Plant-Growth-Promoting Rhizobacteria and Known Interactions with Plant Phytophagous Insects: A Meta-Analysis
Abstract
1. Introduction
2. Results
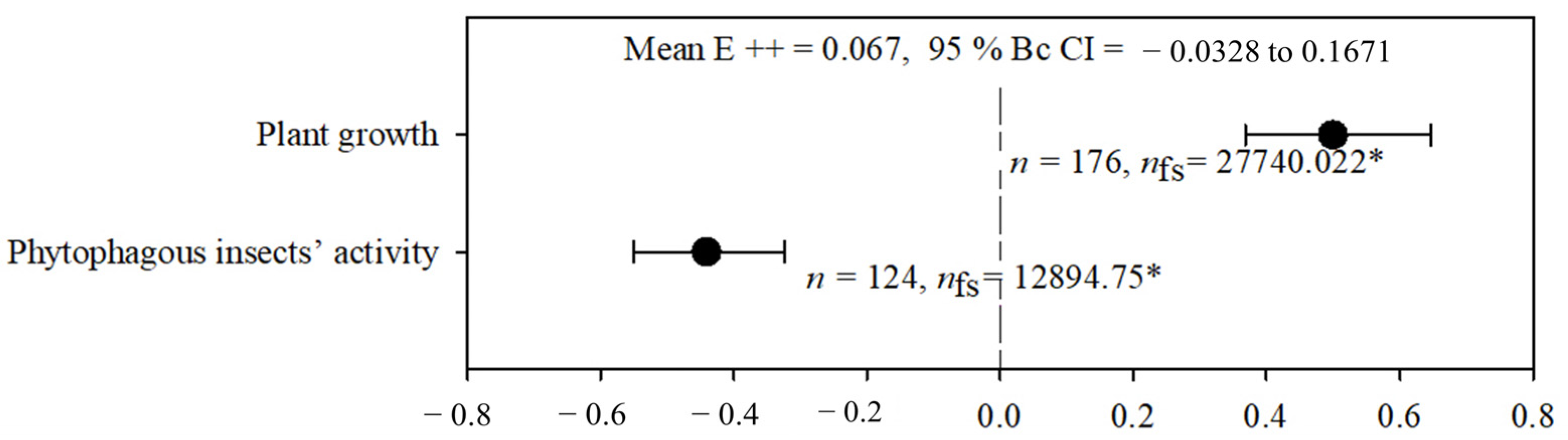
2.1. Heterogeneity and General Effects
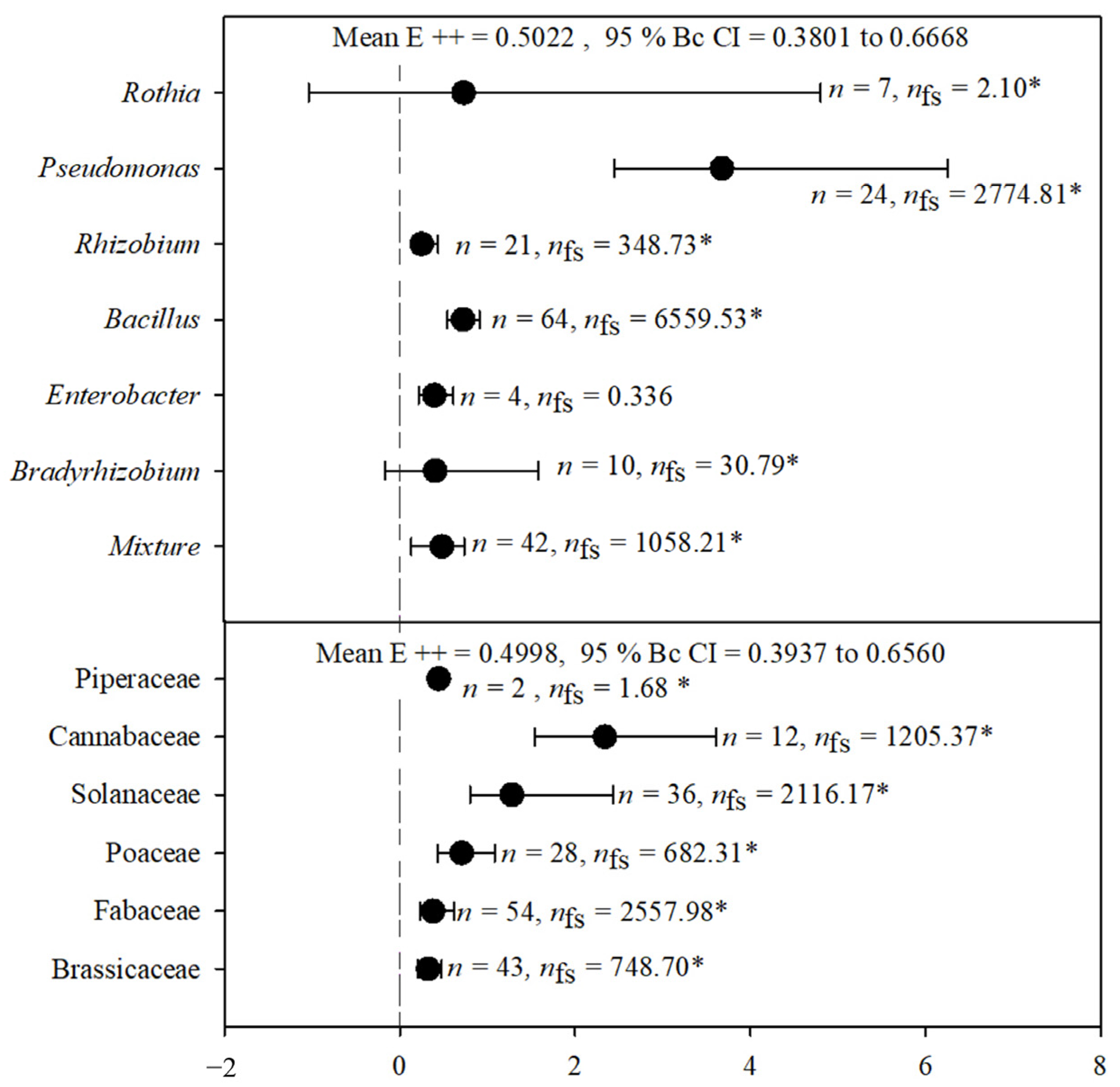
2.2. Plant Growth
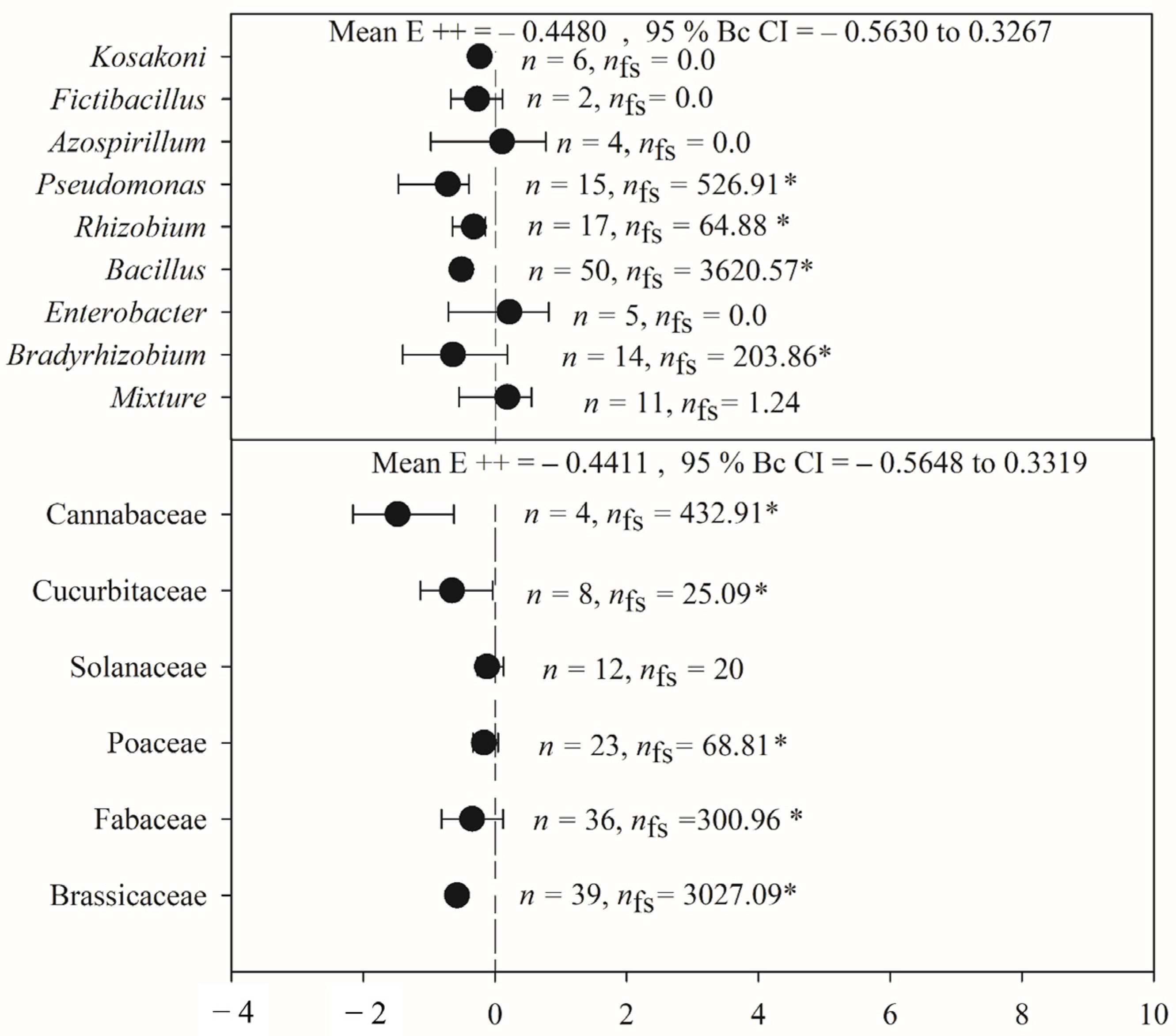
2.3. Phytophagous Insect Activity
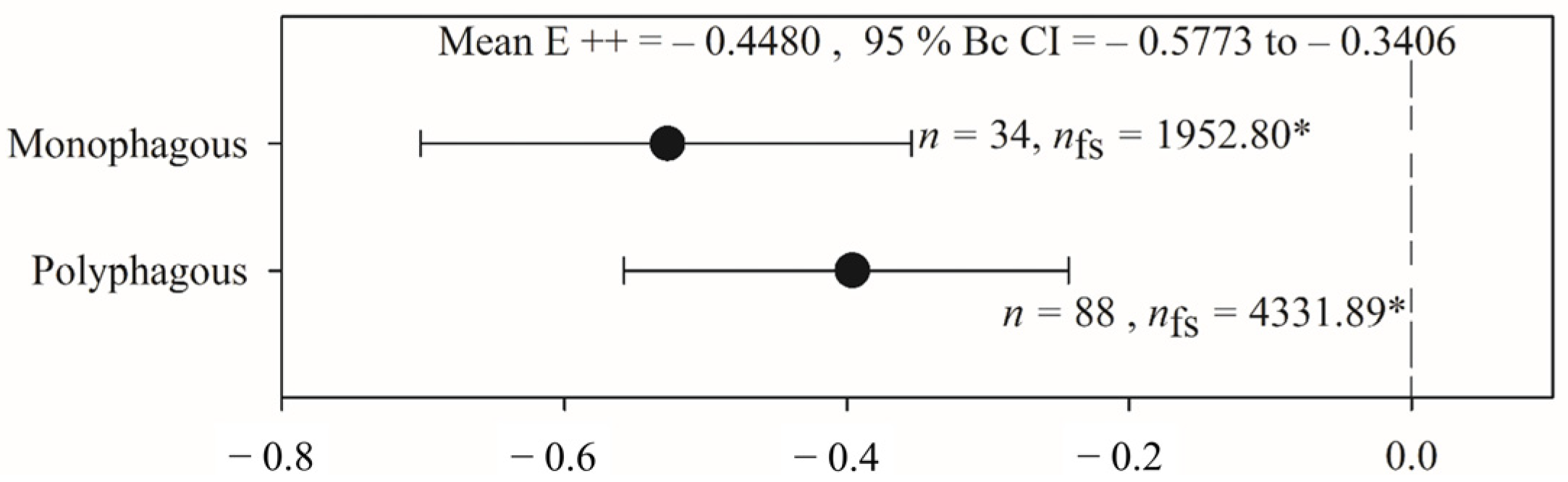
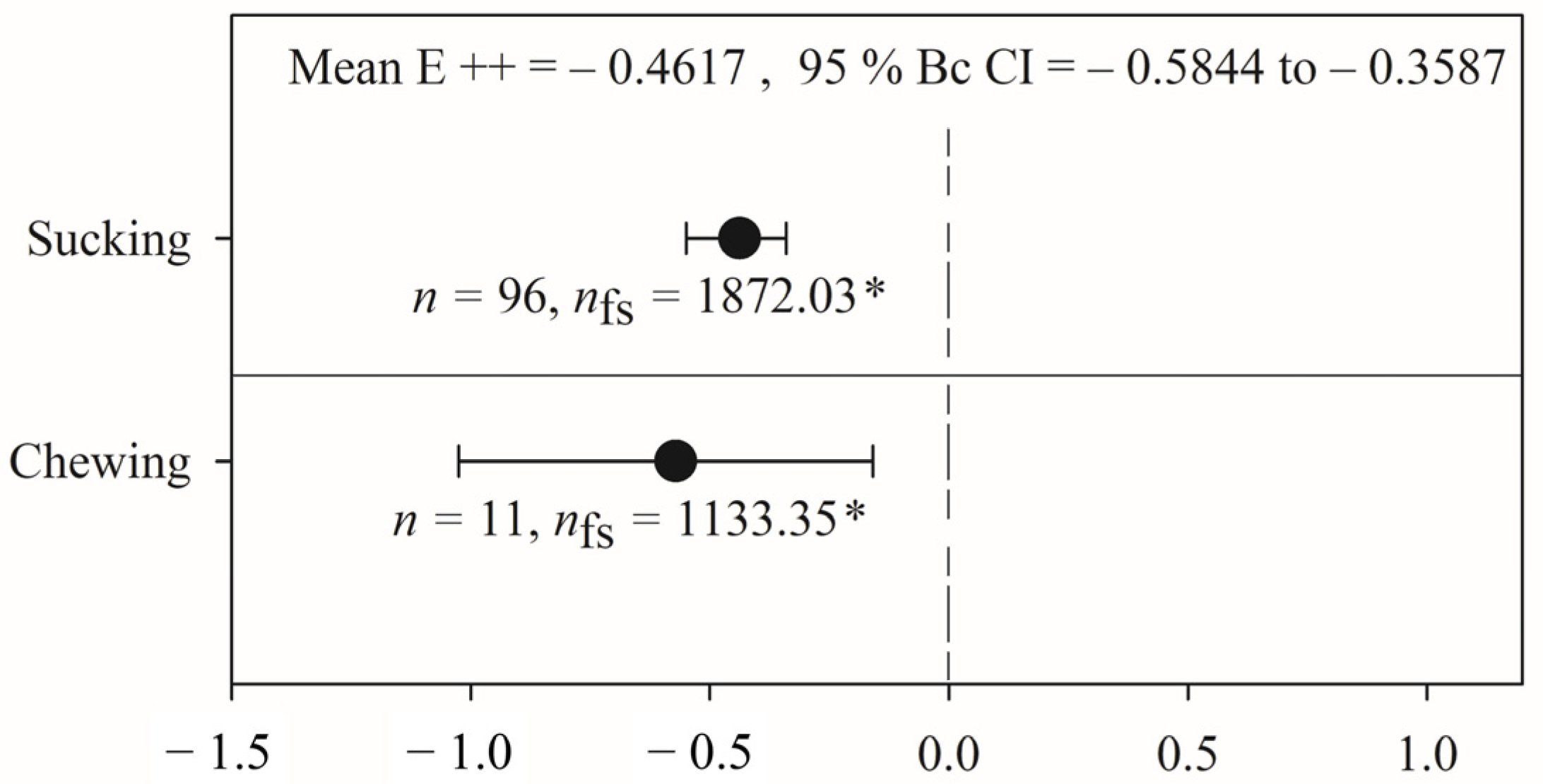
2.4. Variation in the Effects of PGPR on Phytophagous Insect Activity Based on Feeding Guild
3. Discussion
3.1. Plant Growth
3.2. Phytophagous Insect Activity
3.3. Variation in the Effects of PGPR on Phytophagous Insect Activity Based on Feeding Guild
4. Materials and Methods
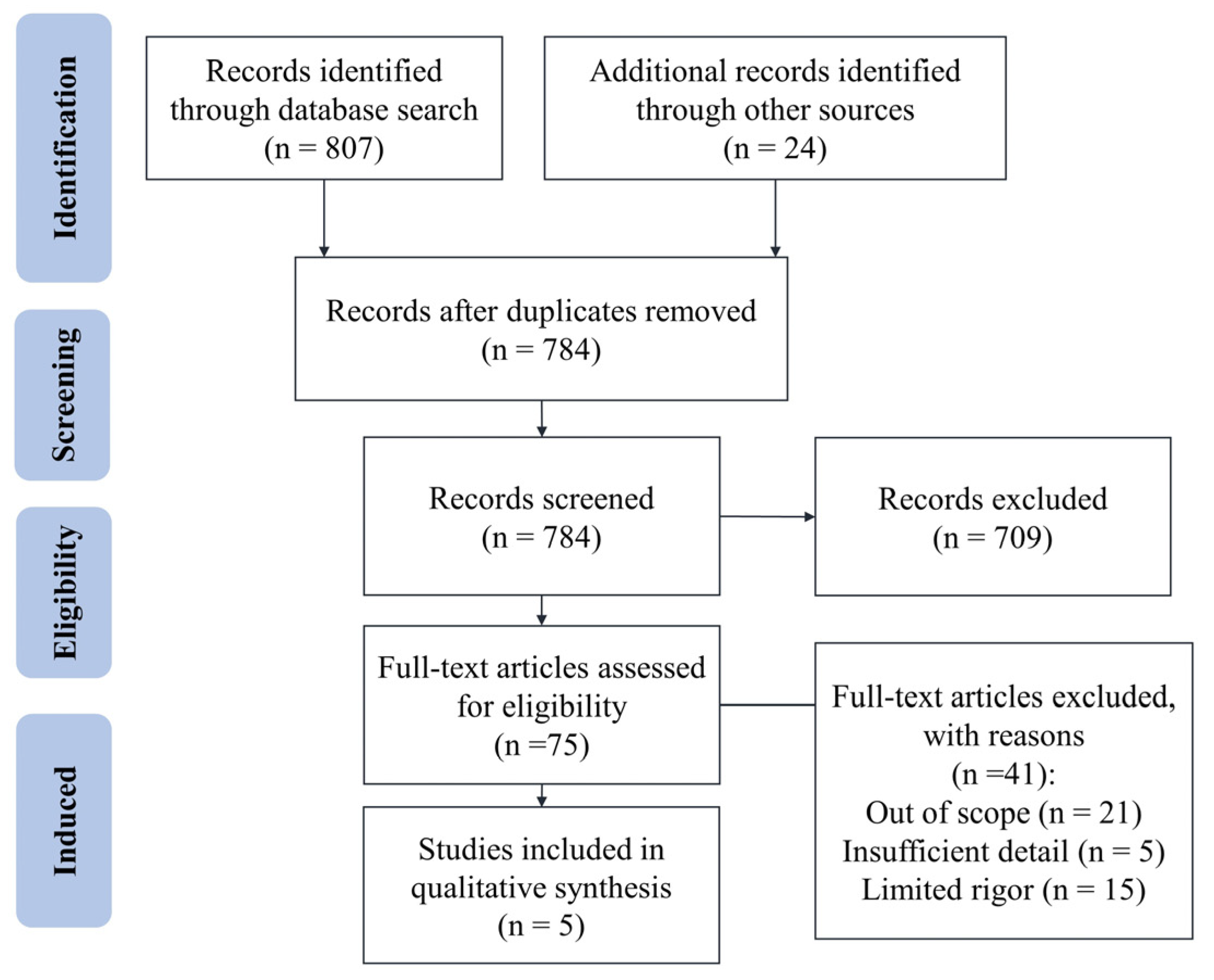
4.1. Data Selection
4.2. Effect Sizes
4.3. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PGPR | Plant-growth-promoting Rhizobacteria |
| N | Nitrogen |
| JA | Jasmonic acid |
| SA | Salicylic acid |
| ISR | Induced systemic resistance |
| SD | Standard deviation |
| SE | Standard error |
| CI | Confidence interval |
| Bc CI | Bias-corrected confidence interval |
| df | Degrees of freedom |
| LOX2 | Lipoxygenase 2 |
References
- Hunter, M.D.; Price, P.W. Playing chutes and ladders: Heterogeneity and the relative roles of bottom-up and top-down forces in natural communities. Ecology 1992, 73, 724–732. [Google Scholar] [CrossRef]
- Ohgushi, T. Indirect interaction webs: Herbivore-induced effects through trait change in plants. Annu. Rev. Ecol. Evol. Syst. 2005, 36, 81–105. [Google Scholar] [CrossRef]
- Poelman, E.H.; Van Loon, J.J.A.; Dicke, M. Consequences of plant defense for biodiversity at higher trophic levels. Trend Plant Sci. 2008, 13, 534–541. [Google Scholar] [CrossRef] [PubMed]
- Nassar, A.H.; El-Tarabily, K.A.; Sivasithamparam, K. Promotion of plant growth by an ACC deaminase-producing Aeromonas sp. isolated from a Salicornia sp. root. Soil Biol. Biochem. 2005, 37, 1177–1184. [Google Scholar]
- Bennett, A.E.; Bever, J.D. Mycorrhizal species differentially alter plant growth and response to herbivory. Ecology 2007, 88, 210–218. [Google Scholar] [CrossRef]
- Laguerre, G.; Depret, G.; Bourion, V.; Duc, G. Rhizobium leguminosarum bv. viciae genotypes interact with pea plants in developmental responses of nodules, roots and shoots. New Phytol. 2007, 176, 680–690. [Google Scholar] [CrossRef]
- Cheplick, G.P.; Stanley, F. Ecology and Evolution of the Grass-Endophyte Symbiosis; Oxford Academic: New York, NY, USA, 2009. [Google Scholar] [CrossRef]
- Martinez-Viveros, O.; Jorquera, M.A.; Crowley, D.E.; Gajardo, G.; Mora, M.L. Mechanisms and practical considerations involved in plant growth promotion by rhizobacteria. J. Soil Sci. Plant Nutr. 2010, 10, 293–319. [Google Scholar] [CrossRef]
- Wilson, K.G.; Stinner, R.E. A potential influence of rhizobium activity on the availability of nitrogen to legume herbivores. Oecologia 1984, 61, 337–341. [Google Scholar] [CrossRef]
- Barton, K.E.; Koricheva, J. The ontogeny of plant defense and herbivory: Characterizing general patterns using meta-analysis. Am. Nat. 2010, 175, 481–493. [Google Scholar] [CrossRef]
- Ballhorn, D.J.; Kautz, S.; Schädler, M. Induced plant defense via volatile production is dependent on rhizobial symbiosis. Oecologia 2013, 172, 833–846. [Google Scholar] [CrossRef]
- Gadhave, K.R.; Finch, P.; Gibson, T.M.; Gange, A.C. Plant growth-promoting Bacillus suppress Brevicoryne brassicae field infestation and trigger density-dependent and density-independent natural enemy responses. J. Pest Sci. 2016, 89, 985–992. [Google Scholar] [CrossRef]
- Kumari, B.; Mallick, M.A.; Solanki, M.K.; Solanki, A.C.; Hora, A.; Guo, W. Plant growth promoting rhizobacteria (PGPR): Modern prospects for sustainable agriculture. In Plant Health Under Biotic Stress; Ansari, R., Mahmood, I., Eds.; Springer: Berlin/Heidelberg, Germany, 2019; pp. 134–141. [Google Scholar] [CrossRef]
- Azevedo, J.L.; Maccheroni, J.W.; Pereira, J.O.; Araujo, W.L. Endophytic microorganisms: A review on insect control and recent advances on tropical plants. Electron. J. Biotech. 2000, 3, 40–65. [Google Scholar] [CrossRef]
- Ryu, C.M.; Farag, M.A.; Hu, C.H.; Reddy, M.S.; Kloepper, J.W. Bacterial volatiles promote growth in Arabidopsis. Proc. Natl. Acad. Sci. USA 2003, 100, 4927–4932. [Google Scholar] [CrossRef]
- Hartley, S.E.; Gange, A.C. Impacts of plant symbiotic fungi on insect herbivores: Mutualism in a multitrophic context. Annu. R. Entomol. 2009, 54, 323–342. [Google Scholar] [CrossRef]
- Schweiger, R.; Heise, A.M.; Persicke, M.; Müller, C. Interactions between the jasmonic and salicylic acid pathways modulate the plant metabolome and affect herbivores of different feeding types. Plant Cell Environ. 2014, 37, 1844–1855. [Google Scholar] [CrossRef]
- Pineda, A.; Zheng, S.J.; Van Loon, J.J.A.; Dicke, M. Helping plants to deal with insects: The role of beneficial soilborne microbes. Trends Plant Sci. 2010, 15, 507–514. [Google Scholar] [CrossRef]
- Ahemad, M.; Kibret, M. Mechanisms and applications of plant growth promoting rhizobacteria: Current perspective. J. King Saud. Uni.—Sci. 2014, 26, 1–20. [Google Scholar] [CrossRef]
- Valenzuela-Soto, J.H.; Estrada-Hernández, M.G.; Ibarra-Laclette, E.; Delano-Frier, J.P. Inoculation of tomato plants (Solanum lycopersicum) with growth-promoting Bacillus subtilis retards whitefly Bemisia tabaci development. Planta 2010, 231, 397–410. [Google Scholar] [CrossRef]
- Kempel, A.; Brandl, R.; Schädler, M. Symbiotic soil microorganisms as players in aboveground plant-herbivore interactions—The role of rhizobia. Oikos 2009, 118, 634–640. [Google Scholar] [CrossRef]
- del Rosario, C.L.; Chiappero, J.; Palermo, T.B. Impact of soil rhizobacteria inoculation and leaf-chewing insect herbivory on Mentha piperita leaf secondary metabolites. J. Chem. Ecol. 2020, 46, 619–630. [Google Scholar] [CrossRef]
- Bashan, Y.; Holguin, G.; de-Bashan, L.E. Azospirillum-plant relationships: Physiological, molecular, agricultural, and environmental advances (1997–2003). Can. J. Microbiol. 2004, 50, 521–577. [Google Scholar] [CrossRef]
- Gou, X.; Reich, P.B.; Qiu, L.; Shao, M.; Wei, G.; Wang, J.; Wei, X. Leguminous plants significantly increase soil N cycling across global climates and ecosystem types. Glob. Chang. Biol. 2023, 29, 4028–4043. [Google Scholar] [CrossRef]
- Ping, L.; Boland, W. Signals from the underground: Bacterial volatiles promote growth in Arabidopsis. Trends Plant Sci. 2004, 9, 263–266. [Google Scholar] [CrossRef] [PubMed]
- Kloepper, J.W.; Leong, J.; Teintze, M.; Schroth, M.N. Enhanced plant growth by siderophores produced by plant growth-promoting rhizobacteria. Nature 1980, 286, 885–886. [Google Scholar] [CrossRef]
- Zahir, Z.A.; Arshad, M.; Frankenberger, W.T. Plant growth promoting rhizobacteria: Applications and perspectives in agriculture. Adv. Agron. 2004, 81, 97–168. [Google Scholar] [CrossRef]
- Bravo, A.; Gill, S.S.; Soberón, M. Mode of action of Bacillus thuringiensis Cry and Cyt toxins and their potential for insect control. Toxicon 2007, 49, 423–435. [Google Scholar] [CrossRef]
- Chen, Y.; Schmelz, E.A.; Wäckers, F.; Ruberson, J.R. Cotton Plant, Gossypium hirsutum L., Defense in Response; Springer: Berlin/Heidelberg, Germany, 2008. [Google Scholar] [CrossRef]
- Vessey, J.K. Plant growth promoting rhizobacteria as biofertilizers. Plant Soil 2003, 255, 571–586. [Google Scholar] [CrossRef]
- Kloepper, J.W.; Ryu, C.M.; Zhang, S. Induced systemic resistance and promotion of plant growth by Bacillus spp. Phytopatho 2004, 94, 1259–1266. [Google Scholar] [CrossRef]
- Gange, A.C.; Eschen, R.; Schroeder, V. The soil microbial community and plant foliar defences against insects. In The Ecology of Plant Secondary Metabolites: From Genes to Global Processes; Cambridge University Press: New York, NY, USA, 2012; pp. 170–188. [Google Scholar] [CrossRef]
- Van Oosten, V.R.; Bodenhausen, N.; Reymond, P.; Van Pelt, J.A.; Van Loon, L.C.; Dicke, M.; Pieterse, C.M.J. Differential effectiveness of microbially induced resistance against herbivorous insects in Arabidopsis. Mol. Plant-Microbe Interact. 2008, 21, 919–930. [Google Scholar] [CrossRef] [PubMed]
- Pineda, A.; Zheng, S.J.; van Loon, J.J.A.; Pieterse, C.M.J.; Dicke, M. Rhizobacteria modify plant-aphid interactions: A case of induced systemic susceptibility. Plant Biol. 2012, 14, 83–90. [Google Scholar] [CrossRef]
- Pieterse, C.M.J.; van Wees, S.C.M.; Ton, J.; van Pelt, J.A.; van Loon, L.C. Signalling in Rhizobacteria-Induced Systemic Resistance in Arabidopsis thaliana. Plant Biol. 2002, 4, 535–544. [Google Scholar] [CrossRef]
- Chowdhury, S.P. Effects of Bacillus amyloliquefaciens FZB42 on lettuce growth and health under pathogen pressure and its impact on the rhizosphere bacterial community. PLoS ONE 2013, 8, e68818. [Google Scholar] [CrossRef]
- Vijayasamundeeswari, A.; Ladhalakshmi, D.; Sankaralingam, A.; Samiyappan, R. Plant growth promoting rhizobacteria of cotton affecting the developmental stages of Helicoverpa armigera. J. Plant Protec. Res. 2009, 49, 239–243. [Google Scholar] [CrossRef]
- Frost, C.J.; Mescher, M.C.; Carlson, J.E.; De Moraes, C.M. Plant Defense Priming against Herbivores: Getting Ready for a Different Battle. Plant Physiol. 2008, 146, 818–824. [Google Scholar] [CrossRef] [PubMed]
- Moran, P.J.; Thompson, G.A. Molecular responses to aphid feeding in Arabidopsis in relation to plant defense pathways. Plant Physiol. 2001, 125, 1074–1085. [Google Scholar] [CrossRef]
- Turan, M.; Ekinci, M.; Yildirim, E.; Gunes, A.; Karagoz, K.; Kotan, R.; Dursan, A. Plant growth-promoting rhizobacteria improved growth, nutrient, and hormone content of cabbage (Brassica oleracea) seedlings. Gesunde Pflanz. 2014, 66, 327–333. [Google Scholar] [CrossRef]
- Dashti, N.; Zhang, F.; Hynes, R.; Smith, D.L. Plant growth promoting rhizobacteria accelerate nodulation and increase nitrogen fixation activity by field grown soybean Glycine max (L.) Merr. under short season conditions. Plant Soil 1998, 200, 205–213. [Google Scholar] [CrossRef]
- Ellis, C.; Karafyllidis, I.; Turner, J.G. Constitutive activation of jasmonate signaling in an Arabidopsis mutant correlates with enhanced resistance to Erysiphe cichoracearum, Pseudomonas syringae, and Myzus persicae. Mol. Plant-Microbe Interact. 2002, 15, 1025–1030. [Google Scholar] [CrossRef]
- Mewis, I.; Appel, H.M.; Hom, A.; Raina, R.; Schultz, J.C. Major signaling pathways modulate Arabidopsis glucosinolate accumulation and response to both phloem-feeding and chewing insects. Plant Physiol. 2005, 138, 1149–1162. [Google Scholar] [CrossRef]
- Herman, M.A.B.; Nault, B.A.; Smart, C.D. Effects of plant growth-promoting rhizobacteria on bell pepper production and green peach aphid infestations in New York. Crop Prot. 2008, 27, 996–1002. [Google Scholar] [CrossRef]
- Dean, J.M.; Mescher, M.C.; De Moraes, C.M. Plant Dependence on Rhizobia for Nitrogen Influences Induced Plant Defenses and Herbivore Performance. Int. J. Mol. Sci. 2014, 15, 1466–1480. [Google Scholar] [CrossRef]
- Srinivasan, M.; Petersen, D.J.; Holl, F.B. Influence of indoleacetic-acid-producing Bacillus isolates on the nodulation of Phaseolus vulgaris by Rhizobium etli under gnotobiotic conditions. Can. J. Microbiol. 1996, 42, 1006–1014. [Google Scholar] [CrossRef]
- Camacho, M.; Santamaria, C.; Temprano, F.; Rodriguez-Navarro, D.N.; Daza, A. Co-inoculation with Bacillus sp. CECT 450 improves nodulation in Phaseolus vulgaris L. Can. J. Microbiol. 2001, 47, 1058–1062. [Google Scholar] [CrossRef]
- Guiñazu, L.B.; Andrés, J.A.; Del Papa, M.F.; Pistorio, M.; Rosas, S.B. Response of alfalfa (Medicago sativa L.) to single and mixed inoculation with phosphate-solubilizing bacteria and Sinorhizobium meliloti. Biol. Fert. Soils 2010, 46, 185–190. [Google Scholar] [CrossRef]
- Mostasso, L.; Mostasso, F.L.; Dias, B.G.; Vargas, M.A.T.; Hungria, M. Selection of bean (Phaseolus vulgaris L.) rhizobial strains for Brazilian Cerrados. Field Crops Res. 2002, 73, 121–132. [Google Scholar] [CrossRef]
- Bonfante, P.; Genre, A. Mechanisms underlying beneficial plant–fungus interactions in mycorrhizal symbiosis. Nat. Commun. 2010, 1, 48. [Google Scholar] [CrossRef]
- Asadi, R.; Afshari, H.M.; Khavazi, K.; Nourgholipour, F.; Otadi, A. Effects of common bean nodulating rhizobia native to Iranian soils on the yield and quality of bean. Iran. J. Soil Water Sci. 2005, 19, 215–225. [Google Scholar] [CrossRef]
- Stajković-Srbinović, O.; Delić, D.; Kuzmanović, D.; Rasulić, N.; Knežević-Vukčević, J.; Simić, A. Improvement of common bean growth by co-inoculation with Rhizobium and plant growth-promoting bacteria. Rom. Biotechnol. Lett. 2011, 16, 5919–5926. [Google Scholar]
- Denno, R.F.; McClure, M.S.; Ott, J.R. Interspecific Interactions in Phytophagous Insects: Competition Reexamined and Resurrected. Ann. Rev. Entomol. 1995, 40, 297–331. [Google Scholar] [CrossRef]
- Rostás, M.; Simon, M.; Hilker, M. Ecological cross-effects of induced plant responses towards herbivores and phytopathogenic fungi. Basic Appl. Ecol. 2003, 4, 43–62. [Google Scholar] [CrossRef]
- Bonte, D.; De Roissart, A.; Vandegehuchte, M.L.; Ballhorn, D.J.; Van Leeuwen, T.; de la Peña, E. Local adaptation of aboveground herbivores towards plant phenotypes induced by soil biota. PLoS ONE 2010, 5, e11174. [Google Scholar] [CrossRef] [PubMed]
- Sprent, J.I. Nitrogen fixation in arid environments. In Nitrogen Fixing Organisms: Pure and Applied Aspects; Chapman and Hall: New York, NY, USA, 1990; pp. 171–188. [Google Scholar] [CrossRef]
- Schädler, M.; Roeder, M.; Brandl, R.; Matthies, D. Interacting effects of elevated CO2, nutrient availability and plant species on a generalist invertebrate herbivore. Glob. Chang. Biol. 2007, 13, 1005–1015. [Google Scholar] [CrossRef]
- De Vos, M.; Van, Z.W.; Koornneef, A.; Korzelius, J.P.; Dicke, M. Herbivore-induced resistance against microbial pathogens in Arabidopsis. Plant Physiol. 2006, 142, 352–363. [Google Scholar] [CrossRef] [PubMed]
- Zarate, S.I.; Kempema, L.A.; Walling, L.L. Silverleaf whitefly induces salicylic acid defenses and suppresses effectual jasmonic acid defenses. Plant Physiol. 2007, 143, 866–875. [Google Scholar] [CrossRef]
- Thaler, J.S. Jasmonate-inducible plant defences cause increased parasitism of herbivores. Nature 1999, 399, 686–688. [Google Scholar] [CrossRef]
- Van Poecke, R.M.P.; Dicke, M. Induced parasitoid attraction by Arabidopsis thaliana: Involvement of the octadecanoid and the salicylic acid pathway. J. Exp. Bot. 2002, 53, 1793–1799. [Google Scholar] [CrossRef] [PubMed]
- Rutz, C.; Hugentobler, U.; Chi, H.; Baumgartner, J.; Oertli, J. Energy-flow in an apple plant-aphid (Aphis pomi Degeer) (Homoptera, Aphididae) ecosystem, with respect to nitrogen-fertilization. Plant Soil 1990, 124, 273–279. [Google Scholar] [CrossRef]
- Schutz, K.; Bonkowski, M.; Scheu, S. Effects of Collembola and fertilizers on plant performance (Triticum aestivum) and aphid reproduction (Rhopalosiphum padi). Basic Appl. Ecol. 2008, 9, 182–188. [Google Scholar] [CrossRef]
- Chau, A.; Heinz, K.; Davies, F. Influences of fertilization on Aphis gossypii and insecticide usage. J. Appl. Entomol. 2005, 129, 89–97. [Google Scholar] [CrossRef]
- Zheng, S.J. Sensitivity and speed of induced defense of cabbage (Brassica oleracea L.): Dynamics of BoLOX expression patterns during insect and pathogen attack. Mol. Plant-Microbe Interact. 2007, 20, 1332–1345. [Google Scholar] [CrossRef]
- Howe, G.A.; Jander, G. Plant immunity to insect herbivores. Ann. Rev. Plant Biol. 2008, 59, 41–66. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Sun, X.; Zhao, H.; Xue, M.; Wang, D. Phenolic compounds induced by Bemisia tabaci and Trialeurodes vaporariorum in Nicotiana tabacum L. and their relationship with the salicylic acid signaling pathway. Arth.-Plant Interact. 2017, 11, 659–667. [Google Scholar] [CrossRef]
- Turlings, T.C.J.; Erb, M. Tritrophic interactions mediated by herbivore-induced plant volatiles: Mechanisms, ecological relevance, and application potential. Ann. R. Entomol. 2018, 63, 433–452. [Google Scholar] [CrossRef] [PubMed]
- Pangesti, N.; Pineda, A.; Dicke, M.; Van Loon, J.J.A. Variation in plant-mediated interactions between rhizobacteria and caterpillars: Potential role of soil composition. Plant Biol. 2015, 17, 474–483. [Google Scholar] [CrossRef]
- Öztürk, B.; Kaya, M.; Demir, M. Does inquiry-based learning model improve learning outcomes? A second-order meta-analysis. J. Pedagog. Res. 2022, 6, 201–216. [Google Scholar] [CrossRef]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) statement. Syst. Rev. 2015, 349, g7647. [Google Scholar]
- Badri, D.V.; Zolla, G.; Bakker, M.G.; Manter, D.K.; Vivanco, J.M. Potential impact of soil microbiomes on the leaf metabolome and on herbivore feeding behavior. New Phytol. 2013, 198, 264–273. [Google Scholar] [CrossRef]
- Ballhorn, D.J.; Schädler, M.; Elias, J.D.; Millar, J.A.; Kautz, S. Friend or Foe-Light Availability Determines the Relationship between Mycorrhizal Fungi, Rhizobia and Lima Bean (Phaseolus lunatus L.). PLoS ONE 2016, 11, e0154116. [Google Scholar] [CrossRef] [PubMed]
- Bell, K.; Naranjo-Guevara, N.; Santos, R.C.D.; Meadow, R.; Bento, J.M.S. Predatory Earwigs are Attracted by Herbivore-Induced Plant Volatiles Linked with Plant Growth-Promoting Rhizobacteria. Insects 2020, 11, 271. [Google Scholar] [CrossRef]
- Brock, A.K.; Berger, B.; Schreiner, M. Plant growth-promoting bacteria Kosakonia radicincitans mediate anti-herbivore defense in Arabidopsis thaliana. Planta 2018, 248, 1383–1392. [Google Scholar] [CrossRef]
- Coy, R.M.; Held, D.W.; Kloepper, J.W. Rhizobacterial treatments of tall fescue and bermudagrass increases tolerance to damage from white grubs. Pest. Manag. Sci. 2019, 75, 3210–3217. [Google Scholar] [CrossRef] [PubMed]
- D’Alessandro, M.; Erb, M.; Ton, J.; Brandenburg, A.; Karlen, D.; Zopfi, J.; Turlings, T.C.J. Volatiles produced by soil-borne endophytic bacteria increase plant pathogen resistance and affect tritrophic interactions. Plant Cell Environ. 2014, 37, 813–826. [Google Scholar] [CrossRef]
- Dean, J.M.; De Moraes, C.M. Plant-rhizobia mutualism influences aphid abundance on soybean. Plant Soil 2009, 323, 187–189. [Google Scholar] [CrossRef]
- Dong, N.D.; Xia, L.H.; Hao, J.C.; Peng, W.Y.; Ya, W.Q.; Ling, J.H.; Hua, G.J. The Plant Growth-Promoting Rhizobacterium Bacillus cereus AR156 Induces Systemic Resistance in Arabidopsis thaliana by Simultaneously Activating Salicylate- and Jasmonate/Ethylene-Dependent Signaling Pathways. Am. Phytopathol. Soc. 2011, 24, 533–542. [Google Scholar] [CrossRef]
- Etesami, H.; Alikhani, A. Bacillus species as the most promising bacterial biocontrol agents in rhizosphere and endorhiza of plants grown in rotation with each other. Eur. J. Plant Pathol. 2018, 150, 497–506. [Google Scholar] [CrossRef]
- Friman, J.; Pineda, A.; Gershenzon, J.; Dicke, M.; Van Loon, J.J.A. Differential effects of the rhizobacterium Pseudomonas simiae on above- and belowground chewing insect herbivores. J. Appl. Entomol. 2021, 145, 250–260. [Google Scholar] [CrossRef]
- Godschal, A.L.; Schädler, M.; Trisel, J.A.; Balkan, M.A.; Ballhorn, D.J. Ants are less attracted to the extrafloral nectar of plants with symbiotic, nitrogen-fixing rhizobia. Ecology 2015, 96, 348–354. [Google Scholar] [CrossRef]
- Heath, K.D.; Lau, J.A. Herbivores alter the fitness benefits of a plant-rhizobium mutualism. Acta Oecol. 2011, 37, 87–92. [Google Scholar] [CrossRef]
- Khoshfarman-Borji, H.; Pahlavan, Y.M.; Bozorg-Amirkalaee, M. Induction of resistance against Brevicoryne brassicae by Pseudomonas putida and salicylic acid in canola. Bull. Entomol. Res. 2020, 10, 1–14. [Google Scholar] [CrossRef]
- Martinuz, A.; Schouten, A.; Menjivar, R.D.; Sikora, R.A. Effectiveness of systemic resistance toward Aphis gossypii (Hom., Aphididae) as induced by combined applications of the endophytes Fusarium oxysporum Fo162 and Rhizobium etli G12. Biol. Control 2012, 62, 206–212. [Google Scholar] [CrossRef]
- Megali, L.; Glauser, G.; Rasmann, S. Fertilization with beneficial microorganisms decreases tomato defenses against insect pests. Agron. Sustain. Dev. 2014, 34, 649–656. [Google Scholar] [CrossRef]
- Muqarab, R.; Bano, A. Plant defence induced by PGPR against Spodoptera litura in tomato. Plant Biol. 2017, 19, 406–412. [Google Scholar] [CrossRef]
- Mustikawati, D.R. Effect of Plant Growth Promoting Rhizobacteria (PGPR) and Liquid Smoke Against Diseases Attacks and Growth of Pepper (Piper nigrum L.). Int. J. Sci. Basic Appl. Res. 2017, 31, 145–155. [Google Scholar]
- Naeem, M.; Aslam, Z.; Khaliq, A.; Ahmed, J.A.; Nawaz, A.; Hussain, M. Plant growth promoting rhizobacteria reduce aphid population and enhance the productivity of bread wheat. Braz. J. Microbiol. 2018, 49, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Nishita, G.; Josh, N. Growth and yield response of chick pea (Cicer arietinum) to seed inoculation with Rhizobium sp. Nat. Sci. 2010, 8, 232–236. [Google Scholar]
- Onwusemu, J.D.; Zebelo, S.; Kloepper, J.W.; Fadamiro, H. Seed inoculation with beneficial rhizobacteria affects European corn borer (Lepidoptera: Pyralidae) oviposition on maize plants. Entomol. Sci. 2018, 21, 48–58. [Google Scholar] [CrossRef]
- Pagnani, G.; Pellegrini, M.; Galieni, A.; D’Egidio, S.; Matteucci, F.; Ricci, A.; Stagnari, F.; Sergi, M.; Lo Sterzo, M.; Pisante, M.; et al. Plant growth-promoting rhizobacteria (PGPR) in Cannabis sativa ‘Finola’ cultivation: An alternative fertilization strategy to improve plant growth and quality characteristics. Ind. Crops Prod. 2018, 123, 75–83. [Google Scholar] [CrossRef]
- Parisi, A.G.; Grimoldi, A.A.; Omacini, M. Endophytic fungi of grasses protect other plants. Fungal Ecol. 2014, 9, 61–64. [Google Scholar] [CrossRef]
- Qi, G.; Zhang, X.; Zhao, X. Endophytic Bacillus subtilis WH2 containing Pinellia ternata agglutinin showed insecticidal activity against whitebacked planthopper Sogatella furcifera. BioControl 2013, 58, 233–246. [Google Scholar] [CrossRef]
- Rashid, M.D.; Harun-O, -R.; Khan, A.; Hossain, M.T.; Chung, Y.R. Induction of Systemic Resistance against Aphids by Endophytic Bacillus velezensis YC7010 via Expressing PHYTOALEXIN DEFICIENT4 in Arabidopsis. Front. Plant Sci. 2017, 8, 211. [Google Scholar] [CrossRef]
- Santos, F.; Fernanda, G.M.; Peñaflor, M.; Pare, P.W.; Sanches, P.A.; Kamiya, A.C.; Tonelli, M.; Nardi, C.; Bento, M.S. A Novel Interaction between Plant-Beneficial Rhizobacteria and Roots: Colonization Induces Corn Resistance against the Root Herbivore Diabrotica speciosa. PLoS ONE 2014, 11, e0113280. [Google Scholar] [CrossRef] [PubMed]
- Senthilraja, G.; Anand, T.; Kennedy, J.S.; Raguchander, T.; Samiyappan, R. Plant growth promoting rhizobacteria (PGPR) and entomopathogenic fungus bioformulation enhance the expression of defense enzymes and pathogenesis-related proteins in groundnut plants against leaf miner insect and collar rot pathogen. Physiol. Mol. Plant Pathol. 2013, 82, 10–19. [Google Scholar] [CrossRef]
- Thamer, S.; Schädler, M.; Bonte, D.; Ballhorn, D.J. Dual benefit from a belowground symbiosis: Nitrogen fixing rhizobia promote growth and defense against a specialist herbivore in a cyanogenic plant. Plant Soil 2011, 341, 209–219. [Google Scholar] [CrossRef]
- Tonelli, M.L.; Noguera, C.M.; Fabra, A. Symbiotic performance and induction of systemic resistance against Cercospora sojina in soybean plants co-inoculated with Bacillus sp. CHEP5 and Bradyrhizobium japonicum E109. Arch. Microbiol. 2017, 199, 1283–1291. [Google Scholar] [CrossRef]
- Whitaker, M.R.L.; Katayama, N.; Ohgushi, T. Plant-rhizobia interactions alter aphid honeydew composition. Arthropod-Plant Interact. 2014, 8, 213–220. [Google Scholar] [CrossRef]
- Wulandari, D.; Aini, L.Q.; Tarno, H. Eating Behavior of Imago Aulacophora similis Oliver on Cucumber (Cucumis sativus L.) with Treatment Plant Growth Promoting Rhizobacteria (PGPR). Res. J. Life Sci. 2020, 7, 192–201. [Google Scholar] [CrossRef]
- Rosenberg, M.S.; Adams, D.C.; Gurevitch, J. MetaWin: Statistical Software for Meta-Analysis, version 2.1; Sinauer: Sunderland, CA, USA, 2002. [Google Scholar]
- Rosenthal, R. The file drawer problem and tolerance for null results. Psychol. Bull. 1979, 86, 638–641. [Google Scholar] [CrossRef]
- Rosenberg, M.S. The file-drawer problem revisited: A general weighted method for calculating fail-safe numbers in meta-analysis. Evolution 2005, 59, 464–468. [Google Scholar] [CrossRef]
| Model | Plant growth | Phytophagous insect activity | ||||
|---|---|---|---|---|---|---|
| Df | QB | p-Value | Df | QB | p-Value | |
| Full model | 297 | 9612.85 | <0.0001 | 297 | 4793.1536 | <0.0001 |
| Bacterial genus | 6 | 308.27 | <0.0001 | 8 | 75.14 | <0.0001 |
| Plant family | 4 | 264.35 | <0.0001 | 6 | 135.46 | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruiz-Santiago, R.R.; Ballina-Gómez, H.S.; Ruíz-Sánchez, E.; Solís-Ramos, L.Y.; Cristóbal-Alejo, J. Plant-Growth-Promoting Rhizobacteria and Known Interactions with Plant Phytophagous Insects: A Meta-Analysis. Stresses 2025, 5, 35. https://doi.org/10.3390/stresses5020035
Ruiz-Santiago RR, Ballina-Gómez HS, Ruíz-Sánchez E, Solís-Ramos LY, Cristóbal-Alejo J. Plant-Growth-Promoting Rhizobacteria and Known Interactions with Plant Phytophagous Insects: A Meta-Analysis. Stresses. 2025; 5(2):35. https://doi.org/10.3390/stresses5020035
Chicago/Turabian StyleRuiz-Santiago, Roberto Rafael, Horacio Salomón Ballina-Gómez, Esaú Ruíz-Sánchez, Laura Yesenia Solís-Ramos, and Jairo Cristóbal-Alejo. 2025. "Plant-Growth-Promoting Rhizobacteria and Known Interactions with Plant Phytophagous Insects: A Meta-Analysis" Stresses 5, no. 2: 35. https://doi.org/10.3390/stresses5020035
APA StyleRuiz-Santiago, R. R., Ballina-Gómez, H. S., Ruíz-Sánchez, E., Solís-Ramos, L. Y., & Cristóbal-Alejo, J. (2025). Plant-Growth-Promoting Rhizobacteria and Known Interactions with Plant Phytophagous Insects: A Meta-Analysis. Stresses, 5(2), 35. https://doi.org/10.3390/stresses5020035






