Coordinated Role of Nitric Oxide, Ethylene, Nitrogen, and Sulfur in Plant Salt Stress Tolerance
Abstract
1. Introduction
2. Salt Stress: An Overview
2.1. Salinity Stress Impacts in Plants
2.1.1. Growth and Development
2.1.2. Photosynthetic Functions
2.1.3. Oxidative Stress and Antioxidant Metabolism
2.1.4. Nutrients Uptake, Assimilation and Related Traits
2.1.5. Crop Yield
3. Nitrogen in Salt Tolerance
4. Sulfur in Salt Tolerance
5. Coordination of N and S in Salt Stress Tolerance
6. Nitric Oxide
6.1. NO Generation and Signaling
6.2. Nitric Oxide in Salt Tolerance
7. Ethylene
7.1. Ethylene Biosynthesis and Signaling
7.2. Ethylene in Salt Tolerance
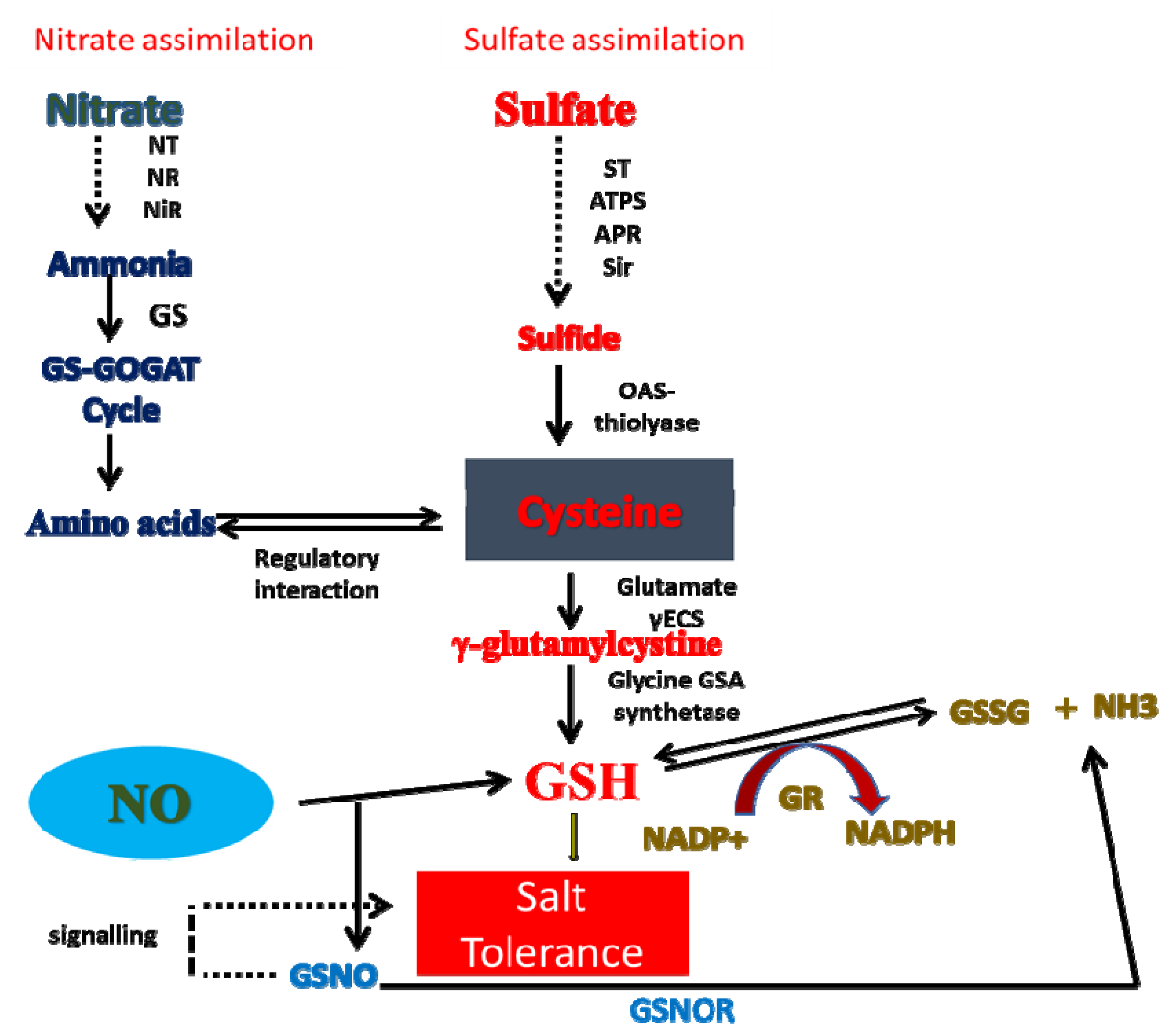
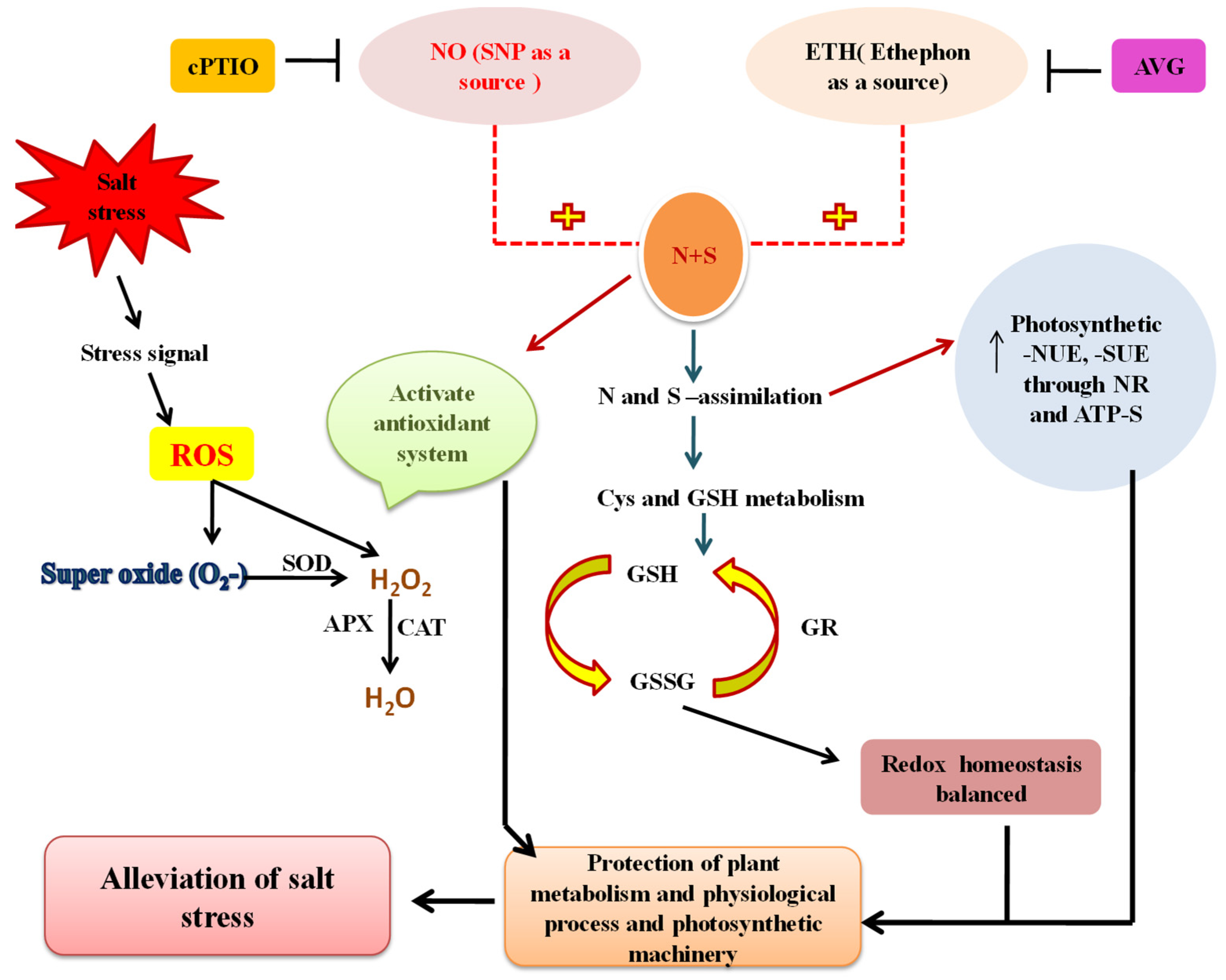
8. Crosstalk of NO with N and S in Salt Tolerance
9. Crosstalk of Ethylene with N and S in Salt Tolerance
10. Conclusions: Bridging the Gaps in Understanding Salinity Tolerance
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sehar, Z.; Masood, A.; Khan, N.A. Nitric oxide reverses glucose-mediated photosynthetic repression in wheat (Triticum aestivum L.) under salt stress. Environ. Exp. Bot. 2019, 161, 277–289. [Google Scholar] [CrossRef]
- Jahan, B.; Al Ajmi, M.F.; Rehman, M.T.; Khan, N.A. Treatment of nitric oxide supplemented with nitrogen and sulfur regulates photosynthetic performance and stomatal behavior in mustard under salt stress. Physiol. Plant. 2020, 168, 490–510. [Google Scholar] [PubMed]
- Khanna, R.R.; Jahan, B.; Iqbal, N.; Khan, N.A.; AlAjmi, M.F.; Rehman, M.T.; Khan, M.I.R. GABA reverses salt-inhibited photosynthetic and growth responses through its influence on NO-mediated nitrogen-sulfur assimilation and antioxidant system in wheat. J. Biotech. 2021, 325, 73–82. [Google Scholar] [CrossRef]
- Machado, R.M.A.; Serralheiro, R.P. Soil salinity: Effect on vegetable crop growth. Management practices to prevent and mitigate soil salinization. Horticulturae 2017, 3, 30. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Nahar, K.; Alam, M.; Bhowmik, P.C.; Hossain, M.; Rahman, M.M.; Fujita, M. Potential use of halophytes to remediate saline soils. Biomed. Res. Int. 2014, 2014, 589341. [Google Scholar] [CrossRef]
- Kumar, P.; Sharma, P.K. Soil salinity and food security in India. Front. Sustain. Syst. 2020, 4, 533781. [Google Scholar] [CrossRef]
- Chokshi, K.; Pancha, I.; Ghosh, A.; Mishra, S. Salinity induced oxidative stress alters the physiological responses and improves the biofuel potential of green microalgae Acutodesmus dimorphus. Bioresour. Technol. 2017, 244, 1376–1383. [Google Scholar] [CrossRef]
- Kamran, M.; Parveen, A.; Ahmar, S.; Malik, Z.; Hussain, S.; Chattha, M.S.; Chen, J.T. An overview of hazardous impacts of soil salinity in crops, tolerance mechanisms, and amelioration through selenium supplementation. Int. J. Mol. Sci. 2020, 21, 148. [Google Scholar] [CrossRef]
- Fatma, M.; Khan, M.I.R.; Masood, A.; Khan, N.A. Coordinate changes in assimilatory sulfate reduction are correlated to salt tolerance: Involvement of phytohormones. Annu. Res. Rev. Biol. 2013, 3, 267–295. [Google Scholar]
- Jahan, B.; Iqbal, N.; Fatma, M.; Sehar, Z.; Masood, A.; Sofo, A.; D’Ippolito, I.; Khan, N.A. Ethylene supplementation combined with split application of nitrogen and sulfur protects salt-inhibited photosynthesis through optimization of proline metabolism and antioxidant system in mustard (Brassica juncea L.). Plants 2021, 10, 1303. [Google Scholar] [CrossRef]
- Liu, C.; Zhao, X.; Yan, J.; Yuan, Z.; Gu, M. Effects of salt stress on growth, photosynthesis, and mineral nutrients of 18 pomegranate (Punica granatum) cultivars. Agronomy 2020, 10, 27. [Google Scholar] [CrossRef]
- Qiao, Y.; Yin, L.; Wang, B.; Ke, Q.; Deng, X.; Wang, S. Melatonin promotes plant growth by increasing nitrogen uptake and assimilation under nitrogen deficient condition in winter wheat. Plant Physiol. Biochem. 2019, 139, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Fan, X.; Miller, A.J. Plant nitrogen assimilation and use efficiency. Annu. Rev. Plant Biol. 2012, 63, 153–182. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, M.; Li, Y.; Wu, A.; Huang, J. Effects of arbuscular mycorrhizal fungi on growth and nitrogen uptake of Chrysanthemum morifolium under salt stress. PLoS ONE 2018, 13, e0196408. [Google Scholar] [CrossRef]
- Hussain, S.J.; Khan, N.A.; Anjum, N.A.; Masood, A.; Khan, M.I.R. Mechanistic elucidation of salicylic acid and sulphur-induced defence systems, nitrogen metabolism, photosynthetic, and growth potential of mungbean (Vigna radiata) under salt stress. J. Plant Growth Regul. 2021, 40, 1000–1016. [Google Scholar] [CrossRef]
- Fatma, M.; Iqbal, N.; Gautam, H.; Sehar, Z.; Sofo, A.; D’Ippolito, I.; Khan, N.A. Ethylene and sulfur coordinately modulate the antioxidant system and ABA accumulation in mustard plants under salt stress. Plants 2021, 10, 180. [Google Scholar] [CrossRef]
- Fatma, M.; Masood, A.; Per, T.S.; Khan, N.A. Nitric oxide alleviates salt stress inhibited photosynthetic performance by interacting with sulfur assimilation in mustard. Front. Plant Sci. 2016, 7, 521. [Google Scholar] [CrossRef]
- Asgher, M.; Per, T.S.; Masood, A.; Fatma, M.; Freschi, L.; Corpas, F.J.; Khan, N.A. Nitric oxide signaling and its crosstalk with other plant growth regulators in plant responses to abiotic stress. Environ. Sci. Pollut. Res. 2017, 24, 2273–2285. [Google Scholar] [CrossRef]
- Per, T.S.; Khan, N.A.; Reddy, P.S.; Masood, A.; Hasanuzzaman, M.; Khan, M.I.R.; Anjum, N.A. Approaches in modulating proline metabolism in plants for salt and drought stress tolerance, Phytohormones, mineral nutrients and transgenics. Plant Physiol. Biochem. 2017, 115, 126–140. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Oku, H.; Nahar, K.; Bhuyan, M.B.; Al Mahmud, J.; Baluska, F.; Fujita, M. Nitric oxide-induced salt stress tolerance in plants: ROS metabolism, signaling, and molecular interactions. Plant Biotechnol. Rep. 2018, 12, 77–92. [Google Scholar] [CrossRef]
- Borbély, P.; Poór, P.; Tari, I. Changes in physiological and photosynthetic parameters in tomato of different ethylene status under salt stress: Effects of exogenous 1-aminocyclopropane-1-carboxylic acid treatment and the inhibition of ethylene signalling. Plant Physiol. Biochem. 2020, 156, 345–356. [Google Scholar] [CrossRef] [PubMed]
- Sehar, Z.; Iqbal, N.; Khan, M.I.R.; Masood, A.; Rehman, M.T.; Hussain, A.; AlAjmi, M.F.; Ahmad, A.; Khan, N.A. Ethylene reduces glucose sensitivity and reverses photosynthetic repression through optimization of glutathione production in salt-stressed wheat (Triticum aestivum L.). Sci. Rep. 2021, 11, 1–12. [Google Scholar]
- Iqbal, N.; Umar, S.; Khan, N.A. Nitrogen availability regulates proline and ethylene production and alleviates salinity stress in mustard (Brassica juncea). J. Plant Physiol. 2015, 178, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Wicke, B.; Smeets, E.; Dornburg, V.; Vashev, B.; Gaiser, T.; Turkenburg, W.; Faaij, A. The global technical and economic potential of bioenergy from salt-affected soils. Energy Environ. Sci. 2011, 4, 2669–2681. [Google Scholar] [CrossRef]
- Rasool, S.; Hameed, A.; Azooz, M.M.; Siddiqi, T.O.; Ahmad, P. Salt stress: Causes, types and responses of plants. In Ecophysiology and Responses of Plants under Salt Stress; Ahmad, P., Azooz, M.M., Prasad, M.N.V., Eds.; Springer Science & Business Media: New York, NY, USA, 2012; pp. 1–24. [Google Scholar]
- Xu, G.Y.; Rocha, P.S.; Wang, M.L.; Xu, M.L.; Cui, Y.C.; Li, L.Y.; Zhu, Y.X.; Xia, X. A novel rice calmodulin-like gene, OsMSR2, enhances drought and salt tolerance and increases ABA sensitivity in Arabidopsis. Planta 2011, 234, 47–59. [Google Scholar] [CrossRef]
- Akbarimoghaddam, H.; Galavi, M.; Ghanbari, A.; Panjehkeh, N. Salinity effects on seed germination and seedling growth of bread wheat cultivars. Trakia J. Sci. 2011, 9, 43–50. [Google Scholar]
- Sharma, P.; Sardana, V.; Banga, S.S. Salt tolerance of Indian mustard (Brassicajuncea L.) at germination and early seedling growth. Environ. Exp. Biol. 2013, 11, 39–46. [Google Scholar]
- Chowdhury, F.T.; Halim, M.A.; Hossain, F.; Akhtar, N. Effects of sodium chloride on germination and seedling growth of Sunflower (Helianthus annuus L.). Jahangirnagar Uni. J. Biol Sci. 2018, 7, 35–44. [Google Scholar] [CrossRef][Green Version]
- Rahneshan, Z.; Nasibi, F.; Moghadam, A.A. Effects of salinity stress on some growth, physiological, biochemical parameters and nutrients in two pistachio (Pistaciavera L.) rootstocks. J. Plant Interact. 2018, 13, 73–82. [Google Scholar] [CrossRef]
- Jaarsma, R.; de Vries, R.S.; de Boer, A.H. Effect of salt stress on growth, Na+ accumulation and proline metabolism in potato (Solanum tuberosum) cultivars. PLoS ONE 2013, 8, e60183. [Google Scholar] [CrossRef]
- Faghire, M.; Mohamed, F.; Taoufiq, K.; Fghire, R.; Bargaz, A.; Mandri, B.; Oufdou, K.; Laury, A.; Drevon, J.J.; Ghoulam, C. Genotypic variation of nodules’ enzymatic activities in symbiotic nitrogen fixation among common bean (Phaseolus vulgaris L.) genotypes grown under salinity constraint. Symbiosis 2013, 60, 115–122. [Google Scholar] [CrossRef]
- Latrach, L.; Farissi, M.; Mouradi, M.; Makoudi, B.; Bouizgaren, A.; Ghoulam, C. Growth and nodulation of alfalfa-rhizobia symbiosis under salinity: Electrolyte leakage, stomatal conductance, and chlorophyll fluorescence. Turk. J. Agric. For. 2014, 38, 320–326. [Google Scholar] [CrossRef]
- Javid, M.; Ford, R.; Norton, R.; Nicolas, M. Sodium and boron exclusion in two Brassica juncea cultivars exposed to the combined treatments of salinity and boron at moderate alkalinity. Biologia 2014, 69, 1157–1163. [Google Scholar] [CrossRef]
- Ghanem, A.E.; Mohamed, E.; Kasem, A.M.; El-Ghamery, A.A. Differential salt tolerance strategies in three halophytes from the same ecological habitat: Augmentation of antioxidant enzymes and compounds. Plants 2021, 10, 1100. [Google Scholar] [CrossRef]
- Li, H.; Chang, J.; Chen, H.; Wang, Z.; Gu, X.; Wei, C.; Zhang, Y.; Ma, J.; Yang, J.; Zhang, X. Exogenous melatonin confers salt stress tolerance to watermelon by improving photosynthesis and redox homeostasis. Front. Plant Sci. 2017, 8, 295. [Google Scholar] [CrossRef]
- Mahdieh, M.; Habibollahi, N.; Amirjani, M.R.; Abnosi, M.H.; Ghorbanpour, M. Exogenous silicon nutrition ameliorates salt-induced stress by improving growth and efficiency of PSII in Oryza sativa L. cultivars. J. Soil Sci. Plant Nutr. 2015, 15, 1050–1060. [Google Scholar] [CrossRef]
- Jiang, C.; Johkan, M.; Hohjo, M.; Tsukagoshi, S.; Maruo, T. A correlation analysis on chlorophyll content and SPAD value in tomato leaves. Hortic. Res. 2017, 71, 37–42. [Google Scholar]
- Mehta, P.; Jajoo, A.; Mathur, S.; Bharti, S. Chlorophyll a fluorescence study revealing effects of high salt stress on Photosystem II in wheat leaves. Plant Physiol. Biochem. 2010, 48, 16–20. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Bosa, K.; Kościelniak, J.; Żuk-Gołaszewska, K. Effects of salt stress on photosystem II efficiency and CO2 assimilation of two Syrian barley landraces. Environ. Exp. Bot. 2011, 73, 64–72. [Google Scholar] [CrossRef]
- Fatma, M.; Iqbal, N.; Sehar, Z.; Alyemeni, M.N.; Kaushik, P.; Khan, N.A.; Ahmad, P. Methyl jasmonate protects the ps ii system by maintaining the stability of chloroplast d1 protein and accelerating enzymatic antioxidants in heat-stressed wheat plants. Antioxidants 2021, 10, 1216. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Singh, V.P.; Prasad, S.M. Responses of photosynthesis, nitrogen and proline metabolism to salinity stress in Solanum lycopersicum under different levels of nitrogen supplementation. Plant Physiol. Biochem. 2016, 109, 72–83. [Google Scholar] [CrossRef]
- Nxele, X.; Klein, A.; Ndimba, B.K. Drought and salinity stress alters ROS accumulation, water retention, and osmolyte content in sorghum plants. S. Afr. J. Bot. 2017, 108, 261–266. [Google Scholar] [CrossRef]
- Aldesuquy, H.; Baka, Z.; Mickky, B. Kinetin and spermine mediated induction of salt tolerance in wheat plants: Leaf area, photosynthesis and chloroplast ultrastructure of flag leaf at ear emergence. Egypt. J. Basic Appl. Sci. 2014, 1, 77–87. [Google Scholar] [CrossRef]
- Wu, X.; Zhu, Z.; Li, X.; Zha, D. Effects of cytokinin on photosynthetic gas exchange, chlorophyll fluorescence parameters and antioxidative system in seedlings of eggplant (Solanum melongena L.) under salinity stress. Acta Physiol. Plant. 2012, 34, 2105–2114. [Google Scholar] [CrossRef]
- Hussain, S.; Bai, Z.; Huang, J.; Cao, X.; Zhu, L.; Zhu, C.; Zhang, J. 1-Methylcyclopropene modulates physiological, biochemical, and antioxidant responses of rice to different salt stress levels. Front. Plant Sci. 2019, 10, 124. [Google Scholar] [CrossRef] [PubMed]
- Khalid, A.; Athar, H.U.R.; Zafar, Z.U.; Akram, A.; Hussain, K.; Manzoor, H.; Al-Qurainy, F.; Ashraf, M. Photosynthetic capacity of canola (Brassica napus L.) plants as affected by glycinebetaine under salt stress. J. Appl. Bot. Food Qual. 2015, 88, 78–86. [Google Scholar]
- Anjum, N.A.; Sofo, A.; Scopa, A.; Roychoudhury, A.; Gill, S.S.; Iqbal, M.; Ahmad, I.; Lukatkin, A.S.; Pereira, E.; Durate, A.C.; et al. Lipids and proteins-major targets of oxidative modifications in abiotic stressed plants. Environ. Sci. Pollut. Res. 2015, 22, 4099–4121. [Google Scholar] [CrossRef]
- Tammam, A.A.; Fakhry, E.M.; El-Sheekh, M. Effect of salt stress on antioxidant system and the metabolism of the reactive oxygen species in Dunaliellasalina and Dunaliellatertiolecta. Afr. J. Biotechnol. 2011, 10, 3795–3808. [Google Scholar]
- AbdElgawad, H.; Zinta, G.; Hegab, M.M.; Pandey, R.; Asard, H.; Abuelsoud, W. High salinity induces different oxidative stress and antioxidant responses in maize seedlings organs. Front. Plant Sci. 2016, 7, 276. [Google Scholar] [CrossRef] [PubMed]
- Sofo, A.; Scopa, A.; Nuzzaci, M.; Vitti, A. Ascorbate peroxidase and catalase activities and their genetic regulation in plants subjected to drought and salinity stresses. Int. J. Mol. Sci. 2015, 16, 13561–13578. [Google Scholar] [CrossRef]
- Weisany, W.; Sohrabi, Y.; Heidari, G.; Siosemardeh, A.; Ghassemi-Golezani, K. Changes in antioxidant enzymes activity and plant performance by salinity stress and zinc application in soybean (‘Glycine max’ L.). Plant Omics 2012, 5, 60–67. [Google Scholar]
- Patel, A.D.; Jadeja, H.; Pandey, A.M. Effect of salinization of soil on growth, water status and nutrient accumulation in seedlings of Acacia auriculiformis (Fabaceae). J. Plant Nutr. 2010, 33, 914–932. [Google Scholar] [CrossRef]
- Parihar, P.; Singh, S.; Singh, R.; Singh, V.P.; Prasad, S.M. Effect of salinity stress on plants and its tolerance strategies, a review. Environ. Sci. Pollut. Res. 2015, 22, 4056–4075. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, M.; Shahzad, S.M.; Imtiaz, M.; Rizwan, M.S.; Arif, M.S.; Kausar, R. Nitrogen nutrition and adaptation of glycophytes to saline environment: A review. Arch. Agron. Soil Sci. 2018, 64, 1181–1206. [Google Scholar] [CrossRef]
- Rasheed, F.; Anjum, N.A.; Masood, A.; Sofo, A.; Khan, N.A. The key roles of salicylic acid and sulfur in plant salinity stress tolerance. J. Plant Growth Regul. 2020, 1–14. [Google Scholar] [CrossRef]
- Wu, H.; Zhang, X.; Giraldo, J.P.; Shabala, S. It is not all about sodium: Revealing tissue specificity and signalling roles of potassium in plant responses to salt stress. Plant Soil 2018, 431, 1–17. [Google Scholar] [CrossRef]
- Wu, H.; Zhu, M.; Shabala, L.; Zhou, M.; Shabala, S. K+ retention in leaf mesophyll, an overlooked component of salinity tolerance mechanism: A case study for barley. J. Integr. Plant Biol. 2015, 57, 171–185. [Google Scholar] [CrossRef]
- Tabassum, T.; Farooq, M.; Ahmad, R.; Zohaib, A.; Wahid, A. Seed priming and transgenerational drought memory improves tolerance against salt stress in bread wheat. Plant Physiol. Biochem. 2017, 118, 362–369. [Google Scholar] [CrossRef]
- Fahad, S.; Hussain, S.; Saud, S.; Hassan, S.; Tanveer, M.; Ihsan, M.Z.; Shah, A.N.; Ullah, A.; Khan, F.; Ullah, S.; et al. A combined application of biochar and phosphorus alleviates heat-induced adversities on physiological, agronomical and quality attributes of rice. Plant Physiol. Biochem. 2016, 103, 191–198. [Google Scholar] [CrossRef]
- Tang, H.; Niu, L.; Wei, J.; Chen, X.; Chen, Y. Phosphorus limitation improved salt tolerance in maize through tissue mass density increase, osmolytes accumulation, and Na+ uptake inhibition. Front. Plant Sci. 2019, 10, 856. [Google Scholar] [CrossRef]
- Zörb, C.; Geilfus, C.M.; Dietz, K.J. Salinity and crop yield. Plant Biol. 2019, 21, 31–38. [Google Scholar] [CrossRef]
- Wani, A.S.; Ahmad, A.; Hayat, S.; Tahir, I. Epibrassinolide and proline alleviate the photosynthetic and yield inhibition under salt stress by acting on antioxidant system in mustard. Plant Physiol. Biochem. 2019, 135, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Nahar, K.; Fujita, M. Plant response to salt stress and role of exogenous protectants to mitigate salt-induced damages. In Ecophysiology and Responses of Plants under Salt Stress; Ahmad, P., Azooz, M.M., Prasad, M.N.V., Eds.; Springer Science & Business Media: New York, NY, USA, 2012; pp. 25–87. [Google Scholar]
- Breseghello, F.; Coelho, A.S.G. Traditional and modern plant breeding methods with examples in rice (Oryza sativa L.). J. Agric. Food Chem. 2013, 61, 8277–8286. [Google Scholar] [CrossRef] [PubMed]
- Ashfaque, F.; Khan, M.I.R.; Khan, N.A. Exogenously applied H2O2 promotes proline accumulation, water relations, photosynthetic efficiency and growth of wheat (Triticumaestivum L.) under salt stress. Annu. Res. Rev. Biol. 2014, 4, 105–120. [Google Scholar] [CrossRef]
- Nazar, R.; Iqbal, N.; Syeed, S.; Khan, N.A. Salicylic acid alleviates decreases in photosynthesis under salt stress by enhancing nitrogen and sulfur assimilation and antioxidant metabolism differentially in two mungbean cultivars. J. Plant Physiol. 2011, 168, 807–815. [Google Scholar] [CrossRef] [PubMed]
- Astolfi, S.; Zuchi, S. Adequate S supply protects barley plants from adverse effects of salinity stress by increasing thiol contents. Acta Physiol. Plant. 2013, 35, 175–181. [Google Scholar] [CrossRef]
- The, C.Y.; Shaharuddin, N.A.; Ho, C.L.; Mahmood, M. Exogenous proline significantly affects the plant growth and nitrogen assimilation enzymes activities in rice (Oryza sativa) under salt stress. Acta Physiol. Plant. 2016, 38, 151. [Google Scholar]
- Fuertes-Mendizábal, T.; Bastías, E.I.; González-Murua, C.; González-Moro, M. Nitrogen assimilation in the highly salt and boron-tolerant ecotype Zea mays L. Amylacea. Plants 2020, 9, 322. [Google Scholar] [CrossRef] [PubMed]
- Goel, P.; Singh, A.K. Abiotic stresses downregulate key genes involved in nitrogen uptake and assimilation in Brassica juncea L. PLoS ONE 2015, 10, e0143645. [Google Scholar] [CrossRef]
- Ahanger, M.A.; Qin, C.; Begum, N.; Maodong, Q.; Dong, X.X.; El-Esawi, M.; El-Sheikh, M.A.; Alatar, A.A.; Zhang, L. Nitrogen availability prevents oxidative effects of salinity on wheat growth and photosynthesis by up-regulating the antioxidants and osmolytes metabolism.; and secondary metabolite accumulation. BMC Plant Biol. 2019, 19, 479. [Google Scholar] [CrossRef]
- Singh, A.; Hussain, I.; Singh, N.B.; Singh, H. Uptake, translocation and impact of green synthesized nanoceria on growth and antioxidant enzymes activity of Solanum lycopersicum L. Ecotoxicol. Environ. Saf. 2019, 182, 109410. [Google Scholar] [CrossRef] [PubMed]
- Coelho, D.G.; Miranda, R.D.S.; Paula-Marinho, S.D.O.; de Carvalho, H.H.; Prisco, J.T.; Gomes-Filho, E. Ammonium nutrition modulates K+ and N uptake, transport and accumulation during salt stress acclimation of sorghum plants. Arch. Agron. Soil Sci. 2020, 66, 1991–2004. [Google Scholar] [CrossRef]
- Soliman, M.; Elkelish, A.; Souad, T.; Alhaithloul, H.; Farooq, M. Brassinosteroid seed priming with nitrogen supplementation improves salt tolerance in soybean. Physiol. Mol. Biol. Plants 2020, 26, 501–511. [Google Scholar] [CrossRef] [PubMed]
- Per, T.S.; Khan, N.A.; Masood, A.; Fatma, M. Methyl jasmonate alleviates cadmium-induced photosynthetic damages through increased S-assimilation and glutathione production in mustard. Front. Plant Sci. 2016, 7, 1933. [Google Scholar] [CrossRef]
- Fatma, M.; Masood, A.; Per, T.S.; Rasheed, F.; Khan, N.A. Interplay between nitric oxide and sulfur assimilation in salt tolerance in plants. Crop J. 2016, 4, 153–161. [Google Scholar] [CrossRef]
- Fatma, M.; Asgher, M.; Masood, A.; Khan, N.A. Excess sulfur supplementation improves photosynthesis and growth in mustard under salt stress through increased production of glutathione. Environ. Exp. Bot. 2014, 107, 55–63. [Google Scholar] [CrossRef]
- Seth, C.S.; Remans, T.; Keunen, E.; Jozefczak, M.; Gielen, H.; Opdenakker, K.; Cuypers, A. Phytoextraction of toxic metals: A central role for glutathione. Plant Cell Environ. 2012, 35, 334–346. [Google Scholar] [CrossRef]
- Kumar, D.; Chattopadhyay, S. Glutathione modulates the expression of heat shock proteins via the transcription factors BZIP10 and MYB21 in Arabidopsis. J. Exp. Bot. 2018, 69, 3729–3743. [Google Scholar] [CrossRef]
- Iqbal, N.; Umar, S.; Khan, N.A.; Corpas, F.J. Nitric oxide and hydrogen sulfide coordinately reduce glucose sensitivity and decrease oxidative stress via ascorbate-glutathione cycle in heat-stressed wheat (Triticum aestivum L.) plants. Antioxidants 2021, 10, 108. [Google Scholar] [CrossRef]
- Mera, R.; Torres, E.; Abalde, J. Sulphate.; more than a nutrient; protects the microalga Chlamydomonas moewusii from cadmium toxicity. Aquat. Toxicol. 2014, 148, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Romero, T.C.; Nortes, T.P.A.; Pedrero, S.F.; Mounzer, O.; Alarcón, C.J.J.; Bayona, G.J.M.; Nicolás, N.E. Assessment of the viability of using saline reclaimed water in grapefruit in medium to long term. Span. J. Agric. Res. 2014, 12, 1137–1148. [Google Scholar] [CrossRef]
- Riffat, A.L.I.A.; Sajid, M.; Ahmad, A. Alleviation of adverse effects of salt stress on growth og maize Zea mays L. by sulfur supplementation. Pak. J. Bot. 2020, 523, 763–773. [Google Scholar]
- de Souza Freitas, W.E.; de Oliveira, A.B.; Mesquita, R.O.; de Carvalho, H.H.; Prisco, J.T.; Gomes-Filho, E. Sulfur-induced salinity tolerance in lettuce is due to a better P and K uptake.; lower Na/K ratio and an efficient antioxidative defense system. Sci. Hortic. 2019, 257, 108764. [Google Scholar] [CrossRef]
- Aziz, A.; Ashraf, M.; Sikandar, S.; Asif, M.; Akhtar, N.; Shahzad, S.M.; Babar, B.H. Optimizing sulfur for improving salt tolerance of sunflower Helianthus annuus L. Soil Environ. 2019, 38, 222–233. [Google Scholar] [CrossRef]
- Coleto, I.; de la Peña, M.; Rodríguez-Escalante, J.; Bejarano, I.; Glauser, G.; Aparicio-Tejo, P.M.; Marino, D. Leaves play a central role in the adaptation of nitrogen and sulfur metabolism to ammonium nutrition in oilseed rape Brassica napus. BMC Plant Biol. 2017, 17, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Jobe, T.O.; Zenzen, I.; Rahimzadeh, K.P.; Kopriva, S. Integration of sulfate assimilation with carbon and nitrogen metabolism in transition from C3 to C4 photosynthesis. J. Exp. Bot. 2019, 70, 4211–4221. [Google Scholar] [CrossRef]
- Prodhan, M.A.; Finnegan, P.M.; Lambers, H. How does evolution in phosphorus-impoverished landscapes impact plant nitrogen and sulfur assimilation? Trends Plant Sci. 2019, 24, 69–82. [Google Scholar] [CrossRef]
- Rais, L.; Masood, A.; Inam, A.; Khan, N. Sulfur and nitrogen co-ordinately improve photosynthetic efficiency, growth and proline accumulation in two cultivars of mustard under salt stress. J. Plant Biochem. Physiol. 2013, 1. [Google Scholar] [CrossRef]
- Zhang, N.N.; Zou, H.; Lin, X.Y.; Pan, Q.; Zhang, W.Q.; Zhang, J.H.; Chen, J. Hydrogen sulfide and rhizobia synergistically regulate nitrogen N assimilation and remobilization during N deficiency-induced senescence in soybean. Plant Cell Environ. 2020, 43, 1130–1147. [Google Scholar] [CrossRef] [PubMed]
- Lancaster, J.R., Jr. Nitric oxide: A brief overview of chemical and physical properties relevant to therapeutic applications. Future Sci. OA 2015, 1. [Google Scholar] [CrossRef]
- Nabi, R.B.S.; Tayade, R.; Hussain, A.; Kulkarni, K.P.; Imran, Q.M.; Mun, B.G.; Yun, B.W. Nitric oxide regulates plant responses to drought, salinity, and heavy metal stress. Environ. Exp. Bot. 2019, 161, 120–133. [Google Scholar] [CrossRef]
- Fancy, N.N.; Bahlmann, A.K.; Loake, G.J. Nitric oxide functions in plant abiotic stress. Plant Cell Environ. 2017, 40, 462–472. [Google Scholar] [CrossRef] [PubMed]
- Akram, N.A.; Iqbal, M.; Muhammad, A.; Ashraf, M.; Al-Qurainy, F.; Shafiq, S. Aminolevulinic acid and nitric oxide regulate oxidative defense and secondary metabolisms in canola (Brassica napus L.) under drought stress. Protoplasma 2018, 255, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, P.; Abass, A.M.; Nasser, A.M.; Wijaya, L.; Alam, P.; Ashraf, M. Mitigation of sodium chloride toxicity in Solanum lycopersicum L. by supplementation of jasmonic acid and nitric oxide. J. Plant Interact. 2018, 13, 64–72. [Google Scholar] [CrossRef]
- Tian, X.; He, M.; Wang, Z.; Zhang, J.; Song, Y.; He, Z.; Dong, Y. Application of nitric oxide and calcium nitrate enhances tolerance of wheat seedlings to salt stress. Plant Growth Regul. 2015, 77, 343–356. [Google Scholar] [CrossRef]
- Gaupels, F.; Kuruthukulangarakoola, G.T.; Durner, J. Upstream and downstream signals of nitric oxide in pathogen defence. Curr. Opin. Plant Biol. 2011, 14, 707–714. [Google Scholar] [CrossRef]
- Martínez-Ruiz, A.; Cadenas, S.; Lamas, S. Nitric oxide signaling: Classical, less classical, and nonclassical mechanisms. Free Radic. Biol. Med. 2011, 51, 17–29. [Google Scholar] [CrossRef]
- Corpas, F.J.; González-Gordo, S.; Cañas, A.; Palma, J.M. Nitric oxide and hydrogen sulfide in plants, which comes first? J. Exp. Bot. 2019, 70, 4391–4404. [Google Scholar] [CrossRef]
- Prochazkova, D.; Haisel, D.; Pavlikova, D. Nitric oxide biosynthesis in plants-the short overview. Plant Soil Environ. 2014, 60, 129–134. [Google Scholar] [CrossRef]
- Planchet, E.; Kaiser, W.M. Nitric oxide NO detection by DAF fluorescence and chemiluminescence: A comparison using abiotic and biotic NO sources. J. Exp. Bot. 2006, 57, 3043–3055. [Google Scholar] [CrossRef]
- Leterrier, M.; Airaki, M.; Palma, J.M.; Chaki, M.; Barroso, J.B.; Corpas, F.J. Arsenic triggers the nitric oxide (NO) and S-nitrosoglutathione (GSNO) metabolism in Arabidopsis. Environ. Pollut. 2012, 166, 136–143. [Google Scholar] [CrossRef]
- Sharma, A.; Kapoor, D.; Wang, J.; Landi, M.; Zheng, B.; Yan, D.; Yuan, H. Nitric oxide mediated mechanisms adopted by plants to cope with salinity. Biol. Plant. 2020, 64, 512–518. [Google Scholar] [CrossRef]
- Astier, J.; Gross, I.; Durner, J. Nitric oxide production in plants, an update. J. Exp. Bot. 2018, 69, 3401–3411. [Google Scholar] [CrossRef]
- Gupta, K.J.; Fernie, A.R.; Kaiser, W.M.; van Dongen, J.T. On the origins of nitric oxide. Trends Plant Sci. 2011, 16, 160–168. [Google Scholar] [CrossRef]
- Baudouin, E. The language of nitric oxide signalling. Plant Biol. 2011, 13, 233–242. [Google Scholar] [CrossRef]
- Wang, Y.; Lin, A.; Loake, G.J.; Chu, C. H2O2-induced leaf cell death and the crosstalk of reactive nitric/oxygen species F. J. Integr. Plant Biol. 2013, 55, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Wang, W.; Wang, B. Recent progress of salinity tolerance research in plants. Russ. J. Genet. 2012, 48, 497–505. [Google Scholar] [CrossRef]
- Vishwakarma, A.; Wany, A.; Pandey, S.; Bulle, M.; Kumari, A.; Kishorekumar, R.; Gupta, K.J. Current approaches to measure nitric oxide in plants. J. Exp. Bot. 2019, 70, 4333–4343. [Google Scholar] [CrossRef] [PubMed]
- Gould, K.S.; Lamotte, O.; Klinguer, A.; Pugin, A.; Wendehenne, D. Nitric oxide production in tobacco leaf cells, a generalized stress response? Plant Cell Environ. 2003, 26, 1851–1862. [Google Scholar] [CrossRef]
- Mur, L.A.; Prats, E.; Pierre, S.; Hall, M.A.; Hebelstrup, K.H. Integrating nitric oxide into salicylic acid and jasmonic acid/ethylene plant defense pathways. Front. Plant Sci. 2013, 4, 215. [Google Scholar] [CrossRef]
- Mata-Pérez, C.; Sánchez-Calvo, B.; Padilla, M.N.; Begara-Morales, J.C.; Valderrama, R.; Corpas, F.J.; Barroso, J.B. Nitro-fatty acids in plant signaling: New key mediators of nitric oxide metabolism. Redox Biol. 2017, 11, 554–561. [Google Scholar] [CrossRef] [PubMed]
- Jagodzik, P.; Tajdel-Zielinska, M.; Ciesla, A.; Marczak, M.; Ludwikow, A. Mitogen-activated protein kinase cascades in plant hormone signaling. Front. Plant Sci. 2018, 9, 1387. [Google Scholar] [CrossRef] [PubMed]
- Asai, S.; Ohta, K.; Yoshioka, H. MAPK signaling regulates nitric oxide and NADPH oxidase-dependent oxidative bursts in Ncotianabenthamiana. Plant Cell 2008, 20, 1390–11406. [Google Scholar] [CrossRef]
- Gadelha, C.G.; de Souza Miranda, R.; Alencar, N.L.M.; Costa, J.H.; Prisco, J.T.; Gomes-Filho, E. Exogenous nitric oxide improves salt tolerance during establishment of Jatropha curcas seedlings by ameliorating oxidative damage and toxic ion accumulation. J. Plant Physiol. 2017, 212, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Yang, R.; Pan, Y.; Ma, M.; Pan, J.; Zhao, Y.; Zhang, L. Nitric oxide contributes to minerals absorption.; proton pumps and hormone equilibrium under cadmium excess in Trifoliumrepens L. plants. Ecotoxicol. Environ. Saf. 2015, 119, 35–46. [Google Scholar] [CrossRef]
- Arora, D.; Bhatla, S.C. Melatonin and nitric oxide regulate sunflower seedling growth under salt stress accompanying differential expression of Cu/Zn SOD and Mn SOD. Free Radic. Biol. Med. 2017, 106, 315–328. [Google Scholar] [CrossRef]
- Babaei, S.; Niknam, V.; Behmanesh, M. Comparative effects of nitric oxide and salicylic acid on salinity tolerance in saffron Crocus sativus. Plant Biosyst. Int. J. Plant Biol. 2021, 155, 73–82. [Google Scholar] [CrossRef]
- Huang, J.; Wei, H.; Li, L.; Yu, S. Transcriptome analysis of nitric oxide-responsive genes in upland cotton Gossypiumhirsutum. PLoS ONE 2018, 13, e0192367. [Google Scholar]
- Maslennikova, D.R.; Allagulova, C.R.; Fedorova, K.A.; Plotnikov, A.A.; Avalbaev, A.M.; Shakirova, F.M. Cytokinins contribute to realization of nitric oxide growth-stimulating and protective effects on wheat plants. Russ. J. Plant Physiol. 2017, 64, 665–671. [Google Scholar] [CrossRef]
- León, J.; Castillo, M.C.; Coego, A.; Lozano-Juste, J.; Mir, R. Diverse functional interactions between nitric oxide and abscisic acid in plant development and responses to stress. J. Exp. Bot. 2014, 65, 907–921. [Google Scholar] [CrossRef]
- Neljubov, D.N. Pflanzen. Beih. Bot. Zentralbl. 1901, 10, 128–139. [Google Scholar]
- Hua, J. Isolation of components involved in ethylene signaling. In Ethylene in Plants, 1st ed.; Wen, C.K., Ed.; Springer: Dordrecht, The Netherlands, 2015; pp. 27–44. [Google Scholar]
- Abeles, F.B.; Morgan, P.W.; Saltveit, M.E., Jr. Ethylene in Plant Biology, 2nd ed.; Academic Press: San Diego, CA, USA, 2012. [Google Scholar]
- Abiri, R.; Shaharuddin, N.A.; Maziah, M.; Yusof, Z.N.B.; Atabaki, N.; Sahebi, M.; Hanafi, M.M. Role of ethylene and the APETALA 2/ethylene response factor superfamily in rice under various abiotic and biotic stress conditions. Environ. Exp. Bot. 2017, 134, 33–44. [Google Scholar] [CrossRef]
- Iqbal, N.; Nazar, R.; Syeed, S.; Masood, A.; Khan, N.A. Exogenously-sourced ethylene increases stomatal conductance, photosynthesis, and growth under optimal and deficient nitrogen fertilization in mustard. J. Exp. Bot. 2011, 62, 4955–4963. [Google Scholar] [CrossRef]
- García, M.J.; Romera, F.J.; Lucena, C.; Alcántara, E.; Pérez-Vicente, R. Ethylene and the regulation of physiological and morphological responses to nutrient deficiencies. Plant Physiol. 2015, 169, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liang, X.; Wan, Q.; Wang, X.; Bi, Y. Ethylene and nitric oxide are involved in maintaining ion homeostasis in Arabidopsis callus under salt stress. Planta 2009, 230, 293–307. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhang, S. Ethylene biosynthesis and regulation in plants. In Ethylene in Plants, 1st ed.; Wen, C.K., Ed.; Springer: Dordrecht, The Netherlands, 2015; pp. 1–25. [Google Scholar]
- Yang, S.F.; Hoffman, N.E. Ethylene biosynthesis and its regulation in higher plants. Annu. Rev. Plant Physiol. 1984, 35, 155–189. [Google Scholar] [CrossRef]
- Chang, C. Q&A: How do plants respond to ethylene and what is its importance? BMC Plant Biol. 2016, 14, 1–7. [Google Scholar]
- Lin, Z.; Zhong, S.; Grierson, D. Recent advances in ethylene research. J. Exp. Bot. 2009, 60, 3311–3336. [Google Scholar] [CrossRef] [PubMed]
- Tsuchisaka, A.; Theologis, A. Unique and overlapping expression patterns among the Arabidopsis 1-amino-cyclopropane-1-carboxylate synthase gene family members. Plant Physiol. 2004, 136, 2982–3000. [Google Scholar] [CrossRef] [PubMed]
- Lyzenga, W.J.; Booth, J.K.; Stone, S.L. The Arabidopsis RING-type E3 ligase XBAT32 mediates the proteasomal degradation of the ethylene biosynthetic enzyme.; 1-aminocyclopropane-1-carboxylate synthase 7. Plant J. 2012, 71, 23–34. [Google Scholar] [CrossRef]
- Ji, Y.; Guo, H. From endoplasmic reticulum (ER) to nucleus: EIN2 bridges the gap in ethylene signaling. Mol. Plant 2013, 6, 11–14. [Google Scholar] [CrossRef]
- Dolgikh, V.A.; Pukhovaya, E.M.; Zemlyanskaya, E.V. Shaping ethylene response: The role of EIN3/EIL1 transcription factors. Front. Plant Sci. 2019, 10, 1030. [Google Scholar] [CrossRef]
- Riyazuddin, R.; Verma, R.; Singh, K.; Nisha, N.; Keisham, M.; Bhati, K.K.; Gupta, R. Ethylene: A master regulator of salinity stress tolerance in plants. Biomolecules 2020, 10, 959. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.I.R.; Jahan, B.; AlAjmi, M.F.; Rehman, M.T.; Khan, N.A. Ethephon mitigates nickel stress by modulating antioxidant system.; glyoxalase system and proline metabolism in Indian mustard. Physiol. Mol. Biol. Plants 2020, 26, 1201–1213. [Google Scholar] [CrossRef]
- Cui, M.; Lin, Y.; Zu, Y.; Efferth, T.; Li, D.; Tang, Z. Ethylene increases accumulation of compatible solutes and decreases oxidative stress to improve plant tolerance to water stress in Arabidopsis. J. Plant Biol. 2015, 58, 193–201. [Google Scholar] [CrossRef]
- Iqbal, N.; Khan, N.A.; Ferrante, A.; Trivellini, A.; Francini, A.; Khan, M.I.R. Ethylene role in plant growth, development and senescence, interaction with other phytohormones. Front. Plant Sci. 2017, 8, 475. [Google Scholar] [CrossRef]
- Al Murad, M.; Razi, K.; Benjamin, L.K.; Lee, J.H.; Kim, T.H.; Muneer, S. Ethylene regulates sulfur acquisition by regulating the expression of sulfate transporter genes in oilseed rape. Physiol. Plant. 2020, 171, 533–545. [Google Scholar] [CrossRef] [PubMed]
- Shakar, M.; Yaseen, M.; Mahmood, R.; Ahmad, I. Calcium carbide induced ethylene modulate biochemical profile of Cucumis sativus at seed germination stage to alleviate salt stress. Sci. Hortic. 2016, 213, 179–185. [Google Scholar] [CrossRef]
- Zhang, X.; Shi, Z.; Tian, Y.; Zhou, Q.; Cai, J.; Dai, T.; Jiang, D. Salt stress increases content and size of glutenin macropolymers in wheat grain. Food Chem. 2016, 197, 516–521. [Google Scholar] [CrossRef]
- Liu, C.; Li, J.; Zhu, P.; Yu, J.; Hou, J.; Wang, C.; Zhao, A. Mulberry EIL3 confers salt and drought tolerances and modulates ethylene biosynthetic gene expression. Peer J. 2019, 7, e6391. [Google Scholar] [CrossRef]
- Shi, S.; Li, S.; Asim, M.; Mao, J.; Xu, D.; Ullah, Z.; Liu, G.; Wang, Q.; Liu, H. The Arabidopsis calcium-dependent protein kinases CDPKs and their roles in Plant growth regulation and abiotic stress responses. Int. J. Mol. Sci. 2018, 19, 1900. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Li, Z.; Wen, X.; Li, W.; Shi, H.; Yang, L.; Zhu, H.; Guo, H. Salt-induced stabilization of EIN3/EIL1 confers salinity tolerance by deterring ROS accumulation in Arabidopsis. PLoS Gen. 2014, 10, e1004664. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Xiang, G.; Sun, Q.; Ni, Y.; Jin, Z.; Gao, S.; Yao, Y. Melatonin enhances salt tolerance by promoting MYB108A-mediated ethylene biosynthesis in grapevines. Hortic. Res. 2019, 6, 1–14. [Google Scholar] [CrossRef]
- Husain, T.; Fatima, A.; Suhel, M.; Singh, S.; Sharma, A.; Prasad, S.M.; Singh, V.P. A brief appraisal of ethylene signaling under abiotic stress in plants. Plant Signal. Behav. 2020, 15, 1782051. [Google Scholar] [CrossRef]
- Fahad, S.; Hussain, S.; Matloob, A.; Khan, F.A.; Khaliq, A.; Saud, S.; Hassan, S.; Shan, D.; Khan, F.; Ullah, N.; et al. Phytohormones and plant responses to salinity stress: A review. Plant Growth Regul. 2015, 75, 391–404. [Google Scholar] [CrossRef]
- Xu, H.; Liu, Q.; Yao, T.; Fu, X. Shedding light on integrative GA signaling. Curr. Opin. Plant Biol. 2014, 21, 89–95. [Google Scholar] [CrossRef]
- Yang, R.; Hong, Y.; Ren, Z.; Tang, K.; Zhang, H.; Zhu, J.K.; Zhao, C. A role for PICKLE in the regulation of cold and salt stress tolerance in Arabidopsis. Front. Plant Sci. 2019, 10, 900. [Google Scholar] [CrossRef]
- Li, N.; Han, X.; Feng, D.; Yuan, D.; Huang, L.J. Signaling crosstalk between salicylic acid and ethylene/jasmonate in plant defense: Do we understand what they are whispering? Int. J. Mol. Sci. 2019, 20, 671. [Google Scholar] [CrossRef]
- Khan, M.I.R.; Asgher, M.; Khan, N.A. Alleviation of salt-induced photosynthesis and growth inhibition by salicylic acid involves glycinebetaine and ethylene in mungbean (Vigna radiata L.). Plant Physiol. Biochem. 2014, 80, 67–74. [Google Scholar] [CrossRef]
- Nazar, R.; Umar, S.; Khan, N.A.; Sareer, O. Salicylic acid supplementation improves photosynthesis and growth in mustard through changes in proline accumulation and ethylene formation under drought stress. S. Afr. J. Bot. 2015, 98, 84–94. [Google Scholar] [CrossRef]
- Zhu, T.; Deng, X.; Zhou, X.; Zhu, L.; Zou, L.; Li, P.; Zhang, D.; Lin, H. Ethylene and hydrogen peroxide are involved in brassinosteroid-induced salt tolerance in tomato. Sci. Rep. 2016, 6, 35392. [Google Scholar] [CrossRef]
- Buet, A.; Galatro, A.; Ramos-Artuso, F.; Simontacchi, M. Nitric oxide and plant mineral nutrition, current knowledge. J. Exp. Bot. 2019, 70, 4461–4476. [Google Scholar] [CrossRef] [PubMed]
- Santolini, J.; André, F.; Jeandroz, S.; Wendehenne, D. Nitric oxide synthase in plants: Where do we stand? Nitric Oxide 2017, 63, 30–38. [Google Scholar] [CrossRef]
- Chamizo-Ampudia, A.; Sanz-Luque, E.; Llamas, A.; Galvan, A.; Fernandez, E. Nitrate reductase regulates plant nitricoxide homeostasis. Trends Plant Sci. 2017, 22, 163–174. [Google Scholar] [CrossRef]
- Dong, F.; Simon, J.; Rienks, M.; Lindermayr, C.; Rennenberg, H. Effects of rhizopheric nitric oxide NO on N uptake in Fagus sylvatica seedlings depend on soil CO2 concentration.; soil N availability and N source. Tree Physiol. 2015, 35, 910–920. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Zheng, N. Molecular mechanism underlying the plant NRT1. 1 dual-affinity nitrate transporter. Front. Physiol. 2015, 18, 6–386. [Google Scholar]
- Wang, J.; Yu, S.X.; Zhang, M.; Cui, X.M. Exogenous nitric oxide-mediated GSH-PC synthesis pathway in tomato under copper stress. Russ. J. Plant Physiol. 2015, 62, 349–359. [Google Scholar] [CrossRef]
- Masood, A.; Iqbal, N.; Khan, M.I.R.; Khan, N.A. The coordinated role of ethylene and glucose in sulfur-mediated protection of photosynthetic inhibition by cadmium. Plant Signal Behav. 2012, 7, 1420–1422. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Saithong, T.; Saerue, S.; Kalapanulak, S.; Sojikul, P.; Narangajavana, J.; Bhumiratana, S. Gene co-expression analysis inferring the crosstalk of ethylene and gibberellin in modulating the transcriptional acclimation of cassava root growth in different seasons. PLoS ONE 2015, 10, e0137602. [Google Scholar]
- Wawrzyńska, A.; Sirko, A. To control and to be controlled: Understanding the Arabidopsis SLIM1 function in sulfur deficiency through comprehensive investigation of the EIL protein family. Front. Plant Sci. 2014, 5, 575. [Google Scholar] [PubMed]


| Soil Types | ECe (dS/m) | ESP | SAR | pH |
|---|---|---|---|---|
| Non-saline soil | <4 | <15 | <15 | 4.5–75 |
| Saline soil | >4 | <15 | <15 | <8.5 |
| Sodic soil | <4 | >15 | >15 | >8.5 |
| Saline-sodic soil | >4 | >15 | >15 | >8.5 |
| Regions | Total Area | Sodic Soils | Percentage | Saline Soils | Percentage |
|---|---|---|---|---|---|
| Africa | 1899.1 | 33.5 | 1.8 | 38.7 | 2.0 |
| Asia-Pacific and Australia | 3107.2 | 248.6 | 8.0 | 195.1 | 6.3 |
| Europe | 2010.8 | 72.7 | 3.6 | 6.7 | 0.3 |
| Latin America | 2038.6 | 50.9 | 2.5 | 60.5 | 3.0 |
| Near East | 1801.9 | 14.1 | 0.8 | 91.5 | 5.1 |
| North America | 1923.7 | 14.5 | 0.8 | 4.6 | 0.2 |
| World’s Total | 12,781.3 | 434.3 | 3.4% | 397.1 | 3.1% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jahan, B.; Rasheed, F.; Sehar, Z.; Fatma, M.; Iqbal, N.; Masood, A.; Anjum, N.A.; Khan, N.A. Coordinated Role of Nitric Oxide, Ethylene, Nitrogen, and Sulfur in Plant Salt Stress Tolerance. Stresses 2021, 1, 181-199. https://doi.org/10.3390/stresses1030014
Jahan B, Rasheed F, Sehar Z, Fatma M, Iqbal N, Masood A, Anjum NA, Khan NA. Coordinated Role of Nitric Oxide, Ethylene, Nitrogen, and Sulfur in Plant Salt Stress Tolerance. Stresses. 2021; 1(3):181-199. https://doi.org/10.3390/stresses1030014
Chicago/Turabian StyleJahan, Badar, Faisal Rasheed, Zebus Sehar, Mehar Fatma, Noushina Iqbal, Asim Masood, Naser A. Anjum, and Nafees A. Khan. 2021. "Coordinated Role of Nitric Oxide, Ethylene, Nitrogen, and Sulfur in Plant Salt Stress Tolerance" Stresses 1, no. 3: 181-199. https://doi.org/10.3390/stresses1030014
APA StyleJahan, B., Rasheed, F., Sehar, Z., Fatma, M., Iqbal, N., Masood, A., Anjum, N. A., & Khan, N. A. (2021). Coordinated Role of Nitric Oxide, Ethylene, Nitrogen, and Sulfur in Plant Salt Stress Tolerance. Stresses, 1(3), 181-199. https://doi.org/10.3390/stresses1030014












