Abstract
Marine macroalgae (commonly known as seaweeds), a rich yet underexplored resource, have emerged as a promising source of bioactive compounds with potent antioxidant properties. While oxidative stress is a critical factor in the pathogenesis of numerous chronic diseases, including neurodegenerative disorders, cardiovascular conditions, and cancer, macroalgae-derived compounds such as polyphenols, carotenoids, peptides, and sulfated polysaccharides have demonstrated the ability to mitigate oxidative damage through multifaceted mechanisms. These compounds neutralize reactive oxygen species and modulate key cellular pathways involved in inflammation and apoptosis. Despite significant advancements, gaps persist in understanding the pharmacokinetics, bioavailability, and clinical applications of these bioactives. Additionally, the inefficiencies of traditional extraction methods call for adopting innovative, environmentally friendly techniques that preserve bioactivity. This review synthesizes current knowledge on the therapeutic potential of macroalgal bioactives, acknowledges the contributions of other marine algae where relevant, highlights challenges in their extraction, and proposes future directions for research and application.
1. Introduction
The oceans, covering more than 70% of the Earth’s surface, are a vast and largely untapped reservoir of biodiversity, hosting an extraordinary array of marine organisms. Among these, marine algae—broadly classified into cyanobacteria, microalgae, and macroalgae—play a critical ecological and biochemical role in marine ecosystems [1]. These photosynthetic organisms serve as a fundamental energy source for marine food webs and synthesize a wide array of secondary metabolites. Evolved as adaptive responses to environmental pressures, many of these metabolites exhibit potent antioxidant properties and have attracted growing attention for their potential to address oxidative stress-related diseases [2,3].
Oxidative stress—an imbalance between the production of reactive oxygen species (ROS) and the body’s antioxidant defenses—is now recognized as a major contributor to the pathogenesis of numerous chronic disorders. Neurodegenerative diseases, cardiovascular conditions, cancer, diabetes, and autoimmune disorders are among the most prominent health challenges linked to oxidative damage. Although endogenous antioxidant systems provide some protection, modern lifestyles and environmental factors exacerbate these conditions, intensifying the search for sustainable and effective therapies.
Marine macroalgae, in particular, are nutrient-dense organisms with a remarkable diversity of bioactive compounds. While thousands of macroalgal species have been documented, many remain unexplored, representing an untapped resource of immense potential. Their biochemical composition can vary significantly by species, habitat, and environmental conditions such as temperature, salinity, and light availability [3]. This variability influences the concentration and functionality of compounds such as polyphenols, carotenoids, sulfated polysaccharides, and phlorotannins—each of which has demonstrated significant antioxidant, anti-inflammatory, and therapeutic properties [4,5]. Recent studies show that these compounds not only neutralize ROS but also modulate key signaling pathways involved in inflammation, apoptosis, and cellular repair, offering a multifaceted approach to disease prevention and management [6,7].
Despite these promising findings, several critical challenges remain. Traditional extraction methods—such as liquid–liquid and solid–liquid extraction—often involve hazardous solvents, extended processing times, and low yields of bioactive molecules. Additionally, these processes may degrade sensitive compounds, reducing their bioactivity and therapeutic potential [8]. In response, novel extraction techniques, including enzyme-assisted, ultrasound-assisted, and microwave-assisted approaches, have gained traction for their enhanced efficiency and alignment with green chemistry principles [9]. However, translating these advancements into therapeutic applications requires a deeper understanding of how macroalgal compounds interact with human biological systems. For example, the molecular mechanisms by which sulfated polysaccharides influence ROS scavenging, inflammation, and overall bioavailability remain insufficiently explored. Bridging these knowledge gaps is crucial to advancing potential therapies.
Moreover, significant strides have been made in identifying macroalgal bioactivities, yet further research is needed to build robust evidence across all stages—from molecular mechanisms to clinical applications. Before clinical trials can be pursued, comprehensive preclinical studies must be conducted to establish toxicity profiles, efficacy, and mechanisms of action. Clarifying long-term impacts and therapeutic windows will be vital to safely and effectively integrate seaweed-derived bioactives into human health solutions. Accordingly, this review primarily explores the therapeutic potential of marine macroalgal bioactives while also acknowledging relevant contributions from other marine algae, such as microalgae and cyanobacteria, when applicable. We synthesize current knowledge on how these compounds mitigate oxidative stress, evaluate recent advances in extraction, and discuss future directions that can harness their full potential.
2. Oxidative Stress and Its Role in Chronic Diseases
2.1. Mechanisms of Oxidative Stress in the Body
Oxidative stress is a relatively recent concept describing a physiological imbalance caused by excess reactive oxygen species (ROS) or insufficient activity of endogenous antioxidant systems [9]. ROS—such as superoxide anion (O2−), hydrogen peroxide (H2O2), hydroxyl radical (OH•), and peroxyl radicals (ROO•)—are byproducts of normal metabolic processes, including oxidative phosphorylation. They can also be generated in the mitochondria via the electron transport chain, as well as by enzymes like NADPH oxidase and xanthine oxidase. However, ROS levels may rise during environmental or physiological stresses, such as exposure to pollutants, ultraviolet radiation, or inflammation [10,11].
The endogenous antioxidant defense system consists of substances produced by the body to prevent the oxidation of biomolecules or to repair oxidative damage. These molecules can neutralize free radicals and mitigate oxidative stress [12]. Primary endogenous antioxidant mechanisms include enzymatic antioxidants such as superoxide dismutase (SOD), which catalyzes the conversion of superoxide radicals into hydrogen peroxide; catalase, which decomposes hydrogen peroxide into water and oxygen; and glutathione peroxidase (GPx), which reduces hydrogen peroxide and lipid hydroperoxides using glutathione as a cofactor [13]. Non-enzymatic antioxidants include glutathione—a tripeptide crucial in redox reactions that help neutralize free radicals—and uric acid, a purine metabolism byproduct that also acts as an antioxidant [10,14]. Nevertheless, under certain conditions—such as aging or chronic diseases—these antioxidant systems become less effective, allowing ROS to accumulate and cause cumulative damage, ultimately contributing to conditions like cancer, diabetes, and neurodegenerative disorders [15,16].
2.2. Link Between Oxidative Stress and Chronic Diseases
ROS can initiate harmful reactions that damage critical biomolecules. For example, lipid peroxidation compromises cell membrane integrity and functionality, while protein oxidation alters protein structure and function. ROS can also induce DNA mutations, disrupting normal cellular processes and contributing to cellular aging and death [10]. Moreover, oxidative stress can trigger inflammatory responses, further fueling the progression of chronic diseases (Figure 1) [17]. The interplay between ROS and inflammatory processes is key to understanding disease mechanisms [18]. Aging—a complex and inevitable process shaped by genetics, environment, and lifestyle—often features oxidative stress as a driving factor. Excess ROS generated in mitochondria and the endoplasmic reticulum disrupts signaling pathways and damages cellular components, leading to abnormal protein accumulation (proteinopathies), mitochondrial dysfunction, and altered cellular senescence, which can promote chronic inflammation associated with aging and, in rare instances, metastasis [19,20].
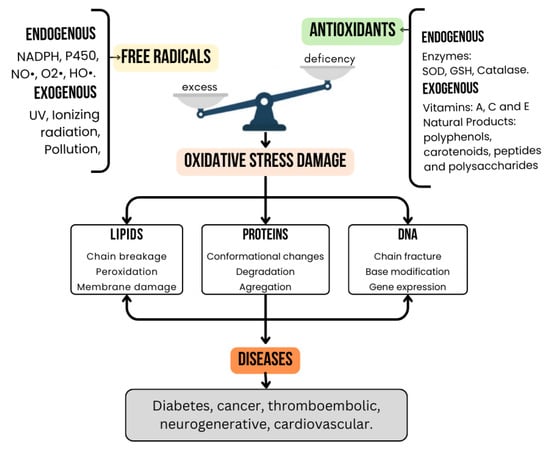
Figure 1.
Oxidative stress imbalance: Excess free radicals and antioxidant deficiency lead to biomolecular damage and chronic diseases.
During oxidative stress, ROS can deregulate multiple metabolic pathways and activate transcription factors such as activator protein 1 (AP-1), mitogen-activated protein kinase (MAPK), and nuclear factor kappa B (NF-kB). This can lead to inappropriate cell death through apoptosis or necrosis [21]. Consequently, chronic inflammation emerges, fueling the development of degenerative and inflammatory diseases, including neurodegenerative disorders, diabetes, cardiovascular diseases, chronic kidney disease, and retinopathies [22]. This vicious cycle—where oxidative stress triggers inflammation and inflammation further exacerbates ROS production—underscores the complexity of effectively mitigating oxidative stress-related diseases.
Conventional therapeutic strategies often center on antioxidant supplementation. However, such approaches face limitations: low specificity, poor bioavailability, and the potential for resistance due to chronic exposure leading to cellular adaptations that diminish efficacy. Additionally, the multifaceted nature of oxidative stress—shaped by genetics, lifestyle, and environmental factors—means that a single antioxidant may not address all underlying mechanisms [7]. Thus, novel antioxidant therapies are needed, particularly those derived from natural, sustainable sources with improved specificity, bioavailability, and safety profiles.
3. Marine Algae as a Source of Bioactive Compounds
Marine macroalgae (seaweeds) are photosynthetic organisms inhabiting diverse aquatic environments, from coastal zones to deeper oceanic regions. Of the approximately 300,000 distinct algal species identified to date, around 6500 are marine [23]. Although the term “marine algae” broadly includes cyanobacteria and microalgae, this review focuses on macroalgae—commonly categorized as Rhodophyta (red algae), Phaeophyta (brown algae), and Chlorophyta (green algae). These seaweeds play a critical role in aquatic food webs and offer various health benefits for humans. They are widely used in the food, pharmaceutical, and cosmetic industries [24,25].
The biochemical diversity of marine macroalgae is vast, as they produce a wide range of compounds with important biological activities [26]. Notably, brown algae contain alginates—used in the food and pharmaceutical industries as thickeners and stabilizers—while red algae yield agar and carrageenans, both employed as gelling agents [27]. Macroalgae also contain a variety of pigments, including chlorophylls (a, b, and c) and fucoxanthin (mainly brown algae), enabling them to absorb light under different oceanic conditions [28]. Additionally, marine macroalgae can be significant sources of polyunsaturated fatty acids (PUFAs), such as eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), known for their cardiovascular benefits [29]. They also supply vitamins A, C, and E, plus minerals like iodine, iron, and calcium [30]. Among other notable secondary metabolites are polyphenols, carotenoids, fucoxanthin, sulfated polysaccharides, and phlorotannins—many of which have gained attention for their potential to mitigate oxidative stress [31].
The health benefits attributed to marine macroalgae are extensive. Omega-3 fatty acids, for instance, are associated with reduced triglycerides, improved blood pressure, and lower heart disease risk [32]. Antioxidants in macroalgae help protect blood vessels from oxidative damage [33]. In the realm of neurological health, omega-3 fatty acids appear to support cognitive function and mood, while antioxidant compounds can reduce inflammation and protect neural cells [34]. Polysaccharides found in macroalgae may strengthen immune responses [35], and vitamins and minerals from seaweeds contribute to overall immune function. Due to their high fiber content and low-calorie density, macroalgae also facilitate weight management by promoting satiety [36,37]. Meanwhile, their antioxidants protect skin from oxidative damage and premature aging, and essential minerals foster healthy hair growth. Iodine, in particular, is critical for thyroid gland function [38,39].
Still, large-scale exploitation of marine macroalgae poses challenges in terms of safety and product quality. Heavy metals (e.g., cadmium, lead, arsenic) and biotoxins may accumulate, posing significant health risks [40]. Additionally, the biochemical composition of macroalgae varies considerably by species, location, season, and environmental factors, complicating the standardization of macroalgae-derived products [41]. To ensure safety and efficacy, establishing quality standards, monitoring for contamination, and advancing scientific research on algal biochemistry are essential. The development of improved extraction and characterization methods, as well as sustainable cultivation practices, will further enable the reliable use of macroalgae as a valuable source of bioactive compounds.
4. Mechanisms of Action of Algal Compounds Against Oxidative Stress
4.1. Phenolic Compounds
Phenolic compounds are a diverse group of secondary metabolites mainly found in plants, though certain marine organisms—particularly macroalgae—also produce them. Chemically, these compounds contain a hydroxyl group attached to a phenol ring, enabling them to capture free radicals and ROS. Compounds with multiple phenolic rings are collectively known as polyphenols. Their classification (phenolic acids, flavonoids, stilbenes, tannins, lignans, halogenated phenols, etc.) typically depends on the number of phenolic rings and their attached functional groups [42,43,44,45].
Among marine macroalgae, bromophenols (BPs) stand out as secondary metabolites characterized by phenolic structures bearing one or more bromine atoms. Many BPs have been isolated from red, brown, and green macroalgae [46]. Phlorotannins, another key group of polyphenols, are particularly abundant in brown macroalgae [47]. For instance, Leyton et al. identified different phloroglucinol isomers (difucofloroethanol, fucodifloroethanol, tetrafucol, and tetrafloroethanol) in Macrocystis pyrifera [48]. Likewise, trimers and tetramers of phloroglucinol have been quantified in Sargassum carpophyllum from coastal areas of Japan [49]. Additional studies on Ascophyllum nodosum, Bifurcaria bifurcata, and Fucus vesiculosus have identified various phlorotannins, flavonoids, phenolic acids, and phloroglucinol derivatives, confirming that phlorotannins represent the most abundant phenolic class in these macroalgae [50]. Other common phenolic compounds in marine macroalgae include different flavonoid subclasses (flavonols, flavanones, isoflavones, dihydrochalcones) and phenolic acids (gallic, protocatechuic, caffeic, chlorogenic, vanillic, p-hydroxybenzoic, salicylic) [51].
Although the present review focuses on macroalgae, some microalgae also produce phenolic compounds—albeit often to a lesser extent or with different profiles. For instance, Andripoulus et al. evaluated the total phenolic content (TPC) of the microalgae Chlorella minutissima, Dunaliella salina, Isochrysis galbana, Nannochloropsis oculata, and Tisochrysis lutea, finding the highest TPC in aqueous extracts of D. salina and I. galbana (8.78 ± 1.49 mg GAE/g DW and 8.13 ± 0.39 mg GAE/g DW, respectively). However, they concluded that pigments, proteins, and fatty acids should be the main focus for these particular microalgae [52].
Mechanistically, phenolic compounds exhibit antioxidant activity through multiple processes (Table 1). First, hydrogen atom transfer (HAT) enables them to donate a hydrogen atom to free radicals, stabilizing those radicals and forming a non-radical species. In turn, the phenolic compound itself becomes a stable antioxidant radical. Another important mechanism is single electron transfer (SET), where the phenolic compound donates a single electron to the free radical, neutralizing reactive species. Sequential proton loss electron transfer (SPLET) involves the phenolic compound donating a proton to a radical—forming an anion—which then donates an electron, ultimately creating a stable molecule. Finally, transition metal chelation allows phenolic compounds to bind transition metals, preventing pro-oxidant reactions that can generate additional free radicals. Collectively, these mechanisms underscore the versatile antioxidant potential of phenolic compounds [53,54].

Table 1.
Antioxidant mechanism of bioactive compounds in algae.
4.2. Carotenoids
Carotenoids are natural pigments widely distributed in nature. Their chemical structure follows the general formula (C5H8)n, with a conjugated double-bond system (the chromophore) that accounts for their characteristic colors (yellow, red, orange, and purple) [55]. They are composed of isoprene (C5) units, which can be classified as C30 (6 units, apocarotenoids), C40 (8 units), C45 (9 units), or C50 (10 units). The molecule is typically symmetrical, linking isoprenes head-to-tail, except at the central junction where they connect tail-to-tail. Some carotenoids feature allenic (C=C=C) or acetylenic (C≡C) groups, observed in various marine organisms [56]. Differences in the position and number of double bonds, as well as in the presence of functional groups, give rise to distinct carotenoid classes—such as carotenes (composed of hydrogen and carbon) and xanthophylls (composed of hydrogen, carbon, and oxygen) [57].
Both marine microalgae and marine macroalgae (seaweeds) can synthesize a variety of carotenoids, including α- and β-carotenes, lycopene, and xanthophylls (e.g., zeaxanthin, lutein, canthaxanthin, astaxanthin, violaxanthin, diadinoxanthin, dinoxanthin, and fucoxanthin) [58,59]. Although some microalgae, such as Dunaliella salina and Chlorella vulgaris, are notable for producing β-carotene, canthaxanthin, and astaxanthin [60,61].
In terms of their antioxidant mechanisms (Table 1), carotenoids can transfer electrons along their polyene chain to quench high-energy radicals [50,62]. They also capture and neutralize singlet oxygen by dissipating its excitation energy harmlessly and returning it to the ground state. Additional processes include hydrogen atom transfer or the formation of adducts with free radicals, thus scavenging peroxyl radicals and preventing oxidative damage. Lastly, carotenoids can form radical cations by donating electrons to high–redox-potential radicals; these intermediates may then react with other biomolecules or be regenerated by antioxidants such as vitamin C and α-tocopherol [63].
4.3. Polysaccharides
Marine macroalgae are distinguished by their high polysaccharide content, typically composed of various monosaccharides and often bearing sulfate groups. These compounds exhibit multiple biological properties [64]. Recent studies show that algae-derived polysaccharides can serve as effective free radical scavengers and antioxidants, helping to prevent oxidative damage in living organisms. In macroalgae, polysaccharides may be located on the cell surface (structural polysaccharides) or stored intracellularly (storage polysaccharides). The most notable structural polysaccharides include alginates, carrageenans, cellulose, sulfated polysaccharides, and fucoidans, whereas laminarin and starch are commonly cited as storage polysaccharides [65].
Polysaccharides exert their antioxidant activity through several key mechanisms (Table 1). First, they act as free radical scavengers, neutralizing reactive oxygen and nitrogen species. Second, they can chelate metal ions such as iron and copper, thereby inhibiting Fenton-type reactions that produce additional radicals. Third, modifying the polysaccharide’s molecular structure—for example, by reducing its molecular weight—can enhance its antioxidant capacity. Finally, interactions with proteins can further improve the effectiveness of these polysaccharides in neutralizing free radicals [66,67].
An important example is fucoidan, a sulfated sugar found in brown algae such as Dictyota menstrualis, Padina boryana, Kjellmaniella crassifolia, and Fucus vesiculosus [68]. While its anticoagulant properties have been studied extensively, a growing body of research highlights its antioxidant potential as well. For instance, fucoidan from Laminaria japonica can prevent the increase of lipid peroxide (LPO) in the serum, liver, and spleen of diabetic mice [69]. Furthermore, fucoidans (homofucans) and heterofucans extracted from Fucus vesiculosus and Padina gymnospora have demonstrated inhibitory effects on hydroxyl and superoxide radicals [70,71]. Interestingly, a higher degree of sulfation appears to improve the radical-scavenging activity of fucoidan [72].
Another noteworthy polysaccharide is carrageenan, the primary structural polysaccharide in numerous red macroalgae, such as Eucheuma, Chondrus, Gigartina, and Fucellaria [73]. It exhibits known antioxidant properties and is made up of alternating units of d-galactose (β(1,3)-d-galactose-4-sulfate) and α(1–4)-3,6-anhydro-d-galactose [74]. For instance, carrageenan from Eucheuma gelatinae—rich in rhamnose, mannose, glucose, fucose, and xylose—displays antioxidant activity (71 ± 5 mg of ascorbic acid equivalent/g of dry weight) and reducing power (8984 ± 5 mg of FeSO4 equivalent/g of dry weight) [75].
4.4. Peptides
Bioactive peptides are biologically active molecules generally embedded within precursor proteins, becoming active once these proteins undergo breakdown. Another type of peptide is actively produced by various microorganisms and other living organisms [76]. Bioactive peptides (BPs) typically consist of 2–40 amino acid residues, and their antioxidant properties are largely determined by their amino acid sequence, composition, structure, and hydrophobicity. Commonly, these BPs are obtained through enzymatic hydrolysis, fermentation, or chemical synthesis [77].
Peptides exert their antioxidant activity primarily through three mechanisms (Table 1). First, metal chelation prevents free radical formation by stabilizing pro-oxidant metals. Second, radical inhibition occurs when peptide structures—often those containing aromatic amino acids or sulfhydryl (SH) groups—donate electrons to stabilize free radicals. Third, physical shielding can act as a barrier that helps prevent lipid peroxidation. The specific sequence and certain amino acids (e.g., histidine, tyrosine, tryptophan, methionine, cysteine, and proline) are crucial contributors to the antioxidant capacity of peptides [78].
Bioactive peptides can be derived from a variety of sources. Among marine macroalgae, Sargassum angustifolium has been proposed as a promising source of bioactive proteins. Jafarirad et al. extracted such peptides from S. angustifolium protein isolates using enzymatic digestion with alcalase, pepsin, and trypsin. The resulting hydrolysates exhibited more potent antioxidant and antimicrobial activities compared to non-hydrolyzed extracts [79]. Similarly, Li et al. worked with Ulva prolifera—an edible green macroalga—to produce an angiotensin-I converting enzyme (ACE) inhibitory peptide. After purifying a protein concentrate and subjecting it to hydrolysis with a neutral protease, they identified a peptide called KAF (IC50 = 0.63 ± 0.26 μM), whose ACE-inhibitory efficacy was mainly due to two conventional hydrogen bonds. They also reported that this peptide activates endothelial nitric oxide synthase (eNOS), promoting nitric oxide (NO) production [80].
Microalgae are also known to produce a range of bioactive peptides. Chlorella, Navicula, and Spirulina, for instance, have been extensively studied as peptide sources [81]. One study identified a hexapeptide (Leu-Asn-Gly-Asp-Val-Trp) from Chlorella ellipsoidea with notable antioxidant properties, scavenging peroxyl, DPPH, and hydroxyl radicals at IC50 values of 0.02, 0.92, and 1.42 mM, respectively. This purified peptide also enhanced cell viability in monkey kidney cells challenged with AAPH-induced cytotoxicity [82].
Despite considerable progress in understanding how algal compounds help combat oxidative stress, multiple gaps remain. In vivo research on phenolic compounds is limited, and their bioavailability and long-term effects are still poorly defined. Bromophenols and phlorotannins likewise need further investigation regarding their disease-specific mechanisms in human systems. Carotenoids face challenges in stability and bioavailability, necessitating innovative delivery systems such as nanoencapsulation. Meanwhile, ongoing difficulties with extraction methods and insufficient pharmacokinetic data hamper the study of polysaccharides. Bioactive peptides, too, show considerable potential but still require scalable production methods and rigorous in vivo validation to confirm efficacy.
5. Therapeutic Potential of Marine Algal Bioactive Compounds
Interest in marine macroalgae as a natural source of health-promoting compounds has increased substantially in recent years. Their rich composition of bioactive molecules underscores their potential for disease prevention and as complementary therapies [83]. This section examines recent advancements in the therapeutic use of macroalgal compounds for specific diseases (Table 2).
5.1. Diabetes
Oxidative stress is central to both the onset and complications of diabetes mellitus, adversely affecting pancreatic beta cells, insulin resistance, and the progression of long-term diabetic conditions through multiple pathways [84]. In the pancreas, hyperglycemia triggers the overproduction of ROS, damaging beta cells and impairing their ability to secrete insulin, which exacerbates hyperglycemia. Oxidative stress also disrupts insulin signaling, reducing glucose uptake by peripheral tissues and further undermining metabolic control [85]. Chronic oxidative stress contributes to diabetic complications, including cardiovascular disease, neuropathy, nephropathy, and retinopathy, by promoting atherosclerosis, nerve damage, and additional tissue injury in the kidneys and eyes [86].

Table 2.
Summary of the Therapeutic Potential of Bioactive Compounds from Marine Algae.
Table 2.
Summary of the Therapeutic Potential of Bioactive Compounds from Marine Algae.
| Therapeutic Area | Algae | Bioactive Compound | Observed Effects | References |
|---|---|---|---|---|
| Diabetes | Polycladia myrica, Halimeda opuntia | Carboxylic acids | Reduction in blood glucose levels, improvement in kidney and liver function, and increase in antioxidants, such as reduced glutathione and superoxide dismutase (SOD). | [86] |
| Caulerpa racemosa | - | Lower glucose levels, weight control, and reduced hepatic fat accumulation. | [87] | |
| Laurencia papillosa | Ethanolic compounds | Reduced preprandial and postprandial glucose levels, interaction with key enzymes, such as pancreatic α-amylase and the insulin receptor. | [88] | |
| Polycladia myrica, Padina antillarum | Glycosides | High α-glucosidase inhibition (IC50: ~12–13 µg/mL). | [89] | |
| Spirulina platensis | acacetin pinocembrin ω-6 PUFA | Decrease in glucose levels, HbA1c percentage as well as TNF-α and IL-6 levels. | [90,91] | |
| Ecklonia cava | Dieckol Fucodiphloroethol G Phlorofucofuroeckol A 6,6′-bieckol 7-Phloroeckol | Inhibition of α-glucosidase and α-amylase. Alleviates postprandial hyperglycemia in streptozotocin-induced diabetic mice. | [92] | |
| Sargassum hemiphyllum | Fucoxanthin | Inhibits α-amylase, sucrase, maltase absorption, enhances insulin release in vitro. | [93] | |
| Ulva pinnatifida | Fucoxanthin | Significant decrease on blood glucose levels promotes translocation and induction of glucose transporter 4 in skeletal muscles of diabetic/obese KK-Ay mice. | [94] | |
| Caulerpa lentillifera | Polyphenols and sterols | Decrease dipeptidyl peptidase-IV and α-glucosidase enzyme. Increases insulin secretion and glucose uptake in 3T3-L1 adipocytes (10 μL CLE). | [95] | |
| Halimeda tuna | Flavonoids and phenol hydroquinine | Inhibits α-amylase IC50 = 0.88 mg/mL and α-glucosidase IC50 = 0.01 mg/mL. | [96] | |
| Cancer | Laminaria japonica | Fucoxanthin | Inhibition of cell migration and enhanced therapeutic efficacy when combined with gefitinib in lung cancer treatment. | [97] |
| Ulva rigida, Chaetomorpha myrica, Gracilaria foliifera | Terpenoids, polyphenols, sulfonates, polysaccharides, fatty acids, chlorophylls, amide proteins, flavonoids, carotenoids | Significant inhibition of human cancer cells (MCF-7), with IC50 values between 13 and 43 µg/mL. | [98] | |
| Lyngbya majuscula | Cucarin-A | Interferes with tubulin polymerization by competitively binding to the colchicine-binding site. | [99] | |
| Dunaliella salina | - | Anti-proliferative activity against the SW480 colon carcinoma cell line. | [100] | |
| Amphiroa anceps | Octadecanoic acid and n-Hexadecanoic acid | A 92% reduction in viability of A549 cancer cells, with no teratogenic effects in zebrafish models. | [101] | |
| Codium decorticatum | Fucoidan | Induction of apoptosis of A549 cancer cells. | [102] | |
| Ulva lactuca Ulva fasciata | Di-isooctyl Phthalate Butylated Hydroxytoluene | U. lactuca extract had strong activity against MCF-7 and Hela cell lines (IC50 10.83 ± 1.0, 12.43 ± 1.3 μg/mL, respectively), while U. fasciata had strong activity against PC3 and HePG2 cell lines (IC50 12.99 ± 1.2, 16.75 ± 1.5 μg/mL. | [103] | |
| Ecklonia cava | Caffeic acid, naringin, catechin hydrate and phloroglucinol | Suppress the growth of CT26 colon cancer by activating apoptosis, suppressing cell proliferation, inhibiting cell migration and enhancing the tumor-suppressing activity. | [104] | |
| Caulerpa racemosa | Caulersin (C2) | Identified as a potent and effective agent in fighting against non-small cell lung cancer (NSCLC). | [105] | |
| Laurencia obtusa | Terpenes and acetogenins | Potential to be used in the treatment of neoplasms, such as gastric adenocarcinoma. | [106] | |
| Costaria costata | Phlorethol CcPh | Inhibitor of the α-NaGalase of cancer cells and, therefore, has high therapeutic potential. | [107] | |
| Thromboembolic Diseases | Padina tetrastromatica, Ulva fasciata | Sulfated polysaccharides PSPS; SPS from P. tetrastromatica, USPS; SPS from U. fasciata | Significant prolongation of coagulation times (APTT and PT), comparable or superior to heparin in vitro and in SD rats. Exert antithrombotic activity through the modulation of the intrinsic coagulation pathway. | [108] |
| Monostroma nitidum | Sulfated polysaccharide MS-1 | Potent anticoagulant and thrombolytic activity by acting on antithrombin-III and factor Xa. | [109] | |
| Rhodomela confervoides | Sulfated polysaccharides | Anticoagulant activity, especially in ethanol extract fraction (prolonging clotting time to 407.97 s at 10 mg/mL. | [110] | |
| Cystoseira humilis and Sargassum vulgare | Sulfated polysaccharides | Procoagulant effects in certain fractions. | [110] | |
| Chlorella sorokiniana | Sulfated polysacharides | Prolonged clotting time more than 38 s at 10 µg/mL in a PTT test. The PT at 200 µg/mL increased clotting time (up to 14 s). | [111] | |
| Caulerpa cupressoide | Sulfated polysaccharides SP1, SP2 y SP3 | Anticoagulant. Potentiation of thrombin inhibition by antithrombin (IC50 = 10 µg/mL). | [112] | |
| Enteromorpha clathrata | Sulfated polysaccharide high arabinose containing | Prolonged the activated partial thromboplastin time and thrombin time in vitro. | [113] | |
| Monostroma angicava | Sulfated polysaccharide PF2 | Anticoagulant activity was mainly attributed to strong potentiation thrombin or factor Xa inhibition. | [114] | |
| Botryocladia occidentalis | Sulfated galactan | Enhance thrombin and factor Xa, inhibition by antihrombin and/or heparin cofactor II. | [115] | |
| Codium divaricatum | Sulfated polysaccharide CP2-1 | High anticoagulant activity assessed by activated partial thromboplastin time and thrombin time. | [116] | |
| Neurodegenerative Diseases | Microalgae | Astaxanthin | AST plays a protective role in neurons and enhances learning, memory, and cognitive abilities. | [117] |
| Sargassum fusiforme | Saringosterol | Improved memory and 81% reduction in beta-amyloid plaques in the hippocampus, with no adverse effects. | [118] | |
| - | Sodium oligomannate GV-971, seaweed-derived | Significant cognitive improvement in patients with mild to moderate Alzheimer’s; approved in China. | [119,120] | |
| Nannochloropsis oceanica | DHA, EPA | Effects against amyloid-beta (Aβ)-induced toxicity in neuronal cells. mitigated Aβ-mediated oxidative stress and upregulated the activity of key antioxidant enzymes, specifically superoxide dismutase SOD, GSH levels, and CAT, in Neuro-2A neuroblastoma cells. | [121] | |
| Sargussum horneri | Fucoxanthin | Anti-Alzheimer’s disease (AD) neuroprotective effects in vitro and in vivo. | [122] | |
| Sargassum macrocarpum | Sargachromenol | Promotes neuronal differentiation of PC12D cells and supports the survival of neuronal PC12D cells via two distinct signaling pathways. | [123] | |
| Laminaria japonica | Fucoidan | Protects against dopaminergic neuron death in vivo and in vitro, via its antioxidative activity. | [124] | |
| Marine red algae | κ-carrageenan oligosaccharides (KOS) | Has immunomodulatory effects and can be used as a potential intervention therapy for inflammatory related neurodegenerative diseases. | [125] |
Several macroalgae have shown antidiabetic effects in experimental models. Polycladia myrica and Halimeda opuntia extracts lowered blood glucose levels, enhanced kidney and liver function, and increased endogenous antioxidants such as reduced glutathione and superoxide dismutase in alloxan-induced diabetic rats, indicating that these macroalgae may help protect pancreatic and hepatic tissues [87]. Caulerpa racemosa supplementation at 500 mg/kg and 1000 mg/kg in a type 2 diabetes rat model diminished blood glucose and organ abnormalities, with the 500 mg/kg dose effectively curtailing weight gain and food intake, possibly by improving satiety and metabolic regulation [88]. Laurencia papillosa ethanolic extract decreased both preprandial and postprandial glucose levels, while molecular analyses suggested a strong affinity for key diabetes-related targets such as α-amylase, the insulin receptor, and SIRT-6 [88]. Additional investigations of Polycladia myrica, Padina antillarum, and Sargassum boveanum have demonstrated potent α-glucosidase inhibition and reduced postprandial glucose in diabetic rats, reinforcing the potential of brown and red macroalgae for blood glucose management [126]. Similar studies of the microalga Spirulina platensis have also shown reductions in glucose, HbA1c, TNF-α, and IL-6 in streptozotocin-induced diabetic rats, indicating that diverse algal species, including microalgae, may exert antidiabetic effects [90,91].
Despite these encouraging results, most research has been limited to animal models, and there remains an incomplete understanding of the specific bioactive agents responsible for the observed outcomes. Variation in extraction protocols, dosages, and species further complicates reproducibility. Future work must clarify the precise molecular mechanisms by which macroalgal compounds modulate insulin signaling, beta-cell function, and glucose metabolism. Expanding the range of underexplored macroalgal species, conducting large-scale clinical trials, and examining long-term effects on cardiovascular, renal, and neurological outcomes will be essential to validate the therapeutic viability of macroalgae for diabetes management. Standardizing extraction and characterization methods will also enhance replicability and facilitate the clinical translation of these natural compounds.
5.2. Cancer
Carcinogenesis arises from a complex interplay of genetic, environmental, and lifestyle factors, where oxidative DNA damage, chronic inflammation, and altered cell signaling pathways are integral to tumor initiation and progression [127,128]. Marine macroalgae produce compounds that may modulate these processes, drawing attention to their potential as complementary or alternative anticancer agents. Studies have investigated various macroalgal derivatives for their ability to inhibit tumor growth, suppress metastasis, and overcome drug resistance.
Fucoxanthin (FX) extracted from Laminaria japonica has shown promise in reducing metastasis and enhancing the efficacy of gefitinib (Gef) in lung cancer cells by inhibiting epithelial–mesenchymal transition (EMT) and PI3K/AKT/NF-κB signaling pathways [97]. Biosynthesized silver nanoparticles from macroalgae such as Ulva rigida, Chaetomorpha myrica, and Gracilaria foliifera have demonstrated potent cytotoxicity against MCF-7 breast cancer cells, surpassing the performance of non-biogenic nanoparticles [98]. While the cyanobacterium Lyngbya majuscula produces Cucarin-A, a selective cytotoxin for multiple human tumor cell lines that disrupts tubulin polymerization [99]. In microalgae, protein hydrolysates from Dunaliella salina have exhibited anti-proliferative activity against SW480 colon carcinoma cells, although further in vivo validation is required [100]. Another relevant example is Amphiroa anceps, whose aqueous extract formulated into liposomes achieved a 92% reduction in A549 lung cancer cell viability at a 100 μg/mL dose, with no teratogenic effects observed in zebrafish [101].
Despite encouraging in vitro and preclinical findings, translating macroalgal anticancer research into clinical applications remains challenging. Discrepancies between in vitro and in vivo outcomes, limited bioavailability, and potential off-target effects require further investigation. Greater emphasis on molecular biology techniques, such as genomics, proteomics, and metabolomics, could elucidate how macroalgal compounds affect specific tumorigenic pathways. Innovations in drug delivery, including nanoparticle- and liposome-based systems, may enhance both the bioavailability and the target specificity of algal-derived molecules. By expanding the scope of research to include multiple cancer types, clarifying mechanisms of action, and exploring next-generation formulations that optimize safety and efficacy, it will be possible to accelerate the development of effective algal-based cancer therapies.
5.3. Thromboembolic Disease
Thromboembolic disease involves the formation of blood clots (thrombi) that can obstruct blood vessels, potentially leading to serious complications such as heart attack, stroke, and pulmonary embolism [129]. Oxidative stress contributes to this condition by inducing endothelial dysfunction, which promotes platelet adhesion and activation. Elevated ROS levels can directly stimulate platelets to aggregate and form clots, and oxidative stress may also modify platelet receptor expression, further enhancing clot formation [130,131]. Research on marine algae indicates that certain macroalgae produce bioactive compounds capable of mitigating these processes, although the available data remain limited.
In one study, Lekshmi et al. examined the anticoagulant and antithrombotic effects of sulfated polysaccharides extracted from Padina tetrastromatica (a brown alga) and Ulva fasciata (a green alga) in rat models, reporting prolongation of coagulation times (APTT and PT) comparable to, or even surpassing, heparin in specific combinations. These findings suggest that such sulfated polysaccharides mainly affect the intrinsic coagulation pathway [108]. Another investigation isolated a homogeneous sulfated polysaccharide, designated MS-1, from the green alga Monostroma nitidum, demonstrating substantial anticoagulant and antithrombotic properties. Comprising α-l-rhamnose-3-/2-linked residues along with xylose, glucose, and glucuronic acid units bearing sulfate groups, MS-1 strongly inhibited thrombin and factor Xa by enhancing antithrombin-III and heparin cofactor-II activity. It also showed thrombolytic capacity by lowering fibrin degradation product levels and promoting arterial recanalization in animal models. These effects, combined with reductions in thrombus size and weight, underscore MS-1’s potential as a marine-derived therapeutic agent for the prevention and treatment of thromboembolic diseases [109].
Subsequent work in Algeria evaluated extracts from five marine algae collected off the Bejaia coast, analyzing phenolic and sugar content as well as the ability to extend clotting times (APTT and PT). Rhodomela confervoides displayed the most pronounced anticoagulant effect, especially in its ethanol extract fraction, where clotting time reached 407.97 s at 10 mg/mL. Certain brown algae also showed anticoagulant activity in various fractions, although species like Cystoseira humilis and Sargassum vulgare exhibited procoagulant outcomes under some conditions. Notably, several algal-derived compounds feature antioxidant activity, which may help to counteract oxidative stress and limit endothelial dysfunction implicated in thrombosis. These findings point to the dual potential of marine algae as anticoagulant or procoagulant agents, contingent on the extraction process and fraction tested [110]. Additional research by Mousavian et al. reported that sulfated polysaccharides isolated from the microalga Chlorella sorokiniana had potent ABTS radical scavenging activity exceeding 90% and demonstrated anticoagulant properties, possibly stemming from a complex interplay between sulfate content, monosaccharide composition, and glycosidic linkages [111].
Despite noteworthy advancements in identifying the antithrombotic potential of marine algae, efforts remain hampered by the limited number of species investigated, inconsistencies across experimental protocols, and a lack of clinical research in human thromboembolic conditions. Variation in extraction methods, doses, and outcome measures also complicates data comparison and reproducibility. Consequently, well-designed clinical trials are needed to assess the safety and efficacy of these bioactive compounds in human patients. Moreover, the establishment of standardized protocols for extracting, purifying, and characterizing novel anticoagulant compounds from macroalgae will be essential for translating these findings into viable therapies.
5.4. Neurodegenerative Diseases
Oxidative stress has a profound, multifaceted impact on neurons, inflicting damage to lipids, proteins, and DNA that compromises cellular integrity and function [132]. Disruption of membrane fluidity through lipid peroxidation impairs the normal activity of membrane-bound proteins, while protein folding deficits favor the accumulation of misfolded aggregates—a key pathological feature in disorders such as Alzheimer’s disease [133]. In Parkinson’s disease, reduced antioxidant enzyme activity and excess iron both contribute to heightened ROS levels, damaging dopaminergic neurons [134]. Similar mechanisms underlie other conditions like amyotrophic lateral sclerosis (ALS), where mutations in the superoxide dismutase gene accelerate ROS production [135], and Huntington’s disease, in which a mutant huntingtin protein drives increased ROS generation and mitochondrial dysfunction [136].
Over recent decades, algae-derived compounds have drawn increasing attention for their potential in treating neurodegenerative disorders. Notable examples include the pigments astaxanthin and fucoxanthin, which display both antioxidant and anti-inflammatory activities, show an ability to cross the blood-brain barrier, and protect neurons by reducing neuroinflammation and oxidative damage [137]. In experimental Alzheimer’s models, astaxanthin mitigated oxidative stress and apoptosis via the SIRT1/PGC-1α pathway, improving cognitive outcomes in mice and PC12 cells [117]. Equally promising is saringosterol, a compound isolated from the brown alga Sargassum fusiforme that selectively activates LXRβ, improving short-term memory and lowering beta-amyloid (Aβ) plaque load in murine models without causing adverse effects like hypertriglyceridemia or hepatic steatosis [118]. Clinical research on a seaweed-derived oligosaccharide known as sodium oligomannate (GV-971) offers further evidence of algal potential, with a phase 3 trial demonstrating cognitive improvements in mild to moderate Alzheimer’s disease and leading to its approval in China for this indication [119,120].
Despite the promising antioxidant and anti-inflammatory properties of macroalgal components such as astaxanthin, fucoxanthin, and saringosterol, most studies remain confined to in vitro and animal models, emphasizing the need for additional validation in human trials. GV-971 stands out as an exception, having reached clinical use; yet the geographical constraints and limited scope of these trials underscore the importance of broader investigations to confirm efficacy across diverse populations. In parallel, there is growing epidemiological evidence linking increased omega-3 polyunsaturated fatty acid intake to reduced risk of Alzheimer’s disease [138]. Aligning with these observations, extracts from the microalga Nannochloropsis oceanica have shown neuroprotective properties in neuronal cells by mitigating Aβ-mediated oxidative stress and enhancing antioxidant enzyme activities—a result partly attributed to their content of docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA) [121].
Moving forward, research must concentrate on dissecting the molecular mechanisms by which algal compounds influence critical processes in neurodegeneration, including protein aggregation and mitochondrial dysfunction. Advanced delivery systems will be crucial to improve bioavailability and targeting specificity, particularly given the challenges posed by the blood-brain barrier. Investigations into lesser-studied algal species may yield novel agents with neuroprotective capabilities, while large-scale clinical trials are needed to firmly establish the safety, efficacy, and durability of these interventions. Addressing these gaps could enable algal-derived bioactives to play an increasingly transformative role in managing and potentially reversing a range of neurodegenerative diseases.
6. Novel Extraction Techniques of Algal Bioactive Compounds
Conventional large-scale methods used to extract biological compounds from algal matrices—such as liquid–liquid extraction (LLE), solid–liquid extraction (SLE), or Soxhlet extraction—require large volumes of water or organic solvents and prolonged processing times. Additionally, the manual nature of these traditional approaches can introduce variability and reduce reproducibility, while the substantial polysaccharide content in algal cell walls often hinders extraction efficiency by binding tightly to many of the targeted molecules [139]. Consequently, there is a growing need for advanced extraction processes that offer enhanced yields, improved reproducibility, and a diminished environmental footprint.
A range of innovative techniques has been investigated for extracting bioactive compounds from algae, including enzyme-assisted (EAE), microwave-assisted (MAE), ultrasound-assisted (UAE), irradiation-assisted extraction (IAE), supercritical fluid extraction (SFE), pressurized liquid extraction (PLE), accelerated solvent extraction (ASE), pressurized hot water extraction (PHWE), deep eutectic solvents (DES), and emerging methods such as pulsed electric field (PEF) and ionic liquids [140]. Table 3 summarizes key features of many of these technologies, which may offer practical alternatives to conventional procedures by increasing extraction efficiency and preserving the bioactivity of target compounds.

Table 3.
Novel Extraction of Algal Bioactive Compounds.
6.1. Assisted Extraction Methods
6.1.1. Enzyme-Assisted Extraction (EAE)
Enzyme-assisted extraction (EAE) employs specific enzymes to degrade cell wall components, thereby releasing intracellular substances and increasing the effective surface area for solvent interaction. This enzymatic approach can lead to higher yields of extracted compounds and shorter processing times compared to traditional methods, owing to the enzymes’ high specificity for particular bonds or molecules [141]. Marine macroalgae are known sources of proteins and peptides with antioxidant properties, yet extracting these molecules can be complicated by large amounts of polysaccharides—such as alginates in brown algae and carrageenans in certain red algae—in the algal cell walls.
In pioneering work, Amano and Noda used enzymes that degrade algal cell walls (e.g., arabinase, cellulase, glucanase, hemicellulase, and xylanase) to facilitate protein extraction from Porphyra yezoensis. They further demonstrated how an enzyme mixture obtained from abalone gut could enhance protein accessibility, setting the stage for subsequent applications in other macroalgae. For instance, applying similar enzymatic treatments to Ulva pertusa, Laminaria japonica, and Callymenia perforata significantly increased the protein content in each species [142]. These findings highlight the value of enzymatic strategies in overcoming cell wall barriers, ultimately boosting the efficiency and scalability of extracting valuable bioactive compounds from macroalgae.
A recent investigation compared EAE to conventional water extraction for releasing bioactive compounds from seven brown macroalgae using eight different enzymes. The results indicated that enzymatic extraction was markedly more efficient, with the specific macroalgal species and enzyme type influencing overall bioactivity. Particularly notable were the antioxidant outcomes from pairing Sargassum boveanum with Viscozymes and Sargassum boveanum with Alcalase, attributed to the liberation of fucoidans during enzymatic hydrolysis. In contrast, Flavourzyme yielded enhanced antimicrobial activity in a greater number of macroalgal species [143]. Another study by Billakanti et al. employed EAE in combination with dimethyl ether extraction to increase total lipid and fucoxanthin yields from Undaria pinnatifida. Alginase lyase was used beforehand to degrade the cell wall polysaccharides, an approach that improved lipid recovery by 15–20% and fucoxanthin yields by 50%. The authors suggest that EAE’s ability to disrupt phenolic–protein complexes in seaweed significantly enhances the extraction of phlorotannins and other phenolic compounds [144]. Elsewhere, Hardouin et al. evaluated the enzymatic extraction of Ulva armoricana (a green macroalga) using six commercial enzyme preparations. Compared to control water extraction, endo-protease treatment substantially boosted yields, chemical composition, and antiviral activity. Levels of organic matter (up to a 2.0-fold increase), neutral sugars (up to 2.7-fold), and proteins (up to 1.75-fold) all rose relative to the control, and extracts produced with a blend of glycosyl hydrolases and an exo-β-1,3(4)-glucanase displayed antiviral activity against herpes simplex virus type 1 (EC50 values of 373.0 ± 20.7 μg/mL and 320.9 ± 33.6 μg/mL). The antiviral effect appears tied to higher concentrations of rhamnose, uronic acids, and sulfate groups [145].
EAE confers multiple advantages in recovering valuable compounds from macroalgae, including marked changes to the physicochemical properties of target molecules. By hydrolyzing complex structures within algal cell walls, enzymes enhance the solubility and overall availability of bioactive substances [146,147]. In some cases, enzymes can also modify the chemical structure of precursor compounds, converting inactive forms into active aglycones, thereby affecting bioactivity, stability, and biological interactions [148]. Although EAE holds promise, several obstacles limit its broader adoption, such as the high specificity and expense of enzymes, variability in algal composition, enzyme stability issues, risk of extract contamination, and environmental considerations tied to enzyme production and disposal. Nonetheless, ongoing progress in enzyme engineering and extraction optimizations, along with the exploration of green solvent systems, suggests a promising future for EAE in isolating high-value compounds from marine algae. Addressing these challenges will likely facilitate the development of a robust and eco-friendly platform for macroalgal extraction on a larger scale.
6.1.2. Microwave-Assisted Extraction (MAE)
Microwave-assisted extraction (MAE) offers a potent and efficient alternative to traditional methods by using microwaves to selectively heat polar molecules in the sample, thus accelerating the release of bioactive compounds into the solvent [149]. This focused heating not only reduces extraction times but often requires less solvent, rendering MAE more environmentally friendly than many conventional approaches. For instance, André et al. demonstrated that microwave-assisted hydrothermal processing of Ulva spp. significantly modified the phytochemical makeup of soluble extracts, with the highest sulfate and protein contents occurring at 160 °C, and the most pronounced antioxidant activity observed at 200 °C [150]. Another study using Sargassum algae reported successful isolation of alginates with higher molecular weights (419–458 kDa) under MAE conditions [151].
Further research by Cantarino et al. compared four techniques—maceration, Soxhlet extraction, ultrasound-assisted extraction, and MAE—for recovering caulerpin, a bisindolic alkaloid, from Caulerpa racemose. Although Soxhlet extraction produced a larger overall yield, MAE delivered substantially higher caulerpin recovery, surpassing the ultrasound method by more than threefold when optimized for solvent type, temperature (90 °C), and extraction time (7 min) [152]. In addition, studies in various microalgal classes, including Chlorophyceae, Bacillariophyceae, Eustigmatophyceae, and Phaeophyceae, highlight MAE’s effectiveness for lipid extraction, often leading to greater yields of unsaturated and essential fatty acids compared to conventional heating [153,154]. Despite challenges, such as equipment cost, the need for precise temperature control, and the risk of degrading heat-sensitive compounds, MAE shows promise for large-scale algae processing—especially if paired with other extraction methods or alternative green solvents that further reduce environmental impact.
6.1.3. Ultrasound-Assisted Extraction (UAE)
Ultrasound-assisted extraction (UAE) employs high-frequency sound waves to disrupt algal cells via cavitation, thereby enhancing solvent penetration and expediting the release of bioactive substances [155]. Studies have shown that UAE can boost yields of phenolics, fucose, and uronic acid from Ascophyllum nodosum beyond those of conventional methods while also facilitating the extraction of higher molecular weight phenolic compounds [156]. Another comparative investigation involving three edible macroalgae—Sargassum wightii, Ulva rigida, and Gracilaria edulis—revealed that UAE at 120 min markedly increased phenolic and flavonoid levels in S. wightii, alongside significant rises in antioxidant activity (by ferric-reducing antioxidant power and DPPH radical scavenging) compared to traditional extraction [157]. Research has also indicated that UAE can outstrip other extraction techniques, achieving enhanced protein recovery rates in marine algae [159] and accelerating lipid or carbohydrate extraction in species such as Nannochloropsis oculata and Rhodosporidium toruloides, respectively [158,161,162].
Although UAE provides notable benefits, including operation under mild conditions and shortened extraction times, it can also induce free radical generation or degrade sensitive compounds when sonication is excessive [163]. The technique’s performance can vary significantly depending on the algal species, target molecules, and ultrasound parameters, necessitating careful optimization to avoid compound degradation. Scaling up UAE to industrial levels is further complicated by the need to maintain uniform ultrasound intensity and cavitation throughout large extraction vessels. This challenge underlines the importance of additional research on cost-effectiveness, scalability, and impacts on product bioactivity, ultimately guiding the broader application of UAE in sustainably extracting valuable compounds from macroalgae.
6.1.4. Irradiation-Assisted Extraction (IAE)
Irradiation-assisted extraction (IAE) has attracted attention for its potential to increase both the efficiency and the selectivity of extracting bioactive compounds from algae. The technique relies on ionizing radiation—typically gamma rays or electron beams—to break chemical bonds within the material, generating free radicals and other reactive species that promote cellular disruption [164]. Enhanced permeability, arising from radiation-induced damage to algal cell structures, facilitates solvent penetration and simplifies the extraction of target molecules. In one study, treating Polysiphonia abscissa with a 20 kGy dose of gamma irradiation improved its antioxidant activity (AA) compared to non-irradiated samples, likely due to higher carbohydrate content in the resulting extracts. Irradiation also aided in characterizing agar-type polysaccharides without laborious fractionation steps [165].
Another case involves laminarin, the primary carbohydrate stored in many macroalgae. Although laminarin already possesses antimicrobial and antitumor properties, gamma irradiation at doses of 40, 100, and 200 kGy effectively degraded laminarin into fragments of 8.5, 7, and 6 kDa, respectively, without substantially altering functional groups except for carbonyl formation. This process improved laminarin’s antioxidant potential by increasing its capacity to chelate metals and inhibit lipid peroxidation, with progressive enhancements in protection factor as the irradiation dose rose [166]. Similarly, work by He et al. examined sulfated polysaccharides from Porphyra yezoensis, generating low molecular weight derivatives (PYSP-20 and PYSP-100) through gamma irradiation (20 and 100 kGy). These derivatives exhibited stronger antiproliferative activity against cell lines such as MDA-MB-231, HeLa, and Hep3B, despite retaining the same sulfate group content and monosaccharide composition as the parent polysaccharide [167].
While IAE can shorten extraction time, improve yields, and facilitate access to novel bioactive molecules, challenges remain. The formation of radiolysis products can potentially alter or contaminate extracts, posing concerns about bioactivity and safety. Issues of scalability and regulatory compliance also demand consideration. Continued research into these facets will be important for realizing the sustainable, large-scale application of IAE in retrieving valuable compounds from macroalgae.
6.2. Emerging Extraction Techniques
A variety of advanced extraction methods have been explored for algae beyond enzyme-, ultrasound-, and microwave-assisted approaches. These include supercritical fluid extraction (SFE), pressurized liquid extraction (PLE), and other techniques employing innovative solvents or mechanical treatments designed to preserve bioactivity and minimize environmental impact. Such approaches can help overcome the drawbacks of conventional processes by reducing solvent usage, operational times, and thermal degradation while maintaining or improving compound yield and selectivity.
6.2.1. Supercritical Fluid Extraction (SFE)
Supercritical fluid extraction (SFE) exploits fluids at conditions exceeding their critical temperature and pressure to isolate target compounds from complex matrices [168]. Carbon dioxide (CO2) is most frequently used because of its low toxicity, relative affordability, and mild supercritical point (31.1 °C and 73.8 bar), although other substances such as ethane, propane, or water can be employed for specific applications [169]. In supercritical form, a fluid blends gas-like diffusivity with liquid-like density, allowing flexible control over solvating power and selectivity by adjusting temperature and pressure.
One study combined three brown macroalgae—Alaria esculenta, Laminaria digitata, and Ascophyllum nodosum—to evaluate how different CO2/ethanol/water mixtures influence SFE’s ability to isolate antioxidative compounds such as tocopherols, carotenoids, and polyphenols. Optimal conditions varied for each class of analyte, ranging from 95/5 (v/v) CO2/EtOH at 60 °C for β-carotene to 16/71/13 (v/v/v) CO2/EtOH/H2O at 80 °C for phloroglucinol [170]. These parameter-specific extractions minimized undesired co-extraction of sugars, proteins, ash, and toxic elements like arsenic. Other reports show that SFE can successfully recover bioactive molecules from macroalgae, exemplified by the two-fold increase in fucosterol purity and antileishmanial potency when using SFE and subsequent purification methods for Lessonia vadosa [171]. Similarly, supercritical CO2 extraction of lyophilized Phormidium valderianum resulted in superior phytochemical profiles, including phenolics, carotenoids, and antioxidant activity, while reducing anatoxin-a content by around 93% compared to hexane-based protocols [172].
Several key advantages characterize SFE. The system’s high recycling capacity reduces solvent waste, and the relatively low operational temperatures protect heat-sensitive compounds. Moreover, extraction rates are typically rapid, and non-toxic solvents like CO2 bolster overall sustainability [173]. However, high initial equipment costs and the need for expertise in thermodynamics and mass transfer can pose barriers. The limited solubility of certain targets in the chosen supercritical medium may also constrain efficiency. Nonetheless, as knowledge expands and operational refinements continue, SFE represents a powerful, eco-friendly strategy for isolating valuable compounds from macroalgae at both laboratory and industrial scales.
6.2.2. Pressurized Liquid Extraction (PLE)
Pressurized liquid extraction (PLE) employs elevated temperatures and pressures to extract compounds from solid matrices, using a solvent heated beyond its normal boiling point. The increased temperature and pressure enhance the solvent’s solvating power and thereby boost extraction efficiency, which in turn shortens processing times and lowers solvent usage when compared to traditional methods [174]. PLE has shown considerable promise in isolating various bioactive substances from algae, including pigments, lipids, polysaccharides, and phenolic compounds. For instance, one study on the microalga Neochloris oleoabundans revealed a link between carotenoid content in PLE extracts and their antiproliferative activity against HT-29 colon cancer cells at 250 μg/mL [175]. Meanwhile, research focusing on macroalgae such as Sargassum muticum demonstrated that PLE—using an ethanol–water mixture (75:25, v/v)—effectively extracted phenolic compounds with antioxidant properties, although the resulting antioxidant activity remained lower than that obtained through centrifugal partition extraction [176]. Another study on Lobophora variegata highlighted PLE’s capability to recover total phenolics and phlorotannins, though a comparison with enzyme-assisted extraction (EAE) indicated that PLE yielded higher phlorotannin levels but fewer overall phenolics [177].
Some limitations must be considered before broad-scale PLE implementation. The high temperatures and pressures employed may degrade thermolabile bioactives, potentially affecting their bioactivity or stability, as can happen with sensitive vitamins, enzymes, or pigments. Nonetheless, optimizing extraction parameters—such as temperature, pressure, solvent composition, and extraction duration—could significantly refine selectivity and yield. Further investigation into alternative or greener solvents may also diminish the environmental footprint of PLE. Moreover, coupling PLE with downstream techniques like chromatography can improve both purification and compound characterization. Advancing these methods is crucial for harnessing PLE’s full potential in sustainably extracting valuable bioactive compounds from macroalgae and, where relevant, microalgae.
6.2.3. Deep Eutectic Solvents (DES)
Deep eutectic solvents (DES) are emerging as viable green alternatives to conventional organic solvents for the extraction of bioactive compounds from natural resources, including marine macroalgae [178]. These solvents form when a hydrogen bond donor (HBD) is combined with a hydrogen bond acceptor (HBA) in specific ratios, producing a eutectic mixture with a melting point significantly lower than that of the individual components. This phenomenon largely stems from strong intermolecular hydrogen bonding between the HBD and HBA, which contributes to DES’s distinctive features—such as tunable polarity, low volatility, and biodegradability. These properties allow precise modification of the solvent’s characteristics by altering the HBD–HBA composition or ratios, thereby targeting specific compounds of interest. Additionally, many DES formulations incorporate bio-based components, reducing the environmental impact.
Recent studies demonstrate the efficacy of DES in extracting compounds from algae. For example, combining a deep eutectic solvent–aqueous two-phase system (DES-ATPS) with ammonium sulfate precipitation was highly effective in purifying R-phycoerythrin from Porphyra yezoensis, achieving a purity of 3.82 and a yield of 69.99% under optimized conditions [179]. Another investigation explored phlorotannin extraction from the brown macroalgae Fucus vesiculosus and Ascophyllum nodosum using natural deep eutectic solvents (NaDES), finding yields comparable to those obtained with acetone or ethanol. A separate screening evaluated free fatty acid (FFA) and pigment recovery from Spirulina, revealing that DES polarity influenced extraction selectivity, with hydrophobic DES facilitating higher FFA yields and hydrophilic DES favoring antioxidant compounds like phycocyanin and carotenoids [180,181].
Despite this promise, DES-based extraction faces hurdles related to high solvent viscosity, which can impede mass transfer and prolong processing times. Scaling up these methods brings further considerations regarding equipment design, process control, and economics. Future research will need to refine DES formulations—potentially reducing viscosity—while optimizing conditions such as temperature, time, and solvent ratio for maximum efficacy. These steps will be essential for expanding the sustainable, large-scale use of DES in extracting bioactive molecules from algae. Continued advancements should strengthen the role of DES as a versatile and eco-friendly platform for recovering high-value compounds from macroalgae and, where appropriate, microalgae.
6.2.4. Pulsed Electric Field (PEF)
Pulsed electric field (PEF) technology has recently emerged as a promising non-thermal method for extracting bioactive compounds from various biological sources, including algae. During PEF treatment, short, high-intensity electric pulses are applied to algal biomass, leading to electroporation—transient membrane permeabilization that disrupts cellular integrity and facilitates the release of intracellular compounds [182]. Multiple studies have demonstrated PEF’s effectiveness in enhancing yields of valuable molecules. For instance, Postma et al. reported that PEF (1–40 pulses, 0.05–5 ms pulses, 7.5–30 kV·cm−1, 0.05–150 kWh·kgDW−1) caused notable releases of ions, carbohydrates, and proteins (including Rubisco) from Chlorella vulgaris [183]. In a similar vein, Einarsdóttir et al. found that combining PEF pretreatment and ethanol extraction significantly boosted the recovery of phenolic compounds and carbohydrates from the macroalga Alaria esculenta, as indicated by higher levels of phenolics, carbohydrate content, and antioxidant activity [184].
Further explorations highlight PEF’s influence on the extraction of carotenoids and phenolic compounds. Kokkalli et al. showed that PEF (1 kV/cm/400 pulses or 3 kV/cm/45 pulses at 100 kJ/kg) markedly impacted carotenoid and phenolic yields from Tetraselmis chuii and Phaeodactylum tricornutum, underscoring the need to optimize parameters such as pulse intensity, duration, and solvent choice [185]. In comparisons between PEF and ultrasound-assisted extraction, Aouir et al. reported equivalent phycocyanin yields from Arthrospira platensis (commonly classified as a microalga), but higher product purity with PEF, suggesting potential advantages in downstream processing [186]. Investigations by Toepfl and Heinz on Spirulina and Chlorella revealed major enhancements in chlorophyll, carotenoid, and protein extraction; a PEF pretreatment at 15 kV/cm and 100 kJ/kg increased carotenoid yields up to 525% for Chlorella vulgaris and 150% for Spirulina platensis compared to ball milling alone [187,188].
Despite its promise, PEF technology also faces notable constraints. The specialized, high-voltage equipment required for generating pulsed fields can entail substantial capital costs, potentially limiting accessibility for smaller-scale operations. Achieving uniform electric field distribution and consistent treatment efficacy poses further challenges when scaling up to industrial levels, particularly given the wide diversity of algal species and target compounds. Careful calibration of pulse strength, duration, and frequency is crucial to avoid excessive permeabilization or cell death, which can generate additional debris and complicate subsequent purification. Future advancements may include more energy-efficient generators, innovative electrode designs, and combined extraction methods (e.g., PEF plus solvent or microwave-assisted extraction) to improve yields and selectivity while minimizing operational complexities and overall costs.
7. Conclusions
This review underscores the significant therapeutic potential of marine macroalgae-derived bioactive compounds for combating oxidative stress-related diseases. Polyphenols, carotenoids, peptides, and sulfated polysaccharides from seaweeds exhibit notable antioxidant, anti-inflammatory, and disease-modulating properties in conditions such as diabetes, cancer, neurodegenerative disorders, and thromboembolic diseases. Although considerable progress has been made in identifying these compounds and elucidating their mechanisms of action, challenges persist in scaling up extraction processes, standardizing characterization, and confirming long-term safety and efficacy in clinical settings. Most studies to date rely on in vitro or animal models, emphasizing the need for rigorous human trials.
Future efforts should focus on advancing sustainable extraction techniques, including novel enzymatic, physical, and chemical approaches, to preserve bioactivity while minimizing environmental impact. Additionally, research on underexplored algae species may unveil new therapeutic molecules, and the development of effective delivery systems could further enhance bioavailability and targeted efficacy. By bridging these knowledge and technology gaps, marine macroalgae can serve as a robust foundation for natural, eco-friendly strategies aimed at preventing and managing oxidative stress-related disorders on a global scale.
Author Contributions
Writing—original draft preparation, I.N.M.-G., S.T.-R. and L.D.C.-O.; visualization, A.M.-A. and E.V.-V.; writing, review and editing F.A.-Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Consejo Nacional de Humanidades, Ciencias y Tecnologías (CONAHCYT) of Mexico, under the postdoctoral scholarship I1200/320/2022 to I.N.M.-G. This research was also funded by the Tecnológico Nacional de México Convocatoria 2024 Proyectos de Investigación Científica, Desarrollo Tecnológico e Innovación under the project “Estudio del potencial inhibitorio de enzimas digestivas de extractos de algas marinas silvestres del norte de Sinaloa” and parcially founded by ProACTI: 8-CAAFE-2024.
Acknowledgments
The authors thank the SNII program and CONAHCYT of México.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Srinivasan, R.; Kannappan, A.; Shi, C.; Lin, X. Marine Bacterial Secondary Metabolites: A Treasure House for Structurally Unique and Effective Antimicrobial Compounds. Mar. Drugs 2021, 19, 530. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazur, M.; Telser, J. Free Radicals and Antioxidants in Normal Physiological Functions and Human Disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef] [PubMed]
- Pereira, L. Algae as a source of bioactive ingredients for the formulation of functional foods and nutraceuticals. In Functional Ingredients from Algae for Foods and Nutraceuticals, 2nd ed.; Dominguez, H., Pereira, L., Kraan, S., Eds.; Woodhead Publishing: Sawston, UK, 2023; pp. 3–114. [Google Scholar] [CrossRef]
- Ganesan, A.R.; Tiwari, U.; Rajauria, G. Seaweed nutraceuticals and their therapeutic role in disease prevention. Food Sci. Hum. Wellness 2019, 8, 252–263. [Google Scholar] [CrossRef]
- Begum, R.; Howlader, S.; Mamun-Or-Rashid, A.N.M.; Rafiquzzaman, S.M.; Ashraf, G.M.; Albadrani, G.M.; Uddin, M.S. Antioxidant and Signal-Modulating Effects of Brown Seaweed-Derived Compounds Against Oxidative Stress-Associated Pathology. Oxid. Med. Cell. Longev. 2021, 1, 9974890. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cel. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Anil-Kumar, N.V.; Zucca, P.; Varoni, E.M.; Dini, L.; Panzarini, E.; Rajkovic, J.; Tsouh Fokou, P.V.; Azzini, E.; Peluso, I.; et al. Lifestyle, Oxidative Stress, and Antioxidants: Back and Forth in the Pathophysiology of Chronic Diseases. Front. Physiol. 2020, 11, 694. [Google Scholar] [CrossRef]
- Bhadange, Y.A.; Carpenter, J.; Saharan, V.K. A Comprehensive Review on Advanced Extraction Techniques for Retrieving Bioactive Components From Natural Sources. ACS Omega 2024, 9, 31274–31297. [Google Scholar] [CrossRef]
- Carvajal, C. Especies Reactivas Del Oxígeno: Formación, Función Y Estrés Oxidativo. Med. Leg. De Costa Rica 2019, 36, 91–100. [Google Scholar]
- Yoshikawa, T.; You, F. Oxidative Stress and Bio-Regulation. Int. J. Mol. Sci. 2024, 25, 3360. [Google Scholar] [CrossRef]
- Perrone, S.; Lembo, C.; Giordano, M.; Petrolini, C.; Cannavò, L.; Gitto, E. Molecular Mechanisms of Oxidative Stress-Related Neonatal Jaundice. J. Biochem. Mol. Toxicol. 2023, 37, e23349. [Google Scholar] [CrossRef]
- Adwas, A.A.; Elsayed, A.; Azab, A.E.; Quwaydir, F.A. Oxidative Stress And Antioxidant Mechanisms In Human Body. J. Appl. Biotechnol. Bioeng. 2019, 6, 43–47. [Google Scholar] [CrossRef]
- Ighodaro, O.M.; Akinloye, O.A. First Line Defense Antioxidants-Superoxide Dismutase (SOD), Catalase (CAT) and Glutathione Peroxidase (GPX): Their Fundamental Role In The Entire Antioxidant Defense Grid. Alex. J. Med. 2018, 54, 287–293. [Google Scholar] [CrossRef]
- Obeme-Nmom, J.I.; Abioye, R.O.; Flores, S.S.R.; Udenigwe, C.C. Regulation Of Redox Enzymes By Nutraceuticals: A Review Of The Roles Of Antioxidant Polyphenols And Peptides. Food Funct. 2024, 11, 10956–10980. [Google Scholar] [CrossRef] [PubMed]
- Jomova, K.; Raptova, R.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Reactive Oxygen Species, Toxicity, Oxidative Stress, and Antioxidants: Chronic Diseases And Aging. Arch. Toxicol. 2023, 97, 2499–2574. [Google Scholar] [CrossRef]
- Jomova, K.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Several Lines of Antioxidant Defense Against Oxidative Stress: Antioxidant Enzymes, Nanomaterials With Multiple Enzyme-Mimicking Activities, and Low-Molecular-Weight Antioxidants. Arch. Toxicol. 2024, 98, 1323–1367. [Google Scholar] [CrossRef]
- García-Sánchez, A.; Miranda-Díaz, A.G.; Cardona-Muñoz, E.G. The Role of Oxidative Stress in Physiopathology and Pharmacological Treatment with Pro- and Antioxidant Properties in Chronic Diseases. Oxid. Med. Cell Longev. 2020, 23, 2082145. [Google Scholar] [CrossRef]
- Yang, J.; Luo, J.; Tian, X.; Zhao, Y.; Li, Y.; Wu, X. Progress in Understanding Oxidative Stress, Aging, and Aging-Related Diseases. Antioxidants 2024, 25, 394. [Google Scholar] [CrossRef]
- Solovev, I.; Sergeeva, A.; Geraskina, A.; Shaposhnikov, M.; Vedunova, M.; Borysova, O.; Moskalev, A. Aging and physiological barriers: Mechanisms of barrier integrity changes and implications for age-related diseases. Mol. Biol. Rep. 2024, 51, 917. [Google Scholar] [CrossRef]
- Chaudhary, M.R.; Chaudhary, S.; Sharma, Y.; Singh, T.A.; Mishra, A.K.; Sharma, S.; Mehdi, M.M. Aging, oxidative stress and degenerative diseases: Mechanisms, complications and emerging therapeutic strategies. Biogerontology 2023, 24, 609–662. [Google Scholar] [CrossRef]
- Sarmiento-Salinas, F.L.; Perez-Gonzalez, A.; Acosta-Casique, A.; Ix-Ballote, A.; Diaz, A.; Treviño, S.; Rosas-Murrieta, N.H.; Millán-Perez-Peña, L.; Maycotte, P. Reactive Oxygen Species: Role in Carcinogenesis, Cancer Cell Signaling and Tumor Progression. Life Sci. 2021, 284, 119942. [Google Scholar] [CrossRef]
- Nakai, K.; Tsuruta, D. What Are Reactive Oxygen Species, Free Radicals, and Oxidative Stress in Skin Diseases? Int. J. Mol. Sci. 2021, 22, 10799. [Google Scholar] [CrossRef] [PubMed]
- Hassan, H.; Ansari, F.A.; Ingle, K.N.; Singh, K.; Bux, F. Commercial Products and Environmental Benefits of Algal Diversity. In Biodiversity and Bioeconomy; Singh, K., Ribeiro, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2024; pp. 475–502. [Google Scholar] [CrossRef]
- Ahmed, N.; Sheikh, M.A.; Ubaid, M.; Chauhan, P.; Kumar, K.; Choudhary, S. Comprehensive Exploration of Marine Algae Diversity, Bioactive Compounds, Health Benefits, Regulatory Issues, and Food and Drug Applications. Meas. Food 2024, 14, 100163. [Google Scholar] [CrossRef]
- Ghallab, D.S.; Ibrahim, R.S.; Mohyeldin, M.M.; Shawky, E. Marine Algae: A Treasure Trove of Bioactive Anti-inflammatory Compounds. Mar. Pollut. Bull. 2024, 199, 116023. [Google Scholar] [CrossRef]
- Stengel, D.B.; Connan, S.; Popper, Z.A. Algal chemodiversity and bioactivity: Sources of natural variability and implications for commercial application. Biotechnol. Adv. 2011, 29, 483–501. [Google Scholar] [CrossRef] [PubMed]
- Flores-Contreras, E.A.; Araújo, R.G.; Rodríguez-Aguayo, A.A.; Guzmán-Román, M.; García-Venegas, J.C.; Nájera-Martínez, E.F.; Sosa-Hernández, J.E.; Iqbal, H.M.N.; Melchor-Martínez, E.M.; Parra-Saldivar, R. Polysaccharides from the Sargassum and Brown Algae Genus: Extraction, Purification, and Their Potential Therapeutic Applications. Plants 2023, 12, 2445. [Google Scholar] [CrossRef]
- Patel, A.K.; Albarico, F.P.J.B.; Perumal, P.K.; Vadrale, A.P.; Nian, C.T.; Chau, H.T.B.; Anwar, C.; Wani, H.M.U.D.; Pal, A.; Saini, R.; et al. Algae as an Emerging Source of Bioactive Pigments. Bioresour. Technol. 2022, 351, 126910. [Google Scholar] [CrossRef]
- Pereira, H.; Barreira, L.; Figueiredo, F.; Custódio, L.; Vizetto-Duarte, C.; Polo, C.; Rešek, E.; Engelen, A.; Varela, J. Polyunsaturated Fatty Acids of Marine Macroalgae: Potential for Nutritional and Pharmaceutical Applications. Mar. Drugs 2012, 9, 1920–1935. [Google Scholar] [CrossRef]
- Arora, N.; Philippidis, G.P. The Prospects of Algae-Derived Vitamins and Their Precursors for Sustainable Cosmeceuticals. Processes 2023, 11, 587. [Google Scholar] [CrossRef]
- Menaa, F.; Wijesinghe, U.; Thiripuranathar, G.; Althobaiti, N.A.; Albalawi, A.E.; Khan, B.A.; Menaa, B. Marine Algae-Derived Bioactive Compounds: A New Wave of Nanodrugs? Mar. Drugs 2021, 19, 484. [Google Scholar] [CrossRef]
- Khan, S.U.; Lone, A.N.; Khan, M.S.; Virani, S.S.; Blumenthal, R.S.; Nasir, K.; Miller, M.; Michos, E.D.; Ballantyne, C.M.; Boden, W.E.; et al. Effect of Omega-3 fatty Acids on Cardiovascular Outcomes: A systematic Review and Meta-analysis. EClinicalMedicine 2021, 38, 100997. [Google Scholar] [CrossRef]
- Pradhan, B.; Nayak, R.; Patra, S.; Jit, B.P.; Ragusa, A.; Jena, M. Bioactive Metabolites from Marine Algae as Potent Pharmacophores against Oxidative Stress-Associated Human Diseases: A Comprehensive Review. Molecules 2021, 26, 37. [Google Scholar] [CrossRef] [PubMed]
- Dighriri, I.M.; Alsubaie, A.M.; Hakami, F.M.; Hamithi, D.M.; Alshekh, M.M.; Khobrani, F.A.; Dalak, F.E.; Hakami, A.A.; Alsueaadi, E.H.; Alsaawi, L.S.; et al. Effects of Omega-3 Polyunsaturated Fatty Acids on Brain Functions: A Systematic Review. Cureus 2022, 14, e30091. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.; Yadav, D.; Lee, P.C.; Jin, J.O. Immunomodulatory effects of polysaccharides from marine algae for treating cancer, infectious disease, and inflammation. Phytother. Res. 2022, 36, 761–777. [Google Scholar] [CrossRef] [PubMed]
- Yadav, R.; Nigam, A.; Mishra, R.; Gupta, S.; Chaudhary, A.A.; Khan, S.-U.-D.; Almuqri, E.A.; Ahmed, Z.H.; Rustagi, S.; Singh, D.P.; et al. Novel Therapeutic Approach for Obesity: Seaweeds as an Alternative Medicine with the Latest Conventional Therapy. Med. Sci. 2024, 12, 55. [Google Scholar] [CrossRef]
- Wan-Loy, C.; Siew-Moi, P. Marine Algae as a Potential Source for Anti-Obesity Agents. Mar. Drugs 2016, 14, 222. [Google Scholar] [CrossRef]
- Jayawardhana, H.H.A.C.K.; Jayawardena, T.U.; Sanjeewa, K.K.A.; Liyanage, N.M.; Nagahawatta, D.P.; Lee, H.-G.; Kim, J.-I.; Jeon, Y.-J. Marine Algal Polyphenols as Skin Protective Agents: Current Status and Future Prospectives. Mar. Drugs 2023, 21, 285. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, J.-E.; Kim, K.H.; Kang, N.J. Beneficial Effects of Marine Algae-Derived Carbohydrates for Skin Health. Mar. Drugs 2018, 16, 459. [Google Scholar] [CrossRef]
- Bilal, M.; Rasheed, T.; Sosa-Hernández, J.E.; Raza, A.; Nabeel, F.; Iqbal, H.M.N. Biosorption: An Interplay between Marine Algae and Potentially Toxic Elements—A Review. Mar. Drugs 2018, 16, 65. [Google Scholar] [CrossRef]
- Øverland, M.; Mydland, L.T.; Skrede, A. Marine Macroalgae as Sources of Protein and Bioactive Compounds in Feed for Monogastric Animals. J Sci Food Agric. 2019, 99, 13–24. [Google Scholar] [CrossRef]
- Jimenez-Lopez, C.; Pereira, A.G.; Lourenço-Lopes, C.; Garcia-Oliveira, P.; Cassani, L.; Fraga-Corral, M.; Prieto, M.A.; Simal-Gandara, J. Main bioactive phenolic compounds in marine algae and their mechanisms of action supporting potential health benefits. Food Chem. 2021, 30, 128262. [Google Scholar] [CrossRef]
- Lv, Q.Z.; Long, J.T.; Gong, Z.F.; Nong, K.Y.; Liang, X.M.; Qin, T.; Huang, W.; Yang, L. Current State of Knowledge on the Antioxidant Effects and Mechanisms of Action of Polyphenolic Compounds. Nat. Prod. Commun. 2021, 16, 7. [Google Scholar] [CrossRef]
- Tziveleka, L.-A.; Tammam, M.A.; Tzakou, O.; Roussis, V.; Ioannou, E. Metabolites with Antioxidant Activity from Marine Macroalgae. Antioxidants 2021, 10, 1431. [Google Scholar] [CrossRef] [PubMed]
- Lang, Y.; Gao, N.; Zang, Z.; Meng, X.; Lin, Y.; Yang, S.; Yang, Y.; Jin, Z.; Li, B. Classification and antioxidant assays of polyphenols: A review. J. Fut. Foods 2024, 4, 193–204. [Google Scholar] [CrossRef]
- Dong, H.; Dong, S.; Erik Hansen, P.; Stagos, D.; Lin, X.; Liu, M. Progress of Bromophenols in Marine Algae from 2011 to 2020: Structure, Bioactivities, and Applications. Mar. Drugs 2020, 18, 411. [Google Scholar] [CrossRef]
- Zheng, H.; Zhao, Y.; Guo, L. A Bioactive Substance Derived from Brown Seaweeds: Phlorotannins. Mar. Drugs 2022, 20, 742. [Google Scholar] [CrossRef]
- Leyton, A.; Pezoa-Conte, R.; Barriga, A.; Buschmann, A.H.; Mäki-Arvela, P.; Mikkola, J.P.; Lienqueo, M.E. Identification and efficient extraction method of phlorotannins from the brown seaweed Macrocystis pyrifera using an orthogonal experimental design. Algal Res. 2016, 16, 201–208. [Google Scholar] [CrossRef]
- Taniguchi, R.; Ito, C.; Keitoku, S.; Miyake, Y.; Itoigawa, M.; Matsui, T.; Shibata, T. Analysis on the structure of phlorethols isolated from the warm-temperate brown seaweed Sargassum carpophyllum and their antioxidant properties. Nat. Prod. Commun. 2022, 17. [Google Scholar] [CrossRef]
- Agregán, R.; Munekata, P.E.S.; Franco, D.; Carballo, J.; Barba, F.J.; Lorenzo, J.M. Antioxidant Potential of Extracts Obtained from Macro- (Ascophyllum nodosum, Fucus vesiculosus and Bifurcaria bifurcata) and Micro-Algae (Chlorella vulgaris and Spirulina platensis) Assisted by Ultrasound. Medicines 2018, 5, 33. [Google Scholar] [CrossRef]
- Cichoński, J.; Chrzanowski, G. Microalgae as a Source of Valuable Phenolic Compounds and Carotenoids. Molecules 2022, 27, 8852. [Google Scholar] [CrossRef]
- Andriopoulos, V.; Gkioni, M.D.; Koutra, E.; Mastropetros, S.G.; Lamari, F.N.; Hatziantoniou, S.; Kornaros, M. Total Phenolic Content, Biomass Composition, and Antioxidant Activity of Selected Marine Microalgal Species with Potential as Aquaculture Feed. Antioxidants 2022, 11, 1320. [Google Scholar] [CrossRef]
- Fernando, I.P.; Kim, M.; Son, K.T.; Jeong, Y.; Jeon, Y.J. Antioxidant Activity of Marine Algal Polyphenolic Compounds: A Mechanistic Approach. J. Med. Food 2016, 19, 615–628. [Google Scholar] [CrossRef] [PubMed]
- Zeb, A. Concept, mechanism, and applications of phenolic antioxidants in foods. J. Food Biochem. 2020, 44, 13394. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, O.V.; do Rosário, R.C.; Teixeira-Costa, B.E. Sources of Carotenoids in Amazonian Fruits. Molecules 2024, 29, 2190. [Google Scholar] [CrossRef] [PubMed]
- Generalić Mekinić, I.; Šimat, V.; Rathod, N.B.; Hamed, I.; Čagalj, M. Algal Carotenoids: Chemistry, Sources, and Application. Foods 2023, 12, 2768. [Google Scholar] [CrossRef]
- Baeza-Morales, A.; Medina-García, M.; Martínez-Peinado, P.; Pascual-García, S.; Pujalte-Satorre, C.; López-Jaén, A.B.; Martínez-Espinosa, R.M.; Sempere-Ortells, J.M. The Antitumour Mechanisms of Carotenoids: A Comprehensive Review. Antioxidants 2024, 13, 1060. [Google Scholar] [CrossRef]
- Ren, Y.; Sun, H.; Deng, J.; Huang, J.; Chen, F. Carotenoid Production from Microalgae: Biosynthesis, Salinity Responses and Novel Biotechnologies. Mar. Drugs 2021, 19, 713. [Google Scholar] [CrossRef]
- Mapelli-Brahm, P.; Gómez-Villegas, P.; Gonda, M.L.; León-Vaz, A.; León, R.; Mildenberger, J.; Rebours, C.; Saravia, V.; Vero, S.; Vila, E.; et al. Microalgae, Seaweeds and Aquatic Bacteria, Archaea, and Yeasts: Sources of Carotenoids with Potential Antioxidant and Anti-Inflammatory Health-Promoting Actions in the Sustainability Era. Mar. Drugs 2023, 21, 340. [Google Scholar] [CrossRef]
- Murthy, K.C.; Vanitha, A.; Rajesha, J.; Swamy, M.M.; Sowmya, P.R.; Ravishankar, G.A. In vivo Antioxidant Activity of Carotenoids from Dunaliella salina—A Green Microalga. Life Sci. 2005, 76, 1381–1390. [Google Scholar] [CrossRef]
- Ahmad, M.T.; Shariff, M.; Yusoff, F.; Goh, Y.M.; Banerjee, S. Applications of Microalga Chlorella vulgaris in Aquaculture. Rev. Aquac. 2020, 12, 328–346. [Google Scholar] [CrossRef]
- Kurniawan, R.; Nurkolis, F.; Taslim, N.A.; Subali, D.; Surya, R.; Gunawan, W.B.; Alisaputra, D.; Mayulu, N.; Salindeho, N.; Kim, B. Carotenoids Composition of Green Algae Caulerpa racemosa and Their Antidiabetic, Anti-Obesity, Antioxidant, and Anti-Inflammatory Properties. Molecules 2023, 28, 3267. [Google Scholar] [CrossRef]
- Pérez-Gálvez, A.; Viera, I.; Roca, M. Carotenoids and Chlorophylls as Antioxidants. Antioxidants 2020, 9, 505. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.-Y.; Huang, X.; Cheong, K.-L. Recent Advances in Marine Algae Polysaccharides: Isolation, Structure, and Activities. Mar. Drugs 2017, 15, 388. [Google Scholar] [CrossRef]
- Moreira, J.B.; Santos, T.D.; Cruz, C.G.; Silveira, J.T.d.; Carvalho, L.F.d.; Morais, M.G.d.; Costa, J.A.V. Algal Polysaccharides-Based Nanomaterials: General Aspects and Potential Applications in Food and Biomedical Fields. Polysaccharides 2023, 4, 371–389. [Google Scholar] [CrossRef]
- Wang, J.; Hu, S.; Nie, S.; Yu, Q.; Xie, M. Reviews on Mechanisms of In Vitro Antioxidant Activity of Polysaccharides. Oxid. Med. Cell Longev. 2016, 2016, 5692852. [Google Scholar] [CrossRef]
- Bai, L.; Xu, D.; Zhou, Y.-M.; Zhang, Y.-B.; Zhang, H.; Chen, Y.-B.; Cui, Y.-L. Antioxidant Activities of Natural Polysaccharides and Their Derivatives for Biomedical and Medicinal Applications. Antioxidants 2022, 11, 2491. [Google Scholar] [CrossRef]
- Usoltseva, R.V.; Anastyuk, S.D.; Ishina, I.A.; Isakov, V.V.; Zvyagintseva, T.N.; Thinh, P.D.; Zadorozhny, P.A.; Dmitrenok, P.S.; Ermakova, S.P. Structural Characteristics and Anticancer Activity in Vitro of Fucoidan from Brown Alga Padina boryana. Carbohydr Polym. 2018, 15, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Li, D.Y.; Xu, R.Y.; Zhou, W.Z.; Sheng, X.B.; Yang, A.Y.; Cheng, J.L. Effects of Fucoidan Extracted from Brown Seaweed on Lipid Peroxidation in Mice. Acta Nutrim. Sin. 2002, 24, 389–392. [Google Scholar]
- Rocha de Souza, M.C.; Marques, C.T.; Guerra Dore, C.M.; Ferreira da Silva, F.R.; Oliveira Rocha, H.A.; Leite, E.L. Antioxidant Activities of Sulfated Polysaccharides from Brown and Red Seaweeds. J. Appl. Phycol. 2007, 19, 153–160. [Google Scholar] [CrossRef]
- El-Sheekh, M.M.; Ward, F.; Deyab, M.A.; Al-Zahrani, M.; Touliabah, H.E. Chemical Composition, Antioxidant, and Antitumor Activity of Fucoidan from the Brown Alga Dictyota dichotoma. Molecules 2023, 28, 7175. [Google Scholar] [CrossRef]
- Li, B.; Lu, F.; Wei, X.; Zhao, R. Fucoidan: Structure and Bioactivity. Molecules 2008, 13, 1671–1695. [Google Scholar] [CrossRef]
- Pacheco-Quito, E.M.; Ruiz-Caro, R.; Veiga, M.D. Carrageenan: Drug Delivery Systems and Other Biomedical Applications. Mar. Drugs 2020, 18, 583. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, A.; Romeis, D.; Hupfeld, E.; Sieber, V. Biocatalytic Conversion of Carrageenans for the Production of 3,6-Anhydro-D-galactose. J. Agric. Food Chem. 2024, 72, 5816–5827. [Google Scholar] [CrossRef] [PubMed]
- Ha, H.T.; Cuong, D.X.; Thuy, L.H.; Thuan, P.T.; Tuyen, D.T.T.; Mo, V.T.; Dong, D.H. Carrageenan of Red Algae Eucheuma gelatinae: Extraction, Antioxidant Activity, Rheology Characteristics, and Physicochemistry Characterization. Molecules 2022, 27, 1268. [Google Scholar] [CrossRef] [PubMed]
- Akbarian, M.; Khani, A.; Eghbalpour, S.; Uversky, V.N. Bioactive Peptides: Synthesis, Sources, Applications, and Proposed Mechanisms of Action. Int. J. Mol. Sci. 2022, 23, 1445. [Google Scholar] [CrossRef]
- Leong, Y.K.; Chang, J.S. Proteins and Bioactive Peptides From Algae: Insights Into Antioxidant, Anti-hypertensive, Anti-diabetic and Anti-cancer Activities. Trends Food Sci. Technol. 2024, 145, 104352. [Google Scholar] [CrossRef]
- López-García, G.; Dublan-García, O.; Arizmendi-Cotero, D.; Gómez Oliván, L.M. Antioxidant and Antimicrobial Peptides Derived from Food Proteins. Molecules 2022, 27, 1343. [Google Scholar] [CrossRef]
- Jafarirad, S.; Nateghi, L.; Moslemi, M.; Afshari, K.P. Functional Properties of the Bioactive Peptides Derived from Sargassum angustifolium algae. Food Meas. 2023, 17, 6588–6599. [Google Scholar] [CrossRef]
- Li, Z.; He, H.; Liu, J.; Gu, H.; Fu, C.; Zeb, A.; Che, T.; Shen, S. Preparation and Vasodilation Mechanism of Angiotensin-I-Converting Enzyme Inhibitory Peptide from Ulva prolifera Protein. Mar. Drugs 2024, 22, 398. [Google Scholar] [CrossRef]
- Fernando, R.; Sun, X.; Rupasinghe, H.P.V. Production of Bioactive Peptides from Microalgae and Their Biological Properties Related to Cardiovascular Disease. Macromol 2024, 4, 582–596. [Google Scholar] [CrossRef]
- Ko, S.C.; Kim, D.; Jeon, Y.J. Protective Effect of a Novel Antioxidative Peptide Purified from a Marine Chlorella ellipsoidea Protein against Free Radical-Induced Oxidative Stress. Food Chem. Toxicol. 2012, 50, 2294–2302. [Google Scholar] [CrossRef]
- Chénais, B. Algae and Microalgae and Their Bioactive Molecules for Human Health. Molecules 2021, 26, 1185. [Google Scholar] [CrossRef] [PubMed]
- Caturano, A.; D’Angelo, M.; Mormone, A.; Russo, V.; Mollica, M.P.; Salvatore, T.; Galiero, R.; Rinaldi, L.; Vetrano, E.; Marfella, R.; et al. Oxidative Stress in Type 2 Diabetes: Impacts from Pathogenesis to Lifestyle Modifications. Curr. Issues Mol. Biol. 2023, 45, 6651–6666. [Google Scholar] [CrossRef] [PubMed]
- Dludla, P.V.; Mabhida, S.E.; Ziqubu, K.; Nkambule, B.B.; Mazibuko-Mbeje, S.E.; Hanser, S.; Basson, A.K.; Pheiffer, C.; Kengne, A.P. Pancreatic β-cell dysfunction in type 2 diabetes: Implications of Inflammation and Oxidative Stress. World J. Diabetes 2023, 14, 130–146. [Google Scholar] [CrossRef] [PubMed]
- Giacco, F.; Brownlee, M. Oxidative Stress and Diabetic Complications. Circ. Res. 2010, 107, 1058–1070. [Google Scholar] [CrossRef]
- Alwaleed, E.A.; Jillany, A.; Kasem, N.R.; Galal, H. Evaluation of the Pancreatoprotective Effect of Algal Extracts on Alloxan-induced Diabetic Rat. Bioact. Carbohydr. Diet. Fibre 2020, 24, 100237. [Google Scholar] [CrossRef]
- El Habitri, N.; Belkacemi, L. Antidiabetic Effect of Oral Supplementation with Caulerpa racemosa Powder. Eur. J. Biol. Res. 2022, 12, 141–152. [Google Scholar] [CrossRef]
- El-Sheekh, M.M.; Kasem, N.R.; Alsoghier, H.M.; Jillany, A.; Galal, H.; Alwaleed, E.A. Efficacy of The Marine Red Alga Laurencia papillosa Extract on Alloxan Stimulated Hyperglycemic Activity in Male Wistar Albino Rats. Bioact. Carbohydr. Diet. Fibre 2024, 31, 100403. [Google Scholar] [CrossRef]
- Alrasheedi, A.A.; Basnawi, A.I.; Althaiban, M.A. Effects of Spirulina platensis Microalgae on Renal Function and Antioxidant Defense in Rats with Streptozotocin-induced Diabetes. J. Funct. Foods 2024, 122, 106485. [Google Scholar] [CrossRef]
- Guldas, M.; Ziyanok-Demirtas, S.; Sahan, Y.; Yildiz, E.; Gurbuz, O. Antioxidant and Anti-diabetic Properties of Spirulina platensis Produced in Turkey. Food Sci Technol. 2020, 41, 615–625. [Google Scholar] [CrossRef]
- Lee, S.H.; Park, M.H.; Heo, S.J.; Kang, S.M.; Ko, S.C.; Han, J.S.; Jeon, Y.J. Dieckol Isolated From Ecklonia cava Inhibits α-glucosidase and α-amylase in vitro and Alleviates Postprandial Hyperglycemia in Streptozotocin-induced Diabetic mice. Food Chem. Toxicol. 2010, 48, 2633–2637. [Google Scholar] [CrossRef]
- Hwang, P.A.; Hung, Y.L.; Tsai, Y.K.; Chien, S.Y.; Kong, Z.L. The Brown Seaweed Sargassum hemiphyllum Exhibits α-amylase and α-glucosidase Inhibitory Activity and Enhances Insulin Release in vitro. Cytotechnology 2015, 67, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, S.; Hosokawa, M.; Miyashita, K. Fucoxanthin Promotes Translocation and Induction of Glucose Transporter 4 in Skeletal Muscles of Diabetic/obese KK-Ay Mice. Phytomedicine 2012, 19, 389–394. [Google Scholar] [CrossRef] [PubMed]
- Sharma, B.R.; Rhyu, D.Y. Anti-diabetic Effects of Caulerpa lentillifera: Stimulation of Insulin Secretion in Pancreatic β-cells and Enhancement of Glucose Uptake in Adipocytes. Asian Pac. J. Trop. Biomed. 2014, 4, 575–580. [Google Scholar] [CrossRef] [PubMed]
- Gazali, M.; Jolanda, O.; Husni, A.; Nurjanah, F.A.; Majid, A.; Syafitri, R. In Vitro α-Amylase and α-Glucosidase Inhibitory Activity of Green Seaweed Halimeda tuna Extract from the Coast of Lhok Bubon, Aceh. Plants 2023, 12, 393. [Google Scholar] [CrossRef]
- Ming, J.X.; Wang, Z.C.; Huang, Y.; Ohishi, H.; Wu, R.J.; Shao, Y.; Wang, H.; Qin, M.Y.; Wu, Z.L.; Li, Y.Y.; et al. Fucoxanthin Extracted from Laminaria Japonica Inhibits Metastasis and Enhances the Sensitivity of Lung Cancer to Gefitinib. J. Ethnopharmacol. 2021, 265, 113302. [Google Scholar] [CrossRef]
- Senthilkumar, D.; Jayanthi, S. Partial Characterization and Anticancer Activities of Purified Glycoprotein Extracted from Green Seaweed Codium decorticatum. J. Funct. Foods 2016, 25, 323–332. [Google Scholar] [CrossRef]
- Abd El-Hack, M.E.; Abdelnour, S.; Alagawany, M.; Abdo, M.; Sakr, M.A.; Khafaga, A.F.; Mahgoub, S.A.; Elnesr, S.S.; Gebriel, M.G. Microalgae in Modern Cancer Therapy: Current knowledge. Biomed. Pharmacother. 2019, 111, 42–50. [Google Scholar] [CrossRef]
- Darvish, M.; Jalili, H.; Ranaei-Siadat, S.O.; Sedighi, M. Potential Cytotoxic Effects of Peptide Fractions from Dunaliella salina protein Hydrolyzed by Gastric Proteases. J. Aquat. Food Prod. Technol. 2017, 27, 165–175. [Google Scholar] [CrossRef]
- Algotiml, R.; Gab-Alla, A.; Seoudi, R.; Abulreesh, H.H.; El-Readi, M.Z.; Elbanna, K. Anticancer and Antimicrobial Activity of Biosynthesized Red Sea Marine Algal Silver Nanoparticles. Scient. Rep. 2022, 12, 2421. [Google Scholar] [CrossRef]
- Janani, G.; Girigoswami, A.; Deepika, B.; Udayakumar, S.; Girigoswami, K. Unveiling the Role of Nano-Formulated Red Algae Extract in Cancer Management. Molecules 2024, 29, 2077. [Google Scholar] [CrossRef]
- Saeed, A.; Abotaleb, S.; Alam, N.; ELMehalawy, A.; Gheda, S. In vitro Assessment of Antimicrobial, Antioxidant and Anticancer Activities of Some Marine Macroalgae. Egypt. J. Bot. 2020, 60, 81–96. [Google Scholar]
- Gong, J.E.; Kim, J.E.; Park, S.H. Anti-tumor Effects of an Aqueous Extract of Ecklonia cava in BALB/cKorl Syngeneic Mice Using Colon Carcinoma CT26 cells. Oncol. Rep. 2023, 49, 128. [Google Scholar] [CrossRef] [PubMed]
- Lau, V.; Nurkolis, F.; Park, M.N.; Heriyanto, D.S.; Taslim, N.A.; Tallei, T.E.; Permatasari, H.K.; Tjandrawinata, R.R.; Moon, S.; Kim, B. Green Seaweed Caulerpa racemosa as a Novel Non-Small Cell Lung Cancer Inhibitor in Overcoming Tyrosine Kinase Inhibitor Resistance: An Analysis Employing Network Pharmacology, Molecular Docking, and In Vitro Research. Mar. Drugs 2024, 22, 272. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, J.R.B.; Rodrigues, R.P.; Mazzuco, A.C.; de Cassia Ribeiro Gonçalves, R.; Bernardino, A.F.; Kuster, R.M.; Kitagawa, R.R. In Vitro and In Silico Evaluation of Red Algae Laurencia obtusa Anticancer Activity. Mar. Drugs 2023, 21, 318. [Google Scholar] [CrossRef] [PubMed]
- Bakunina, I.; Imbs, T.; Likhatskaya, G.; Grigorchuk, V.; Zueva, A.; Malyarenko, O.; Ermakova, S. Effect of Phlorotannins from Brown Algae Costaria costata on α-N-Acetylgalactosaminidase Produced by Duodenal Adenocarcinoma and Melanoma Cells. Mar. Drugs 2023, 21, 33. [Google Scholar] [CrossRef]
- Lekshmi, V.S.; Kurup, G.M. Anticoagulant Activities of Sulfated Polysaccharides From the Edible Marine Algae Padina tetrastromatica and Ulva fasciata: A combined in vitro and in vivo Approach. J. Pharmacogn. Phytochem. 2019, 8, 693–698. [Google Scholar]
- Cao, S.; He, X.; Qin, L.; He, M.; Yang, Y.; Liu, Z.; Mao, W. Anticoagulant and Antithrombotic Properties in Vitro and in Vivo of a Novel Sulfated Polysaccharide from Marine Green Alga Monostroma nitidum. Mar. Drugs 2019, 17, 247. [Google Scholar] [CrossRef]
- Saidani, K.; Ziani, N.; Touati, N.; Merzouk, H.; Bedjou, F. Anticoagulant Activity of Sulfated Polysaccharides and Polyphenols Extracted from Marine Algae. Curr. Bioact. Comp. 2021, 17, 246–255. [Google Scholar] [CrossRef]
- Mousavian, Z.; Safavi, M.; Azizmohseni, F.; Hadizadeh, M.; Mirdamadi, S. Characterization, Antioxidant and Anticoagulant Properties of Exopolysaccharide from Marine Microalgae. AMB Express 2022, 12, 27. [Google Scholar] [CrossRef]
- Rodrigues, J.A.G.; Queiroz, I.N.L.D.; Quinderé, A.L.G.; Vairo, B.C.; Mourão, P.A.D.S.; Benevides, N.M.B. An Antithrombin-dependent Sulfated Polysaccharide Isolated from the Green Alga Caulerpa cupressoides has in vivo Anti-and Prothrombotic Effects. Ciência Rural 2011, 41, 634–639. [Google Scholar] [CrossRef]
- Qi, X.; Mao, W.; Gao, Y.; Chen, Y.; Chen, Y.; Zhao, C.; Shan, J. Chemical Characteristic of an Anticoagulant-active Sulfated Polysaccharide from Enteromorpha clathrata. Carbohydr. Polyms. 2012, 90, 1804–1810. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Liu, X.; He, X.; Wang, S.; Cao, S.; Xia, Z.; Mao, W. Structure and Anticoagulant Property of a Sulfated Polysaccharide Isolated from the Green Seaweed Monostroma angicava. Carbohydr. Polym. 2017, 159, 195–206. [Google Scholar] [CrossRef]
- Melo, F.R.; Mourão, P.A. An Algal Sulfated Galactan has an Unusual Dual Effect on Venous Thrombosis Due to Activation of Factor XII and Inhibition of the Coagulation Proteases. Thromb. Haemost. 2008, 99, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Mao, W.; Yan, M.; Liu, X.; Xia, Z.; Wang, S.; Xiao, B.; Chen, C.; Zhang, L.; Cao, S. Structural Characterization and Anticoagulant Activity of a Sulfated Polysaccharide from the Green Alga Codium divaricatum. Carbohydr. Polym. 2015, 5, 175–182. [Google Scholar] [CrossRef]
- Liu, N.; Lyu, X.; Zhang, X.; Zhang, F.; Chen, Y.; Li, G. Astaxanthin Attenuates Cognitive Deficits in Alzheimer’s Disease Models by Reducing Oxidative Stress Via the SIRT1/PGC-1α Signaling Pathway. Cell Biosci. 2023, 13, 173. [Google Scholar] [CrossRef]
- Bogie, J.; Hoeks, C.; Schepers, M.; Tiane, A.; Cuypers, A.; Leijten, F.; Chintapakorn, Y.; Suttiyut, T.; Pornpakakul, S.; Struik, D.; et al. Dietary Sargassum fusiforme Improves Memory and Reduces Amyloid Plaque Load in an Alzheimer’s Disease Mouse Model. Sci. Rep. 2019, 9, 4908. [Google Scholar] [CrossRef]
- Xiao, S.; Chan, P.; Wang, T.; Hong, Z.; Wang, S.; Kuang, W.; He, J.; Pan, X.; Zhou, Y.; Ji, Y.; et al. A 36-week Multicenter, Randomized, Double-blind, Placebo-controlled, Parallel-group, Phase 3 Clinical Trial of Sodium Oligomannate for Mild-to-moderate Alzheimer’s Dementia. Alzheimers Res. Ther. 2021, 13, 62. [Google Scholar] [CrossRef]
- Syed, Y.Y. Sodium Oligomannate: First Approval. Drugs 2020, 80, 441–444, Erratum in Drugs 2020, 80, 445–446.. [Google Scholar] [CrossRef]
- Lai, Y.-J. Omega-3 Fatty Acid Obtained from Nannochloropsis oceanica Cultures Grown Under Low Urea Protect Against Aβ-induced Neural Damage. J. Food Sci. Technol. 2015, 52, 2982–2989. [Google Scholar] [CrossRef]
- Yang, M.; Jin, L.; Wu, Z.; Xie, Y.; Zhang, P.; Wang, Q.; Yan, S.; Chen, B.; Liang, H.; Naman, C.B.; et al. PLGA-PEG Nanoparticles Facilitate In Vivo Anti-Alzheimer’s Effects of Fucoxanthin, a Marine Carotenoid Derived from Edible Brown Algae. J. Agric. Food Chem. 2021, 69, 9764–9777. [Google Scholar] [CrossRef]
- Tsang, C.K.; Ina, A.; Goto, T.; Kamei, Y. Sargachromenol, a novel nerve growth factor-potentiating substance isolated from Sargassum macrocarpum, promotes neurite outgrowth and survival via distinct signaling pathways in PC12D cells. Neuroscience 2005, 132, 633–643. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.; Zhang, Q.; Wang, H.; Cui, Y.; Sun, Z.; Yang, J.; Zheng, Y.; Jia, J.; Yu, F.; Wang, X.; et al. Fucoidan Protects Against Dopaminergic Neuron Death in vivo and in vitro. Eur. J. Pharmacol. 2009, 617, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.A.; Xu, L.; Jin, L.M.; Wang, B.X.; Fu, C.Z.; Bai, Y.; Wu, H.G. κ-Carrageenan Oligosaccharides Inhibit the Inflammation of Lipopolysaccharide-Activated Microglia Via TLR4/NF-κB and p38/JNK MAPKs Pathways. Neurochem Res. 2022, 47, 295–304. [Google Scholar] [CrossRef]
- Moheimanian, N.; Mirkhani, H.; Purkhosrow, A.; Sohrabipour, J.; Jassbi, A.R. In Vitro and In Vivo Antidiabetic, α-Glucosidase Inhibition and Antibacterial Activities of Three Brown Algae, Polycladia myrica, Padina antillarum, and Sargassum boveanum, and a Red Alga, Palisada perforata from the Persian Gulf. Iran J. Pharm. Res. 2023, 22, 133731. [Google Scholar] [CrossRef] [PubMed]
- Baba, A.I.; Câtoi, C. Carcinogenesis. In Comparative Oncology. Bucharest (RO): The Publishing House of the Romanian Academy; 2007. Available online: https://www.ncbi.nlm.nih.gov/books/NBK9552/ (accessed on 14 December 2024).
- Afzal, S.; Abdul; Manap, A.S.; Attiq, A.; Albokhadaim, I.; Kandeel, M.; Alhojaily, S.M. From Imbalance to Impairment: The Central Role of Reactive Oxygen Species in Oxidative Stress-induced Disorders and Therapeutic Exploration. Front. Pharmacol. 2023, 14, 1269581. [Google Scholar] [CrossRef]
- Lichota, A.; Szewczyk, E.M.; Gwozdzinski, K. Factors Affecting the Formation and Treatment of Thrombosis by Natural and Synthetic Compounds. Int. J. Mol. Sci. 2020, 21, 7975. [Google Scholar] [CrossRef]
- Masselli, E.; Pozzi, G.; Vaccarezza, M.; Mirandola, P.; Galli, D.; Vitale, M.; Carubbi, C.; Gobbi, G. ROS in Platelet Biology: Functional Aspects and Methodological Insights. Int. J. Mol. Sci. 2020, 21, 4866. [Google Scholar] [CrossRef]
- Li, P.; Ma, X.; Huang, G. Understanding Thrombosis: The Critical Role of Oxidative Stress. Hematology 2024, 29, 2301633. [Google Scholar] [CrossRef]
- Olufunmilayo, E.O.; Gerke-Duncan, M.B.; Holsinger, R.M.D. Oxidative Stress and Antioxidants in Neurodegenerative Disorders. Antioxidants 2023, 12, 517. [Google Scholar] [CrossRef]
- Perluigi, M.; Di Domenico, F.; Butterfield, D.A. Oxidative Damage in Neurodegeneration: Roles in the Pathogenesis and Progression of Alzheimer Disease. Physiol. Rev. 2024, 104, 103–197. [Google Scholar] [CrossRef]
- Dionísio, P.A.; Amaral, J.D.; Rodrigues, C.M.P. Oxidative Stress and Regulated Cell Death in Parkinson’s Disease. Ageing Res. Rev. 2021, 67, 101263. [Google Scholar] [CrossRef]
- Hemerková, P.; Vališ, M. Role of Oxidative Stress in the Pathogenesis of Amyotrophic Lateral Sclerosis: Antioxidant Metalloenzymes and Therapeutic Strategies. Biomolecules 2021, 11, 437. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, F.; Fan, X.; Chen, M.; Xu, X.; Xu, Q.; Zhu, H.; Xu, A.; Pouladi, M.A.; Xu, X. CHCHD2 up-regulation in Huntington Disease Mediates a Compensatory Protective Response Against Oxidative Stress. Cell Death Dis. 2024, 15, 126. [Google Scholar] [CrossRef] [PubMed]
- Guardado Yordi, E.; Pérez Martínez, A.; Radice, M.; Scalvenzi, L.; Abreu-Naranjo, R.; Uriarte, E.; Santana, L.; Matos, M.J. Seaweeds as Source of Bioactive Pigments with Neuroprotective and/or Anti-Neurodegenerative Activities: Astaxanthin and Fucoxanthin. Mar. Drugs 2024, 22, 327. [Google Scholar] [CrossRef] [PubMed]
- Calon, F.; Cole, G. Neuroprotective Action of Omega-3 Polyunsaturated Fatty Acids Against Neurodegenerative Diseases: Evidence from Animal Studies. Prostaglandins Leukot. Essent. Fat. Acids 2007, 77, 287–293. [Google Scholar] [CrossRef]
- Quitério, E.; Grosso, C.; Ferraz, R.; Delerue-Matos, C.; Soares, C. A Critical Comparison of the Advanced Extraction Techniques Applied to Obtain Health-Promoting Compounds from Seaweeds. Mar. Drugs 2022, 20, 677. [Google Scholar] [CrossRef]
- Michalak, I.; Chojnacka, K. Algal Extracts: Technology and Advances. Eng. Life Sci. 2014, 14, 581–591. [Google Scholar] [CrossRef]
- Streimikyte, P.; Viskelis, P.; Viskelis, J. Enzymes-Assisted Extraction of Plants for Sustainable and Functional Applications. Int. J. Mol. Sci. 2022, 23, 2359. [Google Scholar] [CrossRef]
- Jeon, Y.-J.; Wijesinghe, W.A.J.P.; Kim, S.-K. Enzyme-assisted Extraction and Recovery of Bioactive Components from Seaweeds. In Hand Book of Marine Macroalgae: Biotechnology and Applied Phycology, 1st ed.; Kim, S.-K., Ed.; John Willy & Sons, Ltd.: New York, NY, USA, 2011. [Google Scholar] [CrossRef]
- Habeebullah, S.; Alagarsamy, S.; Sattari, Z.; Al-Haddad, S.; Fakhraldeen, S.; Al-Ghumaim, A.; Al-Yamani, F. Enzyme-assisted extraction of bioactive compounds from brown seaweeds and characterization. J. Appl. Phycol. 2020, 32, 615–629. [Google Scholar] [CrossRef]
- Billakanti, J.M.; Catchpole, O.; Fenton, T.; Mitchell, K. Extraction of Fucoxanthin from Undaria pinnatifida Using Enzymatic Pre-treatment Followed by DME and EtOH co-solvent Extraction. In Proceedings of the 10th International Symposium on Supercritical Fluids, San Francisco, CA, USA, 13–16 May 2012; King, J., Ed.; CASSS: Emeryville, CA, USA, 2012. [Google Scholar]
- Hardouin, K.; Bedoux, G.; Burlot, A.S.; Donnay-Moreno, C.; Bergé, J.P.; Nyvall-Collén, P.; Bourgougnon, N. Enzyme-assisted Extraction (EAE) for the Production of Antiviral and Antioxidant Extracts from the Green Seaweed Ulva armoricana (Ulvales, Ulvophyceae). Algal Res. 2016, 16, 233–239. [Google Scholar] [CrossRef]
- Nayak, N.; Bhujle, R.R.; Nanje-Gowda, N.A.; Chakraborty, S.; Siliviru, K.; Subbiah, J.; Brennan, C. Advances in the Novel and Green-assisted Techniques for Extraction of Bioactive Compounds from Millets: A comprehensive review. Heliyon 2024, 10, e30921. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Barrutia, M.B.; Iriondo-DeHond, A.; Arcia, P.; Cozzano Ferreira, S.; Castillo, M. Enzyme assisted extraction of health promoting compounds from extruded brewers’ spent grain (BSG). In Proceedings of the XXV Jornadas Internacionales de Nutrición Práctica, Online, 20–22 April 2021; Available online: http://hdl.handle.net/10261/253416 (accessed on 28 December 2024).
- Barbosa, P.P.M.; Ruviaro, A.R.; Macedo, G.A. Conditions of Enzyme-assisted Extraction to Increase the Recovery of Flavanone Aglycones from Pectin Waste. J. Food Sci. Technol. 2021, 58, 4303–4312. [Google Scholar] [CrossRef] [PubMed]
- López-Salazar, H.; Camacho-Díaz, B.H.; Ocampo, M.A.; Jiménez-Aparicio, A.R. Microwave-assisted Extraction of Functional Compounds from Plants: A Review. Bioresources 2023, 18, 6614. [Google Scholar] [CrossRef]
- André, J.; Flórez-Fernández, N.; Domínguez, H.; Torres, M.D. Microwave-assisted Extraction of Ulva spp. Including a Stage of Selective Coagulation of Ulvan Stimulated by a Bio-ionic Liquid. Int. J. Biol. Macromol. 2023, 225, 952–963. [Google Scholar] [CrossRef]
- Nesic, A.; De Bonis, M.V.; Dal Poggetto, G.; Ruocco, G.; Santagata, G. Microwave Assisted Extraction of Raw Alginate as a Sustainable and Cost-Effective Method to Treat Beach-Accumulated Sargassum Algae. Polymers 2023, 15, 2979. [Google Scholar] [CrossRef]
- Cantarino, S.J.; Coutinho, R.; Soares, A.R.; Duarte, H.M.; Martinez, S.T. Microwave irradiation is a suitable method for caulerpin extraction from the green algae Caulerpa racemosa (Chlorophyta, Caulerpaceae). Nat. Prod. Res. 2022, 36, 2149–2153. [Google Scholar] [CrossRef]
- Balasubramanian, S.; Allen, J.D.; Kanitkar, A.; Boldor, D. Oil Extraction from Scenedesmus obliquus Using a Continuous Microwave System–Design, Optimization, and Quality Characterization. Bioresour Technol. 2011, 102, 3396–3403. [Google Scholar] [CrossRef]
- Kapoore, R.V.; Butler, T.O.; Pandhal, J.; Vaidyanathan, S. Microwave-Assisted Extraction for Microalgae: From Biofuels to Biorefinery. Biology 2018, 7, 18. [Google Scholar] [CrossRef]
- Kumar, K.; Srivastav, S.; Sharanagat, V.S. Ultrasound assisted extraction (UAE) of Bioactive Compounds From Fruit and Vegetable Processing by-products: A review. Ultrason. Sonochem. 2021, 70, 105325. [Google Scholar] [CrossRef]
- Kadam, S.U.; Tiwari, B.K.; Smyth, T.J.; O’Donnell, C.P. Optimization of ultrasound assisted extraction of bioactive components from brown seaweed Ascophyllum nodosum using response surface methodology. Ultrason. Sonochem. 2015, 23, 308–316. [Google Scholar] [CrossRef]
- Kumar, Y.; Singhal, S.; Tarafdar, A.; Pharande, A.; Ganesan, M.; Badgujar, P.C. Ultrasound Assisted Extraction of Selected Edible Macroalgae: Effect on Antioxidant Activity and Quantitative Assessment of Polyphenols by Liquid Chromatography with Tandem Mass Spectrometry (LC-MS/MS). Algal Res. 2020, 52, 102114. [Google Scholar] [CrossRef]
- Carreira-Casais, A.; Otero, P.; Garcia-Perez, P.; Garcia-Oliveira, P.; Pereira, A.G.; Carpena, M.; Soria-Lopez, A.; Simal-Gandara, J.; Prieto, M.A. Benefits and Drawbacks of Ultrasound-Assisted Extraction for the Recovery of Bioactive Compounds from Marine Algae. Int. J. Environ. Res. Public Health 2021, 18, 9153. [Google Scholar] [CrossRef] [PubMed]
- Vernès, L.; Abert-Vian, M.; El Maâtaoui, M.; Tao, Y.; Bornard, I.; Chemat, F. Application of Ultrasound for Green Extraction of Proteins from Spirulina. Mechanism, Optimization, Modeling, and Industrial Prospects. Ultrason. Sonochem. 2019, 54, 48–60. [Google Scholar] [CrossRef] [PubMed]
- Mahali, M.; Sibi, G. Extraction Methods and Functional Properties of Protein from Arthospira platensis for Bioavailability of Algal Proteins. Int. J. Pharm. Chem. 2019, 5, 20. [Google Scholar] [CrossRef]
- Zhao, G.; Chen, X.; Wang, L.; Zhou, S.; Feng, H.; Chen, W.N.; Lau, R. Ultrasound Assisted Extraction of Carbohydrates from Microalgae as Feedstock for Yeast Fermentation. Bioresour. Technol. 2013, 128, 337–344. [Google Scholar] [CrossRef]
- Adam, F.; Abert-Vian, M.; Peltier, G.; Chemat, F. “Solvent-free” Ultrasound-assisted Extraction of Lipids from Fresh Microalgae Cells: A Green, Clean and Scalable Process. Bioresour. Technol. 2012, 114, 457–465. [Google Scholar] [CrossRef]
- Jadhav, H.B.; Gogate, P.; Annapure, U. Analyzing the Repercussions of Ultrasound on Triacylglycerols in Food. Food Chem. Adv. 2023, 2, 100332. [Google Scholar] [CrossRef]
- Madureira, J.; Barros, L.; Cabo Verde, S.; Margaça, F.M.A.; Santos-Buelga, C.; Ferreira, I.C.F.R. Ionizing Radiation Technologies to Increase the Extraction of Bioactive Compounds from Agro-Industrial Residues: A Review. J. Agric. Food Chem. 2020, 68, 11054–11067. [Google Scholar] [CrossRef]
- Rojo del Olmo, L.; Pablo, M.J.; Croce, M.E.; Perez, M.B.; Parodi, R.R.; Andreucetti, N.A. Uso de la irradiación gamma en la extracción e identificación de compuestos bioactivos presentes en algas rojas. J. Argen. Chem. Soc. 2012, 99, 1–3. [Google Scholar]
- Choi, J.I.; Kim, H.J.; Lee, J.W. Structural Feature and Antioxidant Activity of Low Molecular Weight Laminarin Degraded by Gamma Irradiation. Food Chem. 2011, 129, 520–523. [Google Scholar] [CrossRef]
- He, D.; Yan, L.; Ma, X.; Cheng, Y.; Wu, S.; Zuo, J.; Park, E.-J.; Liu, J.; Wu, M.; Choi, J.-I.; et al. Gamma-irradiation Degraded Sulfated Polysaccharide from a New Red Algal Strain Pyropia yezoensis Sookwawon 104 with In Vitro Antiproliferative Activity. Oncol. Lett. 2020, 20, 91. [Google Scholar] [CrossRef] [PubMed]
- Herzyk, F.; Piłakowska-Pietras, D.; Korzeniowska, M. Supercritical Extraction Techniques for Obtaining Biologically Active Substances from a Variety of Plant Byproducts. Foods 2024, 13, 1713. [Google Scholar] [CrossRef] [PubMed]
- Samuel, H.S.; Nweke-Maraizu, U.; Etim, E.E. Supercritical Fluids: Properties, Formation and Applications. J. Eng. Ind. Res. 2023, 4, 176–188. [Google Scholar] [CrossRef]
- Gondo, T.F.; Jönsson, M.; Karlsson, E.N.; Sandahl, M.; Turner, C. Extractability, Selectivity, and Comprehensiveness in Supercritical Fluid Extraction of Seaweed Using Ternary Mixtures of Carbon Dioxide, Ethanol, and Water. J. Chromatogr. A 2023, 1706, 464267. [Google Scholar] [CrossRef]
- Becerra, M.; Boutefnouchet, S.; Cordoba, O.; Vitorino, G.P.; Brehu, L.; Lamour, I.; Laimay, F.; Efstathiou, A.; Smirlis, D.; Michel, S.; et al. Antileishmanial Activity of Fucosterol Recovered from Lessonia vadosa Searles (Lessoniaceae) by SFE, PSE and CPC. Phytochem. Lett. 2015, 11, 418–423. [Google Scholar] [CrossRef]
- Chatterjee, D.; Bhattacharjee, P. Supercritical Carbon Dioxide Extraction of Antioxidant Rich Fraction from Phormidium valderianum: Optimization of Experimental Process Parameters. Algal Res. 2014, 3, 49–54. [Google Scholar] [CrossRef]
- Cheriyan, B.V.; Karunakar, K.K.; Anandakumar, R.; Murugathirumal, A. Eco-Friendly Extraction Technologies: A Comprehensive Review of Modern Green Analytical Methods. Sustain. Chem. Clim. Action 2024, 6, 100054. [Google Scholar] [CrossRef]
- Barp, L.; Višnjevec, A.M.; Moret, S. Pressurized Liquid Extraction: A Powerful Tool to Implement Extraction and Purification of Food Contaminants. Foods 2023, 12, 2017. [Google Scholar] [CrossRef]
- Castro-Puyana, M.; Pérez-Sánchez, A.; Valdés, A.; Ibrahim, O.H.M.; Suárez-Alvarez, S.; Ferragut, J.A.; García-Cañas, V. Pressurized Liquid Extraction of Neochloris oleoabundans for the Recovery of Bioactive Carotenoids with Anti-proliferative Activity Against Human Colon Cancer Cells. Food Res. Int. 2017, 99, 1048–1055. [Google Scholar] [CrossRef]
- Anaëlle, T.; Leon, E.S.; Laurent, V.; Elena, I.; Mendiola, J.A.; Stéphane, C.; Nelly, K.; Stéphane, L.B.; Luc, M.; Valérie, S.P. Green Improved Processes to Extract Bioactive Phenolic Compounds from Brown Macroalgae Using Sargassum muticum as Model. Talanta 2013, 104, 44–52. [Google Scholar] [CrossRef]
- Gümüş Yılmaz, G.; Gómez Pinchetti, J.L.; Cifuentes, A.; Herrero, M.; Ibáñez, E. Comparison of Extraction Techniques and Surfactants for the Isolation of Total Polyphenols and Phlorotannins from the Brown Algae Lobophora variegata. Anal. Lett. 2019, 52, 2724–2740. [Google Scholar] [CrossRef]
- Ristivojević, P.; Krstić Ristivojević, M.; Stanković, D.; Cvijetić, I. Advances in Extracting Bioactive Compounds from Food and Agricultural Waste and By-Products Using Natural Deep Eutectic Solvents: A Circular Economy Perspective. Molecules 2024, 29, 4717. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wang, Q.; Hou, Y. Efficient Purification of R-phycoerythrin from Marine Algae (Porphyra yezoensis) Based on a Deep Eutectic Solvents Aqueous Two-Phase System. Mar. Drugs 2020, 18, 618. [Google Scholar] [CrossRef] [PubMed]
- Obluchinskaya, E.D.; Daurtseva, A.V.; Pozharitskaya, O.N.; Flisyuk, E.V.; Shikov, A.N. Natural Deep Eutectic Solvents as Alternatives for Extracting Phlorotannins from Brown Algae, Pharm. Chem. J. 2019, 53, 243–247. [Google Scholar] [CrossRef]
- Wils, L.; Leman-Loubière, C.; Bellin, N.; Clément-Larosière, B.; Pinault, M.; Chevalier, S.; Enguehard-Gueiffier, C.; Bodet, C.; Boudesocque-Delaye, L. Natural deep eutectic solvent formulations for spirulina: Preparation, Intensification, and Skin Impact. Algal Res. 2021, 56, 102317. [Google Scholar] [CrossRef]
- Bocker, R.; Silva, E.K. Pulsed Electric Field Assisted Extraction of Natural Food Pigments and Colorings from Plant Matrices. Food Chem. X 2022, 15, 100398. [Google Scholar] [CrossRef]
- Postma, P.R.; Fernandes, D.A.; Timmermans, R.A.H.; Vermuë, M.H.; Barbosa, M.J.; Eppink, M.H.M.; Eppink, R.H.; Olivieri, G. Pulsed Electric Field for Protein Release of the Microalgae Chlorella vulgaris and Neochloris oleoabundans. Algal Res. 2017, 24, 181–187. [Google Scholar] [CrossRef]
- Einarsdóttir, R.; Þórarinsdóttir, K.A.; Aðalbjörnsson, B.V. Extraction of Bioactive Compounds from Alaria esculenta with Pulsed Electric Field. J. Appl. Phycol. 2022, 34, 597–608. [Google Scholar] [CrossRef]
- Kokkali, M.; Martí-Quijal, F.J.; Taroncher, M.; Ruiz, M.J.; Kousoulaki, K.; Barba, F.J. Improved Extraction Efficiency of Antioxidant Bioactive Compounds from Tetraselmis chuii and Phaedoactylum tricornutum Using Pulsed Electric Fields. Molecules. 2020, 25, 3921. [Google Scholar] [CrossRef]
- Aouir, A.; Amiali, M.; Kirilova-Gachovska, T.; Benchabane, A.; Bitam, A. The Effect of Pulsed Electric Field (PEF) and Ultrasoud (US) Technologies on the Extraction of Phycopiliproteins from Arthrospira platensis. In Proceedings of the IEEE Canada International Humanitarian Technology Conference, Ottawa, ON, Canada, 31 May–4 June 2015; IEEE: Piscataway, NJ, USA; pp. 1–4. [Google Scholar] [CrossRef]
- Toepfl, S.; Heinz, V.; Knorr, D. Applications of Pulsed Electric Fields Technology for the Food Industry. In Pulsed Electric Fields Technology for the Food Industry, 2nd ed.; Raso, J., Heinz, V., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 197–221. [Google Scholar]
- Toepfl, S. Pulsed Electric Fields (PEF) for Permeabilization of Cell Membranes in Food- and Bioprocessing. Applications, Process and Equipment Design and Cost Analysis. Ph.D. Thesis, Technische Universität Berlin, Berlin, Germany, 2006. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).