Abstract
Current research on the effects of childhood trauma largely focuses on maltreatment. In the current study, we used diffusion tensor imaging (DTI) to determine the association between potentially traumatic exposures not related to maltreatment and fractional anisotropy (FA) in 184 youth aged 9–14 years. The Trauma History Profile was used to determine how many traumatic events in different categories were experienced and create low- and high-trauma groups. FA values were compared between groups in twelve a priori chosen regions of interest (ROIs). Five of the twelve regions showed significantly lower FA in the high-trauma groups when compared to the low-trauma groups, including the body of the corpus callosum, the total corpus callosum, bilateral posterior thalamic radiation, and the left cingulate gyrus projection of the cingulum bundle. Group differences were also observed across a range of behaviors. However, FA was not associated with posttraumatic stress symptomology. The results support the hypothesis that the high-trauma group had lower FA compared to the low-trauma group. The significant ROIs represent a subset of regions identified in studies of adults exposed to traumatic childhood events or children with a history of maltreatment. These results, obtained from typically developing youth, underline the importance of examining childhood trauma exposure in future developmental studies.
1. Introduction
By the age of 16, more than two thirds of children in community samples experienced at least one Criterion A trauma [1]. The prevalence and severity of these traumas vary by the type of trauma experienced [1], and the impacts of different trauma types also varies [2,3]. The consequences of childhood trauma for adults have been studied using a variety of neuroimaging modalities, but the neurological correlates of trauma not related to child maltreatment remain understudied in childhood. In the analyses reported here, we examined the association between childhood traumatic events unrelated to maltreatment and white matter (WM) microstructural integrity in a youth sample by using diffusion tensor imaging (DTI).
Current trauma research pertaining to childhood largely focuses on maltreatment, defined as emotional, physical, and sexual abuse, and emotional and physical neglect [4]. Research suggests that different forms of maltreatment are associated with different effects on the brain [5,6,7,8]. Because different forms of abuse are associated with WM integrity in different regions of the brain, we chose to examine how traumatic exposures not related to child maltreatment impact WM integrity to determine whether there are similar effects of other forms of trauma exposure on WM in children.
The transition into adolescence is a critical period for the development of white matter [9,10] and is considered a vulnerable period for the effects of trauma [11,12]. Childhood trauma and adversity are associated with behavioral changes in youth [13] and believed to play a role in psychopathology across one’s lifespan [12,14,15,16]. White matter microstructural differences may be one mechanism involved in this process.
To examine the potential effects of trauma unrelated to child maltreatment, data from a large youth study (N = 184; ages 9–14 years) were examined. Twelve brain regions of interest (ROIs) were selected a priori based on childhood maltreatment research: these included the body, genu, and splenium of the corpus callosum; the whole corpus callosum; the bilateral posterior thalamic radiations (including the optic radiations); bilateral cingulate gyri; the bilateral parahippocampal cingulum; and the bilateral UF. We hypothesized that the participants in the high-trauma group (with 2 or more traumas) would have lower FA in our selected ROIs when compared to the participants in the low-trauma group (with 0 traumas or 1 trauma). We also hypothesized that FA would be negatively associated with posttraumatic stress (PTS) symptomology.
2. Results
2.1. Participants Description
A total of 184 participants were included in the final analysis (91 females). The participants endorsed experiencing a range of trauma types (0–10 trauma types, mean = 2.245) based on the THP. As mentioned previously, the participants were divided into high- (with two or more traumas) and low (with fewer than two traumas)-trauma groups, and these groups did not differ by age, sex, or race (Table 1). The distributions of the number of trauma categories endorsed and the trauma types are shown in Figure 1. The low-trauma group consistently differed from the high-trauma group in terms of the self-report and parental-report behavioral measures (Table 2). Somatic complaints (p = 0.0261), attention problems (p = 0.0029), and thought problems (p-value = 0.023) were notably different in the CBCL-Parent report. The groups had self-reported differences in emotional, conduct, and overall scores in the SDQ-Child assessment. In terms of the TSCC, the groups differed in terms of measures of depression (p < 0.001), anxiety (p < 0.001), and post-traumatic stress (p < 0.001).

Table 1.
Participant characteristics.
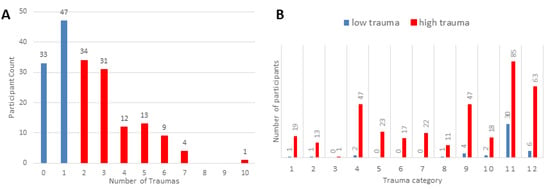
Figure 1.
(A) Histogram depicting the number of trauma categories endorsed. (B) Distribution of the different trauma types endorsed by participants. Trauma category questions: 1. Were you in a disaster, like an earthquake, wildfire, hurricane, tornado or flood? 2. Were you in a bad accident, like a serious car accident or fall? 3. Were you in a place where a war was going on around you? 4. Were you hit, punched, or kicked very hard? (not play fighting) 5. Did you see a family member being hit, punched or kicked very hard? (not play fighting) 6. Were you beaten up, shot at, or threatened to be hurt badly in your school neighborhood or town? 7. Did you see someone who was beaten up, shot at or killed? 8. Did you see a dead body? (does not include funerals) 9. Did you see or hear about the violent death or serious injury of a loved one or friend? 10. Did you have a painful or scary medical treatment when you were very sick or badly injured? 11. Has anyone close to you died? 12. Other than the things described above, has anything else ever happened to you that was really scary or upsetting?

Table 2.
Comparison of behavioral measures between groups.
Brain Region Analysis: Trauma-and-FA Association
Five of the twelve ROIs showed significantly lower FA in the high-trauma group compared to the low-trauma group (see Table 3 and Figure 2).

Table 3.
Statistical tests for FA differences between groups.
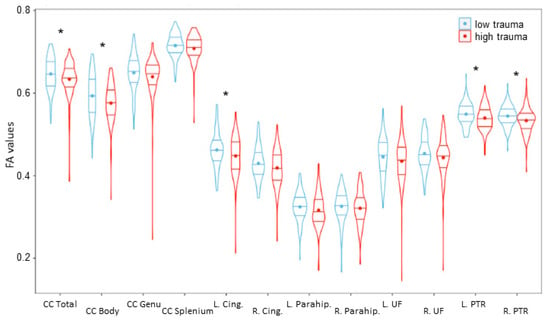
Figure 2.
FA values for each of the 12 ROIs examined. Significant differences (corrected p-value < 0.05) are denoted by *. The low-trauma group is depicted in blue on the left, and the high-trauma group is depicted in red on the right. CC—corpus callosum; L/R Cing.—left/right cingulate; L/R Parahip—left/right parahippocampus; L/R UF—left/right uncinate fasciculus; L/R PTR—left/right posterior thalamic radiation.
2.2. Corpus Callosum
The total corpus callosum exhibited significantly lower FA for the participants in the high-trauma group compared to those in the low-trauma group (F(1,180) = 4.671, p = 0.032). FA in the entire corpus callosum was negatively correlated with the number of traumas (r(182) = −0.183, p-value = 0.012). FA in the body of the corpus callosum was significantly lower (F(1,180) = 5.990, p = 0.015) in the high-trauma group in comparison to the low-trauma group. FA values in the body of the corpus callosum were significantly negatively correlated with the number of traumas (r(182) = −0.199, p-value = 0.006). No group differences for FA were observed in the splenium (F(1,180) = 2.34, p = 0.127) or the genu (F(1,180) = 0.397, p = 0.529), but FA in the splenium was significantly negatively correlated with the number of traumas (r(182) = −0.145, p-value = 0.049). There was no significant effect of gender in the corpus callosum models.
2.3. Posterior Thalamic Radiation (Including Optic Radiation)
Left and right posterior thalamic radiation exhibited significant decreases in FA for the adolescents in the high-trauma group (left: F(1,180) = 5.469, p = 0.020; right: F(1,180) = 7.009, p = 0.009). FA in the left and right thalamic radiation was significantly negatively correlated with the number of traumas (left: r(182) = −0.173, p = 0.019; right: r(182) = −0.243, p = 0.001). There was no significant effect of gender in the thalamic radiation models.
2.4. Cingulate Gyrus
The left cingulate gyrus exhibited a significant decrease in FA in the high-trauma group (F(1,179) = 4.507, p = 0.035). The degree of FA in the left cingulate gyrus was significantly lower in females than in males (F(1,179) = 6.976, p = 0.009). Left cingulate gyrus FA was negatively correlated with the number of traumas (r(182) = −0.204, p = 0.005). A group difference by trauma was trending towards significance in regard to FA of the right cingulate gyrus when controlling for gender (F(1,179) = 3.362, p = 0.068). FA in the right portion of the cingulate gyrus was also significantly lower in females than in males (F(1,179) = 6.616, p = 0.011). FA in the right cingulate gyrus was significantly negatively correlated with the number of traumas (r(182) = −0.189, p = 0.010).
2.5. Parahippocampal Cingulum
There was no significant difference in FA between groups in the right parahippocampal cingulum when controlling for age (F(1,181) = 0.525, p = 0.449). There was also no significant difference in FA between groups in the left parahippocampal cingulum when controlling for gender (F(1,181) = 1.793, p = 0.182). FA was not significantly correlated with the number of traumas in the left or right parahippocampal cingulum (right: r(182) = −0.092, p = 0.209; left: r(182) = −0.110, p = 0.133).
2.6. Uncinate Fasciculus (UF)
No significant differences in FA between groups were observed in the bilateral UF (right: F(1,182) = 2.118, p = 0.147; left: F(1,182) = 1.833, p = 0.177). There were no significant effects of age, gender, or site in the UF model. FA in the UF was not significantly correlated with the number of traumas (right: r(182) = −0.114, p = 0.122; left: r(182) = −0.115, p = 0.116).
2.7. Brain Region Analysis: PTS and FA Association
Posttraumatic stress symptomology was not associated with FA in any analyses (see Table 4) (p’s > 0.135).

Table 4.
Statistical tests for FA association with PTS.
3. Discussion
The current analysis examined the association between exposure to past traumas and white matter integrity in youth. Trauma exposure was associated with group differences in both self-reported behavioral measures such as the SDQ and TSCC and guardian-reported behavioral measures such as the CBCL. Lower FA was identified in youth with high trauma exposure in comparison to youth with low-trauma exposure in five regions of the brain, namely, the total corpus callosum, the body of the corpus callosum, the left cingulate gyrus, and bilateral posterior thalamic radiation. However, FA was not associated with current posttraumatic stress symptoms. Our results suggest exposure to non-abusive traumas in youth negatively affects white matter integrity in a subset of brain regions (identified in the literature) of children who experienced child maltreatment and adults who experienced childhood trauma.
The corpus callosum has been widely examined in association with childhood maltreatment (for a review, see [17]). Our findings of group differences in the whole corpus callosum and the body of the corpus callosum are consistent with previous observations in childhood maltreatment cases [7,16,18]. Differences in the partitioning of the corpus callosum should be noted, as some childhood trauma studies examined the corpus callosum in seven separate sections [7,19], while others examined it in three separate sections [16,18], and one study examined the splenium of the corpus callosum as a portion of the forceps major but found no significant differences in FA [20]. Despite differences in partitioning, most significant differences appear in the body [7,16,18], followed by the genu [16,18] and, occasionally, the splenium [21]. Our results are consistent with the patterns identified in childhood maltreatment studies.
The cingulate gyrus results are consistent with previous DTI studies that examined multiple types of lifetime traumas among adults [22]. Low FA in the left cingulate gyrus is consistent with a pattern of significant observations in the left hemisphere across DTI studies [8,16,23]. This may suggest greater sensitivity to traumatic events in the left hemisphere, and it is supported by our results. As a word of caution, tract-based spatial statistics have been shown to more easily identify tracts in the left hemisphere than in the right hemisphere, specifically in the cingulum [24]. The ease of identifying the left hemisphere cingulum tract may lead to an increase in the signal-to-noise ratio and thus increased statistical power.
Results regarding the thalamic radiation are mixed. One study reported significant FA reduction in the anterior thalamic radiation [5] in a group of healthy adults exposed to physical neglect as children. Another retrospective study reported insignificant microstructural changes in the posterior thalamic radiation when comparing lithium-treated vs. untreated adults with bipolar disorder exposed to adverse childhood events [25]. Lower FA in the posterior thalamic radiation is associated with anorexia nervosa [26]. Lower FA is also associated with sensory processing disorder [27,28] and coordination difficulties in children [29]. Our results add to the current mixed knowledge about the thalamic radiation but suggest more research is needed to understand the effects of trauma exposure on this region.
The insignificant results regarding the parahippocampal cingulum are inconsistent with current early-life stress and childhood maltreatment research. Childhood abuse and neglect have repeatedly been associated with reductions in FA in the hippocampal projection of the cingulum [5,8,20]. When combined, these results may indicate that this effect is specific to abuse and neglect. Alternatively, our failure to find FA group differences in the parahippocampal cingulum may instead indicate that dMRI may not be the most sensitive modality for detecting differences in this region, particularly for youth. Our results mirror the history of volumetric studies, which consistently report hippocampal volume reductions in adults but fail to replicate this with similar parameters in children [4,30,31]. Volumetric differences revealing sex–volume interactions may partially explain the differences observed in youth, as demonstrated previously in the Developmental Chronnecto-genomics (Dev-CoG) sample [32], and it may also explain the insignificance of the parahippocampal cingulum FA measures in the current analysis. However, gender was tested in each model and did not explain the lack of group difference in this WM tract.
Similarly, insignificant FA differences between groups in the UF may indicate effects that are specific to abuse and neglect. Retrospective childhood maltreatment studies have repeatedly reported FA differences in the UF [5,33], but such differences were not observed in our study. There is evidence that FA changes in the UF result from prolonged exposure to adversity in childhood [23]. Prolonged exposure effects in UF FA have also been reported in service members deployed in combat zones for at least three months [34]. Duration of adversity was not assessed in our study. Regardless of short- or long-term exposure effects, the UF is a tract that connects areas of the limbic system (temporal regions: the amygdala and hippocampus) with the frontal cortex [35,36]. The UF plays a role in emotional appraisal, emotion regulation, and memory [36] and is associated with the development of PTSD [35]. Our results support a differentiation between the effects of childhood maltreatment and other forms of trauma on connectivity between limbic structures.
3.1. Posttraumatic Stress Symptoms
The insignificance of the association of FA with posttraumatic stress assessed via the TSCC may be a result of insensitivity to specific PTS symptoms. A small study of children with maltreatment-related PTSD reported lower FA in the corpus callosum when compared to matched healthy controls [7]. In a study of service members with mild TBI and PTSD, the significant reduction observed in FA of the parahippocampal cingulum was not related to total PTSD severity, but it was significantly related to the re-experiencing symptoms (nightmares, flashbacks, etc. [34,37]). These findings suggest a more specialized association with PTS symptoms than what can be obtained with the TSCC or may indicate a limited range of PTS severity in this typically developing sample. For example, FA may be altered in different regions in association with the wide array of symptoms captured by the TSCC. We cannot rule out an association between individual symptoms and FA, and future studies may benefit from testing individual symptoms separately. However, it is important to point out that this sample consists of children without a psychiatric diagnosis, and, as such, the insensitivity to PTS severity may reflect the absence of PTSD. Alternatively, the FA changes may be associated with compensatory changes that allowed the individuals to adapt to past trauma exposure.
Regardless, marked behavioral differences between the groups were observed (see Table 2) and correspond to psychological and emotional problems reported by the children or parents. Although there were no main effects of posttraumatic stress on FA, the behavioral differences observed indicate that other traumas not related to maltreatment also have considerable impacts on behavioral outcomes among children and support a potential role for interventions following exposure to traumatic events.
3.2. Limitations
Our study has limitations that should be mentioned. Although the design of this study, with trauma assessed during childhood, reduces recall bias that may be present in adult studies of childhood trauma, prospective longitudinal studies would more accurately capture the effects of trauma on brain development and further reduce the limitations of retrospective recall. Furthermore, the THP does not capture the number of events within a single trauma category, the severity of the traumatic event(s), or the timing of these traumatic exposures relative to developmental milestones. Thus, trauma exposure may have been underestimated for certain individuals. Despite this, significant differences between the low- and high-trauma groups were observed in terms of the brain and behavioral measures, suggesting that the number of trauma categories provides an adequate assessment of early-life stress in children.
This study’s cross-sectional design also limits our ability to understand if the experienced traumas caused the changes in white matter reported here or if other factors that are linked to increased trauma exposure, such as poverty [38], were the causative factors underlying these changes. Some studies concerning natural disasters have provided support for the direct influence of traumatic experiences on brain measures [39,40], allowing researchers to compare individuals with or without PTSD within a tightly specified timeframe relative to the traumatic event, yet these studies also did not capture brain measures prior to the disaster. Prospective longitudinal studies on children are needed to identify the factors that most influence development, including capturing conditions that may reduce harmful effects due to trauma exposure and identifying factors that increase resilience.
While the THP questions directly asking about child abuse were removed by request of the IRB, two of the retained questions may have incorporated child abuse (questions 4 and 12). Therefore, we cannot rule out the possibility that some of the results incorporate data on children with a history of abuse. However, these categories did not dominate the representation of traumas in either the low- or high-trauma groups. Also, these children were not recruited through referrals for a history of abuse or neglect and thus likely differ in their trauma histories in comparison to studies that directly recruited children from clinics designed to treat children who have experienced neglect or abuse. Because this study identified ROIs based on prior maltreatment studies, other regions that are influenced by non-maltreatment-related trauma may still be impacted. It is important for future studies to consider whole-brain analyses to determine if non-maltreatment trauma influences other brain networks not examined here.
Further, our results may have been influenced by other factors such as poverty, nutrition, genetic predisposition, education, and other forms of early-life stress. Our study controlled for age, but future studies may benefit from examining trauma through the lens of developmental patterns in combination with examining the role of the recency and duration of trauma effects.
3.3. Conclusions
The current analysis provides evidence for an association between trauma exposure and white matter integrity in typically developing youth. The reductions in FA observed in this study indicate trauma exposure is associated with brain structural changes. In addition, this study revealed broad differences in behavior, wherein children in the high-trauma group reported more behavioral difficulties (e.g., increased anxiety and depression) in this otherwise typically developing sample. The results indicate that future research should examine non-abusive traumas and move beyond simply controlling for and excluding these traumas from analyses. With the plethora of evidence for the effects of early-life stress due to abuse on microstructural and behavioral development, the current work indicates that early-life stress due to other traumas experienced in childhood deserves the same attention to detail.
4. Materials and Methods
4.1. Participants
Participants were recruited from two sites, namely, the Mind Research Network (MRN) in New Mexico and the University of Nebraska Medical Center (UNMC) in Nebraska, as part of the longitudinal Dev-CoG study funded by the National Science Foundation, as described in [41]. Consent was obtained from the parents of participants, and child assent was obtained from each participant prior to completion of the study protocols. Both protocols were approved by each site’s institutional review board before the study was conducted. The Dev-CoG study enrolled typically developing youth and acquired 211 baseline dMRI scans. Data from the baseline session were used for the current analysis. Preprocessing yielded good-quality data from 203 participants. Nineteen datasets were excluded due to missing or incomplete data on the Trauma History Profile (n = 8), the trauma symptoms checklist for children (n = 3), or the demographics questionnaire (n = 8, for control variables).
4.2. Assessments
The Trauma History Profile (THP [42]) is a 12-item, self-report questionnaire that captures the number of different lifetime traumatic events experienced, arranged in different categories. This measure does not allow the investigation of the number of traumatic incidences; thus, hereafter, references to “traumas” refer to traumatic event categories, not individual traumatic incidences or child abuse traumas. Traumas investigated included disasters; bad accidents; war; being hit, punched, or kicked very hard; witnessing a family member being hit, punched, or kicked very hard; being beaten up, shot at, or threatened; having witnessed someone being beaten up, shot at, or killed; seeing a dead body (not at a funeral); witnessing or learning about the death of a loved one; having a painful or scary medical treatment; experiencing the death of someone close; and having been exposed to any other potentially traumatic event.
Posttraumatic stress (PTS) symptomology was measured using the posttraumatic stress symptoms subscale of the Trauma Symptoms Checklist for Children (TSCC; [43]). The TSCC is a Likert-type self-report scale used to measure internalized distress on five clinical scales: anger, anxiety, depression, dissociation, and posttraumatic stress.
The self-report Strengths and Difficulties Questionnaire (SDQ-C; [44]) and parental-report (SDQ-P; [45]) were used to examine behavioral differences between trauma groups. The Child Behavior Checklist—Parent Report/Adaptive Functioning (CBCL-PR; [46]) was also used to examine differences in behavior between the groups. Full-scale intelligence scores collected via the Wechsler Abbreviated Scale of Intelligence (Second Edition; WASI-II; [47]) were used as a covariate in models since intelligence has previously been associated with white matter microstructure [48]. Data on handedness were collected based on parent reports in the demographics questionnaire by asking the parents about the handedness of their child (right, left, or ambidextrous).
4.3. Diffusion Imaging Acquisition Parameters and Processing
MRN participants were scanned with a Siemens 3T TrioTim using a 32-channel radio frequency coil. UNMC participants were scanned with a Siemens 3T Magnetom Skyra using a 32-channel radio frequency head coil. The dMRI parameters included field of view (FOV) = 224 × 224, resolution = 2.0 × 2.0 × 2.0 mm3, flip angle = 84/157 deg (MRN) and 90/180 deg (UNMC), echo time (TE) = 108 ms, TR = 4000 ms, multiband acceleration factor = 3, axial slices = 72, b = 800, 1600, 2400 s/mm2, and directions = 165.
dMRI data were collected with phase-reversed blips, producing images with EPI distortion occurring in one of two opposite directions. Eight equally spaced volumes with b = 0 (four with phase encoding in the anterior–posterior direction and four with phase encoding in the posterior–anterior direction) were used along with the FSL (v6.0.1) tool topup [49] to estimate the susceptibility-induced off-resonance field.
dMRI volumes were then corrected for eddy-current-induced distortions and head movements using the FSL tool eddy [50]. Additional features for advanced motion correction were enabled to correct motion-induced signal dropout [51] and intra-volume (slice-to-volume) movement [52].
Fractional anisotropy (FA) maps were made using the AFNI (v.19.1.00) tool 3dDWItoDT [53] and registered to MNI space using nonlinear registration with FSL scripts optimized for FA registration.
4.4. ROI Selection and FA Value Extraction
Twelve brain regions of interest were chosen based on DTI studies of prior childhood maltreatment and PTSD [5,7,16,20,23,34]. The twelve regions examined included the body, genu, and splenium of the corpus callosum; the bilateral sections of the thalamic radiation; bilateral cingulate gyri; bilateral parahippocampal cingulum; and bilateral UF (Figure 3). The corpus callosum was also examined as a whole.
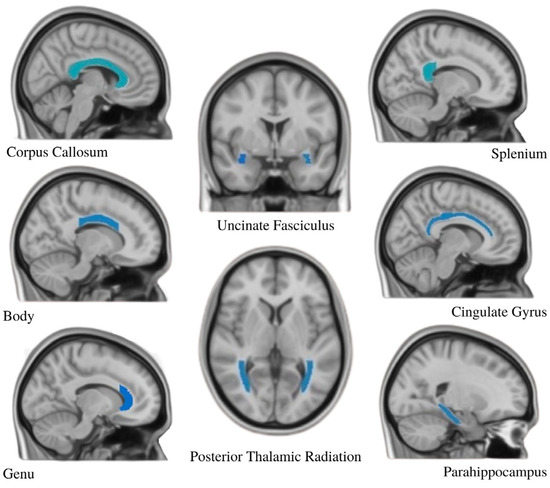
Figure 3.
The JHU ICBM-DTI-81 atlas was used to identify 12 ROIs (shown in blue) to examine the effects of trauma on white matter integrity assessed with fractional anisotropy. Bilateral tracts were analyzed for all ROIs except the corpus callosum (body, genu, and splenium).
Masks for each ROI were created using the JHU ICBM-DTI-81 atlas, a stereotaxic white matter atlas created specifically for accurate examination of white matter [54]. Masks were binarized with the fslmaths function in FSL. Mean FA values for each ROI were extracted using the fslstats command in FSL.
4.5. Statistics and Grouping
Participants were separated into groups based on the number of traumas they had experienced. Because measures of types of experienced traumas do not capture a measure of trauma severity that could be interpreted as a meaningful continuous variable, we used a median split to define the two groups, as carried out in past adult studies that used the adverse childhood experiences questionnaire [55]. Participants who endorsed having experienced zero or one traumas were placed into the low-trauma group, and participants who had experienced two or more traumas were placed into the high-trauma group. Groups were used to compare FA values and ancillary behavioral measures. Posttraumatic stress symptomology was examined for its association with FA regardless of group.
The extracted ROI data were analyzed using R (v4.0.2). ANCOVA models were used to examine group (low trauma vs. high trauma) differences in FA, while controlling for the effects of age and site. Each model was tested for effects of gender and intelligence. Gender and intelligence were only included as control variables if they individually had significant effects on the model result. Models were tested for normality, heteroscedasticity, and linearity for each group. Data were transformed when necessary, with a lambda indicated by a Box–Cox transformation test, and subsequently de-transformed for presentation on the original scale. Bonferroni correction was used to control for multiple comparisons. Kruskal–Wallis tests were used to determine whether there were any gender or site distribution differences across groups. Linear correlations between traumatic events and FA were also analyzed. Please see Supplementary Materials for the results when including SES in the model (Supplementary Table S1) and the description of the final models (Supplementary Text).
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/stresses5010019/s1. Table S1: Statistical tests for FA Differences Between Groups (Models with SES).
Author Contributions
Conceptualization, Y.-P.W., T.W.W., V.D.C. and J.M.S.; data curation, A.R., H.P. and V.D.C.; formal analysis, A.R., H.P., P.M.S., V.D.C. and J.M.S.; funding acquisition, Y.-P.W., T.W.W., V.D.C. and J.M.S.; investigation, A.R., Y.-P.W., T.W.W., V.D.C. and J.M.S.; methodology, A.R., H.P., Y.-P.W., T.W.W., V.D.C. and J.M.S.; project administration, T.W.W., V.D.C. and J.M.S.; resources, Y.-P.W., T.W.W., V.D.C. and J.M.S.; supervision, J.M.S.; visualization, A.R. and J.M.S.; writing—original draft, A.R.; writing—review and editing, A.R., H.P., P.M.S., Y.-P.W., T.W.W., V.D.C. and J.M.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Science Foundation under grant number #1539067, awarded to V.D.C., T.W.W., Y.-P.W., and J.M.S.; the National Institutes of Health [(R21MH118765 to P.M.S. and J.M.S., K23AA025094 to P.M.S., R01MH121101, R01MH116782, R01MH118013, and P20-GM144641 to T.W.W.; R01EB020407 and R01MH118695 to V.D.C.; R01AA029605 to J.M.S.], and At Ease, USA.
Data Availability Statement
Data will be made available upon reasonable request made to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Available online: https://www.apa.org/pi/families/resources/update.pdf (accessed on 10 December 2024).
- Hetzel-Riggin, M.; Roby, R. Trauma Type and Gender Effects on PTSD, General Distress, and Peritraumatic Dissociation. J. Loss Trauma 2012, 18, 41–53. [Google Scholar] [CrossRef]
- Kira, I.; Lewandowski, L.; Somers, C.L.; Yoon, J.S.; Chiodo, L. The Effects of Trauma Types, Cumulative Trauma, and PTSD on IQ in Two Highly Traumatized Adolescent Groups. Psychol. Trauma Theory Res. Pract. Policy 2012, 4, 128–139. [Google Scholar] [CrossRef]
- De Bellis, M.D.; Keshavan, M.S.; Shifflett, H.; Iyengar, S.; Beers, S.R.; Hall, J.; Moritz, G. Brain structures in pediatric maltreatment-related posttraumatic stress disorder: A sociodemographically matched study. Biol. Psychiatry 2002, 52, 1066–1078. [Google Scholar] [CrossRef]
- Tendolkar, I.; Martensson, J.; Kuhn, S.; Klumpers, F.; Fernandez, G. Physical neglect during childhood alters white matter connectivity in healthy young males. Hum. Brain Mapp. 2018, 39, 1283–1290. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Jeong, B.; Polcari, A.; Rohan, M.L.; Teicher, M.H. Reduced fractional anisotropy in the visual limbic pathway of young adults witnessing domestic violence in childhood. Neuroimage 2012, 59, 1071–1079. [Google Scholar] [CrossRef]
- Jackowski, A.P.; Douglas-Palumberi, H.; Jackowski, M.; Win, L.; Schultz, R.T.; Staib, L.W.; Krystal, J.H.; Kaufman, J. Corpus callosum in maltreated children with posttraumatic stress disorder: A diffusion tensor imaging study. Psychiatry Res. 2008, 162, 256–261. [Google Scholar] [CrossRef]
- Choi, J.; Jeong, B.; Rohan, M.L.; Polcari, A.M.; Teicher, M.H. Preliminary evidence for white matter tract abnormalities in young adults exposed to parental verbal abuse. Biol. Psychiatry 2009, 65, 227–234. [Google Scholar] [CrossRef]
- Blakemore, S.J. Imaging brain development: The adolescent brain. Neuroimage 2012, 61, 397–406. [Google Scholar] [CrossRef]
- Sowell, E.R.; Thompson, P.M.; Holmes, C.J.; Batth, R.; Jernigan, T.L.; Toga, A.W. Localizing age-related changes in brain structure between childhood and adolescence using statistical parametric mapping. Neuroimage 1999, 9, 587–597. [Google Scholar] [CrossRef]
- Edwards, V.J.; Holden, G.W.; Felitti, V.J.; Anda, R.F. Relationship between multiple forms of childhood maltreatment and adult mental health in community respondents: Results from the adverse childhood experiences study. Am. J. Psychiatry 2003, 160, 1453–1460. [Google Scholar] [CrossRef]
- Heim, C.; Binder, E.B. Current research trends in early life stress and depression: Review of human studies on sensitive periods, gene-environment interactions, and epigenetics. Exp. Neurol. 2012, 233, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Cisler, J.M.; Begle, A.M.; Amstadter, A.B.; Resnick, H.S.; Danielson, C.K.; Saunders, B.E.; Kilpatrick, D.G. Exposure to interpersonal violence and risk for PTSD, depression, delinquency, and binge drinking among adolescents: Data from the NSA-R. J. Trauma. Stress 2012, 25, 33–40. [Google Scholar] [CrossRef]
- Duncan, N.W.; Hayes, D.J.; Wiebking, C.; Tiret, B.; Pietruska, K.; Chen, D.Q.; Rainville, P.; Marjanska, M.; Ayad, O.; Doyon, J.; et al. Negative childhood experiences alter a prefrontal-insular-motor cortical network in healthy adults: A preliminary multimodal rsfMRI-fMRI-MRS-dMRI study. Hum. Brain Mapp. 2015, 36, 4622–4637. [Google Scholar] [CrossRef]
- Lu, S.; Pan, F.; Gao, W.; Wei, Z.; Wang, D.; Hu, S.; Huang, M.; Xu, Y.; Li, L. Neural correlates of childhood trauma with executive function in young healthy adults. Oncotarget 2017, 8, 79843–79853. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Wei, Z.; Gao, W.; Wu, W.; Liao, M.; Zhang, Y.; Li, W.; Li, Z.; Li, L. White matter integrity alterations in young healthy adults reporting childhood trauma: A diffusion tensor imaging study. Aust. N. Z. J. Psychiatry 2013, 47, 1183–1190. [Google Scholar] [CrossRef]
- Daniels, J.K.; Lamke, J.P.; Gaebler, M.; Walter, H.; Scheel, M. White matter integrity and its relationship to PTSD and childhood trauma--a systematic review and meta-analysis. Depress. Anxiety 2013, 30, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Rinne-Albers, M.A.; van der Werff, S.J.; van Hoof, M.J.; van Lang, N.D.; Lamers-Winkelman, F.; Rombouts, S.A.; Vermeiren, R.R.; van der Wee, N.J. Abnormalities of white matter integrity in the corpus callosum of adolescents with PTSD after childhood sexual abuse: A DTI study. Eur. Child Adolesc. Psychiatry 2016, 25, 869–878. [Google Scholar] [CrossRef]
- Teicher, M.H.; Anderson, C.M.; Ohashi, K.; Polcari, A. Childhood maltreatment: Altered network centrality of cingulate, precuneus, temporal pole and insula. Biol. Psychiatry 2014, 76, 297–305. [Google Scholar] [CrossRef]
- Huang, H.; Gundapuneedi, T.; Rao, U. White matter disruptions in adolescents exposed to childhood maltreatment and vulnerability to psychopathology. Neuropsychopharmacology 2012, 37, 2693–2701. [Google Scholar] [CrossRef]
- Ceponiene, R.; Rinne, T.; Naatanen, R. Maturation of cortical sound processing as indexed by event-related potentials. Clin. Neurophysiol. 2002, 113, 870–882. [Google Scholar] [CrossRef]
- Fani, N.; King, T.Z.; Jovanovic, T.; Glover, E.M.; Bradley, B.; Choi, K.; Ely, T.; Gutman, D.A.; Ressler, K.J. White matter integrity in highly traumatized adults with and without post-traumatic stress disorder. Neuropsychopharmacology 2012, 37, 2740–2746. [Google Scholar] [CrossRef] [PubMed]
- Eluvathingal, T.J.; Chugani, H.T.; Behen, M.E.; Juhasz, C.; Muzik, O.; Maqbool, M.; Chugani, D.C.; Makki, M. Abnormal brain connectivity in children after early severe socioemotional deprivation: A diffusion tensor imaging study. Pediatrics 2006, 117, 2093–2100. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.T.; Yeatman, J.D.; Wandell, B.A.; Buonocore, M.H.; Amaral, D.G.; Nordahl, C.W. Diffusion properties of major white matter tracts in young, typically developing children. Neuroimage 2014, 88, 143–154. [Google Scholar] [CrossRef]
- Poletti, S.; Mazza, E.; Bollettini, I.; Locatelli, C.; Cavallaro, R.; Smeraldi, E.; Benedetti, F. Adverse childhood experiences influence white matter microstructure in patients with schizophrenia. Psychiatry Res. 2015, 234, 35–43. [Google Scholar] [CrossRef]
- Frieling, H.; Fischer, J.; Wilhelm, J.; Engelhorn, T.; Bleich, S.; Hillemacher, T.; Dorfler, A.; Kornhuber, J.; de Zwaan, M.; Peschel, T. Microstructural abnormalities of the posterior thalamic radiation and the mediodorsal thalamic nuclei in females with anorexia nervosa—A voxel based diffusion tensor imaging (DTI) study. J. Psychiatr. Res. 2012, 46, 1237–1242. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.S.; Gratiot, M.; Owen, J.P.; Brandes-Aitken, A.; Desai, S.S.; Hill, S.S.; Arnett, A.B.; Harris, J.; Marco, E.J.; Mukherjee, P. White Matter Microstructure is Associated with Auditory and Tactile Processing in Children with and without Sensory Processing Disorder. Front. Neuroanat. 2015, 9, 169. [Google Scholar] [CrossRef]
- Payabvash, S.; Palacios, E.M.; Owen, J.P.; Wang, M.B.; Tavassoli, T.; Gerdes, M.; Brandes-Aitken, A.; Marco, E.J.; Mukherjee, P. Diffusion tensor tractography in children with sensory processing disorder: Potentials for devising machine learning classifiers. NeuroImage Clin. 2019, 23, 101831. [Google Scholar] [CrossRef]
- Zwicker, J.G.; Missiuna, C.; Harris, S.R.; Boyd, L.A. Developmental coordination disorder: A pilot diffusion tensor imaging study. Pediatr. Neurol. 2012, 46, 162–167. [Google Scholar] [CrossRef]
- Bremner, J.D. The relationship between cognitive and brain changes in posttraumatic stress disorder. Ann. N. Y. Acad. Sci. 2006, 1071, 80–86. [Google Scholar] [CrossRef]
- De Bellis, M.D.; Keshavan, M.S.; Clark, D.B.; Casey, B.J.; Giedd, J.N.; Boring, A.M.; Frustaci, K.; Ryan, N.D.A.E. Bennett Research Award. Developmental traumatology. Part II: Brain development. Biol. Psychiatry 1999, 45, 1271–1284. [Google Scholar] [CrossRef]
- Badura-Brack, A.S.; Mills, M.S.; Embury, C.M.; Khanna, M.M.; Klanecky Earl, A.; Stephen, J.M.; Wang, Y.P.; Calhoun, V.D.; Wilson, T.W. Hippocampal and parahippocampal volumes vary by sex and traumatic life events in children. J. Psychiatry Neurosci. 2020, 45, 190013. [Google Scholar] [CrossRef] [PubMed]
- Hanson, J.L.; Knodt, A.R.; Brigidi, B.D.; Hariri, A.R. Lower structural integrity of the uncinate fasciculus is associated with a history of child maltreatment and future psychological vulnerability to stress. Dev. Psychopathol. 2015, 27, 1611–1619. [Google Scholar] [CrossRef]
- Costanzo, M.E.; Jovanovic, T.; Pham, D.; Leaman, S.; Highland, K.B.; Norrholm, S.D.; Roy, M.J. White matter microstructure of the uncinate fasciculus is associated with subthreshold posttraumatic stress disorder symptoms and fear potentiated startle during early extinction in recently deployed Service Members. Neurosci. Lett. 2016, 618, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Koch, S.B.J.; van Zuiden, M.; Nawijn, L.; Frijling, J.L.; Veltman, D.J.; Olff, M. Decreased uncinate fasciculus tract integrity in male and female patients with PTSD: A diffusion tensor imaging study. J. Psychiatry Neurosci. 2017, 42, 331–342. [Google Scholar] [CrossRef]
- Schmahmann, J.D.; Pandya, D.N.; Wang, R.; Dai, G.; D’Arceuil, H.E.; de Crespigny, A.J.; Wedeen, V.J. Association fibre pathways of the brain: Parallel observations from diffusion spectrum imaging and autoradiography. Brain 2007, 130 Pt 3, 630–653. [Google Scholar] [CrossRef]
- Costanzo, M.E.; Chou, Y.Y.; Leaman, S.; Pham, D.L.; Keyser, D.; Nathan, D.E.; Coughlin, M.; Rapp, P.; Roy, M.J. Connecting combat-related mild traumatic brain injury with posttraumatic stress disorder symptoms through brain imaging. Neurosci. Lett. 2014, 577, 11–15. [Google Scholar] [CrossRef]
- Lacey, R.E.; Howe, L.D.; Kelly-Irving, M.; Bartley, M.; Kelly, Y. The Clustering of Adverse Childhood Experiences in the Avon Longitudinal Study of Parents and Children: Are Gender and Poverty Important? J. Interpers. Violence 2022, 37, 2218–2241. [Google Scholar] [CrossRef]
- Wu, J.; Yuan, Y.; Cao, C.; Zhang, K.; Wang, L.; Zhang, L. The relationship between response inhibition and posttraumatic stress symptom clusters in adolescent earthquake survivors: An event-related potential study. Sci. Rep. 2015, 5, 8844. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Wu, J.; Sun, X.; Zhang, K. Enhanced mismatch negativity in adolescents with posttraumatic stress disorder (PTSD). Int. J. Psychophysiol. 2011, 79, 231–235. [Google Scholar] [CrossRef]
- Stephen, J.M.; Solis, I.; Janowich, J.; Stern, M.; Frenzel, M.R.; Eastman, J.A.; Mills, M.S.; Embury, C.M.; Coolidge, N.M.; Heinrichs-Graham, E.; et al. The Developmental Chronnecto-Genomics (Dev-CoG) study: A multimodal study on the developing brain. Neuroimage 2020, 225, 117438. [Google Scholar] [CrossRef]
- Pynoos, R.; Steinberg, A. UCLA Trauma History Profile; National Child Traumatic Stress Network: Los Angeles, CA, USA, 2002. [Google Scholar]
- Briere, J. Trauma Symptom Checklist for Children: Professional Manual; Psychological Assessment Resources, Inc.: Odessa, FL, USA, 1996. [Google Scholar]
- Goodman, R.; Meltzer, H.; Bailey, V. The Strengths and Difficulties Questionnaire: A pilot study on the validity of the self-report version. Eur. Child Adolesc. Psychiatry 1998, 7, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Goodman, R. The Strengths and Difficulties Questionnaire: A research note. J. Child Psychol. Psychiatry 1997, 38, 581–586. [Google Scholar] [CrossRef] [PubMed]
- Achenbach, T. Manual for Child Behavior Checklist/4-18 and 1991 Profile; University of Vermont Department of Psychiatry: Burlington, VT, USA, 1991. [Google Scholar]
- Wechsler, D. Wechsler Abbreviated Scale of Intelligence, 2nd ed.; Pearson: Bloomington, MN, USA, 2011. [Google Scholar]
- Bathelt, J.; Scerif, G.; Nobre, A.C.; Astle, D.E. Whole-brain white matter organization, intelligence, and educational attainment. Trends Neurosci. Educ. 2019, 15, 38–47. [Google Scholar] [CrossRef]
- Andersson, J.L.; Skare, S.; Ashburner, J. How to correct susceptibility distortions in spin-echo echo-planar images: Application to diffusion tensor imaging. Neuroimage 2003, 20, 870–888. [Google Scholar] [CrossRef]
- Andersson, J.L.R.; Sotiropoulos, S.N. An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. Neuroimage 2016, 125, 1063–1078. [Google Scholar] [CrossRef]
- Andersson, J.L.R.; Graham, M.S.; Zsoldos, E.; Sotiropoulos, S.N. Incorporating outlier detection and replacement into a non-parametric framework for movement and distortion correction of diffusion MR images. Neuroimage 2016, 141, 556–572. [Google Scholar] [CrossRef]
- Andersson, J.L.R.; Graham, M.S.; Drobnjak, I.; Zhang, H.; Filippini, N.; Bastiani, M. Towards a comprehensive framework for movement and distortion correction of diffusion MR images: Within volume movement. Neuroimage 2017, 152, 450–466. [Google Scholar] [CrossRef] [PubMed]
- Taylor, P.A.; Saad, Z.S. FATCAT: (an efficient) Functional and Tractographic Connectivity Analysis Toolbox. Brain Connect. 2013, 3, 523–535. [Google Scholar] [CrossRef] [PubMed]
- Mori, S.; Oishi, K.; Jiang, H.; Jiang, L.; Li, X.; Akhter, K.; Hua, K.; Faria, A.V.; Mahmood, A.; Woods, R.; et al. Stereotaxic white matter atlas based on diffusion tensor imaging in an ICBM template. Neuroimage 2008, 40, 570–582. [Google Scholar] [CrossRef]
- Felitti, V.J.; Anda, R.F.; Nordenberg, D.; Williamson, D.F.; Spitz, A.M.; Edwards, V.; Koss, M.P.; Marks, J.S. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study. Am. J. Prev. Med. 1998, 14, 245–258. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).