Surface-to-Volume Ratio Affects the Toxicity of Nanoinks in Daphnids
Abstract
1. Introduction
2. Results
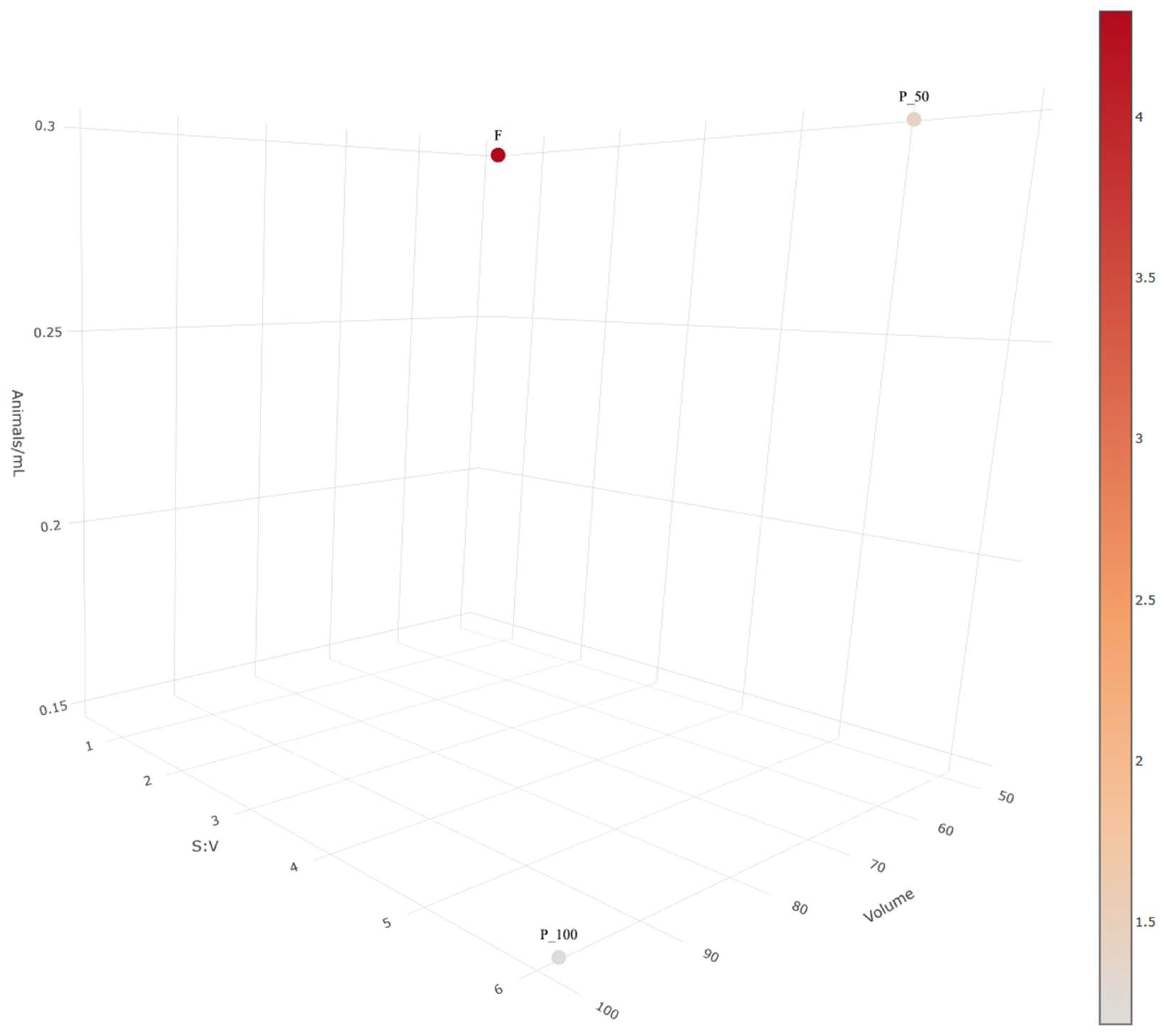
2.1. Acute Exposure to Silver Nanoparticle Ink in Different Vessels
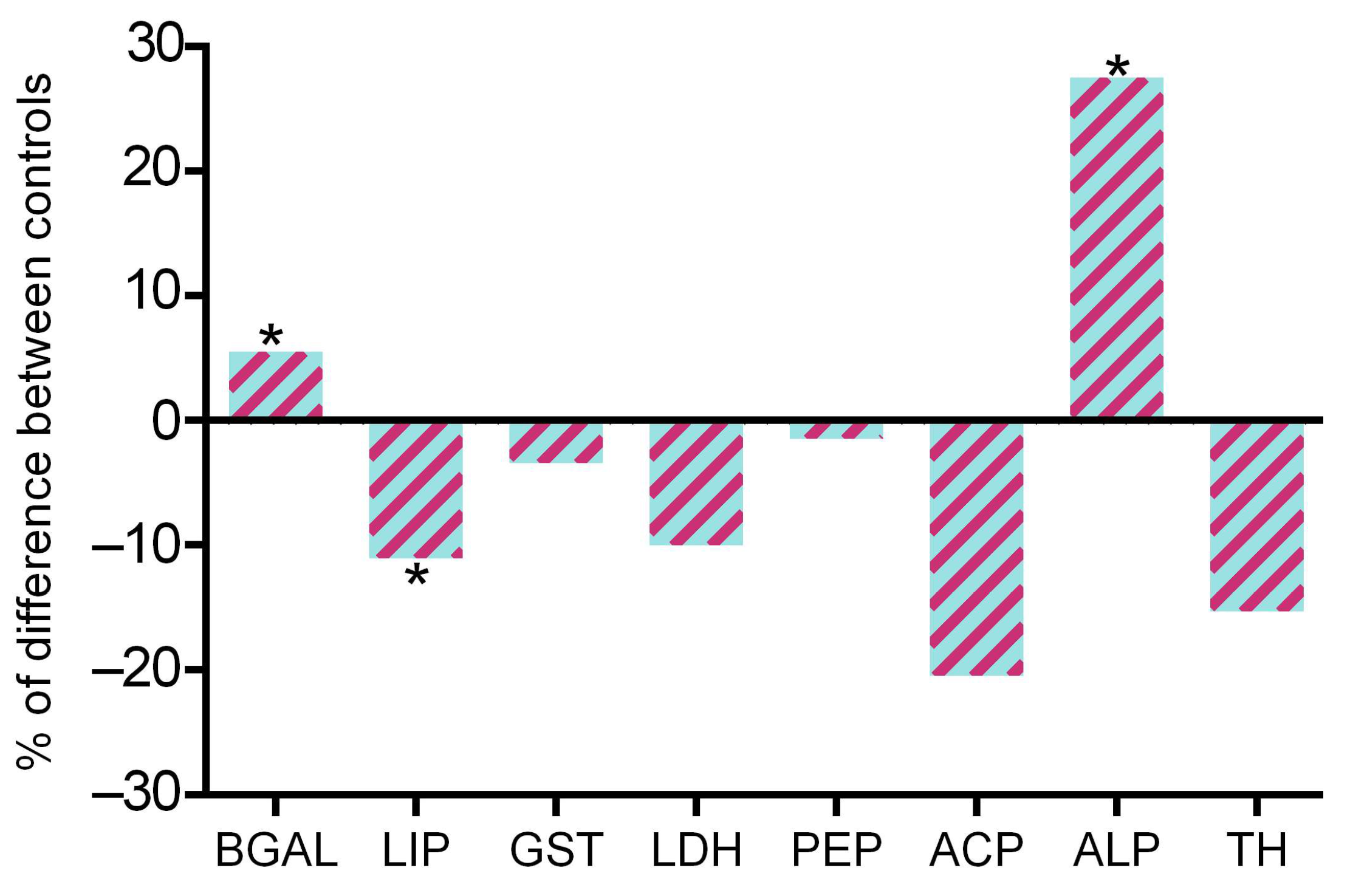
2.2. Acute Biochemical Responses to Nanomaterials
2.3. Feeding and Microscope Images
3. Discussion
4. Materials and Methods
4.1. Culturing Daphnids and Exposures
4.2. Exposure of Daphnids to Silver Nanoink and Markers of Physiology
4.3. Feeding Assay
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nanoparticle Analysis Market. Available online: https://www.gminsights.com/industry-analysis/nanoparticle-analysis-market (accessed on 31 May 2023).
- Stark, W.J.; Stoessel, P.R.; Wohlleben, W.; Hafner, A. Industrial applications of nanoparticles. Chem. Soc. Rev. 2015, 44, 5793–5805. [Google Scholar] [CrossRef] [PubMed]
- Mohajerani, A.; Burnett, L.; Smith, J.V.; Kurmus, H.; Milas, J.; Arulrajah, A.; Horpibulsuk, S.; Kadir, A.A. Nanoparticles in Construction Materials and Other Applications, and Implications of Nanoparticle Use. Materials 2019, 12, 3052. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.A.; Jacob, M.V.; Antunes, E. A critical review on silver nanoparticles: From synthesis and applications to its mitigation through low-cost adsorption by biochar. J. Environ. Manag. 2021, 281, 111918. [Google Scholar] [CrossRef] [PubMed]
- Efthimiou, I.; Kalamaras, G.; Papavasileiou, K.; Anastasi-Papathanasi, N.; Georgiou, Y.; Dailianis, S.; Deligiannakis, Y.; Vlastos, D. ZnO, Ag and ZnO-Ag nanoparticles exhibit differential modes of toxic and oxidative action in hemocytes of mussel Mytilus galloprovincialis. Sci. Total Environ. 2021, 767, 144699. [Google Scholar] [CrossRef] [PubMed]
- Avila, A.M.; Bebenek, I.; Bonzo, J.A.; Bourcier, T.; Davis Bruno, K.L.; Carlson, D.B.; Dubinion, J.; Elayan, I.; Harrouk, W.; Lee, S.L.; et al. An FDA/CDER perspective on nonclinical testing strategies: Classical toxicology approaches and new approach methodologies (NAMs). Regul. Toxicol. Pharmacol. RTP 2020, 114, 104662. [Google Scholar] [CrossRef]
- Westmoreland, C.; Bender, H.J.; Doe, J.E.; Jacobs, M.N.; Kass, G.E.N.; Madia, F.; Mahony, C.; Manou, I.; Maxwell, G.; Prieto, P.; et al. Use of New Approach Methodologies (NAMs) in regulatory decisions for chemical safety: Report from an EPAA Deep Dive Workshop. Regul. Toxicol. Pharmacol. RTP 2022, 135, 105261. [Google Scholar] [CrossRef]
- Reyes, V.; Ventura, M.; Amarillo, P. Ecotoxicological Assessment of Water and Sediment in Areas of Taal Lake with Heavy Aquaculture Practices Using Allium cepa and Daphnia magna Assay. Philipp. J. Sci. 2022, 151, 969–974. [Google Scholar] [CrossRef]
- Connors, K.A.; Brill, J.L.; Norberg-King, T.; Barron, M.G.; Carr, G.; Belanger, S.E. Daphnia magna and Ceriodaphnia dubia Have Similar Sensitivity in Standard Acute and Chronic Toxicity Tests. Environ. Environ. Toxicol. Chem. 2022, 41, 134–147. [Google Scholar] [CrossRef]
- Duchet, C.; Mitchell, C.J.; McIntyre, J.K.; Stark, J.D. Chronic toxicity of three formulations of neonicotinoid insecticides and their mixture on two daphniid species: Daphnia magna and Ceriodaphnia dubia. Aquat. Toxicol. 2023, 254, 106351. [Google Scholar] [CrossRef]
- Sanpradit, P.; Peerakietkhajorn, S. Disturbances in growth, oxidative stress, energy reserves and the expressions of related genes in Daphnia magna after exposure to ZnO under thermal stress. Sci. Total Environ. 2023, 869, 161682. [Google Scholar] [CrossRef]
- Kim, H.; Kim, D.; An, Y.-J. Microplastics enhance the toxicity and phototoxicity of UV filter avobenzone on Daphnia magna. J. Hazard. Mater. 2023, 445, 130627. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Chen, Y.; Huo, D.; Gao, J.; Xu, Y.; Yang, R.; Yang, Y.; Yu, G. Combined methods elucidate the multi-organ toxicity of cylindrospermopsin (CYN) on Daphnia magna. Environ. Pollut. 2023, 324, 121250. [Google Scholar] [CrossRef] [PubMed]
- Marambio-Jones, C.; Hoek, E.M.V. A review of the antibacterial effects of silver nanomaterials and potential implications for human health and the environment. J. Nanopart. Res. 2010, 12, 1531–1551. [Google Scholar] [CrossRef]
- Navarro, E.; Baun, A.; Behra, R.; Hartmann, N.B.; Filser, J.; Miao, A.J.; Quigg, A.; Santschi, P.H.; Sigg, L. Environmental behavior and ecotoxicity of engineered nanoparticles to algae, plants, and fungi. Ecotoxicology 2008, 17, 372–386. [Google Scholar] [CrossRef]
- Navarro, E.; Piccapietra, F.; Wagner, B.; Marconi, F.; Kaegi, R.; Odzak, N.; Sigg, L.; Behra, R. Toxicity of Silver Nanoparticles to Chlamydomonas reinhardtii. Environ. Sci. Technol. 2008, 42, 8959–8964. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, L.M.; Dickson, H.; Klanjscek, T.; Keller, A.A.; McCauley, E.; Nisbet, R.M. Environmental Feedbacks and Engineered Nanoparticles: Mitigation of Silver Nanoparticle Toxicity to Chlamydomonas reinhardtii by Algal-Produced Organic Compounds. PloS ONE 2013, 8, e74456. [Google Scholar] [CrossRef]
- Yin, L.Y.; Cheng, Y.W.; Espinasse, B.; Colman, B.P.; Auffan, M.; Wiesner, M.; Rose, J.; Liu, J.; Bernhardt, E.S. More than the Ions: The Effects of Silver Nanoparticles on Lolium multiflorum. Environ. Sci. Technol. 2011, 45, 2360–2367. [Google Scholar] [CrossRef]
- Zhao, Z.L.; Xu, L.M.; Wang, Y.; Li, B.H.; Zhang, W.M.; Li, X.C. Toxicity mechanism of silver nanoparticles to Chlamydomonas reinhardtii: Photosynthesis, oxidative stress, membrane permeability, and ultrastructure analysis. Environ. Sci. Pollut. Res. 2021, 28, 15032–15042. [Google Scholar] [CrossRef]
- Lekamge, S.; Miranda, A.F.; Abraham, A.; Li, V.; Shukla, R.; Bansal, V.; Nugegoda, D. The Toxicity of Silver Nanoparticles (AgNPs) to Three Freshwater Invertebrates with Different Life Strategies: Hydra vulgaris, Daphnia carinata, and Paratya australiensis. Front. Environ. Sci. 2018, 6, 152. [Google Scholar] [CrossRef]
- Tortella, G.R.; Rubilar, O.; Duran, N.; Diez, M.C.; Martinez, M.; Parada, J.; Seabra, A.B. Silver nanoparticles: Toxicity in model organisms as an overview of its hazard for human health and the environment. J. Hazard. Mater. 2020, 390, 121974. [Google Scholar] [CrossRef]
- Michalaki, A.; Grintzalis, K. Acute and Transgenerational Effects of Non-Steroidal Anti-Inflammatory Drugs on Daphnia magna. Toxics 2023, 11, 320. [Google Scholar] [CrossRef]
- Sibiya, A.; Gopi, N.; Jeyavani, J.; Mahboob, S.; Al-Ghanim, K.A.; Sultana, S.; Mustafa, A.; Govindarajan, M.; Vaseeharan, B. Comparative toxicity of silver nanoparticles and silver nitrate in freshwater fish Oreochromis mossambicus: A multi-biomarker approach. Comp. Biochem. Phys. C 2022, 259, 109391. [Google Scholar] [CrossRef] [PubMed]
- Pecoraro, R.; Marino, F.; Salvaggio, A.; Capparucci, F.; Di Caro, G.; Iaria, C.; Salvo, A.; Rotondo, A.; Tibullo, D.; Guerriero, G.; et al. Evaluation of Chronic Nanosilver Toxicity to Adult Zebrafish. Front. Physiol. 2017, 8, 1011. [Google Scholar] [CrossRef] [PubMed]
- Din, S.Z.U.; Shah, K.D.; Bibi, N.; Mahboub, H.H.; Kakakhel, M.A. Recent Insights into the Silver Nanomaterials: An Overview of Their Transformation in the Food Webs and Toxicity in the Aquatic Ecosystem. Water Air Soil Pollut. 2023, 234, 114. [Google Scholar] [CrossRef]
- Andrade, V.S.; Ale, A.; Antezana, P.E.; Desimone, M.F.; Cazenave, J.; Gutierrez, M.F. Ecotoxicity of nanosilver on cladocerans and the role of algae provision. Environ. Sci. Pollut. Res. 2023, 30, 27137–27149. [Google Scholar] [CrossRef] [PubMed]
- Manikandan, N.A.; McCann, R.; Kakavas, D.; Rochfort, K.D.; Sreenilayam, S.P.; Alkan, G.; Stornetta, T.; McGivern, A.R.; Grintzalis, K.; Friedrich, B.; et al. Production of Silver Nano-Inks and Surface Coatings for Anti-Microbial Food Packaging and Its Ecological Impact. Int. J. Mol. Sci. 2023, 24, 5341. [Google Scholar] [CrossRef]
- Cui, R.; Chae, Y.; An, Y.J. Dimension-dependent toxicity of silver nanomaterials on the cladocerans Daphnia magna and Daphnia galeata. Chemosphere 2017, 185, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Zhou, Y.; Wang, C.J.; Li, S.G.; Wang, X.K. Toxic Effects and Molecular Mechanism of Different Types of Silver Nanoparticles to the Aquatic Crustacean Daphnia magna. Environ. Sci. Technol. 2017, 51, 12868–12878. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, F.; Gallego-Urrea, J.A.; Jurkschat, K.; Crossley, A.; Hassellov, M.; Taylor, C.; Soares, A.M.V.M.; Loureiro, S. Silver nanoparticles and silver nitrate induce high toxicity to Pseudokirchneriella subcapitata, Daphnia magna and Danio rerio. Sci. Total Environ. 2014, 466, 232–241. [Google Scholar] [CrossRef]
- Seitz, F.; Bundschuh, M.; Rosenfeldt, R.R.; Schulz, R. Nanoparticle toxicity in Daphnia magna reproduction studies: The importance of test design. Aquat. Toxicol. 2013, 126, 163–168. [Google Scholar] [CrossRef]
- Reilly, K.; Ellis, L.-J.A.; Davoudi, H.H.; Supian, S.; Maia, M.T.; Silva, G.H.; Guo, Z.; Martinez, D.S.T.; Lynch, I. Daphnia as a model organism to probe biological responses to nanomaterials—From individual to population effects via adverse outcome pathways. Front. Toxicol. 2023, 5, 25. [Google Scholar] [CrossRef] [PubMed]
- Seitz, F.; Rosenfeldt, R.R.; Storm, K.; Metreveli, G.; Schaumann, G.E.; Schulz, R.; Bundschuh, M. Effects of silver nanoparticle properties, media pH and dissolved organic matter on toxicity to Daphnia magna. Ecotoxicol. Environ. Saf. 2015, 111, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.; Lee, B.T.; Kim, H.A.; Kim, K.W.; Kim, S.D.; Hwang, Y.S. Citrate coated silver nanoparticles change heavy metal toxicities and bioaccumulation of Daphnia magna. Chemosphere 2016, 143, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Auclair, J.; Gagne, F. Shape-Dependent Toxicity of Silver Nanoparticles on Freshwater Cnidarians. Nanomaterials 2022, 12, 3107. [Google Scholar] [CrossRef]
- Gutierrez, M.F.; Ale, A.; Andrade, V.; Bacchetta, C.; Rossi, A.; Cazenave, J. Metallic, metal oxide, and metalloid nanoparticles toxic effects on freshwater microcrustaceans: An update and basis for the use of new test species. Water Environ. Res. 2021, 93, 2505–2526. [Google Scholar] [CrossRef]
- Kelpsiene, E.; Torstensson, O.; Ekvall, M.T.; Hansson, L.A.; Cedervall, T. Long-term exposure to nanoplastics reduces life-time in Daphnia magna. Sci. Rep. 2020, 10, 5979. [Google Scholar] [CrossRef]
- Liu, S.; Cui, M.M.; Li, X.M.; Thuyet, D.Q.; Fan, W.H. Effects of hydrophobicity of titanium dioxide nanoparticles and exposure scenarios on copper uptake and toxicity in Daphnia magna. Water Res. 2019, 154, 162–170. [Google Scholar] [CrossRef]
- Ebert, D. Ecology, Epidemiology, and Evolution of Parasitism in Daphnia; National Library of Medicine: Bethesda, MD, USA, 2005. [Google Scholar]
- Levard, C.; Hotze, E.M.; Lowry, G.V.; Brown, G.E. Environmental Transformations of Silver Nanoparticles: Impact on Stability and Toxicity. Environ. Sci. Technol. 2012, 46, 6900–6914. [Google Scholar] [CrossRef]
- Lekamge, S.; Miranda, A.F.; Ball, A.S.; Shukla, R.; Nugegoda, D. The toxicity of coated silver nanoparticles to Daphnia carinata and trophic transfer from alga Raphidocelis subcapitata. PLoS ONE 2019, 14, e0214398. [Google Scholar] [CrossRef]
- Grintzalis, K.; Dai, W.; Panagiotidis, K.; Belavgeni, A.; Viant, M.R. Miniaturising acute toxicity and feeding rate measurements in Daphnia magna. Ecotoxicol. Environ. Saf. 2017, 139, 352–357. [Google Scholar] [CrossRef]
- Baumann, J.; Sakka, Y.; Bertrand, C.; Koser, J.; Filser, J. Adaptation of the Daphnia sp. acute toxicity test: Miniaturization and prolongation for the testing of nanomaterials. Environ. Sci. Pollut. Res. 2014, 21, 2201–2213. [Google Scholar] [CrossRef] [PubMed]
- Hahn, M.A.; Effertz, C.; Bigler, L.; von Elert, E. 5α-cyprinol sulfate, a bile salt from fish, induces diel vertical migration in Daphnia. eLife 2019, 8, e44791. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.E.; Kim, S.; Ahn, J.H.; Youn, P.; Kang, J.S.; Park, K.; Yi, J.; Ryu, D.Y. Induction of oxidative stress and apoptosis by silver nanoparticles in the liver of adult zebrafish. Aquat. Toxicol. 2010, 100, 151–159. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, J.W.; Li, X.H.; Shao, J.P.; Peijnenburg, W.J.G.M. Aquatic toxicity of nanosilver colloids to different trophic organisms: Contributions of particles and free silver ion. Environ. Toxicol. Chem. 2012, 31, 2408–2413. [Google Scholar] [CrossRef] [PubMed]
- Poynton, H.C.; Lazorchak, J.M.; Impellitteri, C.A.; Blalock, B.J.; Rogers, K.; Allen, H.J.; Loguinov, A.; Heckrnan, J.L.; Govindasmawy, S. Toxicogenomic Responses of Nanotoxicity in Daphnia magna Exposed to Silver Nitrate and Coated Silver Nanoparticles. Environ. Sci. Technol. 2012, 46, 6288–6296. [Google Scholar] [CrossRef]
- Lee, W.S.; Kim, E.; Cho, H.J.; Kang, T.; Kim, B.; Kim, M.Y.; Kim, Y.S.; Song, N.W.; Lee, J.S.; Jeong, J. The Relationship between Dissolution Behavior and the Toxicity of Silver Nanoparticles on Zebrafish Embryos in Different Ionic Environments. Nanomaterials 2018, 8, 652. [Google Scholar] [CrossRef]
- Devasena, T.; Iffath, B.; Kumar, R.R.; Muninathan, N.; Baskaran, K.; Srinivasan, T.; John, S.T. Insights on the Dynamics and Toxicity of Nanoparticles in Environmental Matrices. Bioinorg. Chem. Appl. 2022, 2022, 4348149. [Google Scholar] [CrossRef]
- Sun-young Park, J.C. Geno- and Ecotoxicity Evaluation of Silver Nanoparticles in Freshwater Crustacean Daphnia magna. Environ. Eng. Res. 2010, 15, 23–27. [Google Scholar] [CrossRef]
- Bianchini, A.; Wood, C.M. Mechanism of acute silver toxicity in Daphnia magna. Environ. Toxicol. Chem. 2003, 22, 1361–1367. [Google Scholar] [CrossRef]
- Niyogi, S.; Wood, C.M. Biotic ligand model, a flexible tool for developing site-specific water quality guidelines for metals. Environ. Sci. Technol. 2004, 38, 6177–6192. [Google Scholar] [CrossRef]
- Janes, N.; Playle, R.C. Modeling Silver-Binding to Gills of Rainbow-Trout (Oncorhynchus-Mykiss). Environ. Toxicol. Chem. 1995, 14, 1847–1858. [Google Scholar] [CrossRef]
- Sgrignani, J.; Magistrato, A. The Structural Role of Mg2+ Ions in a Class I RNA Polymerase Ribozyme: A Molecular Simulation Study. J. Phys. Chem. B 2012, 116, 2259–2268. [Google Scholar] [CrossRef]
- Ulm, L.; Krivohlavek, A.; Jurasin, D.; Ljubojevic, M.; Sinko, G.; Crnkovic, T.; Zuntar, I.; Sikic, S.; Vrcek, I.V. Response of biochemical biomarkers in the aquatic crustacean Daphnia magna exposed to silver nanoparticles. Environ. Sci. Pollut. Res. 2015, 22, 19990–19999. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, S.; Lee, S. Differentiation of the toxicities of silver nanoparticles and silver ions to the Japanese medaka (Oryzias latipes) and the cladoceran Daphnia magna. Nanotoxicology 2011, 5, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Conine, A.L.; Frost, P.C. Variable toxicity of silver nanoparticles to Daphnia magna: Effects of algal particles and animal nutrition. Ecotoxicology 2017, 26, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Andrei, J.; Pain-Devin, S.; Felten, V.; Devin, S.; Giamberini, L.; Mehennaoui, K.; Cambier, S.; Gutleb, A.C.; Guerold, F. Silver nanoparticles impact the functional role of Gammarus roeseli (Crustacea Amphipoda). Environ. Pollut. 2016, 208, 608–618. [Google Scholar] [CrossRef] [PubMed]
- Dedourge-Geffard, O.; Palais, F.; Biagianti-Risbourg, S.; Geffard, O.; Geffard, A. Effects of metals on feeding rate and digestive enzymes in Gammarus fossarum: An in situ experiment. Chemosphere 2009, 77, 1569–1576. [Google Scholar] [CrossRef] [PubMed]
- Romer, I.; Gavin, A.J.; White, T.A.; Merrifield, R.C.; Chipman, J.K.; Viant, M.R.; Lead, J.R. The critical importance of defined media conditions in Daphnia magna nanotoxicity studies. Toxicol. Lett. 2013, 223, 103–108. [Google Scholar] [CrossRef]
- Khan, F.R.; Paul, K.B.; Dybowska, A.D.; Valsami-Jones, E.; Lead, J.R.; Stone, V.; Fernandes, T.F. Accumulation Dynamics and Acute Toxicity of Silver Nanoparticles to Daphnia magna and Lumbriculus variegatus: Implications for Metal Modeling Approaches. Environ. Sci. Technol. 2015, 49, 4389–4397. [Google Scholar] [CrossRef]
- Zhao, C.M.; Wang, W.X. Biokinetic Uptake and Efflux of Silver Nanoparticles in Daphnia magna. Environ. Sci. Technol. 2010, 44, 7699–7704. [Google Scholar] [CrossRef]
- Kontoghiorghes, G.J. Advances on Chelation and Chelator Metal Complexes in Medicine. Int. J. Mol. Sci. 2020, 21, 2499. [Google Scholar] [CrossRef] [PubMed]
- Van Acker, S.A.B.E.; van Balen, G.P.; van den Berg, D.J.; Bast, A.; van der Vijgh, W.J.F. Influence of iron chelation on the antioxidant activity of flavonoids. Biochem. Pharmacol. 1998, 56, 935–943. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, M.T.; Mira, M.L.; Florencio, M.H.; Jennings, K.R. Iron and copper chelation by flavonoids: An electrospray mass spectrometry study. J. Inorg. Biochem. 2002, 92, 105–111. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kakavas, D.; Panagiotidis, K.; Rochfort, K.D.; Grintzalis, K. Surface-to-Volume Ratio Affects the Toxicity of Nanoinks in Daphnids. Stresses 2023, 3, 488-499. https://doi.org/10.3390/stresses3020035
Kakavas D, Panagiotidis K, Rochfort KD, Grintzalis K. Surface-to-Volume Ratio Affects the Toxicity of Nanoinks in Daphnids. Stresses. 2023; 3(2):488-499. https://doi.org/10.3390/stresses3020035
Chicago/Turabian StyleKakavas, Dimitrios, Konstantinos Panagiotidis, Keith D. Rochfort, and Konstantinos Grintzalis. 2023. "Surface-to-Volume Ratio Affects the Toxicity of Nanoinks in Daphnids" Stresses 3, no. 2: 488-499. https://doi.org/10.3390/stresses3020035
APA StyleKakavas, D., Panagiotidis, K., Rochfort, K. D., & Grintzalis, K. (2023). Surface-to-Volume Ratio Affects the Toxicity of Nanoinks in Daphnids. Stresses, 3(2), 488-499. https://doi.org/10.3390/stresses3020035







