Beneficial Microorganisms as a Sustainable Alternative for Mitigating Biotic Stresses in Crops
Abstract
1. Introduction
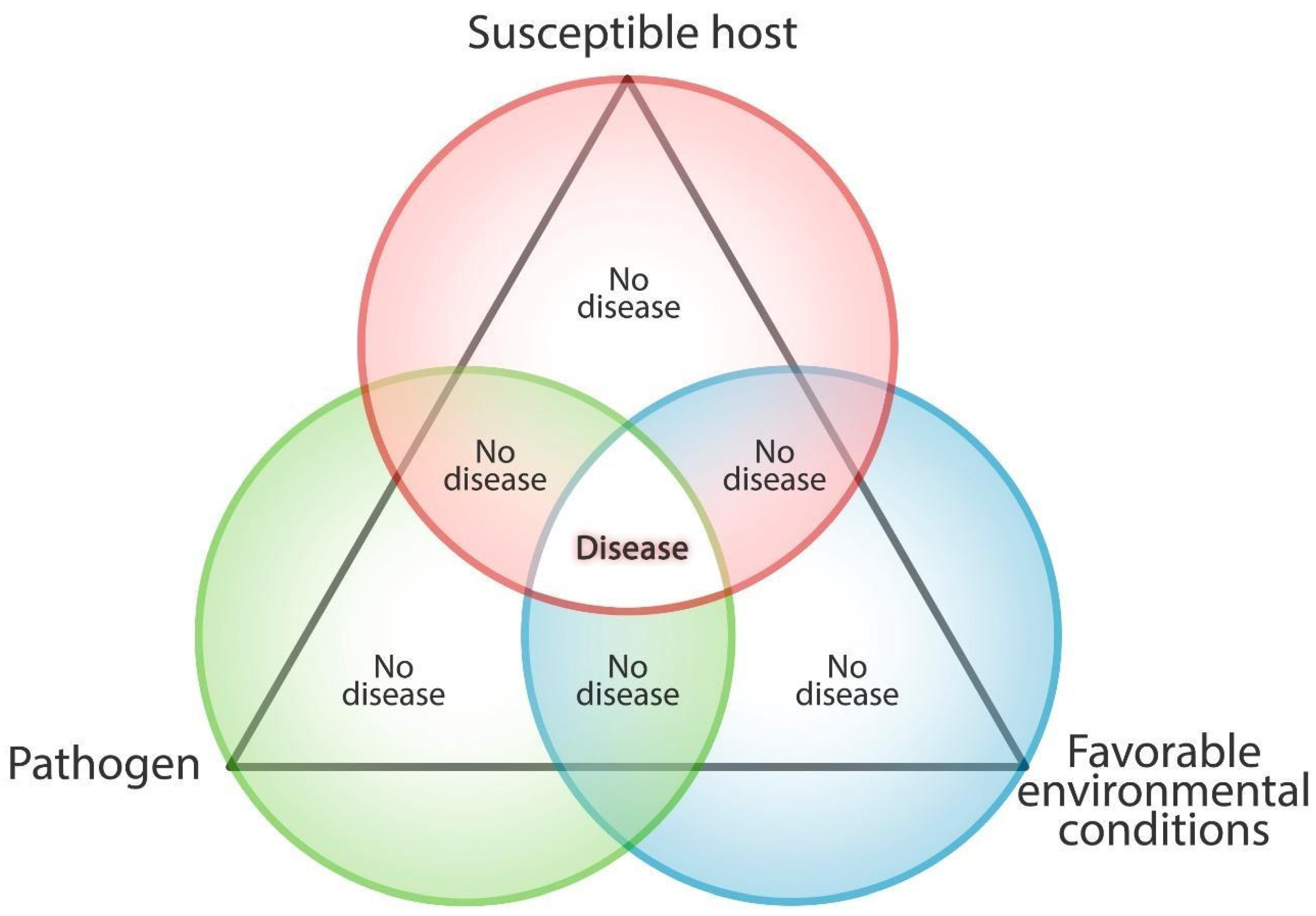
2. Plants Biotic Stress: Interactions between Plants and Pathogens
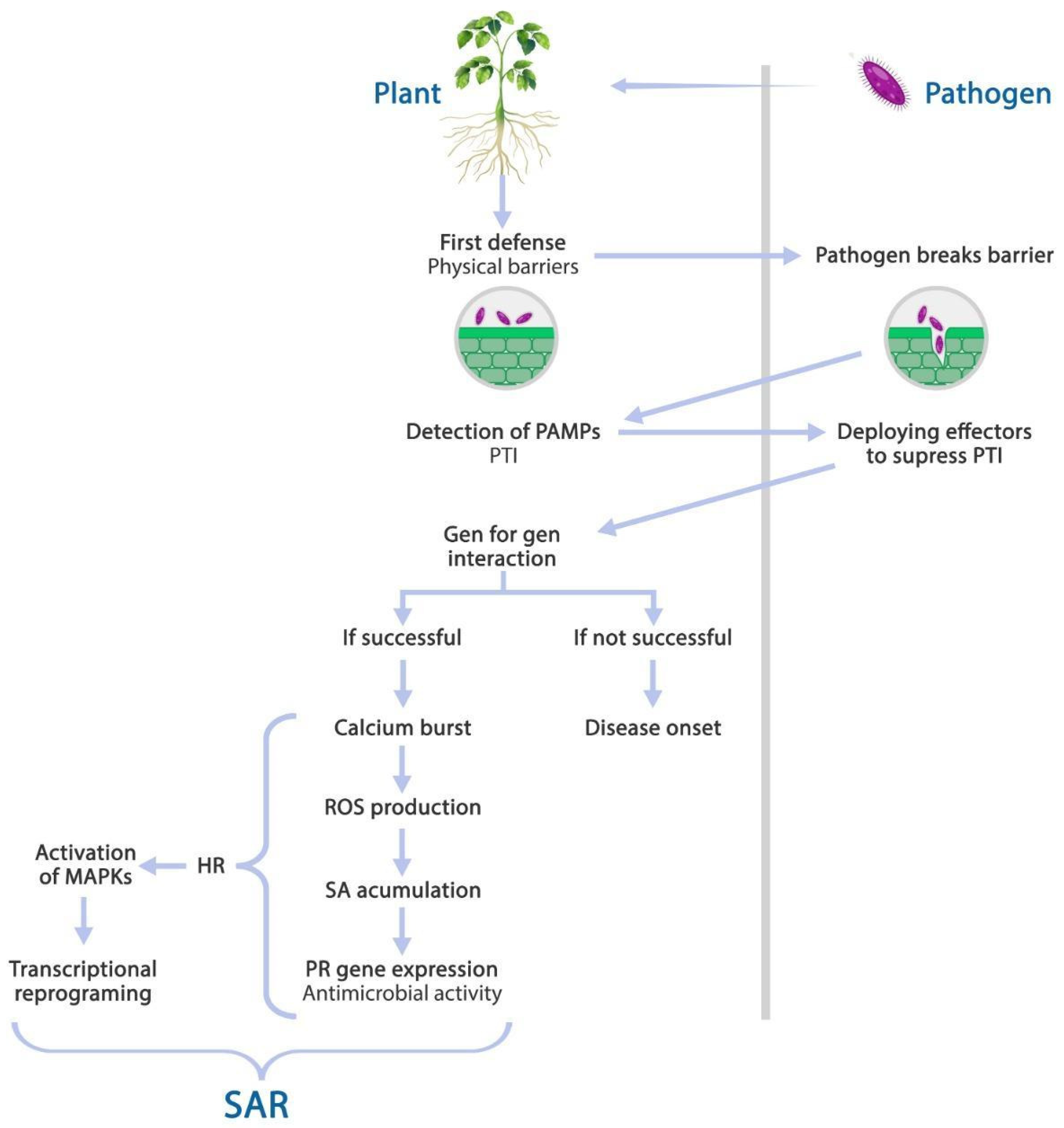
3. Plants’ Response to Biotic Stress: Systemic Acquired Resistance (SAR)
4. Interactions between Plants and Plant Growth-Promoting Microorganisms for Mitigating Biotic Stresses
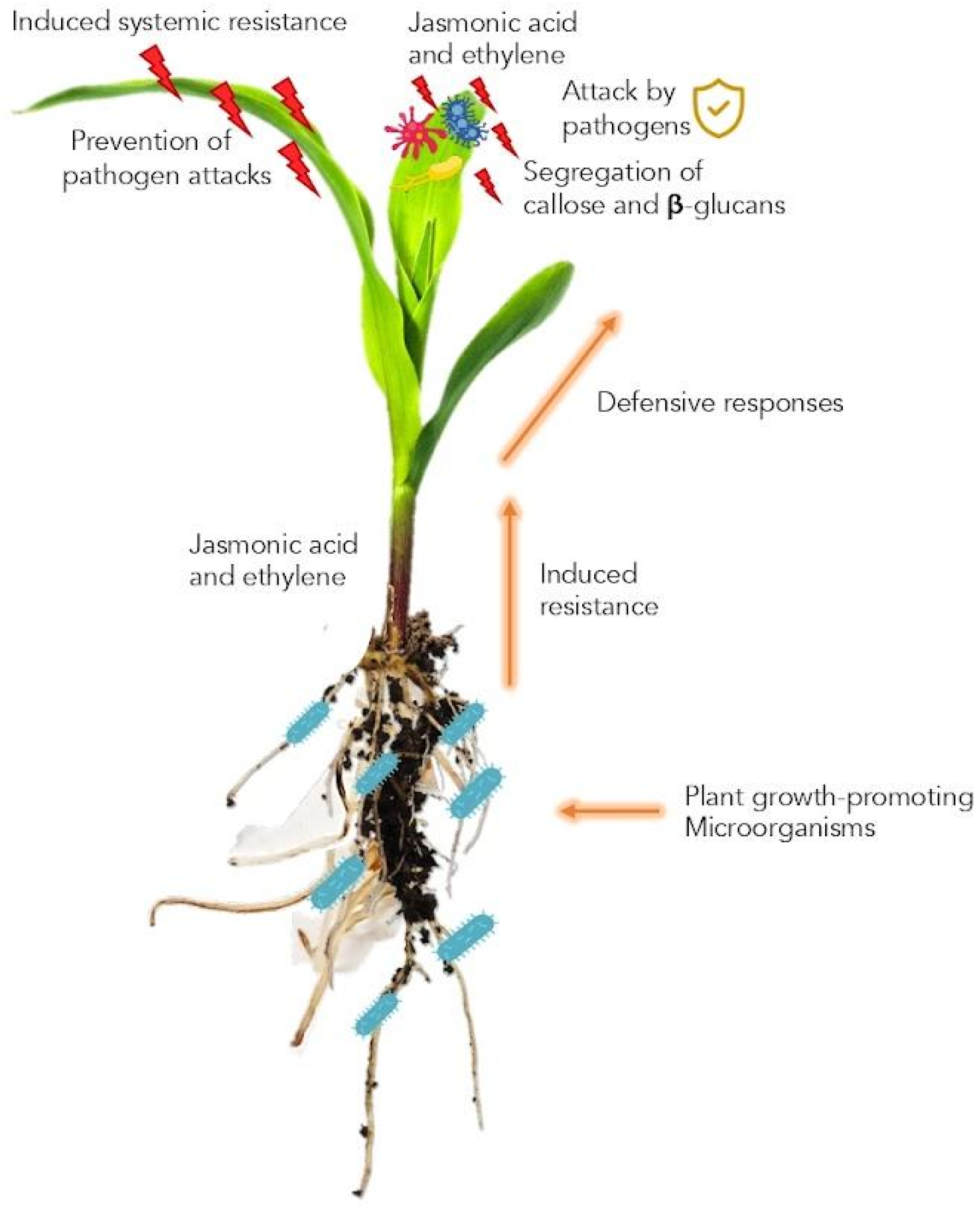
5. Induced Systemic Resistance (ISR)
6. Induced Systemic Resistance as a Biocontrol Mechanism
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tian, Z.; Wang, J.W.; Li, J.; Han, B. Designing future crops: Challenges and strategies for sustainable agriculture. Plant J. 2021, 105, 1165–1178. [Google Scholar] [CrossRef] [PubMed]
- Lassalle, G. Monitoring natural and anthropogenic plant stressors by hyperspectral remote sensing: Recommendations and guidelines based on a meta-review. Sci. Total Environ. 2021, 788, 147758. [Google Scholar] [CrossRef] [PubMed]
- Taiz, L.; Zeiger, E. Plant Physiology. 5th Edition. 2010. Available online: https://scholar.google.es/scholar?hl=es&as_sdt=0%2C5&q=Taiz%2C+L.+and+Zeiger%2C+E.+%282010%29+Plant+Physiology.+5th+Edition&btnG= (accessed on 8 November 2022).
- Verma, S.; Nizam, S.; Verma, P.K. Biotic and Abiotic Stress Signaling in Plants. Stress Signaling in Plants: Genomics and Proteomics Perspective. In Stress Signaling in Plants: Genomics and Proteomics Perspective; Sarwat, M., Ahmad, A., Abdin, M., Eds.; Springer: New York, NY, USA, 2013; Volume 1, pp. 25–50. Available online: https://link.springer.com/chapter/10.1007/978-1-4614-6372-6_2 (accessed on 8 November 2022).
- Agrios, G.N. Plant Pathology, 5th ed.; Academic Press: Cambridge, MA, USA, 2009; p. 952. ISBN 978-0120445653. [Google Scholar]
- Andersen, E.J.; Ali, S.; Byamukama, E.; Yen, Y.; Nepal, M.P. Disease Resistance Mechanisms in Plants. Genes 2018, 9, 339. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, D.P.; Kumar, A.; Mandhania, S.S.; Srivastava, P.; Kumar, R.S. MAIZE AS FODDER? Available online: www.maizeindia.org (accessed on 5 December 2021).
- De los Santos-Villalobos, S.; Parra-Cota, F.I. Current trends in plant growth-promoting microorganisms research for sustainable food security. Curr. Res. Microb. Sci. 2021, 2, 100016. [Google Scholar] [CrossRef]
- Zhao, C.; Liu, B.; Piao, S.; Wang, X.; Lobell, D.B.; Huang, Y.; Huang, M.T.; Yao, Y.T.; Bassu, S.; Ciais, P.; et al. Temperature Increase Reduces Global Yields of Major Crops in Four Independent Estimates. Proc. Natl. Acad. Sci. USA 2017, 114, 9326–9331. Available online: https://www.pnas.org/doi/abs/10.1073/pnas.1701762114 (accessed on 8 November 2022). [CrossRef] [PubMed]
- Varshney, R.K.; Bansal, K.C.; Aggarwal, P.K.; Datta, S.K.; Craufurd, P.Q. Agricultural Biotechnology for Crop Improvement in a Variable Climate: Hope or Hype? Trends Plant Sci. 2011, 16, 363–371. Available online: http://www.cell.com/article/S1360138511000525/fulltext (accessed on 8 November 2022). [CrossRef]
- Ghini, R.; Bettiol, W.; Hamada, E. Diseases in tropical and plantation crops as affected by climate changes: Current knowledge and perspectives. Plant Pathol. 2011, 60, 122–132. [Google Scholar] [CrossRef]
- Sarker, A.; Ansary, M.W.R.; Hossain, M.N.; Islam, T. Prospect and Challenges for Sustainable Management of Climate Change-Associated Stresses to Soil and Plant Health by Beneficial Rhizobacteria. Stresses 2021, 1, 200–222. [Google Scholar] [CrossRef]
- Dean, R.; Van Kan, J.A.L.; Pretorius, Z.A.; Hammond-Kosack, K.E.; Di Pietro, A.; Spanu, P.D.; Rudd, J.J.; Dickman, M.; Kahmann, R.; Ellis, J.; et al. The Top 10 fungal pathogens in molecular plant pathology. Mol. Plant Pathol. 2012, 13, 414–430. [Google Scholar] [CrossRef]
- Nazarov, P.A.; Baleev, D.N.; Ivanova, M.I.; Sokolova, L.M.; Karakozova, M.V. Infectious plant diseases: Etiology, current status, problems and prospects in plant protection. Acta Nat. 2020, 12, 46–59. [Google Scholar] [CrossRef]
- Fernandez, J.; Orth, K. Rise of a Cereal Killer: The Biology of Magnaporthe oryzae Biotrophic Growth. Trends Microbiol. 2018, 26, 582–597. [Google Scholar] [CrossRef] [PubMed]
- Hua, L.; Yong, C.; Zhanquan, Z.; Boqiang, L.; Guozheng, Q.; Shiping, T. Pathogenic mechanisms and control strategies of Botrytis cinerea causing post-harvest decay in fruits and vegetables. Food Qual. Saf. 2018, 2, 111–119. [Google Scholar] [CrossRef]
- FAO. FAO Wheat Rust Disease Global Programme 2014–2017. Available online: https://www.fao.org/agriculture/crops/wheatrust (accessed on 27 December 2022).
- Figueroa, M.; Hammond-Kosack, K.E.; Solomon, P.S. A review of wheat diseases-A field perspective. Mol. Plant Pathol. 2018, 19, 1523–1536. [Google Scholar] [CrossRef]
- Montoya-Martínez, A.C.; Parra-Cota, F.I.; De los Santos-Villalobos, S. Beneficial Microorganisms in Sustainable Agriculture: Harnessing Microbes’ Potential to Help Feed the World. Plants 2022, 11, 372. [Google Scholar] [CrossRef] [PubMed]
- Santoyo, G.; Moreno-Hagelsieb, G.; Orozco-Mosqueda, M.C.; Glick, B.R. Plant growth-promoting bacterial endophytes. Microbiol. Res. 2016, 183, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Schlechter, R.O.; Miebach, M.; Remus-Emsermann, M.N. Driving factors of epiphytic bacterial communities: A review. J. Adv. Res. 2019, 19, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Knief, C.; Delmotte, N.; Chaffron, S.; Stark, M.; Innerebner, G.; Wassmann, R.; Von Mering, C.; Vorholt, J.A. Metaproteogenomic analysis of microbial communities in the phyllosphere and rhizosphere of rice. ISME J. 2011, 6, 1378–1390. [Google Scholar] [CrossRef] [PubMed]
- Compant, S.; Samad, A.; Faist, H.; Sessitsch, A. A review on the plant microbiome: Ecology, functions, and emerging trends in microbial application. J. Adv. Res. 2019, 190, 29–37. [Google Scholar] [CrossRef]
- Hakim, S.; Naqqash, T.; Nawaz, M.S.; Laraib, I.; Siddique, M.J.; Zia, R.; Mirza, M.S.; Imran, A. Rhizosphere Engineering With Plant Growth-Promoting Microorganisms for Agriculture and Ecological Sustainability. Front. Sustain. Food Syst. 2021, 5, 617157. [Google Scholar] [CrossRef]
- Santoyo, G. How plants recruit their microbiome? New insights into beneficial interactions. J. Adv. Res. 2022, 40, 45–58. [Google Scholar] [CrossRef]
- Wirthmueller, L.; Maqbool, A.; Banfield, M. On the front line: Structural insights into plant-pathogen interactions. Nat. Rev. Genet. 2013, 11, 761–776. [Google Scholar] [CrossRef] [PubMed]
- Velásquez, A.C.; Castroverde, C.D.M.; He, S.Y. Plant–Pathogen Warfare under Changing Climate Conditions. Curr. Biol. 2018, 28, R619–R634. [Google Scholar] [CrossRef] [PubMed]
- Berg, G.; Grube, M.; Schloter, M.; Smalla, K. Unraveling the plant microbiome: Looking back and future perspectives. Front. Microbiol. 2014, 5, 148. [Google Scholar] [CrossRef] [PubMed]
- Nifakos, K.; Tsalgatidou, P.C.; Thomloudi, E.-E.; Skagia, A.; Kotopoulis, D.; Baira, E.; Delis, C.; Papadimitriou, K.; Markellou, E.; Venieraki, A.; et al. Genomic Analysis and Secondary Metabolites Production of the Endophytic Bacillus velezensis Bvel1: A Biocontrol Agent against Botrytis cinerea Causing Bunch Rot in Post-Harvest Table Grapes. Plants 2021, 10, 1716. [Google Scholar] [CrossRef] [PubMed]
- Tronsmo, A.M.; Collinge, D.B.; Djurle, A.; Munk, L.; Yuen, J.; Tronsmo, A. Plant Pathology and Plant Diseases; CAB International: Boston, MA, USA, 2020. [Google Scholar]
- Rangel Sánchez, G.; Castro Mercado, E.; Beltran Peña, E.; Reyes de la Cruz, H.; García Pineda, E. El acido salicílico y su participación en la resistencia a patógenos en plantas. Biológicas 2010, 12, 90–95. [Google Scholar]
- Lamb, C.J.; Lawton, M.A.; Dron, M.; Dixon, R.A. Signals and Transduction Mechanisms for Activation of Plant Defenses Against Microbial Attack. Cell 1989, 56, 215–224. Available online: https://pubmed.ncbi.nlm.nih.gov/2643475/ (accessed on 21 November 2022). [CrossRef]
- Matei, A.; Doehlemann, G. Cell biology of corn smut disease—Ustilago maydis as a model for biotrophic interactions. Curr. Opin. Microbiol. 2016, 34, 60–66. [Google Scholar] [CrossRef]
- Lamour, K.; Stam, R.; Jupe, J.; Huitema, E. The oomycete broad-host-range pathogen Phytophthora capsici. Mol. Plant Pathol. 2011, 13, 329–337. [Google Scholar] [CrossRef]
- Williamson, B.; Tudzynski, B.; Tudzynski, P.; Van Kan, J.A.L. Botrytis cinerea: The cause of grey mould disease. Mol. Plant Pathol. 2007, 8, 561–580. [Google Scholar] [CrossRef]
- Villa-Rodriguez, E.; Lugo-Enríquez, C.; Santos-Villalobos, S.D.L.; Parra-Cota, F.I.; Figueroa-López, P. First Report of Cochliobolus sativus Causing Spot Blotch on Durum Wheat (Triticum durum) in The Yaqui Valley, Mexico. Plant Dis. 2016, 100, 2329. [Google Scholar] [CrossRef]
- Bartholomew, E.S.; Xu, S.; Zhang, Y.; Yin, S.; Feng, Z.; Chen, S.; Sun, L.; Yang, S.; Wang, Y.; Liu, P.; et al. A Chitinase CsChi23 Promoter Polymorphism Underlies Cucumber Resistance against Fusarium oxysporum f. sp. cucumerinum. New Phytol. 2022, 236, 1471–1486. Available online: https://onlinelibrary.wiley.com/doi/full/10.1111/nph.18463 (accessed on 15 December 2022). [CrossRef] [PubMed]
- Burkhanova, G.F.; Veselova, S.V.; Sorokan, A.V.; Blagova, D.K.; Nuzhnaya, T.V.; Maksimov, I.V. Strains of Bacillus ssp. Regulate Wheat Resistance to Septoria nodorum Berk. Appl. Biochem. Microbiol. 2017, 53, 346–352. Available online: https://link.springer.com/article/10.1134/S0003683817030048 (accessed on 17 December 2022). [CrossRef]
- Lian, L.; Xie, L.; Zheng, L.; Lin, Q. Induction of Systemic Resistance in Tobacco against Tobacco Mosaic Virus by Bacillus spp. Biocontrol Sci. Technol. 2011, 21, 281–292. Available online: https://www.tandfonline.com/doi/abs/10.1080/09583157.2010.543667 (accessed on 17 December 2022). [CrossRef]
- Fürst, U.; Zeng, Y.; Albert, M.; Witte, A.K.; Fliegmann, J.; Felix, G. Perception of Agrobacterium Tumefaciens Flagellin by FLS2XL Confers Resistance to Crown Gall Disease. Nat. Plants 2020, 6, 22–27. Available online: https://www.nature.com/articles/s41477-019-0578-6 (accessed on 16 January 2022). [CrossRef] [PubMed]
- Lahoz, E.; Contillo, R.; Porrone, F. Induction of Systemic Resistance to Erysiphe orontii Cast in Tobacco by Application on Roots of an Isolate of Gliocladium roseum Bainier. J. Phytopathol. 2004, 152, 465–470. Available online: https://onlinelibrary.wiley.com/doi/full/10.1111/j.1439-0434.2004.00876.x (accessed on 18 December 2022). [CrossRef]
- Parra-Cota, F.I.; García-Pereyra, J.; Aviña-Martínez, G.N.; De los Santos-Villalobos, S. First Report of Fusarium Wilt on Citrus Sinensis Var. Valencia in the Yaqui Valley, Mexico. Mex. J. Phytopathol. 2018, 37, 193–201. Available online: https://www.smf.org.mx/rmf/ojs/index.php/RMF/article/view/139 (accessed on 18 December 2022).
- Garcia Pereyra, J.; Aviña Martínez, G.N.; De los Santos Villalobos, S.; Garcia Montelongo, A.M.; Alejandre-Iturbide, G.; Rubio Graciano, R.B. Biological Control of Erwinia Amylovora in Apple Trees Employing Antibacterial Agents. AshEse J. Agric. Sci. 2020, 3, 117–126. Available online: http://www.ashese.co.uk/ajas-issues/biological-control-of-erwinia-amylovora-in-apple-trees-employing-antibacterial-agents (accessed on 18 December 2022).
- Silva, M.S.S.; Arraes, F.B.M.; Campos, M.; Grossi-de-Sa, M.F.; Fernandez, D.; Cândido, E.D.; Cardoso, M.H.; Franco, O.L.; Grossi-de-Sa, M. Review: Potential biotechnological assets related to plant immunity modulation applicable in engineering disease-resistant crops. Plant Sci. 2018, 270, 72–84. [Google Scholar] [CrossRef]
- Castro-Moretti, F.R.; Gentzel, I.N.; Mackey, D.; Alonso, A.P. Metabolomics as an emerging tool for the study of plant–pathogen interactions. Metabolites 2020, 10, 52. [Google Scholar] [CrossRef]
- Glazebrook, J. Contrasting mechanisms of defense against biotrophic and necrotrophic pathogens. Annu. Rev. Phytopathol. 2005, 43, 205–227. [Google Scholar] [CrossRef]
- Chisholm, S.T.; Coaker, G.; Day, B.; Staskawicz, B.J. Host-microbe interactions: Shaping the evolution of the plant immune response. Cell 2006, 124, 803–814. [Google Scholar] [CrossRef]
- Keen, N.T. Gene-for-gene complementarity in plant-pathogen interactions. Annu. Rev. Genet. 1990, 24, 447–463. [Google Scholar] [CrossRef] [PubMed]
- Bigeard, J.; Colcombet, J.; Hirt, H. Signaling mechanisms in pattern-triggered immunity (PTI). Mol. Plant 2015, 8, 521–539. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Smigel, A.; Tsai, Y.C.; Braam, J.; Berkowitz, G.A. Innate immunity signaling: Cytosolic Ca2+ elevation is linked to downstream nitric oxide generation through the action of calmodulin or a calmodulin-like protein. Plant Physiol. 2008, 148, 818–828. [Google Scholar] [CrossRef] [PubMed]
- Kovtun, Y.; Chiu, W.L.; Tena, G.; Sheen, J. Functional analysis of oxidative stress-activated mitogen-activated protein kinase cascade in plants. Proc. Natl. Acad. Sci. USA 2000, 97, 2940–2945. [Google Scholar] [CrossRef] [PubMed]
- Noman, A.; Aqeel, M.; Qari, S.H.; Al Surhanee, A.A.; Yasin, G.; Alamri, S.; Hashem, M.; Al-Saadi, A.M. Plant hypersensitive response vs pathogen ingression: Death of few gives life to others. Microb. Pathog. 2020, 145, 104224. [Google Scholar] [CrossRef]
- Reichheld, J.; Vernoux, T.; Lardon, F.; Van Montagu, M.; Inzé, D. Specific checkpoints regulate plant cell cycle progression in response to oxidative stress. Plant J. 1999, 17, 647–656. [Google Scholar] [CrossRef]
- Khurana, S.M.P.; Pandey, S.K.; Sarkar, D.; Chanemougasoundharam, A. Apoptosis in plant disease response: A close encounter of the pathogen kind. Curr. Sci. 2005, 88, 740–752. [Google Scholar]
- Dangl, J.L.; Dietrich, R.A.; Richberg, M.H. Death Don’t Have No Mercy: Cell Death Programs in Plant-Microbe Interactions. Plant Cell 1996, 8, 1793. [Google Scholar] [CrossRef]
- Dempsey, D.A.; Klessig, D.F. SOS—Too Many Signals for Systemic Acquired Resistance? Trends Plant Sci. 2012, 17, 538–545. Available online: https://pubmed.ncbi.nlm.nih.gov/22749315/ (accessed on 21 November 2022). [CrossRef]
- Forouhar, F.; Yang, Y.; Kumar, D.; Chen, Y.; Fridman, E.; Park, S.W.; Chiang, Y.; Acton, T.B.; Montelione, G.T.; Pichersky, E.; et al. Structural and biochemical studies identify tobacco SABP2 as a methyl salicylate esterase and implicate it in plant innate immunity. Proc. Natl. Acad. Sci. USA 2005, 102, 1773–1778. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Puentes, L.N. Systemic acquired resistance induced by salicylic acid. Biotecnol. En El Sect. Agropecu. Y Agroind. 2012, 10, 257–267. [Google Scholar]
- Chakraborty, S.; Moeder, W.; Yoshioka, K. Plant Immunity. Ref. Modul. Life Sci. 2017, 1–8. Available online: https://linkinghub.elsevier.com/retrieve/pii/B9780128096338121545 (accessed on 21 November 2022).
- Pieterse, C.M.J.; Zamioudis, C.; Berendsen, R.L.; Weller, D.M.; Van Wees, S.C.M.; Bakker, P.A.H.M. Induced Systemic Resistance by Beneficial Microbes. Annu. Rev. Phytopathol. 2014, 52, 347–375. Available online: https://www.annualreviews.org/doi/abs/10.1146/annurev-phyto-082712-102340 (accessed on 10 November 2022). [CrossRef] [PubMed]
- Niderman, T.; Genetet, I.; Bruyere, T.; Gees, R.; Stintzi, A.; Legrand, M.; Fritig, B.; Mosinger, E. Pathogenesis-Related PR-1 Proteins Are Antifungal. Plant Physiol. 1995, 108, 17–27. [Google Scholar] [CrossRef]
- Bigeard, J.; Hirt, H. Nuclear Signaling of Plant MAPKs. Front. Plant Sci. 2018, 9, 469. [Google Scholar] [CrossRef]
- Moore, J.W.; Loake, G.; Spoel, S.H. Transcription Dynamics in Plant Immunity. Plant Cell 2011, 23, 2809–2820. [Google Scholar] [CrossRef]
- Schlaeppi, K.; Mauch, F. Indolic secondary metabolites protect Arabidopsis from the oomycete pathogen Phytophthora brassicae. Plant Signal. Behav. 2010, 5, 1099–1101. [Google Scholar] [CrossRef]
- Iakimova, E.T.; Sobiczewski, P.; Michalczuk, L.; Węgrzynowicz-Lesiak, E.; Mikiciński, A.; Woltering, E.J. Morphological and biochemical characterization of Erwinia amylovora-induced hypersensitive cell death in apple leaves. Plant Physiol. Biochem. 2013, 63, 292–305. [Google Scholar] [CrossRef]
- Moya-Elizondo, E.A.; Jacobsen, B.J. Integrated management of Fusarium crown rot of wheat using fungicide seed treatment, cultivar resistance, and induction of systemic acquired resistance (SAR). Biol. Control. 2016, 92, 153–163. [Google Scholar] [CrossRef]
- Köhl, J.; Kolnaar, R.; Ravensberg, W.J. Mode of Action of Microbial Biological Control Agents Against Plant Diseases: Relevance Beyond Efficacy. Front. Plant Sci. 2019, 10, 845. [Google Scholar] [CrossRef]
- Fatima, S.; Anjum, T. Identification of a Potential ISR Determinant from Pseudomonas aeruginosa PM12 against Fusarium Wilt in Tomato. Front. Plant Sci. 2017, 8, 848. [Google Scholar] [CrossRef]
- Poveda, J.; Hermosa, R.; Monte, E.; Nicolás, C. The Trichoderma harzianum Kelch Protein ThKEL1 Plays a Key Role in Root Colonization and the Induction of Systemic Defense in Brassicaceae Plants. Front. Plant Sci. 2019, 10, 1478. [Google Scholar] [CrossRef] [PubMed]
- Etesami, H.; Maheshwari, D.K. Use of Plant Growth Promoting Rhizobacteria (PGPRs) with Multiple Plant Growth Promoting Traits in Stress Agriculture: Action Mechanisms and Future Prospects. Ecotoxicol. Environ. Saf. 2018, 156, 225–246. Available online: https://pubmed.ncbi.nlm.nih.gov/29554608/ (accessed on 21 November 2022). [CrossRef] [PubMed]
- Mhlongo, M.I.; Piater, L.A.; Madala, N.E.; Labuschagne, N.; Dubery, I.A. The Chemistry of Plant–Microbe Interactions in the Rhizosphere and the Potential for Metabolomics to Reveal Signaling Related to Defense Priming and Induced Systemic Resistance. Front. Plant Sci. 2018, 9, 112. [Google Scholar] [CrossRef] [PubMed]
- Mar Vázquez, M.; César, S.; Azcón, R.; Barea, J.M. Interactions between arbuscular mycorrhizal fungi and other microbial inoculants (Azospirillum, Pseudomonas, Trichoderma) and their effects on microbial population and enzyme activities in the rhizosphere of maize plants. Appl. Soil Ecol. 2000, 15, 261–272. [Google Scholar] [CrossRef]
- Goswami, D.; Thakker, J.N.; Dhandhukia, P.C. Portraying Mechanics of Plant Growth Promoting Rhizobacteria (PGPR): A Review. Cogent Food Agric. 2016, 2, 1127500. Available online: https://www.tandfonline.com/doi/abs/10.1080/23311932.2015.1127500 (accessed on 5 December 2021). [CrossRef]
- Fernandes, C.; Domingues, D.; Cecato, U.; Biserra, T.T.; Mamédio, D.; Galbeiro, S. Azospirillum spp. on grasses and forage crops. Review. Rev. Mex. De Cienc. Pecu. 2020, 11, 223–240. [Google Scholar] [CrossRef]
- Yang, J.; Lan, L.; Jin, Y.; Yu, N.; Wang, D.; Wang, E. Mechanisms underlying legume–rhizobium symbioses. J. Integr. Plant Biol. 2021, 64, 244–267. [Google Scholar] [CrossRef]
- Mommer, L.; Kirkegaard, J.; Van Ruijven, J. Root–Root Interactions: Towards A Rhizosphere Framework. Trends Plant Sci. 2016, 21, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Sasse, J.; Martinoia, E.; Northen, T. Feed Your Friends: Do Plant Exudates Shape the Root Microbiome? Trends Plant Sci. 2018, 23, 25–41. [Google Scholar] [CrossRef]
- Fahad, S.; Hussain, S.; Bano, A.; Saud, S.; Hassan, S.; Shan, D.; Khan, F.A.; Khan, F.; Chen, Y.; Wu, C.; et al. Potential role of phytohormones and plant growth-promoting rhizobacteria in abiotic stresses: Consequences for changing environment. Environ. Sci. Pollut. Res. 2014, 22, 4907–4921. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.-W.; Mu, W.; Zsu, B.-Y.; Du, Y.-C.; Liu, F. Antagonistic Activities of Volatiles from Four Strains of Bacillus spp. and Paenibacillus spp. Against Soil-Borne Plant Pathogens. Agric. Sci. China 2008, 7, 1104–1114. [Google Scholar] [CrossRef]
- Schenk, S.T.; Hernández-Reyes, C.; Samans, B.; Stein, E.; Neumann, C.; Schikora, M.; Reichelt, M.; Mithöfer, A.; Becker, A.; Kogel, K.-H.; et al. N-Acyl-Homoserine Lactone Primes Plants for Cell Wall Reinforcement and Induces Resistance to Bacterial Pathogens via the Salicylic Acid/Oxylipin Pathway. Plant Cell 2014, 26, 2708–2723. [Google Scholar] [CrossRef] [PubMed]
- Rojas Padilla, J.; Encinas, L.A.C.; Montoya, R.I.R.; De Los Santos Villalobos, S. Growth promotion on wheat (Triticum turgidum L. subsp. durum) by co-inoculation of native Bacillus strains isolated from the Yaqui Valley, Mexico. Nova Sci. 2020, 12. Epub 2 July 2020. Available online: https://doi.org/10.21640/ns.v12i24.2136 (accessed on 16 December 2022).
- Peñafiel-Jaramillo, M.F.; Sánchez-Sepúlveda, E.; Cruz-Rosero, N.; Belezaca-Pinargote, C.; Prieto-Encalada, H.G.; Martínez, H.F.C. Activación de resistencia sistémica inducida en vid “Thompson Seedless”, en respuesta Pseudomonas veronii R4. Cienc. Y Tecnol. 2016, 9, 1–9. Available online: https://revistas.uteq.edu.ec/index.php/cyt/article/view/161 (accessed on 26 April 2022). [CrossRef]
- Toral, L.; Rodríguez, M.; Béjar, V.; Sampedro, I. Crop Protection against Botrytis cinerea by Rhizhosphere Biological Control Agent Bacillus velezensis XT1. Microorganisms 2020, 8, 992. Available online: https://doi.org/10.3390/microorganisms8070992 (accessed on 23 August 2022). [CrossRef] [PubMed]
- Villa-Rodríguez, E.; Parra-Cota, F.; Castro-Longoria, E.; López-Cervantes, J.; De Los Santos-Villalobos, S. Bacillus subtilis TE3: A promising biological control agent against Bipolaris sorokiniana, the causal agent of spot blotch in wheat (Triticum turgidum L. subsp. durum). Biol. Control 2019, 132, 135–143. [Google Scholar] [CrossRef]
- Lee, G.; Lee, S.-H.; Kim, K.M.; Ryu, C.-M. Foliar application of the leaf-colonizing yeast Pseudozyma churashimaensis elicits systemic defense of pepper against bacterial and viral pathogens. Sci. Rep. 2017, 7, 39432. Available online: www.nature.com/scientificreports (accessed on 17 December 2022). [CrossRef]
- Léon-Kloosterziel, K.M.; Verhagen, B.W.M.; Keurentjes, J.J.B.; Van Pelt, J.A.; Rep, M.; Van Loon, L.C.; Pieterse, C.M. Colonization of the Arabidopsis rhizosphere by fluorescent Pseudomonas spp. activates a root-specific, ethylene-responsive PR-5 gene in the vascular bundle. Plant Mol. Biol. 2005, 57, 731–748. Available online: http://www.bio.uu.nl/$fytopath (accessed on 17 December 2022). [CrossRef]
- Jaimes-Suárez, Y.Y.; Velandia, C.A.M.; Prado, A.M.C. Inducción de resistencia sistémica contra Fusarium oxysporum en tomate por Trichoderma koningiopsis Th003. Acta Biológica Colomb. 2009, 14, 111–120. Available online: https://revistas.unal.edu.co/index.php/actabiol/article/view/1344 (accessed on 1 March 2022).
- Félix-Pablos, C.M.; Parra-Cota, F.I.; Santoyo, G.; Orozco-Mosqueda, M.; Del, C.; De los Santos-Villalobos, S. Draft genome sequence of Bacillus sp. strain FSQ1, a biological control agent against white mold in common bean (Phaseolus vulgaris L.). Curr. Res. Microb. Sci. 2022, 3, 100138. [Google Scholar] [CrossRef]
- Ortega-Urquieta, M.E.; Valenzuela-Ruíz, V.; Mitra, D.; Hyder, S.; Elsheery, N.I.; Kumar Das Mohapatra, P.; Parra-Cota, F.I.; De los Santos-Villalobos, S. Draft Genome Sequence of Priestia sp. Strain TSO9, a Plant Growth-Promoting Bacterium Associated with Wheat (Triticum turgidum subsp. durum) in the Yaqui Valley, Mexico. Plants 2022, 11, 2231. Available online: https://www.mdpi.com/2223-7747/11/17/2231/htm (accessed on 18 December 2022). [CrossRef] [PubMed]
- Valenzuela-Ruiz, V.; Robles-Montoya, R.I.; Parra-Cota, F.I.; Santoyo, G.; Orozco-Mosqueda, M.D.C.; Rodríguez-Ramírez, R.; Santos-Villalobos, S.D.L. Draft genome sequence of Bacillus paralicheniformis TRQ65, a biological control agent and plant growth-promoting bacterium isolated from wheat (Triticum turgidum subsp. durum) rhizosphere in the Yaqui Valley, Mexico. 3 Biotech 2019, 9, 436. Available online: https://link.springer.com/article/10.1007/s13205-019-1972-5 (accessed on 18 December 2022). [CrossRef] [PubMed]
- Valenzuela-Ruiz, V.; Parra-Cota, F.I.; Santoyo, G.; De los Santos-Villalobos, S. Potential biocontrol mechanisms of Bacillus sp. TSO2 against Bipolaris sorokiniana, spot blotch in wheat. Mex. J. Phytopathol. 2022, 40, 230–239. Available online: https://doi.org/10.18781/R.MEX.FIT.2201-1 (accessed on 18 December 2022). [CrossRef]
- Villa-Rodriguez, E.; Lugo-Enríquez, C.; Ferguson, S.; Parra-Cota, F.I.; Cira-Chávez, L.A.; De los Santos-Villalobos, S. Trichoderma harzianum sensu lato TSM39: A wheat microbiome fungus that mitigates spot blotch disease of wheat (Triticum turgidum L. subsp. durum) caused by Bipolaris sorokiniana. Biol. Control. 2022, 175, 105055. [Google Scholar] [CrossRef]
- Morales-Cedeño, L.R.; Santos-Villalobos, S.D.L.; Santoyo, G. Functional and Genomic Analysis of Rouxiella badensis SER3 as a Novel Biocontrol Agent of Fungal Pathogens. Front. Microbiol. 2021, 12, 2184. [Google Scholar] [CrossRef]
- Khoshru, B.; Mitra, D.; Khoshmanzar, E.; Myo, E.M.; Uniyal, N.; Mahakur, B.; Das Mohapatra, P.K.; Panneerselvam, P.; Boutaj, H.; Alizadeh, M.; et al. Current scenario and future prospects of plant growth-promoting rhizobacteria: An economic valuable resource for the agriculture revival under stressful conditions. J. Plant Nutr. 2020, 43, 3062–3092. Available online: https://www.tandfonline.com/doi/abs/10.1080/01904167.2020.1799004 (accessed on 8 November 2022). [CrossRef]
- Cesari, A.; Paulucci, N.; López-Gómez, M.; Hidalgo-Castellanos, J.; Plá, C.L.; Dardanelli, M.S. Restrictive water condition modifies the root exudates composition during peanut-PGPR interaction and conditions early events, reversing the negative effects on plant growth. Plant Physiol. Biochem. 2019, 142, 519–527. [Google Scholar] [CrossRef]
- Dutta, S.; Podile, A.R. Plant Growth Promoting Rhizobacteria (PGPR): The bugs to debug the root zone. Crit. Rev. Microbiol. 2010, 36, 232–244. Available online: https://www.tandfonline.com/doi/abs/10.3109/10408411003766806 (accessed on 8 November 2022). [CrossRef]
- Basu, A.; Prasad, P.; Das, S.N.; Kalam, S.; Sayyed, R.Z.; Reddy, M.S.; El Enshasy, H. Plant Growth Promoting Rhizobacteria (PGPR) as Green Bioinoculants: Recent Developments, Constraints, and Prospects. Sustainability 2021, 13, 1140. Available online: https://www.mdpi.com/2071-1050/13/3/1140/htm (accessed on 8 November 2022).
- De Souza, R.; Ambrosini, A.; Passaglia, L.M.P. Plant growth-promoting bacteria as inoculants in agricultural soils. Genet Mol Biol. 2015, 38, 401–419. [Google Scholar] [CrossRef] [PubMed]
- Ketta, H.A.; Elkhateeb, N.M.; Saleh, M.M.; Kamel, S.M. Efficiency Assessment of Combinations Between Rhizobium leguminosarum and Trichoderma spp. for Controlling of Pea (Pisum sativum L.) Damping-off Disease. Egypt. J. Phytopathol. 2021, 49, 1–14. Available online: https://ejp.journals.ekb.eg/article_139637.html (accessed on 1 January 2023). [CrossRef]
- Sibponkrung, S.; Kondo, T.; Tanaka, K.; Tittabutr, P.; Boonkerd, N.; Yoshida, K.-I.; Teaumroong, N. Co-Inoculation of Bacillus velezensis Strain S141 and Bradyrhizobium Strains Promotes Nodule Growth and Nitrogen Fixation. Microorganisms 2020, 8, 678. Available online: https://www.mdpi.com/2076-2607/8/5/678/htm (accessed on 1 January 2023). [CrossRef] [PubMed]
- Timm, C.M.; Carter, K.R.; Carrell, A.A.; Jun, S.-R.; Jawdy, S.S.; Vélez, J.M.; Gunter, L.E.; Yang, Z.; Nookaew, I.; Engle, N.L.; et al. Abiotic Stresses Shift Belowground Populus-Associated Bacteria Toward a Core Stress Microbiome. Msystems 2018, 3, e00070-17. Available online: https://doi.org/10.1128/mSystems.00070-17 (accessed on 8 November 2022). [CrossRef] [PubMed]
- Liu, H.; Brettell, L.E.; Qiu, Z.; Singh, B.K. Microbiome-Mediated Stress Resistance in Plants. Trends Plant Sci. 2020, 25, 733–743. Available online: https://pubmed.ncbi.nlm.nih.gov/32345569/ (accessed on 8 November 2022). [CrossRef]
- Chi, F.; Shen, S.-H.; Cheng, H.-P.; Jing, Y.-X.; Yanni, Y.G.; Dazzo, F.B. Ascending Migration of Endophytic Rhizobia, from Roots to Leaves, inside Rice Plants and Assessment of Benefits to Rice Growth Physiology. Appl. Environ. Microbiol. 2005, 71, 7271–7278. Available online: https://journals.asm.org/doi/10.1128/AEM.71.11.7271-7278.2005 (accessed on 8 November 2022). [CrossRef]
- Buscaill, P.; Van der Hoorn, R.A.L. Defeated by the Nines: Nine Extracellular Strategies to Avoid Microbe-Associated Molecular Patterns Recognition in Plants. Plant Cell 2021, 33, 2116–2130. Available online: https://academic.oup.com/plcell/article/33/7/2116/6237920 (accessed on 8 November 2022). [CrossRef]
- Jones, J.D.G.; Dangl, J.L. The plant immune system. Nature 2006, 444, 323–329. Available online: https://www.nature.com/articles/nature05286 (accessed on 8 November 2022). [CrossRef]
- Lebeis, S.L.; Paredes, S.H.; Lundberg, D.S.; Breakfield, N.; Gehring, J.; McDonald, M.; Malfatti, S.; Glavina del rio, T.; Jones, C.D.; Tringe, S.G.; et al. Salicylic acid modulates colonization of the root microbiome by specific bacterial taxa. Science 2015, 349, 860–864. Available online: https://www.science.org/doi/10.1126/science.aaa8764 (accessed on 8 November 2022). [CrossRef]
- Nakagawa, T.; Kawaguchi, M. Shoot-applied MeJA Suppresses Root Nodulation in Lotus japonicus. Plant Cell Physiol. 2006, 47, 176–180. Available online: https://academic.oup.com/pcp/article/47/1/176/1867512 (accessed on 8 November 2022).
- Ronald, P.C.; Beutler, B. Plant and animal sensors of conserved microbial signatures. Science 2010, 330, 1061–1064. Available online: https://www.science.org/doi/10.1126/science.1189468 (accessed on 8 November 2022). [CrossRef]
- Hacquard, S.; Spaepen, S.; Garrido-Oter, R.; Schulze-Lefert, P. Interplay Between Innate Immunity and the Plant Microbiota. Annu. Rev. Phytopathol. 2017, 55, 565–589. Available online: https://www.annualreviews.org/doi/abs/10.1146/annurev-phyto-080516-035623 (accessed on 8 November 2022). [CrossRef] [PubMed]
- Macho, A.P.; Zipfel, C. Plant PRRs and the activation of innate immune signaling. Mol. Cell 2014, 54, 263–272. Available online: https://pubmed.ncbi.nlm.nih.gov/24766890/ (accessed on 8 November 2022). [CrossRef] [PubMed]
- Burbano-Figueroa, Ó. Resistencia de plantas a patógenos: Una revisión sobre los conceptos de resistencia vertical y horizontal. Rev. Argent. De Microbiol. 2020, 52, 245–255. Available online: https://www.elsevier.es/es-revista-revista-argentina-microbiologia-372-articulo-resistencia-plantas-patogenos-una-revision-S0325754120300328 (accessed on 8 November 2022). [CrossRef] [PubMed]
- Shah, J.; Zeier, J. Long-distance communication and signal amplification in systemic acquired resistance. Front. Plant Sci. 2013, 4, 30. Available online: https://pubmed.ncbi.nlm.nih.gov/23440336/ (accessed on 8 November 2022). [CrossRef] [PubMed]
- Millet, Y.A.; Danna, C.H.; Clay, N.K.; Songnuan, W.; Simon, M.D.; Werck-Reichhart, D.; Ausubel, F.M. Innate Immune Responses Activated in Arabidopsis Roots by Microbe-Associated Molecular Patterns. Plant Cell 2010, 22, 973–990. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2861455/ (accessed on 8 November 2022). [CrossRef] [PubMed]
- Lambais, M.R.; Barrera, S.E.; Santos, E.C.; Crowley, D.E.; Jumpponen, A. Phyllosphere Metaproteomes of Trees from the Brazilian Atlantic Forest Show High Levels of Functional Redundancy. Microb. Ecol. 2016, 73, 123–134. Available online: https://link.springer.com/article/10.1007/s00248-016-0878-6 (accessed on 8 November 2022). [CrossRef]
- Heil, M.; Baldwin, I.T. Fitness costs of induced resistance: Emerging experimental support for a slippery concept. Trends Plant Sci. 2002, 7, 61–67. [Google Scholar] [CrossRef]
- De Kesel, J.; Conrath, U.; Flors, V.; Luna, E.; Mageroy, M.H.; Mauch-Mani, B.; Pastor, V.; Pozo, M.J.; Pieterse, C.M.J.; Ton, J.; et al. The Induced Resistance Lexicon: Do’s and Don’ts. Trends Plant Sci. 2021, 26, 685–691. [Google Scholar] [CrossRef]
- Hammerschmidt, R.; M’Etraux, J.-P.; Van Loon, L. Inducing Resistance: A Summary of Papers Presented at the First International Symposium on Induced Resistance to Plant Diseases, Corfu, May 2000. Eur. J. Plant Pathol. 2001, 107, 1–6. [Google Scholar] [CrossRef]
- Heil, M.; Bostock, R.M. Induced Systemic Resistance (ISR) Against Pathogens in the Context of Induced Plant Defences. Ann. Bot. 2002, 89, 503. Available online: https://doi.org/10.1093/aob/mcf076 (accessed on 1 May 2022). [CrossRef]
- Martinez-Medina, A.; Flors, V.; Heil, M.; Mauch-Mani, B.; Pieterse, C.M.; Pozo, M.J.; Ton, J.; Van Dam, N.M.; Conrath, U. Recognizing Plant Defense Priming. Trends Plant Sci. 2016, 21, 818–822. [Google Scholar] [CrossRef] [PubMed]
- Hilker, M.; Schwachtje, J.; Baier, M.; Balazadeh, S.; Bäurle, I.; Geiselhardt, S.; Hincha, D.K.; Kunze, R.; Mueller-Roeber, B.; Rillig, M.C.; et al. Priming and memory of stress responses in organisms lacking a nervous system. Biol. Rev. 2015, 91, 1118–1133. Available online: https://onlinelibrary.wiley.com/doi/full/10.1111/brv.12215 (accessed on 27 October 2022). [CrossRef] [PubMed]
- Rasmann, S.; De Vos, M.; Jander, G. Plant Signaling & Behavior Ecological role of transgenerational resistance against biotic threats. Plant Signal. Behav. 2012, 7, 447–449. Available online: https://www.tandfonline.com/action/journalInformation?journalCode=kpsb20 (accessed on 8 November 2022). [PubMed]
- Sen, S. Role of Phytoalexins in Plant-Microbe Interactions and Human Health. Int. J. Sci. Res. Manag. 2017, 5, 18033–18056. [Google Scholar] [CrossRef]
- Song, H.; Wang, P.; Li, C.; Han, S.; Lopez-Baltazar, J.; Zhang, X.; Wang, X. Identification of lipoxygenase (LOX) genes from legumes and their responses in wild type and cultivated peanut upon Aspergillus flavus infection. Sci. Rep. 2016, 6, 35245. Available online: https://www.nature.com/articles/srep35245 (accessed on 28 April 2022). [CrossRef]
- Chen, Z.; Chen, X.; Yan, H.; Li, W.; Li, Y.; Cai, R.; Xiang, Y. The Lipoxygenase Gene Family in Poplar: Identification, Classification, and Expression in Response to MeJA Treatment. PLoS ONE 2015, 10, e0125526. Available online: https://pubmed.ncbi.nlm.nih.gov/25928711/ (accessed on 1 May 2022). [CrossRef]
- Liu, X.M.; Zhang, H. The effects of bacterial volatile emissions on plant abiotic stress tolerance. Front. Plant Sci. 2015, 6, 774. [Google Scholar] [CrossRef]
- Sofía, L.; Vanegas, C.; Barrera, C.C.; Bernal, A.J. De la raíz a la hoja: Capacidad de Bacilos Rizosféricos como Potenciales Inductores de Resistencia Sistémica (ISR) en Plantas de Arabidopsis Thaliana. Available online: https://repositorio.uniandes.edu.co/handle/1992/45022 (accessed on 8 November 2022).
- Yan, Z.; Reddy, M.S.; Ryu, C.-M.; McInroy, J.A.; Wilson, M.; Kloepper, J.W. Induced Systemic Protection Against Tomato Late Blight Elicited by Plant Growth-Promoting Rhizobacteria. Phytopathology 2002, 92, 1329–1333. Available online: https://apsjournals.apsnet.org/doi/10.1094/PHYTO.2002.92.12.1329 (accessed on 8 November 2022). [CrossRef]
- Pieterse, C.M.J.; Leon-Reyes, A.; Van der Ent, S.; Van Wees, S.C.M. Networking by small-molecule hormones in plant immunity. Nat. Chem. Biol. 2009, 5, 308–316. Available online: https://www.nature.com/articles/nchembio.164 (accessed on 9 November 2022). [CrossRef]
- Gao, L.; Zhao, W.; Qu, H.; Wang, Q.; Lingxia, Z. The yellow-fruited tomato 1 (yft1) mutant has altered fruit carotenoid accumulation and reduced ethylene production as a result of a genetic lesion in ETHYLENE INSENSITIVE2. Theor. Appl. Genet. 2016, 129, 717–728. Available online: http://faostat.fao.org/ (accessed on 17 December 2022). [CrossRef]
- Huang, Y.; Wang, S.; Shi, L.; Xu, F. JASMONATE RESISTANT 1 negatively regulates root growth under boron deficiency in Arabidopsis. J. Exp. Bot. 2021, 72, 3108–3121. Available online: https://pubmed.ncbi.nlm.nih.gov/33530106/ (accessed on 17 December 2022). [CrossRef] [PubMed]
- Tungadi, T.; Watt, L.G.; Groen, S.C.; Murphy, A.; Du, Z.; E Pate, A.; Westwood, J.H.; Fennell, T.G.; Powell, G.; Carr, J. Infection of Arabidopsis by cucumber mosaic virus triggers jasmonate-dependent resistance to aphids that relies partly on the pattern-triggered immunity factor BAK1. Mol. Plant Pathol. 2021, 22, 1082–1091. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo, O.; Piqueras, R.; Sánchez-Serrano, J.J.; Solano, R. ETHYLENE RESPONSE FACTOR1 Integrates Signals from Ethylene and Jasmonate Pathways in Plant Defense. Plant Cell 2003, 15, 165–178. Available online: www.plantcell.org/cgi/doi/10.1105/tpc.007468 (accessed on 17 December 2022). [CrossRef] [PubMed]
- Katsir, L.; Schilmiller, A.L.; Staswick, P.E.; He, S.Y.; Howe, G.A. COI1 is a critical component of a receptor for jasmonate and the bacterial virulence factor coronatine. Proc. Natl. Acad. Sci. USA 2008, 105, 7100–7105. Available online: https://www.pnas.org/doi/abs/10.1073/pnas.0802332105 (accessed on 17 December 2022). [CrossRef] [PubMed]
- Staswick, P.E.; Tiryaki, I.; Rowe, M.L. Jasmonate Response Locus JAR1 and Several Related Arabidopsis Genes Encode Enzymes of the Firefly Luciferase Superfamily That Show Activity on Jasmonic, Salicylic, and Indole-3-Acetic Acids in an Assay for Adenylation. Plant Cell 2002, 14, 1405–1415. Available online: https://academic.oup.com/plcell/article/14/6/1405/6009765 (accessed on 17 December 2022). [CrossRef]
- Liu, M.; Sun, W.; Ma, Z.; Zheng, T.; Huang, L.; Wu, Q.; Zhao, G.; Tang, Z.; Bu, T.; Li, C.; et al. Genome-wide investigation of the AP2/ERF gene family in tartary buckwheat (Fagopyum Tataricum). BMC Plant Biol. 2019, 19, 84. Available online: https://bmcplantbiol.biomedcentral.com/articles/10.1186/s12870-019-1681-6 (accessed on 17 December 2022). [CrossRef]
- Kim, D.S.; Hwang, B.K. An important role of the pepper phenylalanine ammonia-lyase gene (PAL1) in salicylic acid-dependent signalling of the defence response to microbial pathogens. J. Exp. Bot. 2014, 65, 2295–2306. Available online: https://doi.org/10.1093/jxb/eru109 (accessed on 18 December 2022). [CrossRef]
- Hayron, M.C.; Jaramillo, M.P.; Belezaca Pinargote, C.; Carranza Patiño, M.; Prieto Benavides, O.; Fernández, R.G. Respuesta de poblaciones microbianas que lideran el crecimiento en raíces y resistencia sistémica inducida. Cienc. Y Tecnol. 2015, 8, 1–11. Available online: https://revistas.uteq.edu.ec/index.php/cyt/article/view/150 (accessed on 18 December 2022).
- Valenzuela Ruiz, V.; Gálvez Gamboa, G.T.; Villa Rodríguez, E.D.; Parra Cota, F.I.; Santoyo, G.; De los Santos-Villalobos, S. Lipopéptidos producidos por agentes de control biológico del género Bacillus: Revisión de herramientas analíticas utilizadas para su estudio. Rev. Mex. De Cienc. Agrícolas 2020, 11, 419–432. Available online: http://www.scielo.org.mx/scielo.php?script=sci_arttext&pid=S2007-09342020000200419&lng=es&nrm=iso&tlng=es (accessed on 6 December 2022). [CrossRef]
- Holtappels, D.; Fortuna, K.; Lavigne, R.; Wagemans, J. The future of phage biocontrol in integrated plant protection for sustainable crop production. Curr. Opin. Biotechnol. 2021, 68, 60–71. [Google Scholar] [CrossRef]
- Botías, C.; Sánchez-Bayo, F. Papel de los plaguicidas en la pérdida de polinizadores. Ecosistemas 2018, 27, 34–41. Available online: https://www.revistaecosistemas.net/index.php/ecosistemas/article/view/1314 (accessed on 6 December 2022). [CrossRef]
- Mazari-Hiriart, M.; Pérez-Ortiz, G.; Orta-Ledesma, M.T.; Armas-Vargas, F.; Tapia, M.A.; Solano-Ortiz, R.; Silva, M.A.; Yañez-Noguez, I.; López-Vidal, Y.; Díaz-Ávalos, C. Final opportunity to rehabilitate an urban river as a water source for Mexico City. PLoS ONE 2014, 9, e102081. Available online: https://pubmed.ncbi.nlm.nih.gov/25054805/ (accessed on 31 January 2022). [CrossRef]
- De Los Santos-Villalobos, S.; María Díaz-Rodríguez, A.; Fernanda Ávila-Mascareño, M.; Denisse Martínez-Vidales, A.; Parra-Cota, F.I. COLMENA: A Culture Collection of Native Microorganisms for Harnessing the Agro-Biotechnological Potential in Soils and Contributing to Food Security. Diversity 2021, 13, 337. Available online: https://www.mdpi.com/1424-2818/13/8/337/htm (accessed on 31 January 2022). [CrossRef]
- Villa-Rodriguez, E.; Moreno-Ulloa, A.; Castro-Longoria, E.; Parra-Cota, F.I.; Santos-Villalobos, S.D.L. Integrated omics approaches for deciphering antifungal metabolites produced by a novel Bacillus species, B. cabrialesii TE3T, against the spot blotch disease of wheat (Triticum turgidum L. subsp. durum). Microbiol. Res. 2021, 251, 126826. Available online: https://pubmed.ncbi.nlm.nih.gov/34298216/ (accessed on 31 January 2022). [CrossRef]
- Peteira Delgado-Oramas, B. La resistencia inducida como alternativa para el manejo de plagas en las plantas de cultivo. Rev. Prot. Veg. 2020, 35, e07. Available online: http://scielo.sld.cu/scielo.php?script=sci_arttext&pid=S1010-27522020000100001&lng=es&tlng=es (accessed on 31 January 2022).
- Li, Y.; Gu, Y.; Li, J.; Xu, M.; Wei, Q.; Wang, Y. Biocontrol agent Bacillus amyloliquefaciens LJ02 induces systemic resistance against cucurbits powdery mildew. Front. Microbiol. 2015, 6, 883. [Google Scholar] [CrossRef] [PubMed]
- Mathys, J.; De Cremer, K.; Timmermans, P.; Van Kerckhove, S.; Lievens, B.; Vanhaecke, M.; Cammue, B.P.A.; De Coninck, B. Genome-Wide Characterization of ISR Induced in Arabidopsis thaliana by Trichoderma hamatum T382 Against Botrytis cinerea Infection. Front. Plant Sci. 2012, 3, 108. Available online: www.frontiersin.org (accessed on 1 January 2023). [CrossRef] [PubMed]
- Mashabela, M.D.; Piater, L.A.; Dubery, I.A.; Tugizimana, F.; Mhlongo, M.I. Rhizosphere Tripartite Interactions and PGPR-Mediated Metabolic Reprogramming towards ISR and Plant Priming: A Metabolomics Review. Biology 2022, 11, 346. Available online: https://www.mdpi.com/2079-7737/11/3/346/htm (accessed on 22 February 2022). [CrossRef]
| Pathogen | Host | Disease | Type | Reference |
|---|---|---|---|---|
| Ustilago maydis | Corn | Smut | Biotrophic | [33] |
| Phytophthora capsici | Tomato, pepper, other solanaceous, and cucurbit plants | Root rot, stem necrosis, foliar blight, and fruit rot | Hemibiotrophic | [34] |
| Botrytis cinerea | Strawberry, grape, raspberry, blackberry | Gray mold, post-harvest rots | Necrotrophic | [35] |
| Bipolaris sorokiniana | Wheat | Blotch spot | Necrotrophic | [36] |
| Fusarium oxysporum | Cucumber | Fusarium wilt | Hemibiotrophic | [37] |
| Septoria nodorum Berk | Wheat | Glume blotch | Hemibiotrophic | [38] |
| Tobacco mosaic virus | Tobacco | Mosaic virus disease | Biotrophic | [39] |
| Agrobacterium tumefaciens | Wild grape species (Vitis riparia) | Formation of tumors, rod-shaped, crown gall disease | Biotrophic | [40] |
| Erysiphe orontii | Tobacco | Powdery mildew diseases | Hemibiotrophic | [41] |
| Fusarium solani | Orange trees (Citrus sinensis L. Osbeck) | Darkening of the vascular system | Hemibiotrophic | [42] |
| Erwinia amylovora | Apple trees | Fire blight disease | Semi-necrotrophic or necrotrophic | [43] |
| Beneficial Microorganism | Host | Function | Reference |
|---|---|---|---|
| Bacillus megaterium, Bacillus paralicheniformis, and Bacillus cabrialesii | Wheat (Triticum turgidum L. subsp. durum) | Growth promotion | [81] |
| Pseudomonas veronii R4 | Vid (Thompson Seedless) | Induced systemic resistance (ISR) defense for leaves and roots | [82] |
| Bacillus velezensis XT1 | Tomato and strawberry plant | Growth promotion and biocontrol | [83] |
| Bacillus cabrialesii | Wheat (Triticum turgidum L. subsp. durum) | Biocontrol | [84] |
| Bacillus subtilis Cohn, Bacillus thuringiensis Berliner | Wheat | Plant resistance | [38] |
| Pseudozyma churashimaensis | Pepper | Elicits systemic defense against bacterial and viral pathogens | [85] |
| Pseudomonas fluorescens WCS417r | Arabidopsis thaliana | Local resistance of roots | [86] |
| Bacillus pumilus EN16, Bacillus subtilis SW1 | Tobacco | Systemic resistance | [39] |
| Trichoderma koningiopsis Th00 | Tomato | Induced systemic resistance (ISR) controlling Fusarium sp. | [87] |
| Gliocladium roseum | Tobacco | Induced systemic resistance (ISR) controlling Erysiphe orontii | [41] |
| Bacillus sp. FSQ1 | Common bean (Phaseolus vulgaris) | Biological control | [88] |
| Priestia sp. TSO9 | Wheat (Triticum turgidum L. subsp. durum) | Plant growth-promoting | [89] |
| Bacillus paralicheniformis TRQ65 | Wheat (Triticum turgidum L. subsp. durum) | Biological control agent and plant growth-promoting | [90] |
| Bacillus sp. TSO2 | Wheat (Triticum turgidum L. subsp. durum) | Biocontrol | [91] |
| Trichoderma harzianum sensu lato TSM39 | Wheat (Triticum turgidum L. subsp. durum) | Biocontrol | [92] |
| Rouxiella badensis SER3 | Strawberry | Biocontrol | [93] |
| Gene | Function | Source | Reference |
|---|---|---|---|
| CsChi23 | Antifungal activity | Cucumber | [81] |
| Npr1 | AS-dependent regulatory factor to RSA | Vid (Thompson Seedless) | [82] |
| Eir1 | Auxin efflux transport, being root-specific, RSI activation | Vid (Thompson Seedless) | [82] |
| Lox2 | Lipoxygenase leading to JA biosynthesis, induced systemic resistance (ISR) | Vid (Thompson Seedless) | [82] |
| Tlp1 | Antifungal activity | Vid (Thompson Seedless) | [82] |
| AtTLP1 | Encodes a thaumatin-like protein with antimicrobial properties | Arabidopsis thaliana | [86] |
| EIN2 | Ethylene signaling, chromoplast development | Solanum lycopersicum (tomato) | [129] |
| JAR1 | JA signaling in root | Arabidopsis thaliana | [130] |
| BAK1 | Co-receptor enabling detection of microbe-associated molecular patterns and induction of PTI | Arabidopsis thaliana | [131] |
| ERF1 | Ethylene and jasmonate pathways in plant defense | Arabidopsis thaliana | [132] |
| LOX | Resistance to biotic and abiotic stress | Arachis duranensis, Arachis ipaënsis, Cajanus cajan, Cicer arietinum, Glycine max, Lotus japonicus, Medicago truncatula | [123] |
| COI1 | JA signaling | Tomato plants | [133] |
| JAR1 | Encodes a JA–amido synthetase that catalyzes the formation of jasmonoyl-l-isoleucine (JA–Ile) | Arabidopsis thaliana | [134] |
| AP2/ERF | Biotic and abiotic stresses responses | Tartary buckwheat (Fagopyum Tataricum) | [135] |
| PAL1 | SA-dependent signaling of the defense response to microbial pathogens | Pepper | [136] |
| R2R3-MYB | Initiate the ISR priming process | Arabidopsis thaliana | [137] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Montelongo, A.M.; Montoya-Martínez, A.C.; Morales-Sandoval, P.H.; Parra-Cota, F.I.; de los Santos-Villalobos, S. Beneficial Microorganisms as a Sustainable Alternative for Mitigating Biotic Stresses in Crops. Stresses 2023, 3, 210-228. https://doi.org/10.3390/stresses3010016
García-Montelongo AM, Montoya-Martínez AC, Morales-Sandoval PH, Parra-Cota FI, de los Santos-Villalobos S. Beneficial Microorganisms as a Sustainable Alternative for Mitigating Biotic Stresses in Crops. Stresses. 2023; 3(1):210-228. https://doi.org/10.3390/stresses3010016
Chicago/Turabian StyleGarcía-Montelongo, Ana María, Amelia C. Montoya-Martínez, Pamela Helue Morales-Sandoval, Fannie Isela Parra-Cota, and Sergio de los Santos-Villalobos. 2023. "Beneficial Microorganisms as a Sustainable Alternative for Mitigating Biotic Stresses in Crops" Stresses 3, no. 1: 210-228. https://doi.org/10.3390/stresses3010016
APA StyleGarcía-Montelongo, A. M., Montoya-Martínez, A. C., Morales-Sandoval, P. H., Parra-Cota, F. I., & de los Santos-Villalobos, S. (2023). Beneficial Microorganisms as a Sustainable Alternative for Mitigating Biotic Stresses in Crops. Stresses, 3(1), 210-228. https://doi.org/10.3390/stresses3010016








