Drought and Elevated CO2 Impacts Photosynthesis and Biochemicals of Basil (Ocimum basilicum L.)
Abstract
:1. Introduction
2. Results and Discussion
2.1. Physiological and Gas Exchange Measurements
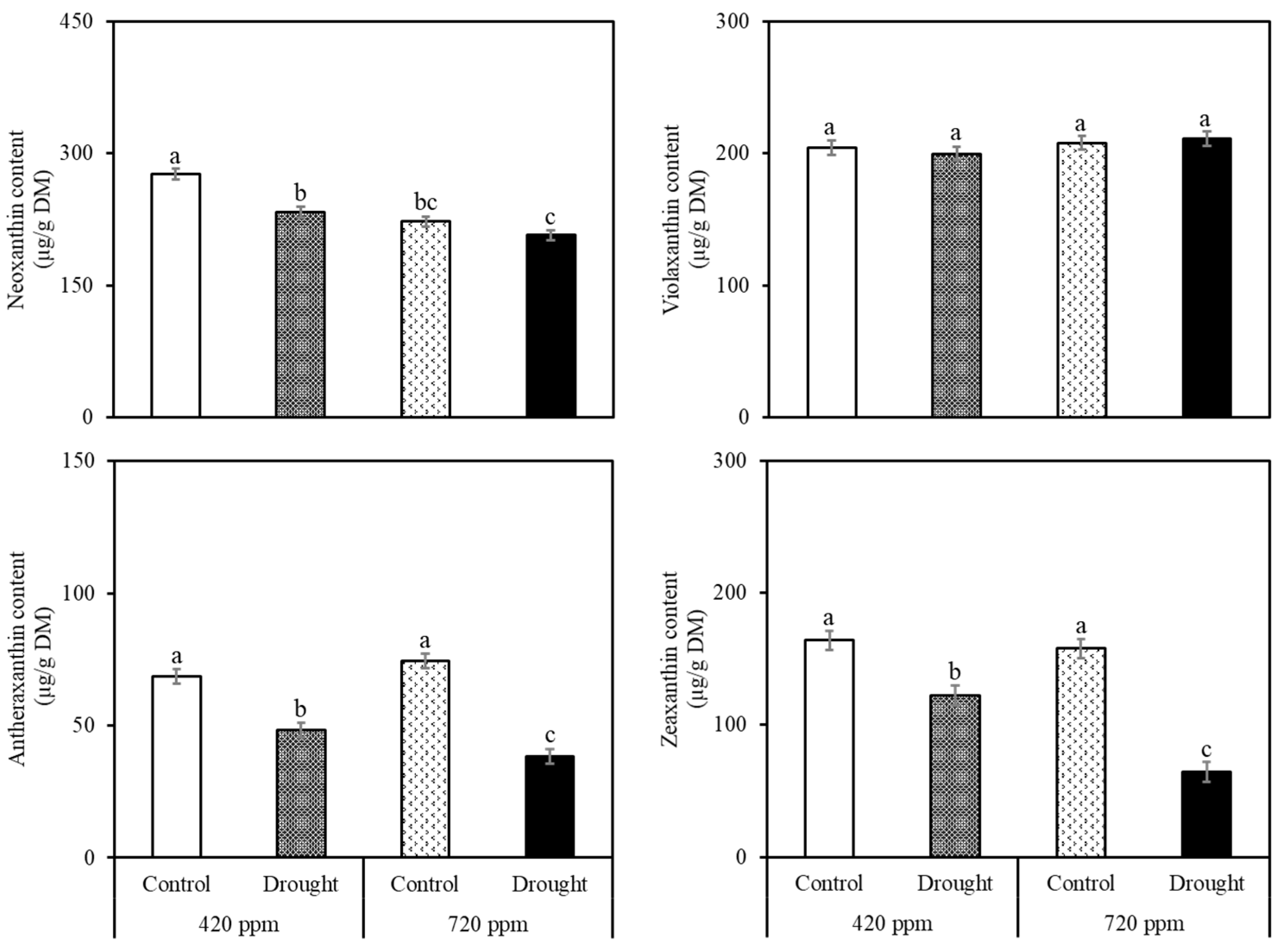
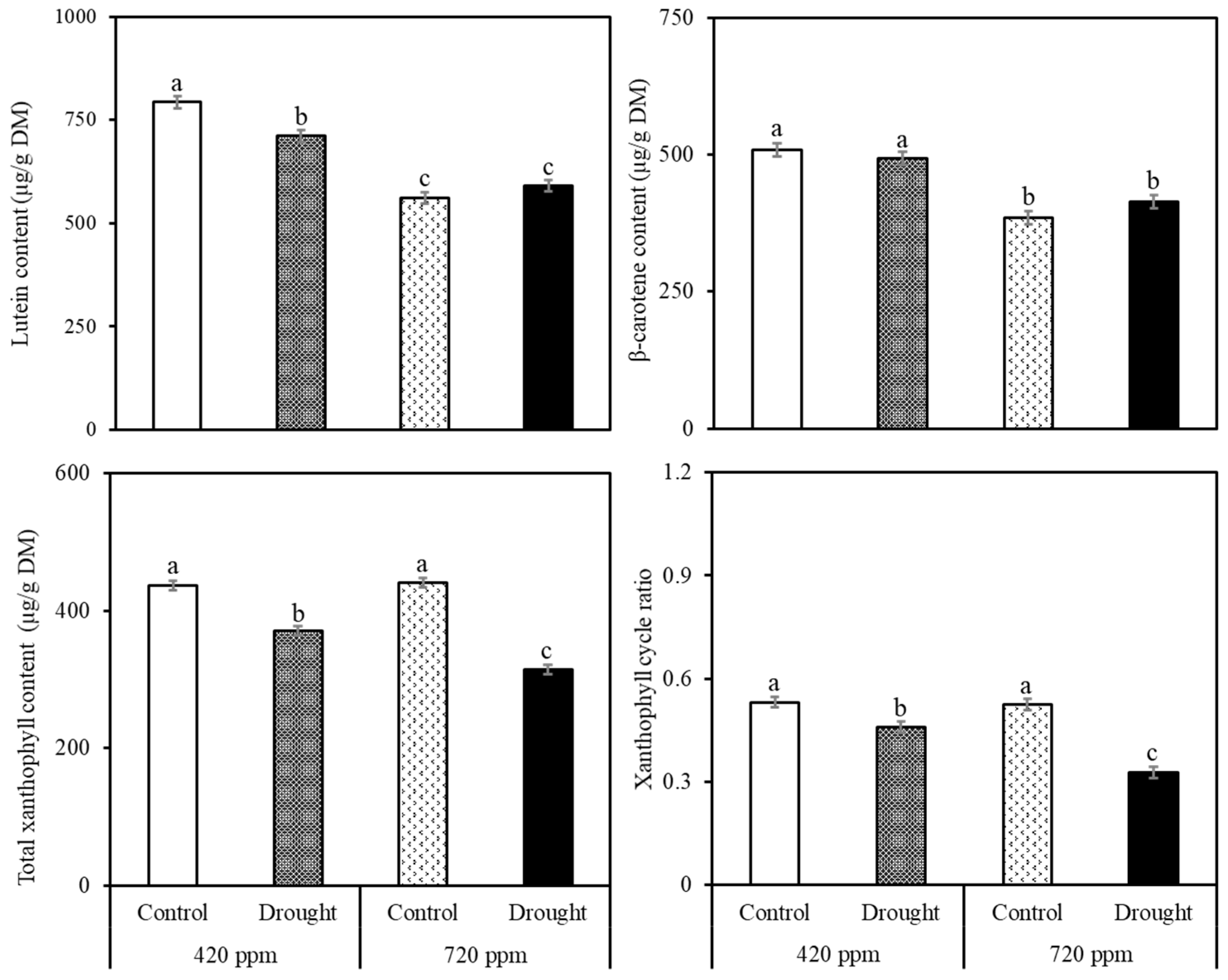
2.2. Carotenoid and Chlorophyll Analysis
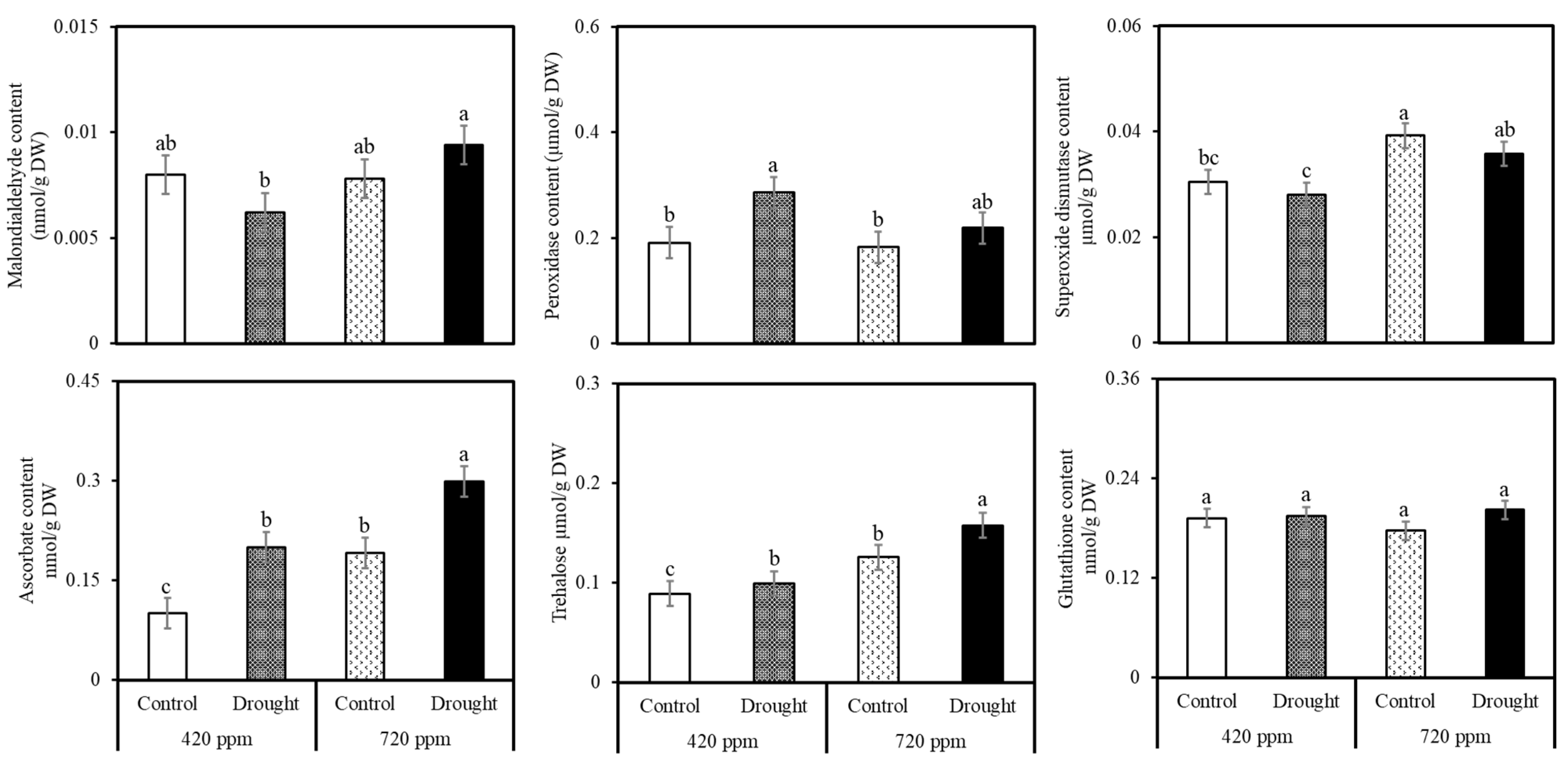
2.3. Biochemical and Phytonutrient Analysis
3. Materials and Methods
3.1. Plant Materials and Growing Condition
3.2. Treatments Application
3.3. Physiological and Gas Exchange Measurements
3.4. Carotenoid and Chlorophyll Analysis
4. Biochemical and Phytonutrient Parameters
4.1. Malondialdehyde (MDA)
4.2. Hydrogen Peroxide (H2O2)
4.3. Superoxide Dismutase (SOD)
4.4. Ascorbic Acid (ASC)
4.5. Trehalose
4.6. Glutathione
4.7. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shanker, A.K.; Maheswari, M.; Yadav, S.K.; Desai, S.; Bhanu, D.; Attal, N.B.; Venkateswarlu, B. Drought stress responses in crops. Funct. Integr. Genom. 2014, 14, 11–22. [Google Scholar] [CrossRef]
- Delgado, J.A.; Groffman, P.M.; Nearing, M.A.; Goddard, T.; Reicosky, D.; Lal, R.; Kitchen, N.R.; Rice, C.W.; Towery, D.; Salon, P. Conservation practices to mitigate and adapt to climate change. J. Soil Water Conserv. 2011, 66, 118A–129A. [Google Scholar] [CrossRef] [Green Version]
- Hirt, H.; Shinozaki, K. Plant Responses to Abiotic Stress; Springer Science & Business Media: Berlin, Germany, 2003; Volume 4, ISBN 3540200371. [Google Scholar]
- Lisar, S.Y.; Motafakkerazad, R.; Hossain, M.M.; Rahman, I.M. Water Stress in Plants: Causes, Effects and Responses. In Water Stress; InTech: Rijeka, Croatia, 2012; Volume 25, pp. 1–14. [Google Scholar]
- IPCC Climate change 2007: The physical science basis. Agenda 2007, 6, 333.
- Salehi-Lisar, S.Y.; Bakhshayeshan-Agdam, H. Drought Stress in Plants: Causes, Consequences, and Tolerance BT—Drought Stress Tolerance in Plants, Vol 1: Physiology and Biochemistry; Hossain, M.A., Wani, S.H., Bhattacharjee, S., Burritt, D.J., Tran, L.-S.P., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 1–16. ISBN 978-3-319-28899-4. [Google Scholar]
- Gornall, J.; Betts, R.; Burke, E.; Clark, R.; Camp, J.; Willett, K.; Wiltshire, A. Implications of climate change for agricultural productivity in the early twenty-first century. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 2973–2989. [Google Scholar] [CrossRef]
- Wery, J.; Silim, S.N.; Knights, E.J.; Malhotra, R.S.; Cousin, R. Screening techniques and sources and tolerance to extremes of moisture and air temperature in cool season food legumes. Euphytica 1994, 73, 73–83. [Google Scholar] [CrossRef]
- Farooq, M.; Wahid, A.; Kobayashi, N.; Fujita, D.; Basra, S.M.A. Plant drought stress: Effects, mechanisms and management. Agron. Sustain. Dev. 2009, 29, 185–212. [Google Scholar] [CrossRef] [Green Version]
- Bhargava, S.; Sawant, K. Drought stress adaptation: Metabolic adjustment and regulation of gene expression. Plant Breed. 2013, 132, 21–32. [Google Scholar] [CrossRef]
- Niu, Y.; Wang, Y.; Li, P.; Zhang, F.; Liu, H.; Zheng, G. Drought stress induces oxidative stress and the antioxidant defense system in ascorbate-deficient vtc1 mutants of Arabidopsis thaliana. Acta Physiol. Plant. 2013, 35, 1189–1200. [Google Scholar] [CrossRef]
- Earl, H.J.; Davis, R.F. Effect of drought stress on leaf and whole canopy radiation use efficiency and yield of maize. Agron. J. 2003, 95, 688–696. [Google Scholar] [CrossRef]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Chen, Z.; Gallie, D.R. Dehydroascorbate reductase affects leaf growth, development, and function. Plant Physiol. 2006, 142, 775–787. [Google Scholar] [CrossRef] [Green Version]
- Asada, K. The water-water cycle in chloroplasts: Scavenging of active oxygens and dissipation of excess photons. Annu. Rev. Plant Biol. 1999, 50, 601–639. [Google Scholar] [CrossRef]
- Vanaja, M.; Yadav, S.K.; Archana, G.; Lakshmi, N.J.; Reddy, P.R.R.; Vagheera, P.; Razak, S.K.A.; Maheswari, M.; Venkateswarlu, B. Response of C4 (maize) and C3 (sunflower) crop plants to drought stress and enhanced carbon dioxide concentration. Plant Soil Environ. 2011, 57, 207–215. [Google Scholar] [CrossRef] [Green Version]
- Damalas, C.A. Improving drought tolerance in sweet basil (Ocimum basilicum) with salicylic acid. Sci. Hortic. 2019, 246, 360–365. [Google Scholar] [CrossRef]
- Makri, O.; Kintzios, S. Ocimum sp.(basil): Botany, cultivation, pharmaceutical properties, and biotechnology. J. Herbs. Spices Med. Plants 2008, 13, 123–150. [Google Scholar] [CrossRef]
- Pushpangadan, P.; George, V. Basil. In Handbook of Herbs and Spices; Elsevier: Boca Raton, FL, USA, 2012; pp. 55–72. [Google Scholar]
- Labra, M.; Miele, M.; Ledda, B.; Grassi, F.; Mazzei, M.; Sala, F. Morphological characterization, essential oil composition and DNA genotyping of Ocimum basilicum L. cultivars. Plant Sci. 2004, 167, 725–731. [Google Scholar] [CrossRef]
- Purushothaman, B.; PrasannaSrinivasan, R.; Suganthi, P.; Ranganathan, B.; Gimbun, J.; Shanmugam, K. A comprehensive review on Ocimum basilicum. J. Nat. Remedies 2018, 18, 71–85. [Google Scholar] [CrossRef] [Green Version]
- Simon, J.E.; Quinn, J.; Murray, R.G. Basil: A Source of Essential Oils. Advances in new crops. In Proceedings of the First National Symposium “New Crops: Research, Development, Economics”, Indianapolis, IN, USA, 23–26 October 1988; pp. 484–489. [Google Scholar]
- Georgiadou, E.C.; Kowalska, E.; Patla, K.; Kulbat, K.; Smolińska, B.; Leszczyńska, J.; Fotopoulos, V. Influence of heavy metals (Ni, Cu, and Zn) on nitro-oxidative stress responses, proteome regulation and allergen production in basil (Ocimum basilicum L.) plants. Front. Plant Sci. 2018, 9, 862. [Google Scholar] [CrossRef] [Green Version]
- Vieira, R.F.; Simon, J.E. Chemical characterization of basil (Ocimum spp.) found in the markets and used in traditional medicine in Brazil. Econ. Bot. 2000, 54, 207–216. [Google Scholar] [CrossRef]
- Bernstein, N.; Kravchik, M.; Dudai, N. Salinity-induced changes in essential oil, pigments and salts accumulation in sweet basil (Ocimum basilicum) in relation to alterations of morphological development. Ann. Appl. Biol. 2010, 156, 167–177. [Google Scholar] [CrossRef]
- Dzida, K. Biological value and essential oil content in sweet basil (Ocimum basilicum L.) depending on calcium fertilization and cultivar. Acta Sci. Pol. Hortorum Cultus 2010, 9, 153–161. [Google Scholar]
- Ahmed, E.A.; Hassan, E.A.; El Tobgy, K.M.K.; Ramadan, E.M. Evaluation of rhizobacteria of some medicinal plants for plant growth promotion and biological control. Ann. Agric. Sci. 2014, 59, 273–280. [Google Scholar] [CrossRef] [Green Version]
- Mijani, S.; Nasrabadi, S.E.; Zarghani, H.; Abadi, M.G. Seed germination and early growth responses of hyssop, sweet basil and oregano to temperature levels. Not. Sci. Biol. 2013, 5, 462–467. [Google Scholar] [CrossRef] [Green Version]
- Walters, K.J.; Currey, C.J. Growth and development of basil species in response to temperature. HortScience 2019, 54, 1915–1920. [Google Scholar] [CrossRef] [Green Version]
- Farooq, M.; Hussain, M.; Wahid, A.; Siddique, K.H.M. Drought stress in plants: An overview. In Plant Responses to Drought Stress; Springer: Berlin, Germany, 2012; pp. 1–33. [Google Scholar]
- Rahbarian, R.; Khavari-Nejad, R.; Ganjeali, A.; Bagheri, A.; Najafi, F. Drought stress effects on photosynthesis, chlorophyll fluorescence and water relations in tolerant and susceptible chickpea (Cicer arietinum L.) genotypes. Acta Biol. Cracoviensia. Ser. Bot. 2011, 53, 47–56. [Google Scholar] [CrossRef]
- Sirousmehr, A.; Arbabi, J.; Asgharipour, M.R. Effect of drought stress levels and organic manures on yield, essential oil content and some morphological characteristics of sweet basil (Ocimum basilicum). Adv. Environ. Biol. 2014, 8, 880–885. [Google Scholar]
- Baghalian, K.; Abdoshah, S.; Khalighi-Sigaroodi, F.; Paknejad, F. Physiological and phytochemical response to drought stress of German chamomile (Matricaria recutita L.). Plant Physiol. Biochem. 2011, 49, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Gomes, M.d.M.d.A.; Lagôa, A.M.M.A.; Medina, C.L.; Machado, E.C.; Machado, M.A. Interactions between leaf water potential, stomatal conductance and abscisic acid content of orange trees submitted to drought stress. Braz. J. Plant Physiol. 2004, 16, 155–161. [Google Scholar] [CrossRef]
- Flexas, J.; Bota, J.; Galmes, J.; Medrano, H.; Ribas-Carbó, M. Keeping a positive carbon balance under adverse conditions: Responses of photosynthesis and respiration to water stress. Physiol. Plant. 2006, 127, 343–352. [Google Scholar] [CrossRef]
- Jaleel, C.A.; Manivannan, P.; Wahid, A.; Farooq, M.; Al-Juburi, H.J.; Somasundaram, R.; Panneerselvam, R. Drought stress in plants: A review on morphological characteristics and pigments composition. Int. J. Agric. Biol 2009, 11, 100–105. [Google Scholar]
- Ashraf, M.; Nawazish, S.; Athar, H.-U.-R. Are chlorophyll fluorescence and photosynthetic capacity potential physiological determinants of drought tolerance in maize (Zea mays L.). Pak. J. Bot. 2007, 39, 1123–1131. [Google Scholar]
- Ahmed, S.; Nawata, E.; Hosokawa, M.; Domae, Y.; Sakuratani, T. Alterations in photosynthesis and some antioxidant enzymatic activities of mungbean subjected to waterlogging. Plant Sci. 2002, 163, 117–123. [Google Scholar] [CrossRef]
- Zhang, J.; Kirkham, M.B. Drought-stress-induced changes in activities of superoxide dismutase, catalase, and peroxidase in wheat species. Plant Cell Physiol. 1994, 35, 785–791. [Google Scholar] [CrossRef]
- Nayyar, H.; Gupta, D. Differential sensitivity of C3 and C4 plants to water deficit stress: Association with oxidative stress and antioxidants. Environ. Exp. Bot. 2006, 58, 106–113. [Google Scholar] [CrossRef]
- Al-Huqail, A.; El-Dakak, R.M.; Sanad, M.N.; Badr, R.H.; Ibrahim, M.M.; Soliman, D.; Khan, F. Effects of Climate Temperature and Water Stress on Plant Growth and Accumulation of Antioxidant Compounds in Sweet Basil (Ocimum basilicum L.) Leafy Vegetable. Scientifica 2020, 2020, 3808909. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heidari, M.; Golpayegani, A. Effects of water stress and inoculation with plant growth promoting rhizobacteria (PGPR) on antioxidant status and photosynthetic pigments in basil (Ocimum basilicum L.). J. Saudi Soc. Agric. Sci. 2012, 11, 57–61. [Google Scholar] [CrossRef] [Green Version]
- Allen, R.D. Dissection of oxidative stress tolerance using transgenic plants. Plant Physiol. 1995, 107, 1049. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al Jaouni, S.; Saleh, A.M.; Wadaan, M.A.M.; Hozzein, W.N.; Selim, S.; AbdElgawad, H. Elevated CO2 induces a global metabolic change in basil (Ocimum basilicum L.) and peppermint (Mentha piperita L.) and improves their biological activity. J. Plant Physiol. 2018, 224, 121–131. [Google Scholar] [CrossRef]
- Saleh, A.M.; Selim, S.; Al Jaouni, S.; AbdElgawad, H. CO2 enrichment can enhance the nutritional and health benefits of parsley (Petroselinum crispum L.) and dill (Anethum graveolens L.). Food Chem. 2018, 269, 519–526. [Google Scholar] [CrossRef]
- Anjum, S.A.; Wang, L.C.; Farooq, M.; Hussain, M.; Xue, L.L.; Zou, C.M. Brassinolide application improves the drought tolerance in maize through modulation of enzymatic antioxidants and leaf gas exchange. J. Agron. Crop Sci. 2011, 197, 177–185. [Google Scholar] [CrossRef]
- Chaves, M.M.; Flexas, J.; Pinheiro, C. Photosynthesis under drought and salt stress: Regulation mechanisms from whole plant to cell. Ann. Bot. 2009, 103, 551–560. [Google Scholar] [CrossRef] [Green Version]
- Kopsell, D.A.; Kopsell, D.E.; Curran-Celentano, J. Carotenoid and chlorophyll pigments in sweet basil grown in the field and greenhouse. HortScience 2005, 40, 1119D-1119. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Race, E.J.; Shrikhande, A.J. Characterization of anthocyanins in grape juices by ion trap liquid chromatography—Mass spectrometry. J. Agric. Food Chem. 2003, 51, 1839–1844. [Google Scholar] [CrossRef] [PubMed]
- El Kelish, A.; Zhao, F.; Heller, W.; Durner, J.; Winkler, J.B.; Behrendt, H.; Traidl-Hoffmann, C.; Horres, R.; Pfeifer, M.; Frank, U. Ragweed (Ambrosia artemisiifolia) pollen allergenicity: SuperSAGE transcriptomic analysis upon elevated CO2 and drought stress. BMC Plant Biol. 2014, 14, 176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghasemzadeh, A.; Jaafar, H.Z.E.; Karimi, E.; Ibrahim, M.H. Combined effect of CO2 enrichment and foliar application of salicylic acid on the production and antioxidant activities of anthocyanin, flavonoids and isoflavonoids from ginger. BMC Complement. Altern. Med. 2012, 12, 229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, D.; Sun, D.; Wang, C.; Li, Y.; Guo, T. Expression of flavonoid biosynthesis genes and accumulation of flavonoid in wheat leaves in response to drought stress. Plant Physiol. Biochem. 2014, 80, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Pérez-López, U.; Robredo, A.; Lacuesta, M.; Mena-Petite, A.; Munoz-Rueda, A. Elevated CO2 reduces stomatal and metabolic limitations on photosynthesis caused by salinity in Hordeum vulgare. Photosynth. Res. 2012, 111, 269–283. [Google Scholar] [CrossRef]
- Ben-Jabeur, M.; Vicente, R.; López-Cristoffanini, C.; Alesami, N.; Djébali, N.; Gracia-Romero, A.; Serret, M.D.; López-Carbonell, M.; Araus, J.L.; Hamada, W. A novel aspect of essential oils: Coating seeds with Thyme essential oil induces drought resistance in Wheat. Plants 2019, 8, 371. [Google Scholar] [CrossRef] [Green Version]
- Saibo, N.J.M.; Lourenço, T.; Oliveira, M.M. Transcription factors and regulation of photosynthetic and related metabolism under environmental stresses. Ann. Bot. 2009, 103, 609–623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Gabbiesh, A.; Kleinwächter, M.; Selmar, D. Influencing the contents of secondary metabolites in spice and medicinal plants by deliberately applying drought stress during their cultivation. Jordan J. Biol. Sci. 2015, 147, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Cartelat, A.; Cerovic, Z.G.; Goulas, Y.; Meyer, S.; Lelarge, C.; Prioul, J.-L.; Barbottin, A.; Jeuffroy, M.-H.; Gate, P.; Agati, G. Optically assessed contents of leaf polyphenolics and chlorophyll as indicators of nitrogen deficiency in wheat (Triticum aestivum L.). Field Crop. Res. 2005, 91, 35–49. [Google Scholar] [CrossRef]
- Van Heerden, P.D.R.; Tsimilli-Michael, M.; Krüger, G.H.J.; Strasser, R.J. Dark chilling effects on soybean genotypes during vegetative development: Parallel studies of CO2 assimilation, chlorophyll a fluorescence kinetics O-J-I-P and nitrogen fixation. Physiol. Plant. 2003, 117, 476–491. [Google Scholar] [CrossRef]
- Fresneau, C.; Ghashghaie, J.; Cornic, G. Drought effect on nitrate reductase and sucrose-phosphate synthase activities in wheat (Triticum durum L.): Role of leaf internal CO2. J. Exp. Bot. 2007, 58, 2983–2992. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balasooriya, H.N.; Dassanayake, K.B.; Seneweera, S.; Ajlouni, S. Interaction of elevated carbon dioxide and temperature on strawberry (Fragaria × ananassa) growth and fruit yield. Int. J. Biol. Biomol. Agric. Food Biotechnol. Eng. World Acad. Sci. Eng. Technol. Int. Sci. Index 2018, 12, 279–287. [Google Scholar]
- Reddy, A.R.; Reddy, K.R.; Hodges, H.F. Interactive effects of elevated carbon dioxide and growth temperature on photosynthesis in cotton leaves. Plant Growth Regul. 1998, 26, 33–40. [Google Scholar] [CrossRef]
- Li, P.; Li, H.; Zong, Y.; Li, F.Y.; Han, Y.; Hao, X. Photosynthesis and metabolite responses of Isatis indigotica Fortune to elevated [CO2]. Crop J. 2017, 5, 345–353. [Google Scholar] [CrossRef]
- Teng, N.; Wang, J.; Chen, T.; Wu, X.; Wang, Y.; Lin, J. Elevated CO2 induces physiological, biochemical and structural changes in leaves of Arabidopsis thaliana. New Phytol. 2006, 172, 92–103. [Google Scholar] [CrossRef]
- Grossman-Clarke, S.; Pinter, P.J.; Kartschall, T.; Kimball, B.A.; Hunsaker, D.J.; Wall, G.W.; Garcia, R.L.; LaMorte, R.L. Modelling a spring wheat crop under elevated CO2 and drought. New Phytol. 2001, 150, 315–335. [Google Scholar] [CrossRef]
- Rajasekaran, L.R.; Blake, T.J. New plant growth regulators protect photosynthesis and enhance growth under drought of jack pine seedlings. J. Plant Growth Regul. 1999, 18, 175–181. [Google Scholar] [CrossRef]
- Acock, B. Effects of carbon dioxide on photosynthesis, plant growth, and other processes. Impact Carbon Dioxide Trace Gases Clim. Chang. Glob. Agric. 1990, 53, 45–60. [Google Scholar]
- Kalisz, A.; Jezdinský, A.; Pokluda, R.; Sękara, A.; Grabowska, A.; Gil, J. Impacts of chilling on photosynthesis and chlorophyll pigment content in juvenile basil cultivars. Hortic. Environ. Biotechnol. 2016, 57, 330–339. [Google Scholar] [CrossRef]
- Gallé, A.; Feller, U. Changes of photosynthetic traits in beech saplings (Fagus sylvatica) under severe drought stress and during recovery. Physiol. Plant. 2007, 131, 412–421. [Google Scholar] [CrossRef] [PubMed]
- Fracheboud, Y.; Leipner, J. The application of chlorophyll fluorescence to study light, temperature, and drought stress. In Practical Applications of Chlorophyll Fluorescence in Plant Biology; Springer: Boston, MA, USA, 2003; pp. 125–150. [Google Scholar]
- Davison, P.A.; Hunter, C.N.; Horton, P. Overexpression of β-carotene hydroxylase enhances stress tolerance in Arabidopsis. Nature 2002, 418, 203–206. [Google Scholar] [CrossRef] [PubMed]
- Santabarbara, S.; Casazza, A.P.; Ali, K.; Economou, C.K.; Wannathong, T.; Zito, F.; Redding, K.E.; Rappaport, F.; Purton, S. The requirement for carotenoids in the assembly and function of the photosynthetic complexes in Chlamydomonas reinhardtii. Plant Physiol. 2013, 161, 535–546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kusama, Y.; Inoue, S.; Jimbo, H.; Takaichi, S.; Sonoike, K.; Hihara, Y.; Nishiyama, Y. Zeaxanthin and echinenone protect the repair of photosystem II from inhibition by singlet oxygen in Synechocystis sp. PCC 6803. Plant Cell Physiol. 2015, 56, 906–916. [Google Scholar] [CrossRef] [PubMed]
- Dhami, N.; Tissue, D.T.; Cazzonelli, C.I. Leaf-age dependent response of carotenoid accumulation to elevated CO2 in Arabidopsis. Arch. Biochem. Biophys. 2018, 647, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Croce, R.; Müller, M.G.; Bassi, R.; Holzwarth, A.R. Carotenoid-to-chlorophyll energy transfer in recombinant major light-harvesting complex (LHCII) of higher plants. I. Femtosecond transient absorption measurements. Biophys. J. 2001, 80, 901–915. [Google Scholar] [CrossRef] [Green Version]
- Scott, K.J. Detection and measurement of carotenoids by UV/VIS spectrophotometry. Curr. Protoc. Food Anal. Chem. 2001, F2.2.1–F2.2.10. [Google Scholar] [CrossRef]
- Cheng, S.-H.; Moore, B.D.; Seemann, J.R. Effects of short-and long-term elevated CO2 on the expression of ribulose-1, 5-bisphosphate carboxylase/oxygenase genes and carbohydrate accumulation in leaves of Arabidopsis thaliana (L.) Heynh. Plant Physiol. 1998, 116, 715–723. [Google Scholar] [CrossRef] [Green Version]
- Van der Kooij, L.A.W.; De Kok, L.J.; Stulen, I. Biomass Production and Carbohydrate Content of Arabidopsis thaliana at Atmospheric CO2 Concentrations from 390 to 1680 μL L−1. Plant Biol. 1999, 1, 482–486. [Google Scholar] [CrossRef]
- Bae, H.; Sicher, R. Changes of soluble protein expression and leaf metabolite levels in Arabidopsis thaliana grown in elevated atmospheric carbon dioxide. Field Crop. Res. 2004, 90, 61–73. [Google Scholar] [CrossRef]
- Melkozernov, A.N.; Blankenship, R.E. Photosynthetic functions of chlorophylls. In Chlorophylls and Bacteriochlorophylls; Springer: Dordrecht, The Netherlands, 2006; pp. 397–412. [Google Scholar]
- Pérez-Gálvez, A.; Viera, I.; Roca, M. Carotenoids and chlorophylls as antioxidants. Antioxidants 2020, 9, 505. [Google Scholar] [CrossRef]
- Gummuluru, S.; Jana, S.; Hobbs, S.L.A. Genotypic variability in physiological characters and its relationship to drought tolerance in durum wheat. Can. J. Plant Sci. 1989, 69, 703–711. [Google Scholar] [CrossRef]
- Mafakheri, A.; Siosemardeh, A.F.; Bahramnejad, B.; Struik, P.C.; Sohrabi, Y. Effect of drought stress on yield, proline and chlorophyll contents in three chickpea cultivars. Aust. J. Crop Sci. 2010, 4, 580–585. [Google Scholar]
- Li, Q.; Liu, B.; Wu, Y.; Zou, Z. Interactive effects of drought stresses and elevated CO2 concentration on photochemistry efficiency of cucumber seedlings. J. Integr. Plant Biol. 2008, 50, 1307–1317. [Google Scholar] [CrossRef]
- Li, Y.; Gupta, G. Photosynthetic changes in soybean with and without nitrogen and increased carbon dioxide. Plant Sci. 1993, 89, 1–4. [Google Scholar] [CrossRef]
- Zhao, X.; Mao, Z.; Xu, J. Gas exchange, chlorophyll and growth responses of Betula Platyphylla seedlings to elevated CO2 and nitrogen. Int. J. Biol. 2010, 2, 143. [Google Scholar] [CrossRef]
- Manivannan, P.; Jaleel, C.A.; Sankar, B.; Kishorekumar, A.; Somasundaram, R.; Lakshmanan, G.M.A.; Panneerselvam, R. Growth, biochemical modifications and proline metabolism in Helianthus annuus L. as induced by drought stress. Colloids Surfaces B Biointerfaces 2007, 59, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Balfagón, D.; Zandalinas, S.I.; Baliño, P.; Muriach, M.; Gómez-Cadenas, A. Involvement of ascorbate peroxidase and heat shock proteins on citrus tolerance to combined conditions of drought and high temperatures. Plant Physiol. Biochem. 2018, 127, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Silva, E.N.; Ferreira-Silva, S.L.; de Vasconcelos Fontenele, A.; Ribeiro, R.V.; Viégas, R.A.; Silveira, J.A.G. Photosynthetic changes and protective mechanisms against oxidative damage subjected to isolated and combined drought and heat stresses in Jatropha curcas plants. J. Plant Physiol. 2010, 167, 1157–1164. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Z.-B.; Liu, X.; Tian, X.-J.; Yue, M. Effects of CO2 laser pretreatment on drought stress resistance in wheat. J. Photochem. Photobiol. B Biol. 2008, 90, 17–25. [Google Scholar] [CrossRef]
- Toussaint, J.; Kraml, M.; Nell, M.; Smith, S.E.; Smith, F.A.; Steinkellner, S.; Schmiderer, C.; Vierheilig, H.; Novak, J. Effect of Glomus mosseae on concentrations of rosmarinic and caffeic acids and essential oil compounds in basil inoculated with Fusarium oxysporum f. sp. basilici. Plant Pathol. 2008, 57, 1109–1116. [Google Scholar] [CrossRef]
- Kwee, E.M.; Niemeyer, E.D. Variations in phenolic composition and antioxidant properties among 15 basil (Ocimum basilicum L.) cultivars. Food Chem. 2011, 128, 1044–1050. [Google Scholar] [CrossRef]
- Bekhradi, F.; Delshad, M.; Marín, A.; Luna, M.C.; Garrido, Y.; Kashi, A.; Babalar, M.; Gil, M.I. Effects of salt stress on physiological and postharvest quality characteristics of different Iranian genotypes of basil. Hortic. Environ. Biotechnol. 2015, 56, 777–785. [Google Scholar] [CrossRef]
- Raja, K.; Read, J.J.; McKinion, J.M. Soil-Plant-Atmosphere-Research (SPAR) facility: A tool for plant research and modeling. Biotronics 2001, 30, 27–50. [Google Scholar]
- Wijewardana, C.; Hock, M.; Henry, B.; Reddy, K.R. Screening corn hybrids for cold tolerance using morphological traits for early-season seeding. Crop Sci. 2015, 55, 851–867. [Google Scholar] [CrossRef] [Green Version]
- Hoagland, D.R.; Arnon, D.I. The water-culture method for growing plants without soil. Circ. Calif. Agric. Exp. Stn. 1950, 347, 32. [Google Scholar]
- Barickman, T.C.; Olorunwa, O.J.; Sehgal, A.; Walne, C.H.; Reddy, K.R.; Gao, W. Yield, Physiological Performance, and Phytochemistry of Basil (Ocimum basilicum L.) under Temperature Stress and Elevated CO2 Concentrations. Plants 2021, 10, 1072. [Google Scholar] [CrossRef]
- Kopsell, D.A.; Kopsell, D.E.; Lefsrud, M.G.; Curran-Celentano, J.; Dukach, L.E. Variation in lutein, β-carotene, and chlorophyll concentrations among Brassica oleracea cultigens and seasons. HortScience 2004, 39, 361–364. [Google Scholar] [CrossRef]
- Barickman, T.C.; Kopsell, D.A.; Sams, C.E. Abscisic acid impacts tomato carotenoids, soluble sugars, and organic acids. HortScience 2016, 51, 370–376. [Google Scholar] [CrossRef] [Green Version]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Mukherjee, S.P.; Choudhuri, M.A. Implications of water stress-induced changes in the levels of endogenous ascorbic acid and hydrogen peroxide in Vigna seedlings. Physiol. Plant. 1983, 58, 166–170. [Google Scholar] [CrossRef]
- Dhindsa, R.S.; Plumb-Dhindsa, P.; Thorpe, T.A. Leaf senescence: Correlated with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. J. Exp. Bot. 1981, 32, 93–101. [Google Scholar] [CrossRef]
- Awasthi, R.; Gaur, P.; Turner, N.C.; Vadez, V.; Siddique, K.H.M.; Nayyar, H. Effects of individual and combined heat and drought stress during seed filling on the oxidative metabolism and yield of chickpea (Cicer arietinum) genotypes differing in heat and drought tolerance. Crop Pasture Sci. 2017, 68, 823–841. [Google Scholar] [CrossRef]
- Trevelyan, W.E.; Harrison, J.S. Studies on yeast metabolism. 1. Fractionation and microdetermination of cell carbohydrates. Biochem. J. 1952, 50, 298–303. [Google Scholar] [CrossRef] [PubMed]
- Brin, M. [89] Transketolase: Clinical aspects. In Methods in enzymology; Elsevier: Cambridge, MA, USA, 1966; Volume 9, pp. 506–514. ISBN 0076-6879. [Google Scholar]
- Einig, W.; Hampp, R. Carbon partitioning in Norway spruce: Amounts of fructose 2, 6-bisphosphate and of intermediates of starch/sucrose synthesis in relation to needle age and degree of needle loss. Trees 1990, 4, 9–15. [Google Scholar] [CrossRef]
- Griffith, O.W. Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal. Biochem. 1980, 106, 207–212. [Google Scholar] [CrossRef]

| Treatment | Chlorophyll 1 | Flavonoids | Anthocyanin | NBI |
|---|---|---|---|---|
| [μg/mL] | [mg/g DM] | [mg/g DM] | ||
Control | 21.468 bc | 420 ppm 0.685 ab | 0.114 b | 32.415 b |
| Drought | 25.744 a | 0.645 b | 0.102 c | 40.890 a |
| 720 ppm | ||||
| Control | 18.978 c | 0.704 ab | 0.113 bc | 28.062 c |
| Drought | 22.027 b | 0.739 a | 0.127 a | 30.391 bc |
| Treatment 2 | *** | ns | *** | *** |
| CO2 | ** | * | ** | *** |
| Trt*CO2 | ns | ns | ns | * |
| Treatment | Pn | gs | Ci | ETR | E | Tleaf | CiCa 1 |
|---|---|---|---|---|---|---|---|
| 420 ppm | |||||||
| Control | 24.475 b | 0.375 a | 295.090 b | 187.340 a | 6.788 a | 30.483 c | 0.704 a |
| Drought | 20.123 b | 0.159 b | 198.400 c | 182.140 a | 4.578 b | 31.710 ab | 0.473 b |
| 720 ppm | |||||||
| Control | 31.513 a | 0.312 a | 530.710 a | 184.980 a | 6.670 a | 31.263 b | 0.737 a |
| Drought | 24.475 b | 0.083 b | 291.480 b | 193.460 a | 2.498 c | 32.400 a | 0.406 b |
| Treatment 2,3 | ** | *** | *** | ns | *** | *** | *** |
| CO2 | ns | * | *** | ns | * | ** | ns |
| Trt*CO2 | ns | ns | *** | ns | * | ns | * |
| Treatment | Fo | Fm | Fs | Fv/Fm | ΦPSII | ΦCO2 | qP | qN |
|---|---|---|---|---|---|---|---|---|
| 420 ppm | ||||||||
| Control | 448.300 a | 840.500 a | 622.100 a | 0.466 b | 0.261 a | 0.0195 b | 0.558 ab | 1.875 b |
| Drought | 453.000 a | 815.400 a | 599.900 a | 0.444 b | 0.264 a | 0.0160 b | 0.593 a | 1.800 b |
| 720 ppm | ||||||||
| Control | 440.800 a | 907.900 a | 674.100 a | 0.513 a | 0259 a | 0.0248 a | 0.507 b | 2.058 a |
| Drought | 457.600 a | 854.100 a | 638.900 a | 0.462 b | 0.249 a | 0.0163 b | 0.538 ab | 1.865 b |
| Treatment 1,2 | ns | ns | ns | * | ns | ** | ns | * |
| CO2 | ns | ns | ns | * | ns | ns | ns | * |
| Trt*CO2 | ns | ns | ns | ns | ns | ns | ns | ns |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barickman, T.C.; Adhikari, B.; Sehgal, A.; Walne, C.H.; Reddy, K.R.; Gao, W. Drought and Elevated CO2 Impacts Photosynthesis and Biochemicals of Basil (Ocimum basilicum L.). Stresses 2021, 1, 223-237. https://doi.org/10.3390/stresses1040016
Barickman TC, Adhikari B, Sehgal A, Walne CH, Reddy KR, Gao W. Drought and Elevated CO2 Impacts Photosynthesis and Biochemicals of Basil (Ocimum basilicum L.). Stresses. 2021; 1(4):223-237. https://doi.org/10.3390/stresses1040016
Chicago/Turabian StyleBarickman, T. Casey, Bikash Adhikari, Akanksha Sehgal, C. Hunt Walne, K. Raja Reddy, and Wei Gao. 2021. "Drought and Elevated CO2 Impacts Photosynthesis and Biochemicals of Basil (Ocimum basilicum L.)" Stresses 1, no. 4: 223-237. https://doi.org/10.3390/stresses1040016
APA StyleBarickman, T. C., Adhikari, B., Sehgal, A., Walne, C. H., Reddy, K. R., & Gao, W. (2021). Drought and Elevated CO2 Impacts Photosynthesis and Biochemicals of Basil (Ocimum basilicum L.). Stresses, 1(4), 223-237. https://doi.org/10.3390/stresses1040016








