Enhancing Horticultural Sustainability in the Face of Climate Change: Harnessing Biostimulants for Environmental Stress Alleviation in Crops
Abstract
1. Introduction
2. Methodology
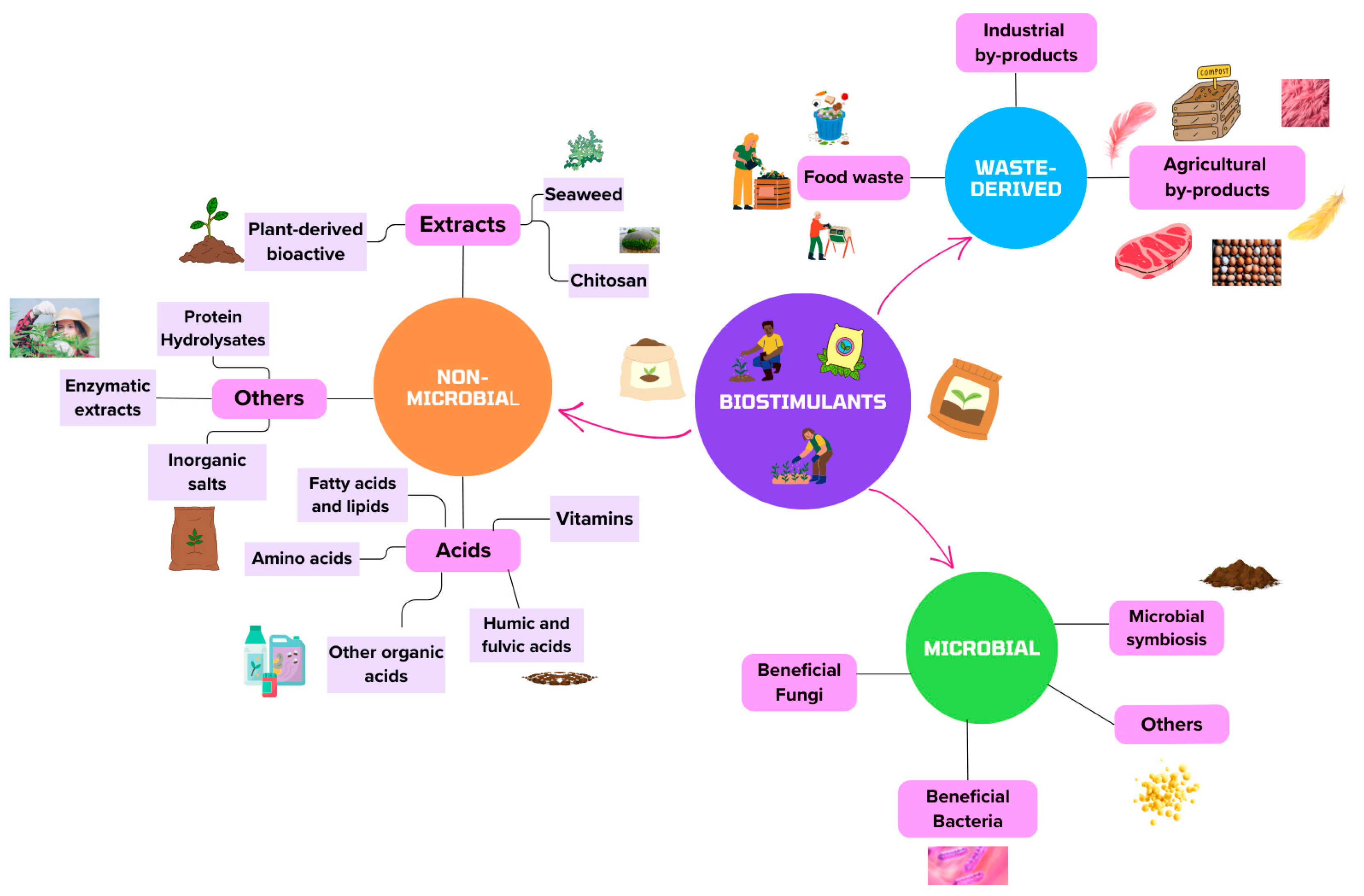
3. Concepts and Types of Biostimulants
3.1. Non-Microbial Biostimulants
3.1.1. Humic Substances (HS)
3.1.2. Seaweed Extract (SWE)
3.1.3. Botanical Derivatives
3.1.4. Protein Hydrolysates and Nitrogen-Containing Compounds
3.1.5. GABA
3.1.6. Selenium
3.1.7. Chitosan
3.2. Microbial Biostimulants
4. Sources of Biostimulants
5. Biostimulants as Growth, Yield, and Quality Enhancers of Fruit Crops
5.1. Strawberry
5.2. Pome and Stone Fruit
5.3. Berries and Vine Crop
5.4. Cherry, Papaya and Olive
| Fruit Crop | Biostimulant | Dosage | Method of Application | Effect of Biostimulants on Fruit Crop | Reference |
|---|---|---|---|---|---|
| Kiwifruit Actinidia chinensis var. deliciosa (A.Chev.) | Humic acid | 4 mL per litre | Foliar and drenching | Increased the yield, TSS, and vitamin C content. | [93] |
| Strawberry (Fragaria ananassa Duch.) | Humic acid | 100 kg per ha | Soil application | Increased the carotenoids, chlorophyll a, and leaf area. | [94] |
| Olive (Olea europaea L.) | Arginine +humic acid | 5 mL per litre | Foliar | Increased the total chlorophyll and fruit protein content. | [95] |
| Grapevine (Vitis vinifera L.) | Seaweed | 1 g/L | Foliar application | Increased the yield, berries number, and anthocyanin content. | [96] |
| Sour orange (Citrus × aurantium L.) | Seaweed extracts | 4 g/L | Fertigation | Increased fruit quality and blossom count per plant. | [97] |
| Mango (Mangifera indica L.) | Seaweed extract | 4 mL/L | Foliar application | Increased the nitrogen, potassium, iron and zinc content in the leaf. | [98] |
| Grape (Vitis vinifera L.) | Protein hydrolysate | 2 g/L | Foliar application | Increased the amino acid and C: N ratio. | [99] |
| Washington Navel Orange Citrus sinensis (L.) Osbeck | Chitosan | 2 g/L | Foliar application | Increased the leaf inflorescence and fruit set. | [100] |
| Pomegranate (Punica granatum L.) | Chitosan | Chitosan at 0.5% | Foliar application | Improved the fruit quality and reduced the fruit cracking. | [101] |
| Cherry (Prunus avium L.) | Chitosan coatings | At 2% | Dipping | Reduced the pectin content and increase the firmness of the fruit. | [102] |
| Grapevine (Vitis vinifera L.) | Protein hydrolysates | 1.6 g per litre | Foliar application | Increased the yield per vine and fruit colour and TSS. | [83,103] |
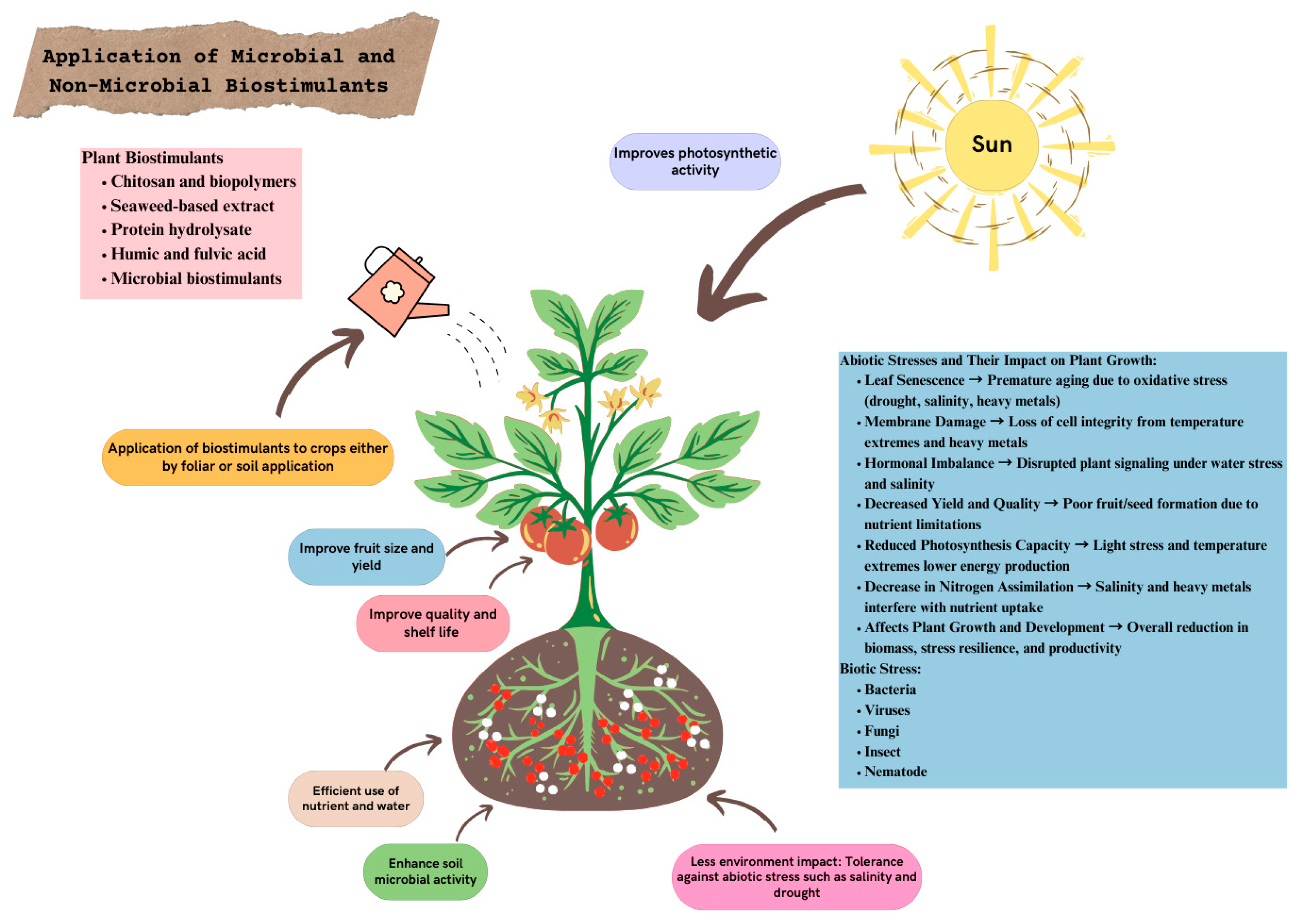
6. Biostimulants as Stress Alleviators in Fruit Crops
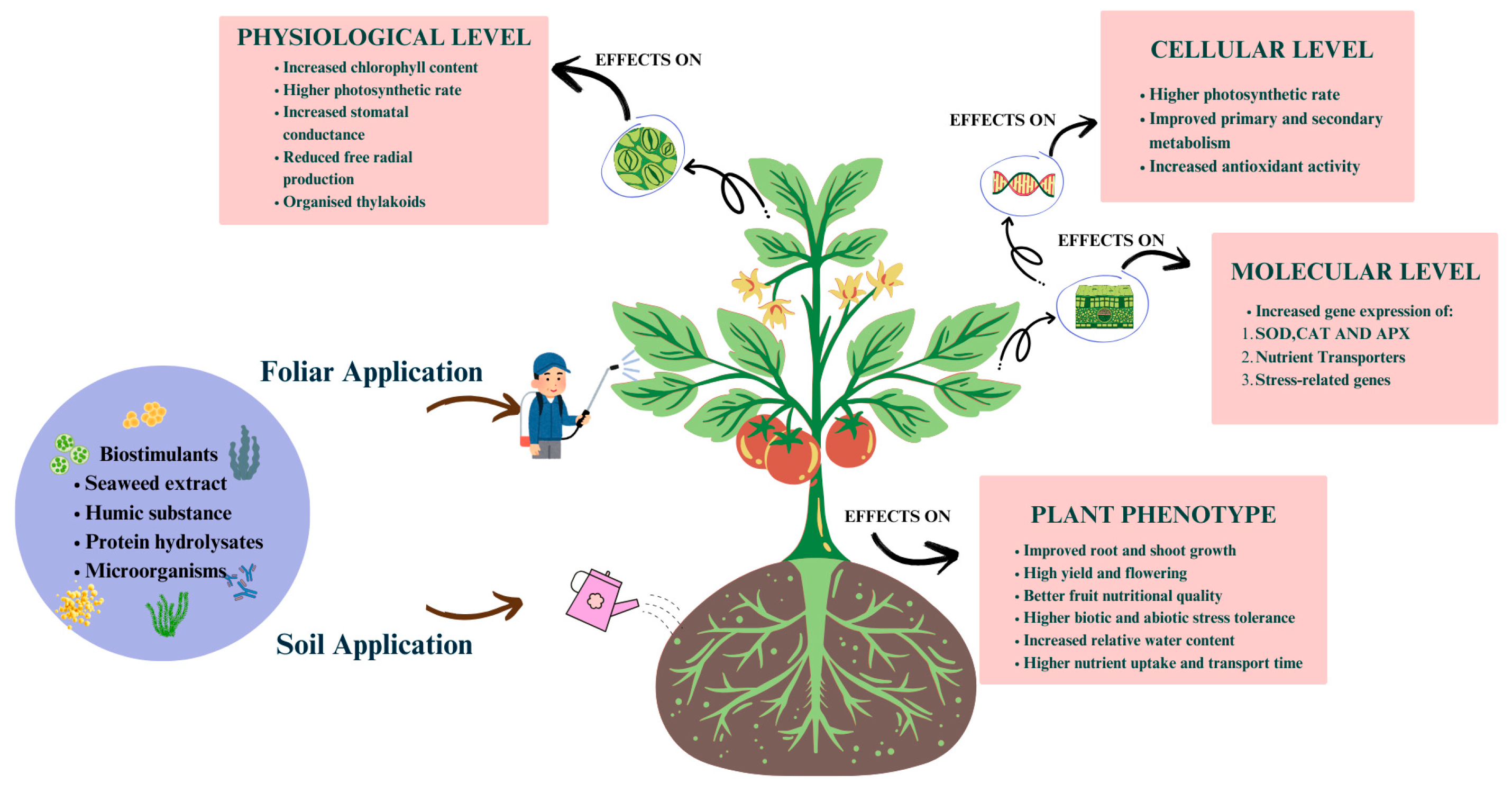
7. Mechanisms of Biostimulants for Stress Tolerance: Molecular and Physiological
7.1. Molecular Mechanisms
7.1.1. Gene Expression Modulation
7.1.2. Antioxidant Defense Activation
7.1.3. Phytohormone Regulation
7.1.4. Metabolic Reconfiguration
7.2. Physiological Mechanisms
7.2.1. Enhanced Nutrient Uptake
7.2.2. Osmotic Adjustment
7.2.3. Stress Acclimation
8. Estimation of Biostimulant Effects on Fruit Crops with the Help of Systematic Techniques
8.1. Enhancing Growth and Yield
8.2. Improving Fruit Quality
8.3. Mitigating Abiotic Stress
| Name of Crop | Biostimulants | Mode of Application | Effects of Biostimulants | References |
|---|---|---|---|---|
| Strawberry (Fragaria × ananassa Duch.) | Chitosan | Foliar | Yield increases 20% | [162] |
| Grape (Vitis vinifera L.) | Chitosan | Dipping | Increases the no. of canes and internodes | [163] |
| Citrus (Citrus spp.) | Sea weed extract | Soil drenching and soil application | Enhances the plant growth | [118,164] |
| Loquat Eriobotrya japonica (Thunb.) Lindl. | AMF | Soil application | Enhances the dry mass of the leaf | [165] |
| (Funneliformis mosseae) | ||||
| Mango (Mangifera indica L.) | Potassium silicate | Drenching | Increases the vegetative and reproductive growth | [166] |
| Tangerine orange (Citrus reticulata) Blanco | Humic acid | Soil application | Increases the plant height, stem diameter | [167] |
| Strawberry (Fragaria × ananassa Duch.) | PGPB B (Bacillus subtili) | Root dipping | Increases the final yield | [168] |
| Apple (Malus domestica Borkh.) | Humic acid | Drenching | Increases the root length | [169] |
| Pear (Pyrus communis L.) | Amino acid | Foliar application | Increases the shoot growth and yield | [170] |
| Almond Prunus dulcis (Mill.) D.A.Webb | Seaweed extract | Foliar spray | Increases the shoot length and shoot biomass | [171] |
| Apricot (Prunus armeniaca L.) | Humic acid | Foliar spray | Increases the vegetative growth and yield | [172] |
9. Biostimulants: Current Challenges, Future Prospects for Sustainable Agriculture
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sible, C.N.; Seebauer, J.R.; Below, F.E. Plant biostimulants: A categorical review, their implications for row crop production, and relation to soil health indicators. Agronomy 2021, 11, 1297. [Google Scholar] [CrossRef]
- Cirillo, V.; Molisso, D.; Aprile, A.M.; Maggio, A.; Rao, R. Systemin peptide application improves tomato salt stress tolerance and reveals common adaptation mechanisms to biotic and abiotic stress in plants. Environ. Exp. Bot. 2022, 199, 104865. [Google Scholar] [CrossRef]
- Ranjan, A.; Sinha, R.; Bala, M.; Pareek, A.; Singla-Pareek, S.L.; Singh, A.K. Silicon-mediated abiotic and biotic stress mitigation in plants: Underlying mechanisms and potential for stress resilient agriculture. Plant Physiol. Biochem. 2021, 163, 15–25. [Google Scholar] [CrossRef]
- Venkateswarlu, B.; Singh, A.K. Climate change adaptation and mitigation strategies in rainfed agriculture. In Climate Change Modelling, Planning and Policy for Agriculture; Singh, A.K., Dagar, J.C., Arunachalam, A.R.G., Shelat, K.N., Eds.; Springer: New Delhi, India, 2015; pp. 1–11. [Google Scholar] [CrossRef]
- Datta, P.; Behera, B.; Rahut, D.B. Climate change and Indian agriculture: A systematic review of farmers’ perception, adaptation, and transformation. Environ. Chall. 2022, 8, 100543. [Google Scholar] [CrossRef]
- Bhupenchandra, I.; Chongtham, S.K.; Devi, E.L.; Choudhary, A.K.; Salam, M.D.; Sahoo, M.R.; Bhutia, T.L.; Devi, S.H.; Thounaojam, A.S.; Behera, C.; et al. Role of biostimulants in mitigating the effects of climate change on crop performance. Front. Plant Sci. 2022, 13, 967665. [Google Scholar] [CrossRef] [PubMed]
- Hatfield, J.L.; Boote, K.J.; Kimball, B.A.; Ziska, L.H.; Izaurralde, R.C.; Ort, D.; Thomson, A.M.; Wolfe, D. Climate impacts on agriculture: Implications for crop production. Agron. J. 2011, 103, 351–370. [Google Scholar] [CrossRef]
- Hussain, S.B.; Karagiannis, E.; Manzoor, M.; Ziogas, V. From Stress to success: Harnessing technological advancements to overcome climate change impacts in citriculture. Crit. Rev. Plant Sci. 2023, 42, 345–363. [Google Scholar] [CrossRef]
- El-Boray, M.; Mostafa, M.; El-Galel, M.A.; Somaa, I. Effect of humic and fulvic acids with some nutrients at different time of application on yield and fruit quality of Anna apple trees. J. Plant Prod. 2015, 6, 307–321. [Google Scholar] [CrossRef]
- Rouphael, Y.; Colla, G. Biostimulants in Agriculture. Front. Plant Sci. 2020, 11, 40. [Google Scholar] [CrossRef]
- Du Jardin, P. Plant biostimulants: Definition, concept, main categories and regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef]
- Balfagón, D.; Gómez-Cadenas, A.; Rambla, J.L.; Granell, A.; de Ollas, C.; Bassham, D.C.; Mittler, R.; Zandalinas, S.I. γ-Aminobutyric acid plays a key role in plant acclimation to a combination of high light and heat stress. Plant Physiol. 2022, 188, 2026–2038. [Google Scholar] [CrossRef] [PubMed]
- Grusak, M.A.; Broadley, M.R.; White, P.J. Plant Macro-and micronutrient minerals. eLS 2016. [Google Scholar] [CrossRef]
- Araújo, W.B.S.; Teixeira, G.C.M.; Prado, R.d.M.; Rocha, A.M.S. Silicon mitigates nutritional stress of nitrogen, phosphorus, and calcium deficiency in two forages plants. Sci. Rep. 2022, 12, 6611. [Google Scholar] [CrossRef]
- Khan, S.-A.; Li, M.-Z.; Wang, S.-M.; Yin, H.-J. Revisiting the role of plant transcription factors in the battle against abiotic stress. Int. J. Mol. Sci. 2018, 19, 1634. [Google Scholar] [CrossRef]
- Azmat, A.; Tanveer, Y.; Yasmin, H.; Hassan, M.N.; Shahzad, A.; Reddy, M.; Ahmad, A. Coactive role of zinc oxide nanoparticles and plant growth promoting rhizobacteria for mitigation of synchronized effects of heat and drought stress in wheat plants. Chemosphere 2022, 297, 133982. [Google Scholar] [CrossRef]
- Ismail, L.M.; Soliman, M.I.; El-Aziz, M.H.A.; Abdel-Aziz, H.M.M. Impact of silica ions and nano silica on growth and productivity of pea plants under salinity stress. Plants 2022, 11, 494. [Google Scholar] [CrossRef] [PubMed]
- El-Badri, A.M.; Hashem, A.M.; Batool, M.; Sherif, A.; Nishawy, E.; Ayaad, M.; Hassan, H.M.; Elrewainy, I.M.; Wang, J.; Kuai, J.; et al. Comparative efficacy of bio-selenium nanoparticles and sodium selenite on morpho-physiochemical attributes under normal and salt stress conditions, besides selenium detoxification pathways in Brassica napus L. J. Nanobiotechnol. 2022, 20, 163. [Google Scholar] [CrossRef] [PubMed]
- Pawar, V.A.; Laware, S.L. Seed priming a critical review. Int. J. Sci. Res. Biol. Sci. 2018, 5, 94–101. [Google Scholar] [CrossRef]
- Rajora, N.; Vats, S.; Raturi, G.; Thakral, V.; Kaur, S.; Rachappanavar, V.; Kumar, M.; Kesarwani, A.K.; Sonah, H.; Sharma, T.R.; et al. Seed priming with melatonin: A promising approach to combat abiotic stress in plants. Plant Stress 2022, 4, 100071. [Google Scholar] [CrossRef]
- Chojnacka, K. Innovative bio-products for agriculture. Open Chem. 2015, 12, 932–993. [Google Scholar] [CrossRef]
- Li, J.; Van Gerrewey, T.; Geelen, D. A meta-analysis of biostimulant yield effectiveness in field trials. Front. Plant Sci. 2022, 13, 836702. [Google Scholar] [CrossRef] [PubMed]
- Singh, I.; Anand, K.V.; Solomon, S.; Shukla, S.K.; Rai, R.; Zodape, S.T.; Ghosh, A. Can we not mitigate climate change using seaweed based biostimulant: A case study with sugarcane cultivation in India. J. Clean. Prod. 2018, 204, 992–1003. [Google Scholar] [CrossRef]
- Patil, H.J.; Solanki, M.K. Microbial Inoculant: Modern Era of Fertilizers and Pesticides. In Microbial Inoculants in Sustainable Agricultural Productivity; Springer: New Delhi, India, 2016; Volume 1, pp. 319–343. [Google Scholar]
- Zulfiqar, F.; Navarro, M.; Ashraf, M.; Akram, N.A.; Munné-Bosch, S. Nanofertilizer use for sustainable agriculture: Advantages and limitations. Plant Sci. 2019, 289, 110270. [Google Scholar] [CrossRef]
- Bhupenchandra, I.; Devi, S.H.; Basumatary, A.; Dutta, S.; Singh, L.K.; Kalita, P.; Bora, S.S.; Devi, R.S.; Saikia, A.; Sharma, P.; et al. Biostimulants: Potential and prospects in agriculture. Int. Res. J. Pure Appl. Chem. 2020, 21, 20–35. [Google Scholar] [CrossRef]
- Khurana, A.; Kumar, V. State of Biofertilizers and Organic Fertilizers in India; Centre for Science and Environment: New Delhi, India, 2022. [Google Scholar]
- Jiang, Y.; Yue, Y.; Wang, Z.; Lu, C.; Yin, Z.; Li, Y.; Ding, X. Plant Biostimulant as an Environmentally Friendly Alternative to Modern Agriculture. J. Agric. Food Chem. 2024, 72, 5107–5121. [Google Scholar] [CrossRef] [PubMed]
- Rathor, P.; Gorim, L.Y.; Thilakarathna, M.S. Plant physiological and molecular responses triggered by humic based biostimulants—A way forward to sustainable agriculture. Plant Soil 2023, 492, 31–60. [Google Scholar] [CrossRef]
- Ali, O.; Ramsubhag, A.; Jayaraman, J. Biostimulant properties of seaweed extracts in plants: Implications towards sustainable crop production. Plants 2021, 10, 531. [Google Scholar] [CrossRef]
- Abdullahi, U.A.; Khandaker, M.M.; Alias, N.; Shaari, E.M.; Alam, A.; Badaluddin, N.A.; Mohd, K.S. Seaweed effects on plant growth and environmental remediation: A review. J. Phytol. 2021, 13, 122–129. [Google Scholar] [CrossRef]
- Battacharyya, D.; Babgohari, M.Z.; Rathor, P.; Prithiviraj, B. Seaweed extracts as biostimulants in horticulture. Sci. Hortic. 2015, 196, 39–48. [Google Scholar] [CrossRef]
- Rakkammal, K.; Maharajan, T.; Ceasar, S.A.; Ramesh, M. Biostimulants and their role in improving plant growth under drought and salinity. Cereal Res. Commun. 2023, 51, 61–74. [Google Scholar] [CrossRef]
- Paul, K.; Sorrentino, M.; Lucini, L.; Rouphael, Y.; Cardarelli, M.; Bonini, P.; Reynaud, H.; Canaguier, R.; Trtílek, M.; Panzarová, K.; et al. Understanding the biostimulant action of vegetal-derived protein hydrolysates by high-throughput plant phenotyping and metabolomics: A case study on tomato. Front. Plant Sci. 2019, 10, 47. [Google Scholar] [CrossRef] [PubMed]
- Agliassa, C.; Mannino, G.; Molino, D.; Cavalletto, S.; Contartese, V.; Bertea, C.M.; Secchi, F. A new protein hydrolysate-based biostimulant applied by fertigation promotes relief from drought stress in Capsicum annuum L. Plant Physiol. Biochem. 2021, 166, 1076–1086. [Google Scholar] [CrossRef] [PubMed]
- Nardi, S.; Pizzeghello, D.; Schiavon, M.; Ertani, A. Plant biostimulants: Physiological responses induced by protein hydrolyzed-based products and humic substances in plant metabolism. Sci. Agric. 2016, 73, 18–23. [Google Scholar] [CrossRef]
- Al-Khayri, J.M.; Abdel-Haleem, M.; Khedr, E.H. Harnessing GABA pathways to improve plant resilience against salt stress. Horticulturae 2024, 10, 1296. [Google Scholar] [CrossRef]
- Kaspal, M.; Kanapaddalagamage, M.H.; Ramesh, S.A. Emerging roles of γ aminobutyric acid (GABA) gated channels in plant stress tolerance. Plants 2021, 10, 2178. [Google Scholar] [CrossRef]
- Abdel Razik, E.S.; Alharbi, B.M.; Pirzadah, T.B.; Alnusairi, G.S.; Soliman, M.H.; Hakeem, K.R. γ-Aminobutyric acid (GABA) mitigates drought and heat stress in sunflower (Helianthus annuus L.) by regulating its physiological, biochemical and molecular pathways. Physiol. Plant. 2021, 172, 505–527. [Google Scholar] [CrossRef]
- Hu, L.; Wang, X.; Zou, Y.; Wu, D.; Gao, G.; Zhong, Z.; Liu, Y.; Hu, S.; Fan, H.; Zhang, B. Effects of inorganic and organic selenium intervention on resistance of radish to arsenic stress. Ital. J. Food Sci. 2022, 34, 44–58. [Google Scholar] [CrossRef]
- Chaudhary, S.; Kumar, S.; Kumar, V.; Sharma, R. Chitosan nanoemulsions as advanced edible coatings for fruits and vegetables: Composition, fabrication and developments in last decade. Int. J. Biol. Macromol. 2020, 152, 154–170. [Google Scholar] [CrossRef]
- Chaudhary, S.; Kumar, V.; Sharma, V.; Sharma, R.; Kumar, S. Chitosan nanoemulsion: Gleam into the futuristic approach for preserving the quality of muscle foods. Int. J. Biol. Macromol. 2021, 250, 126187. [Google Scholar] [CrossRef]
- Backer, R.; Rokem, J.S.; Ilangumaran, G.; Lamont, J.; Praslickova, D.; Ricci, E.; Subramanian, S.; Smith, D.L. Plant growth-promoting rhizobacteria: Context, mechanisms of action, and roadmap to commercialization of biostimulants for sustainable agriculture. Front. Plant Sci. 2018, 9, 1473. [Google Scholar] [CrossRef]
- Vasseur-Coronado, M.; du Boulois, H.D.; Pertot, I.; Puopolo, G. Selection of plant growth promoting rhizobacteria sharing suitable features to be commercially developed as biostimulant products. Microbiol. Res. 2021, 245, 126672. [Google Scholar] [CrossRef]
- Crovadore, J.; Cochard, B.; Grizard, D.; Chablais, R.; Baillarguet, M.; Comby, M.; Lefort, F. Draft genome sequence of Bacillus licheniformis strain UASWS1606, a plant biostimulant for agriculture. Genome Announc. 2020, 9, e00740-20. [Google Scholar] [CrossRef]
- García-García, A.L.; García-Machado, F.J.; Borges, A.A.; Morales-Sierra, S.; Boto, A.; Jiménez-Arias, D. Pure Organic Active Compounds Against Abiotic Stress: A Biostimulant Overview. Front. Plant Sci. 2020, 11, 575829. [Google Scholar] [CrossRef] [PubMed]
- Roy, D. Role of Biostimulants towards Sustainable Agriculture: A Review. Food Sci. Rep. 2024, 5, 47–52. [Google Scholar]
- Etemadian, Y.; Ghaemi, V.; Shaviklo, A.R.; Pourashouri, P.; Mahoonak, A.R.S.; Rafipour, F. Development of animal/plant-based protein hydrolysate and its application in food, feed and nutraceutical industries: State of the art. J. Clean. Prod. 2020, 278, 123219. [Google Scholar] [CrossRef]
- Sedlar, T.; Cakarevic, J.; Tomic, J.; Popovic, L. Vegetable byproducts as new sources of functional proteins. Plant Foods Hum. Nutr. 2021, 76, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Mikula, K.; Konieczka, M.; Taf, R.; Skrzypczak, D.; Izydorczyk, G.; Moustakas, K.; Kulazynski, M.; Chojnacka, K.; Witek-Krowiak, A. Tannery waste as a renewable source of nitrogen for production of multicomponent fertilizers with biostimulating properties. Environ. Sci. Pollut. Res. Int. 2023, 30, 8759–8777. [Google Scholar] [CrossRef]
- Yang, L.; Wen, K.-S.; Ruan, X.; Zhao, Y.-X.; Wei, F.; Wang, Q. Response of plant secondary metabolites to environmental factors. Molecules 2018, 23, 762. [Google Scholar] [CrossRef]
- Noman, A.; Aqeel, M.; Qasim, M.; Haider, I.; Lou, Y. Plant-insect-microbe interaction: A love triangle between enemies in ecosystem. Sci. Total. Environ. 2020, 699, 134181. [Google Scholar] [CrossRef]
- Shahrajabian, M.H.; Chaski, C.; Polyzos, N.; Tzortzakis, N.; Petropoulos, S.A. Sustainable agriculture systems in vegetable production using chitin and chitosan as plant biostimulants. Biomolecules 2021, 11, 819. [Google Scholar] [CrossRef]
- Stanley-Raja, V.; Senthil-Nathan, S.; Chanthini, K.M.; Sivanesh, H.; Ramasubramanian, R.; Karthi, S.; Shyam-Sundar, N.; Vasantha-Srinivasan, P.; Kalaivani, K. Biological activity of Chitosan inducing resistance efficiency of rice (Oryza sativa L.) after treatment with fungal based chitosan. Sci. Rep. 2021, 11, 20488. [Google Scholar] [CrossRef] [PubMed]
- Jindo, K.; Martim, S.A.; Navarro, E.C.; Pérez-Alfocea, F.; Hernandez, T.; Garcia, C.; Aguiar, N.O.; Canellas, L.P. Root growth promotion by humic acids from composted and non-composted urban organic wastes. Plant Soil 2011, 353, 209–220. [Google Scholar] [CrossRef]
- Huang, S.; Ma, Q.; Hou, Q.; Zuo, T.; Zhang, Z.; Ni, W. Identification and quantitative chemical analysis of betaines in different organic wastes and their bioconversion composts. Bioresour. Technol. 2021, 328, 124857. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Zheng, X.; Luo, L.; Ni, Y.; Yao, L.; Ni, W. Biostimulants in bioconversion compost of organic waste: A novel booster in sustainable agriculture. J. Clean. Prod. 2021, 319, 128704. [Google Scholar] [CrossRef]
- Magnabosco, P.; Masi, A.; Shukla, R.; Bansal, V.; Carletti, P. Advancing the Impact of Plant Biostimulants to Sustainable Agriculture through Nanotechnologies. Chem. Biol. Technol. Agric. 2023, 10, 117. [Google Scholar] [CrossRef]
- Drobek, M.; Frąc, M.; Cybulska, J. Plant Biostimulants: Importance of the quality and yield of horticultural crops and the improvement of plant tolerance to abiotic stress—A review. Agronomy 2019, 9, 335. [Google Scholar] [CrossRef]
- Osman, H.S.; Rady, A.M.; Awadalla, A.; Omara, A.E.-D.; Hafez, E.M. Improving the Antioxidants System, Growth, and Sugar Beet Quality Subjected to Long-Term Osmotic Stress by Phosphate Solubilizing Bacteria and Compost Tea. Int. J. Plant Prod. 2021, 16, 119–135. [Google Scholar] [CrossRef]
- Kisvarga, S.; Barna, D.; Kovács, S.; Csatári, G.; Tóth, I.O.; Fári, M.G.; Makleit, P.; Veres, S.; Alshaal, T.; Bákonyi, N. Fermented Alfalfa Brown Juice Significantly Stimulates the Growth and Development of Sweet Basil (Ocimum basilicum L.). Plants. Agron. 2020, 10, 657. [Google Scholar] [CrossRef]
- Kaur, M.; Bhari, R.; Singh, R.S. Chicken feather waste-derived protein hydrolysate as a potential biostimulant for cultivation of mung beans. Biologia 2021, 76, 1807–1815. [Google Scholar] [CrossRef]
- Kok, A.D.X.; Wan Abdullah, W.M.A.N.; Tang, C.N.; Low, L.Y.; Yuswan, M.H.; Ong-Abdullah, J.; Tan, N.P.; Lai, K.S. Sodium lig-nosulfonate improves shoot growth of Oryza sativa via enhancement of photosynthetic activity and reduced accumulation of reactive oxygen species. Sci. Rep. 2021, 11, 13226. [Google Scholar] [CrossRef]
- Raguraj, S.; Kasim, S.; Jaafar, N.M.; Nazli, M.H.; Amali, R.K.A. A comparative study of tea waste derived humic-like substances with lignite-derived humic substances on chemical composition, spectroscopic properties and biological activity. Environ. Sci. Pollut. Res. Int. 2022, 29, 60631–60640. [Google Scholar] [CrossRef] [PubMed]
- Yoruklu, H.C.; Ozkaya, B.; Demir, A. Optimization of liquid fertilizer production from waste seaweed: A design of experiment based statistical approach. Chemosphere 2022, 286, 131885. [Google Scholar] [CrossRef] [PubMed]
- López-Corona, B.E.; Mondaca-Fernández, I.; Gortáres-Moroyoqui, P.; Meza-Montenegro, M.M.; Balderas-Cortés, J.d.J.; Ruiz-Alvarado, C.; Rueda-Puente, E.O. Ecofisiología y bioquímica de Salicornia bigelovii (Torr.) Por efecto de quitosano-aib bajo condiciones del desierto de Sonora. Polibot’ Anica 2020, 49, 75–92. [Google Scholar] [CrossRef]
- Aghaeifard, F.; Booshehri, A.A.; Shirani, H.; Tehranifar, A. Influence of humic acid and salicylic acid on fruit quality, yield, and nutrient uptake in strawberry. Sci. Hortic. 2016, 211, 153–158. [Google Scholar] [CrossRef]
- Rana, V.S.; Lingwal, K.; Sharma, S.; Rana, N.; Pawar, R.; Kumar, V.; Sharma, U. Enhancement in growth, yield and nutritive characteristics of strawberry (Fragaria × ananassa Duch.) by the application of biostimulant: Seaweed extract. Acta Physiol. Plant. 2023, 45, 122. [Google Scholar] [CrossRef]
- Soppelsa, S.; Kelderer, M.; Casera, C.; Bassi, M.; Robatscher, P.; Matteazzi, A.; Andreotti, C. Foliar applications of biostimulants promote growth, yield and fruit quality of strawberry plants grown under nutrient limitation. Agronomy 2019, 9, 483. [Google Scholar] [CrossRef]
- Flores-Félix, J.D.; Silva, L.R.; Rivera, L.P.; Marcos-García, M.; García-Fraile, P.; Martínez-Molina, E.; Mateos, P.F.; Velázquez, E.; Andrade, P.; Rivas, R. Plants probiotics as a tool to produce highly functional fruits: The case of Phyllobacterium and Vitamin C in strawberries. PLoS ONE 2015, 10, e0122281. [Google Scholar] [CrossRef]
- Malaguti, D.; Matassi, G.; Guidi, B. Seaweed extract effect on apple tree growth and fruit color. J. Plant Nutr. 2002, 25, 1333–1347. [Google Scholar] [CrossRef]
- Soppelsa, S.; Kelderer, M.; Casera, C.; Bassi, M.; Robatscher, P.; Andreotti, C. Use of biostimulants for organic apple production: Effects on tree growth, yield, and fruit quality at harvest and during storage. Front. Plant Sci. 2018, 9, 1342. [Google Scholar] [CrossRef]
- Yang, S.; Wang, H.; Wang, G.; Wang, J.; Gu, A.; Xue, X.; Chen, R. Effects of Seaweed-Extract-Based Organic Fertilizers on the Levels of Mineral Elements, Sugar–Acid Components and Hormones in Fuji Apples. Agronomy 2023, 13, 969. [Google Scholar] [CrossRef]
- Karlidag, H.; Esitken, A.; Yildirim, E.; Donmez, M.F.; Turan, M. Effects of root inoculation of plant growth promoting rhizobacteria (PGPR) on yield, growth, and nutrient uptake in apple. Sci. Hortic. 2007, 114, 16–20. [Google Scholar] [CrossRef]
- Al-Saif, A.M.; Sas-Paszt, L.; Awad, R.M.; Mosa, W.F. Apricot (Prunus armeniaca) performance under foliar application of humic acid, brassinosteroids, and seaweed extract. Horticulturae 2023, 9, 519. [Google Scholar] [CrossRef]
- Kavino, M.; Harish, S.; Kumar, N.; Saravanakumar, D.; Damodaran, T.; Soorianathasundaram, K. Impact of grafting on product quality of fruit vegetables. Sci. Hortic. 2010, 127, 172–179. [Google Scholar] [CrossRef]
- Garza-Alonso, C.A.; Olivares-Sáenz, E.; González-Morales, S.; la Fuente, M.C.-D.; Juárez-Maldonado, A.; González-Fuentes, J.A.; Tortella, G.; Valdés-Caballero, M.V.; Benavides-Mendoza, A. Strawberry biostimulation: From mechanisms of action to plant growth and fruit quality. Plants 2022, 11, 3463. [Google Scholar] [CrossRef]
- Popescu, G.; Popescu, M. Humic acids improve fruit yield and quality in grapevines. J. Appl. Bot. 2018, 92, 321–328. [Google Scholar]
- Ferrara, G.; Brunetti, G. Foliar applications of humic acids in grapevine to improve production and quality. Agron. J. 2010, 102, 151–157. [Google Scholar] [CrossRef]
- Parrado, J.; Escobar, I.; Benítez, C.; Friaza, V.; García-Martínez, A. Chemical characterization of humic acids and their effect on red wine quality. Food Chem. 2007, 101, 1012–1018. [Google Scholar] [CrossRef]
- Frioni, T.; Tombesi, S.; Silvestroni, O.; Lanari, V.; Bellincontro, A.; Palliotti, A. Seaweed extracts in viticulture: Effects on red grape quality and anthocyanins content. J. Sci. Food Agric. 2018, 98, 3744–3750. [Google Scholar] [CrossRef]
- Malhotra, S.K. Horticultural crops and climate change: A review. Indian J. Agric. Sci. 2017, 87, 12–22. [Google Scholar] [CrossRef]
- Boselli, M.; Bahouaoui, M.; Lachhab, N.; Sanzani, S.; Ferrara, G.; Ippolito, A. Protein hydrolysates effects on grapevine (Vitis vinifera L., cv. Corvina) performance and water stress tolerance. Sci. Hortic. 2019, 258, 108784. [Google Scholar] [CrossRef]
- Monteiro, E.; Gonçalves, B.; Cortez, I.; Castro, I. The role of biostimulants as alleviators of biotic and abiotic stresses in grapevine: A review. Plants 2022, 11, 396. [Google Scholar] [CrossRef] [PubMed]
- Garde-Cerdán, T.; Rubio-Bretón, P.; Arias-Gil, M.; Martínez-Cutillas, A. Phenylalanine sprays increase wine phenolic compounds and aroma precursors. Food Res. Int. 2014, 62, 684–691. [Google Scholar] [CrossRef]
- Esitken, A.; Pirlak, L.; Turan, M.; Sahin, F. Effects of floral and foliar application of plant growth promoting rhizobacteria (PGPR) on yield, growth and nutrition of sweet cherry. Sci. Hortic. 2006, 110, 324–327. [Google Scholar] [CrossRef]
- Vercammen, J.; Soppelsa, S.; Robatscher, P.; Andreotti, C. Effect of seaweed extract on fruit quality of sweet cherry. Hortic. Sci. 2008, 45, 153–160. [Google Scholar]
- Kaiser, N.; Matthys, C.; Andreotti, C. Potassium silicate in cherry cultivation: Fruit firmness and quality improvements. Acta Hortic. 2014, 1028, 97–105. [Google Scholar] [CrossRef]
- Yang, G.; Wang, Y.; Wang, S.; Zhao, X. Drilling of Super Large Granular Slow-Release Humic Acid Compound Fertilizer Improves Simultaneously Environmental and Economic Benefits in Peach Orchard. Agric. Ecosyst. Environ. 2023, 348, 108437. [Google Scholar] [CrossRef]
- Colavita, G.M.; Sottile, F.; Di Lorenzo, R.; Pisciotta, A. Influence of foliar sprays of seaweed extracts on yield and quality of plums. Acta Hortic. 2011, 901, 281–288. [Google Scholar] [CrossRef]
- Vázquez-Hernández, M.C.; Olalde-Portugal, V.; López-Jiménez, A.; Herrera-Estrella, A. Effect of AMF on yield and quality of papaya fruits. J. Hortic. Sci. Biotechnol. 2011, 86, 573–578. [Google Scholar]
- Chouliaras, V.; Bantis, F.; Kormas, I. Seaweed extract effects on oil content and maturation rate in olive trees. Sci. Hortic. 2009, 120, 452–456. [Google Scholar] [CrossRef]
- Manyaku, A.; Witbooi, H.; Laubscher, C.P. The Significance of Organic Horticulture in Mitigating Climate Change and Promoting the Production of Healthier Fruits and Vegetables. Appl. Sci. 2024, 14, 4966. [Google Scholar] [CrossRef]
- Rostami, M.; Shokouhian, A.; Mohebodini, M. Effect of humic acid, nitrogen concentrations and application method on the morphological, yield and biochemical characteristics of strawberry ‘Paros’. Int. J. Fruit Sci. 2022, 22, 203–214. [Google Scholar] [CrossRef]
- Nargesi, M.M.; Sedaghathoor, S.; Hashemabadi, D. Effect of foliar application of amino acid, humic acid and fulvic acid on the oil content and quality of olive. Saudi J. Biol. Sci. 2022, 29, 3473–3481. [Google Scholar] [CrossRef]
- Taskos, D.; Stamatiadis, S.; Yvin, J.-C.; Jamois, F. Effects of an Ascophyllum nodosum (L.) Le Jol. extract on grapevine yield and berry composition of a Merlot vineyard. Sci. Hortic. 2019, 250, 27–32. [Google Scholar] [CrossRef]
- Al-Shatri, A.H.N.; Pakyürek, M.; Yaviç, A. Effect of Seaweed application on the vegetative growth of strawberry cv. Albion grown under Iraq ecological conditions. Appl. Ecol. Environ. Res. 2020, 18, 1211–1225. [Google Scholar] [CrossRef]
- Al-Marsoumi, F.H.; Al-Hadethi, M.E. Efect of humic acid and seaweed extract spray in leaf mineral content of mango seedlings. Plant Archiv. 2020, 20, 827–830. [Google Scholar]
- El-Sese, A.M.; Mohamed, A.K.A.; Abou-Zaid, E.A.; Abd-El-Ghany, A.M.M. Impact of foliar application with seaweed extract, amino acids and vitamins on yield and berry quality of some Grapevine cultivars. SVU-Int. J. Agric. Sci. 2020, 2, 73–84. [Google Scholar] [CrossRef]
- Mohamed, S.A.; Ahmed, H.S. Study effect of Chitosan and gibberellic acid on growth, flowering, fruit set, yield and fruit quality of Washington navel orange trees. Mid. East J. 2019, 8, 255–267. [Google Scholar]
- Soleimanzadeh, A.; Mizani, S.; Mirzaei, G.; Bavarsad, E.T.; Farhoodi, M.; Esfandiari, Z.; Rostami, M. Recent advances in characterizing the physical and functional properties of active packaging films containing pomegranate peel. Food Chem. X 2024, 22, 101416. [Google Scholar] [CrossRef]
- Xin, Y.; Jin, Z.; Chen, F.; Lai, S.; Yang, H. Effect of chitosan coatings on the evolution of sodium carbonate-soluble pectin during sweet cherry softening under non-isothermal conditions. Int. J. Biol. Macromol. 2020, 154, 267–275. [Google Scholar] [CrossRef]
- Colla, G.; Nardi, S.; Cardarelli, M.; Ertani, A.; Lucini, L.; Canaguier, R.; Rouphael, Y. Protein Hydrolysates as Biostimulants in Horticulture. Sci. Hortic. 2015, 196, 28–38. [Google Scholar] [CrossRef]
- Sangiorgio, D.; Cellini, A.; Donati, I.; Pastore, C.; Onofrietti, C.; Spinelli, F. Facing climate change: Application of microbial biostimulants to mitigate stress in horticultural crops. Agronomy 2020, 10, 794. [Google Scholar] [CrossRef]
- Asif, A.; Ali, M.; Qadir, M.; Karthikeyan, R.; Singh, Z.; Khangura, R.; Di Gioia, F.; Ahmed, Z.F.R. Enhancing crop resilience by harnessing the synergistic effects of biostimulants against abiotic stress. Front. Plant Sci. 2023, 14, 1276117. [Google Scholar] [CrossRef]
- Rana, V.S.; Sharma, S.; Rana, N.; Sharma, U. Sustainable production through biostimulants under fruit orchards. CABI Agric. Biosci. 2022, 3, 38. [Google Scholar] [CrossRef]
- Andreotti, C.; Rouphael, Y.; Colla, G.; Basile, B. Rate and timing of application of biostimulant substances to enhance fruit tree tolerance toward environmental stresses and fruit quality. Agronomy 2022, 12, 603. [Google Scholar] [CrossRef]
- Franzoni, G.; Cocetta, G.; Prinsi, B.; Ferrante, A.; Espen, L. Biostimulants on crops: Their impact under abiotic stress conditions. Horticulturae 2022, 8, 189. [Google Scholar] [CrossRef]
- Rojas-Rodríguez, M.L.; Ramírez-Gil, J.G.; González-Concha, L.F.; Balaguera-López, H.E. Biostimulants Improve Yield and Quality in Preharvest without Impinging on the Postharvest Quality of Hass Avocado and Mango Fruit: Evaluation under Organic and Traditional Systems. Agronomy 2023, 13, 1917. [Google Scholar] [CrossRef]
- Karaca, U.; Sabir, A. Sustainable mitigation of alkaline stress in grapevine rootstocks (Vitis spp.) by plant growth-promoting rhizobacteria. Erwerbs-Obstbau 2017, 60, 211–220. [Google Scholar] [CrossRef]
- Soltaniband, V.; Brégard, A.; Gaudreau, L.; Dorais, M. Biostimulants promote plant development, crop productivity, and fruit quality of protected strawberries. Agronomy 2022, 12, 1684. [Google Scholar] [CrossRef]
- Ziogas, V.; Bravos, N.; Hussain, S.B. Preharvest foliar application of Si–Ca-based biostimulant affects postharvest quality and shelf-life of clementine mandarin (Citrus clementina Hort. Ex Tan). Horticulturae 2022, 8, 996. [Google Scholar] [CrossRef]
- Świerczyński, S.; Antonowicz, A.; Bykowska, J. The effect of the foliar application of biostimulants and fertilisers on the growth and physiological parameters of maiden apple trees cultivated with limited mineral fertilisation. Agronomy 2021, 11, 1216. [Google Scholar] [CrossRef]
- Harhash, M.M.; Saad, R.M.; Mosa, W.F.A. Response of ”wonderful” pomegranate cultivar to the foliar application of some biostimulants. Plant Arch. 2021, 21, 09725210. [Google Scholar]
- Bodin, E.; Bellée, A.; Dufour, M.-C.; André, O.; Corio-Costet, M.-F. Grapevine Stimulation: A Multidisciplinary Approach to Investigate the Effects of Biostimulants and a Plant Defense Stimulator. J. Agric. Food Chem. 2020, 68, 15085–15096. [Google Scholar] [CrossRef]
- Spann, T.M.; Little, H.A. Applications of a Commercial Extract of the Brown Seaweed Ascophyllum nodosum Increases Drought Tolerance in Container-grown ‘Hamlin’ Sweet Orange Nursery Trees. HortScience 2011, 46, 577–582. [Google Scholar] [CrossRef]
- Samuels, L.J.; Setati, M.E.; Blancquaert, E.H. Towards a better understanding of the potential benefits of seaweed based biostimulants in Vitis vinifera L. cultivars. Plants 2022, 11, 348. [Google Scholar] [CrossRef] [PubMed]
- Pavlou, A.; Efstathiou, E.; Christodoulou, S.; Stylianou, S.; Manganaris, G. The effect of preharvest applications with biostimulants on qualitative properties and postharvest performance of loquat fruit. Acta Hortic. 2022, 129–136. [Google Scholar] [CrossRef]
- Dong, C.; Wang, G.; Du, M.; Niu, C.; Zhang, P.; Zhang, X.; Ma, D.; Ma, F.; Bao, Z. Biostimulants promote plant vigor of tomato and strawberry after transplanting. Sci. Hortic. 2020, 267, 109355. [Google Scholar] [CrossRef]
- Kiczorowski, P. Influence of NPK minerals and biostimulants on the growth, yield, and fruit nutritional value in cv. ’šampion’ apple trees growing on different rootstocks. Acta Sci. Pol. Hortorum Cultus 2019, 18, 197–205. [Google Scholar] [CrossRef]
- Irani, H.; ValizadehKaji, B.; Naeini, M.R. Biostimulant-induced drought tolerance in grapevine is associated with physiological and biochemical changes. Chem. Biol. Technol. Agric. 2021, 8, 5. [Google Scholar] [CrossRef]
- Yaghubi, K.; Ghaderi, N.; Vafaee, Y.; Javadi, T. Potassium silicate alleviates deleterious effects of salinity on two strawberry cultivars grown under soilless pot culture. Sci. Hortic. 2016, 213, 87–95. [Google Scholar] [CrossRef]
- Zouari, I.; Mechri, B.; Tekaya, M.; Dabbaghi, O.; Cheraief, I.; Mguidiche, A.; Annabi, K.; Laabidi, F.; Attia, F.; Hammami, M.; et al. Olive oil quality influenced by biostimulant foliar fertilizers. Braz. J. Biol. Sci. 2020, 7, 3–18. [Google Scholar] [CrossRef]
- Shereni, C. Use of Biostimulants as an Alternate Approach to Achieve Plant Performance and Fruit Quality. Ph.D. Thesis, Stellenbosch University, Western Cape, South Africa, 2019. [Google Scholar]
- Hidangmayum, A.; Dwivedi, P.; Katiyar, D.; Hemantaranjan, A. Application of chitosan on plant responses with special reference to abiotic stress. Physiol. Mol. Biol. Plants 2019, 25, 313–326. [Google Scholar] [CrossRef]
- Mrid, R.B.; Benmrid, B.; Hafsa, J.; Boukcim, H.; Sobeh, M.; Yasri, A. Secondary metabolites as biostimulant and bioprotectant agents: A review. Sci. Total Environ. 2021, 777, 146204. [Google Scholar] [CrossRef]
- Fleming, T.R.; Fleming, C.C.; Levy, C.C.B.; Repiso, C.; Hennequart, F.; Nolasco, J.B.; Liu, F. Biostimulants enhance growth and drought tolerance in Arabidopsis thaliana and exhibit chemical priming action. Ann. Appl. Biol. 2019, 174, 153–165. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, J.; Gong, Z.; Zhu, J. Abiotic Stress Responses in Plants. Nat. Rev. Genet. 2021, 23, 104–119. [Google Scholar] [CrossRef] [PubMed]
- Poveda, J. Cyanobacteria in plant health: Biological strategy against abiotic and biotic stresses. Crop. Prot. 2021, 141, 105450. [Google Scholar] [CrossRef]
- Nephali, L.; Piater, L.A.; Dubery, I.A.; Patterson, V.; Huyser, J.; Burgess, K.; Tugizimana, F. Biostimulants for plant growth and mitigation of abiotic stresses: A metabolomics perspective. Metabolites 2020, 10, 505. [Google Scholar] [CrossRef]
- Kaushal, P.; Ali, N.; Saini, S.; Pati, P.K.; Pati, A.M. Physiological and molecular insight of microbial biostimulants for sustainable agriculture. Front. Plant Sci. 2023, 14, 1041413. [Google Scholar] [CrossRef]
- Lahlali, R.; Ezrari, S.; Radouane, N.; Belabess, Z.; Jiang, Y.; Mokrini, F.; Tahiri, A.; Peng, G. Bacillus spp.-Mediated Drought Stress Tolerance in Plants: Current and Future Prospects. In Bacilli in Climate Resilient Agriculture and Bioprospecting; Springer: Berlin/Heidelberg, Germany, 2022; pp. 487–518. [Google Scholar] [CrossRef]
- Balmer, A.; Pastor, V.; Glauser, G.; Mauch-Mani, B. Tricarboxylates induce defense priming against bacteria in Arabidopsis thaliana. Front. Plant Sci. 2018, 9, 1221. [Google Scholar] [CrossRef]
- Cha, J.-Y.; Kang, S.-H.; Ali, I.; Lee, S.C.; Ji, M.G.; Jeong, S.Y.; Shin, G.-I.; Kim, M.G.; Jeon, J.-R.; Kim, W.-Y. Humic acid enhances heat stress tolerance via transcriptional activation of Heat-Shock Proteins in Arabidopsis. Sci. Rep. 2020, 10, 15042. [Google Scholar] [CrossRef]
- Laxa, M.; Liebthal, M.; Telman, W.; Chibani, K.; Dietz, K.-J. The role of the plant antioxidant system in drought tolerance. Antioxidants 2019, 8, 94. [Google Scholar] [CrossRef]
- Buqori, D.M.A.I.; Sugiharto, B.; Suherman; Siswoyo, T.A.; Hariyono, K. Mitigating drought stress by application of drought-tolerant Bacillus spp. enhanced root architecture, growth, antioxidant and photosynthetic genes expression in sugarcane. Sci. Rep. 2025, 15, 5259. [Google Scholar] [CrossRef]
- Hou, X.; Kong, Y.; Teng, Z.; Yang, C.; Li, Y.; Zhu, Z. Integrating genes and metabolites: Unraveling mango’s drought resilience mechanisms. BMC Plant Biol. 2024, 24, 208. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.I.R.; Poor, P.; Janda, T. Salicylic Acid: A Versatile Signaling Molecule in Plants. J. Plant Growth Regul. 2022, 41, 1887–1890. [Google Scholar] [CrossRef]
- Reyes-Hernández, S.J.; Zamora-Briseño, J.A.; Cerqueda-García, D.; Castaño, E.; Rodríguez-Zapata, L.C. Alterations in the sap-associated microbiota of Carica papaya in response to drought stress. Symbiosis 2020, 81, 93–100. [Google Scholar] [CrossRef]
- Albasri, H.M.; Mawad, A.M.; Aldaby, E.S. Enhancing Abiotic Stress Tolerance in Fruit Trees Using Microbial Biostimulants. J. Pure Appl. Microbiol. 2024, 18, 1454–1470. [Google Scholar] [CrossRef]
- Zhou, Y.; Sommer, M.L.; Hochholdinger, F. Cold response and tolerance in cereal roots. J. Exp. Bot. 2021, 72, 7474–7481. [Google Scholar] [CrossRef] [PubMed]
- Afonso, S.; Oliveira, I.; Ribeiro, C.; Vilela, A.; Meyer, A.S.; Gonçalves, B. Exploring the role of biostimulants in sweet cherry (Prunus avium L.) fruit quality traits. Agriculture 2024, 14, 1521. [Google Scholar] [CrossRef]
- Khalid, F.; Rasheed, Y.; Asif, K.; Ashraf, H.; Maqsood, M.F.; Shahbaz, M.; Zulfiqar, U.; Sardar, R.; Haider, F.U. Plant Biostimulants: Mechanisms and Applications for Enhancing Plant Resilience to Abiotic Stresses. J. Soil Sci. Plant Nutr. 2024, 24, 6641–6690. [Google Scholar] [CrossRef]
- Alvarez, I.Z.; Ahmed, M.; McSorley, G.; Dunlop, M.; Lucas, I.; Hu, Y. An overview of biostimulant activity and plant responses under abiotic and biotic stress conditions. Syst. Microbiol. Biomanuf. 2023, 4, 39–55. [Google Scholar] [CrossRef]
- Awasthi, S.; Chauhan, R.; Srivastava, S. The importance of beneficial and essential trace and ultratrace elements in plant nutrition, growth, and stress tolerance. In Plant Nutrition and Food Security in the Era of Climate Change; Academic Press: Cambridge, MA, USA, 2022; pp. 27–46. [Google Scholar] [CrossRef]
- Wang, M.; Wang, R.; Mur, L.A.J.; Ruan, J.; Shen, Q.; Guo, S. Functions of silicon in plant drought stress responses. Hortic. Res. 2021, 8, 254. [Google Scholar] [CrossRef]
- Himanshu; Sharma, S.; Rana, V.S.; Thakur, A.; Kumar, A.; Sharma, N. Unlocking the sustainable role of melatonin in fruit production and stress tolerance: A review. CABI Agric. Biosci. 2024, 5, 103. [Google Scholar] [CrossRef]
- Ali, A.Y.A.; Ibrahim, M.E.H.; Zhou, G.; Nimir, N.E.A.; Jiao, X.; Zhu, G.; Elsiddig, A.M.I.; Suliman, M.S.E.; Elradi, S.B.M.; Yue, W. Exogenous jasmonic acid and humic acid increased salinity tolerance of sorghum. Agron. J. 2020, 112, 871–884. [Google Scholar] [CrossRef]
- Malerba, M.; Cerana, R. Chitin- and chitosan-based derivatives in plant protection against biotic and abiotic stresses and in recovery of contaminated soil and water. Polysaccharides 2020, 1, 21–30. [Google Scholar] [CrossRef]
- Chanda, M.-J.; Merghoub, N.; EL Arroussi, H. Microalgae polysaccharides: The new sustainable bioactive products for the development of plant bio-stimulants? World J. Microbiol. Biotechnol. 2019, 35, 177. [Google Scholar] [CrossRef]
- Mannino, G.; Ricciardi, M.; Gatti, N.; Serio, G.; Vigliante, I.; Contartese, V.; Gentile, C.; Bertea, C.M. Changes in the Phytochemical Profile and Antioxidant Properties of Prunus persica Fruits after the Application of a Commercial Biostimulant Based on Seaweed and Yeast Extract. Int. J. Mol. Sci. 2022, 23, 15911. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.-C.; Chen, C.-H.; Chang, Y.-S.; Lin, K.-H.; Wu, C.-W. Effect of Biostimulants on Plant Growth and Leaf Functional Compounds of Passiflora Plants. HortScience 2024, 59, 1682–1690. [Google Scholar] [CrossRef]
- Kaya, C.; Şenbayram, M.; Akram, N.A.; Ashraf, M.; Alyemeni, M.N.; Ahmad, P. Sulfur-enriched leonardite and humic acid soil amendments enhance tolerance to drought and phosphorus deficiency stress in maize (Zea mays L.). Sci. Rep. 2020, 10, 6432. [Google Scholar] [CrossRef] [PubMed]
- Hussain, H.A.; Men, S.; Hussain, S.; Chen, Y.; Ali, S.; Zhang, S.; Zhang, K.; Li, Y.; Xu, Q.; Liao, C.; et al. Interactive effects of drought and heat stresses on morpho-physiological attributes, yield, nutrient uptake and oxidative status in maize hybrids. Sci. Rep. 2019, 9, 3890. [Google Scholar] [CrossRef]
- Abidi, W.; Akrimi, R.; Gouiaa, M. Mitigating drought stress by plant-derived biostimulant in Arbequina olive (Olea europeae L.) cultivar conducted in super high density. Acta Physiol. Plant. 2023, 45, 132. [Google Scholar] [CrossRef]
- Wang, X.; Wang, X.; Huang, J.; Huang, J.; Peng, S.; Peng, S.; Xiong, D.; Xiong, D. Leaf rolling precedes stomatal closure in rice (Oryza sativa) under drought conditions. J. Exp. Bot. 2023, 74, 6650–6661. [Google Scholar] [CrossRef]
- Kilic, N. Improvement in Plant Growth, Yield, and Fruit Quality with Biostimulant Treatment in Organic Strawberry Cultivation. HortScience 2024, 59, 1165–1171. [Google Scholar] [CrossRef]
- Rady, M.M.; El-Yazal, M.A.S. Metabolic changes in polyamines, phenylethylamine, and arginine during bud break in apple flower buds under foliar-applied dormancy-breaking agents. Int. J. Empir. Educ. Res. 2018, 1, 1–18. [Google Scholar] [CrossRef]
- Mosa, W.F.A.; Sas-Paszt, L.; Głuszek, S.; Górnik, K.; Anjum, M.A.; Saleh, A.A.; Abada, H.S.; Awad, R.M. Effect of some biostimulants on the vegetative growth, yield, fruit quality attributes and nutritional status of apple. Horticulturae 2022, 9, 32. [Google Scholar] [CrossRef]
- Dekhane, S.S.; Kumar, N.; Pisal, R.R. Assessment of the Effect of Different Bio-Stimulants on the Growth, Quality, and Yield of Strawberries in a Sub-Tropical Climatic Region: A Case Study. J. Exp. Agric. Int. 2023, 45, 58–63. [Google Scholar] [CrossRef]
- Ma, Y.; Freitas, H.; Dias, M.C. Strategies and prospects for biostimulants to alleviate abiotic stress in plants. Front. Plant Sci. 2022, 13, 1024243. [Google Scholar] [CrossRef] [PubMed]
- Mahfouze, S.A.; Mahfouze, H.A.; Helmi, R.Y.; Fathallah, F.B.; Aboud, K.A.; Ottai, M.E. The role of chitosan in improving cold stress tolerance in strawberry varieties. Jordan J. Biol. Sci. 2024, 17, 645–655. [Google Scholar] [CrossRef]
- Gornik, K.; Grzesik, M.; Romanowska-Duda, B. The effect of chitosan on rooting of grapevine cuttings and on subsequent plant growth under drought and temperature stress. J. Fruit Ornamental Plant Res. 2008, 16, 333–343. [Google Scholar]
- Khan, A.S.; Munir, M.; Shaheen, T.; Tassawar, T.; Rafiq, M.A.; Ali, S.; Anwar, R.; Rehman, R.N.; Hasan, M.U.; Malik, A.U. Supplemental foliar applied mixture of amino acids and seaweed extract improved vegetative growth, yield and quality of citrus fruit. Sci. Hortic. 2022, 296, 110903. [Google Scholar] [CrossRef]
- Camprubi, A.; Solari, J.; Bonini, P.; Garcia-Figueres, F.; Colosimo, F.; Cirino, V.; Lucini, L.; Calvet, C. Plant performance and metabolomic profile of loquat in response to mycorrhizal inoculation, Armillaria mellea, and their interaction. Agronomy 2020, 10, 899. [Google Scholar] [CrossRef]
- Ioppolo, A.; Laudicina, V.A.; Badalucco, L.; Saiano, F.; Palazzolo, E. Wastewaters from citrus processing industry as natural biostimulants for soil microbial community. J. Environ. Manag. 2020, 273, 111137. [Google Scholar] [CrossRef]
- Hameed, A.; Fatma, S.; Wattoo, J.I.; Yaseen, M.; Ahmad, S. Accumulative effects of humic acid and multinutrient foliar fertilizers on the vegetative and reproductive attributes of citrus (Citrus reticulata cv. kinnow mandarin). J. Plant Nutr. 2018, 41, 2495–2506. [Google Scholar] [CrossRef]
- Rana, V.S.; Lingwal, K.; Sharma, S.; Rana, N.; Pawar, R.; Kumar, V.; Sharma, U. Biostimulatory effect of seaweed extract on the fruiting and runner production of strawberry. Emerg. Life Sci. Res. 2022, 8, 132–141. [Google Scholar] [CrossRef]
- Moenne, A.; González, A. Chitosan-, alginate-carrageenan-derived oligosaccharides stimulate defense against biotic and abiotic stresses, and growth in plants: A historical perspective. Carbohydr. Res. 2021, 503, 108298. [Google Scholar] [CrossRef] [PubMed]
- Pascoalino, L.A.; Reis, F.S.; Barros, L.; Rodrigues, M.Â.; Correia, C.M.; Vieira, A.L.; Ferreira, I.C.F.R.; Barreira, J.C.M. Effect of plant biostimulants on nutritional and chemical profiles of almond and hazelnut. Appl. Sci. 2021, 11, 7778. [Google Scholar] [CrossRef]
- Cirillo, A.; Izzo, L.; Ciervo, A.; Ledenko, I.; Cepparulo, M.; Piscitelli, A.; Di Vaio, C. Optimizing Apricot Yield and Quality with Biostimulant Interventions: A Comprehensive Analysis. Horticulturae 2024, 10, 447. [Google Scholar] [CrossRef]
- Khan, W.; Rayirath, U.P.; Subramanian, S.; Jithesh, M.N.; Rayorath, P.; Hodges, D.M.; Critchley, A.T.; Craigie, J.S.; Norrie, J.; Prithiviraj, B. Seaweed extracts as biostimulants of plant growth and development. J. Plant Growth Regul. 2009, 28, 386–399. [Google Scholar] [CrossRef]
- Dragovoz, I.V.; Yavorskaya, V.K.; Antoniuk, V.P.; Kurchii, B.A. Hormonal substances produced by microorganism association from ginseng roots. Physiol. Biochem. Cultiv. Plant 2009, 41, 393–399. [Google Scholar]
- Li, J.; Lardon, R.; Mangelinckx, S.; Geelen, D. A practical guide to the discovery of biomolecules with biostimulant activity. J. Exp. Bot. 2024, 75, 3797–3817. [Google Scholar] [CrossRef]
- Vijayan, S.R.; Santhiyagu, P.; Singamuthu, M.; Ahila, N.K.; Jayaraman, R.; Ethiraj, K. Synthesis and characterization of silver and gold nanoparticles using aqueous extract of seaweed, Turbinaria conoides, and their antimicrofouling activity. Sci. World J. 2014, 938272. [Google Scholar] [CrossRef]
| Source of Biostimulant | Example | Activity | |
|---|---|---|---|
| Hydrolysis product | Enzymatic (alfalfa, hay, pulses, fruits, and vegetables) and chemicals (feathers, bone meal, collagen from skin, animal tissues, and fish waste) | Enhanced productivity | |
| Improved leaves’ nutrient content and uptake | [1,21,30,32,33,43] | ||
| Enhanced protein levels | |||
| Defense against both abiotic and biotic stressors. | |||
| Enhanced soil fertility by promoting the growth of soil microbes. | |||
| Aerobic digestion product | Animals and plants and lignin biomass | Induced the effect of auxin. | |
| Enhanced nutrient availability. | |||
| Biopreparations from marine algae | Ascophyllum nodosum, Sargassm wightii, Ecklonia maxima, Gelidium pectinutum | Enhanced antioxidant capacity and ability to scavenge free radicals. | |
| Chelation effect. | |||
| Enhanced mitigation from abiotic and biotic stress. | |||
| Prolonged shelf life | |||
| Enhanced plant resilience to heat. | |||
| Defended against the stress of drought. | |||
| Consortia of useful fungus | Rhizophagus intraradices, Rhymbocarpus aggregatus, Glomus spp., Trichoderma spp., Heteroconium chaetospira | Enhanced plant growth and yield, both individually and in symbiosis. |
| Biostimulants | Sources | Effect of Biostimulants | Maximum Increases in Germination/Yield/Biomass (%) | Dosages/Concentration | Treatment Conditions | References |
|---|---|---|---|---|---|---|
| Compost tea | Composting compounds from organic waste | Enhance sugar beet growth and improve its quality. | 4% improvement in yield. | 500 ppm | Foliar application was used in the field testing. | [60] |
| Deproteinized leaf juice | Byproducts of leaf protein isolation from alfalfa | Enhance photosynthesis in sweet basil and promote the growth of roots and leaves. | 50.3% growth increase in shoot length. | 50, 100, 250 ppm | Foliar spray, pot trails. | [61] |
| Protein hydrolysate | Chicken Feather Waste | Enhance soil fertility and yield in mung bean. | 40% improvement in germination. | 10, 20, 30 mL | Potting trials; irrigation. | [62] |
| Lignin salts | Pulp mill byproducts | Reduce the buildup of reactive oxygen species and increase rice’s photosynthesis. | 32.36% growth in shoot height. | 100, 200, 300, 400 ppm | Added to the rhizosphere. | [63] |
| Humus-like substance | Tea waste | Stimulate the growth of tea trees. | 117% improvement in plant-dried weight. | 20, 40, 80, 160 ppm | Field trials; soil applications. | [64] |
| Seaweed extract | Seaweed waste | Stimulate cress seed germination and growth. | 25% increase in germination percentage. | 100, 1000, 2500, 5000, 10,000 ppm | Potting trials; irrigation. | [65] |
| Chitosan | Exoskeleton of insects, shrimps and crabs | Promote Salicornia bigelovii root development. | 35% increase in biomass. | 5000, 10,000 ppm | Field trials; root dipping. | [66] |
| Stress | Name of Crop | Biostimulant | Mode of Application | Effect of Biostimulants on Plant | Reference |
|---|---|---|---|---|---|
| Cold stress | Avocado (Persea americana Mill.) | Fulvic acid | Dipping | Decreased the chilling injury and increase the shelf life. | [109] |
| Grapevine (Vitis vinifera L.) | PGPB | Root immersion | Increased metabolic activity. | [110] | |
| Strawberry (Fragaria × ananassa Duch.) | Amino acid | Foliar spray | Delayed the spring frost injury. | [111] | |
| Grapefruit (Citrus × paradisi Macfad.) | Methyl Jasmonates | Dipping | Reduced the chilling injury. | [112] | |
| Heat stress | Apple (Malus domestica Borkh.) | Humic acid | Foliar | Reduced the sunburn | [113] |
| Pomegranate (Punica granatum L.) | Kaolin | Foliar spray | Reduced the sunburn and fruit cracking. | [114] | |
| Drought stress | Grapevines (Vitis vinifera L.) | AMF (Glomus mosseae) | Soil application | Increased leaf water potential and stomatal conductance. | [115] |
| Citrus (Citrus spp.) | Seaweed extract | Soil drenching | Increased the stem water potential and growth. | [116] | |
| Grape (Vitis vinifera L.) | Chitosan | Dipping | Increased the rooting in cutting and no. of new canes | [117] | |
| Loquat Eriobotrya japonica (Thunb.) Lindl. | AMF (Funneliformis mosseae) | Soil application | Increased the osmatic adjustment in the root. | [118] | |
| Salinity stress | Strawberry (Fragaria × ananassa Duch.) | PGPB | Root dipping | Decreased the sodium and chloride content in roots and leaf. | [119] |
| Apple (Malus domestica Borkh.) | AMF (Glomus versiforme) | Drenching | Reduced the ROS effect and increased the root length. | [120] | |
| Grapevines (Vitis vinifera L.) | Potassium silicate | Drenching | Increased the plant height and yield. | [121] | |
| Strawberry (Fragaria × ananassa Duch.) | Potassium silicate | Drenching | Decreased the proline ion content and increased the fruit yield. | [122] | |
| Nutritional | Olive (Olea europaea L.) | Humic acid | Drenching | Increased the shoot growth. | [123] |
| Pear (Pyrus communis L.) | Amino acid | Foliar spray | Increased the Fe, Cu, Zn and Mn content in the leaf. | [124] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, M.; Subahan, G.M.; Sharma, S.; Singh, G.; Sharma, N.; Sharma, U.; Kumar, V. Enhancing Horticultural Sustainability in the Face of Climate Change: Harnessing Biostimulants for Environmental Stress Alleviation in Crops. Stresses 2025, 5, 23. https://doi.org/10.3390/stresses5010023
Singh M, Subahan GM, Sharma S, Singh G, Sharma N, Sharma U, Kumar V. Enhancing Horticultural Sustainability in the Face of Climate Change: Harnessing Biostimulants for Environmental Stress Alleviation in Crops. Stresses. 2025; 5(1):23. https://doi.org/10.3390/stresses5010023
Chicago/Turabian StyleSingh, Manya, Gudammagari Mabu Subahan, Sunny Sharma, Gurpreet Singh, Neha Sharma, Umesh Sharma, and Vineet Kumar. 2025. "Enhancing Horticultural Sustainability in the Face of Climate Change: Harnessing Biostimulants for Environmental Stress Alleviation in Crops" Stresses 5, no. 1: 23. https://doi.org/10.3390/stresses5010023
APA StyleSingh, M., Subahan, G. M., Sharma, S., Singh, G., Sharma, N., Sharma, U., & Kumar, V. (2025). Enhancing Horticultural Sustainability in the Face of Climate Change: Harnessing Biostimulants for Environmental Stress Alleviation in Crops. Stresses, 5(1), 23. https://doi.org/10.3390/stresses5010023






