Varietal Differences of Yield, Morphological, and Biochemical Parameters of Allium cepa L. under Precipitation Excess in Different Phenological Phases
Abstract
:1. Introduction
2. Results and Discussion
2.1. Morphological Characteristics
2.2. Biochemical Parameters
2.2.1. General Patterns and Changes of Biochemical Parameters
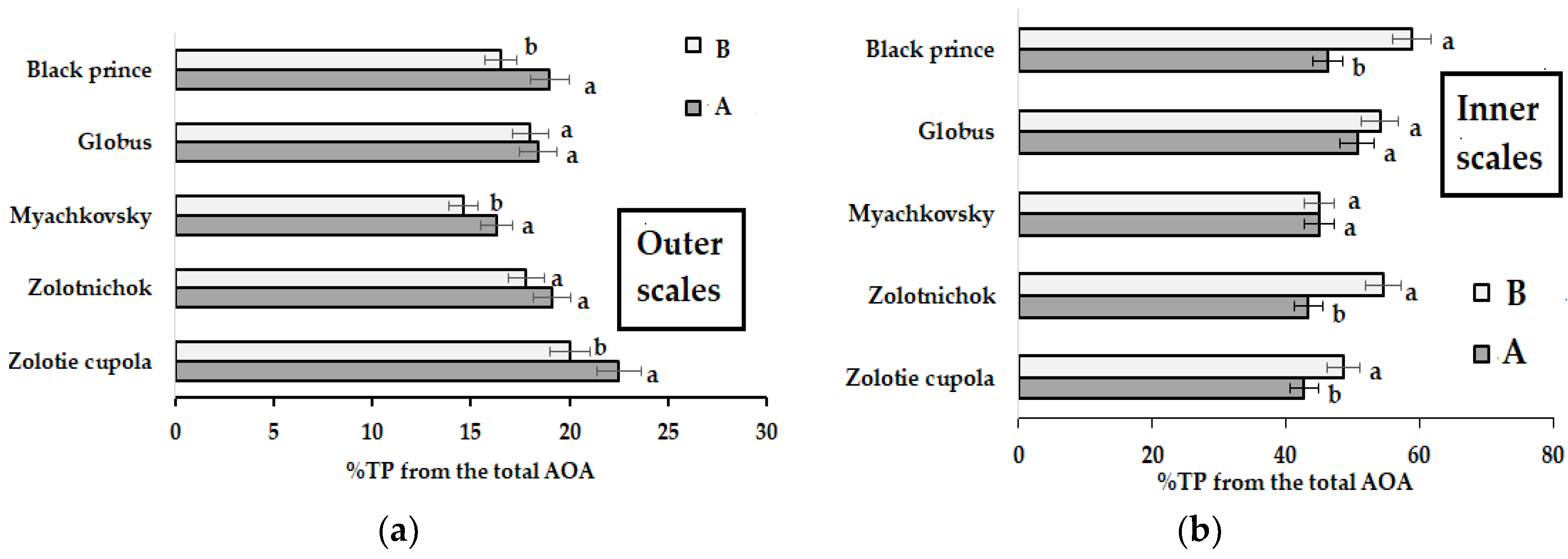
2.2.2. Antioxidant Activity and Phenolics Content
2.2.3. Proline
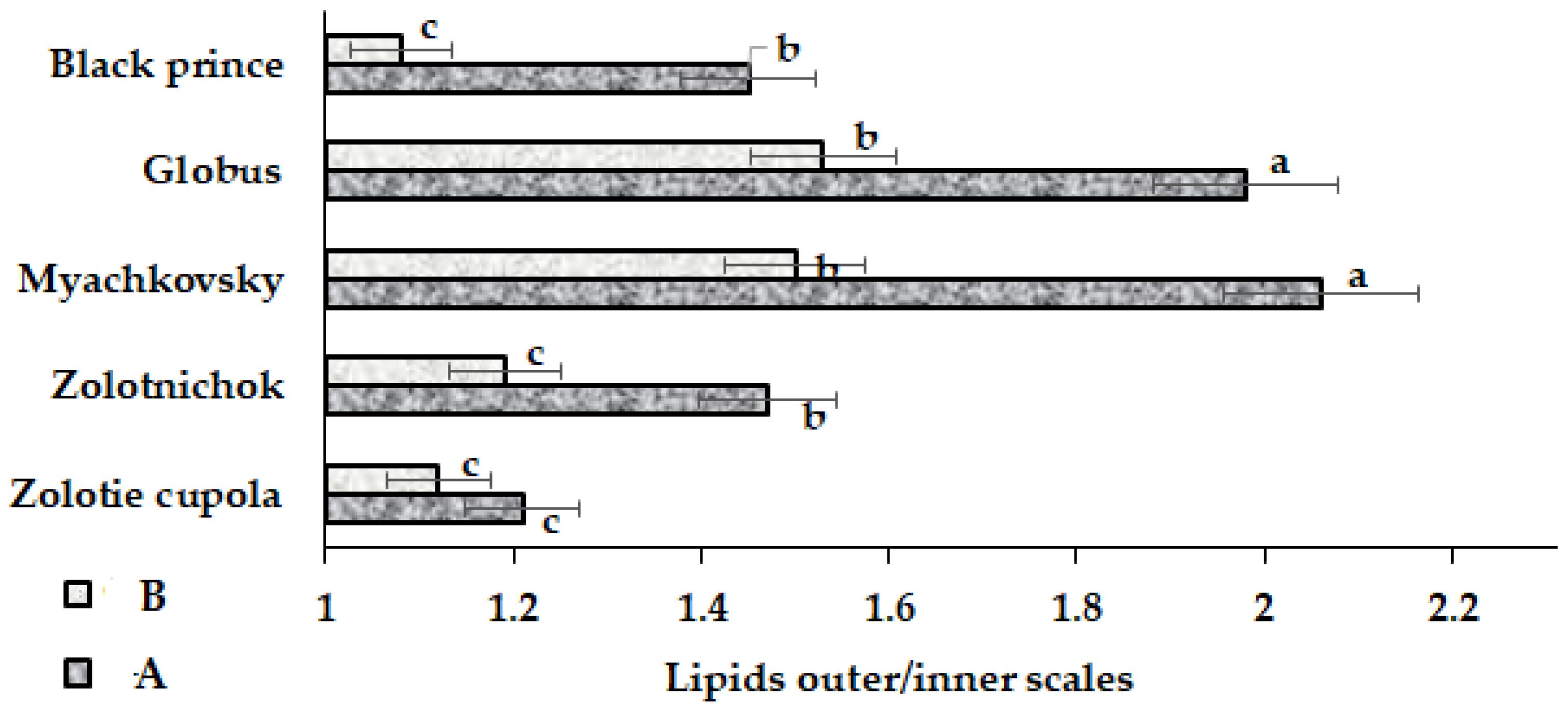
2.2.4. Non-Polar Lipophylic Compounds (Lipids)
2.2.5. Malonic Dialdehyde (MDA)
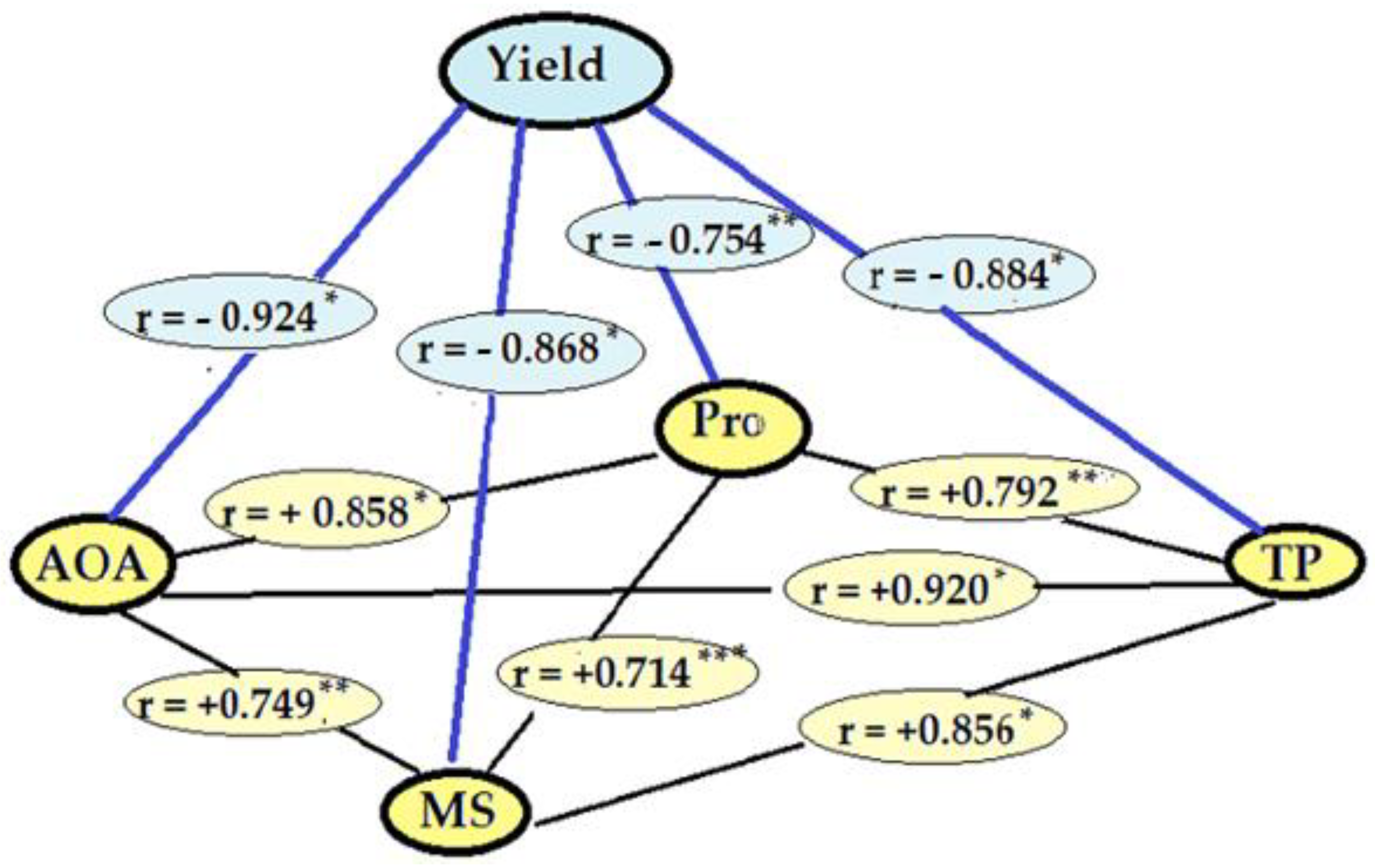
2.2.6. Correlations
3. Material and Methods
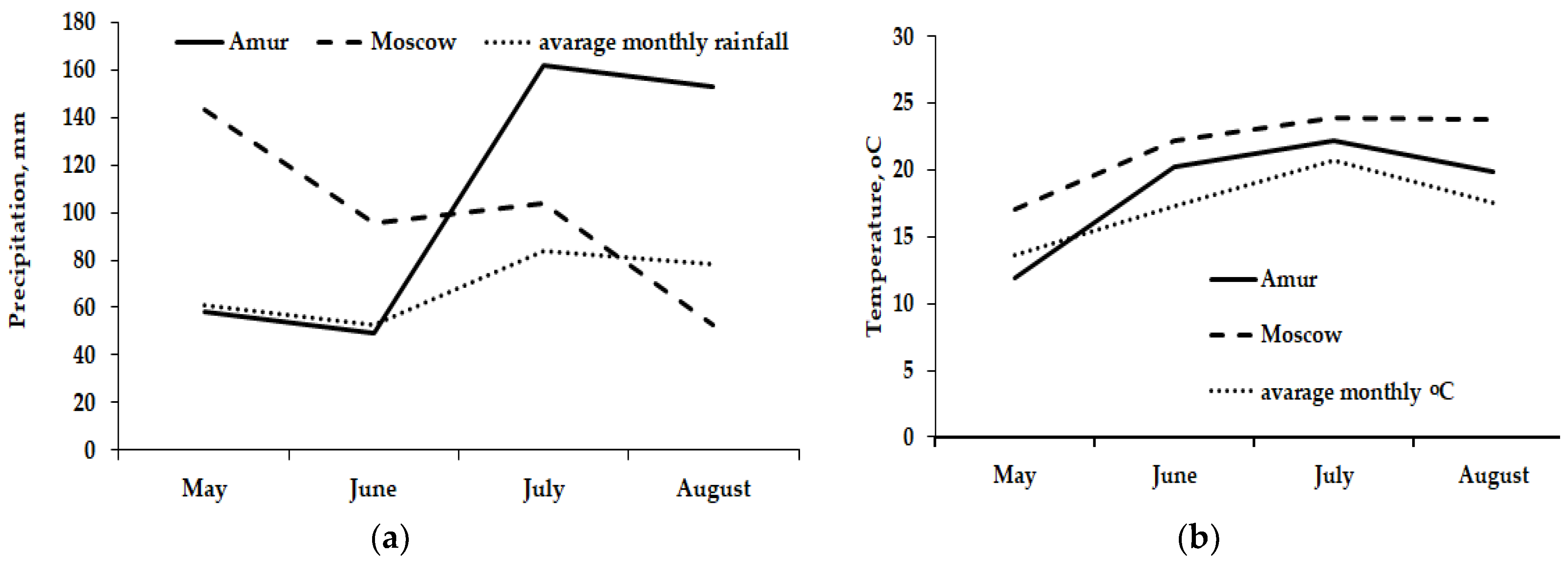
3.1. Experimental Conditions
3.2. Sample Preparation
3.3. Bulb Shape Index (BSI)
3.4. Dry Matter
3.5. Antioxidant Activity (AOA)
3.6. Total Polyphenols (TP)
3.7. Proline
3.8. Malonic Dialdehyde (MDA)
3.9. Sugars
3.10. Non-Polar Lipophilic Compounds (Lipids)
3.11. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- FAO (Food and Agriculture Organization of the United Nations). FAOSTAT Statistics Division. Rome, Italy. 2020. Available online: http://www.fao.org/faostat/en/#data (accessed on 29 May 2023).
- Golubkina, N.; Caruso, G. Allium cepa. In Nutritional Composition and Antioxidant Properties of Fruits and Vegetables; Jaiswal, A., Ed.; Academic Press: Dublin, Ireland, 2020; Chapter 5; pp. 73–87. [Google Scholar] [CrossRef]
- Ghodke, P.H.; Shirsat, D.V.; Thangasamy, A.; Mahajan, V.; Salunkhe, V.N.; Khade, Y.; Singh, M. Effect of water logging stress at specific growth stages in onion crop. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 3438–3448. [Google Scholar] [CrossRef] [Green Version]
- Pasley, H.R.; Huber, I.; Castellano, M.J.; Archontoulis, S.V. Modeling flood-induced stress in soybeans. Front. Plant Sci. 2020, 11, 62. [Google Scholar] [CrossRef] [Green Version]
- Nishiuchi, S.; Yamauchi, T.; Takahashi, H.; Kotula, L.; Nakazono, M. Mechanisms for coping with submergence and waterlogging in rice. Rice 2012, 5, 2. [Google Scholar] [CrossRef] [Green Version]
- Posso, D.A.; Borella, J.; Reissig, G.N.; Bacarin, M.A. Root flooding induced changes in the dynamic dissipation of the photosynthetic energy of common bean plants. Acta Physiol. Plant. 2018, 40, 212. [Google Scholar] [CrossRef]
- Garcia, N.; da-Silva, C.J.; Cocco, K.L.T.; Pomagualli, D.; de Oliveira, F.K.; da Silva, J.V.L.; de Oliveira, A.C.B.; de Amarante, L. Waterlogging tolerance of five soybean genotypes through different physiological and biochemical mechanisms. Environ. Experim. Bot. 2020, 172, 103975. [Google Scholar] [CrossRef]
- Dubey, S.; Kuruwanshi, V.B.; Bhagat, K.P.; Ghodke, P.H. Impact of Excess Moisture in Onion Genotypes (Allium cepa L.) under Climate Change Scenario. Int. J. Curr. Microbiol. Appl. Sci. 2021, 10, 166–175. [Google Scholar] [CrossRef]
- Dubey, S.; Kuruwanshi, V.B.; Ghodke, P.H.; Mahajan, V. Biochemical and yield evaluation of onion (Allium cepa L.) genotypes under waterlogging condition. Int. J. Chem. Stud. 2020, 8, 2036–2040. [Google Scholar] [CrossRef]
- Wakchaure, G.C.; Minhas, P.S.; Kumar, S.; Khapte, P.S.; Rane, J.; Reddy, K.S. Bulb productivity and quality of monsoon onion (Allium cepa L.) as affected by transient waterlogging at different growth stages and its alleviation with plant growth regulators. Agr. Water Manag. 2023, 278, 108136. [Google Scholar] [CrossRef]
- Yiu, J.C.; Liu, C.W.; Fang, D.Y.; Lai, Y.S. Waterlogging tolerance of Welsh onion (Allium fistulosum L.) enhanced by exogenous spermidine and spermine. Plant Physiol. Biochem. 2009, 47, 710–716. [Google Scholar] [CrossRef]
- Pál, M.; Tajti, J.; Szalai, G.; Peeva, V.; Végh, B.; Janda, T. Interaction of polyamines, abscisic acid and proline under osmotic stress in the leaves of wheat plants. Sci. Rep. 2018, 8, 12839. [Google Scholar] [CrossRef] [Green Version]
- Gedam, P.A.; Shirsat, D.V.; Arunachalam, T.; Ghosh, S.; Gawande, S.J.; Mahajan, V.; Gupta, A.J.; Singh, M. Screening of onion (Allium cepa L.) genotypes for waterlogging tolerance. Front. Plant Sci. 2022, 12, 727262. [Google Scholar] [CrossRef]
- Sagar, N.A.; Pareek, S.; Benkeblia, N.; Xiao, J. Onion (Allium cepa L.) bioactives: Chemistry, pharmacotherapeutic functions, and industrial applications. Food Front. 2022, 3, 380–412. [Google Scholar] [CrossRef]
- Caruso, G.; Conti, S.; Villari, G.; Borrelli, C.; Melchionna, G.; Minutolo, M.; Russo, G.; Amalfitano, C. Effects of transplanting time and plant density on yield, quality and antioxidant content of onion (Allium cepa L.) in southern Italy. Sci. Hortic. 2014, 166, 111–120. [Google Scholar] [CrossRef]
- Galsurker, O.; Doron-Faigenboim, A.; Teper-Bamnolker, P.; Daus, A.; Fridman, Y.; Lers, A.; Eshel, D. Cellular and molecular changes associated with onion skin formation suggest involvement of programmed cell death. Front. Plant Sci. 2017, 7, 2031. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pelter, G.Q.; Mittelstadt, R.; Leib, B.G.; Redulla, C.A. Effects of water stress at specific growth stages on onion bulb yield and quality. Agric. Water Manag. 2004, 68, 107–115. [Google Scholar] [CrossRef]
- Wakchaure, G.C.; Khapte, P.S.; Kumar, S.; Kumar, P.S.; Sabatino, L.; Kumar, P. Exogenous growth regulators and water stress enhance long-term storage quality characteristics of onion. Agronomy 2023, 13, 297. [Google Scholar] [CrossRef]
- Kurganova, O.P.; Yavkina, E.N. Sitnikova GV Review of hydrological peculiarities of the flooding in the Amur region with the purpose to establish a complex of sanitary anti- epidemic actions of minimization of social implication. Rospotrebnadzor Act. Emerg. 2014, 1, 29–32. [Google Scholar]
- Dobrutskaya, E.G.; Antoshkin, A.A.; Agafonov, A.F.; Dubova, M.B. Evaluation of A. cepa breeding material diversity by adaptability, productivity and bulb quality. Gavrish 2008, 4, 33–37. (In Russian) [Google Scholar]
- Toscano, S.; Trivellini, A.; Cocetta, G.; Bulgari, R.; Francini, A.; Romano, D.; Ferrante, A. Effect of preharvest abiotic stresses on the accumulation of bioactive compounds in horticultural produce. Front. Plant Sci. 2019, 10, 1212. [Google Scholar] [CrossRef] [Green Version]
- Jeandet, P.; Formela-Luboińska, M.; Labudda, M.; Morkunas, I. The role of sugars in plant responses to stress and their regulatory function during development. Int. J. Mol. Sci. 2022, 23, 5161. [Google Scholar] [CrossRef]
- Zeng, F.-S.; Yao, Y.-F.; Wang, L.-F.; Li, W.-J. Polysaccharides as antioxidants and prooxidants in managing the double-edged sword of reactive oxygen species. Biomed. Pharmacother. 2023, 159, 114221. [Google Scholar] [CrossRef]
- Hayat, S.; Hayat, Q.; Alyemeni, M.N.; Wani, A.S.; Pichtel, J.; Ahmad, A. Role of proline under changing environments: A review. Plant Signal. Behav. 2012, 7, 1456–1466. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, U.K.; Islam, M.N.; Siddiqui, M.N.; Cao, X.; Khan, M.A.R. Proline, a multifaceted signalling molecule in plant responses to abiotic stress: Understanding the physiological mechanisms. Plant Biol. 2022, 24, 227–239. [Google Scholar] [CrossRef]
- Kijowska-Oberc, J.; Staszak, A.M.; Wawrzyniak, M.K.; Ratajczak, E. Changes in proline levels during seed development of orthodox and recalcitrant seeds of genus Acer in a climate change scenario. Forests 2020, 11, 1362. [Google Scholar] [CrossRef]
- Xie, L.-J.; Zhou, Y.; Chen, Q.W.-F.; Xiao, S. New insights into the role of lipids in plant hypoxia responses. Progress Lipid Res. 2021, 81, 101072. [Google Scholar] [CrossRef]
- Hou, Q.; Ufer, G.; Bartels, D. Lipid signaling in plant responses to abiotic stress. Plant Cell Environ. 2016, 39, 1029–1048. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, J.; Sharif, R.; Xu, X.; Chen, X. Mechanisms of waterlogging tolerance in plants: Research progress and prospects. Front. Plant Sci. 2021, 11, 627331. [Google Scholar] [CrossRef] [PubMed]
- Bello, M.O.; Olabanji, I.O.; Abdul-Hammed, M.; Okunade, T.D. Characterization of domestic onion wastes and bulb (Allium cepa L.): Fatty acids and metal contents. Int. Food Ress. J. 2012, 20, 2153–2158. [Google Scholar]
- Tsiaganis, M.C.; Laskari, K.; Melissari, E. Fatty acid composition of Allium species lipids. J. Food Comp. Anal. 2006, 19, 620–627. [Google Scholar] [CrossRef]
- Alché, J.D. A concise appraisal of lipid oxidation and lipoxidation in higher plants. Redox Biol. 2019, 23, 101136. [Google Scholar] [CrossRef]
- Tian, L.; Zhang, Y.; Chen, P.; Zhang, F.; Li, J.; Yan, F.; Dong, Y.; Feng, B. How does the waterlogging regime affect crop yield? A global meta-analysis. Front. Plant Sci. 2021, 12, 634898. [Google Scholar] [CrossRef] [PubMed]
- ISO (3166)004-97; Method of Dry Matter Determination. Standartinform: Moscow, Russia, 2012.
- Golubkina, N.A.; Kekina, H.G.; Molchanova, A.V.; Antoshkina, M.S.; Nadezhkin, S.M.; Soldatenko, A.V. Plants Antioxidants and Methods of Their Determination; Infra-M: Moscow, Russia, 2020; (In Russian). [Google Scholar] [CrossRef]
- Ábrahám, E.; Hourton-Cabassa, C.; Erdei, L.; Szabados, L. Methods for determination of proline in plants. In Plant Stress Tolerance. Methods in Molecular Biology (Methods and Protocols); Sunkar, R., Ed.; Humana Press: Totowa, NJ, USA, 2010; Volume 639, Chapter 20, pp. 317–331. [Google Scholar] [CrossRef]
- Heath, R.L.; Parker, L. Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Swamy, P.M. Laboratory Manual on Biotechnology; Rastogi: New Delhi, India, 2008; p. 617. [Google Scholar]



| Parameter | Water Stress | Zolotie Cupola | Zolot-Nichok | Myach-Kovsky | Globus | Black Prince | M ± SD | CV (%) |
|---|---|---|---|---|---|---|---|---|
| Leaf length (cm) | A | 55.4 c | 58.5 bc | 52.1 c | 51.8 c | 58.7 bc | 56.1 ± 3.2 | 5.7 |
| B | 67.1 a | 69.5 a | 66.8 a | 69.0 a | 68.1 ab | 68.1 ± 1.2 | 1.8 | |
| Leaves number | A | 8.2 abc | 7.5 d | 6.0 d | 6.3 d | 7.4 cd | 7.1 ± 0.9 | 13.0 |
| B | 10.2 a | 10.1 a | 9.8 ab | 10.3 a | 10.2 q | 10.1 ± 0.2 | 2.0 | |
| Bulb diameter (cm) | A | 5.1 b | 4.7 b | 5.3 b | 4.9 b | 4.9 b | 5.0 ± 0.2 | 4.0 |
| B | 7.3 a | 7.2 a | 7.2 a | 6.8 a | 7.3 a | 7.2 ± 0.2 | 2.8 | |
| Bulb height (cm) | A | 4.2 cd | 4.8 bc | 3.9 d | 5.0 abc | 5.0 abc | 4.6 ± 0.5 | 10.9 |
| B | 5.2 ab | 5.8 a | 4.3 cd | 6.0 a | 5.7 ab | 5.4 ± 0.7 | 13.0 | |
| Bulb shape index | A | 0.80 b | 1.00 a | 0.70 c | 1.00 a | 1.00 a | 0.9 ± 0.1 | 11.1 |
| B | 0.72 c | 0.81 ab | 0.59 d | 0.87 a | 0.78 bc | 0.75 ± 0.1 | 13.3 | |
| Bulb weight (g) | A | 68.9 b | 67.5 b | 65.5 b | 78.4 b | 70.0 b | 70.1 ± 6.7 | 9.3 |
| B | 127.0 a | 141.2 a | 114.3 a | 129.3 a | 137.1 a | 129.8 ± 10.4 | 8.0 |
| Parameter | Water Stress | Zolotie Cupola | Zolot-Nichok | Myach-Kovsky | Globus | Black Prince | M ± SD | CV (%) |
|---|---|---|---|---|---|---|---|---|
| Dry matter (%) | A | 13.6 ab | 10.2 c | 13.4 ab | 13.8 ab | 12.5 bc | 12.7 ± 1.5 | 11.8 |
| B | 15.0 a | 15.0 a | 15.5 a | 14.1 a | 14.6 a | 14.8 ± 0.5 | 3.4 | |
| Monosaccharides (%) | A | 3.4 a | 3.0 ab | 3.5 a | 3.5 a | 3.0 ab | 3.3 ± 0.3 | 9.1 |
| B | 2.4 c | 2.2 c | 2.3 c | 2.6 bc | 2.6 bc | 2.4 ± 0.2 | 8.3 | |
| Total sugar (%) | A | 7.9 bc | 6.5 d | 6.9 cd | 6.7 cd | 6.4 d | 6.9 ± 0.6 | 8.7 |
| B | 10.9 a | 8.25 b | 11.1 a | 11.0 a | 9.1 ab | 10.1 ± 1.3 | 12.9 |
| Parameter | Water Stress | Zolotie Cupola | Zolot-Nichok | Myach-Kovsky | Globus | Black Prince | M ± SD | CV (%) |
|---|---|---|---|---|---|---|---|---|
| AOA Inner scales | A | 45.4 a | 45.9 a | 42.6 ab | 35.9 bc | 36.9 bc | 41.3 ± 4.2 | 10.2 |
| B | 28.6 d | 28.0 d | 34.0 c | 28.1 d | 27.0 d | 29.2 ± 7.1 | 24.3 | |
| AOA Outer scales | A | 121.7 b | 129.5 b | 157.8 a | 145.1 ab | 148.7 a | 140.6 ± 13.1 | 9.3 |
| B | 129.2 b | 138.1 ab | 168.4 a | 145.4 ab | 157.0 a | 147.6 ± 13.8 | 9.8 | |
| AOA outer/ inner scales | A | 2.68 | 2.82 | 3.70 | 4.04 | 4.03 | 3.39 ± 0.64 | 18.9 |
| B | 4.52 | 4.93 | 4.95 | 5.17 | 5.81 | 5.08 ± 0.47 | 9.2 | |
| TP Inner scales | A | 19.4 a | 19.9 a | 19.2 a | 18.2 a | 17.1 a | 18.8 ± 1.0 | 5.3 |
| B | 13.9 b | 15.3 b | 15.3 b | 15.2 b | 15.9 b | 15.1 ± 2.1 | 13.9 | |
| TP Outer scales | A | 27.4 a | 24.7 a | 25.8 a | 26.7 a | 28.3 a | 26.6 ± 1.2 | 3.8 |
| B | 25.9 a | 24.6 a | 24.6 a | 26.1 a | 25.9 a | 25.4 ± 1.1 | 3.1 |
| Parameter | Water Stress | Zolotie Cupola | Zolot-Nichok | Myach-Kovsky | Globus | Black Prince | M ± SD | CV (%) |
|---|---|---|---|---|---|---|---|---|
| Inner Scales proline | A | 1.92 b | 1.66 c | 2.30 a | 1.37 c | 0.97 d | 1.64 ± 0.51 | 31.1 |
| B | 0.95 d | 0.96 d | 1.34 c | 0.95 d | 0.73 e | 0.99 ± 0.22 | 22.2 | |
| A/B proline ratio | A | 2.02 | 1.73 | 1.72 | 1.44 | 1.33 | 1.65 ± 0.24 | 14.5 |
| Seeds proline | B | 1.13 a | 1.12 a | 0.86 b | 0.89 b | 1.12 a | 1.01 ± 0.10 | 10.0 |
| Seeds/bulb proline ratio | A | 0.54 | 0.67 | 0.37 | 0.65 | 1.15 | 0.68 ± 0.19 | 27.9 |
| B | 1.08 | 1.17 | 0.64 | 0.94 | 1.53 | 1.07 ± 0.23 | 21.5 |
| Parameter | Water Stress | Zolotie Cupola | Zolot-Nichok | Myach-Kovsky | Globus | Black Prince | M ± SD | CV (%) |
|---|---|---|---|---|---|---|---|---|
| Inner scales | A | 19.3 a | 18.2 a | 12.7 c | 12.7 c | 18.8 bc | 16.4 ± 3.3 | 20.1 |
| B | 19.7 a | 19.3 a | 15.3 b | 15.8 b | 22.0 a | 18.4 ± 3.0 | 16.3 | |
| Outer scales | A | 23.4 ab | 26.7 ab | 26.1 ab | 25.1 ab | 27.3 a | 25.7 ± 1.4 | 5.4 |
| B | 22.1 b | 22.9 b | 23.0 ab | 24.2 ab | 24.5 ab | 23.3 ± 1.7 | 7.3 |
| Water Stress | Zolotie Cupola | Zolot-Nichok | Myach-Kovsky | Globus | Black Prince | M ± SD | CV (%) | |
|---|---|---|---|---|---|---|---|---|
| Inner scales | A | 0.82 a | 0.86 a | 0.94 a | 0.86 a | 0.84 a | 0.86 ± 0.04 | 4.7 |
| B | 0.80 a | 0.81 a | 0.86 a | 0.86 a | 0.80 a | 0.83 ± 0.04 | 4.8 | |
| Outer scales | A | 0.5 ab | 0.46 b | 0.56 a | 0.48 ab | 0.50 a | 0.49 ± 0.04 | 8.2 |
| B | 0.48 ab | 0.44 bc | 0.42 bc | 0.38 c | 0.47 ab | 0.43 ± 0.05 | 11.6 |
| Parameter | Moscow Region | Amur Region |
|---|---|---|
| Geographical coordinates | 55°39.51′ N, 37°12.23′ E | 50°19′09″ N, 127°44′12″ E |
| Climate | Temperate continental | Monsoon |
| Mean annual temperature (°C) | +6.3 | −1.3 |
| Mean annual precipitation (mm) | 708 | 550 |
| Mean annual radiation (hours) | 1731 | 2400 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Golubkina, N.; Romanova, O.; Romanov, V.; Krivenkov, L.; Shevchenko, T.; Murariu, O.C.; Vecchietti, L.; Hamburda, S.B.; Caruso, G. Varietal Differences of Yield, Morphological, and Biochemical Parameters of Allium cepa L. under Precipitation Excess in Different Phenological Phases. Stresses 2023, 3, 541-554. https://doi.org/10.3390/stresses3030038
Golubkina N, Romanova O, Romanov V, Krivenkov L, Shevchenko T, Murariu OC, Vecchietti L, Hamburda SB, Caruso G. Varietal Differences of Yield, Morphological, and Biochemical Parameters of Allium cepa L. under Precipitation Excess in Different Phenological Phases. Stresses. 2023; 3(3):541-554. https://doi.org/10.3390/stresses3030038
Chicago/Turabian StyleGolubkina, Nadezhda, Olga Romanova, Valery Romanov, Leonid Krivenkov, Tatiana Shevchenko, Otilia Cristina Murariu, Lorenzo Vecchietti, Silvia Brindusa Hamburda, and Gianluca Caruso. 2023. "Varietal Differences of Yield, Morphological, and Biochemical Parameters of Allium cepa L. under Precipitation Excess in Different Phenological Phases" Stresses 3, no. 3: 541-554. https://doi.org/10.3390/stresses3030038







