Endoplasmic Reticulum Stress Signaling in the Regulation of Hepatic Pathological Responses
Abstract
1. Introduction
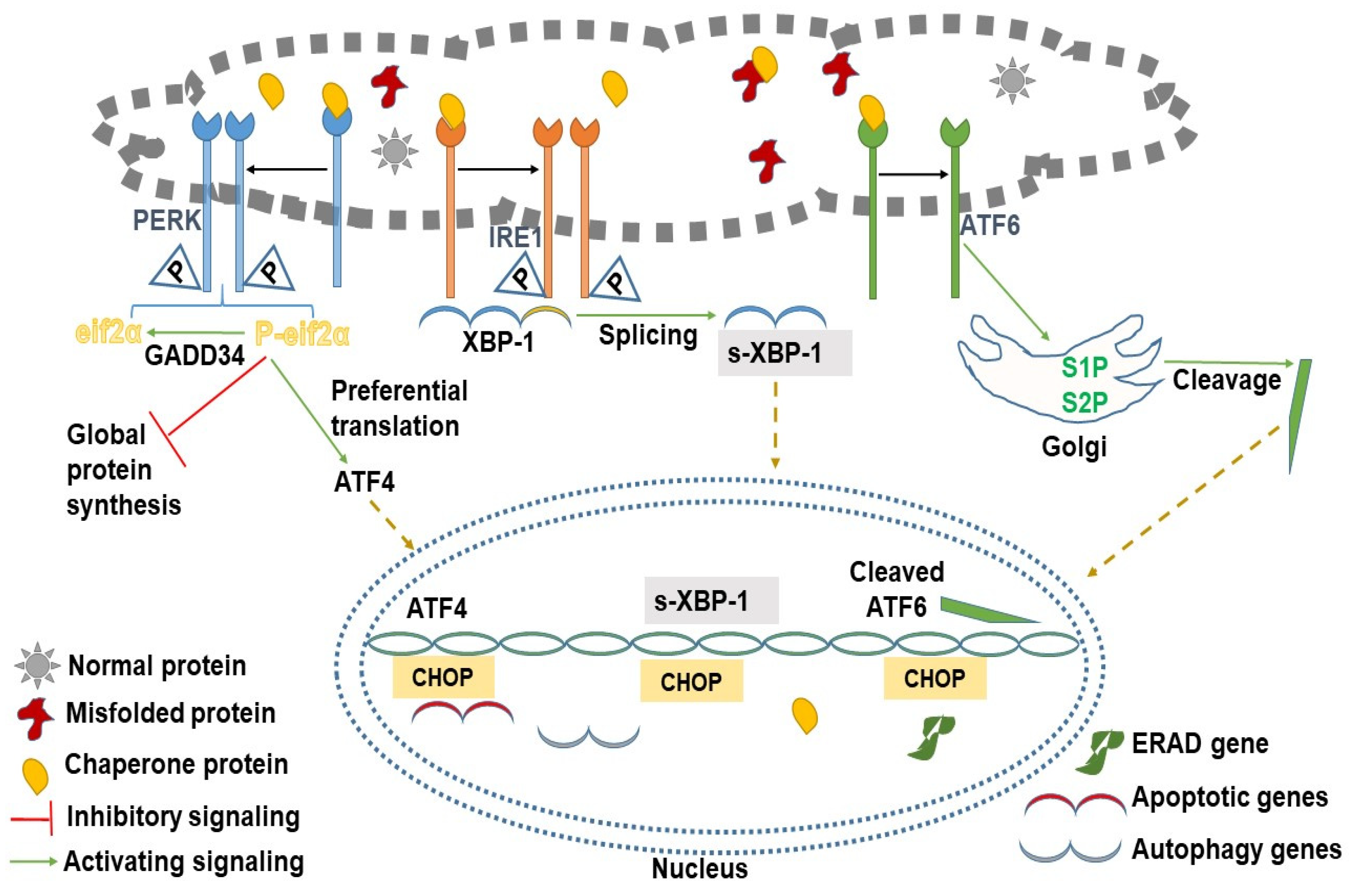
2. Branches of UPR and Their Signal Transduction
2.1. Protein Kinase R-like ER Kinase (PERK)
2.2. Inositol-Requiring Enzyme Type 1 (IRE1)
2.3. Activating Transcription Factor 6 (ATF6)
3. All Roads Lead to CHOP
4. Self-Regulation of ER Stress
5. ERAD and ER-Phagy Two Dual Dynamos of ER Quality-Control
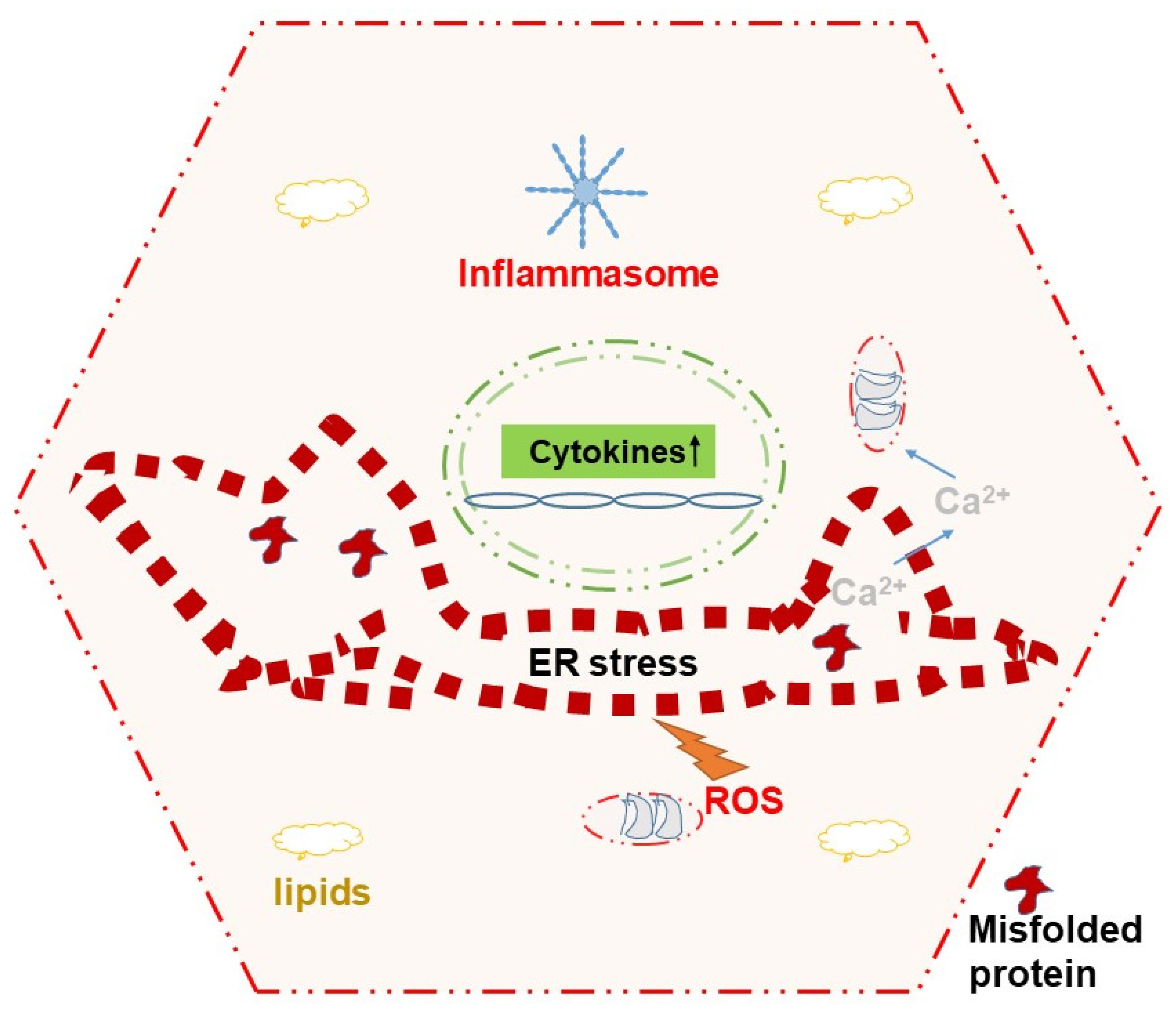
6. ER Stress Relays Signals to Mitochondria for Inducing Cell Death
7. ER Stress and Cytokines Cross Talk to Modulate Cellular Physiology
8. Involvement of ER Stress in Liver Diseases
9. Therapies Targeting ER Stress in Liver Disease
| Agent | Major Mechanism | Target UPR Protein | Ref. |
|---|---|---|---|
| Asiatic acid | Autophagy induction | All | [168] |
| Astragaloside | AMPK activation | PERK-CHOP | [169] |
| Berberine | Reduction in inflammation | CHOP, ATF4, XBP1 | [170] |
| Resveratrol | Inhibit HSC activation | CHOP | [175] |
| Salubrinal | Inhibt NFκB activation | PERK | [176] |
| Allin, capsaicin gingerol | Prevent steatosis | Multiple | [177] |
| Sinularolide | Prevent HCC | PERK-CHOP | [187] |
| Piperlongumine | Prevent HCC | CHOP | [188,191] |
| Kaempferol | Prevent NASH and HCC | CHOP | [190,192] |
| 4µ8c | Prevent HCC | XBP1s | [186] |
| UDCA, TUDCA, | Multipotent including Steatosis, NASH, PBC, etc. | Multiple | [193] |
| 4-PBA | Counter urea cycle disorders Inhibit steatosis | Multiple | [194] [195] |
10. Conclusions
11. Future Directions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ozcan, L.; Tabas, I. Role of endoplasmic reticulum stress in metabolic disease and other disorders. Annu. Rev. Med. 2012, 63, 317–328. [Google Scholar] [CrossRef] [PubMed]
- Baral, A.; Park, P.H. Leptin Induces Apoptotic and Pyroptotic Cell Death via NLRP3 Inflammasome Activation in Rat Hepatocytes. Int. J. Mol. Sci. 2021, 22, 12589. [Google Scholar] [CrossRef] [PubMed]
- Kil, Y.-S.; Baral, A.; Jeong, B.-S.; Laatikainen, P.; Liu, Y.; Han, A.-R.; Hong, M.-J.; Kim, J.-B.; Choi, H.; Park, P.-H.; et al. Combining NMR and MS to Describe Pyrrole-2-Carbaldehydes in Wheat Bran of Radiation. J. Agric. Food Chem. 2022, 70, 13002–13014. [Google Scholar] [CrossRef] [PubMed]
- Porter, K.R.; Claude, A.; Fullam, E.F. A Study of Tissue Culture Cells by Electron Microscopy: Methods and Preliminary Observations. J. Exp. Med. 1945, 81, 233–246. [Google Scholar] [CrossRef] [PubMed]
- Howell, S.H. Endoplasmic reticulum stress responses in plants. Annu. Rev. Plant Biol. 2013, 64, 477–499. [Google Scholar] [CrossRef] [PubMed]
- Shergalis, A.G.; Hu, S.; Bankhead, A., 3rd; Neamati, N. Role of the ERO1-PDI interaction in oxidative protein folding and disease. Pharmacol. Ther. 2020, 210, 107525. [Google Scholar] [CrossRef]
- Ellgaard, L.; Sevier, C.S.; Bulleid, N.J. How Are Proteins Reduced in the Endoplasmic Reticulum? Trends Biochem. Sci. 2018, 43, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.Y.; Schröder, M.; Kaufman, R.J. Ligand-independent Dimerization Activates the Stress Response Kinases IRE1 and PERK in the Lumen of the Endoplasmic Reticulum*. J. Biol. Chem. 2000, 275, 24881–24885. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Shen, J.; Prywes, R. The luminal domain of ATF6 senses endoplasmic reticulum (ER) stress and causes translocation of ATF6 from the ER to the Golgi. J. Biol. Chem. 2002, 277, 13045–13052. [Google Scholar] [CrossRef]
- Ye, J.; Rawson, R.B.; Komuro, R.; Chen, X.; Davé, U.P.; Prywes, R.; Brown, M.S.; Goldstein, J.L. ER stress induces cleavage of membrane-bound ATF6 by the same proteases that process SREBPs. Mol. Cell 2000, 6, 1355–1364. [Google Scholar] [CrossRef]
- Harding, H.P.; Zhang, Y.; Ron, D. Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature 1999, 397, 271–274. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Tirasophon, W.; Shen, X.; Michalak, M.; Prywes, R.; Okada, T.; Yoshida, H.; Mori, K.; Kaufman, R.J. IRE1-mediated unconventional mRNA splicing and S2P-mediated ATF6 cleavage merge to regulate XBP1 in signaling the unfolded protein response. Genes Dev. 2002, 16, 452–466. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, H.; Okada, T.; Haze, K.; Yanagi, H.; Yura, T.; Negishi, M.; Mori, K. ATF6 activated by proteolysis binds in the presence of NF-Y (CBF) directly to the cis-acting element responsible for the mammalian unfolded protein response. Mol. Cell. Biol. 2000, 20, 6755–6767. [Google Scholar] [CrossRef] [PubMed]
- Gill, A.; Gao, N.; Lehrman, M.A. Rapid activation of glycogen phosphorylase by the endoplasmic reticulum unfolded protein response. J. Biol. Chem. 2002, 277, 44747–44753. [Google Scholar] [CrossRef] [PubMed]
- B’Chir, W.; Chaveroux, C.; Carraro, V.; Averous, J.; Maurin, A.C.; Jousse, C.; Muranishi, Y.; Parry, L.; Fafournoux, P.; Bruhat, A. Dual role for CHOP in the crosstalk between autophagy and apoptosis to determine cell fate in response to amino acid deprivation. Cell. Signal. 2014, 26, 1385–1391. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.; Strudwick, N.; Suwara, M.; Sutcliffe, L.K.; Mihai, A.D.; Ali, A.A.; Watson, J.N.; Schröder, M. An initial phase of JNK activation inhibits cell death early in the endoplasmic reticulum stress response. J. Cell Sci. 2016, 129, 2317–2328. [Google Scholar] [CrossRef] [PubMed]
- Rutkowski, D.T.; Arnold, S.M.; Miller, C.N.; Wu, J.; Li, J.; Gunnison, K.M.; Mori, K.; Sadighi Akha, A.A.; Raden, D.; Kaufman, R.J. Adaptation to ER stress is mediated by differential stabilities of pro-survival and pro-apoptotic mRNAs and proteins. PLoS Biol. 2006, 4, e374. [Google Scholar] [CrossRef]
- Jiang, H.-Y.; Jiang, L.; Wek, R.C. The Eukaryotic Initiation Factor-2 Kinase Pathway Facilitates Differential GADD45a Expression in Response to Environmental Stress*. J. Biol. Chem. 2007, 282, 3755–3765. [Google Scholar] [CrossRef]
- Gupta, M.; Gupta, S.K.; Balliet, A.G.; Hollander, M.C.; Fornace, A.J.; Hoffman, B.; Liebermann, D.A. Hematopoietic cells from Gadd45a- and Gadd45b-deficient mice are sensitized to genotoxic-stress-induced apoptosis. Oncogene 2005, 24, 7170–7179. [Google Scholar] [CrossRef]
- Tanaka, N.; Takahashi, S.; Hu, X.; Lu, Y.; Fujimori, N.; Golla, S.; Fang, Z.-Z.; Aoyama, T.; Krausz, K.W.; Gonzalez, F.J. Growth arrest and DNA damage-inducible 45α protects against nonalcoholic steatohepatitis induced by methionine- and choline-deficient diet. Biochim. Biophys. Acta (BBA)—Mol. Basis Dis. 2017, 1863, 3170–3182. [Google Scholar] [CrossRef]
- Yao, W.; Cai, H.; Li, X.; Li, T.; Hu, L.; Peng, T. Endoplasmic reticulum stress links hepatitis C virus RNA replication to wild-type PGC-1α/liver-specific PGC-1α upregulation. J. Virol. 2014, 88, 8361–8374. [Google Scholar] [CrossRef]
- Stein, D.; Slobodnik, Z.; Tam, B.; Einav, M.; Akabayov, B.; Berstein, S.; Toiber, D. 4-phenylbutyric acid-Identity crisis; can it act as a translation inhibitor? Aging Cell 2022, 21, e13738. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Pan, E.; Zhu, J.; Xu, L.; Chen, X.; Li, J.; Liang, L.; Hu, Y.; Xia, J.; Chen, J.; et al. Cisplatin Enhances Hepatitis B Virus Replication and PGC-1α Expression through Endoplasmic Reticulum Stress. Sci. Rep. 2018, 8, 3496. [Google Scholar] [CrossRef]
- Boslem, E.; Reibe, S. Therapeutic blockade of ER stress and inflammation prevents NASH and progression to HCC. Sci. Adv. 2023, 9, eadh0831. [Google Scholar] [CrossRef]
- Kim, J.Y.; Garcia-Carbonell, R.; Yamachika, S.; Zhao, P.; Dhar, D.; Loomba, R.; Kaufman, R.J.; Saltiel, A.R.; Karin, M. ER Stress Drives Lipogenesis and Steatohepatitis via Caspase-2 Activation of S1P. Cell 2018, 175, 133–145.e15. [Google Scholar] [CrossRef] [PubMed]
- Kon, K.; Ikejima, K.; Uchiyama, A.; Arai, K.; Yamashina, S.; Watanabe, S. Inhibition of endoplasmic reticulum stress by 4-phenylbutyrate prevents steatohepatitis progression and tumorigenesis in NASH-HCC model mice: 1412. Hepatology 2012, 56, 856A. [Google Scholar]
- Sasaki, M.; Yoshimura-Miyakoshi, M.; Sato, Y.; Nakanuma, Y. A possible involvement of endoplasmic reticulum stress in biliary epithelial autophagy and senescence in primary biliary cirrhosis. J. Gastroenterol. 2015, 50, 984–995. [Google Scholar] [CrossRef] [PubMed]
- Okamura, K.; Kimata, Y.; Higashio, H.; Tsuru, A.; Kohno, K. Dissociation of Kar2p/BiP from an ER sensory molecule, Ire1p, triggers the unfolded protein response in yeast. Biochem. Biophys. Res. Commun. 2000, 279, 445–450. [Google Scholar] [CrossRef]
- Pobre, K.F.R.; Poet, G.J. The endoplasmic reticulum (ER) chaperone BiP is a master regulator of ER functions: Getting by with a little help from ERdj friends. J. Biol. Chem. 2019, 294, 2098–2108. [Google Scholar] [CrossRef]
- Wei, J.; Hendershot, L.M. Characterization of the Nucleotide Binding Properties and ATPase Activity of Recombinant Hamster BiP Purified from Bacteria (∗). J. Biol. Chem. 1995, 270, 26670–26676. [Google Scholar] [CrossRef]
- Ichhaporia, V.P.; Kim, J.; Kavdia, K.; Vogel, P.; Horner, L.; Frase, S.; Hendershot, L.M. SIL1, the endoplasmic-reticulum-localized BiP co-chaperone, plays a crucial role in maintaining skeletal muscle proteostasis and physiology. Dis. Models Mech. 2018, 11, dmm033043. [Google Scholar] [CrossRef] [PubMed]
- Vattem, K.M.; Wek, R.C. Reinitiation involving upstream ORFs regulates ATF4 mRNA translation in mammalian cells. Proc. Natl. Acad. Sci. USA 2004, 101, 11269–11274. [Google Scholar] [CrossRef] [PubMed]
- Ryczek, N.; Łyś, A.; Makałowska, I. The Functional Meaning of 5′UTR in Protein-Coding Genes. Int. J. Mol. Sci. 2023, 24, 2976. [Google Scholar] [CrossRef] [PubMed]
- Fusakio, M.E.; Willy, J.A.; Wang, Y.; Mirek, E.T.; Al Baghdadi, R.J.; Adams, C.M.; Anthony, T.G.; Wek, R.C. Transcription factor ATF4 directs basal and stress-induced gene expression in the unfolded protein response and cholesterol metabolism in the liver. Mol. Biol. Cell 2016, 27, 1536–1551. [Google Scholar] [CrossRef] [PubMed]
- Averous, J.; Bruhat, A.; Jousse, C.; Carraro, V.; Thiel, G.; Fafournoux, P. Induction of CHOP Expression by Amino Acid Limitation Requires Both ATF4 Expression and ATF2 Phosphorylation*. J. Biol. Chem. 2004, 279, 5288–5297. [Google Scholar] [CrossRef] [PubMed]
- Shamu, C.E.; Walter, P. Oligomerization and phosphorylation of the Ire1p kinase during intracellular signaling from the endoplasmic reticulum to the nucleus. EMBO J. 1996, 15, 3028–3039. [Google Scholar] [CrossRef] [PubMed]
- Bertolotti, A.; Wang, X.; Novoa, I.; Jungreis, R.; Schlessinger, K.; Cho, J.H.; West, A.B.; Ron, D. Increased sensitivity to dextran sodium sulfate colitis in IRE1beta-deficient mice. J. Clin. Investig. 2001, 107, 585–593. [Google Scholar] [CrossRef] [PubMed]
- Siwecka, N.; Rozpędek-Kamińska, W. The Structure, Activation and Signaling of IRE1 and Its Role in Determining Cell Fate. Biomedicines 2021, 9, 156. [Google Scholar] [CrossRef] [PubMed]
- Back, S.H.; Lee, K.; Vink, E.; Kaufman, R.J. Cytoplasmic IRE1alpha-mediated XBP1 mRNA splicing in the absence of nuclear processing and endoplasmic reticulum stress. J. Biol. Chem. 2006, 281, 18691–18706. [Google Scholar] [CrossRef]
- Gómora-García, J.C.; Gerónimo-Olvera, C.; Pérez-Martínez, X.; Massieu, L. IRE1α RIDD activity induced under ER stress drives neuronal death by the degradation of 14-3-3 θ mRNA in cortical neurons during glucose deprivation. Cell Death Discov. 2021, 7, 131. [Google Scholar] [CrossRef]
- Oikawa, D.; Kimata, Y.; Kohno, K. Self-association and BiP dissociation are not sufficient for activation of the ER stress sensor Ire1. J. Cell Sci. 2007, 120 Pt 9, 1681–1688. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Shen, J.; Arenzana, N.; Tirasophon, W.; Kaufman, R.J.; Prywes, R. Activation of ATF6 and an ATF6 DNA binding site by the endoplasmic reticulum stress response. J. Biol. Chem. 2000, 275, 27013–27020. [Google Scholar] [CrossRef] [PubMed]
- Thuerauf, D.J.; Morrison, L.; Glembotski, C.C. Opposing Roles for ATF6α and ATF6β in Endoplasmic Reticulum Stress Response Gene Induction*. J. Biol. Chem. 2004, 279, 21078–21084. [Google Scholar] [CrossRef] [PubMed]
- Luethy, J.D.; Fargnoli, J.; Park, J.S.; Fornace, A.J., Jr.; Holbrook, N.J. Isolation and characterization of the hamster gadd153 gene. Activation of promoter activity by agents that damage DNA. J. Biol. Chem. 1990, 265, 16521–16526. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Z.; Lawson, B.; Brewer, J.W.; Zinszner, H.; Sanjay, A.; Mi, L.J.; Boorstein, R.; Kreibich, G.; Hendershot, L.M.; Ron, D. Signals from the stressed endoplasmic reticulum induce C/EBP-homologous protein (CHOP/GADD153). Mol. Cell. Biol. 1996, 16, 4273–4280. [Google Scholar] [CrossRef] [PubMed]
- Maytin, E.V.; Ubeda, M.; Lin, J.C.; Habener, J.F. Stress-inducible transcription factor CHOP/gadd153 induces apoptosis in mammalian cells via p38 kinase-dependent and -independent mechanisms. Exp. Cell Res. 2001, 267, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.; Xu, W.; Reed, J.C. Cell death and endoplasmic reticulum stress: Disease relevance and therapeutic opportunities. Nat. Rev. Drug Discov. 2008, 7, 1013–1030. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, J.D.; Luethy, J.D.; Carlson, S.G.; Sollott, S.J.; Holbrook, N.J. Calcium ionophore A23187 induces expression of the growth arrest and DNA damage inducible CCAAT/enhancer-binding protein (C/EBP)-related gene, gadd153. Ca2+ increases transcriptional activity and mRNA stability. J. Biol. Chem. 1992, 267, 20465–20470. [Google Scholar] [CrossRef] [PubMed]
- Bruhat, A.; Jousse, C.; Wang, X.-Z.; Ron, D.; Ferrara, M.; Fafournoux, P. Amino Acid Limitation Induces Expression of CHOP, a CCAAT/Enhancer Binding Protein-related Gene, at Both Transcriptional and Post-transcriptional Levels *. J. Biol. Chem. 1997, 272, 17588–17593. [Google Scholar] [CrossRef] [PubMed]
- Oyadomari, S.; Mori, M. Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ. 2004, 11, 381–389. [Google Scholar] [CrossRef]
- Dai, X.; Ding, Y.; Liu, Z.; Zhang, W.; Zou, M.H. Phosphorylation of CHOP (C/EBP Homologous Protein) by the AMP-Activated Protein Kinase Alpha 1 in Macrophages Promotes CHOP Degradation and Reduces Injury-Induced Neointimal Disruption In Vivo. Circ. Res. 2016, 119, 1089–1100. [Google Scholar] [CrossRef]
- Chikka, M.R.; McCabe, D.D.; Tyra, H.M.; Rutkowski, D.T. C/EBP homologous protein (CHOP) contributes to suppression of metabolic genes during endoplasmic reticulum stress in the liver. J. Biol. Chem. 2013, 288, 4405–4415. [Google Scholar] [CrossRef]
- Liu, K.; Zhao, C. A beneficial adaptive role for CHOP in driving cell fate selection during ER stress. EMBO Rep. 2024, 25, 228–253. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Tian, M.; Ding, C.; Yu, S. The C/EBP Homologous Protein (CHOP) Transcription Factor Functions in Endoplasmic Reticulum Stress-Induced Apoptosis and Microbial Infection. Front. Immunol. 2018, 9, 3083. [Google Scholar] [CrossRef] [PubMed]
- Marciniak, S.J.; Yun, C.Y.; Oyadomari, S.; Novoa, I.; Zhang, Y.; Jungreis, R.; Nagata, K.; Harding, H.P.; Ron, D. CHOP induces death by promoting protein synthesis and oxidation in the stressed endoplasmic reticulum. Genes Dev. 2004, 18, 3066–3077. [Google Scholar] [CrossRef] [PubMed]
- Barone, M.V.; Crozat, A.; Tabaee, A.; Philipson, L.; Ron, D. CHOP (GADD153) and its oncogenic variant, TLS-CHOP, have opposing effects on the induction of G1/S arrest. Genes Dev. 1994, 8, 453–464. [Google Scholar] [CrossRef]
- DeZwaan-McCabe, D.; Riordan, J.D.; Arensdorf, A.M.; Icardi, M.S.; Dupuy, A.J.; Rutkowski, D.T. The stress-regulated transcription factor CHOP promotes hepatic inflammatory gene expression, fibrosis, and oncogenesis. PLoS Genet. 2013, 9, e1003937. [Google Scholar] [CrossRef] [PubMed]
- Toriguchi, K.; Hatano, E.; Tanabe, K.; Takemoto, K.; Nakamura, K.; Koyama, Y.; Seo, S.; Taura, K.; Uemoto, S. Attenuation of steatohepatitis, fibrosis, and carcinogenesis in mice fed a methionine-choline deficient diet by CCAAT/enhancer-binding protein homologous protein deficiency. J. Gastroenterol. Hepatol. 2014, 29, 1109–1118. [Google Scholar] [CrossRef] [PubMed]
- Ji, C.; Mehrian-Shai, R.; Chan, C.; Hsu, Y.H.; Kaplowitz, N. Role of CHOP in hepatic apoptosis in the murine model of intragastric ethanol feeding. Alcohol. Clin. Exp. Res. 2005, 29, 1496–1503. [Google Scholar] [CrossRef]
- Tamaki, N.; Hatano, E.; Taura, K.; Tada, M.; Kodama, Y.; Nitta, T.; Iwaisako, K.; Seo, S.; Nakajima, A.; Ikai, I.; et al. CHOP deficiency attenuates cholestasis-induced liver fibrosis by reduction of hepatocyte injury. Am. J. Physiol. Gastrointest. Liver Physiol. 2008, 294, G498–G505. [Google Scholar] [CrossRef]
- Cazanave, S.C.; Mott, J.L.; Bronk, S.F.; Werneburg, N.W.; Fingas, C.D.; Meng, X.W.; Finnberg, N.; El-Deiry, W.S.; Kaufmann, S.H.; Gores, G.J. Death receptor 5 signaling promotes hepatocyte lipoapoptosis. J. Biol. Chem. 2011, 286, 39336–39348. [Google Scholar] [CrossRef] [PubMed]
- Allagnat, F.; Fukaya, M.; Nogueira, T.C.; Delaroche, D.; Welsh, N.; Marselli, L.; Marchetti, P.; Haefliger, J.A.; Eizirik, D.L.; Cardozo, A.K. C/EBP homologous protein contributes to cytokine-induced pro-inflammatory responses and apoptosis in β-cells. Cell Death Differ. 2012, 19, 1836–1846. [Google Scholar] [CrossRef]
- Willy, J.A.; Young, S.K.; Stevens, J.L.; Masuoka, H.C.; Wek, R.C. CHOP links endoplasmic reticulum stress to NF-κB activation in the pathogenesis of nonalcoholic steatohepatitis. Mol. Biol. Cell 2015, 26, 2190–2204. [Google Scholar] [CrossRef]
- Campos, G.; Schmidt-Heck, W.; Ghallab, A.; Rochlitz, K.; Pütter, L.; Medinas, D.B.; Hetz, C.; Widera, A.; Cadenas, C.; Begher-Tibbe, B.; et al. The transcription factor CHOP, a central component of the transcriptional regulatory network induced upon CCl4 intoxication in mouse liver, is not a critical mediator of hepatotoxicity. Arch. Toxicol. 2014, 88, 1267–1280. [Google Scholar] [CrossRef] [PubMed]
- Novoa, I.; Zeng, H.; Harding, H.P.; Ron, D. Feedback inhibition of the unfolded protein response by GADD34-mediated dephosphorylation of eIF2alpha. J. Cell Biol. 2001, 153, 1011–1022. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Nishio, N.; Ito, S.; Tanaka, Y.; Sun, Y.; Isobe, K. Growth arrest and DNA damage-inducible protein (GADD34) enhanced liver inflammation and tumorigenesis in a diethylnitrosamine (DEN)-treated murine model. Cancer Immunol. Immunother. 2015, 64, 777–789. [Google Scholar] [CrossRef] [PubMed]
- Clarke, H.J.; Chambers, J.E.; Liniker, E.; Marciniak, S.J. Endoplasmic Reticulum Stress in Malignancy. Cancer Cell 2014, 25, 563–573. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Frank, C.L.; Korth, M.J.; Sopher, B.L.; Novoa, I.; Ron, D.; Katze, M.G. Control of PERK eIF2alpha kinase activity by the endoplasmic reticulum stress-induced molecular chaperone P58IPK. Proc. Natl. Acad. Sci. USA 2002, 99, 15920–15925. [Google Scholar] [CrossRef] [PubMed]
- Bailly-Maitre, B.; Fondevila, C.; Kaldas, F.; Droin, N.; Luciano, F.; Ricci, J.E.; Croxton, R.; Krajewska, M.; Zapata, J.M.; Kupiec-Weglinski, J.W.; et al. Cytoprotective gene bi-1 is required for intrinsic protection from endoplasmic reticulum stress and ischemia-reperfusion injury. Proc. Natl. Acad. Sci. USA 2006, 103, 2809–2814. [Google Scholar] [CrossRef]
- Pinkaew, D.; Chattopadhyay, A. Fortilin binds IRE1α and prevents ER stress from signaling apoptotic cell death. Nat. Commun. 2017, 8, 18. [Google Scholar] [CrossRef]
- Nakato, R.; Ohkubo, Y.; Konishi, A.; Shibata, M.; Kaneko, Y.; Iwawaki, T.; Nakamura, T.; Lipton, S.A.; Uehara, T. Regulation of the unfolded protein response via S-nitrosylation of sensors of endoplasmic reticulum stress. Sci. Rep. 2015, 5, 14812. [Google Scholar] [CrossRef] [PubMed]
- Shemorry, A.; Harnoss, J.M. Caspase-mediated cleavage of IRE1 controls apoptotic cell commitment during endoplasmic reticulum stress. Elife 2019, 8, e47084. [Google Scholar] [CrossRef] [PubMed]
- Grey, M.J.; Cloots, E.; Simpson, M.S.; LeDuc, N.; Serebrenik, Y.V.; De Luca, H.; De Sutter, D.; Luong, P.; Thiagarajah, J.R.; Paton, A.W.; et al. IRE1β negatively regulates IRE1α signaling in response to endoplasmic reticulum stress. J. Cell Biol. 2020, 219, e201904048. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.-Y.; Wu, L.; Liu, Y.-F.; Tang, M.-Y.; Tang, J.-Y.; Deng, Y.-Q.; Liu, L.; Nie, B.-B.; Zou, Z.-K.; Huang, L. IRE1α: From the function to the potential therapeutic target in atherosclerosis. Mol. Cell. Biochem. 2024, 479, 1079–1092. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Yang, Z.; Zhang, K.; Fang, D.; Sun, F. SUMOylation represses the transcriptional activity of the Unfolded Protein Response transducer ATF6. Biochem. Biophys. Res. Commun. 2017, 494, 446–451. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, S.G.; Ishigaki, S.; Oslowski, C.M.; Lu, S.; Lipson, K.L.; Ghosh, R.; Hayashi, E.; Ishihara, H.; Oka, Y.; Permutt, M.A.; et al. Wolfram syndrome 1 gene negatively regulates ER stress signaling in rodent and human cells. J. Clin. Investig. 2010, 120, 744–755. [Google Scholar] [CrossRef] [PubMed]
- Tsukumo, Y.; Tomida, A.; Kitahara, O.; Nakamura, Y.; Asada, S.; Mori, K.; Tsuruo, T. Nucleobindin 1 Controls the Unfolded Protein Response by Inhibiting ATF6 Activation*. J. Biol. Chem. 2007, 282, 29264–29272. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, H.; Uemura, A.; Mori, K. pXBP1(U), a negative regulator of the unfolded protein response activator pXBP1(S), targets ATF6 but not ATF4 in proteasome-mediated degradation. Cell Struct. Funct. 2009, 34, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Wang, Y.; Yang, J.; Klöting, N.; Liu, C.; Dai, J.; Jin, S.; Chen, L.; Liu, S.; Liu, Y.; et al. EMC10 modulates hepatic ER stress and steatosis in an isoform specific manner. J. Hepatol. 2024, in press. [Google Scholar] [CrossRef]
- Brodsky, J.L.; Werner, E.D.; Dubas, M.E.; Goeckeler, J.L.; Kruse, K.B.; McCracken, A.A. The requirement for molecular chaperones during endoplasmic reticulum-associated protein degradation demonstrates that protein export and import are mechanistically distinct. J. Biol. Chem. 1999, 274, 3453–3460. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Sun, S.; Wang, H.; Liu, M.; Long, Q.; Yin, L.; Kersten, S.; Zhang, K.; Qi, L. Hepatic Sel1L-Hrd1 ER-associated degradation (ERAD) manages FGF21 levels and systemic metabolism via CREBH. EMBO J. 2018, 37, e99277. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Shi, G.; Sha, H.; Ji, Y.; Han, X.; Shu, X.; Ma, H.; Inoue, T.; Gao, B.; Kim, H.; et al. IRE1α is an endogenous substrate of endoplasmic-reticulum-associated degradation. Nat. Cell Biol. 2015, 17, 1546–1555. [Google Scholar] [CrossRef] [PubMed]
- Reggiori, F.; Molinari, M. ER-phagy: Mechanisms, regulation, and diseases connected to the lysosomal clearance of the endoplasmic reticulum. Physiol. Rev. 2022, 102, 1393–1448. [Google Scholar] [CrossRef] [PubMed]
- Duwaerts, C.C.; Maiers, J.L. ER Disposal Pathways in Chronic Liver Disease: Protective, Pathogenic, and Potential Therapeutic Targets. Front. Mol. Biosci. 2022, 8, 804097. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.; Li, L.; Ren, Z.; Li, J.; Wang, R.; Ding, H. FAM134B-mediated ER-phagy alleviates endoplasmic reticulum stress of rat soleus muscle in response to acute exercise. Gen. Physiol. Biophys. 2022, 41, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Forrester, A.; De Leonibus, C.; Grumati, P.; Fasana, E.; Piemontese, M.; Staiano, L.; Fregno, I.; Raimondi, A.; Marazza, A.; Bruno, G.; et al. A selective ER-phagy exerts procollagen quality control via a Calnexin-FAM134B complex. EMBO J. 2019, 38, e99847. [Google Scholar] [CrossRef] [PubMed]
- D’Erasmo, L.; Di Costanzo, A.; Gallo, A.; Bruckert, E.; Arca, M. ApoCIII: A multifaceted protein in cardiometabolic disease. Metab. Clin. Exp. 2020, 113, 154395. [Google Scholar] [CrossRef] [PubMed]
- Rao, R.V.; Castro-Obregon, S.; Frankowski, H.; Schuler, M.; Stoka, V.; del Rio, G.; Bredesen, D.E.; Ellerby, H.M. Coupling Endoplasmic Reticulum Stress to the Cell Death Program: AN Apaf-1-Independent Intrinsic Pathway*. J. Biol. Chem. 2002, 277, 21836–21842. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Pandey, P.; Mishra, N.; Kumar, S.; Narula, N.; Kharbanda, S.; Saxena, S.; Kufe, D. Targeting of the c-Abl tyrosine kinase to mitochondria in endoplasmic reticulum stress-induced apoptosis. Mol. Cell. Biol. 2001, 21, 6233–6242. [Google Scholar] [CrossRef] [PubMed]
- Lodish, H.F.; Kong, N.; Wikström, L. Calcium is required for folding of newly made subunits of the asialoglycoprotein receptor within the endoplasmic reticulum. J. Biol. Chem. 1992, 267, 12753–12760. [Google Scholar] [CrossRef]
- Xiao, W.C.; Zhang, J.; Chen, S.L.; Shi, Y.J.; Xiao, F.; An, W. Alleviation of palmitic acid-induced endoplasmic reticulum stress by augmenter of liver regeneration through IP3R-controlled Ca2+ release. J. Cell. Physiol. 2018, 233, 6148–6157. [Google Scholar] [CrossRef]
- Santulli, G.; Xie, W.; Reiken, S.R.; Marks, A.R. Mitochondrial calcium overload is a key determinant in heart failure. Proc. Natl. Acad. Sci. USA 2015, 112, 11389–11394. [Google Scholar] [CrossRef]
- Waterhouse, N.J.; Finucane, D.M.; Green, D.R.; Elce, J.S.; Kumar, S.; Alnemri, E.S.; Litwack, G.; Khanna, K.; Lavin, M.F.; Watters, D.J. Calpain activation is upstream of caspases in radiation-induced apoptosis. Cell Death Differ. 1998, 5, 1051–1061. [Google Scholar] [CrossRef] [PubMed]
- Norberg, E.; Gogvadze, V.; Ott, M.; Horn, M.; Uhlén, P.; Orrenius, S.; Zhivotovsky, B. An increase in intracellular Ca2+ is required for the activation of mitochondrial calpain to release AIF during cell death. Cell Death Differ. 2008, 15, 1857–1864. [Google Scholar] [CrossRef]
- Mathiasen, I.S.; Sergeev, I.N.; Bastholm, L.; Elling, F.; Norman, A.W.; Jäättelä, M. Calcium and calpain as key mediators of apoptosis-like death induced by vitamin D compounds in breast cancer cells. J. Biol. Chem. 2002, 277, 30738–30745. [Google Scholar] [CrossRef]
- Mohsin, A.A.; Thompson, J.; Hu, Y.; Hollander, J.; Lesnefsky, E.J.; Chen, Q. Endoplasmic reticulum stress-induced complex I defect: Central role of calcium overload. Arch. Biochem. Biophys. 2020, 683, 108299. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Yang, Y.; Shan, S.; Huang, Z.; Liu, Z.; Yong, H.; Liu, Z.; Zhang, C.; Song, F. Increased IP3R-3 degradation induced by acrylamide promoted Ca2+-dependent calpain activation and axon damage in rats. Toxicol. Lett. 2023, 383, 162–176. [Google Scholar] [CrossRef]
- Chen, Q.; Thompson, J.; Hu, Y.; Lesnefsky, E.J. Tunicamycin-Induced Endoplasmic Reticulum Stress Damages Complex I in Cardiac Mitochondria. Life 2022, 12, 1209. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Guan, Z.; Gao, Y.; Bai, Y.; Zhan, X.; Ji, X.; Xu, J.; Zhou, H.; Rao, Z. ER stress promotes mitochondrial calcium overload and activates the ROS/NLRP3 axis to mediate fatty liver ischemic injury. Hepatol. Commun. 2024, 8, e0399. [Google Scholar] [CrossRef]
- Ghosh, S.; Chowdhury, S.; Das, A.K.; Sil, P.C. Taurine ameliorates oxidative stress induced inflammation and ER stress mediated testicular damage in STZ-induced diabetic Wistar rats. Food Chem. Toxicol. 2019, 124, 64–80. [Google Scholar] [CrossRef]
- Lebeaupin, C.; Proics, E.; de Bieville, C.H.; Rousseau, D.; Bonnafous, S.; Patouraux, S.; Adam, G.; Lavallard, V.J.; Rovere, C.; Le Thuc, O.; et al. ER stress induces NLRP3 inflammasome activation and hepatocyte death. Cell Death Dis. 2015, 6, e1879. [Google Scholar] [CrossRef] [PubMed]
- Baral, A. Mechanisms of Inflammasome Activation and Involvement in Liver Disease. J. Mol. Pathol. 2024, 5, 171–186. [Google Scholar] [CrossRef]
- Negrin, K.A.; Roth Flach, R.J.; DiStefano, M.T.; Matevossian, A.; Friedline, R.H.; Jung, D.; Kim, J.K.; Czech, M.P. IL-1 signaling in obesity-induced hepatic lipogenesis and steatosis. PLoS ONE 2014, 9, e107265. [Google Scholar] [CrossRef] [PubMed]
- Kamari, Y.; Shaish, A.; Vax, E.; Shemesh, S.; Kandel-Kfir, M.; Arbel, Y.; Olteanu, S.; Barshack, I.; Dotan, S.; Voronov, E.; et al. Lack of interleukin-1α or interleukin-1β inhibits transformation of steatosis to steatohepatitis and liver fibrosis in hypercholesterolemic mice. J. Hepatol. 2011, 55, 1086–1094. [Google Scholar] [CrossRef] [PubMed]
- Petrasek, J.; Bala, S.; Csak, T.; Lippai, D.; Kodys, K.; Menashy, V.; Barrieau, M.; Min, S.Y.; Kurt-Jones, E.A.; Szabo, G. IL-1 receptor antagonist ameliorates inflammasome-dependent alcoholic steatohepatitis in mice. J. Clin. Investig. 2012, 122, 3476–3489. [Google Scholar] [CrossRef]
- Kandel-Kfir, M.; Almog, T.; Shaish, A.; Shlomai, G.; Anafi, L.; Avivi, C.; Barshack, I.; Grosskopf, I.; Harats, D.; Kamari, Y. Interleukin-1α deficiency attenuates endoplasmic reticulum stress-induced liver damage and CHOP expression in mice. J. Hepatol. 2015, 63, 926–933. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhao, N.; Zhu, H.; Zhu, S.; Pan, S.; Xu, J.; Zhang, X.; Zhang, Y.; Wang, J. Circulating interleukin-1β promotes endoplasmic reticulum stress-induced myocytes apoptosis in diabetic cardiomyopathy via interleukin-1 receptor-associated kinase-2. Cardiovasc. Diabetol. 2015, 14, 125. [Google Scholar] [CrossRef]
- Dasgupta, D.; Ghosh, S.; Dey, I.; Majumdar, S.; Chowdhury, S.; Das, S.; Banerjee, S.; Saha, M.; Ghosh, A.; Roy, N.; et al. Influence of polymorphisms in TNF-α and IL1β on susceptibility to alcohol induced liver diseases and therapeutic potential of miR-124-3p impeding TNF-α/IL1β mediated multi-cellular signaling in liver microenvironment. Front. Immunol. 2023, 14, 1241755. [Google Scholar] [CrossRef]
- Chae, M.K.; Park, S.G.; Song, S.O.; Kang, E.S.; Cha, B.S.; Lee, H.C.; Lee, B.W. Pentoxifylline attenuates methionine- and choline-deficient-diet-induced steatohepatitis by suppressing TNF-α expression and endoplasmic reticulum stress. Exp. Diabetes Res. 2012, 2012, 762565. [Google Scholar] [CrossRef]
- Hu, P.; Han, Z.; Couvillon, A.D.; Kaufman, R.J.; Exton, J.H. Autocrine tumor necrosis factor alpha links endoplasmic reticulum stress to the membrane death receptor pathway through IRE1alpha-mediated NF-kappaB activation and down-regulation of TRAF2 expression. Mol. Cell. Biol. 2006, 26, 3071–3084. [Google Scholar] [CrossRef]
- Liu, J.; Ren, F.; Cheng, Q.; Bai, L.; Shen, X.; Gao, F.; Busuttil, R.W.; Kupiec-Weglinski, J.W.; Zhai, Y. Endoplasmic reticulum stress modulates liver inflammatory immune response in the pathogenesis of liver ischemia and reperfusion injury. Transplantation 2012, 94, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, H.; Umemura, A.; Taniguchi, K.; Font-Burgada, J.; Dhar, D.; Ogata, H.; Zhong, Z.; Valasek, M.A.; Seki, E.; Hidalgo, J.; et al. ER stress cooperates with hypernutrition to trigger TNF-dependent spontaneous HCC development. Cancer Cell 2014, 26, 331–343. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, Y.; Suzuki, O.; Haruyama, T.; Akaike, T. Interferon-gamma induces reactive oxygen species and endoplasmic reticulum stress at the hepatic apoptosis. J. Cell. Biochem. 2003, 89, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Shan, B.; Dai, J.; Xia, Z.; Cai, J.; Chen, T.; Lv, S.; Feng, Y.; Zheng, L.; Wang, Y.; et al. Dual role for inositol-requiring enzyme 1α in promoting the development of hepatocellular carcinoma during diet-induced obesity in mice. Hepatology 2018, 68, 533–546. [Google Scholar] [CrossRef] [PubMed]
- Henkel, A.S.; LeCuyer, B.; Olivares, S.; Green, R.M. Endoplasmic Reticulum Stress Regulates Hepatic Bile Acid Metabolism in Mice. Cell. Mol. Gastroenterol. Hepatol. 2017, 3, 261–271. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.M.; Xu, X.S. Kupffer Cell-Derived TNF-α Triggers the Apoptosis of Hepatic Stellate Cells through TNF-R1/Caspase 8 due to ER Stress. BioMed Res. Int. 2020, 2020, 8035671. [Google Scholar] [CrossRef] [PubMed]
- Khanal, S.; Bok, E.; Kim, J.; Park, G.H.; Choi, D.Y. Dopaminergic neuroprotective effects of inosine in MPTP-induced parkinsonian mice via brain-derived neurotrophic factor upregulation. Neuropharmacology 2023, 238, 109652. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.; Dainty, S.; Strudwick, N.; Mihai, A.D.; Watson, J.N.; Dendooven, R.; Paton, A.W.; Paton, J.C.; Schröder, M. Endoplasmic reticulum stress causes insulin resistance by inhibiting delivery of newly synthesized insulin receptors to the cell surface. Mol. Biol. Cell 2020, 31, 2597–2629. [Google Scholar] [CrossRef] [PubMed]
- Schulze-Bergkamen, H.; Brenner, D.; Krueger, A.; Suess, D.; Fas, S.C.; Frey, C.R.; Dax, A.; Zink, D.; Büchler, P.; Müller, M.; et al. Hepatocyte growth factor induces Mcl-1 in primary human hepatocytes and inhibits CD95-mediated apoptosis via Akt. Hepatology 2004, 39, 645–654. [Google Scholar] [CrossRef]
- Nogueira, J.P.; Cusi, K. Role of Insulin Resistance in the Development of Nonalcoholic Fatty Liver Disease in People With Type 2 Diabetes: From Bench to Patient Care. Diabetes Spectr. 2024, 37, 20–28. [Google Scholar] [CrossRef]
- Utzschneider, K.M.; Kahn, S.E. The Role of Insulin Resistance in Nonalcoholic Fatty Liver Disease. J. Clin. Endocrinol. Metab. 2006, 91, 4753–4761. [Google Scholar] [CrossRef] [PubMed]
- Lebeaupin, C.; Vallée, D.; Rousseau, D.; Patouraux, S.; Bonnafous, S.; Adam, G.; Luciano, F.; Luci, C.; Anty, R.; Iannelli, A.; et al. Bax inhibitor-1 protects from nonalcoholic steatohepatitis by limiting inositol-requiring enzyme 1 alpha signaling in mice. Hepatology 2018, 68, 515–532. [Google Scholar] [CrossRef]
- Shreya, S.; Grosset, C.F. Unfolded Protein Response Signaling in Liver Disorders: A 2023 Updated Review. Int. J. Mol. Sci. 2023, 24, 14066. [Google Scholar] [CrossRef]
- Lebeaupin, C.; Vallée, D.; Hazari, Y.; Hetz, C.; Chevet, E.; Bailly-Maitre, B. Endoplasmic reticulum stress signalling and the pathogenesis of non-alcoholic fatty liver disease. J. Hepatol. 2018, 69, 927–947. [Google Scholar] [CrossRef]
- Gentile, C.L.; Pagliassotti, M.J. The role of fatty acids in the development and progression of nonalcoholic fatty liver disease. J. Nutr. Biochem. 2008, 19, 567–576. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-h.; Li, C.-y.; Muhammad, I.; Zhang, X.-y. Fatty acid composition in serum correlates with that in the liver and non-alcoholic fatty liver disease activity scores in mice fed a high-fat diet. Environ. Toxicol. Pharmacol. 2016, 44, 140–150. [Google Scholar] [CrossRef] [PubMed]
- Anderson, S.G.; Sanders, T.A.; Cruickshank, J.K. Plasma fatty acid composition as a predictor of arterial stiffness and mortality. Hypertension 2009, 53, 839–845. [Google Scholar] [CrossRef]
- Han, M.S.; Park, S.Y.; Shinzawa, K.; Kim, S.; Chung, K.W.; Lee, J.-H.; Kwon, C.H.; Lee, K.-W.; Lee, J.-H.; Park, C.K.; et al. Lysophosphatidylcholine as a death effector in the lipoapoptosis of hepatocytes. J. Lipid Res. 2008, 49, 84–97. [Google Scholar] [CrossRef]
- Kakisaka, K.; Cazanave, S.C.; Fingas, C.D.; Guicciardi, M.E.; Bronk, S.F.; Werneburg, N.W.; Mott, J.L.; Gores, G.J. Mechanisms of lysophosphatidylcholine-induced hepatocyte lipoapoptosis. Am. J. Physiol.-Gastrointest. Liver Physiol. 2012, 302, G77–G84. [Google Scholar] [CrossRef]
- Branković, M.; Jovanović, I.; Dukić, M.; Radonjić, T.; Oprić, S.; Klašnja, S.; Zdravković, M. Lipotoxicity as the Leading Cause of Non-Alcoholic Steatohepatitis. Int. J. Mol. Sci. 2022, 23, 5146. [Google Scholar] [CrossRef]
- Mendez-Sanchez, N.; Cruz-Ramon, V.C.; Ramirez-Perez, O.L.; Hwang, J.P.; Barranco-Fragoso, B.; Cordova-Gallardo, J. New Aspects of Lipotoxicity in Nonalcoholic Steatohepatitis. Int. J. Mol. Sci. 2018, 19, 2034. [Google Scholar] [CrossRef] [PubMed]
- Fang, D.L.; Wan, Y.; Shen, W.; Cao, J.; Sun, Z.X.; Yu, H.H.; Zhang, Q.; Cheng, W.H.; Chen, J.; Ning, B. Endoplasmic reticulum stress leads to lipid accumulation through upregulation of SREBP-1c in normal hepatic and hepatoma cells. Mol. Cell. Biochem. 2013, 381, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Hasnain, S.Z.; Lourie, R.; Das, I.; Chen, A.C.; McGuckin, M.A. The interplay between endoplasmic reticulum stress and inflammation. Immunol. Cell Biol. 2012, 90, 260–270. [Google Scholar] [CrossRef] [PubMed]
- Taub, R. Blocking NF-kappaB in the liver: The good and bad news. Hepatology 1998, 27, 1445–1446. [Google Scholar] [CrossRef] [PubMed]
- Suetsugu, H.; Iimuro, Y.; Uehara, T.; Nishio, T.; Harada, N.; Yoshida, M.; Hatano, E.; Son, G.; Fujimoto, J.; Yamaoka, Y. Nuclear factor {kappa}B inactivation in the rat liver ameliorates short term total warm ischaemia/reperfusion injury. Gut 2005, 54, 835–842. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Karin, M. NF-kappaB signaling, liver disease and hepatoprotective agents. Oncogene 2008, 27, 6228–6244. [Google Scholar] [CrossRef]
- Ahmadi, A.; Niknahad, H.; Li, H.; Mobasheri, A.; Manthari, R.K.; Azarpira, N.; Mousavi, K.; Khalvati, B.; Zhao, Y.; Sun, J.; et al. The inhibition of NFкB signaling and inflammatory response as a strategy for blunting bile acid-induced hepatic and renal toxicity. Toxicol. Lett. 2021, 349, 12–29. [Google Scholar] [CrossRef]
- Henkel, A.S.; Dewey, A.M.; Anderson, K.A.; Olivares, S.; Green, R.M. Reducing endoplasmic reticulum stress does not improve steatohepatitis in mice fed a methionine- and choline-deficient diet. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 303, G54–G59. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, K.; Paul, S.; Libby, A. HERV1-env Induces Unfolded Protein Response Activation in Autoimmune Liver Disease: A Potential Mechanism for Regulatory T Cell Dysfunction. J. Immunol. 2023, 210, 732–744. [Google Scholar] [CrossRef]
- Heneghan, M.A.; Yeoman, A.D.; Verma, S.; Smith, A.D.; Longhi, M.S. Autoimmune hepatitis. Lancet 2013, 382, 1433–1444. [Google Scholar] [CrossRef]
- Krawitt, E.L. Autoimmune hepatitis: Classification, heterogeneity, and treatment. Am. J. Med. 1994, 96 (Suppl. S1), S23–S26. [Google Scholar] [CrossRef] [PubMed]
- Mima, S.; Sekiya, C.; Kanagawa, H.; Uchida, T. Ursodeoxycholic acid (UDCA) therapy for autoimmune hepatitis. Int. Hepatol. Commun. 1994, 2, 207–212. [Google Scholar] [CrossRef]
- Czaja, A.J.; Carpenter, H.A.; Lindor, K.D. Ursodeoxycholic acid as adjunctive therapy for problematic type 1 autoimmune hepatitis: A randomized placebo-controlled treatment trial. Hepatology 1999, 30, 1381–1386. [Google Scholar] [CrossRef]
- Nakamura, K.; Yoneda, M.; Yokohama, S.; Tamori, K.; Sato, Y.; Aso, K.; Aoshima, M.; Hasegawa, T.; Making, I. Efficacy of ursodeoxycholic acid in Japanese patients with type 1 autoimmune hepatitis. J. Gastroenterol. Hepatol. 1998, 13, 490–495. [Google Scholar] [CrossRef] [PubMed]
- Iorga, A.; Dara, L. Cell death in drug-induced liver injury. Adv. Pharmacol. 2019, 85, 31–74. [Google Scholar] [PubMed]
- Iorga, A.; Dara, L.; Kaplowitz, N. Drug-Induced Liver Injury: Cascade of Events Leading to Cell Death, Apoptosis or Necrosis. Int. J. Mol. Sci. 2017, 18, 1018. [Google Scholar] [CrossRef] [PubMed]
- Fredriksson, L.; Wink, S.; Herpers, B.; Benedetti, G.; Hadi, M.; de Bont, H.; Groothuis, G.; Luijten, M.; Danen, E.; de Graauw, M.; et al. Drug-induced endoplasmic reticulum and oxidative stress responses independently sensitize toward TNFα-mediated hepatotoxicity. Toxicol. Sci. Off. J. Soc. Toxicol. 2014, 140, 144–159. [Google Scholar] [CrossRef] [PubMed]
- Ye, H.; Chen, C.; Wu, H.; Zheng, K.; Martín-Adrados, B.; Caparros, E.; Francés, R.; Nelson, L.J.; Gómez Del Moral, M.; Asensio, I.; et al. Genetic and pharmacological inhibition of XBP1 protects against APAP hepatotoxicity through the activation of autophagy. Cell Death Dis. 2022, 13, 143. [Google Scholar] [CrossRef] [PubMed]
- Na, M.; Yang, X.; Deng, Y.; Yin, Z.; Li, M. Endoplasmic reticulum stress in the pathogenesis of alcoholic liver disease. PeerJ 2023, 11, e16398. [Google Scholar] [CrossRef] [PubMed]
- Farfán Labonne, B.E.; Gutiérrez, M.; Gómez-Quiroz, L.E.; Konigsberg Fainstein, M.; Bucio, L.; Souza, V.; Flores, O.; Ortíz, V.; Hernández, E.; Kershenobich, D.; et al. Acetaldehyde-induced mitochondrial dysfunction sensitizes hepatocytes to oxidative damage. Cell Biol. Toxicol. 2009, 25, 599–609. [Google Scholar] [CrossRef]
- Mandrekar, P.; Szabo, G. Signalling pathways in alcohol-induced liver inflammation. J. Hepatol. 2009, 50, 1258–1266. [Google Scholar] [CrossRef] [PubMed]
- Hill, D.B.; Marsano, L.; Cohen, D.; Allen, J.; Shedlofsky, S.; McClain, C.J. Increased plasma interleukin-6 concentrations in alcoholic hepatitis. J. Lab. Clin. Med. 1992, 119, 547–552. [Google Scholar] [CrossRef] [PubMed]
- Mannaa, F.A.; Abdel-Wahhab, K.G. Physiological potential of cytokines and liver damages. Hepatoma Res. 2016, 2, 131–143. [Google Scholar] [CrossRef]
- Nowak, A.J.; Relja, B. The Impact of Acute or Chronic Alcohol Intake on the NF-κB Signaling Pathway in Alcohol-Related Liver Disease. Int. J. Mol. Sci. 2020, 21, 9407. [Google Scholar] [CrossRef] [PubMed]
- Tilg, H.; Moschen, A.R.; Szabo, G. Interleukin-1 and inflammasomes in alcoholic liver disease/acute alcoholic hepatitis and nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. Hepatology 2016, 64, 955–965. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Shen, G.; Xu, L.; Liu, X.; Brown, J.M.; Feng, D.; Ross, R.A.; Gao, B.; Liangpunsakul, S.; Ju, C. IL-1 receptor like 1 protects against alcoholic liver injury by limiting NF-κB activation in hepatic macrophages. J. Hepatol. 2017, 68, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Shuda, M.; Kondoh, N.; Imazeki, N.; Tanaka, K.; Okada, T.; Mori, K.; Hada, A.; Arai, M.; Wakatsuki, T.; Matsubara, O.; et al. Activation of the ATF6, XBP1 and grp78 genes in human hepatocellular carcinoma: A possible involvement of the ER stress pathway in hepatocarcinogenesis. J. Hepatol. 2003, 38, 605–614. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Qiao, S.; Xiang, Y.; Cui, M.; Yao, X.; Lin, R.; Zhang, X. Endoplasmic reticulum stress: Multiple regulatory roles in hepatocellular carcinoma. Biomed. Pharmacother. 2021, 142, 112005. [Google Scholar] [CrossRef] [PubMed]
- Bobrovnikova-Marjon, E.; Grigoriadou, C.; Pytel, D.; Zhang, F.; Ye, J.; Koumenis, C.; Cavener, D.; Diehl, J.A. PERK promotes cancer cell proliferation and tumor growth by limiting oxidative DNA damage. Oncogene 2010, 29, 3881–3895. [Google Scholar] [CrossRef]
- Vandewynckel, Y.P.; Laukens, D.; Bogaerts, E.; Paridaens, A.; Van den Bussche, A.; Verhelst, X.; Van Steenkiste, C.; Descamps, B.; Vanhove, C.; Libbrecht, L.; et al. Modulation of the unfolded protein response impedes tumor cell adaptation to proteotoxic stress: A PERK for hepatocellular carcinoma therapy. Hepatol. Int. 2015, 9, 93–104. [Google Scholar] [CrossRef]
- Khaled, J.; Kopsida, M.; Lennernäs, H.; Heindryckx, F. Drug Resistance and Endoplasmic Reticulum Stress in Hepatocellular Carcinoma. Cells 2022, 11, 632. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Xiao, Y.; Yu, J.; Xia, T.; Liu, B.; Guo, Y.; Deng, J.; Chen, S.; Wang, C.; Guo, F. Liver-specific Gene Inactivation of the Transcription Factor ATF4 Alleviates Alcoholic Liver Steatosis in Mice. J. Biol. Chem. 2016, 291, 18536–18546. [Google Scholar] [CrossRef]
- Koo, J.H.; Lee, H.J.; Kim, W.; Kim, S.G. Endoplasmic Reticulum Stress in Hepatic Stellate Cells Promotes Liver Fibrosis via PERK-Mediated Degradation of HNRNPA1 and Up-regulation of SMAD2. Gastroenterology 2016, 150, 181–193.e188. [Google Scholar] [CrossRef]
- Guo, H.L.; Hassan, H.M.; Ding, P.P.; Wang, S.J.; Chen, X.; Wang, T.; Sun, L.X.; Zhang, L.Y.; Jiang, Z.Z. Pyrazinamide-induced hepatotoxicity is alleviated by 4-PBA via inhibition of the PERK-eIF2α-ATF4-CHOP pathway. Toxicology 2017, 378, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Uzi, D.; Barda, L.; Scaiewicz, V.; Mills, M.; Mueller, T.; Gonzalez-Rodriguez, A.; Valverde, A.M.; Iwawaki, T.; Nahmias, Y.; Xavier, R.; et al. CHOP is a critical regulator of acetaminophen-induced hepatotoxicity. J. Hepatol. 2013, 59, 495–503. [Google Scholar] [CrossRef]
- Pavlović, N.; Calitz, C.; Thanapirom, K.; Mazza, G.; Rombouts, K.; Gerwins, P.; Heindryckx, F. Inhibiting IRE1α-endonuclease activity decreases tumor burden in a mouse model for hepatocellular carcinoma. eLife 2020, 9, e55865. [Google Scholar] [CrossRef]
- Wu, S.; Du, R.; Gao, C.; Kang, J.; Wen, J.; Sun, T. The role of XBP1s in the metastasis and prognosis of hepatocellular carcinoma. Biochem. Biophys. Res. Commun. 2018, 500, 530–537. [Google Scholar] [CrossRef]
- Pang, X.; Qiao, Q.; Vonglorkham, S.; Feng, Z.; Pang, L.; Chen, S.; Wang, D.; Lao, L.; Lin, X.; Wei, J. Asiatic acid ameliorates acute hepatic injury by reducing endoplasmic reticulum stress and triggering hepatocyte autophagy. Biomed. Pharmacother. 2020, 129, 110375. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Zhou, D.L.; Wei, X.H.; Zhong, R.Y.; Xu, J.; Sun, L. Astragaloside IV attenuates free fatty acid-induced ER stress and lipid accumulation in hepatocytes via AMPK activation. Acta Pharmacol. Sin. 2017, 38, 998–1008. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, X.; Zhao, D.; Wang, X.; Gurley, E.C.; Liu, R.; Li, X.; Hylemon, P.B.; Chen, W.; Zhou, H. Berberine inhibits free fatty acid and LPS-induced inflammation via modulating ER stress response in macrophages and hepatocytes. PLoS ONE 2020, 15, e0232630. [Google Scholar]
- Song, M.; Zhang, H.; Chen, Z.; Yang, J.; Li, J.; Shao, S.; Liu, J. Shikonin reduces hepatic fibrosis by inducing apoptosis and inhibiting autophagy via the platelet-activating factor-mitogen-activated protein kinase axis. Exp. Ther. Med. 2021, 21, 28. [Google Scholar] [CrossRef] [PubMed]
- Tamitani, M.; Yamamoto, T.; Yamamoto, N.; Fujisawa, K.; Tanaka, S.; Nakamura, Y.; Uchinoumi, H.; Oda, T.; Okuda, S.; Takami, T.; et al. Dantrolene prevents hepatic steatosis by reducing cytoplasmic Ca2+ level and ER stress. Biochem. Biophys. Rep. 2020, 23, 100787. [Google Scholar] [CrossRef] [PubMed]
- Utili, R.; Boitnott, J.K.; Zimmerman, H.J. Dantrolene-Associated Hepatic Injury: Incidence and character. Gastroenterology 1977, 72 Pt 1, 610–616. [Google Scholar] [CrossRef] [PubMed]
- Pasrija, D.; Gupta, S.; Hassinger, A. Dantrolene-Induced Hepatitis: A Rare Culprit in the PICU. J. Pediatr. Intensive Care 2021, 10, 152–154. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Sheng, L.; Li, J.; Qian, J.; Wu, G.; Wang, Z.; Zhang, Y. Resveratrol Alleviates Hepatic Fibrosis in Associated with Decreased Endoplasmic Reticulum Stress-Mediated Apoptosis and Inflammation. Inflammation 2022, 45, 812–823. [Google Scholar] [CrossRef]
- Kuo, T.F.; Tatsukawa, H.; Matsuura, T.; Nagatsuma, K.; Hirose, S.; Kojima, S. Free fatty acids induce transglutaminase 2-dependent apoptosis in hepatocytes via ER stress-stimulated PERK pathways. J. Cell. Physiol. 2012, 227, 1130–1137. [Google Scholar] [CrossRef] [PubMed]
- Yun, Y.-R.; Lee, J.-E. Alliin, capsaicin, and gingerol attenuate endoplasmic reticulum stress-induced hepatic steatosis in HepG2 cells and C57BL/6N mice. J. Funct. Foods 2022, 95, 105186. [Google Scholar] [CrossRef]
- Huang, Q.; Wang, T.; Yang, L.; Wang, H.Y. Ginsenoside Rb2 Alleviates Hepatic Lipid Accumulation by Restoring Autophagy via Induction of Sirt1 and Activation of AMPK. Int. J. Mol. Sci. 2017, 18, 1063. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Tan, J.; Miao, Y.; Li, M.; Zhang, Q. Crosstalk of autophagy and apoptosis: Involvement of the dual role of autophagy under ER stress. J. Cell. Physiol. 2017, 232, 2977–2984. [Google Scholar] [CrossRef]
- Cabrera, D.; Arab, J.P.; Arrese, M. UDCA, NorUDCA, and TUDCA in Liver Diseases: A Review of Their Mechanisms of Action and Clinical Applications. Handb. Exp. Pharmacol. 2019, 256, 237–264. [Google Scholar]
- Kars, M.; Yang, L.; Gregor, M.F.; Mohammed, B.S.; Pietka, T.A.; Finck, B.N.; Patterson, B.W.; Horton, J.D.; Mittendorfer, B.; Hotamisligil, G.S.; et al. Tauroursodeoxycholic Acid may improve liver and muscle but not adipose tissue insulin sensitivity in obese men and women. Diabetes 2010, 59, 1899–1905. [Google Scholar] [CrossRef]
- Ben Mosbah, I.; Alfany-Fernández, I.; Martel, C.; Zaouali, M.A.; Bintanel-Morcillo, M.; Rimola, A.; Rodés, J.; Brenner, C.; Roselló-Catafau, J.; Peralta, C. Endoplasmic reticulum stress inhibition protects steatotic and non-steatotic livers in partial hepatectomy under ischemia-reperfusion. Cell Death Dis. 2010, 1, e52. [Google Scholar] [CrossRef]
- Szaruga, M.; Janssen, D.A.; de Miguel, C.; Hodgson, G.; Fatalska, A.; Pitera, A.P.; Andreeva, A.; Bertolotti, A. Activation of the integrated stress response by inhibitors of its kinases. Nat. Commun. 2023, 14, 5535. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Rivera, D.; Delvaeye, T.; Roelandt, R.; Nerinckx, W.; Augustyns, K.; Vandenabeele, P.; Bertrand, M.J.M. When PERK inhibitors turn out to be new potent RIPK1 inhibitors: Critical issues on the specificity and use of GSK2606414 and GSK2656157. Cell Death Differ. 2017, 24, 1100–1110. [Google Scholar] [CrossRef]
- Zucchi, E.; Musazzi, U.M.; Fedele, G.; Martinelli, I.; Gianferrari, G.; Simonini, C.; Fini, N.; Ghezzi, A.; Caputo, M.; Sette, E.; et al. Effect of tauroursodeoxycholic acid on survival and safety in amyotrophic lateral sclerosis: A retrospective population-based cohort study. eClinicalMedicine 2023, 65, 102256. [Google Scholar] [CrossRef]
- Kopsida, M.; Clavero, A.L.; Khaled, J.; Balgoma, D.; Luna-Marco, C.; Chowdhury, A.; Nyman, S.S.; Rorsman, F.; Ebeling Barbier, C.; Bergsten, P.; et al. Inhibiting the endoplasmic reticulum stress response enhances the effect of doxorubicin by altering the lipid metabolism of liver cancer cells. Mol. Metab. 2024, 79, 101846. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.J.; Su, J.H.; Tsao, C.Y.; Hung, C.T.; Chao, H.H.; Lin, J.J.; Liao, M.H.; Yang, Z.Y.; Huang, H.H.; Tsai, F.J.; et al. Sinulariolide induced hepatocellular carcinoma apoptosis through activation of mitochondrial-related apoptotic and PERK/eIF2α/ATF4/CHOP pathway. Molecules 2013, 18, 10146–10161. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liu, J.M.; Xiong, X.X.; Qiu, X.Y.; Pan, F.; Liu, D.; Lan, S.J.; Jin, S.; Yu, S.B.; Chen, X.Q. Piperlongumine selectively kills hepatocellular carcinoma cells and preferentially inhibits their invasion via ROS-ER-MAPKs-CHOP. Oncotarget 2015, 6, 6406–6421. [Google Scholar] [CrossRef]
- Han, B.; Yu, Y.Q.; Yang, Q.L.; Shen, C.Y.; Wang, X.J. Kaempferol induces autophagic cell death of hepatocellular carcinoma cells via activating AMPK signaling. Oncotarget 2017, 8, 86227–86239. [Google Scholar] [CrossRef]
- Wang, H.; Chen, L.; Zhang, X.; Xu, L.; Xie, B.; Shi, H.; Duan, Z.; Zhang, H.; Ren, F. Kaempferol protects mice from d-GalN/LPS-induced acute liver failure by regulating the ER stress-Grp78-CHOP signaling pathway. Biomed. Pharmacother. 2019, 111, 468–475. [Google Scholar] [CrossRef]
- Zhang, Q.; Chen, W.; Lv, X.; Weng, Q.; Chen, M.; Cui, R.; Liang, G.; Ji, J. Piperlongumine, a Novel TrxR1 Inhibitor, Induces Apoptosis in Hepatocellular Carcinoma Cells by ROS-Mediated ER Stress. Front. Pharmacol. 2019, 10, 1180. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Ren, F.; Zhang, L.; Zhang, X.; Yang, R.; Xie, B.; Li, Z.; Hu, Z.; Duan, Z.; Zhang, J. Kaempferol induces apoptosis in HepG2 cells via activation of the endoplasmic reticulum stress pathway. Mol. Med. Rep. 2016, 13, 2791–2800. [Google Scholar] [CrossRef] [PubMed]
- Reardon, J.; Hussaini, T.; Alsahafi, M.; Azalgara, V.M.; Erb, S.R.; Partovi, N.; Yoshida, E.M. Ursodeoxycholic Acid in Treatment of Non-cholestatic Liver Diseases: A Systematic Review. J. Clin. Transl. Hepatol. 2016, 4, 192–205. [Google Scholar] [PubMed]
- Brusilow, S.W. Phenylacetylglutamine may replace urea as a vehicle for waste nitrogen excretion. Pediatr. Res. 1991, 29, 147–150. [Google Scholar] [CrossRef]
- Lee, H.Y.; Marahatta, A.; Bhandary, B.; Kim, H.-R.; Chae, H.-J. 4-Phenylbutyric acid regulates CCl4-induced acute hepatic dyslipidemia in a mouse model: A mechanism-based PK/PD study. Eur. J. Pharmacol. 2016, 777, 104–112. [Google Scholar] [CrossRef]
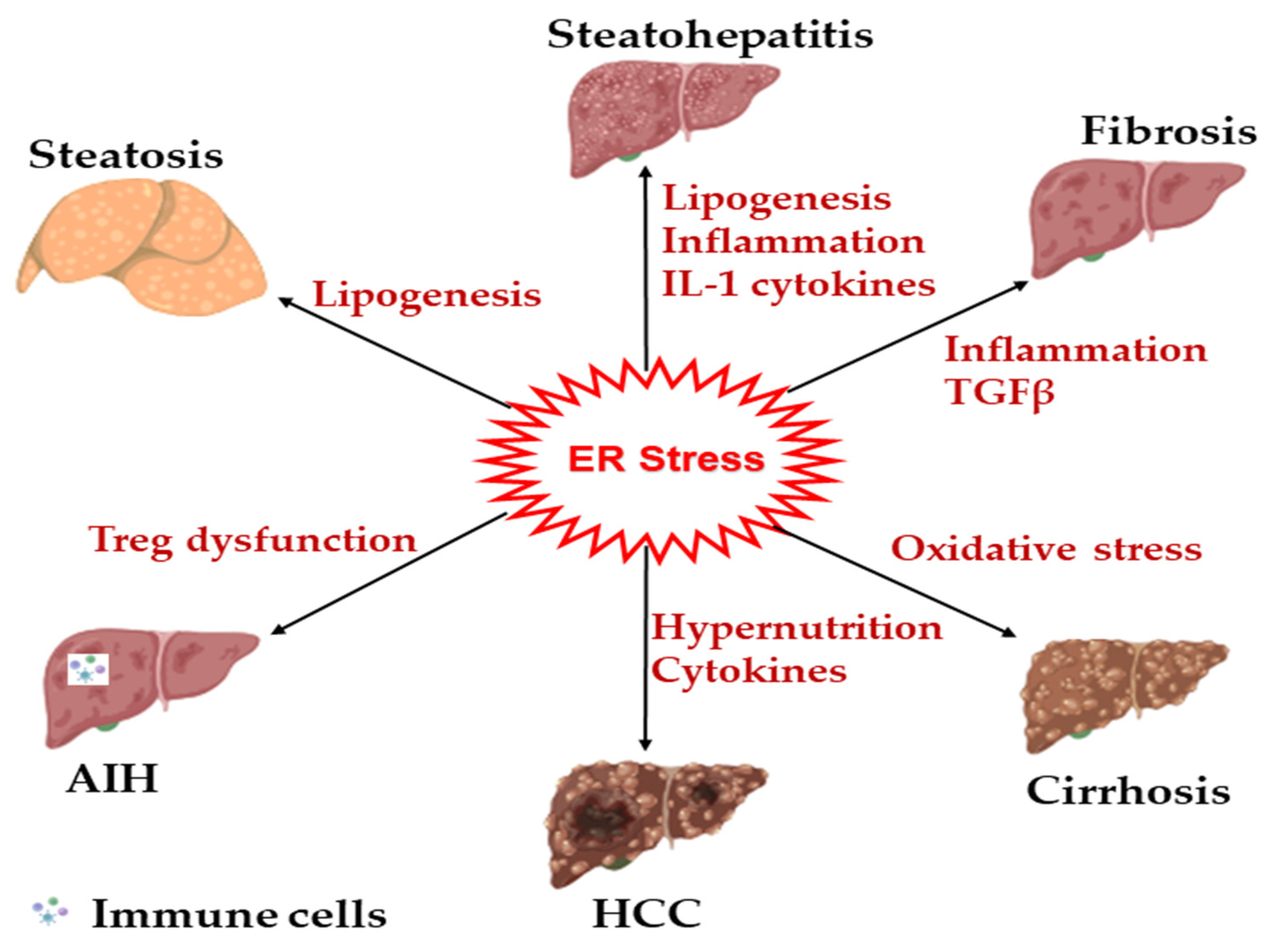

| Disease | Mechanism | Main UPR Protein | Ref. |
|---|---|---|---|
| Steatosis | Upregulation of lipogenic genes | ATF4 | [162] |
| Steatohepatitis | Inflammation and steatosis | PERK-CHOP | [58] |
| Fibrosis | SMAD2 upregulation in HSC | CHOP | [163] |
| Cirrhosis | Hepatic apoptosis and inflammation | CHOP | [58] |
| AIH | Enhanced IL17A and Treg suppression | ATF6α | [139] |
| PBC | Accumulation of hydrophobic bile acids | CHOP, IRE1α | [27] |
| DILI | Hepatocyte apoptosis, necrosis | CHOP, ATF6α | [164,165] |
| HCC | Metabolic dysfunction and inflammation | IRE1α XBP1 | [166,167] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baral, A. Endoplasmic Reticulum Stress Signaling in the Regulation of Hepatic Pathological Responses. Stresses 2024, 4, 481-504. https://doi.org/10.3390/stresses4030031
Baral A. Endoplasmic Reticulum Stress Signaling in the Regulation of Hepatic Pathological Responses. Stresses. 2024; 4(3):481-504. https://doi.org/10.3390/stresses4030031
Chicago/Turabian StyleBaral, Ananda. 2024. "Endoplasmic Reticulum Stress Signaling in the Regulation of Hepatic Pathological Responses" Stresses 4, no. 3: 481-504. https://doi.org/10.3390/stresses4030031
APA StyleBaral, A. (2024). Endoplasmic Reticulum Stress Signaling in the Regulation of Hepatic Pathological Responses. Stresses, 4(3), 481-504. https://doi.org/10.3390/stresses4030031






