Algae Polysaccharides (Carrageenan and Alginate)—A Treasure-Trove of Antiviral Compounds: An In Silico Approach to Identify Potential Candidates for Inhibition of S1-RBD Spike Protein of SARS-CoV2
Abstract
:1. Introduction
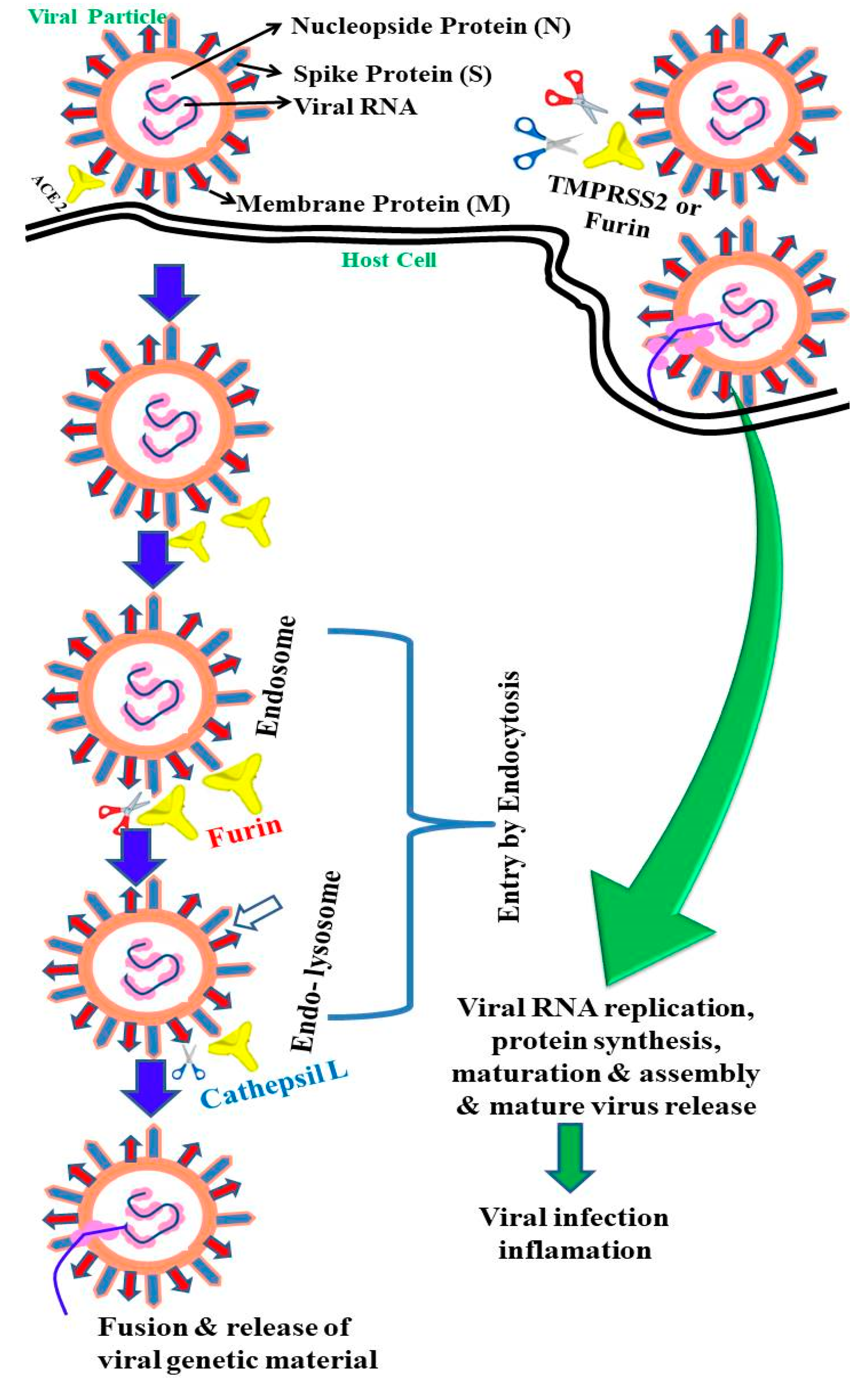
2. Epidemiology and Life Cycle of SARS-CoV-2
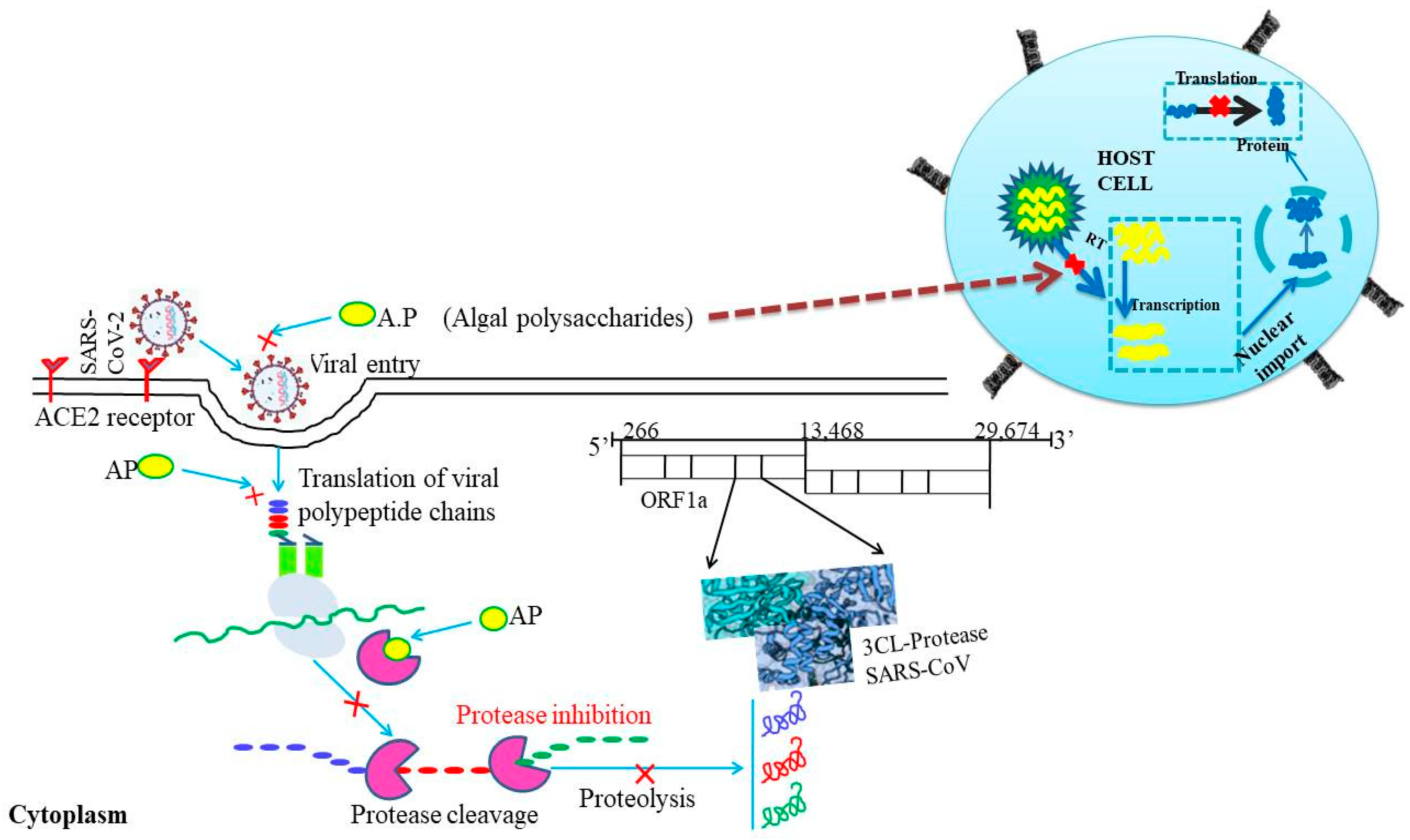
3. Algal Polysaccharides and Their Potential as Antiviral Agents
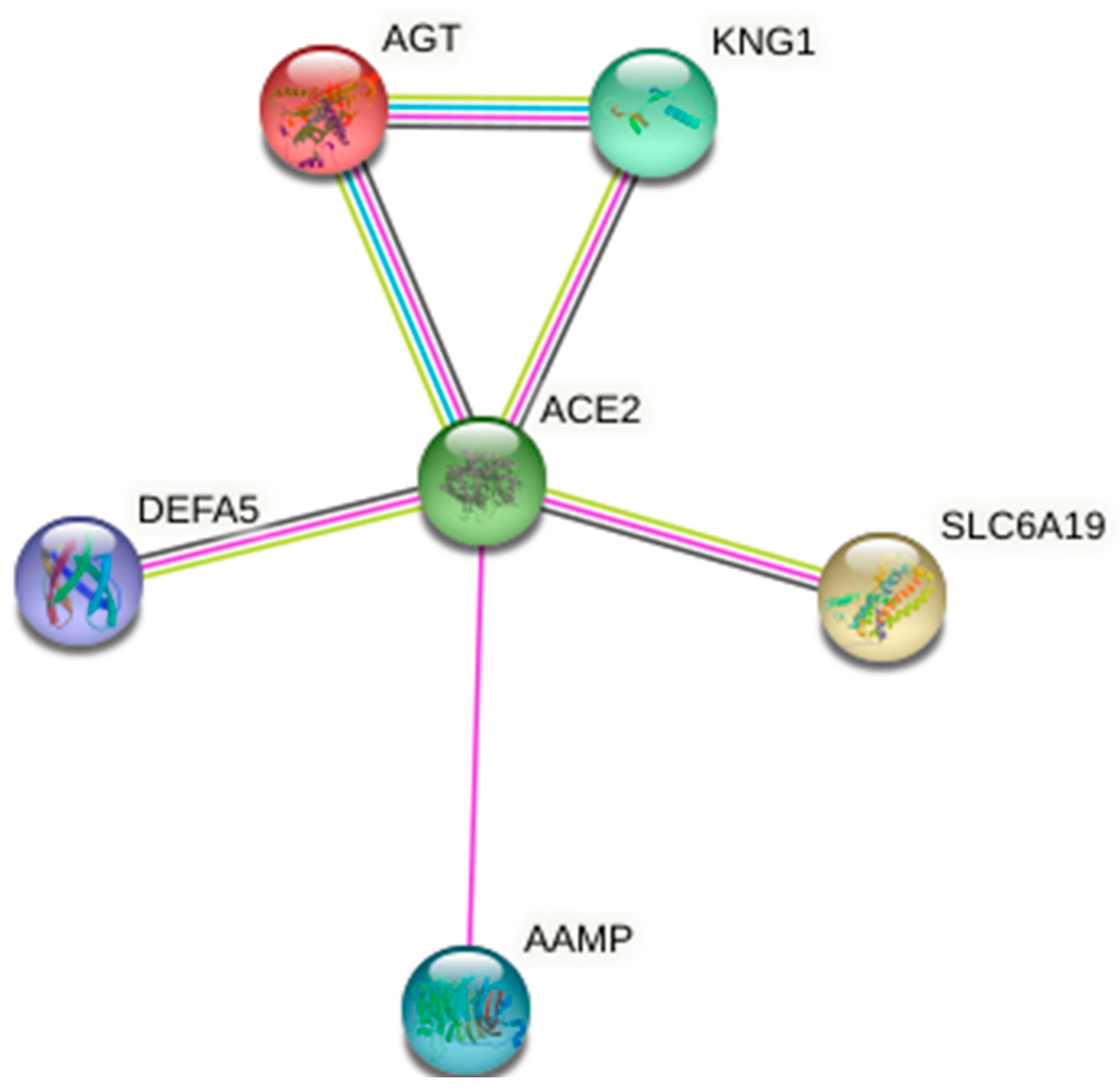
4. ACE2 Host Receptor for S1-RBD of SARS-CoV-2: Its Protein Network
5. In Silico Approaches for Prediction of the Potentiality of Algal Metabolites (Alginic Acid and Carrageenan) against SARS-CoV-2
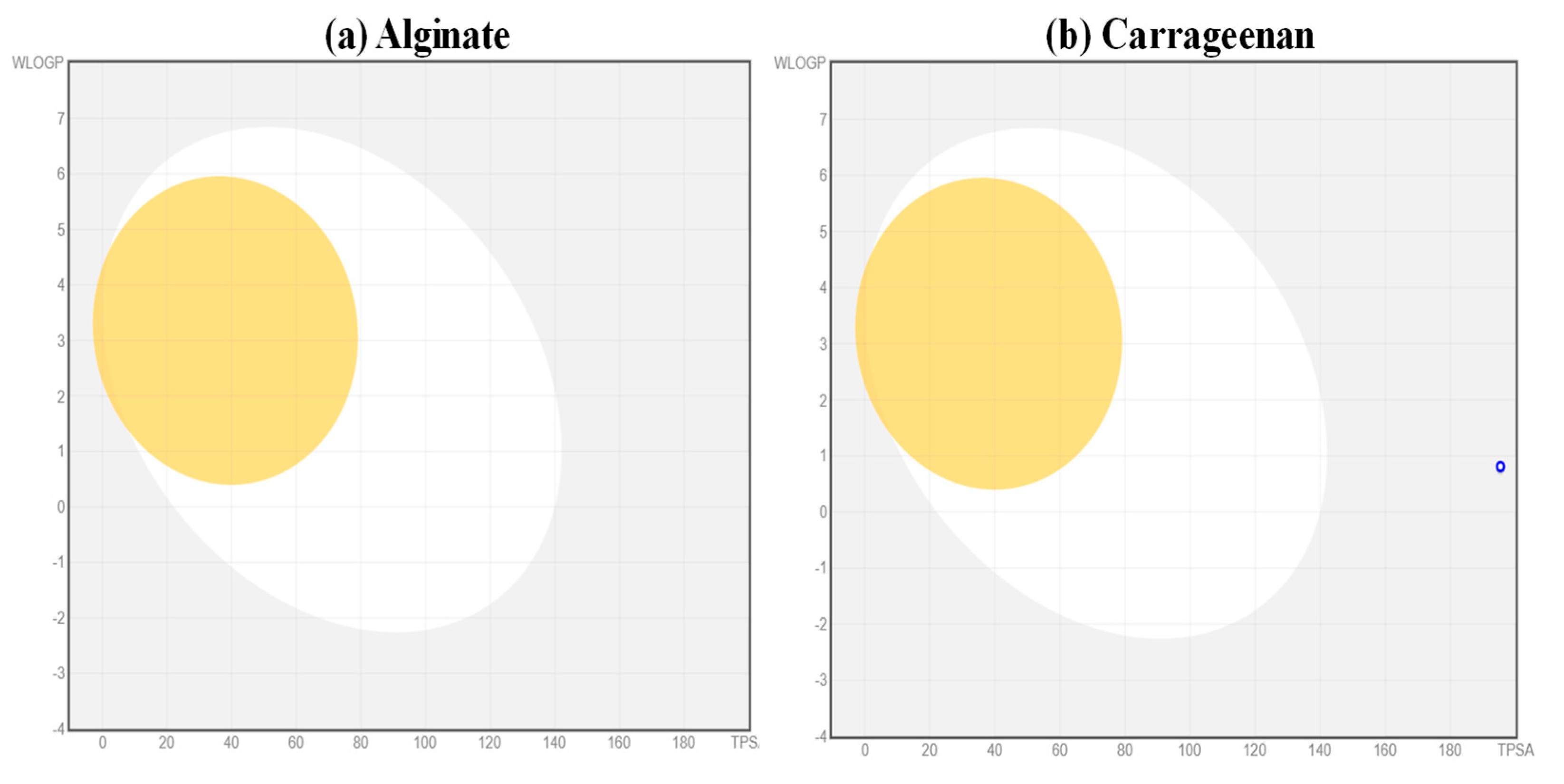
5.1. Analysis of Pharmacokinetic Properties of the Ligands
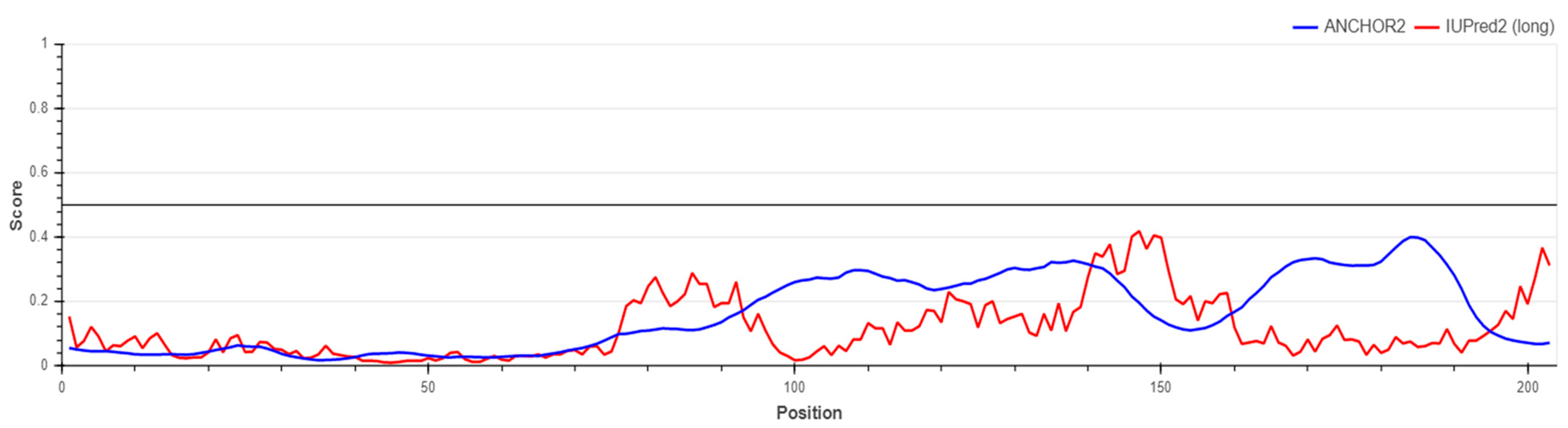
5.2. Prediction of S1-RBD Spike Protein Stability
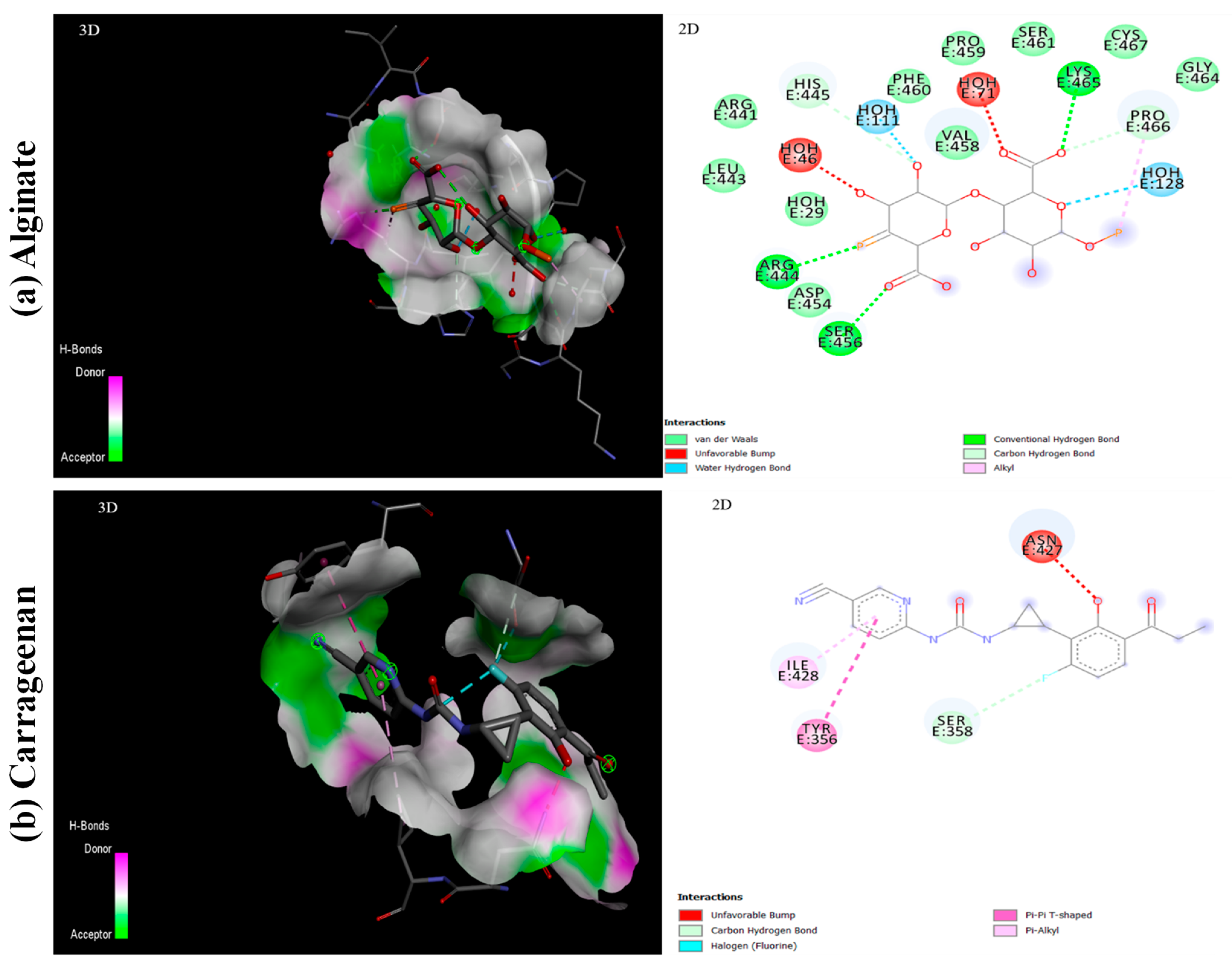
5.3. Interaction of Ligands with S1-RBD of SARS-CoV-2
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumar, A.; Singh, R.P.; Kumar, I.; Yadav, P.; Singh, S.K.; Kaushalendra; Singh, P.K.; Gupta, R.K.; Singh, S.M.; Kesawat, M.S.; et al. Algal Metabolites Can Be an Immune Booster against COVID-19 Pandemic. Antioxidants 2022, 11, 452. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, S.; Vijayakumar, S.; Praseetha, P. Cyanobacterial metabolites as novel drug candidates in corona viral therapies: A review. Chronic. Dis. Transl. Med. 2022, 8, 172–183. [Google Scholar] [PubMed]
- Silva, S.C.; Ferreira, I.C.F.R.; Dias, M.M.; Barreiro, M.F. Microalgae-Derived Pigments: A 10-Year Bibliometric Review and Industry and Market Trend Analysis. Molecules 2020, 25, 3406. [Google Scholar] [CrossRef] [PubMed]
- Chakdar, H.; Jadhav, S.D.; Dhar, D.W.; Pabbi, S. Potential applications of blue green algae. J. Sci. Ind. Res. 2012, 71, 13–20. [Google Scholar]
- Novoveská, L.; Ross, M.E.; Stanley, M.S.; Pradelles, R.; Wasiolek, V.; Sassi, J.F. Microalgal carotenoids: A review of production, current markets, regulations, and future direction. Mar. Drugs 2019, 17, 640. [Google Scholar] [CrossRef] [Green Version]
- Chin, Y.W.; Balunas, M.J.; Chai, H.B.; Kinghorn, A.D. Drug discovery from natural sources. AAPS J. 2006, 8, E239–E253. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, T.N.B.T.; Feisal, N.A.S.; Kamaludin, N.H.; Cheah, W.Y.; How, V.; Bhatnagar, A.; Ma, Z.; Show, P.L. Biological active metabolites from microalgae for healthcare and pharmaceutical industries: A comprehensive review. Bioresour. Technol. 2023, 21, 128661. [Google Scholar] [CrossRef]
- Yeo, C.; Kaushal, S.; Yeo, D. Enteric involvement of coronaviruses: Is faecal-oral transmission of SARS-CoV-2 possible? Lancet Gastroenterol. Hepatol. 2020, 5, 335–337. [Google Scholar] [CrossRef] [Green Version]
- Dini, I. The Potential of Algae in the Nutricosmetic Sector. Molecules 2023, 28, 4032. [Google Scholar] [CrossRef]
- Brogi, S. Computational Approaches for Drug Discovery. Molecules 2019, 24, 3061. [Google Scholar] [CrossRef] [Green Version]
- Kaur, M.; Bhatia, S.; Gupta, U.; Decker, E.; Tak, Y.; Bali, M.; Gupta, V.K.; Dar, R.A.; Bala, S. Microalgal bioactive metabolites as promising implements in nutraceuticals and pharmaceuticals: Inspiring therapy for health benefits. Phytochem. Rev. 2023, 14, 1–31. [Google Scholar] [CrossRef]
- Perumal, P.K.; Dong, C.D.; Chauhan, A.S.; Anisha, G.S.; Kadri, M.S.; Chen, C.W.; Singhania, R.R.; Patel, A.K. Advances in oligosaccharides production from algal sources and potential applications. Biotechnol. Adv. 2023, 67, 108195. [Google Scholar] [CrossRef] [PubMed]
- Kumari, A.; GarimaBharadvaja, N. A comprehensive review on algal nutraceuticals as prospective therapeutic agent for different diseases. 3 Biotech 2023, 13, 44. [Google Scholar] [CrossRef] [PubMed]
- Dewi, I.C.; Falaise, C.; Hellio, C.; Bourgougnon, N.; Mouget, J.L. Anticancer, antiviral, antibacterial, and antifungal properties in microalgae. In Microalgae in Health and Disease Prevention. In Microalgae in Health and Disease Prevention; Academic Press: Cambridge, MA, USA, 2018; pp. 235–261. [Google Scholar]
- Joshi, V.; Bharadvaja, N. Current Prospects and Clinical Status of Microalgae Derived Chemotherapeutics. Rev. Bras. Farmacogn. 2023, 33, 445–470. [Google Scholar] [CrossRef]
- Singh, U.; Gandhi, H.A.; Nikita; Bhattacharya, J.; Tandon, R.; Tiwari, G.L.; Tandon, R. Cyanometabolites: Molecules with immense antiviral potential. Arch. Microbiol. 2023, 205, 164. [Google Scholar] [CrossRef]
- Ibañez, E.; Cifuentes, A. Benefits of using algae as natural sources of functional ingredients. J. Sci. Food Agric. 2013, 93, 703–709. [Google Scholar] [CrossRef] [Green Version]
- Ganesan, A.R.; Tiwari, U.; Rajauria, G. Seaweed nutraceuticals and their therapeutic role in disease prevention. Food Sci. Hum. Wellness. 2019, 8, 252–263. [Google Scholar] [CrossRef]
- Sharaf, M.; Amara, A.; Aboul-Enein, A.; Helmi, S.; Ballot, A.; Astani, A.; Schnitzler, P. Molecular authentication and characterization of the antiherpetic activity of the cyanobacterium Arthrospira fusiformis. Die Pharm. Int. J. Pharm. Sci. 2010, 65, 132–136. [Google Scholar]
- Silva, T.; Salomon, P.S.; Hamerski, L.; Walter, J.; Menezes, R.B.; Siqueira, J.E.; Santos, A.; Santos, J.A.M.; Ferme, N.; Guimarães, T.; et al. Inhibitory effect of microalgae and cyanobacteria extracts on influenza virus replication and neuraminidase activity. Peer J. 2018, 6, e5716. [Google Scholar] [CrossRef] [Green Version]
- Shih, S.R.; Tsai, K.N.; Li, Y.S.; Chueh, C.C.; Chan, E.C. Inhibition of enterovirus 71-induced apoptosis by allophycocyanin isolated from a blue-green alga Spirulina platensis. J. Med. Virol. 2003, 70, 119–125. [Google Scholar] [CrossRef]
- Dey, B.; Lerner, D.L.; Lusso, P.; Boyd, M.R.; Elder, J.H.; Berger, E.A. Multiple antiviral activities of cyanovirin-N: Blocking of human immunodeficiency virus type 1 gp120 interaction with CD4 and co-receptor and inhibition of diverse enveloped viruses. J. Virol. 2000, 74, 4562–4569. [Google Scholar] [CrossRef] [PubMed]
- Kachko, A.; Loesgen, S.; Shahzad-ul-Hussan, S.; Tan, W.; Zubkova, I.; Takeda, K.; Wells, F.; Rubin, S.; Bewley, C.A.; Major, M.E. Inhibition of hepatitis C virus by the cyanobacterial protein Microcystis viridis lectin: Mechanistic differences between the high-mannose specific lectins MVL, CV-N., and GNA. Mol. Pharm. 2013, 10, 4590–4602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Keefe, B.R.; Smee, D.F.; Turpin, J.A.; Saucedo, C.J.; Gustafson, K.R.; Mori, T.; Blakeslee, D.; Buckheit, R.; Boyd, M.R. Potent anti-influenza activity of cyanovirin-N and interactions with viral hemagglutinin. Antimicrob. Agents Chemother. 2003, 47, 2518–2525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, R.; Parihar, P.; Singh, M.; Bajguz, A.; Kumar, J.; Singh, S.; Singh, V.P.; Prasad, S.M. Uncovering potential applications of cyanobacteria and algal metabolites in biology, agriculture and medicine: Current status and future prospects. Front. Microbiol. 2017, 8, 459–537. [Google Scholar]
- Elaya Perumal, U.; Sundararaj, R. Algae: A potential source to prevent and cure the novel coronavirus–A review. Int. J. Emerg. Technol. 2020, 11, 479–483. [Google Scholar]
- Khan, R.J.; Jha, R.K.; Amera, G.M.; Jain, M.; Singh, E.; Pathak, A.; Singh, R.P.; Muthukumaran, J.; Singh, A.K. Targeting SARS-CoV-2: A systematic drug repurposing approach to identify promising inhibitors against 3C-like proteinase and 2′-O-ribose methyltransferase. J. Biomol. Struct. Dyn. 2021, 39, 2679–2692. [Google Scholar]
- Hui, D.S.; Azhar, E.I.; Madani, T.A.; Ntoumi, F.; Kock, R.; Dar, O.; Ippolito, G.; Mchugh, T.D.; Memish, Z.A.; Drosten, C.; et al. The continuing 2019-nCoV epidemic threat of novel coronaviruses to global health—The latest 2019 novel coronavirus outbreak in Wuhan, China. Int. J. Infec. Dis. 2020, 91, 264–266. [Google Scholar] [CrossRef] [Green Version]
- Sahin, A.R.; Erdogan, A.; Agaoglu, P.M.; Dineri, Y.; Cakirci, A.Y.; Senel, M.E.; Okyay, R.A.; Tasdogan, A.M. 2019 novel coronavirus (COVID-19) outbreak: A review of the current literature. EJMO 2020, 4, 1–7. [Google Scholar]
- Grubaugh, N.D.; Ladner, J.T.; Lemey, P.; Pybus, O.G.; Rambaut, A.; Holmes, E.C.; Andersen, K.G. Tracking virus outbreaks in the twenty-first century. Nat. Microbiol. 2019, 4, 10–19. [Google Scholar] [CrossRef]
- Guan, W.J.; Ni, Z.Y.; Hu, Y.; Liang, W.H.; Ou, C.Q.; He, J.X.; Liu, L.; Shan, H.; Lei, C.L.; Hui, D.S.; et al. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef]
- Nowruz, B.; Haghighat, S.; Fahimi, H.; Mohammadi, E. Nostoc cyanobacteria species: A new and rich source of novel bioactive compounds with pharmaceutical potential. J. Pharm. Health Serv. Res. 2018, 9, 5–12. [Google Scholar] [CrossRef]
- Thuan, N.H.; An, T.T.; Shrestha, A.; Canh, N.X.; Sohng, J.K.; Dhakal, D. Recent advances in exploration and biotechnological production of bioactive compounds in three cyanobacterial genera: Nostoc, Lyngbya, and Microcystis. Front. Chem. 2019, 7, 604. [Google Scholar] [CrossRef] [PubMed]
- de Jesus Raposo, M.F.; de Morais, R.M.S.C.; de Morais, A.M.M.B. Health applications of bioactive compounds from marine microalgae. Life Sci. 2013, 93, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Mayer, A.; Guerrero, A.J.; Rodríguez, A.D.; Taglialatela-Scafati, O.; Nakamura, F.; Fusetani, N. Marine pharmacology in 2014–2015: Marine compounds with antibacterial, antidiabetic, antifungal, anti-inflammatory, antiprotozoal, antituberculosis, antiviral, and anthelmintic activities; affecting the immune and nervous systems, and other miscellaneous mechanisms of action. Mar. Drugs 2020, 18, 5. [Google Scholar]
- Soares, A.R.; Robaina, M.; Mendes, G.S.; Silva, T.S.; Gestinari, L.; Pamplona, O.S.; Yoneshigue-Valentin, Y.; Kaiser, C.R.; Romanos, M.T.V. Antiviral activity of extracts from Brazilian seaweeds against herpes simplex virus. Rev. Bras. Farmacogn. 2012, 22, 714–723. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Riverol, A.; Ramos, A.P.; Díaz, L.F.M.; López, Y.T.; Llanes, D.M.; del Barrio Alonso, G. Antiviral activity of an aqueous extract from the red alga Laurencia obtusa against influenza A and B viruses. Rev. Cubana. Med. Trop. 2014, 66, 273–285. [Google Scholar]
- Zaid, S.A.A.L.; Hamed, N.N.E.D.; Abdel-Wahab, K.S.E.D.; Abo El-Magd, E.K.; Salah El-Din, R.A.L. Antiviral activities and phytochemical constituents of Egyptian marine seaweeds (Cystoseira myrica (SG Gmelin) C. Agardh and Ulva Lactuca Linnaeus) aqueous extract. Egypt. J. Hosp. Med. 2016, 64, 422–429. [Google Scholar] [CrossRef]
- El-Sheekh, M.M.; Shabaan, M.T.; Hassan, L.; Morsi, H.H. Antiviral activity of algae biosynthesized silver and gold nanoparticles against Herpes Simplex (HSV-1) virus in-vitro using cell-line culture technique. Int. J. Environ. Health Res. 2020, 32, 616–627. [Google Scholar]
- Pinto, A.M.V.; Leite, J.P.G.; Ferreira, W.J.; Cavalcanti, D.N.; Villaça, R.C.; Giongo, V.; Teixeira, V.L.; Paixão, I.C.N.D.P. Marine natural seaweed products as potential antiviral drugs against bovine viral diarrhea virus. Rev. Bras. Farmacogn. 2012, 22, 813–817. [Google Scholar] [CrossRef] [Green Version]
- Bhuiyan, F.R.; Howlader, S.; Raihan, T.; Hasan, M. Plants metabolites: Possibility of natural therapeutics against the COVID-19 pandemic. Front. Med. 2020, 7, 444. [Google Scholar]
- Lan, J.; Ge, J.; Yu, J.; Shan, S.; Zhou, H.; Fan, S.; Zhang, Q.; Shi, X.; Wang, Q.; Zhang, L.; et al. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature 2020, 581, 215–220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Peng, H.; Wang, L.; Zhao, Y.; Zeng, L.; Gao, H.; Liu, Y. Infants born to mothers with a new coronavirus (COVID-19). Front. Pediatr. 2020, 8, 104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phan, L.T.; Nguyen, T.V.; Luong, Q.C.; Nguyen, T.V.; Nguyen, H.T.; Le, H.Q.; Nguyen, T.T.; Cao, T.M.; Pham, Q.D. Importation and human-to-human transmission of a novel Coronavirus in Vietnam. N. Engl. J. Med. 2020, 382, 872–874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirano, T.; Murakami, M. COVID-19: A new virus, but a familiar receptor and cytokine release syndrome. Immunity 2020, 52, 731–733. [Google Scholar]
- Coperchini, F.; Chiovato, L.; Croce, L.; Magri, F.; Rotondi, M. The cytokine storm in COVID-19: An overview of the involvement of the chemokine/chemokine-receptor system. Cytokine Growth Factor Rev. 2020, 53, 25–32. [Google Scholar]
- Liang, Y.; Wang, M.L.; Chien, C.S.; Yarmishyn, A.A.; Yang, Y.P.; Lai, W.Y.; Luo, Y.H.; Lin, Y.T.; Chen, Y.J.; Chang, P.C.; et al. Highlight of immune pathogenic response and hematopathologic effect in SARS-CoV, MERS-CoV and SARS-Cov-2 infection. Front. Immunol. 2020, 11, 1022. [Google Scholar] [CrossRef]
- Tang, X.; Wu, C.; Li, X.; Song, Y.; Yao, X.; Wu, X.; Duan, Y.; Zhang, H.; Wang, Y.; Qian, Z.; et al. On the origin and continuing evolution of SARS-CoV-2. Natl. Sci. Rev. 2020, 3, 036. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Hou, Y.; Shen, J.; Huang, Y.; Martin, W.; Cheng, F. Network-based drug repurposing for novel coronavirus 2019-nCoV/SARS-CoV-2. Cell Discov. 2020, 6, 14. [Google Scholar] [CrossRef]
- Xu, J.; Zhao, S.; Teng, T.; Abdalla, A.E.; Zhu, W.; Xie, L.; Wang, Y.; Guo, X. Systematic comparison of two animal-to-human transmitted human Coronaviruses: SARS-CoV-2 and SARS-CoV. Viruses 2020, 12, 244. [Google Scholar]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.H.; Nitsche, A.; et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 2020, 2, 52. [Google Scholar] [CrossRef] [PubMed]
- Shereen, M.A.; Khan, S.; Kazmi, A.; Bashir, N.; Siddique, R. COVID-19 infection: Origin, transmission, and characteristics of human coronaviruses. J. Adv. Res. 2020, 24, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Xue, S.; Yang, H.; Chen, C. Recent progress in the discovery of inhibitors targeting coronavirus proteases. Virol. Sin. 2016, 31, 24–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, W.; Li, T. COVID-19: Towards understanding of pathogenesis. Cell Res. 2020, 30, 367–369. [Google Scholar] [PubMed]
- Rockx, B.; Kuiken, T.; Herfst, S.; Bestebroer, T.; Lamers, M.M.; Oude Munnink, B.B.; De Meulder, D.; Van Amerongen, G.; Van Den Brand, J.; Okba, N.M.; et al. Comparative pathogenesis of COVID-19, MERS, and SARS in a nonhuman primate model. Science 2020, 368, 1012–1015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Liu, Q.; Guo, D. Emerging coronaviruses: Genome structure, replication, and pathogenesis. J. Med. Virol. 2020, 92, 418–423. [Google Scholar]
- Kim, D.; Lee, J.Y.; Yang, J.S.; Kim, J.W.; Kim, V.N.; Chang, H. The architecture of SARS-CoV-2 transcriptome. Cell 2020, 181, 914–921. [Google Scholar] [CrossRef]
- Al-Horani, R.A.; Kar, S.; Aliter, K.F. Potential Anti-COVID-19 Therapeutics that Block the Early Stage of the Viral Life Cycle: Structures, Mechanisms, and Clinical Trials. Int. J. Mol. Sci. 2020, 21, 5224. [Google Scholar]
- Özçelik, B.; Kartal, M.; Orhan, I. Cytotoxicity, antiviral and antimicrobial activities of alkaloids, flavonoids, and phenolic acids. Pharm. Biol. 2011, 49, 396–402. [Google Scholar] [CrossRef]
- Cho, J.K.; Curtis-Long, M.J.; Lee, K.H.; Kim, D.W.; Ryu, H.W.; Yuk, H.J.; Park, K.H. Geranylated flavonoids displaying SARS-CoV papain-like protease inhibition from the fruits of Paulownia tomentosa. Bioorganic Med. Chem. 2013, 21, 3051–3057. [Google Scholar] [CrossRef]
- Ryu, Y.B.; Jeong, H.J.; Kim, J.H.; Kim, Y.M.; Park, J.Y.; Kim, D.; Naguyen, T.T.H.; Park, S.J.; Chang, J.S.; Park, K.H.; et al. Biflavonoids from Torreya nucifera displaying SARS-CoV 3CLpro inhibition. Bioorganic Med. Chem. 2010, 18, 7940–7947. [Google Scholar] [CrossRef]
- Yang, C.W.; Lee, Y.Z.; Hsu, H.Y.; Shih, C.; Chao, Y.S.; Chang, H.Y.; Lee, S.J. Targeting coronaviral replication and cellular JAK2 mediated dominant NF- kB activation for comprehensive and ultimate inhibition of coronaviral activity. Sci. Rep. 2017, 7, 4105. [Google Scholar] [CrossRef] [Green Version]
- Pradhan, B.; Ki, J.S. Biological activity of algal derived carrageenan: A comprehensive review in light of human health and disease. Int. J. Biol. Macromol. 2023, 21, 124085. [Google Scholar] [CrossRef]
- Andrew, M.; Jayaraman, G. Marine sulfated polysaccharides as potential antiviral drug candidates to treat Corona Virus disease (COVID-19). Carbohydr. Res. 2021, 505, 108326. [Google Scholar]
- Pradhan, B.; Bhuyan, P.P.; Ki, J.-S. Immunomodulatory, Antioxidant, Anticancer, and Pharmacokinetic Activity of Ulvan, a Seaweed-Derived Sulfated Polysaccharide: An Updated Comprehensive Review. Mar. Drugs 2023, 21, 300. [Google Scholar] [CrossRef]
- Wang, W.; Wang, S.X.; Guan, H.S. The antiviral activities and mechanisms of marine polysaccharides: An overview. Mar. Drugs 2012, 10, 2795–2816. [Google Scholar] [CrossRef]
- Cosenza, V.A.; Navarro, D.A.; Pujol, C.A.; Damonte, E.B.; Stortz, C.A. Partial and total C-6 oxidation of gelling carrageenans. Modulation of the antiviral activity with the anionic character. Carbohydr. Polym. 2015, 128, 199–206. [Google Scholar] [CrossRef]
- Zia, K.M.; Tabasum, S.; Nasif, M.; Sultan, N.; Aslam, N.; Noreen, A.; Zuber, M. A review on synthesis, properties and applications of natural polymer based carrageenan blends and composites. Int. J. Biol. Macromol. 2017, 96, 282–301. [Google Scholar] [CrossRef]
- Pacheco-Quito, E.M.; Ruiz-Caro, R.; Veiga, M.D. Carrageenan: Drug delivery systems and other biomedical applications. Mar. Drugs 2020, 18, 583. [Google Scholar] [CrossRef]
- Álvarez-Viñas, M.; Souto, S.; Flórez-Fernández, N.; Torres, M.D.; Bandín, I.; Domínguez, H. Antiviral activity of carrageenans and processing implications. Mar. Drugs 2021, 19, 437. [Google Scholar] [CrossRef]
- Campo, V.L.; Kawano, D.F.; da Silva Jr, D.B.; Carvalho, I. Carrageenans: Biological properties, chemical modifications and structural analysis—A review. Carbohyd. Polym. 2009, 77, 167–180. [Google Scholar] [CrossRef]
- Boulho, R.; Marty, C.; Freile-Pelegrín, Y.; Robledo, D.; Bourgougnon, N.; Bedoux, G. Antiherpetic (HSV-1) activity of carrageenans from the red seaweed Solieria chordalis (Rhodophyta, Gigartinales) extracted by microwave-assisted extraction (MAE). J. Appl. Phycol. 2017, 29, 2219–2228. [Google Scholar]
- Gomaa, H.H.A.; Elshoubaky, G.A. Antiviral activity of sulfated polysaccharides carrageenan from some marine seaweeds. Int. J. Curr. Pharm. Rev. Res. 2016, 7, 34–42. [Google Scholar]
- Abu-Galiyun, E.; Huleihel, M.; Levy-Ontman, O. Antiviral bioactivity of renewable polysaccharides against Varicella zoster. Cell Cycle. 2019, 18, 3540–3549. [Google Scholar] [CrossRef]
- Baba, M.; Snoeck, R.; Pauwels, R.; De Clercq, E. Sulfated polysaccharides are potent and selective inhibitors of various enveloped viruses, including herpes simplex virus, cytomegalovirus, vesicular stomatitis virus, and human immunodeficiency virus. Antimicrob. Agents Chemother. 1988, 32, 1742–1745. [Google Scholar] [CrossRef] [Green Version]
- Yamada, T.; Ogamo, A.; Saito, T.; Uchiyama, H.; Nakagawa, Y. Preparation of O-acylated low-molecular-weight carrageenans with potent anti-HIV activity and low anticoagulant effect. Carbohydr. Polym. 2000, 41, 115–120. [Google Scholar] [CrossRef]
- Klimyte, E.M.; Smith, S.E.; Oreste, P.; Lembo, D.; Dutch, R.E. Inhibition of Human Metapneumovirus Binding to Heparan Sulfate Blocks Infection in Human Lung Cells and Airway Tissues. J. Virol. 2016, 90, 9237–9250. [Google Scholar] [CrossRef] [Green Version]
- Besednova, N.N.; Zvyagintseva, T.N.; Kuznetsova, T.A.; Makarenkova, I.D.; Smolina, T.P.; Fedyanina, L.N.; Kryzhanovsky, S.P.; Zaporozhets, T.S. Marine algae metabolites as promising therapeutics for the prevention and treatment of HIV/AIDS. Metabolites 2019, 9, 87. [Google Scholar] [CrossRef] [Green Version]
- Damonte, E.; Matulewicz, M.; Cerezo, A. Sulfated Seaweed Polysaccharides as Antiviral Agents. Curr. Med. Chem. 2012, 11, 2399–2419. [Google Scholar]
- Shi, Q.; Wang, A.; Lu, Z.; Qin, C.; Hu, J.; Yin, J. Overview on the antiviral activities and mechanisms of marine polysaccharides from seaweeds. Carbohydr. Res. 2017, 453, 1–9. [Google Scholar]
- Bansal, S.; Jonsson, C.B.; Taylor, S.L.; Manuel Figueroa, J.; Vanesa, A.; Palacios, C.; César Vega, J. Iota-carrageenan and Xylitol inhibit SARS-CoV-2 in cell culture. bioRxiv 2020. [Google Scholar] [CrossRef]
- Jang, Y.; Shin, H.; Lee, M.K.; Kwon, O.S.; Shin, J.S.; Kim, Y.; Kim, C.W.; Lee, H.R.; Kim, M. Antiviral activity of lambda-carrageenan against influenza viruses and severe acute respiratory syndrome coronavirus 2. Sci. Rep. 2021, 11, 821. [Google Scholar]
- Morokutti-Kurz, M.; Fröba, M.; Graf, P.; Große, M.; Grassauer, A.; Auth, J.; Schubert, U.; Prieschl-Grassauer, E. Iota-carrageenan neutralizes SARS-CoV-2 and inhibits viral replication in vitro. PLoS ONE 2021, 16, e0237480. [Google Scholar] [CrossRef]
- Song, S.; Peng, H.; Wang, Q.; Liu, Z.; Dong, X.; Wen, C.; Ai, C.; Zhang, Y.; Wang, Z.; Zhu, B. Inhibitory activities of marine sulfated polysaccharides against SARS-CoV-2. Food Funct. 2020, 11, 7415–7420. [Google Scholar]
- Davidson, I.W.; Sutherland, I.W.; Lawson, C.J. Purification and properties of an alginate lysase from a marine bacterium. Biochem. J. 1976, 159, 703–707. [Google Scholar] [CrossRef] [Green Version]
- Meena, R.; Chhatbar, M.; Prasad, K.; Siddhanta, A.K. Development of a robust hydrogel system based on agar and sodium alginate blend. Poly. Int. 2008, 57, 329–336. [Google Scholar] [CrossRef]
- Smidsrød, O.; Skja, G. Alginate as immobilization matrix for cells. Trends Biotechnol. 1990, 8, 71–78. [Google Scholar] [CrossRef]
- Ratner, B.D.; Hoffman, A.S.; Schoen, F.J.; Lemons, J.E. Biomaterials Science: An Introduction to Materials in Medicine; Academic Press: Toronto, ON, Canada, 2012. [Google Scholar]
- Mastromarino, P.; Petruzziello, R.; Macchia, S.; Rieti, S.; Nicoletti, R.; Orsi, N. Antiviral activity of natural and semisynthetic polysaccharides on the early steps of rubella virus infection. J. Antimicrob. Chemother. 1997, 39, 339–345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bandyopadhyay, S.S.; Navid, M.H.; Ghosh, T.; Schnitzler, P.; Ray, B. Structural features and in vitro antiviral activities of sulfated polysaccharides from Sphacelaria indica. Phytochemistry 2011, 72, 276–283. [Google Scholar]
- Sinha, S.; Astani, A.; Ghosh, T.; Schnitzler, P.; Ray, B. Polysaccharides from Sargassum tenerrimum: Structural features, chemical modification and anti-viral activity. Phytochemistry 2010, 71, 235–242. [Google Scholar]
- Fabra, M.J.; Falcó, I.; Randazzo, W.; Sánchez, G.; López-Rubio, A. Antiviral and antioxidant properties of active alginate edible films containing phenolic extracts. Food Hydrocoll. 2018, 81, 96–103. [Google Scholar] [CrossRef]
- Falcó, I.; Flores-Meraz, P.L.; Randazzo, W.; Sánchez, G.; López-Rubio, A.; Fabra, M.J. Antiviral activity of alginate-oleic acid based coatings incorporating green tea extract on strawberries and raspberries. Food Hydrocoll. 2019, 87, 611–618. [Google Scholar] [CrossRef] [Green Version]
- Beyerstedt, B.; Casaro, E.B.; Range, E.B. COVID-19: Angiotensin-converting enzyme 2 (ACE2) expression and tissue susceptibility to SARS-CoV-2 infection. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 905–919. [Google Scholar] [PubMed]
- Winterhalter, C.; Nicolle, R.; Louis, A.; Radvanyi, F.; Elati, M. PEPPER: Cytoscape app for protein complex expansion using protein–protein interaction networks. Bioinformatics 2014, 30, 3419–3420. [Google Scholar] [PubMed] [Green Version]
- Vilar, S.; Sobarzo-Sánchez, E.; Santana, L.; Uriarte, E. Molecular Docking and Drug Discovery in β-Adrenergic Receptors. Curr. Med. Chem. 2017, 24, 4340–4359. [Google Scholar] [CrossRef]
- Srivastava, A.K.; Kumar, V.; Roy, B.K. Insights from the molecular docking of curcumin to the virulent factors of Helicobacter pylori. Bioinformation 2015, 11, 447–453. [Google Scholar]
- Srivastava, A.K.; Singh, D.; Roy, B.K. Structural interactions of curcumin biotransformed molecules with the N-terminal residues of cytotoxic-associated gene a protein provide insights into suppression of oncogenic activities. Interdiscip. Sci. Comput. Life Sci. 2017, 9, 116–129. [Google Scholar] [CrossRef]
- Srivastava, A.K.; Tewari, M.; Shukla, H.S.; Roy, B.K. In-silico Profiling of the Potentiality of Curcumin and Conventional Drugs for CagA Oncoprotein Inactivation. Arch. Pharm. 2015, 348, 548–555. [Google Scholar] [CrossRef]
- Nagle, V.; Gaikwad, M.; Pawar, Y.; Dasgupta, S. Marine red alga Porphyridium sp. as a source of sulfated polysaccharides (SPs) for combating against COVID-19. Preprints 2020, 2020040168. [Google Scholar]
- Tran, N.M.; Dufresne, M.; Helle, F.; Hoffmann, T.W.; François, C.; Brochot, E.; Paullier, P.; Legallais, C.; Duverlie, G.; Castelain, S. Alginate hydrogel protects encapsulated hepatic HuH-7 cells against hepatitis C virus and other viral infections. PLoS ONE 2014, 9, e109969. [Google Scholar]
- Daina, A.; Zoete, V. A BOILED-egg to predict gastrointestinal absorption and brain penetration of small molecules. Chem. Med. Chem. 2016, 11, 1117–1121. [Google Scholar] [CrossRef] [Green Version]
- Mészáros, B.; Erdös, G.; Dosztányi, Z. IUPred2A: Context-dependent prediction of protein disorder as a function of redox state and protein binding. Nucleic Acids Res. 2018, 46, W329–W337. [Google Scholar] [PubMed] [Green Version]
- Mohankumar, T.; Chandramohan, V.; Lalithamba, H.S.; Jayaraj, R.L.; Kumaradhas, P.; Sivanandam, M.; Hunday, G.; Vijayakumar, R.; Balakrishnan, R.; Manimaran, D.; et al. Design and molecular dynamic investigations of 7, 8-dihydroxyflavone derivatives as potential neuroprotective agents against alpha-synuclein. Sci. Rep. 2020, 10, 599. [Google Scholar] [PubMed] [Green Version]
- Asiamah, I.; Obiri, S.A.; Tamekloe, W.; Armah, F.A.; Borquaye, L.S. Applications of molecular docking in natural products-based drug discovery. Sci. Afr. 2023, 20, e01593. [Google Scholar] [CrossRef]
| Algal Polysaccharides | Source | Viral Diseases | Mechanism of Actions |
|---|---|---|---|
| Carrageenan | Red algae | Influenza virus Human immunodeficiency virus Herpes simplex virus | The compound inhibits the binding or entry of the virus to the host cell |
| Alginate | Brown algae | Human immunodeficiency virus Hepatitis B Virus | Compounds inhibit adhesion of virus to the host cell and also inhibit replication inside the cell |
| Fucan | Brown algae | Human immunodeficiency virus Herpes simplex virus | By blockage of reverse transcriptase |
| Agar | Red algae | Influenza virus | By the partial blockage of the adhesion to the endothelial cells |
| Laminaran | Brown algae | Human immunodeficiency virus | By the blockage of reverse transcriptase |
| Galactan | Red algae | Human immunodeficiency virus Herpes simplex virus | By inhibiting adhesion of the virus to the host cells and inhibition of replication |
| Ulvans | Green algae | Human and avian influenza viruses | Inhibit viral reproduction |
| Interactors | Functions | Score |
|---|---|---|
| AGT | Major component of the renin–angiotensin system (RAS), an efficient regulator of blood pressure, body fluid, and electrolyte homeostasis. | 0.998 |
| KNG1 | Plays an essential function in blood coagulation. | 0.885 |
| SLC6A19 | Transporter, helps in amino acids resorption. | 0.999 |
| AAMP | Involved in angiogenesis and cell migration. | 0.491 |
| DEFA5 | Defensins kill microbes by permeabilizing their plasma membrane. | 0.692 |
| Compounds | Caco-2 Permeability | AMES Toxicity | Carcinogens | Rat Acute Toxicity LD50 (mol/kg) |
|---|---|---|---|---|
| Alginate | Caco2- | Non-AMES toxic | Non-carcinogens | 2.1445 |
| Carrageenan | Caco2- | Non-AMES toxic | Non-carcinogens | 2.6262 |
| Compounds | ACE |
|---|---|
| Alginate | −12,131.2 |
| Carrageenan | −162,339.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rohilla, D.; Srivastava, A.K.; Singh, R.P.; Yadav, P.; Singh, S.K.; Kumar, D.; Bhardwaj, N.; Kesawat, M.S.; Pandey, K.D.; Kumar, A. Algae Polysaccharides (Carrageenan and Alginate)—A Treasure-Trove of Antiviral Compounds: An In Silico Approach to Identify Potential Candidates for Inhibition of S1-RBD Spike Protein of SARS-CoV2. Stresses 2023, 3, 555-569. https://doi.org/10.3390/stresses3030039
Rohilla D, Srivastava AK, Singh RP, Yadav P, Singh SK, Kumar D, Bhardwaj N, Kesawat MS, Pandey KD, Kumar A. Algae Polysaccharides (Carrageenan and Alginate)—A Treasure-Trove of Antiviral Compounds: An In Silico Approach to Identify Potential Candidates for Inhibition of S1-RBD Spike Protein of SARS-CoV2. Stresses. 2023; 3(3):555-569. https://doi.org/10.3390/stresses3030039
Chicago/Turabian StyleRohilla, Dikshansha, Akhileshwar Kumar Srivastava, Rahul Prasad Singh, Priya Yadav, Sandeep Kumar Singh, Dharmendra Kumar, Nikunj Bhardwaj, Mahipal Singh Kesawat, Kapil Deo Pandey, and Ajay Kumar. 2023. "Algae Polysaccharides (Carrageenan and Alginate)—A Treasure-Trove of Antiviral Compounds: An In Silico Approach to Identify Potential Candidates for Inhibition of S1-RBD Spike Protein of SARS-CoV2" Stresses 3, no. 3: 555-569. https://doi.org/10.3390/stresses3030039
APA StyleRohilla, D., Srivastava, A. K., Singh, R. P., Yadav, P., Singh, S. K., Kumar, D., Bhardwaj, N., Kesawat, M. S., Pandey, K. D., & Kumar, A. (2023). Algae Polysaccharides (Carrageenan and Alginate)—A Treasure-Trove of Antiviral Compounds: An In Silico Approach to Identify Potential Candidates for Inhibition of S1-RBD Spike Protein of SARS-CoV2. Stresses, 3(3), 555-569. https://doi.org/10.3390/stresses3030039









