Maize Morphophysiological Changes Modulated by Cover Crops Rotation in Northeast Brazil
Abstract
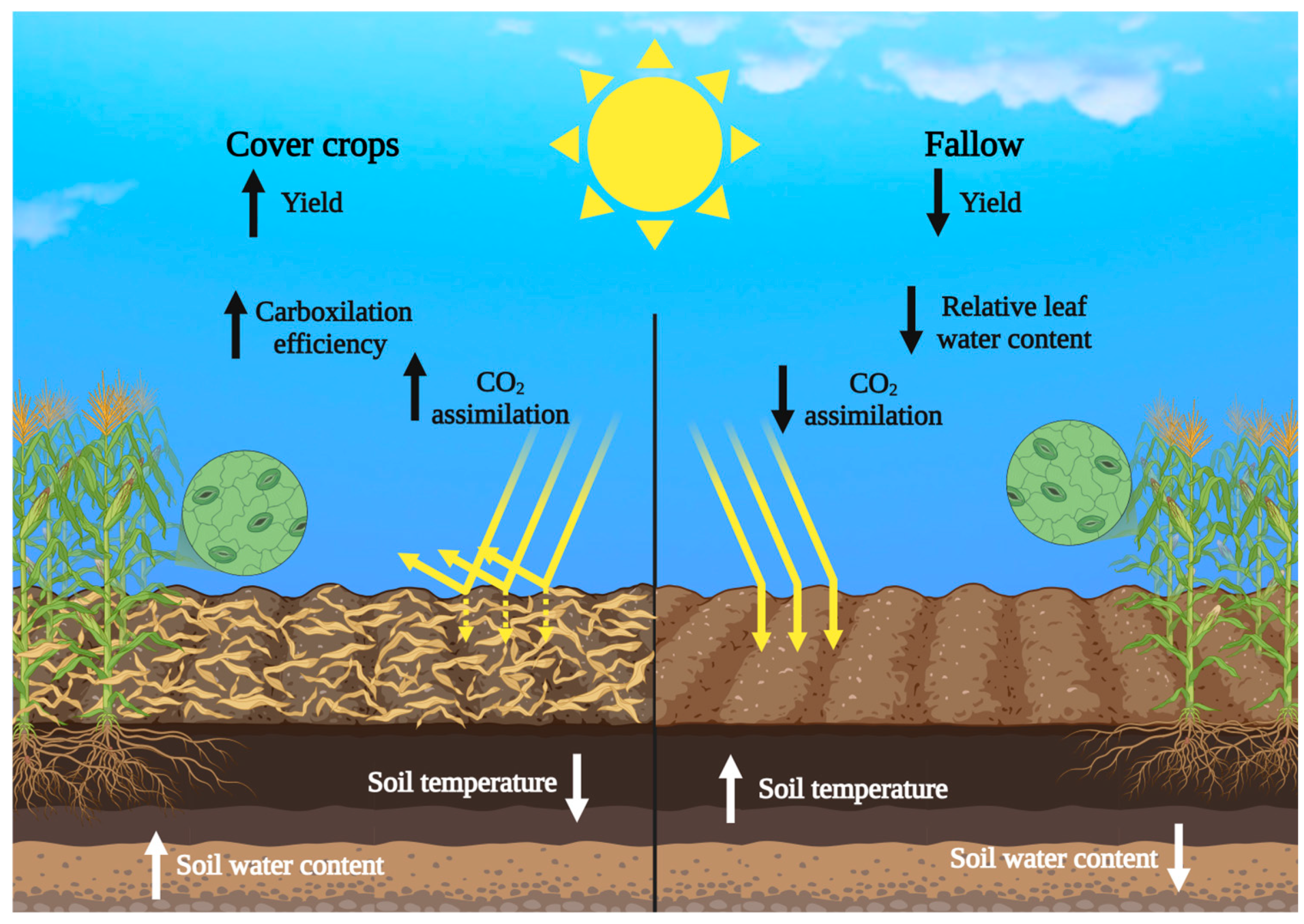
1. Introduction
2. Results
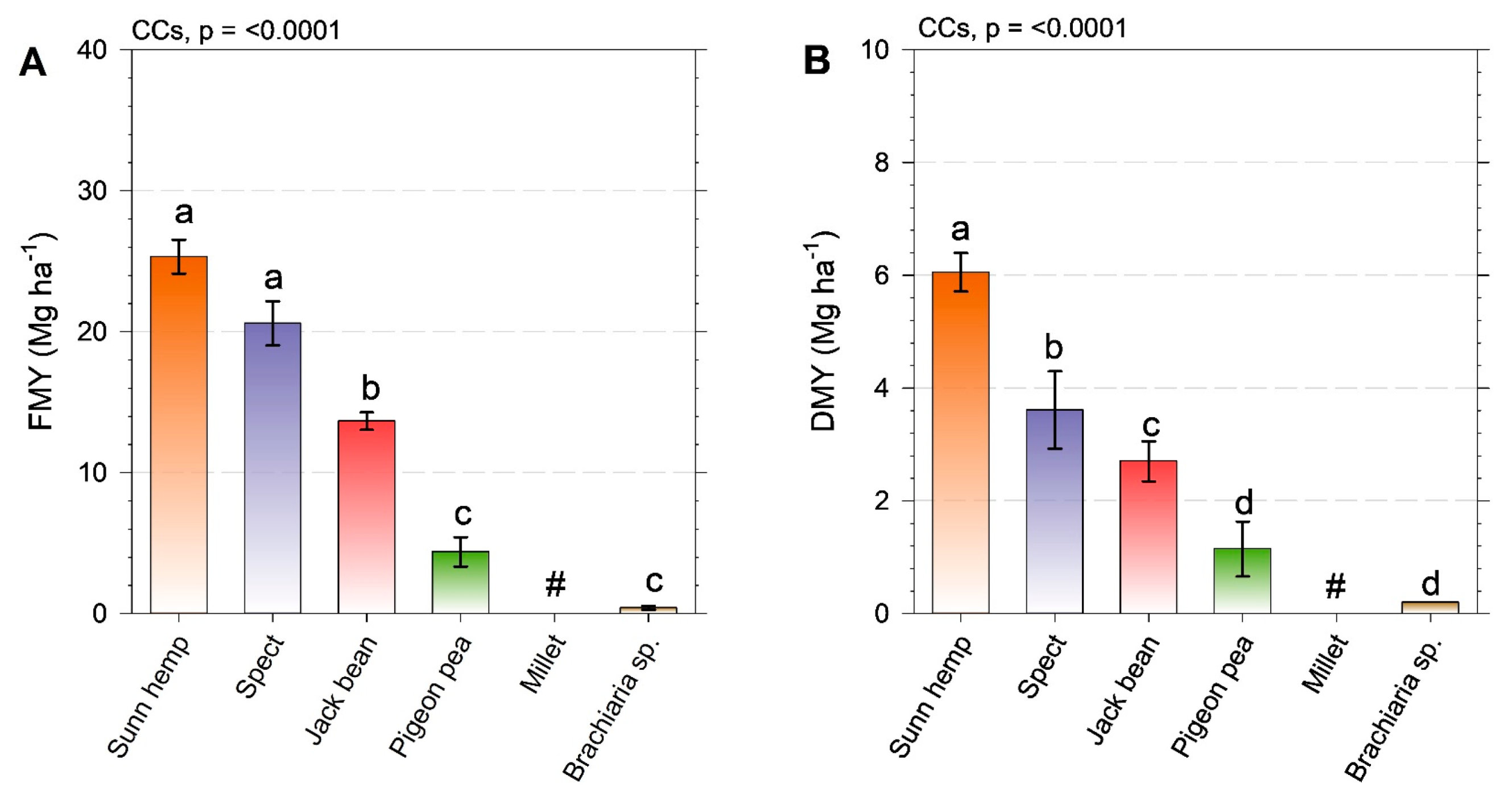
2.1. Cover Crop Yield
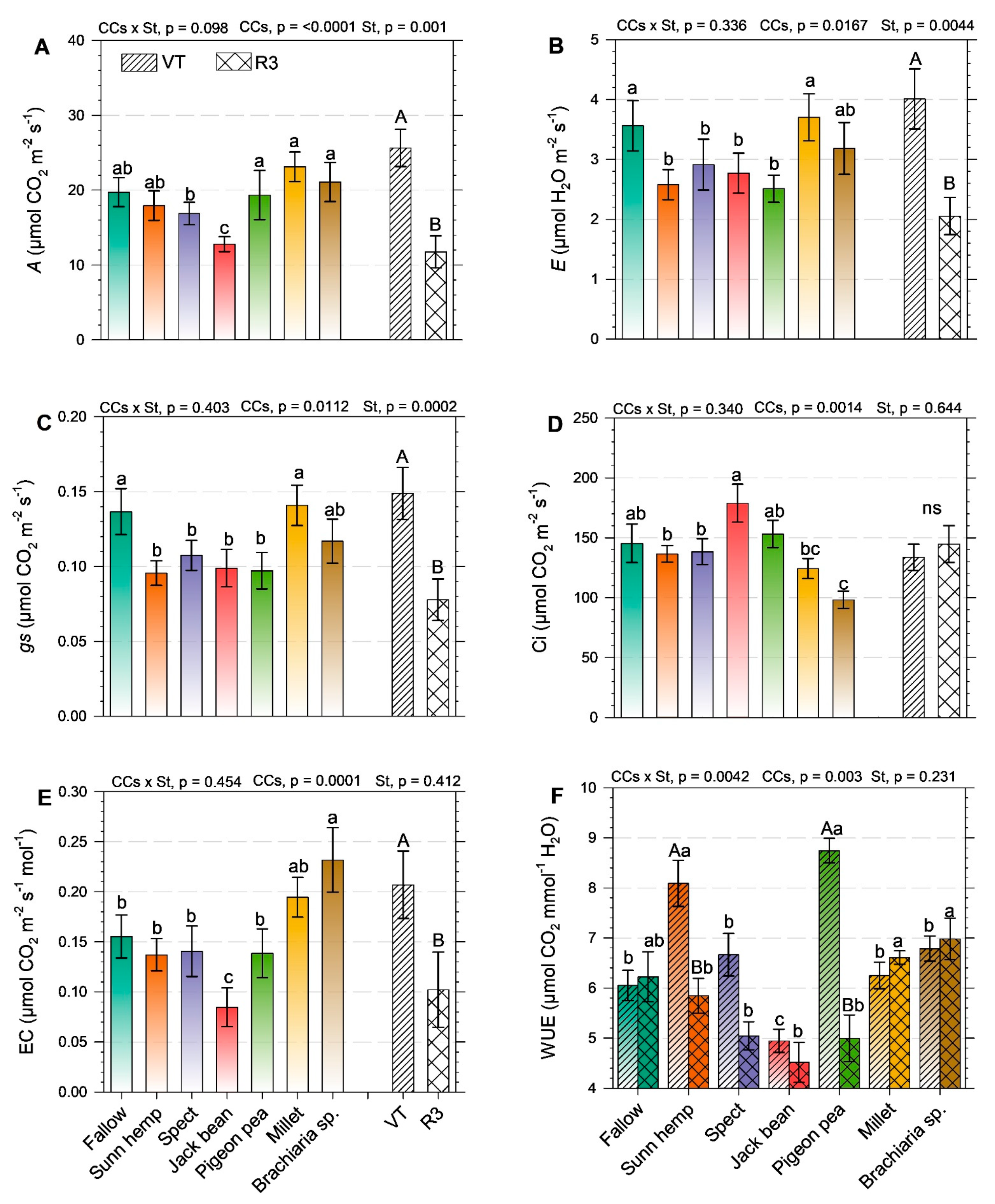
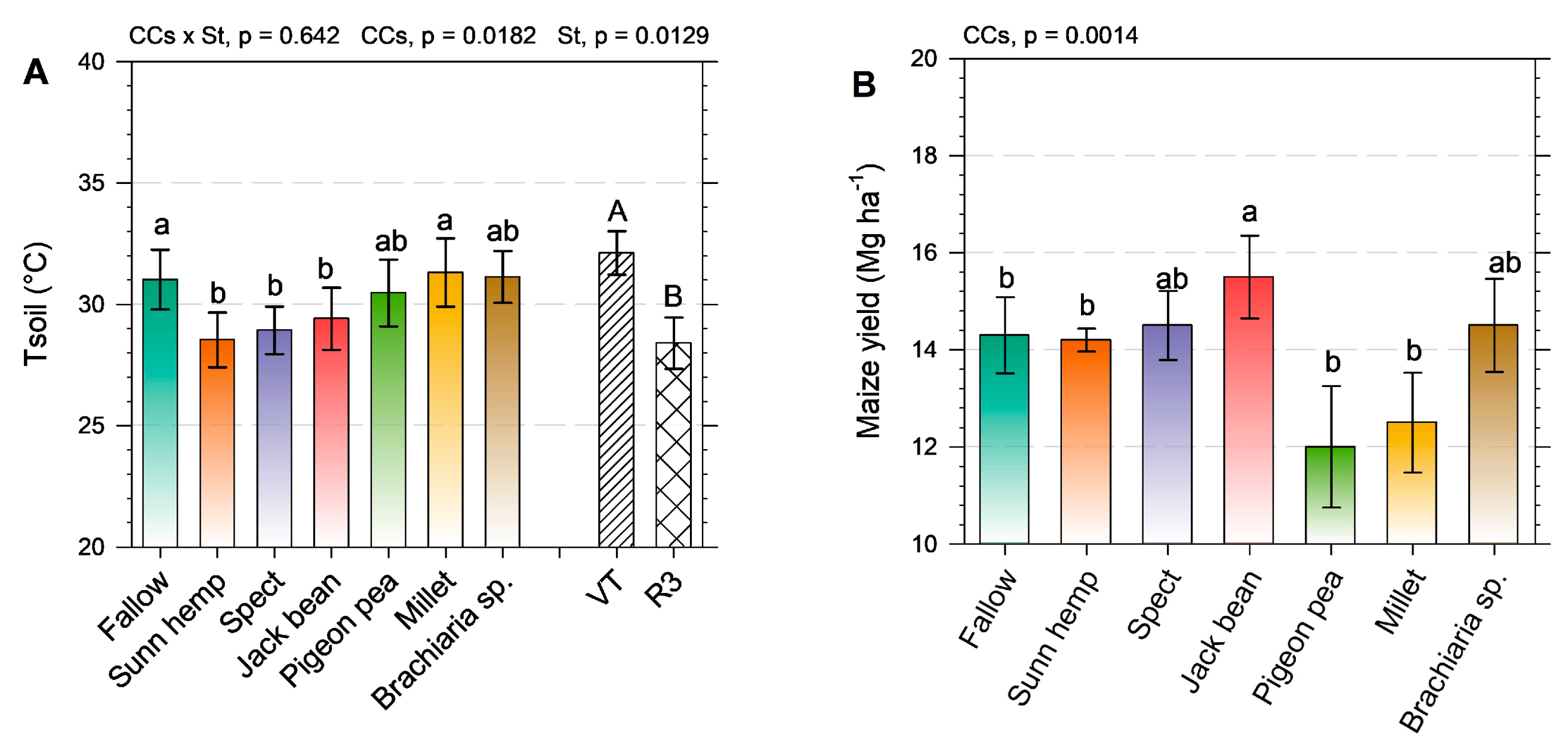
2.2. Physiological Aspects and Yield of Green Maize
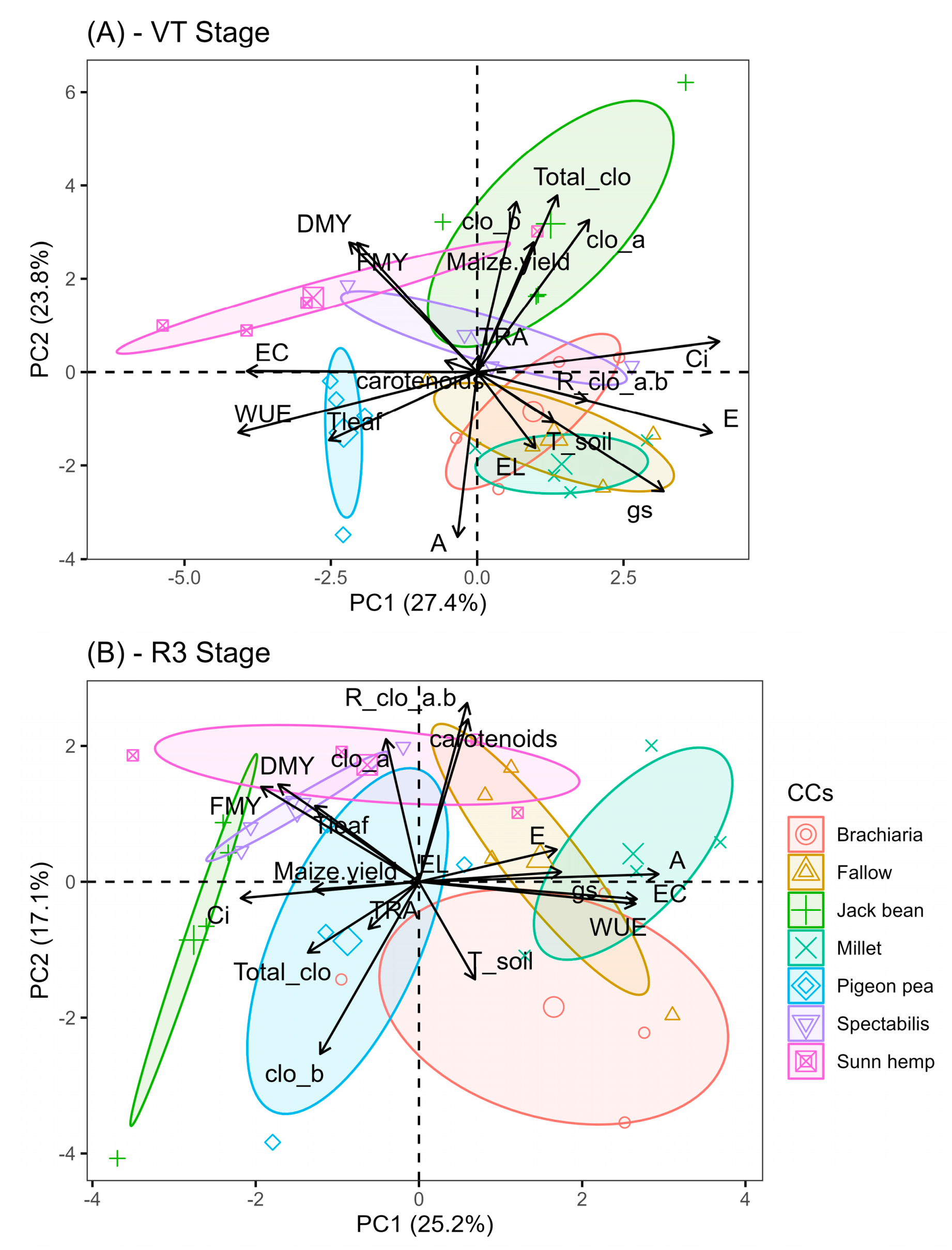
2.3. Multivariate Comparisons
3. Discussion
4. Materials and Methods
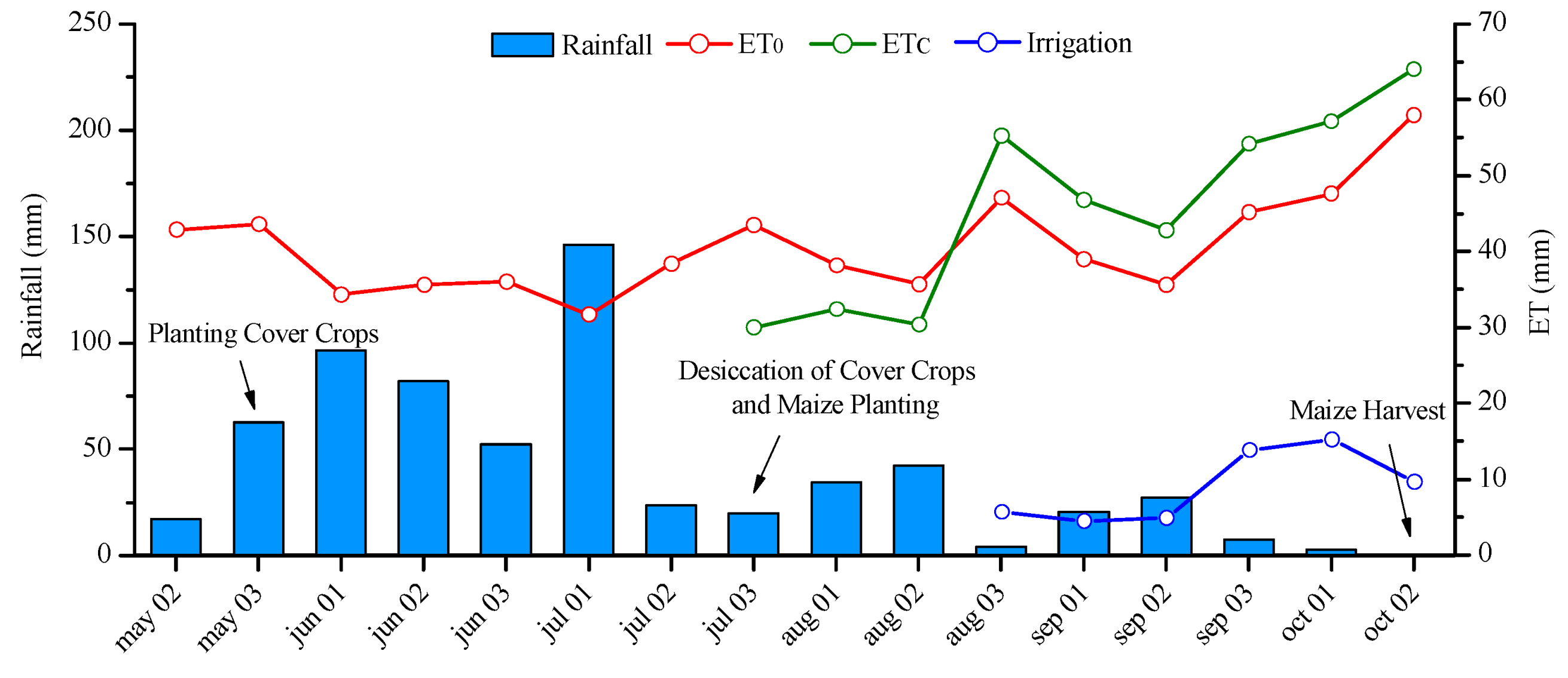
4.1. Research Site
4.2. Experimental Design and Crop Management
4.3. Experimental Evaluations
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lara, S.G.; Saldivar, S.O.S. Corn History and Culture. In Corn: Chem Technol, 3rd ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2019; pp. 1–18. [Google Scholar] [CrossRef]
- Instituto Brasileiro de Geografia e Estatística (2022) Área Plantada ou Destinada á Colheita. Available online: https://sidra.ibge.gov.br/tabela/5457#resultado (accessed on 15 August 2023).
- de Lima, C.I.S.; Silva, F.D.d.S.; de Freitas, I.G.F.; Pinto, D.D.C.; Costa, R.L.; Gomes, H.B.; Silva, E.H.d.L.; da Silva, L.L.; Silva, V.d.P.R.d.; Silva, B.K.d.N. Método Alternativo de Zoneamento Agroclimático do Milho para o Estado de Alagoas. Rev. Bras. Meteorol. 2020, 35, 1057–1067. [Google Scholar] [CrossRef]
- da Luz, J.H.S.; da Silva, M.B.; Barbosa, L.D.N.S.; de Souza, J.W.G.; Farias, M.R.d.S.; dos Santos, J.K.; De Gois, M.G.J.L.; Paulino, S.S.; Silva, R.B.; Silva, D.M.R.; et al. Short-Term Agronomic and Economic Responses to the Adoption of Cover Crops for Corn Rotation in the Brazilian Semiarid Region. Sustainability 2023, 15, 15091. [Google Scholar] [CrossRef]
- Cavalcante, V.S.; Santos, V.R.; Neto, A.L.S.; Santos, M.A.L.; Santos, C.G.; Costa, L.C. Biomassa e extração de nutrientes por plantas de cobertura. Rev. Bras. Eng. Agríc Ambient 2012, 16, 512–528. [Google Scholar] [CrossRef]
- Jafarikouhini, N.; Kazemeini, S.A.; Sinclair, T.R. Sweet corn nitrogen accumulation, leaf photosynthesis rate, and radiation use efficiency under variable nitrogen fertility and irrigation. Field Crop. Res. 2020, 257, 107913. [Google Scholar] [CrossRef]
- Boulet, A.K.; Alarcão, C.; Ferreira, C.; Kalantari, Z.; Veiga, A.; Campos, L.; Ferreira, A.; Hessel, R. Agro-ecological services delivered by legume cover crops grown in succession with grain corn crops in the Mediterranean region. Open Agric. 2021, 6, 609–626. [Google Scholar] [CrossRef]
- Silveira, B.d.S.; Torres, J.L.R.; Júnior, V.O.; Favaro, J.H.d.S.; Costa, L.L.; de O Charlo, H.C. Cover crops in the production of green and sweet corn. Hortic. Bras. 2021, 39, 94–101. [Google Scholar] [CrossRef]
- Sigdel, S.; Chatterjee, A. Do Cover Crop And Soil-Mediated Legacy Influence Succeeding Wheat Production? Commun. Soil Sci. Plant Anal. 2020, 51, 1514–1524. [Google Scholar] [CrossRef]
- Tosi, M.; Ogilvie, C.M.; Spagnoletti, F.N.; Fournier, S.; Martin, R.C.; Dunfield, K.E. Cover Crops Modulate the Response of Arbuscular Mycorrhizal Fungi to Water Supply: A Field Study in Corn. Plants 2023, 12, 1015. [Google Scholar] [CrossRef]
- Vujić, S.; Krstić, D.; Mačkić, K.; Čabilovski, R.; Radanović, Z.; Zhan, A.; Ćupina, B. Effect of winter cover crops on water soil storage, total forage production, and quality of silage corn. Eur. J. Agron. 2021, 130, 126366. [Google Scholar] [CrossRef]
- Renato, N.d.S.; Silva, J.B.L.; Sediyama, G.C.; Pereira, E.G. Influência dos métodos para cálculo de graus-dia em condições de aumento de temperatura para as culturas de milho e feijão. Rev. Bras. Meteorol. 2013, 28, 382–388. [Google Scholar] [CrossRef]
- Cavalcante, V.S.; Barboza, J.T.V.; Costa, L.C.; Santos, V.R.S.; Nunes, M.J. Produção de adubos verdes e a utilização dos resíduos no cultivo da cebolinha. ABA-Agroecologia 2015, 10, 24–31. [Google Scholar]
- dos Santos, V.R.; Costa, L.C.; Rocha, A.M.S.; dos Santos, C.G.; dos Santos, M.A.L.; Rabêlo, F.H.S.; Prado, R.d.M. Biomass accumulation, extraction and nutrient use efficiency by cover crops. Res. Soc. Dev. 2020, 9. [Google Scholar] [CrossRef]
- Marshall, A.J.; Gallaher, R.N.; Wang, K.H.; McSorley, R. Partitioning of dry matter and minerals in sunn hemp. In Proceedings of the 25th Annual Southern Conservation Tillage Conference for Sustainable Agriculture, Auburn, AL, USA, 24–26 June 2002; pp. 310–313. [Google Scholar]
- Oliveira, T.P.; Braz, M.G.; Smaniotto, A.O.; DA Silva, D.F.P.; Cruz, S.C.S. Advancing nitrogen fertilization of corn using Brachiaria ruziziensis as cover crop. Rev. Caatinga 2021, 34, 9–19. [Google Scholar] [CrossRef]
- Taiz, L.; Zeiger, E.; Muller, I.M.; Murphy, A. Physiology and Plant Development, 6th ed.; Artmed Editora: Rio Grande do Sul, Brazil, 2017; pp. 168–254. [Google Scholar]
- Aker, A.M.; Passos, A.M.A.; Marcolan, A.L.; Santos, F.C.; Cipriani, H.N.; Vargas, L.A. Plantas de cobertura sobre atributos agronômicos do milho na região sudoeste da Amazônia. Rev. Bras. Milho Sorgo 2016, 15, 531–542. [Google Scholar] [CrossRef]
- Shashishekhar, A.J.; Patil, V.D.; Jawale, S.A.; Saptute, U. Effect of soil fertility levels on chlorophyll content of maize crop. J. Pharmacogn. Phytochem. 2017, 6, 95–97. [Google Scholar]
- Teixeira, C.M.; de Carvalho, G.J.; Silva, C.A.; de Andrade, M.J.B.; Pereira, J.M. Liberação de macronutrientes das palhadas de milheto solteiro e consorciado com feijão-de-porco sob cultivo de feijão. Rev. Bras. Cienc. Solo 2010, 34, 497–506. [Google Scholar] [CrossRef]
- Thapa, V.R.; Ghimire, R.; Acosta-Martínez, V.; Marsalis, M.A.; Schipanski, M.E. Cover crop biomass and species composition affect soil microbial community structure and enzyme activities in semiarid cropping systems. Appl. Soil Ecol. 2020, 157, 103735. [Google Scholar] [CrossRef]
- Sun, D.B.; Sun, G.D.; Wang, Q.S. Effects of No-tillage on the Photosynthesis and Transpiration of Maize in the Semi-arid Area of Northern Shanxi Province. Chin. J. Agrometeorol. 2010, 31, 235–239. [Google Scholar]
- Portugal, J.R.; Arf, O.; Peres, A.R.; Gitti, D.C.; Garcia, N.F.S. Coberturas vegetais, doses de nitrogênio e inoculação com Azospirillum brasilense em milho no Cerrado. Rev. Ciênc. Agron. 2017, 48, 639–649. [Google Scholar]
- Santos, P.M.; Da Cruz, P.G.; De Araujo, L.C.; Pezzopane, J.R.M.; Valle, C.B.D.; Pezzopane, C.D.G. Response mechanisms of Brachiaria brizantha cultivars to water deficit stress. Rev. Bras. Zootec. 2013, 42, 767–773. [Google Scholar] [CrossRef]
- Dias, M.B.d.C.; Costa, K.A.d.P.; Severiano, E.d.C.; Bilego, U.O.; Neto, A.E.F.; Almeida, D.P.; Brand, S.C.; Vilela, L. Brachiaria and Panicum maximum in an integrated crop–livestock system and a second-crop maize system in succession with soybean. J. Agric. Sci. 2020, 158, 206–217. [Google Scholar] [CrossRef]
- de Oliveira, S.M.; de Almeida, R.E.M.; Junior, C.P.; Reis, A.F.d.B.; Souza, L.F.N.; Favarin, J.L. Contribution of corn intercropped with Brachiaria species to nutrient cycling1. Pesqui. Agropecu. Trop. 2019, 49. [Google Scholar] [CrossRef]
- Anderson, R.L. Improving resource-use-efficiency with no-till and crop diversity. Renew. Agric. Food Syst. 2016, 32, 105–108. [Google Scholar] [CrossRef]
- Vendig, I.; Guzman, A.; De La Cerda, G.; Esquivel, K.; Mayer, A.C.; Ponisio, L.; Bowles, T.M. Quantifying direct yield benefits of soil carbon increases from cover cropping. Nat. Sustain. 2023, 6, 1125–1134. [Google Scholar] [CrossRef]
- Sinclair, T.R. Improved carbon and nitrogen assimilation for increased yield. In Soybeans: Improvement, Production, and Uses, 3rd ed.; ASA, CSSA, SSSA: Madison, WI, USA, 2004; Volume 16, pp. 537–568. [Google Scholar] [CrossRef]
- Zhang, H.; Ghahramani, A.; Ali, A.; Erbacher, A. Cover cropping impacts on soil water and carbon in dryland cropping system. PLoS ONE 2023, 18, e0286748. [Google Scholar] [CrossRef]
- Barros, A.H.C.; Filho, J.C.A.; Silva, A.B.; Santiago, G.A.C.F. Climatologia do Estado de Alagoas, 2nd ed.; Embrapa Solos: Recife, Brazil, 2012; p. 32. [Google Scholar]
- Santos, H.G.; Jacomine, P.K.T.; Anjos, L.H.C.; Oliveira, V.A.; Lumbreras, J.F.; Coelho, M.R.; Almeida, J.A.; Araujo Filho, J.C.; Oliveira, J.B.; Cunha, T.J.F. Sistema Brasileiro de Classificação de Solos, 5th ed.; Embrapa Solos: Brasilia, DF, Brazil, 2018; p. 355. [Google Scholar]
- Soil Survey Staff. Keys to Soil Taxonomy, 13th ed.; United States Department of Agriculture, Natural Re-sources Conservation Service: Washington, DC, USA, 2022. Available online: https://www.nrcs.usda.gov/sites/default/files/2022-09/Keys-to-Soil-Taxonomy (accessed on 25 February 2023).
- Pereira, a.R.; Angelocci, l.R.; Sentelhas, p.C. Agrometeorologia: Fundamentos e Aplicações Práticas; Agropecuária: Guaíba, Brazil, 2002; p. 478. [Google Scholar]
- Wellburn, A.R. The Spectral Determination of Chlorophylls a and b, as well as Total Carotenoids, Using Various Solvents with Spectrophotometers of Different Resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Barrs, H.D.; Weatherley, P.E. A Re-Examination of the Relative Turgidity Technique for Estimating Water Deficits in Leaves. Aust. J. Biol. Sci. 1962, 15, 413–428. [Google Scholar] [CrossRef]
- Campos, P.S.; Thi, A.T.P. Effects of an abscisic acid pretreatment on membrane leakage and lipid composition of Vigna unguiculata leaf discs subjected to osmotic stress. Plant Sci. 1997, 130, 11–18. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Souza, J.W.G.d.; Luz, J.H.S.d.; Silva, D.M.R.; Silva, R.B.; Costa, B.R.d.S.; Melo, A.F.; Santos, H.R.d.; Marques, I.C.d.S.; Sousa, J.I.d.; Vanderley, M.B.; et al. Maize Morphophysiological Changes Modulated by Cover Crops Rotation in Northeast Brazil. Stresses 2024, 4, 699-713. https://doi.org/10.3390/stresses4040045
Souza JWGd, Luz JHSd, Silva DMR, Silva RB, Costa BRdS, Melo AF, Santos HRd, Marques ICdS, Sousa JId, Vanderley MB, et al. Maize Morphophysiological Changes Modulated by Cover Crops Rotation in Northeast Brazil. Stresses. 2024; 4(4):699-713. https://doi.org/10.3390/stresses4040045
Chicago/Turabian StyleSouza, José Wilker Germano de, João Henrique Silva da Luz, Dayane Mércia Ribeiro Silva, Ricardo Barros Silva, Bruno Richardson dos Santos Costa, Alan Fontes Melo, Hugo Rodrigues dos Santos, Isabelly Cristina da Silva Marques, Jadielson Inácio de Sousa, Mariana Bernardino Vanderley, and et al. 2024. "Maize Morphophysiological Changes Modulated by Cover Crops Rotation in Northeast Brazil" Stresses 4, no. 4: 699-713. https://doi.org/10.3390/stresses4040045
APA StyleSouza, J. W. G. d., Luz, J. H. S. d., Silva, D. M. R., Silva, R. B., Costa, B. R. d. S., Melo, A. F., Santos, H. R. d., Marques, I. C. d. S., Sousa, J. I. d., Vanderley, M. B., Barbosa, L. d. N. S., Farias, M. R. d. S., Farias, E. d. S. F., Paulino, S. S., Santos Neto, A. L. d., Pavinato, P. S., Silva, J. V., & Santos, V. R. d. (2024). Maize Morphophysiological Changes Modulated by Cover Crops Rotation in Northeast Brazil. Stresses, 4(4), 699-713. https://doi.org/10.3390/stresses4040045





