacity to alleviate metal-induced toxicity. Biopriming rice seeds with Pseudomonas fluorescens improved germination performance by enhancing amylase enzyme activity, stability, and efficiency under metal stress [84]. Similarly, the root endophyte Piriformospora indica has been shown to increase Cd tolerance in rice by promoting plant growth and biomass while reducing reactive oxygen species accumulation and cell damage [85]. This effect is partially attributed to the sequestration of Cd within fungal structures, which minimizes its toxic effects on hosts tissues. Moreover, priming with polyamines such as spermine (SPM) has been shown to mitigate chromium toxicity in rice seedlings. SPM application enhanced germination, biomass production, and photosynthetic efficiency under Cr stress. It also improved hormonal and nutrient homeostasis, reinforced antioxidant defenses, and reduced oxidative stress indicators such as hydrogen peroxide, superoxide radical, and malondialdehyde. Additionally, SPM upregulated salicylic acid-responsive genes, suggesting its involvement in the activation of key stress signaling pathways [86]. Collectively, these findings highlight the multifaceted potential of seed priming in enhancing heavy metal tolerance in cereal crops through improved physiological and biochemical mechanisms.

Table 2.
Priming methods and their role in enhancing stress resilience in cereal seeds.
Table 2.
Priming methods and their role in enhancing stress resilience in cereal seeds.
| Stress Type | Priming Method | Seed Species | Effects | Ref. |
|---|---|---|---|---|
| Salt stress | Chitosan priming | Triticum durum L. | -Improved germination percentage, seedling length, and fresh/dry mass. -Increased GABA shunt metabolites (GABA, glutamate, alanine). -Reduced oxidative damage (lower MDA levels than untreated seeds). | [87] |
| Chitosan nanoparticles and chitosan priming | Oryza sativa L. | -Enhanced germination potential and seedling vigor. -Improved morphological, physiological, and biochemical responses. -Increased antioxidant activity. | [73] | |
| Ascorbic acid, potassium silicate, proline, spermidine, and saline water | Triticum durum L. | -Enhanced germination percentage and rate. | [71] | |
| Salicylic acid | Zea mays L. | -Improved growth, root dry matter, leaf relative water content, and free proline content. | [88] | |
| Salicylic acid (SA), Gibberellic acid (GA), and Sodium chloride | Hordeum vulgare L. | SA: Improved antioxidant defense mechanisms; -Increased proline, sugar, and ascorbic acid levels. -Reduced ROS accumulation and lipid peroxidation. -Supported seed adaptation to salinity stress. GA: Most effective for improving germination, shoot/root growth, and photosynthesis under salinity. | [76] | |
| Kinetin, Gibberellic acid, Iron, Auxin, and Potassium nitrate | Triticum aestivum L. | -Improved germination rate and seedling growth under salt stress. -Enhanced coleoptile and radicle growth. -Stimulated root development and seedling growth. | [89] | |
| Osmopriming with Ca2+ and K+ | Chenopodium quinoa | -Enhanced salinity tolerance through antioxidant enzyme activation (CAT, APX, SOD, GPX, PPO). -Increased antioxidant metabolites (phenolics, flavonoids, ascorbic acid), proline, glycine betaine, soluble carbohydrates. -Improved K+/Na+ homeostasis and Na+ exclusion. | [90] | |
| Zinc oxide nanoparticles + 24-epibrassinolide | Zea mays L. | -Improved root length, root surface area, stem diameter, relative leaf water content, total chlorophyll content, photosynthetic rate, and uptake of Zn and K+. -Reduced Na+ accumulation and Na+/K+ ratio. | [91] | |
| Hydrogen peroxide | Zea mays L. | -Enhanced germination, growth, and physiological traits. -Improved antioxidant defense (APX, CAT, POD, AsA). -Increased leaf water status, soluble proteins, amino acids, proline, sugars, IAA, and GA. -Reduced MDA and H2O2 levels. -Limited Na+ and Cl− uptake while improving Ca2+, K+, and Mg2+ content. | [92] | |
| Glutathione + Zinc | Zea mays L. | -Improved germination and seedling emergence -Enhanced antioxidant defense. -Reduced ROS and MDA levels. -Improved nutrient uptake (K+, Ca2+), and reduced Na+ accumulation and toxicity. -Increased K+/Na+ and Ca2+/Na+ ratios | [93] | |
| Biopriming with Bacillus sp. | Oryza sativa L. | -Enhanced germination percentage, seedling growth, and photosynthetic pigment content. | [94] | |
| Priming with PVP-coated silver nanoparticles | Hordeum vulgare L. | -Restored seed germination under salt stress; -Reduced ROS accumulation. -Increased antioxidant enzymes (SOD, CAT, GR, GPX). -Upregulation of antioxidant genes (HvSOD, HvCAT, HvGR, HvGPX). | [95] | |
| Drought stress | Selenium | Chenopodium quinoa | -Increased main panicle length, panicle weight, and grain weight. -Enhanced gas exchange parameters (photosynthesis, stomatal conductance). -Increased chlorophyll content, total phenol content, and water relations. -Improved grain quality (P, K, proteins). | [96] |
| Biopriming with Trichoderma harzianum | Oryza sativa L. | -Improved drought tolerance by reducing leaf rolling, increasing chlorophyll content, leaf area index, membrane stability index, and relative water content. Proline levels were minimized. -Enhanced morphological, physiological, and biochemical responses and delayed drought effects during tillering stage. | [97] | |
| Biopriming with Bacillus subtilis | Triticum aestivum L. | -Improved germination and growth. -Increased chlorophyll a and b, carotenoids, water-holding capacity, and salicylic acid content were observed. -Reduced proline, lipid peroxidation, and electrolyte leakage. | [98] | |
| Zinc Oxide Nanoparticles | Triticum aestivum L. | -Enhanced antioxidant enzyme activities (POX, CAT, GR), total phenolics, flavonoids, and sugars under drought. -ROS detoxification was improved. | [99] | |
| γ-Aminobutyric acid (GABA) seed priming | Triticum aestivum L. | -Improved germination, seedling biomass, water content, and photosystem efficiency. -Boosted antioxidant enzyme activities, leaf free proline, glycine betaine, soluble phenolics, and endogenous GABA levels. | [100] | |
| Halopriming with NaCl | Zea mays L. | -Improved seed germination uniformity, speed, and overall vigor (increased germination percentage, index, and seedling dry weight). -Improved water use efficiency. | [101] | |
| Ethephon seed priming | Triticum aestivum L. | -Enhanced drought stress memory at the tillering stage by maintaining leaf water; -Decreased MDA levels; -Improved root-to-leaf ABA signaling, ROS scavenging, and osmotic regulation; -Upregulated genes in ethylene-mediated pathways (carbon metabolism, glutathione metabolism, phenylpropanoid biosynthesis). | [102] | |
| Hydropriming | Chenopodium quinoa Willd | -Enhanced growth and seed yield. -Increase pigments and proline contents. | [103] | |
| Osmopriming with KNO3, Mg(NO3)2, GA3, Hydropriming | Zea mays L. | -Improved the antioxidative defense system. -Improved morpho-physiological traits such as total chlorophyll, and chlorophyll a and b. -Increased proline and catalase activity and decreased MDA content. | [104] | |
| Copper nanoparticle priming | Zea mays L. | -Enhanced drought tolerance in maize by increasing leaf water content, biomass, and yield components. -Elevated anthocyanin, chlorophyll, and carotenoid contents. -ROS accumulation was reduced through activation of antioxidant enzymes. | [105] | |
| Silver oxide nanoparticle | Triticum aestivum L. | -Significant improvement in germination, seedling growth, and biomass under drought conditions; -Positive correlation between root length and other traits; -Increased chlorophyll content; -Identification of 261 single-nucleotide polymorphisms and key genes (TraesCS1A02G049700) linked to drought tolerance. | [106] | |
| Cobalt (Co) toxicity | ZnO nanoparticle priming | Zea mays L. | -Improved plant growth, biomass, and photosynthesis. -Reduced ROS and MDA levels, limited Co uptake, stabilized ultrastructures and photosynthetic machinery, and enhanced nutrient content and antioxidant enzyme activities. | [107] |
| Cadmium (Cd) toxicity | Multiwall carbon nanotubes (MWCNTs) priming | Zea mays L. | -Improved seed germination rate, root and shoot growth, and antioxidant enzyme activities (POD, SOD, CAT). | [108] |
| Bacillus subtilis NA2, Aspergillus niger PMI-118, and L-proline priming | Triticum aestivum L. | -Improved plant biomass, shoot length, root length, chlorophyll, total sugars, proteins, and ascorbic acid. -Reduced antioxidant enzyme activities (CAT, APX) and oxidative stress (H2O2). | [109] | |
| Copper (Cu) toxicity | Silicon, melatonin, salicylic acid, glycine betaine, and ascorbic acid priming | Triticum aestivum L. | -Inhanced plant growth, biomass, and photosynthetic traits. -Reduced oxidative stress (MDA, H2O2), and boosted antioxidant enzymes. -Improved proline metabolism, AsA-GSH cycle, and gene expression. | [110] |
| Copper (Cu) toxicity | Seed priming with silver ions | Oryza sativa L. | -Increased fresh biomass, reduced Cu content in roots and shoots, and improved nutrient uptake (Ca, Fe, Mg, Mn). -Improved root cell viability, maintained root morphology, reduced malondialdehyde accumulation, and activated key signaling pathways (MAPK, phytohormone) for defense response. | [111] |
| Arsenic (As) toxicity | Seed priming with zinc | Oryza sativa L. | -Restored seedling growth, reduce As uptake, and limite oxidative stress through modulating redox homeostasis. -Reduced ROS production and protected antioxidant enzymes. | [112] |
| Aluminum (Al) toxicity | Seed priming with 24-epibrassinolide | Oryza sativa (rice) | -Enhanced seed germination, root and shoot length, and biomass. -Reduced MDA and H2O2 levels, enhanced antioxidant enzyme activities (SOD, CAT, APX), and improved photosynthetic pigments. | [113] |
| Lead (Pb) toxicity | Seed priming with calcium and salicylic acid | Triticum aestivum L. | -Alleviated oxidative stress, restored osmoprotectants, reduced Pb ion content, and enhanced antioxidant enzyme activities. They also downregulated genes overexpressed under Pb stress, suggesting protective mechanisms. | [114] |
| Lead (Pb) toxicity | Seed priming with nano–graphene oxide, nano–molybdenum, nano–selenium, nano–zinc oxide, and nano–silica. | Hordeum vulgare L. | -Improved plant growth and biomass. -Reduced oxidative stress, decreased MDA and H2O2 accumulation, enhanced enzymatic and non-enzymatic antioxidants, and supported better gas exchange and gene expression. | [115] |
5. Molecular Signaling Pathways and Gene Regulatory Networks Mediated by Seed Priming in Cereal Crops to Enhance Stress Tolerance
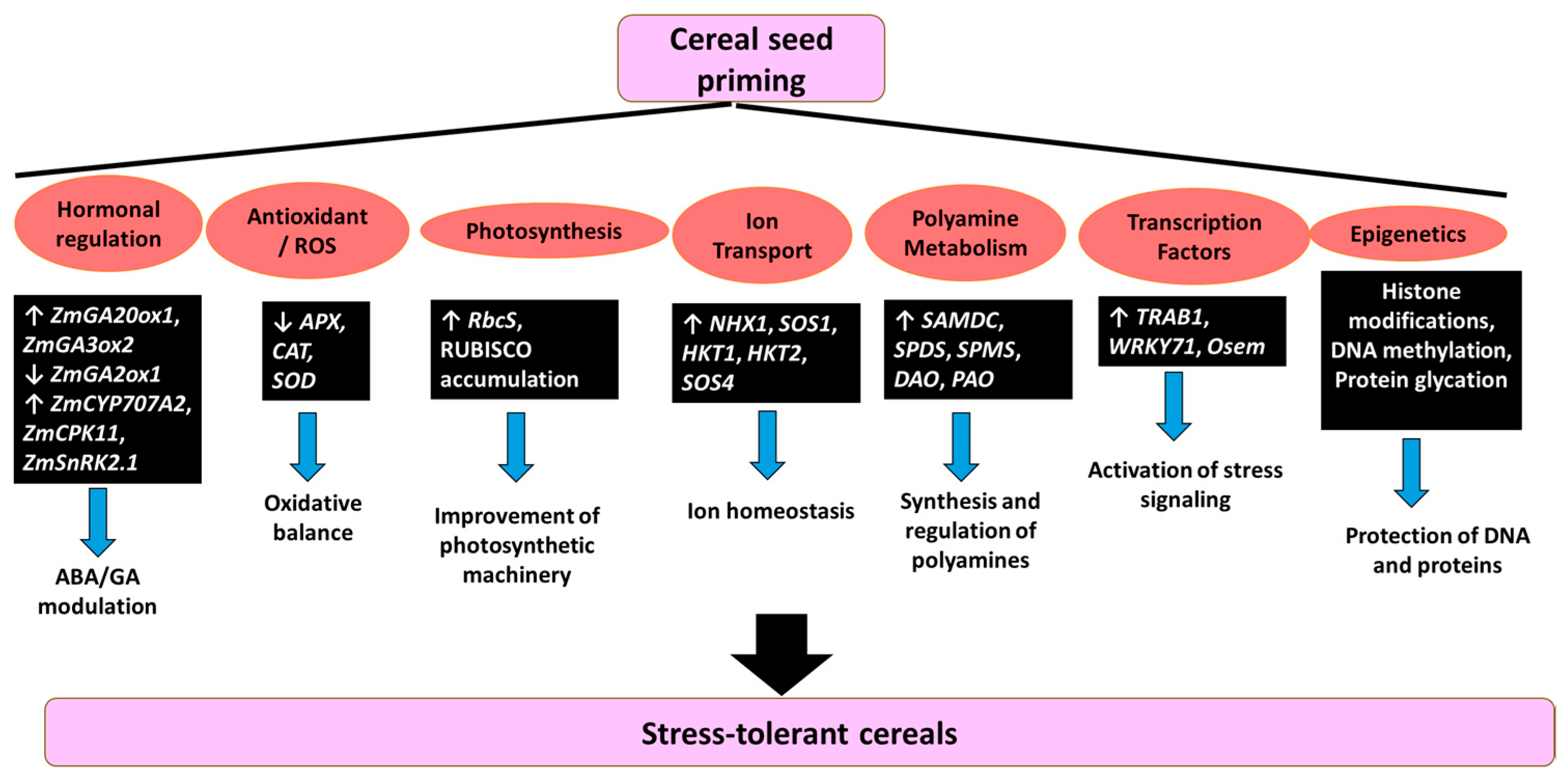
Seed priming in cereal crops activates a complex and multilayered molecular network that enhances tolerance to abiotic stresses. This network involves a coordinated interplay between hormonal regulation, redox homeostasis, transcriptional reprogramming, and epigenetic modifications [12]. Rather than acting through isolated mechanisms, these interconnected responses precondition seeds to respond more efficiently and robustly to environmental challenges, ultimately enhancing seedling vigor and crop establishment (Figure 2). A central mechanism through which priming confers stress tolerance is hormonal modulation, especially the dynamic balance between ABA and GA, two key regulators of germination and stress signaling. In maize, for instance, priming with SA and H2O2 under chilling stress promotes GA biosynthesis by upregulating ZmGA20ox1 and ZmGA3ox2, while simultaneously downregulating the catabolic gene ZmGA2ox1. This hormonal shift favors GA accumulation, enhancing germination and early growth. At the same time, ABA catabolism is stimulated through ZmCYP707A2, and its signaling is fine-tuned via ZmCPK11 and ZmSnRK2.1, thus alleviating ABA-mediated inhibition [116]. In rice, PEG priming under nano-ZnO stress has shown to improve growth, reduce oxidative stress markers such as MDA, and lower antioxidant enzyme activities (SOD, POD, CAT). The reduced expression of antioxidant-related genes (APX, CAT, SOD) suggests that primed plants are better preconditioned and less reliant on stress-induced responses. Structural integrity in mesophyll and root tissues was also preserved, as evidenced by ultrastructural analysis [117]. Under salinity stress, polyamine-based priming enhances both enzymatic and non-enzymatic antioxidant systems. This includes upregulation of RbcS and accumulation of RUBISCO, improving photosynthetic performance. Additionally, stress-related transcription factors (TRAB1, WRKY71, Osem), ABA biosynthesis (NCED3), and ion transport (NHX1) genes are upregulated, contributing to hormonal and ionic homeostasis. Polyamine metabolism is also stimulated through increased expression of SAMDC, SPDS, SPMS, DAO, and PAO, providing metabolic support for adaptation [118]. In wheat, seed priming leads to the strong induction of salt-responsive genes such as TaNHX1, TaSOS1, TaSOS4, TaHKT1, and TaHKT2, which are involved in ion transport, photosynthetic pigment biosynthesis, and oxidative stress mitigation [119]. Similar responses occur in rice, where OsSOS1, OsNHX1, and OsHKT1 contribute to ionic balance and stress tolerance under saline conditions [120]. Beyond stress perception and early seedling responses, seed priming also influences seed maturation and storage. During desiccation, their chromatin structure is at risk due to changes in DNA helical conformation. Priming helps mitigate desiccation-induced damage through epigenetic mechanisms such as histone modifications, DNA methylation, and protein glycation. These processes preserve DNA and protein integrity, playing a protective role in seed longevity and viability [121].
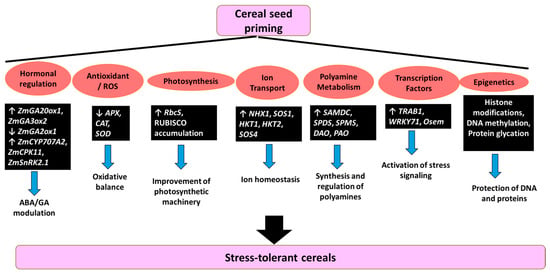
Figure 2.
Molecular mechanisms and regulatory pathways mediated by seed Priming for enhanced abiotic stress tolerance in cereal crops.
6. Conclusions
Seed priming offers a practical, low-cost, and environmentally sustainable strategy to enhance the performance of cereal crops under abiotic stress conditions. This review highlights how different priming techniques—such as hormonal, osmotic, or nano-based approaches—activate critical physiological and molecular responses, including improved hormonal balance, enhanced antioxidant capacity, and regulation of stress-related gene expression. These changes collectively improve seed germination, early seedling vigor, and stress resilience. To translate these benefits into real-world agricultural practices, future research must focus on standardizing priming protocols based on crop species, developmental stage, and specific stress types. In addition, the integration of omics technologies (transcriptomics, proteomics, epigenomics) will be essential for identifying reliable biomarkers and understanding the long-term stability and transgenerational effects of priming.
Author Contributions
Conceptualization, methodology, and investigation, I.J., A.E., R.B.L., M.A.-E.-M., and M.A.; writing—original draft preparation, I.J.; writing—review and editing, I.J., A.E., R.B.L., M.A.-E.-M., and M.A. All authors have read and agreed to the published version of the manuscript.
Funding
This project received no external funding.
Data Availability Statement
No new data were created or analyzed in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Food and Agriculture Organization. Agricultural Production Statistics 2010–2023; Food and Agriculture Organization: Rome, Italy, 2024. [Google Scholar]
- Guo, H.; Wu, H.; Sajid, A.; Li, Z. Whole Grain Cereals: The Potential Roles of Functional Components in Human Health. Crit. Rev. Food Sci. Nutr. 2022, 62, 8388–8402. [Google Scholar] [CrossRef]
- Nahi, O.; Siankevich, S. Upcycling of Cereal Byproducts: A Sustainable Opportunity to Valorize Wasted Nutrients and Derive Bioactive Compounds for Humans and Animals Nutrition and Health. Chimia 2023, 77, 858–866. [Google Scholar] [CrossRef]
- Noort, M.W.J.; Renzetti, S.; Linderhof, V.; du Rand, G.E.; Marx-Pienaar, N.J.M.M.; de Kock, H.L.; Magano, N.; Taylor, J.R.N. Towards Sustainable Shifts to Healthy Diets and Food Security in Sub-Saharan Africa with Climate-Resilient Crops in Bread-Type Products: A Food System Analysis. Foods 2022, 11, 135. [Google Scholar] [CrossRef] [PubMed]
- Jeyasri, R.; Muthuramalingam, P.; Satish, L.; Pandian, S.K.; Chen, J.T.; Ahmar, S.; Wang, X.; Mora-Poblete, F.; Ramesh, M. An Overview of Abiotic Stress in Cereal Crops: Negative Impacts, Regulation, Biotechnology and Integrated Omics. Plants 2021, 10, 1472. [Google Scholar] [CrossRef]
- Hossain, A.; Skalicky, M.; Brestic, M.; Maitra, S.; Alam, M.A.; Syed, M.A.; Hossain, J.; Sarkar, S.; Saha, S.; Bhadra, P.; et al. Consequences and Mitigation Strategies of Abiotic Stresses in Wheat (Triticum aestivum L.) under the Changing Climate. Agronomy 2021, 11, 241. [Google Scholar] [CrossRef]
- Mthiyane, P.; Aycan, M.; Mitsui, T. Strategic Advancements in Rice Cultivation: Combating Heat Stress through Genetic Innovation and Sustainable Practices—A Review. Stresses 2024, 4, 452–480. [Google Scholar] [CrossRef]
- Sharma, V.; Garg, N. Organic Solutes in Cereals Under Abiotic Stress. In Sustainable Remedies for Abiotic Stress in Cereals; Springer Nature: Berlin/Heidelberg, Germany, 2022; pp. 29–50. [Google Scholar] [CrossRef]
- Hameed, A.; Hussain, S.; Nisar, F.; Rasheed, A.; Shah, S.Z. Seed Priming as an Effective Technique for Enhancing Salinity Tolerance in Plants: Mechanistic Insights and Prospects for Saline Agriculture with a Special Emphasis on Halophytes. Seeds 2025, 4, 14. [Google Scholar] [CrossRef]
- Thakur, M.; Sharma, P.; Anand, A. Seed priming-induced early vigor in crops: An alternate strategy for abiotic stress tolerance. In Priming and Pretreatment of Seeds and Seedlings: Implication in Plant Stress Tolerance and Enhancing Productivity in Crop Plants; Springer: Berlin/Heidelberg, Germany, 2019; pp. 163–180. [Google Scholar]
- Ali, K.; Mubasher, H.M.; Sher, A.; Sattar, A.; Manaf, A. Seed Priming for Abiotic Stress Tolerance. In Climate-Resilient Agriculture; Springer International Publishing: Berlin/Heidelberg, Germany, 2023; Volume 2, pp. 641–665. [Google Scholar] [CrossRef]
- Jatana, B.S.; Grover, S.; Ram, H.; Baath, G.S. Seed Priming: Molecular and Physiological Mechanisms Underlying Biotic and Abiotic Stress Tolerance. Agronomy 2024, 14, 2901. [Google Scholar] [CrossRef]
- Liu, X.; Quan, W.; Bartels, D. Stress Memory Responses and Seed Priming Correlate with Drought Tolerance in Plants: An Overview. Planta 2022, 255, 1–14. [Google Scholar] [CrossRef]
- Koushal, S.; Mankar, A.A.; Anbarasan, S.; Kumar, V.; Kumari, J.; Nagarjuna, S.; Jahan, R.; Kishan Kumar, R.; Satapathy, S.N. Mechanism and Methodologies of Seed Priming: Enhancing Germination and Crop Resilience. Plant Cell Biotechnol. Mol. Biol. 2024, 25, 185–194. [Google Scholar] [CrossRef]
- Kubala, S.; Lechowska, K.; Wojtyla, L.; Kosmala, A.; Quinet, M.; Lutts, S.; Garnczarska, M. Transcriptome and proteome changes accompanying increased vigor of osmoprimed rape (Brassica napus L.) seeds. BioTechnologia. J. Biotechnol. Comput. Biol. Bionanotechnol. 2013, 3, 94. [Google Scholar]
- El-Maarouf-bouteau, H. The Seed and the Metabolism Regulation. Biology 2022, 11, 168. [Google Scholar] [CrossRef]
- Nonogaki, H.; Bassel, G.W.; Bewley, J.D. Germination-Still a Mystery. Plant Sci. 2010, 179, 574–581. [Google Scholar] [CrossRef]
- Jacobsen, J.V.; Gubler, F.; Chandler, P.M. Gibberellin Action in Germinated Cereal Grains. In Plant Hormones; Springer: Berlin/Heidelberg, Germany, 1995; pp. 246–271. [Google Scholar] [CrossRef]
- Ma, Z.; Bykova, N.V.; Igamberdiev, A.U. Cell Signaling Mechanisms and Metabolic Regulation of Germination and Dormancy in Barley Seeds. Crop J. 2017, 5, 459–477. [Google Scholar] [CrossRef]
- Farooq, M.; Usman, M.; Nadeem, F.; Rehman, H.U.; Wahid, A.; Basra, S.M.A.; Siddique, K.H.M. Seed Priming in Field Crops: Potential Benefits, Adoption and Challenges. Crop Pasture Sci. 2019, 70, 731–771. [Google Scholar] [CrossRef]
- Corbineau, F.; Taskiran-Özbingöl, N.; El-Maarouf-Bouteau, H. Improvement of Seed Quality by Priming: Concept and Biological Basis. Seeds 2023, 2, 101–115. [Google Scholar] [CrossRef]
- Rhaman, M.S.; Tania, S.S.; Imran, S.; Rauf, F.; Kibria, M.G.; Ye, W.; Hasanuzzaman, M.; Murata, Y. Seed Priming with Nanoparticles: An Emerging Technique for Improving Plant Growth, Development, and Abiotic Stress Tolerance. J. Soil. Sci. Plant Nutr. 2022, 22, 4047–4062. [Google Scholar] [CrossRef]
- Yadav, N.; Bora, S.; Devi, B.; Upadhyay, C.; Singh, P. Nanoparticle-Mediated Defense Priming: A Review of Strategies for Enhancing Plant Resilience against Biotic and Abiotic Stresses. Plant Physiol. Biochem. 2024, 213, 108796. [Google Scholar] [CrossRef]
- Singh, H.; Jassal, R.K.; Kang, J.S.; Sandhu, S.S.; Kang, H.; Grewal, K. Seed Priming Techniques in Field Crops—A Review. Agric. Rev. 2015, 36, 251–264. [Google Scholar] [CrossRef]
- Afzal, S.; Sharma, D.; Singh, N.K. Eco-Friendly Synthesis of Phytochemical-Capped Iron Oxide Nanoparticles as Nano-Priming Agent for Boosting Seed Germination in Rice (Oryza sativa L.). Environ. Sci. Pollut. Res. 2021, 28, 40275–40287. [Google Scholar] [CrossRef]
- Sharma, D.; Afzal, S.; Singh, N.K. Nanopriming with Phytosynthesized Zinc Oxide Nanoparticles for Promoting Germination and Starch Metabolism in Rice Seeds. J. Biotechnol. 2021, 336, 64–75. [Google Scholar] [CrossRef]
- Batool, R.; Umer, M.J.; Shabbir, M.Z.; Wang, Y.; Ahmed, M.A.; Guo, J.; He, K.; Zhang, T.; Bai, S.; Chen, J.; et al. Seed Myco-Priming Improves Crop Yield and Herbivory Induced Defenses in Maize by Coordinating Antioxidants and Jasmonic Acid Pathway. BMC Plant Biol. 2022, 22, 1–17. [Google Scholar] [CrossRef]
- Biswas, S.; Seal, P.; Majumder, B.; Biswas, A.K. Efficacy of Seed Priming Strategies for Enhancing Salinity Tolerance in Plants: An Overview of the Progress and Achievements. Plant Stress. 2023, 9, 100186. [Google Scholar] [CrossRef]
- Chen, S.; Liu, H.; Yangzong, Z.; Gardea-Torresdey, J.L.; White, J.C.; Zhao, L. Seed Priming with Reactive Oxygen Species-Generating Nanoparticles Enhanced Maize Tolerance to Multiple Abiotic Stresses. Environ. Sci. Technol. 2023, 57, 19932–19941. [Google Scholar] [CrossRef]
- Faisal, S.; Muhammad, S.; Luqman, M.; Hasnain, M.; Rasool, A.; Awan, M.U.F.; Khan, Z.I.; Hussain, I. Effects of Priming on Seed Germination, Physico-Chemistry and Yield of Late Sown Wheat Crop (Triticum aestivum L.). Pol. J. Environ. Stud. 2023, 32, 1113–1124. [Google Scholar] [CrossRef]
- Janah, I.; Elhasnaoui, A.; Abouloifa, H.; Ait-El-Mokhtar, M.; Ben Laouane, R. Hormonal Priming to Increase Germination of Stevia rebaudiana Bertoni Seeds in Saline Environments. Int. J. Plant Biol. 2025, 16, 2. [Google Scholar] [CrossRef]
- Khalequzzaman; Ullah, H.; Himanshu, S.K.; Islam, N.E.T.; Tisarum, R.; Cha-um, S.; Datta, A. Seed Priming Improves Germination, Yield, and Water Productivity of Cotton Under Drought Stress. J. Soil. Sci. Plant Nutr. 2023, 23, 2418–2432. [Google Scholar] [CrossRef]
- Paparella, S.; Araújo, S.S.; Rossi, G.; Wijayasinghe, M.; Carbonera, D.; Balestrazzi, A. Seed Priming: State of the Art and New Perspectives. Plant Cell Rep. 2015, 34, 1281–1293. [Google Scholar] [CrossRef]
- Jisha, K.C.; Vijayakumari, K.; Puthur, J.T. Seed Priming for Abiotic Stress Tolerance: An Overview. Acta Physiol. Plant. 2013, 35, 1381–1396. [Google Scholar] [CrossRef]
- Sen, A.; Puthur, J.T. Influence of Different Seed Priming Techniques on Oxidative and Antioxidative Responses during the Germination of Oryza sativa Varieties. Physiol. Mol. Biol. Plants 2020, 26, 551–565. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, X.; Gao, G.; Ali, I.; Wu, X.; Tang, M.; Chen, L.; Jiang, L.; Liang, T. Effects of Various Seed Priming on Morphological, Physiological, and Biochemical Traits of Rice under Chilling Stress. Front. Plant Sci. 2023, 14, 1146285. [Google Scholar] [CrossRef]
- Subedi, R.; Maharjan, B.; Adhikari, R. Effect of Different Priming Methods in Rice (Oryza sativa). J. Agric. Environ. 2015, 16, 152–160. [Google Scholar] [CrossRef]
- Nawaz, F.; Ashraf, M.Y.; Ahmad, R.; Waraich, E.A. Selenium (Se) Seed Priming Induced Growth and Biochemical Changes in Wheat under Water Deficit Conditions. Biol. Trace Elem. Res. 2013, 151, 284–293. [Google Scholar] [CrossRef]
- Alam, A.U.; Ullah, H.; Himanshu, S.K.; Tisarum, R.; Cha-um, S.; Datta, A. Seed Priming Enhances Germination and Morphological, Physio-Biochemical, and Yield Traits of Cucumber under Water-Deficit Stress. J. Soil. Sci. Plant Nutr. 2023, 23, 3961–3978. [Google Scholar] [CrossRef]
- Sun, H.; Li, L.; Wang, X.; Wu, S.; Wang, X. Ascorbate-Glutathione Cycle of Mitochondria in Osmoprimed Soybean Cotyledons in Response to Imbibitional Chilling Injury. J. Plant Physiol. 2011, 168, 226–232. [Google Scholar] [CrossRef]
- Jisha, K.C.; Puthur, J.T. Seed Priming with BABA (β-Amino Butyric Acid): A Cost-Effective Method of Abiotic Stress Tolerance in Vigna radiata (L.) Wilczek. Protoplasma 2016, 253, 277–289. [Google Scholar] [CrossRef]
- Thornton, J.M.; Collins, A.R.S.; Powell, A.A. The Effect of Aerated Hydration on DNA Synthesis in Embryos of Brassica oleracea L. Seed Sci. Res. 1993, 3, 195–199. [Google Scholar] [CrossRef]
- Jian, H.; Wang, J.; Wang, T.; Wei, L.; Li, J.; Liu, L. Identification of Rapeseed MicroRNAs Involved in Early Stage Seed Germination under Salt and Drought Stresses. Front. Plant Sci. 2016, 7, 658. [Google Scholar] [CrossRef]
- Oshunsanya, S.O.; Nwosu, N.J.; Li, Y. Abiotic Stress in Agricultural Crops Under Climatic Conditions. In Sustainable Agriculture, Forest and Environmental Management; Springer: Singapore, 2019; pp. 71–100. [Google Scholar] [CrossRef]
- Balfagón, D.; Zandalinas, S.I.; Gómez-Cadenas, A. High Temperatures Change the Perspective: Integrating Hormonal Responses in Citrus Plants under Co-Occurring Abiotic Stress Conditions. Physiol. Plant 2019, 165, 183–197. [Google Scholar] [CrossRef]
- Abdel Latef, A.A.H. Sustainable Remedies for Abiotic Stress in Cereals; Springer Nature: Berlin/Heidelberg, Germany, 2022. [Google Scholar] [CrossRef]
- Fahad, S.; Noor, M.; Adnan, M.; Khan, M.A.; Rahman, I.U.; Alam, M.; Nasim, W. Abiotic stress and rice grain quality. In Advances in Rice Research for Abiotic Stress Tolerance; Woodhead Publishing: Sawston, UK, 2019; pp. 571–583. [Google Scholar]
- Djanaguiraman, M.; Prasad, P.V.; Ciampitti, I.A.; Talwar, H.S. Impacts of abiotic stresses on sorghum physiology. In Sorghum in the 21st Century: Food–Fodder–Feed–Fuel for a Rapidly Changing World; Springer: Singapore, 2020; pp. 157–188. [Google Scholar]
- Bali, A.S.; Sidhu, G.P.S. Abiotic stress-induced oxidative stress in wheat. In Wheat Production in Changing Environments: Responses, Adaptation and Tolerance; Springer: Berlin/Heidelberg, Germany, 2019; pp. 225–239. [Google Scholar]
- Salika, R.; Riffat, J. Abiotic stress responses in maize: A review. Acta Physiol. Plant. 2021, 43, 130. [Google Scholar] [CrossRef]
- Li, P.; Yang, X.; Wang, H.; Pan, T.; Wang, Y.; Xu, Y.; Yang, Z. Genetic control of root plasticity in response to salt stress in maize. Theor. Appl. Genet. 2021, 134, 1475–1492. [Google Scholar] [CrossRef]
- Thiruvengadam, R.; Venkidasamy, B.; Easwaran, M.; Chi, H.Y.; Thiruvengadam, M.; Kim, S.H. Dynamic Interplay of Reactive Oxygen and Nitrogen Species (ROS and RNS) in Plant Resilience: Unveiling the Signaling Pathways and Metabolic Responses to Biotic and Abiotic Stresses. Plant Cell Rep. 2024, 43, 1–24. [Google Scholar] [CrossRef]
- Calixto, C.P.G. Molecular Aspects of Heat Stress Sensing in Land Plants. Plant J. 2025, 121, e70069. [Google Scholar] [CrossRef]
- Chakraborty, S.; Roychoudhury, A. Morphological, Architectural and Biochemical Modifications of Cereal Crops During Abiotic Stress. In Omics Approach to Manage Abiotic Stress in Cereals; Springer: Singapore, 2022; pp. 1–20. [Google Scholar] [CrossRef]
- Rane, J.; Singh, A.K.; Kumar, M.; Boraiah, K.M.; Meena, K.K.; Pradhan, A.; Vara Prasad, P.V. The Adaptation and Tolerance of Major Cereals and Legumes to Important Abiotic Stresses. Int. J. Mol. Sci. 2021, 22, 12970. [Google Scholar] [CrossRef]
- Shahzadi, A.; Noreen, Z.; Alamery, S.; Zafar, F.; Haroon, A.; Rashid, M.; Aslam, M.; Younas, A.; Attia, K.A.; Mohammed, A.A.; et al. Effects of Biochar on Growth and Yield of Wheat (Triticum aestivum L.) under Salt Stress. Sci. Rep. 2024, 14, 20024. [Google Scholar] [CrossRef]
- Ahmad, A.; Aslam, Z.; Javed, T.; Hussain, S.; Raza, A.; Shabbir, R.; Mora-Poblete, F.; Saeed, T.; Zulfiqar, F.; Ali, M.M.; et al. Screening of Wheat (Triticum aestivum L.) Genotypes for Drought Tolerance through Agronomic and Physiological Response. Agronomy 2022, 12, 287. [Google Scholar] [CrossRef]
- Nazir, A.; Rafique, F.; Ahmed, K.; Khan, S.A.; Khan, N.; Akbar, M.; Zafar, M. Evaluation of Heavy Metals Effects on Morpho-Anatomical Alterations of Wheat (Triticum aestivum L.) Seedlings. Microsc. Res. Tech. 2021, 84, 2517–2529. [Google Scholar] [CrossRef]
- Shahzad, H.; Ullah, S.; Iqbal, M.; Bilal, H.M.; Shah, G.M.; Ahmad, S.; Zakir, A.; Ditta, A.; Farooqi, M.A.; Ahmad, I. Salinity Types and Level-Based Effects on the Growth, Physiology and Nutrient Contents of Maize (Zea mays). Ital. J. Agron. 2019, 14, 199–207. [Google Scholar] [CrossRef]
- Cao, L.; Lu, X.; Wang, G.; Zhang, P.; Fu, J.; Wang, Z.; Wei, L.; Wang, T. Transcriptional Regulatory Networks in Response to Drought Stress and Rewatering in Maize (Zea mays L.). Mol. Genet. Genom. 2021, 296, 1203–1219. [Google Scholar] [CrossRef]
- Oudghiri, M.; Yamani, B.; Benlemlih, N.; El Aammouri, S.; Abid, N.; Brhadda, N.; Bouhassoun, S.; Ziri, R.; Chriqui, A.; Aoujil, F.Z.; et al. Effect of Heavy Metals on the Morphological and Physiological Responses of the Torro Plus Variant of Zea Mays. J. Environ. Earth Sci. 2025, 7, 165–179. [Google Scholar] [CrossRef]
- Torun, H.; Novák, O.; Mikulík, J.; Strnad, M.; Ayaz, F.A. The Effects of Exogenous Salicylic Acid on Endogenous Phytohormone Status in Hordeum vulgare L. under Salt Stress. Plants 2022, 11, 618. [Google Scholar] [CrossRef]
- Ferioun, M.; Srhiouar, N.; Bouhraoua, S.; El Ghachtouli, N.; Louahlia, S. Physiological and Biochemical Changes in Moroccan Barley (Hordeum vulgare L.) Cultivars Submitted to Drought Stress. Heliyon 2023, 9, e13643. [Google Scholar] [CrossRef]
- Selim, S.; Abuelsoud, W.; Al-Sanea, M.M.; AbdElgawad, H. Elevated CO2 Differently Suppresses the Arsenic Oxide Nanoparticles-Induced Stress in C3 (Hordeum vulgare) and C4 (Zea maize) Plants via Altered Homeostasis in Metabolites Specifically Proline and Anthocyanin Metabolism. Plant Physiol. Biochem. 2021, 166, 235–245. [Google Scholar] [CrossRef]
- Rodríguez Coca, L.I.; García González, M.T.; Gil Unday, Z.; Jiménez Hernández, J.; Rodríguez Jáuregui, M.M.; Fernández Cancio, Y. Effects of Sodium Salinity on Rice (Oryza sativa L.) Cultivation: A Review. Sustainability 2023, 15, 1804. [Google Scholar] [CrossRef]
- Bhandari, U.; Gajurel, A.; Khadka, B.; Thapa, I.; Chand, I.; Bhatta, D.; Poudel, A.; Pandey, M.; Shrestha, S.; Shrestha, J. Morpho-Physiological and Biochemical Response of Rice (Oryza sativa L.) to Drought Stress: A Review. Heliyon 2023, 9, e13744. [Google Scholar] [CrossRef]
- Bari, M.A.; El-Shehawi, A.M.; Elseehy, M.M.; Naheen, N.N.; Rahman, M.M.; Kabir, A.H. Molecular Characterization and Bioinformatics Analysis of Transporter Genes Associated with Cd-Induced Phytotoxicity in Rice (Oryza sativa L.). Plant Physiol. Biochem. 2021, 167, 438–448. [Google Scholar] [CrossRef]
- Azeem, M.; Sultana, R.; Mahmood, A.; Qasim, M.; Siddiqui, Z.S.; Mumtaz, S.; Javed, T.; Umar, M.; Adnan, M.Y.; Siddiqui, M.H. Ascorbic and Salicylic Acids Vitalized Growth, Biochemical Responses, Antioxidant Enzymes, Photosynthetic Efficiency, and Ionic Regulation to Alleviate Salinity Stress in Sorghum bicolor. J. Plant Growth Regul. 2023, 42, 5266–5279. [Google Scholar] [CrossRef]
- Amoah, J.N.; Antwi-Berko, D. Comparative Physiological, Biochemical and Transcript Response to Drought in Sorghum Genotypes. Biotechnol. J. Int. 2020, 24, 1–14. [Google Scholar] [CrossRef]
- Singh, A.; Parihar, S.; Shekhawat, G.S. Cd-Induced Cytotoxicity and Its HO-1 and ROS Quenching Enzyme-Mediated Regulation in 2–3 Leaf Stage Seedlings of Sorghum Bicolor: An Important Millet Crop of the Arid & Semi-Arid Regions. J. Trace Elem. Miner. 2024, 9, 100165. [Google Scholar] [CrossRef]
- Feghhenabi, F.; Hadi, H.; Khodaverdiloo, H.; van Genuchten, M.T. Seed Priming Alleviated Salinity Stress during Germination and Emergence of Wheat (Triticum aestivum L.). Agric. Water Manag. 2020, 231, 106022. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, R.; Xing, Y.; Jiang, B.; Li, B.; Xu, X.; Zhou, Y. The Efficacy of Different Seed Priming Agents for Promoting Sorghum Germination under Salt Stress. PLoS ONE 2021, 16, e0245505. [Google Scholar] [CrossRef] [PubMed]
- Soni, A.T.; Rookes, J.E.; Arya, S.S. Chitosan Nanoparticles as Seed Priming Agents to Alleviate Salinity Stress in Rice (Oryza sativa L.) Seedlings. Polysaccharides 2023, 4, 129–141. [Google Scholar] [CrossRef]
- Yang, X.; Xiong, J.; Du, X.; Sun, M.; Ding, L.; Mei, W.; Sun, Z.; Feng, N.; Zheng, D.; Shen, X. Effects of Exogenous Spermidine on Seed Germination and Physiological Metabolism of Rice Under NaCl Stress. Plants 2024, 13, 3599. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, F.; Kamal, A.; Singh, A.; Ashfaque, F.; Alamri, S.; Siddiqui, M.H.; Khan, M.I.R. Seed priming with gibberellic acid induces high salinity tolerance in Pisum sativum through antioxidants, secondary metabolites and up-regulation of antiporter genes. Plant Biol. 2021, 23, 113–121. [Google Scholar] [CrossRef]
- Ellouzi, H.; Zorrig, W.; Amraoui, S.; Oueslati, S.; Abdelly, C.; Rabhi, M.; Siddique, K.H.M.; Hessini, K. Seed Priming with Salicylic Acid Alleviates Salt Stress Toxicity in Barley by Suppressing ROS Accumulation and Improving Antioxidant Defense Systems, Compared to Halo- and Gibberellin Priming. Antioxidants 2023, 12, 1779. [Google Scholar] [CrossRef]
- Choi, J.-Y.; Ju, Y.-H.; Nakamichi, A.; Cho, S.-W.; Woo, S.-H.; Sakagami, J.-I. Effect of Seed Hydropriming on the Elongation of Plumule and Radicle During the Germination Process and Changes in Enzyme Activity Under Water-Deficient Conditions. Plants 2024, 13, 3537. [Google Scholar] [CrossRef]
- Ren, M.; Tan, B.; Xu, J.; Yang, Z.; Zheng, H.; Tang, Q.; Wang, W. Priming methods affected deterioration speed of primed rice seeds by regulating reactive oxygen species accumulation, seed respiration and starch degradation. Front. Plant Sci. 2023, 14, 1267103. [Google Scholar] [CrossRef]
- Ali, L.G.; Nulit, R.; Ibrahim, M.H.; Yien, C.Y.S. Efficacy of KNO3, SiO2 and SA Priming for Improving Emergence, Seedling Growth and Antioxidant Enzymes of Rice (Oryza sativa), under Drought. Sci. Rep. 2021, 11, 3864. [Google Scholar] [CrossRef]
- Tounekti, T.; Mahdhi, M.; Al-Faifi, Z.; Khemira, H. Priming Improves Germination and Seed Reserve Utilization, Growth, Antioxidant Responses and Membrane Stability at Early Seedling Stage of Saudi Sorghum Varieties under Drought Stress. Not. Bot. Horti Agrobot. Cluj. Napoca 2020, 48, 938–953. [Google Scholar] [CrossRef]
- Mim, M.F.; Chowdhury, M.Z.H.; Rohman, M.M.; Naz, A.; Bhuiyan, A.U.A.; Mohi-Ud-Din, M.; Haque, M.A.; Islam, S.M.N. Metarhizium Anisopliae (MetA1) Seed Priming Improves Photosynthesis, Growth, Plant Defense and Yield of Wheat under Drought Stress. Plant Physiol. Biochem. 2024, 217, 109239. [Google Scholar] [CrossRef]
- Hussain, A.; Rizwan, M.; Ali, Q.; Ali, S. Seed Priming with Silicon Nanoparticles Improved the Biomass and Yield While Reduced the Oxidative Stress and Cadmium Concentration in Wheat Grains. Environ. Sci. Pollut. Res. 2019, 26, 7579–7588. [Google Scholar] [CrossRef]
- Nawaz, F.; Naeem, M.; Akram, A.; Ashraf, M.Y.; Ahmad, K.S.; Zulfiqar, B.; Sardar, H.; Shabbir, R.N.; Majeed, S.; Shehzad, M.A.; et al. Seed Priming with KNO3 Mediates Biochemical Processes to Inhibit Lead Toxicity in Maize (Zea mays L.). J. Sci. Food Agric. 2017, 97, 4780–4789. [Google Scholar] [CrossRef]
- Al-Hazmi, N.E.; Naguib, D.M. Amylase Properties and Its Metal Tolerance during Rice Germination Improved by Priming with Rhizobacteria. Rhizosphere 2022, 22, 100518. [Google Scholar] [CrossRef]
- Dabral, S.; Yashaswee; Varma, A.; Choudhary, D.K.; Bahuguna, R.N.; Nath, M. Biopriming with Piriformospora Indica Ameliorates Cadmium Stress in Rice by Lowering Oxidative Stress and Cell Death in Root Cells. Ecotoxicol. Environ. Saf. 2019, 186, 109741. [Google Scholar] [CrossRef]
- Basit, F.; Bhat, J.A.; Ulhassan, Z.; Noman, M.; Zhao, B.; Zhou, W.; Kaushik, P.; Ahmad, A.; Ahmad, P.; Guan, Y. Seed Priming with Spermine Mitigates Chromium Stress in Rice by Modifying the Ion Homeostasis, Cellular Ultrastructure and Phytohormones Balance. Antioxidants 2022, 11, 1704. [Google Scholar] [CrossRef] [PubMed]
- Al-Quraan, N.A.; Samarah, N.H.; Rasheed, E.I. The Role of Chitosan Priming in Induction of GABA Shunt Pathway during Wheat Seed Germination under Salt Stress. Biol. Plant 2023, 67, 234–248. [Google Scholar] [CrossRef]
- Islam, A.T.M.T.; Ullah, H.; Himanshu, S.K.; Tisarum, R.; Cha-um, S.; Datta, A. Effect of Salicylic Acid Seed Priming on Morpho-Physiological Responses and Yield of Baby Corn under Salt Stress. Sci. Hortic. 2022, 304, 111304. [Google Scholar] [CrossRef]
- Hadia, E.; Slama, A.; Romdhane, L.; Cheikh M’Hamed, H.; Fahej, M.A.S.; Radhouane, L. Seed Priming of Bread Wheat Varieties with Growth Regulators and Nutrients Improves Salt Stress Tolerance Particularly for the Local Genotype. J. Plant Growth Regul. 2023, 42, 304–318. [Google Scholar] [CrossRef]
- Mamedi, A.; Sharifzadeh, F.; Maali-Amiri, R.; Divargar, F.; Rasoulnia, A. Seed Osmopriming with Ca2+ and K+ Improves Salt Tolerance in Quinoa Seeds and Seedlings by Amplifying Antioxidant Defense and Ameliorating the Osmotic Adjustment Process. Physiol. Mol. Biol. Plants 2022, 28, 251–274. [Google Scholar] [CrossRef]
- Ahmad, A.; Tola, E.K.; Alshahrani, T.S.; Seleiman, M.F. Enhancement of Morphological and Physiological Performance of Zea mays L. under Saline Stress Using ZnO Nanoparticles and 24-Epibrassinolide Seed Priming. Agronomy 2023, 13, 771. [Google Scholar] [CrossRef]
- Chattha, M.U.; Hassan, M.U.U.; Khan, I.; Nawaz, M.; Shah, A.N.; Sattar, A.; Hashem, M.; Alamri, S.; Aslam, M.T.; Alhaithloul, H.A.S.; et al. Hydrogen Peroxide Priming Alleviates Salinity Induced Toxic Effect in Maize by Improving Antioxidant Defense System, Ionic Homeostasis, Photosynthetic Efficiency and Hormonal Crosstalk. Mol. Biol. Rep. 2022, 49, 5611–5624. [Google Scholar] [CrossRef] [PubMed]
- Kasana, R.A.; Iqbal, M.; Ali, Q.; Saeed, F.; Rizwan, M.; Perveen, R.; Yong, J.W.H. Synergistic Effects of Glutathione and Zinc Seed Priming in Alleviating Salt Stress on Maize Seed Germination, Metabolite Levels, Seedling Vigor, and Nutrient Acquisition. Plant Stress. 2025, 15, 100767. [Google Scholar] [CrossRef]
- Dutta, B.; Datta, A.; Dey, A.; Ghosh, A.K.; Bandopadhyay, R. Establishment of Seed Biopriming in Salt Stress Mitigation of Rice Plants by Mangrove Derived Bacillus Sp. Biocatal. Agric. Biotechnol. 2023, 48, 102626. [Google Scholar] [CrossRef]
- Cembrowska-Lech, D.; Rybak, K. Nanopriming of Barley Seeds—A Shotgun Approach to Improve Germination under Salt Stress Conditions by Regulating of Reactive Oxygen Species. Plants 2023, 12, 405. [Google Scholar] [CrossRef]
- Raza, M.A.S.; Aslam, M.U.; Valipour, M.; Iqbal, R.; Haider, I.; Mustafa, A.E.Z.M.A.; Elshikh, M.S.; Ali, I.; Roy, R.; Elshamly, A.M.S. Seed Priming with Selenium Improves Growth and Yield of Quinoa Plants Suffering Drought. Sci. Rep. 2024, 14, 886. [Google Scholar] [CrossRef]
- Singh, P.; Singh, R.; Madhu, G.S.; Singh, V.P. Seed Biopriming with Trichoderma harzianum for Growth Promotion and Drought Tolerance in Rice (Oryza sativus). Agric. Res. 2023, 12, 154–162. [Google Scholar] [CrossRef]
- Lastochkina, O.; Garshina, D.; Ivanov, S.; Yuldashev, R.; Khafizova, R.; Allagulova, C.; Fedorova, K.; Avalbaev, A.; Maslennikova, D.; Bosacchi, M. Seed Priming with Endophytic Bacillus subtilis Modulates Physiological Responses of Two Different Triticum aestivum L. Cultivars Under Drought Stress. Plants 2020, 9, 1810. [Google Scholar] [CrossRef]
- Rizk, R.; Ahmed, M.; Abdul-Hamid, D.; Zedan, M.; Tóth, Z.; Decsi, K. Resulting Key Physiological Changes in Triticum aestivum L. Plants Under Drought Conditions After Priming the Seeds with Conventional Fertilizer and Greenly Synthesized Zinc Oxide Nanoparticles from Corn Wastes. Agronomy 2025, 15, 211. [Google Scholar] [CrossRef]
- Al Ghafri, S.H.; Al-Busaidi, W.M.; Farooq, M. Enhancing Drought Tolerance in Bread Wheat Through GABA Seed Priming and Optimized Storage Temperatures. J. Plant Growth Regul. 2025. [Google Scholar] [CrossRef]
- El-Sanatawy, A.M.; Ash-Shormillesy, S.M.A.I.; Qabil, N.; Awad, M.F.; Mansour, E. Seed Halo-Priming Improves Seedling Vigor, Grain Yield, and Water Use Efficiency of Maize under Varying Irrigation Regimes. Water 2021, 13, 2115. [Google Scholar] [CrossRef]
- Yang, H.; Hu, W.; Zhao, J.; Huang, X.; Zheng, T.; Fan, G. Genetic Improvement Combined with Seed Ethephon Priming Improved Grain Yield and Drought Resistance of Wheat Exposed to Soil Water Deficit at Tillering Stage. Plant Growth Regul. 2021, 95, 399–419. [Google Scholar] [CrossRef]
- Nadali, F.; Asghari, H.R.; Abbasdokht, H.; Dorostkar, V.; Bagheri, M. Improved Quinoa Growth, Physiological Response, and Yield by Hydropriming Under Drought Stress Conditions. Gesunde Pflanz. 2021, 73, 53–66. [Google Scholar] [CrossRef]
- Thongbam, S.; Sinam, V.; Mentada, B.A.S.; Kalangutkar, A.M.; Siddique, A. Priming-Mediated Triggering of Antioxidative Response to Induce Drought Tolerance in Maize (Zea mays L.). Plant Sci. Today 2023, 10, 247–252. [Google Scholar] [CrossRef]
- Van Nguyen, D.; Nguyen, H.M.; Le, N.T.; Nguyen, K.H.; Nguyen, H.T.; Le, H.M.; Nguyen, A.T.; Dinh, N.T.T.; Hoang, S.A.; Van Ha, C. Copper Nanoparticle Application Enhances Plant Growth and Grain Yield in Maize Under Drought Stress Conditions. J. Plant Growth Regul. 2022, 41, 364–375. [Google Scholar] [CrossRef]
- Elkelish, A.; Alqudah, A.M.; Alammari, B.S.; Alsubeie, M.S.; Hamed, S.M.; Thabet, S.G. Exploring genetic determinants of silver oxide nanoparticle-induced seed priming for drought tolerance in wheat. Genet. Resour. Crop Evol. 2025, 72, 3203–3218. [Google Scholar] [CrossRef]
- Salam, A.; Khan, A.R.; Liu, L.; Yang, S.; Azhar, W.; Ulhassan, Z.; Zeeshan, M.; Wu, J.; Fan, X.; Gan, Y. Seed Priming with Zinc Oxide Nanoparticles Downplayed Ultrastructural Damage and Improved Photosynthetic Apparatus in Maize under Cobalt Stress. J. Hazard. Mater. 2022, 423, 127021. [Google Scholar] [CrossRef]
- Chen, J.; Zeng, X.; Yang, W.; Xie, H.; Ashraf, U.; Mo, Z.; Liu, J.; Li, G.; Li, W. Seed Priming with Multiwall Carbon Nanotubes (MWCNTs) Modulates Seed Germination and Early Growth of Maize Under Cadmium (Cd) Toxicity. J. Soil. Sci. Plant Nutr. 2021, 21, 1793–1805. [Google Scholar] [CrossRef]
- Bashir, S.; Javed, S.; Al-Anazi, K.M.; Farah, M.A.; Ali, S. Bioremediation of Cadmium Toxicity in Wheat (Triticum aestivum L.) Plants Primed with L-Proline, Bacillus subtilis and Aspergillus niger. Int. J. Environ. Res. Public Health 2022, 19, 12683. [Google Scholar] [CrossRef]
- Ma, J.; Zou, M.; Peijnenburg, W.; Chen, F. Priming Agents Combat Copper Stress in Wheat (Triticum aestivum L.) under Hydroponic Conditions: Insights in Impacts on Morpho–Physio–Biochemical Traits and Health Risk Assessment. Ecotoxicol. Environ. Saf. 2025, 291, 117899. [Google Scholar] [CrossRef]
- Mu, C.; Huang, D.; Wang, M.; Li, Y.; Wang, X.; Si, D.; Cheng, C.; Ge, C.; Zhao, L.; Zhou, D. Seed Priming with Silver Ions Improves Growth and Physicochemical Features of Rice Plants (Oryza sativa L.) under Copper Stress. ACS Agric. Sci. Technol. 2024, 4, 711–722. [Google Scholar] [CrossRef]
- Choudhury, S.; Moulick, D.; Mazumder, M.K.; Pattnaik, B.K.; Ghosh, D.; Vemireddy, L.R.; Aldhahrani, A.; Soliman, M.M.; Gaber, A.; Hossain, A. An In Vitro and In Silico Perspective Study of Seed Priming with Zinc on the Phytotoxicity and Accumulation Pattern of Arsenic in Rice Seedlings. Antioxidants 2022, 11, 1500. [Google Scholar] [CrossRef] [PubMed]
- Basit, F.; Liu, J.; An, J.; Chen, M.; He, C.; Zhu, X.; Li, Z.; Hu, J.; Guan, Y. Seed Priming with Brassinosteroids Alleviates Aluminum Toxicity in Rice via Improving Antioxidant Defense System and Suppressing Aluminum Uptake. Environ. Sci. Pollut. Res. 2022, 29, 10183–10197. [Google Scholar] [CrossRef] [PubMed]
- Sobhy, S.E.; Abo-Kassem, E.E.M.; Sewelam, N.A.; Saad-Allah, K.M.; Aseel, D.G.; Saleh, A.A.; Hafez, E.E. Growth, Physiological and Molecular Response of Calcium and Salicylic Acid Primed Wheat under Lead Stress. Mol. Biol. Rep. 2025, 52, 133. [Google Scholar] [CrossRef] [PubMed]
- AL-Huqail, A.A.; Alatawi, A.; Alghanem, S.M.S.; Khan, K.A.; Abeed, A.H.A. Innovative Approach Using Different Nano—Primers to Enhance Stress Tolerance in Barley (Hordeum vulgare L.) Under Lead Toxicity. J. Soil. Sci. Plant Nutr. 2025. [Google Scholar] [CrossRef]
- Li, Z.; Xu, J.; Gao, Y.; Wang, C.; Guo, G.; Luo, Y.; Huang, Y.; Hu, W.; Sheteiwy, M.S.; Guan, Y.; et al. The Synergistic Priming Effect of Exogenous Salicylic Acid and H2O2 on Chilling Tolerance Enhancement during Maize (Zea mays L.) Seed Germination. Front. Plant Sci. 2017, 8, 1153. [Google Scholar] [CrossRef]
- Salah, S.M.; Yajing, G.; Dongdong, C.; Jie, L.; Aamir, N.; Qijuan, H.; Weimin, H.; Mingyu, N.; Jin, H. Seed Priming with Polyethylene Glycol Regulating the Physiological and Molecular Mechanism in Rice (Oryza sativa L.) under Nano-ZnO Stress. Sci. Rep. 2015, 5, 14278. [Google Scholar] [CrossRef]
- Paul, S.; Roychoudhury, A. Seed Priming with Spermine and Spermidine Regulates the Expression of Diverse Groups of Abiotic Stress-Responsive Genes during Salinity Stress in the Seedlings of Indica Rice Varieties. Plant Gene 2017, 11, 124–132. [Google Scholar] [CrossRef]
- Alzahrani, O.; Abouseadaa, H.; Abdelmoneim, T.K.; Alshehri, M.A.; El-Mogy, M.M.; El-Beltagi, H.S.; Atia, M.A.M. Agronomical, Physiological and Molecular Evaluation Reveals Superior Salt-Tolerance in Bread Wheat through Salt-Induced Priming Approach. Not. Bot. Horti Agrobot. Cluj. Napoca 2021, 49, 1–21. [Google Scholar] [CrossRef]
- Hidayah, A.; Nisak, R.R.; Susanto, F.A.; Nuringtyas, T.R.; Yamaguchi, N.; Purwestri, Y.A. Seed Halopriming Improves Salinity Tolerance of Some Rice Cultivars During Seedling Stage. Bot. Stud. 2022, 63, 24. [Google Scholar] [CrossRef]
- Forti, C.; Shankar, A.; Singh, A.; Balestrazzi, A.; Prasad, V.; Macovei, A. Hydropriming and Biopriming Improve Medicago truncatula Seed Germination and Upregulate DNA Repair and Antioxidant Genes. Genes 2020, 11, 242. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).