Supplementary Light on the Development of Lettuce and Cauliflower Seedlings
Abstract
1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Experimental Conditions and Species Tested
3.2. Experiment Preparation and Treatments
3.3. Evaluation of the Biometric and Biochemical Parameters of the Seedlings
3.4. Experimental Design and Statistical Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pessoa, H.P.; Machado Júnior, R. Folhosas: Em destaque no cenário nacional [Boadleaves: Featured on the national scene]. Rev. Campo Neg. 2021. [Google Scholar]
- National Supply Company (CONAB). Banco de Dados ProHort [ProHort Database]. 2022. Available online: http://dw.ceasa.gov.br (accessed on 27 May 2023).
- Lyra, R. Conheça os Benefícios da Couve-Flor Para a Saúde [Know the Benefits of Cauliflower for Health]; Centrais de Abastecimento do Espírito Santo: Vitória, Brazil, 2015. Available online: https://ceasa.es.gov.br/conheca-os-beneficios-da-couve-flor-para-a-sa (accessed on 14 July 2023).
- Corwin, D.L. Climate change impacts on soil salinity in agricultural areas. Eur. J. Soil Sci. 2023, 72, 842–862. [Google Scholar] [CrossRef]
- Etae, N.; Wamae, Y.; Khummueng, W.; Utaipan, T.; Ruangrak, E. Effects of artificial light sources on growth and phytochemicals content in green oak lettuce. Hort. Bras. 2020, 38, 204–210. [Google Scholar] [CrossRef]
- Bantis, F.; Smirnakou, S.; Ozounis, T.; Koukounaras, A.; Ntagkas, N.; Radoglou, K. Current status and recent achievements in the field of horticulture with the use of light-emitting diodes (LEDs). Sci. Hort. 2018, 235, 437–451. [Google Scholar] [CrossRef]
- Colantoni, A.; Monarca, D.; Marucci, A.; Cecchini, M.; Zambon, I.; Di Battista, F.; Maccario, D.; Saporito, M.G.; Beruto, M. Solar Radiation Distribution inside a Greenhouse Prototypal with Photovoltaic Mobile Plant and Effects on Flower Growth. Sustainability 2018, 10, 855. [Google Scholar] [CrossRef]
- Paik, I.; Huq, E. Plant photoreceptors: Multi-functional sensory proteins and their signaling networks. Semin. Cell Dev. Biol. 2019, 92, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Chang, Y.; Chen, G.; Lin, H. The research led to a supplementary lighting system for plants. Optik 2016, 127, 7193–7201. [Google Scholar] [CrossRef]
- Cardia, L.H.B.; Bortolassi Junior, M.R. Influência da Iluminação Artificial no Cultivo de Rabanete [Influence of Artificial Lighting on Radish Cultivation]; Monograph; UniCesumar Maringá: Maringá, Brazil, 2019. [Google Scholar]
- Freitas, I.S. Suplementação Luminosa com Lâmpadas LED no Cultivo de Microverdes em Ambiente Protegido [Light Supplementation with LED Lamps in Microgreen Cultivation in Protected Environment]. Master’s Thesis, Universidade de São Paulo, Piracicaba, Brazil, 2020. [Google Scholar] [CrossRef]
- Maluf, G.E.G.M.; Paula, A.C.C.F.F.; Alvarenga, A.A.; Maluf, H.J.G.M. Efeito da iluminação noturna complementar a 18 cm de altura no crescimento de mudas de alface (Lactuca sativa L.) [Effect of night complementary lighting at a height of 18 cm in the growth of lettuce (Lactuca sativa L.) seedlings]. In Proceedings of the IV Semana de Ciência e Tecnologia do IFMG, Bambuí, Brazil, 4 December 2011. [Google Scholar]
- Chen, X.L.; Li, Y.L.; Guo, W.Z. Red and blue wavelengths affect the morphology, energy use efficiency and nutritional content of lettuce (Lactuca sativa L.). Sci. Rep. 2021, 11, 8374. [Google Scholar] [CrossRef]
- Lima, S.C.; Pedroza, J.P.; Almeida, B.G.; Melo, D.F.; Carvalho, R.O. Produção de alface cultivada sob iluminação de diodos emissores de luz [Lettuce production under light-emitting diodes lighting]. In Proceedings of the Congresso Técnico Científico da Engenharia e da Agronomia, Palmas, Brazil, 12 September 2019. [Google Scholar]
- Paniagua-Pardo, G.; Hernández-Aguilar, C.; Rico-Maertínez, F.; Domínguez-Pacheco, F.A.; Martínez-Ortíz, E.; Martínez-González, C.L. Efecto de la luz LED de alta intensidad sobre la germinación y el crecimiento de plántulas de brócoli (Brassica oleracea L.). Polibotánica 2015, 40, 199–212. [Google Scholar] [CrossRef]
- Jones-Baumgardt, C.; Llewellyn, D.; Ying, Q.; Zheng, Y. Intensity of Sole-source Light-emitting Diodes Affects Growth, Yield, and Quality of Brassicaceae Microgreens. HortScience 2019, 54, 1168–1174. [Google Scholar] [CrossRef]
- He, J.; Qin, L.; Teo, L.J.L.; Wei, C.T. Nitrate accumulation, productivity and photosynthesis of Brassica alboglabra grown under low light with supplemental LED lighting in the tropical greenhouse. J. Plant Nutr. 2019, 42, 1740–1749. [Google Scholar] [CrossRef]
- Razzak, M.A.; Asaduzzaman, M.; Tanaka, H.; Asao, T. Effects of supplementing green light to red and blue light on the growth and yield of lettuce in plant factories. Sci. Hortic. 2022, 305, 111429. [Google Scholar] [CrossRef]
- Lee, M.J.; Son, K.H.; Oh, M.M. Increase in biomass and bioactive compounds in lettuce under various ratios of red to far-red LED light supplemented with blue LED light. Hortic. Environ. Biotechnol. 2016, 57, 139–147. [Google Scholar] [CrossRef]
- Legendre, R.; van Iersel, M.W. Supplemental Far-Red Light Stimulates Lettuce Growth: Disentangling Morphological and Physiological Effects. Plants 2021, 10, 166. [Google Scholar] [CrossRef]
- Chung, H.; Chang, M.; Wu, C.; Fang, W. Quantitative Evaluation of Electric Light Recipes for Red Leaf Lettuce Cultivation in Plant Factories. HortTechnology 2018, 28, 755–763. [Google Scholar] [CrossRef]
- Li, Y.; Liu, N.; Ji, F.; He, D. Optimal red:blue ratio of full spectrum LEDs for hydroponic pakchoi cultivation in plant factory. Int. J. Agric. Biol. Eng. 2022, 15, 72–77. [Google Scholar] [CrossRef]
- Arias, L.A.; Berli, F.; Fontana, A.; Bottini, R.; Piccoli, P. Climate Change Effects on Grapevine Physiology and Biochemistry: Benefits and Challenges of High Altitude as an Adaptation Strategy. Front. Plant Sci. 2022, 13, 835425. [Google Scholar] [CrossRef]
- Hooks, T.; Sun, L.; Kong, Y.; Masabni, J.; Niu, G. Short-Term Pre-Harvest Supplemental Lighting with Different Light Emitting Diodes Improves Greenhouse Lettuce Quality. Horticulture 2022, 8, 435. [Google Scholar] [CrossRef]
- Amoozgar, A.; Mohmmadi, A.; Sabzalian, M.R. Impact of light-emitting diode irradiation on photosynthesis, phytochemical composition and mineral element content of lettuce cv. grizzly. Photosynthetica 2017, 55, 85–95. [Google Scholar] [CrossRef]
- Monostori, I.; Heilmann, M.; Kocsy, G.; Rakszegi, M.; Ahres, M.; Altenbach, S.B.; Szalai, G.; Pál, M.; Toldi, D.; Simon-Sarkadi, L.; et al. LED Lighting—Modification of Growth, Metabolism, Yield and Flour Composition in Wheat by Spectral Quality and Intensity. Front. Plant Sci. 2018, 9, 605. [Google Scholar] [CrossRef]
- Li, Y.; Wu, L.; Jiang, H.; He, R.; Song, S.; Su, W.; Liu, H. Supplementary Far-Red and Blue Lights Influence the Biomass and Phytochemical Profiles of Two Lettuce Cultivars in Plant Factory. Molecules 2021, 26, 7405. [Google Scholar] [CrossRef] [PubMed]
- Larcher, W. Ecofisiologia Vegetal [Plant Ecophysiology]; RiMa: São Carlos, Brazil, 2004. [Google Scholar]
- Taiz, L.; Zeiger, E. Fisiologia Vegetal [Plant Physiology]; Artmed: Porto Alegre, Brazil, 2013. [Google Scholar]
- Zheng, Y.J.; Zhang, Y.T.; Liu, H.C.; Li, Y.M.; Liu, Y.L.; Hao, Y.W.; Lei, B.F. Supplemental blue light increases growth and quality of greenhouse pak choi depending on cultivar and supplemental light intensity. J. Integr. Agric. 2018, 17, 2245–2256. [Google Scholar] [CrossRef]
- Poudel, P.R.; Kataoka, I.; Mochioka, R. Effect of red-and blue-light-emitting diodes on growth and morphogenesis of grapes. Plant Cell Tissue Organ Cult. 2008, 92, 147–153. [Google Scholar] [CrossRef]
- Rahman, M.M.; Field, D.L.; Ahmed, S.M.; Hasan, M.T.; Basher, M.K.; Alameh, K. LED Illumination for High-Quality High-Yield Crop Growth in Protected Cropping Environments. Plants 2021, 10, 2470. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Xu, A.; Cheng, Z.M. Effects of light emitting diode lights on plant growth, development and traits a meta-analysis. Hortic. Plant J. 2021, 7, 552–564. [Google Scholar] [CrossRef]
- Pandey, P.; Veazie, P.; Whipker, B.; Young, S. Predicting foliar nutrient concentrations and nutrient deficiencies of hydroponic lettuce using hyperspectral imaging. Biosyst. Eng. 2023, 230, 458–469. [Google Scholar] [CrossRef]
- Sharma, A.; Hazarika, M.; Heisnam, P.; Pandey, H.; Devadas, V.S.; Wangsu, M.; Kartha, B.D. Factors Affecting Production, Nutrient Translocation Mechanisms, and LED Emitted Lightin Growth of Microgreen Plants in Soilless Culture. ACS Agric. Sci. Technol. 2023, 3, 701–719. [Google Scholar] [CrossRef]
- Almeida, H.J.; Carmona, V.M.V.; Cavalcante, V.S.; Filho, A.B.C.; Prado, R.M.; Flores, R.A.; Borges, B.M.M.N.; Mauad, M. Nutritional and Visual Diagnosis in Broccoli (Brassica oleracea L. var. italica) Plants: Disorders in Physiological Activity, Nutritional Efficiency and Metabolism of Carbohydrates. Agronomy 2020, 10, 1572. [Google Scholar] [CrossRef]
- Gómez, C.; Mitchell, C.A. Growth Responses of Tomato Seedlings to Different Spectra of Supplemental Lighting. HortScience 2015, 50, 112–118. [Google Scholar] [CrossRef]
- Randall, W.C.; Lopez, R.G. Comparison of Supplemental Lighting from High-pressure Sodium Lamps and Light-emitting Diodes during Bedding Plant Seedling Production. HortScience 2014, 49, 589–595. [Google Scholar] [CrossRef]
- Martinazzo, E.G.; Anese, S.; Wandscheer, A.C.D.; Pastorini, L.H. Efeito do sombreamento sobre o crescimento inicial e teor de clorofila foliar de Eugenia uniflora Linn (Pitanga)—Família Myrtaceae [Shading effect on the initial growth and leaf chroroplyll content of Eugenia uniflora Linn (Pitanga)—Myrtaceae family]. Rev. Bras. Biociênc. 2007, 5, 162–164. [Google Scholar]
- Malavolta, E.; Vitti, G.C.; Oliveira, S.A. Avaliação do Estado Nutricional das Plantas: Princípios e Aplicações [Evaluation of the Nutritional State of Plants: Principles and Applications], 2nd ed.; Potafos: Piracicaba, Brazil, 1997. [Google Scholar]
- Pereira, G.A.; Arruda, H.S.; Pastore, G.M. Modification and validation of Folin-Ciocalteu assay for faster and safer analysis of total phenolic content in food samples. Braz. J. Food Res. 2018, 9, 125–140. [Google Scholar] [CrossRef]
- Alrajhi, A.A.; Alsahli, A.S.; Alhelal, I.M.; Rihan, H.Z.; Fuller, M.P.; Alsadon, A.A.; Ibrahim, A.A. The Effect of LED Light Spectra on the Growth, Yield and Nutritional Value of Red and Green Lettuce (Lactuca sativa). Plants 2023, 12, 463. [Google Scholar] [CrossRef]
- Kume, A.; Akitsu, T.; Nasahara, K.N. Why is chlorophyll b only used in light-harvesting systems? J. Plant Res. 2018, 131, 961–972. [Google Scholar] [CrossRef]
- Kähkönen, M.P.; Hopia, A.I.; Vuorela, H.J.; Rauha, J.P.; Pihlaja, K.; Kujala, T.S.; Heinonen, M. Antioxidant activity of plant extracts containing phenolic compounds. J. Agric. Food Chem. 1999, 47, 3954–3962. [Google Scholar] [CrossRef] [PubMed]
- Kaur, C.; Kapoor, H.C. Antioxidants in fruits and vegetables—The millennium’s health. Int. J. Food Sci. Technol. 2001, 36, 703–725. [Google Scholar]
- Koleva, I.I.; Van Beek, T.A.; Linssen, J.P.H.; Groot, A.; Evstatieva, L.N. Screening of plant extracts for antioxidant activity: A comparative study on three testing methods. Phytochem. Anal 2002, 13, 8–17. [Google Scholar] [CrossRef]
- Melo, E.A.; Maciel, M.I.S.; Lima, V.L.A.G.; Leal, F.L.L.; Caetano, A.C.S.; Nascimento, R.J. Capacidade antioxidante de hortaliças usualmente consumidas [Antioxidant capacity of commonly consumed vegetables]. Ciênc. Tecnol. Alim. 2006, 26, 639–644. [Google Scholar] [CrossRef]
- Lee, D.W. Simulating forest shade to study the development ecology of tropical plants: Juvenile growth in three vines in India. J. Trop. Ecol. 1988, 4, 281–292. [Google Scholar] [CrossRef]
- Engel, V.L.; Poggiani, F. Estudo da concentração de clorofila nas folhas e seu espectro de absorção de luz em função do sombreamento em mudas de quatro espécies florestais nativas [Study of chlorophyll concentration in leaves and its light absorption spectrum as a function of shading in seedlings of four native forest species]. Rev. Bras. Fisiol. Veg. 1991, 3, 39–45. [Google Scholar]
- Frede, K.; Schreiner, M.; Baldermann, S. Light quality-induced changes of carotenoid composition in pak choi Brassica rapa ssp. chinensis. J. Photochem. Photobiol. B 2019, 193, 18–30. [Google Scholar] [CrossRef] [PubMed]
- Gobbo Neto, L.; Lopes, N.P. Plantas medicinais: Fatores de influência no conteúdo de metabólitos secundários [Medicinal plants: Influencing factors on the content of secondary metabolites]. Quím. Nova 2007, 30, 374–381. [Google Scholar] [CrossRef]
- Sarruge, J.R. Soluções nutritivas [Nutritive solutions]. Summa Phytopathol. 1975, 1, 213–233. [Google Scholar]
- Naznin, M.T.; Lefsrud, M.; Azad, M.O.K.; Park, C.H. Effect of Different Combinations of Red and Blue LED Light on Growth Characteristics and Pigment Content of In Vitro Tomato Plantlets. Agriculture 2019, 9, 196. [Google Scholar] [CrossRef]
- Naznin, M.T.; Lefsrud, M.; Gravel, V.; Azad, X.O.K.; Alsanius, B.W. Different ratios of red and blue LEDs enhance Romaine lettuce production and antioxidant capacity in urban controlled environment. Acta Hortic. 2022, 1337, 137–142. [Google Scholar] [CrossRef]
- Vaštakaitė-Kairienė, V.; Brazaitytė, A.; Miliauskienė, J.; Runkle, E.S. Red to Blue Light Ratio and Iron Nutrition Influence Growth, Metabolic Response, and Mineral Nutrients of Spinach Grown Indoors. Sustainability 2022, 14, 12564. [Google Scholar] [CrossRef]
- Matic, P.; Sabljic, M.; Jakobek, L. Validation of Spectrophotometric Methods for the Determination of Total Polyphenol and Total Flavonoid Content. J. AOAC Int. 2017, 100, 1795–1803. [Google Scholar] [CrossRef]
- Ross, C.W. Plant Physiology Laboratory Manual; Wadsworth Publishing Company: Belmont, MA, USA, 1974. [Google Scholar]
- Lichtenthaler, H.; Wellburn, A. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem. Soc. Transac. 1983, 603, 591–592. [Google Scholar] [CrossRef]
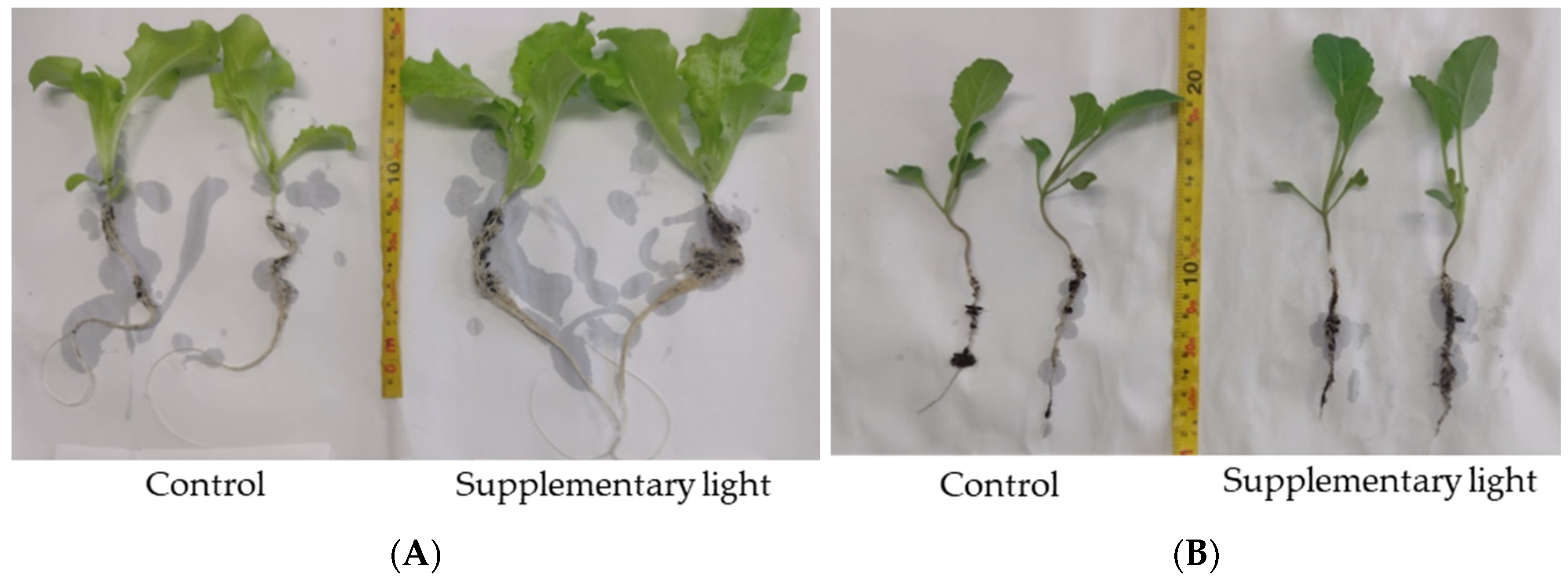
| Treatment | Root Dry Weight (mg) | Root Length (cm) | Aerial Dry Weight (mg) | Cumulative Leaf Area (cm2) |
|---|---|---|---|---|
| Control | 20.40 ± 0.44 | 19.5 ± 2.88 | 59.7 ± 2.91 | 3899 ± 742 |
| Supplemental light | 69.41 ± 1.57 * | 25.3 ± 5.59 * | 182.9 ± 1.11 * | 9256 ± 1392 * |
| CV (%) | 25.7 | 19.9 | 18.2 | 17.0 |
| Treatment | Root Dry Weight (mg) | Root Length (cm) | Aerial Dry Weight (mg) | Cumulative Leaf Area (cm2) |
|---|---|---|---|---|
| Control | 1.53 ± 0.55 | 8.8 ± 0.92 | 5.53 ± 0.99 | 1438 ± 610 |
| Supplemental light | 4.45 ± 0.26 * | 11.0 ± 2.61 * | 12.6 ± 3.69 * | 2690 ± 299 * |
| CV (%) | 32.1 | 13.3 | 30.0 | 23.3 |
| Treatment | Control | Supplemental Lighting | tcalculated 1 | CV (%) | |
|---|---|---|---|---|---|
| N | g·kg−1 (d.b.) | 44.6 ± 0.2 * | 38.4 ± 1.2 | 7.56 | 8.93 |
| P | 8.6 ± 0.1 * | 7.4 ± 0.5 | 4.80 | 9.44 | |
| K | 65.5 ± 1.1 * | 49.2 ± 4.2 | 7.43 | 16.95 | |
| Ca | 8.6 ± 0.1 ns | 8.0 ± 0.8 | 1.54 | 6.89 | |
| Mg | 4.9 ± 0.1 * | 3.7 ± 0.4 | 5.35 | 16.58 | |
| S | 2.9 ± 0.1 * | 2.1 ± 0.1 | 16.00 | 19.00 | |
| Zn | mg·kg−1 (d.b.) | 94.4 ± 4.1 * | 78.9 ± 3.2 | 5.99 | 10.93 |
| Cu | 18.7 ± 6.1 ns | 21.2 ± 1.1 | 0.79 | 19.24 | |
| Mn | 128.7 ± 15.7 ns | 160.7 ± 16.1 | 2.76 | 15.78 | |
| Fe | 362.7 ± 7.8 * | 209.0 ± 7.8 | 27.81 | 31.12 | |
| B | 17.9 ± 0.8 ns | 16.2 ± 0.1 | 6.45 | 6.45 | |
| Treatment | Control | Supplemental Lighting | tcalculated 1 | CV (%) | |
|---|---|---|---|---|---|
| N | g·kg−1 (d.b.) | 36.9 ± 2.1 | 43.3 ± 1.0 * | 5.67 | 9.85 |
| P | 5.0 ± 0.1 ns | 5.3 ± 0.2 | 2.68 | 4.24 | |
| K | 50.5 ± 0.4 ns | 49.5 ± 0.4 | 4.00 | 1.29 | |
| Ca | 12.9 ± 0.1 ns | 14.9 ± 0.2 | 3.32 | 9.73 | |
| Mg | 6.9 ± 0.1 | 9.3 ± 0.2 * | 18.43 | 16.90 | |
| S | 8.7 ± 0.1 ns | 8.7 ± 0.5 | 0.19 | 3.44 | |
| Zn | mg·kg−1 (d.b.) | 39.3 ± 2.2 | 52.6 ± 2.8 * | 7.46 | 17.38 |
| Cu | 6.6 ± 0.8 ns | 10.3 ± 3.8 | 1.92 | 37.06 | |
| Mn | 78.4 ± 0.8 | 107.7 ± 7.7 * | 7.56 | 18.81 | |
| Fe | 105.1 ± 4.9 ns | 155.1 ± 30.0 | 3.29 | 25.97 | |
| B | 16.9 ± 0.3 | 20.3 ± 1.0 * | 6.60 | 11.03 | |
| Treatment | Chlorophyll-a (mg∙kg−1 d.b.) | Chlorophyll-b (mg∙kg−1 d.b.) | Total Chlorophyll (mg∙kg−1 d.b.) | a/b Chlorophyll Ratio | Phenolics (mg∙100 g−1 d.b.) | Flavonoids (mg∙100 g−1 d.b.) |
|---|---|---|---|---|---|---|
| Control | 175 ± 12 | 82 ± 18 | 258 ± 30 | 2.13 ± 0.17 * | 27.2 ± 4.18 | 29.1 ± 0.89 |
| Suppl. Lighting | 203 ± 4 * | 136 ± 4 * | 340 ± 9 * | 1.49 ± 0.07 | 29.4 ± 1.05 * | 37.9 ± 2.67 * |
| CV (%) | 4.7 | 12.2 | 7.4 | 7.1 | 10.8 | 5.9 |
| Treatment | Chlorophyll-a (mg∙kg−1 d.b.) | Chlorophyll-b (mg∙kg−1 d.b.) | Total Chlorophyll (mg∙kg−1 d.b.) | a/b Chlorophyll Ratio | Phenolics (mg∙100 g−1 d.b.) | Flavonoids (mg∙100 g−1 d.b.) |
|---|---|---|---|---|---|---|
| Control | 93.0 ± 34.5 ns | 42.8 ± 17.7 ns | 135.8 ± 52.2 ns | 2.17 ± 0.37 ns | 198.4 ± 28.6 * | 28.1 ± 8.6 ns |
| Suppl. Lighting | 138.7 ± 32.1 | 69.3 ± 17.5 | 208.0 ± 49.5 | 2.00 ± 0.23 | 136.8 ± 14.0 | 21.5 ± 1.4 |
| CV (%) | 28.8 | 31.4 | 29.7 | 25.8 | 13.4 | 25 |
| Period | Temperature (°C) | Insolation Time (h) | RH (%) | |||
|---|---|---|---|---|---|---|
| Average | Minimum | Maximum | ||||
| Climatological normal | 24–31 May | 14.3 | 11.3 | 18.9 | 36.2 | 82.9 |
| 1–30 June | 13.0 | 9.7 | 17.5 | 140.6 | 62.7 | |
| Experiment | 24–31 May | 13.2 | 10.4 | 16.7 | 18.6 | 85.7 |
| 1–30 June | 11.0 | 7.9 | 15.2 | 119.0 | 82.5 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rizzon, A.A.; Silvestre, W.P.; Vicenço, C.B.; Rota, L.D.; Pauletti, G.F. Supplementary Light on the Development of Lettuce and Cauliflower Seedlings. Stresses 2024, 4, 94-106. https://doi.org/10.3390/stresses4010006
Rizzon AA, Silvestre WP, Vicenço CB, Rota LD, Pauletti GF. Supplementary Light on the Development of Lettuce and Cauliflower Seedlings. Stresses. 2024; 4(1):94-106. https://doi.org/10.3390/stresses4010006
Chicago/Turabian StyleRizzon, Adilson Antonio, Wendel Paulo Silvestre, Camila Bonatto Vicenço, Luciana Duarte Rota, and Gabriel Fernandes Pauletti. 2024. "Supplementary Light on the Development of Lettuce and Cauliflower Seedlings" Stresses 4, no. 1: 94-106. https://doi.org/10.3390/stresses4010006
APA StyleRizzon, A. A., Silvestre, W. P., Vicenço, C. B., Rota, L. D., & Pauletti, G. F. (2024). Supplementary Light on the Development of Lettuce and Cauliflower Seedlings. Stresses, 4(1), 94-106. https://doi.org/10.3390/stresses4010006






