Morphological and Physiological Response of Different Lettuce Genotypes to Salt Stress
Abstract
:1. Introduction
2. Results
2.1. Morphological Traits
2.1.1. Leaf Number
2.1.2. Fresh Mass (FM)
2.1.3. Dry Mass (DM)
2.2. Physiological Traits
2.2.1. Chlorophyll Index
2.2.2. Flavonoid Index
2.2.3. Nitrogen Balance Index
2.2.4. Anthocyanin Index
2.2.5. Salt Tolerance Coefficient Index
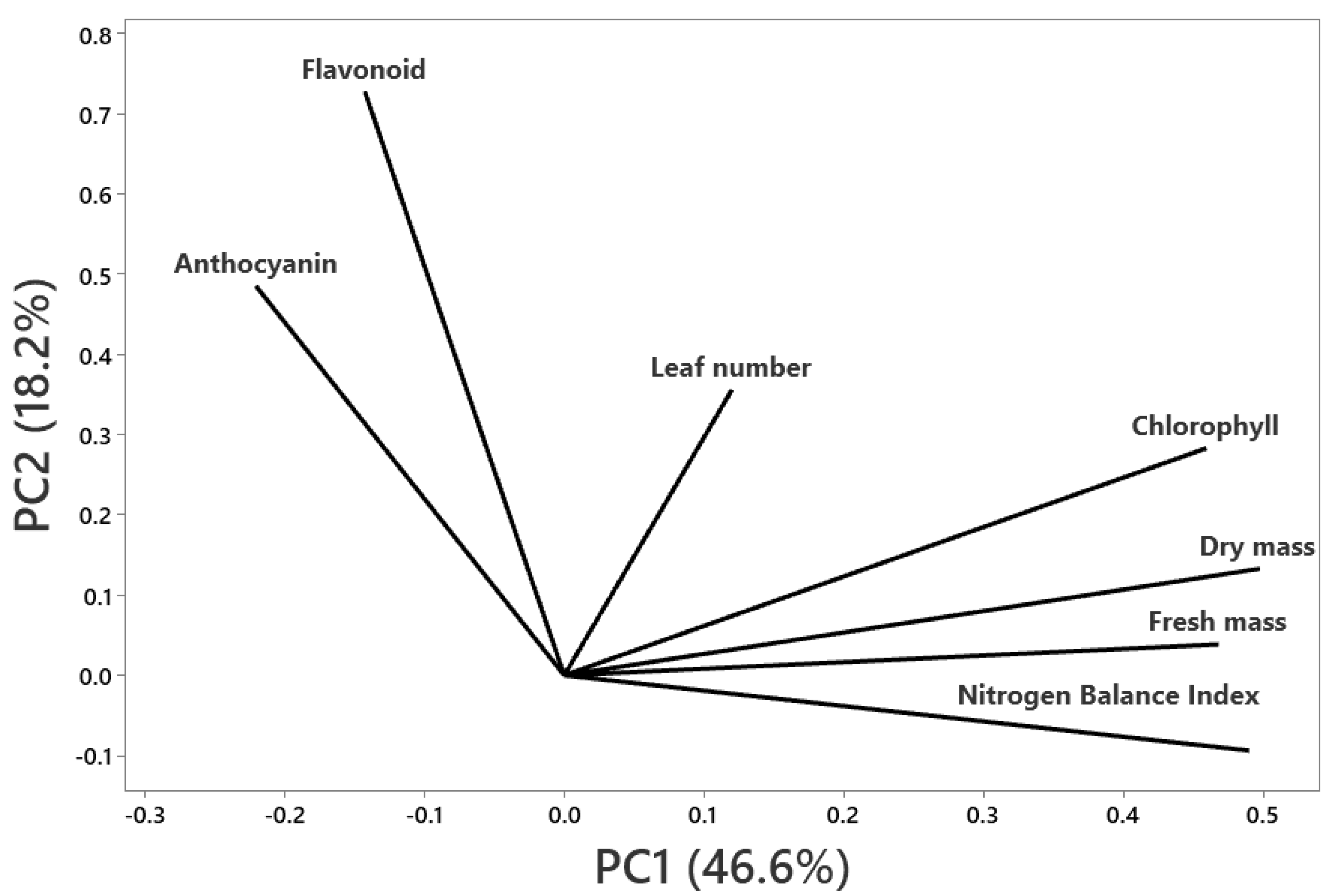
2.3. Principal Component Analysis
3. Discussion
3.1. Response of Morphological Traits under Salt Stress
3.2. Response of Physiological Traits under Salt Stress
4. Materials and Methods
4.1. Growth Condition and Plant Materials
4.2. Salt Treatment
4.3. Morphological Traits Measurement
4.4. Physiological Traits Measurement
4.5. Salt Tolerance Evaluation
5. Data Analysis
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Buhaug, H.; von Uexkull, N. Vicious circles: Violence, vulnerability, and climate change. Annu. Rev. Environ. Resour. 2021, 46, 545–568. [Google Scholar] [CrossRef]
- Xu, C.; Mou, B. Evaluation of lettuce genotypes for salinity tolerance. HortScience 2015, 50, 1441–1446. [Google Scholar] [CrossRef] [Green Version]
- Rouphael, Y.; Petropoulos, S.A.; Cardarelli, M.; Colla, G. Salinity as eustressor for enhancing quality of vegetables. Sci. Hortic. 2018, 234, 361–369. [Google Scholar] [CrossRef]
- Zhu, J. Plant salt stress. eLS 2007. [Google Scholar] [CrossRef]
- Machado, R.M.A.; Serralheiro, R.P. Soil salinity: Effect on vegetable crop growth. Management practices to prevent and mitigate soil salinization. Horticulturae 2017, 3, 30. [Google Scholar]
- Abogadallah, G.M. Insights into the significance of antioxidative defense under salt stress. Plant Signal. Behav. 2010, 5, 369–374. [Google Scholar] [CrossRef]
- Grattan, S.R.; Grieve, C.M. Salinity–mineral nutrient relations in horticultural crops. Sci. Hortic. 1998, 78, 127–157. [Google Scholar] [CrossRef]
- Shrivastava, P.; Kumar, R. Soil salinity: A serious environmental issue and plant growth promoting bacteria as one of the tools for its alleviation. Saudi J. Biol. Sci. 2015, 22, 123–131. [Google Scholar] [CrossRef] [Green Version]
- Pimentel, D.; Berger, B.; Filiberto, D.; Newton, M.; Wolfe, B.; Karabinakis, E.; Clark, S.; Poon, E.; Abbett, E.; Nandagopal, S. Water resources: Agricultural and environmental issues. Bioscience 2004, 54, 909–918. [Google Scholar] [CrossRef] [Green Version]
- Isayenkov, S.V. Physiological and molecular aspects of salt stress in plants. Cytol. Genet. 2012, 46, 302–318. [Google Scholar] [CrossRef] [Green Version]
- Orcutt, D.M.; Nilsen, E.T. Physiology of Plants under Stress: Soil and Biotic Factors; John Wiley & Sons: Hoboken, NJ, USA, 2000; Volume 2, ISBN 0471170089. [Google Scholar]
- Maas, E.V. Plant growth response to salt stress. In Towards the Rational Use of High Salinity Tolerant Plants; Springer: Berlin/Heidelberg, Germany, 1993; pp. 279–291. [Google Scholar]
- De Pascale, S.; Barbieri, G. Effects of soil salinity from long-term irrigation with saline-sodic water on yield and quality of winter vegetable crops. Sci. Hortic. 1995, 64, 145–157. [Google Scholar] [CrossRef]
- Kim, M.J.; Moon, Y.; Tou, J.C.; Mou, B.; Waterland, N.L. Nutritional value, bioactive compounds and health benefits of lettuce (Lactuca sativa L.). J. Food Compos. Anal. 2016, 49, 19–34. [Google Scholar] [CrossRef]
- Mou, B. Genetic variation of beta-carotene and lutein contents in lettuce. J. Am. Soc. Hortic. Sci. 2005, 130, 870–876. [Google Scholar] [CrossRef] [Green Version]
- Humphries, J.M.; Khachik, F. Distribution of lutein, zeaxanthin, and related geometrical isomers in fruit, vegetables, wheat, and pasta products. J. Agric. Food Chem. 2003, 51, 1322–1327. [Google Scholar] [CrossRef]
- Nicolle, C.; Cardinault, N.; Gueux, E.; Jaffrelo, L.; Rock, E.; Mazur, A.; Amouroux, P.; Rémésy, C. Health effect of vegetable-based diet: Lettuce consumption improves cholesterol metabolism and antioxidant status in the rat. Clin. Nutr. 2004, 23, 605–614. [Google Scholar] [CrossRef]
- Pérez-López, U.; Sgherri, C.; Miranda-Apodaca, J.; Micaelli, F.; Lacuesta, M.; Mena-Petite, A.; Quartacci, M.F.; Muñoz-Rueda, A. Concentration of phenolic compounds is increased in lettuce grown under high light intensity and elevated CO2. Plant Physiol. Biochem. 2018, 123, 233–241. [Google Scholar] [CrossRef]
- Mohammadi, P.; Khoshgoftarmanesh, A.H. The effectiveness of synthetic zinc (Zn)-amino chelates in supplying Zn and alleviating salt-induced damages on hydroponically grown lettuce. Sci. Hortic. 2014, 172, 117–123. [Google Scholar] [CrossRef]
- Kim, H.-J.; Fonseca, J.M.; Choi, J.-H.; Kubota, C.; Kwon, D.Y. Salt in irrigation water affects the nutritional and visual properties of romaine lettuce (Lactuca sativa L.). J. Agric. Food Chem. 2008, 56, 3772–3776. [Google Scholar] [CrossRef]
- Flowers, T.J. Improving crop salt tolerance. J. Exp. Bot. 2004, 55, 307–319. [Google Scholar] [CrossRef]
- Sanders, D. Lettuce|NC State Extension Publications. Available online: https://content.ces.ncsu.edu/lettuce# (accessed on 14 July 2021).
- Parida, A.K.; Das, A.B. Salt tolerance and salinity effects on plants: A review. Ecotoxicol. Environ. Saf. 2005, 60, 324–349. [Google Scholar] [CrossRef]
- Wijewardana, C.; Hock, M.; Henry, B.; Reddy, K.R. Screening corn hybrids for cold tolerance using morphological traits for early—Season seeding. Crop Sci. 2015, 55, 851–867. [Google Scholar] [CrossRef] [Green Version]
- Orlovsky, N.; Japakova, U.; Zhang, H.; Volis, S. Effect of salinity on seed germination, growth and ion content in dimorphic seeds of Salicornia europaea L.(Chenopodiaceae). Plant Divers. 2016, 38, 183–189. [Google Scholar] [CrossRef] [Green Version]
- Li, Y. Effect of salt stress on seed germination and seedling growth of three salinity plants. Pak. J. Biol. Sci. 2008, 11, 1268–1272. [Google Scholar] [CrossRef] [Green Version]
- Panuccio, M.R.; Jacobsen, S.E.; Akhtar, S.S.; Muscolo, A. Effect of saline water on seed germination and early seedling growth of the halophyte quinoa. AoB Plants 2014, 6, plu047. [Google Scholar] [CrossRef]
- Lazof, D.B.; Bernstein, N. Effects of salinization on nutrient transport to lettuce leaves: Consideration of leaf developmental stage. New Phytol. 1999, 144, 85–94. [Google Scholar] [CrossRef]
- Ekinci, M.; Yildirim, E.; Dursun, A.; Turan, M. Mitigation of salt stress in lettuce (Lactuca sativa L. var. Crispa) by seed and foliar 24-epibrassinolide treatments. HortScience 2012, 47, 631–636. [Google Scholar] [CrossRef]
- Maksimovic, I.; Ilin, Z. Effects of salinity on vegetable growth and nutrients uptake. Irrig. Syst. Pract. Chall. Environ. 2012, 9. [Google Scholar] [CrossRef] [Green Version]
- Ahanger, M.A.; Tomar, N.S.; Tittal, M.; Argal, S.; Agarwal, R.M. Plant growth under water/salt stress: ROS production; antioxidants and significance of added potassium under such conditions. Physiol. Mol. Biol. Plants 2017, 23, 731–744. [Google Scholar] [CrossRef]
- Bilgin, O.; Baser, I.; Korkut, K.Z.; Balkan, A.; Saglam, N. The impacts on seedling root growth of water and salinity stress in maize (Zea mays indentata Sturt.). Bulg. J. Agric. Sci. 2008, 14, 313–320. [Google Scholar]
- Al-Maskri, A.; Al-Kharusi, L.; Al-Miqbali, H.; Khan, M.M. Effects of salinity stress on growth of lettuce (Lactuca sativa) under closed-recycle nutrient film technique. Int. J. Agric. Biol. 2010, 12, 377–380. [Google Scholar]
- Garrido, Y.; Tudela, J.A.; Marín, A.; Mestre, T.; Martínez, V.; Gil, M.I. Physiological, phytochemical and structural changes of multi-leaf lettuce caused by salt stress. J. Sci. Food Agric. 2014, 94, 1592–1599. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.K.; Bhandari, S.R.; Jo, J.S.; Song, J.W.; Cho, M.C.; Yang, E.Y.; Lee, J.G. Response to salt stress in lettuce: Changes in chlorophyll fluorescence parameters, phytochemical contents, and antioxidant activities. Agronomy 2020, 10, 1627. [Google Scholar] [CrossRef]
- Qados, A.M.S.A. Effect of salt stress on plant growth and metabolism of bean plant Vicia faba (L.). J. Saudi Soc. Agric. Sci. 2011, 10, 7–15. [Google Scholar]
- Awang, Y.B.; Atherton, J.G.; Taylor, A.J. Salinity effects on strawberry plants grown in rockwool. I. Growth and leaf water relations. J. Hortic. Sci. 1993, 68, 783–790. [Google Scholar]
- Moncada, A.; Vetrano, F.; Miceli, A. Alleviation of Salt Stress by Plant Growth-Promoting Bacteria in Hydroponic Leaf Lettuce. Agronomy 2020, 10, 1523. [Google Scholar] [CrossRef]
- Shannon, M.C.; McCreight, J.D. Salt tolerance of lettuce introductions. HortScience 1984, 19, 673–675. [Google Scholar]
- Adhikari, N.D.; Simko, I.; Mou, B. Phenomic and physiological analysis of salinity effects on lettuce. Sensors 2019, 19, 4814. [Google Scholar] [CrossRef] [Green Version]
- Agüero, M.V.; Ponce, A.G.; Moreira, M.R.; Roura, S.I. Lettuce quality loss under conditions that favor the wilting phenomenon. Postharvest Biol. Technol. 2011, 59, 124–131. [Google Scholar] [CrossRef]
- Ashraf, M.; Harris, P.J.C. Photosynthesis under stressful environments: An overview. Photosynthetica 2013, 51, 163–190. [Google Scholar] [CrossRef]
- Salama, S.; Trivedi, S.; Busheva, M.; Arafa, A.A.; Garab, G.; Erdei, L. Effects of NaCl salinity on growth, cation accumulation, chloroplast structure and function in wheat cultivars differing in salt tolerance. J. Plant Physiol. 1994, 144, 241–247. [Google Scholar] [CrossRef]
- Silveira, J.A.G.; Carvalho, F.E.L. Proteomics, photosynthesis and salt resistance in crops: An integrative view. J. Proteom. 2016, 143, 24–35. [Google Scholar] [CrossRef]
- Taffouo, V.D.; Nouck, A.H.; Dibong, S.D.; Amougou, A. Effects of salinity stress on seedlings growth, mineral nutrients and total chlorophyll of some tomato (Lycopersicum esculentum L.) cultivars. Afr. J. Biotechnol. 2010, 9, 33. [Google Scholar]
- Ashraf, M. The effect of NaCl on water relations, chlorophyll, and protein and proline contents of two cultivars of blackgram (Vigna mungo L.). Plant Soil 1989, 119, 205–210. [Google Scholar] [CrossRef]
- Tuna, A.L.; Kaya, C.; Ashraf, M.; Altunlu, H.; Yokas, I.; Yagmur, B. The effects of calcium sulphate on growth, membrane stability and nutrient uptake of tomato plants grown under salt stress. Environ. Exp. Bot. 2007, 59, 173–178. [Google Scholar] [CrossRef]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef] [Green Version]
- Bartha, C.; Fodorpataki, L.; del Carmen Martinez-Ballesta, M.; Popescu, O.; Carvajal, M. Sodium accumulation contributes to salt stress tolerance in lettuce cultivars. J. Appl. Bot. Food Qual. 2015, 88, 42–48. [Google Scholar]
- Bettaieb, I.; Hamrouni-Sellami, I.; Bourgou, S.; Limam, F.; Marzouk, B. Drought effects on polyphenol composition and antioxidant activities in aerial parts of Salvia officinalis L. Acta Physiol. Plant. 2011, 33, 1103–1111. [Google Scholar] [CrossRef]
- Nogués, S.; Baker, N.R. Effects of drought on photosynthesis in Mediterranean plants grown under enhanced UV—B radiation. J. Exp. Bot. 2000, 51, 1309–1317. [Google Scholar] [CrossRef] [Green Version]
- Taffouo, V.D.; Nouck, A.E.; Nyemene, K.P.J.; Tonfack, B.; Meguekam, T.L.; Youmbi, E. Effects of salt stress on plant growth, nutrient partitioning, chlorophyll content, leaf relative water content, accumulation of osmolytes and antioxidant compounds in pepper (Capsicum annuum L.) cultivars. Not. Bot. Horti Agrobot. Cluj-Napoca 2017, 45, 481–490. [Google Scholar]
- Padilla, F.M.; Peña-Fleitas, M.T.; Gallardo, M.; Thompson, R.B. Evaluation of optical sensor measurements of canopy reflectance and of leaf flavonols and chlorophyll contents to assess crop nitrogen status of muskmelon. Eur. J. Agron. 2014, 58, 39–52. [Google Scholar] [CrossRef]
- Liu, W.; Zhu, D.-W.; Liu, D.-H.; Geng, M.-J.; Zhou, W.-B.; Mi, W.-J.; Yang, T.-W.; Hamilton, D. Influence od Nitrogen on the Primary and Secondary Metabolism and Synthesis of Flavonoids in Chrysanthemum Morifolium Ramat. J. Plant Nutr. 2010, 33, 240–254. [Google Scholar] [CrossRef]
- Katerji, N.; Mastrorilli, M.; Van Hoorn, J.W.; Lahmer, F.Z.; Hamdy, A.; Oweis, T. Durum wheat and barley productivity in saline–drought environments. Eur. J. Agron. 2009, 31, 1–9. [Google Scholar] [CrossRef]
- Cartelat, A.; Cerovic, Z.G.; Goulas, Y.; Meyer, S.; Lelarge, C.; Prioul, J.-L.; Barbottin, A.; Jeuffroy, M.-H.; Gate, P.; Agati, G. Optically assessed contents of leaf polyphenolics and chlorophyll as indicators of nitrogen deficiency in wheat (Triticum aestivum L.). Field Crop. Res. 2005, 91, 35–49. [Google Scholar] [CrossRef]
- Tremblay, N.; Wang, Z.; Cerovic, Z.G. Sensing crop nitrogen status with fluorescence indicators. A review. Agron. Sustain. Dev. 2012, 32, 451–464. [Google Scholar] [CrossRef] [Green Version]
- Viršilė, A.; Brazaitytė, A.; Vaštakaitė-Kairienė, V.; Miliauskienė, J.; Jankauskienė, J.; Novičkovas, A.; Samuolienė, G. Lighting intensity and photoperiod serves tailoring nitrate assimilation indices in red and green baby leaf lettuce. J. Sci. Food Agric. 2019, 99, 6608–6619. [Google Scholar] [CrossRef]
- Sarabi, B.; Fresneau, C.; Ghaderi, N.; Bolandnazar, S.; Streb, P.; Badeck, F.-W.; Citerne, S.; Tangama, M.; David, A.; Ghashghaie, J. Stomatal and non-stomatal limitations are responsible in down-regulation of photosynthesis in melon plants grown under the saline condition: Application of carbon isotope discrimination as a reliable proxy. Plant Physiol. Biochem. 2019, 141, 1–19. [Google Scholar] [CrossRef]
- Deuner, C.; Meneghello, G.E.; Castellanos, C.I.S.; Borges, C.T.; Deuner, S. Biochemical responses in lettuce subjected to salinity and the application of purple lettuce extract. Sci. Plena 2020, 16. [Google Scholar] [CrossRef]
- Veitch, N.C.; Grayer, R.J. Flavonoids and their glycosides, including anthocyanins. Nat. Prod. Rep. 2011, 28, 1626–1695. [Google Scholar] [CrossRef]
- Llorach, R.; Martínez-Sánchez, A.; Tomás-Barberán, F.A.; Gil, M.I.; Ferreres, F. Characterisation of polyphenols and antioxidant properties of five lettuce varieties and escarole. Food Chem. 2008, 108, 1028–1038. [Google Scholar] [CrossRef]
- Kennedy, B.; De Filippis, L.F. Physiological and oxidative response to NaCl of the salt tolerant Grevillea ilicifolia and the salt sensitive Grevillea arenaria. J. Plant Physiol. 1999, 155, 746–754. [Google Scholar] [CrossRef]
- Pérez-López, U.; Miranda-Apodaca, J.; Lacuesta, M.; Mena-Petite, A.; Muñoz-Rueda, A. Growth and nutritional quality improvement in two differently pigmented lettuce cultivars grown under elevated CO2 and/or salinity. Sci. Hortic. 2015, 195, 56–66. [Google Scholar] [CrossRef]
- Barickman, T.C.; Adhikari, B.; Sehgal, A.; Walne, C.H.; Reddy, K.R.; Gao, W. Drought and Elevated Carbon Dioxide Impact the Morphophysiological Profile of Basil (Ocimum basilicum L.). Crops 2021, 1, 118–128. [Google Scholar] [CrossRef]

| Type | Variety | LN 1 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Control | Salt | % 3 | ||||||||
| Butterhead | Burpee Bibb | 12.2 | ± | 1.4 | a 2 | 10.9 | ± | 1.6 | a | −10.7 |
| Buttercrunch-1 | 12.7 | ± | 1.8 | a | 12.4 | ± | 3.4 | a | −2.4 | |
| Buttercrunch-2 | 12.9 | ± | 3.6 | a | 12.9 | ± | 2.1 | a | 0.0 | |
| Hybrid Bibb | 15.5 | ± | 2 | a | 12.3 | ± | 0.3 | a | −20.6 | |
| PI 273606 | 11.9 | ± | 1 | a | 11.3 | ± | 2.3 | a | −5.0 | |
| PI 342448 | 10.8 | ± | 1 | ab | 11.6 | ± | 1.7 | a | 7.4 | |
| PI 342515 | 14.7 | ± | 0.9 | a | 12.6 | ± | 0.8 | a | −14.3 | |
| PI 358020 | 12.7 | ± | 1.5 | a | 12.2 | ± | 1.8 | a | −3.9 | |
| PI 372908 | 12.8 | ± | 2 | a | 11.8 | ± | 2 | a | −7.8 | |
| PI 615052 | 11.8 | ± | 2 | a | 11.5 | ± | 1.6 | a | −2.5 | |
| PI 634671 | 12.1 | ± | 1.3 | a | 10.8 | ± | 2.3 | ab | −10.7 | |
| Crisphead | Crispino | 8.4 | ± | 1.4 | hijk | 8.2 | ± | 2.7 | jk | −2.4 |
| Iceberg | 10.3 | ± | 0.2 | abc | 10 | ± | 1.9 | abcde | −2.9 | |
| Parris Island | 9.9 | ± | 0.9 | abcde | 10.1 | ± | 2.2 | abcd | 2.02 | |
| PI 177423 | 10.2 | ± | 2 | abcd | 9.1 | ± | 1.2 | abcd | −11 | |
| PI 536803 | 8.1 | ± | 1.8 | k | 7.6 | ± | 1.8 | k | −6.2 | |
| PI 536822 | 8.5 | ± | 2 | ghijk | 8.3 | ± | 3.3 | jk | −2.4 | |
| PI 593426 | 9.1 | ± | 1.6 | cdefgh | 7.7 | ± | 0.8 | k | −15 | |
| PI 600773 | 9.4 | ± | 0.5 | bcdefg | 9.2 | ± | 1.2 | abcdef | −2.1 | |
| PI 635075 | 9.1 | ± | 2.2 | cdefgh | 8.9 | ± | 1.2 | defghi | −2.2 | |
| PI 635077 | 8.6 | ± | 2.9 | abcdef | 9.4 | ± | 1.4 | cdefg | 9.3 | |
| Prizehead | 10.7 | ± | 2.2 | ab | 10.0 | ± | 1.2 | abcde | −6.5 | |
| Type | Variety | LN 1 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Control | Salt | % 3 | ||||||||
| Leaf | PI 171675 | 11.4 | ± | 1.8 | a 2 | 8 | ± | 0.7 | k | −30 |
| PI 171676 | 8 | ± | 2.1 | k | 7.8 | ± | 1.2 | k | −2.5 | |
| PI 175737 | 9.6 | ± | 0.6 | abcde | 8 | ± | 1 | k | −17 | |
| PI 212099 | 9.5 | ± | 2.4 | abcde | 9.6 | ± | 1.9 | abcde | 1.05 | |
| PI 358018 | 13.3 | ± | 2.6 | a | 10.9 | ± | 1.4 | ab | −18 | |
| PI 601339 | 10.6 | ± | 1.1 | ab | 9.4 | ± | 1.7 | abcde | −11 | |
| PI 601488 | 10.3 | ± | 3 | abcd | 9.3 | ± | 2.3 | bcdef | −9.7 | |
| Ruby | 9.4 | ± | 1.4 | bcdef | 7.6 | ± | 2.1 | k | −19 | |
| Romaine | Burgundy Delight | 8.8 | ± | 2.3 | efghij | 9.4 | ± | 0.3 | abcde | 6.82 |
| Green Forest | 10.9 | ± | 0.8 | a | 9.5 | ± | 1.5 | abcde | −13 | |
| PI 278066 | 10.2 | ± | 2.4 | abcd | 9.7 | ± | 1.8 | abcde | −4.9 | |
| PI 289023 | 11.6 | ± | 0.6 | a | 12.4 | ± | 1.6 | a | 6.9 | |
| PI 536708 | 11.0 | ± | 1.4 | a | 12.2 | ± | 1.2 | a | 10.9 | |
| PI 612664 | 9.8 | ± | 0.8 | abcde | 11.3 | ± | 1.0 | a | 15.3 | |
| PI 613577 | 11.3 | ± | 2 | a | 8.3 | ± | 1.3 | ijk | −27 | |
| PI 274366 | 10.3 | ± | 1.6 | abc | 10.2 | ± | 0.9 | abcd | −1 | |
| Type | Variety | FM 1 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Control | Salt | % 3 | ||||||||
| Butterhead | Burpee Bibb | 58.8 | ± | 15 | abcde 2 | 54.3 | ± | 20 | abcde | −7.7 |
| Buttercrunch-1 | 39.9 | ± | 21 | efg | 63.8 | ± | 31 | abcd | 59.9 | |
| Buttercrunch-2 | 49.5 | ± | 34 | abcde | 68 | ± | 13 | abc | 37.4 | |
| Hybrid Bibb | 61.4 | ± | 38 | abc | 45.5 | ± | 12 | cde | −26 | |
| PI 273606 | 43.6 | ± | 25 | cde | 33.2 | ± | 22 | def | −24 | |
| PI 342448 | 12.6 | ± | 19 | fg | 14.3 | ± | 24 | efd | 13.5 | |
| PI 342515 | 41.9 | ± | 24 | cde | 47 | ± | 23 | bcde | 12.2 | |
| PI 358020 | 33.5 | ± | 30 | abcde | 45.3 | ± | 32 | abcde | 35.2 | |
| PI 372908 | 44.4 | ± | 19 | abcde | 37.8 | ± | 21 | abcde | −15 | |
| PI 615052 | 19.3 | ± | 21 | cde | 14.6 | ± | 49 | cdef | −24 | |
| PI 634671 | 25.1 | ± | 10 | bcde | 30.2 | ± | 25 | bcde | 20.3 | |
| Crisphead | Crispino | 53.4 | ± | 5.5 | ab | 56.7 | ± | 9.3 | abc | 6.18 |
| Iceberg | 45.5 | ± | 15 | abcd | 43.2 | ± | 20 | abcde | −5.1 | |
| Parris Island | 33.2 | ± | 26 | bcd | 55.4 | ± | 56 | abcde | 66.9 | |
| PI 177423 | 33.8 | ± | 52 | abcde | 40.3 | ± | 13 | abcd | 19.2 | |
| PI 536803 | 47.1 | ± | 22 | abcd | 42.5 | ± | 23 | abcde | −9.8 | |
| PI 536822 | 47.2 | ± | 16 | abcd | 33.3 | ± | 26 | abcde | −29 | |
| PI 593426 | 65.3 | ± | 19 | ab | 60.1 | ± | 14 | ab | −8 | |
| PI 600773 | 52.9 | ± | 18 | abc | 63.1 | ± | 40 | abcd | 19.3 | |
| PI 635075 | 54.1 | ± | 20 | abcd | 47.3 | ± | 9.9 | bcde | −13 | |
| PI 635077 | 54.6 | ± | 9.6 | abcd | 74.9 | ± | 11 | a | 37.2 | |
| Prizehead | 44.1 | ± | 5.3 | bcd | 47.0 | ± | 27 | abcde | 6.58 | |
| Type | Variety | FM 1 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Control | Salt | % 3 | ||||||||
| Leaf | PI 171675 | 71.6 | ± | 28 | ab 2 | 20.2 | ± | 5.5 | f | −72 |
| PI 171676 | 15 | ± | 23 | def | 30.6 | ± | 21 | cdef | 104 | |
| PI 175737 | 28.9 | ± | 13 | cdef | 30 | ± | 2.3 | cde | 3.81 | |
| PI 212099 | 26 | ± | 29 | cdef | 77.1 | ± | 23 | a | 197 | |
| PI 358018 | 39.6 | ± | 33 | abcd | 33.9 | ± | 11 | cde | −14 | |
| PI 601339 | 37.3 | ± | 15 | de | 34.7 | ± | 6.5 | cde | −7 | |
| PI 601488 | 51.9 | ± | 25 | abcde | 31.7 | ± | 14 | cde | −39 | |
| Ruby | 67.4 | ± | 6.9 | a | 43.7 | ± | 17 | bcde | −35 | |
| Romaine | Burgundy Delight | 29.9 | ± | 31 | bcdef | 44.2 | ± | 26 | abcde | 47.8 |
| Green Forest | 62.6 | ± | 33 | abcde | 44 | ± | 22 | abcde | −30 | |
| PI 278066 | 54.5 | ± | 24 | abcde | 36.5 | ± | 16 | cdef | −33 | |
| PI 289023 | 40.6 | ± | 5.5 | bcde | 39.8 | ± | 9.1 | cdef | −2 | |
| PI 536708 | 62.8 | ± | 11 | abc | 65.7 | ± | 3.6 | a | 4.62 | |
| PI 612664 | 30.1 | ± | 9.5 | de | 34 | ± | 11 | cdef | 13 | |
| PI 613577 | 45.5 | ± | 18 | cde | 38.6 | ± | 13 | cdef | −15 | |
| PI 274366 | 32.6 | ± | 18 | cdef | 25.3 | ± | 6.4 | ef | −22 | |
| Type | Variety | DM 1 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Control | Salt | % 3 | ||||||||
| Butterhead | Burpee Bibb | 7.5 | ± | 2.8 | abc 2 | 4.9 | ± | 1.4 | cdef | −35 |
| Buttercrunch-1 | 6.4 | ± | 1.8 | bcd | 8.6 | ± | 2.9 | abc | 34.4 | |
| Buttercrunch-2 | 7.2 | ± | 4.5 | abcde | 7.8 | ± | 1 | bcde | 8.33 | |
| Hybrid Bibb | 9.5 | ± | 2.8 | A | 6.6 | ± | 1.1 | cd | −31 | |
| PI 273606 | 5.2 | ± | 3.1 | de | 3.8 | ± | 2.8 | ef | −27 | |
| PI 342448 | 1.6 | ± | 1.8 | F | 1.5 | ± | 2.5 | f | −6.3 | |
| PI 342515 | 5.2 | ± | 2.9 | def | 4.2 | ± | 3.4 | def | −19 | |
| PI 358020 | 4.5 | ± | 3.4 | def | 6 | ± | 4.5 | abcde | 33.3 | |
| PI 372908 | 5.4 | ± | 2.2 | def | 3.9 | ± | 2.6 | def | −28 | |
| PI 615052 | 2.3 | ± | 2.1 | ef | 3.5 | ± | 3.7 | def | 52.2 | |
| PI 634671 | 2.8 | ± | 1.4 | ef | 2.9 | ± | 4.3 | def | 3.57 | |
| Crisphead | Crispino | 7.5 | ± | 0.8 | ab | 7.7 | ± | 1.1 | ab | 2.67 |
| Iceberg | 6.6 | ± | 1.7 | abc | 6.1 | ± | 1.3 | bc | −7.6 | |
| Parris Island | 6.1 | ± | 2.6 | abc | 8 | ± | 3.7 | abcd | 31.1 | |
| PI 177423 | 5.1 | ± | 5.1 | abcde | 4.6 | ± | 1.0 | cde | −9.8 | |
| PI 536803 | 7.3 | ± | 1.9 | bcde | 5.3 | ± | 1.6 | def | −27 | |
| PI 536822 | 5.1 | ± | 2.7 | bcdef | 3.5 | ± | 3.0 | cdef | −31 | |
| PI 593426 | 8.4 | ± | 1.8 | abcde | 7.1 | ± | 1.5 | abcd | −15 | |
| PI 600773 | 7.8 | ± | 1.7 | abcd | 7.1 | ± | 2.0 | abcde | −9 | |
| PI 635075 | 7.1 | ± | 2.4 | abcde | 7 | ± | 0.5 | bcde | −1.4 | |
| PI 635077 | 7.4 | ± | 0.8 | bcd | 7.9 | ± | 0.8 | abc | 6.76 | |
| Prizehead | 5.9 | ± | 0.6 | cd | 5.5 | ± | 2.0 | cd | −6.8 | |
| Type | Variety | DM 1 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Control | Salt | % 3 | ||||||||
| Leaf | PI 171675 | 7.9 | ± | 3 | abc 2 | 1.8 | ± | 0.5 | f | −77 |
| PI 171676 | 1.9 | ± | 2 | f | 3 | ± | 4 | bcde | 57.9 | |
| PI 175737 | 3.2 | ± | 1.5 | ef | 3.4 | ± | 0.1 | ef | 6.25 | |
| PI 212099 | 3 | ± | 2.8 | cdef | 4.9 | ± | 1.9 | bcde | 63.3 | |
| PI 358018 | 5.8 | ± | 3.6 | abcde | 4.6 | ± | 0.9 | cde | −21 | |
| PI 601339 | 5.7 | ± | 1.5 | cdef | 4.8 | ± | 1.4 | cd | −16 | |
| PI 601488 | 8.3 | ± | 2.8 | abcde | 5.3 | ± | 0.4 | cd | −36 | |
| Ruby | 7.6 | ± | 1.8 | abcd | 5.5 | ± | 2.7 | abcd | −28 | |
| Romaine | Burgundy Delight | 4.3 | ± | 3.2 | cdef | 5.6 | ± | 2.6 | bcde | 30.2 |
| Green Forest | 10.1 | ± | 3.8 | a | 7 | ± | 2.4 | abc | −31 | |
| PI 278066 | 7.4 | ± | 2.5 | abcd | 5.7 | ± | 1.1 | cde | −23 | |
| PI 289023 | 5.7 | ± | 0.7 | bcde | 4.9 | ± | 1.0 | cde | −14 | |
| PI 536708 | 8.6 | ± | 2.6 | abcd | 8.2 | ± | abc | −4.7 | ||
| PI 612664 | 3.6 | ± | 1.2 | de | 3.7 | ± | 3.5 | cdef | 2.78 | |
| PI 613577 | 5.1 | ± | 1.5 | cde | 4 | ± | 1.7 | def | −22 | |
| PI 274366 | 4.0 | ± | 1.8 | def | 3.6 | ± | 0.7 | def | −10 | |
| Type | Variety | CI 1 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Control | Salt | % 3 | ||||||||
| Butterhead | Burpee Bibb | 14 | ± | 5.1 | abcd 2 | 11.3 | ± | 2.8 | e | −18 |
| Buttercrunch-1 | 9.8 | ± | 2.5 | e | 11.7 | ± | 1.1 | e | 19.4 | |
| Buttercrunch-2 | 13 | ± | 1.5 | de | 10.9 | ± | 1.4 | e | −17 | |
| Hybrid Bibb | 14 | ± | 4.4 | bcde | 12.4 | ± | 3.7 | de | −8.1 | |
| PI 273606 | 7 | ± | 3.4 | efgh | 5.6 | ± | 1.8 | fghi | −20 | |
| PI 342448 | 6.3 | ± | 2.1 | fghi | 7.5 | ± | 1.4 | fghi | 19 | |
| PI 342515 | 6.6 | ± | 1.2 | fghi | 6.5 | ± | 1.2 | fghi | −1.5 | |
| PI 358020 | 5.8 | ± | 2.7 | ghi | 4.1 | ± | 3.1 | i | −29 | |
| PI 372908 | 8.2 | ± | 3.7 | e | 7 | ± | 0.9 | efgh | −15 | |
| PI 615052 | 7.5 | ± | 1.2 | efgh | 8.2 | ± | 3.5 | efgh | 9.33 | |
| PI 634671 | 3.7 | ± | 3.3 | i | 4.5 | ± | 3.6 | ghi | 21.6 | |
| Crisphead | Crispino | 7.3 | ± | 1.1 | efg | 7.3 | ± | 1.9 | fghi | 0 |
| Iceberg | 7.2 | ± | 2.1 | efgh | 6.5 | ± | 5.3 | fghi | −9.7 | |
| Parris Island | 13 | ± | 3 | 6 | 14.3 | ± | 2.9 | abcd | 14.4 | |
| PI 177423 | 9.5 | ± | 1.6 | e | 7.4 | ± | 3.5 | ef | −22 | |
| PI 536803 | 6.6 | ± | 2.7 | efgh | 8.3 | ± | 1.4 | e | 25.8 | |
| PI 536822 | 5.6 | ± | 2.3 | fghi | 3.7 | ± | 3.2 | i | −34 | |
| PI 593426 | 5.2 | ± | 1.5 | ghi | 7.5 | ± | 1.8 | efgh | 44.2 | |
| PI 600773 | 15 | ± | 1.1 | abcd | 14.4 | ± | 0.6 | abcd | −0.7 | |
| PI 635075 | 14 | ± | 3 | abcd | 11.4 | ± | 3 | de | −16 | |
| PI 635077 | 11 | ± | 4.1 | e | 13.8 | ± | 3.7 | bcde | 25.5 | |
| Prizehead | 6.8 | ± | 3.2 | efgh | 5.4 | ± | 1 | fghi | −21 | |
| Type | Variety | CI 1 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Control | Salt | % 3 | ||||||||
| Leaf | PI 171675 | 5.5 | ± | 4.1 | fghi 2 | 4 | ± | 2.5 | i | −27 |
| PI 171676 | 7.1 | ± | 5.3 | efgh | 5.4 | ± | 1 | ghi | −24 | |
| PI 175737 | 4.2 | ± | 5.3 | ghi | 4.8 | ± | 4.3 | ghi | 14.3 | |
| PI 212099 | 4.1 | ± | 2.1 | i | 4.9 | ± | 7.2 | ghi | 19.5 | |
| PI 358018 | 7.1 | ± | 0.6 | fghi | 6.4 | ± | 2.4 | fghi | −9.9 | |
| PI 601339 | 9.5 | ± | 3 | e | 6.6 | ± | 2.4 | efgh | −31 | |
| PI 601488 | 11.4 | ± | 2.7 | e | 9.6 | ± | 2.6 | e | −16 | |
| Ruby | 6.4 | ± | 2.2 | fghi | 4.3 | ± | 0.9 | hi | −33 | |
| Romaine | Burgundy Delight | 13.4 | ± | 1.5 | abcd | 15.3 | ± | 1.4 | ab | 14.2 |
| Green Forest | 15.4 | ± | 2.2 | a | 11.3 | ± | 1.6 | e | −27 | |
| PI 278066 | 8.5 | ± | 1.4 | e | 5.8 | ± | 2.6 | efgh | −32 | |
| PI 289023 | 8 | ± | 3.7 | e | 5.6 | ± | 3.1 | efgh | −30 | |
| PI 536708 | 14.9 | ± | 0.8 | abc | 13.6 | ± | 4.7 | abcd | −8.7 | |
| PI 612664 | 9 | ± | 4.7 | e | 11.5 | ± | 4.6 | e | 27.8 | |
| PI 613577 | 9.6 | ± | 3.9 | e | 9.4 | ± | 0.5 | e | −2.1 | |
| PI 274366 | 4.1 | ± | 1.2 | i | 2.6 | ± | 1.8 | i | −37 | |
| Type | Variety | FI 1 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Control | Salt | % 3 | ||||||||
| Butterhead | Burpee Bibb | 0.9 | ± | 0.1 | fg 2 | 0.9 | ± | 0.3 | fg | 0 |
| Buttercrunch-1 | 1.3 | ± | 0.1 | abcd | 1.2 | ± | 0.2 | abcde | −7.7 | |
| Buttercrunch-2 | 1.3 | ± | 0.2 | abcd | 1.2 | ± | 0 | abcde | −7.7 | |
| Hybrid Bibb | 1.6 | ± | 0.2 | abcd | 1.5 | ± | 0.1 | a | −6.3 | |
| PI 273606 | 1.1 | ± | 0.3 | defg | 1 | ± | 0.2 | efg | −9.1 | |
| PI 342448 | 1.1 | ± | 0.2 | cdef | 1.3 | ± | 0.3 | abcde | 18.2 | |
| PI 342515 | 1.2 | ± | 0.1 | abcde | 1.1 | ± | 0 | cdef | −8.3 | |
| PI 358020 | 1.3 | ± | 0.1 | abcde | 1.3 | ± | 0.3 | abcde | 0 | |
| PI 372908 | 1.2 | ± | 0.1 | abcde | 1.1 | ± | 0.2 | defg | −8.3 | |
| PI 615052 | 1.1 | ± | 0.1 | defg | 1.2 | ± | 0.4 | abcde | 9.09 | |
| PI 634671 | 1.2 | ± | 0.2 | defg | 1.1 | ± | 0.2 | efg | −8.3 | |
| Crisphead | Crispino | 1 | ± | 0.1 | g | 0.8 | ± | 0.1 | g | −20 |
| Iceberg | 1.4 | ± | 0.2 | abc | 1.4 | ± | 0 | abc | 0 | |
| Parris Island | 1.5 | ± | 0 | a | 1.4 | ± | 0.1 | a | −6.7 | |
| PI 177423 | 1 | ± | 0.2 | efg | 1.1 | ± | 0.1 | cdef | 10 | |
| PI 536803 | 1 | ± | 0.1 | efg | 1 | ± | 0 | fg | 0 | |
| PI 536822 | 1.1 | ± | 0.1 | efg | 1.2 | ± | 0.2 | abcde | 9.09 | |
| PI 593426 | 0.8 | ± | 0.1 | g | 0.8 | ± | 0.1 | g | 0 | |
| PI 600773 | 1 | ± | 0.2 | fg | 1 | ± | 0.1 | efg | 0 | |
| PI 635075 | 1 | ± | 0.1 | fg | 1 | ± | 0.2 | efg | 0 | |
| PI 635077 | 1 | ± | 0.4 | fg | 0.9 | ± | 0.2 | fg | −10 | |
| Prizehead | 1.4 | ± | 0.1 | abc | 1.4 | ± | 0.2 | abc | 0 | |
| Type | Variety | Fl 1 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Control | Salt | % 3 | ||||||||
| Leaf | PI 171675 | 1 | ± | 0.2 | abcde 2 | 1.1 | ± | 0.2 | cdef | 10 |
| PI 171676 | 1.4 | ± | 0.2 | abc | 1.1 | ± | 0 | bcdef | −21 | |
| PI 175737 | 1.2 | ± | 0.1 | abcde | 1 | ± | 0.1 | efg | −17 | |
| PI 212099 | 1.1 | ± | 0.2 | efg | 1 | ± | 0.2 | efg | −9.1 | |
| PI 358018 | 1.2 | ± | 0.2 | bcdef | 1.3 | ± | 0.2 | abcd | 8.33 | |
| PI 601339 | 1.4 | ± | 0.4 | a | 1.3 | ± | 0.3 | abcd | −7.1 | |
| PI 601488 | 0.8 | ± | 0.1 | g | 1 | ± | 0.2 | fg | 25 | |
| Ruby | 1.1 | ± | 0.2 | cdefg | 1.3 | ± | 0.1 | abcde | 18.2 | |
| Romaine | Burgundy Delight | 1.6 | ± | 0.1 | a | 1.5 | ± | 0.2 | a | −6.3 |
| Green Forest | 1.3 | ± | 0.1 | abc | 1.4 | ± | 0 | ab | 7.69 | |
| PI 278066 | 1.4 | ± | 0.3 | abc | 1.9 | ± | 0.1 | a | 35.7 | |
| PI 289023 | 1.2 | ± | 0.2 | cdefg | 1.3 | ± | 0.8 | abcde | 8.33 | |
| PI 536708 | 1.3 | ± | 0.1 | abcde | 1.2 | ± | 0.2 | abcde | −7.7 | |
| PI 612664 | 1.2 | ± | 0.2 | abcde | 1.2 | ± | 0.2 | abcde | 0 | |
| PI 613577 | 1.5 | ± | 0.3 | a | 1.4 | ± | 0.2 | a | −6.7 | |
| PI 274366 | 1.1 | ± | 0.2 | abc | 1.4 | ± | 0.2 | a | 27.3 | |
| Type | Variety | NB 1 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Control | Salt | % 3 | ||||||||
| Butterhead | Burpee Bibb | 15 | ± | 8.6 | ab 2 | 12.8 | ± | 5.6 | bc | −16 |
| Buttercrunch-1 | 7.2 | ± | 1.8 | de | 10.7 | ± | 3.2 | bcde | 48.6 | |
| Buttercrunch-2 | 11 | ± | 2.1 | cde | 9.7 | ± | 1.1 | cde | −12 | |
| Hybrid Bibb | 7.6 | ± | 5.4 | de | 9.1 | ± | 4 | de | 19.7 | |
| PI 273606 | 6.8 | ± | 10 | de | 6.5 | ± | 3.8 | de | −4.4 | |
| PI 342448 | 5.3 | ± | 1.4 | de | 5.3 | ± | 3.6 | de | 0 | |
| PI 342515 | 6.3 | ± | 0.3 | de | 7.2 | ± | 0.8 | de | 14.3 | |
| PI 358020 | 5.2 | ± | 1.4 | de | 3.2 | ± | 4.5 | e | −38 | |
| PI 372908 | 7.2 | ± | 3.6 | de | 8.9 | ± | 3.9 | de | 23.6 | |
| PI 615052 | 9.7 | ± | 1.7 | de | 6.9 | ± | 8.3 | de | −29 | |
| PI 634671 | 3.3 | ± | 5.5 | e | 4.5 | ± | 5.4 | e | 36.4 | |
| Crisphead | Crispino | 7.9 | ± | 1.3 | cde | 8.4 | ± | 1.2 | de | 6.33 |
| Iceberg | 5.2 | ± | 5 | e | 4.6 | ± | 6.3 | e | −12 | |
| Parris Island | 9.6 | ± | 1.7 | de | 10.9 | ± | 1.4 | bcde | 13.5 | |
| PI 177423 | 8.6 | ± | 1.6 | de | 6.6 | ± | 4.7 | de | −23 | |
| PI 536803 | 6.5 | ± | 1.9 | de | 9.5 | ± | 2.5 | de | 46.2 | |
| PI 536822 | 4.8 | ± | 2.7 | de | 3.4 | ± | 7.7 | e | −29 | |
| PI 593426 | 6.5 | ± | 2.7 | de | 9.1 | ± | 2.4 | de | 40 | |
| PI 600773 | 15 | ± | 0.2 | ab | 15.8 | ± | 1.0 | ab | 3.27 | |
| PI 635075 | 18 | ± | 2.6 | a | 11.3 | ± | 2.7 | bcd | −37 | |
| PI 635077 | 13 | ± | 11 | bc | 13.7 | ± | 3.7 | b | 7.03 | |
| Prizehead | 4.9 | ± | 3.7 | e | 4.1 | ± | 2.7 | e | −16 | |
| Type | Variety | NB 1 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Control | Salt | % 3 | ||||||||
| Leaf | PI 171675 | 6 | ± | 4.8 | de 2 | 5.9 | ± | 3.8 | de | −1.7 |
| PI 171676 | 4.9 | ± | 5.5 | e | 6 | ± | 1.2 | de | 22.4 | |
| PI 175737 | 4 | ± | 4.2 | e | 5.5 | ± | 2.8 | de | 37.5 | |
| PI 212099 | 3.9 | ± | 4.8 | e | 6.1 | ± | 8.5 | de | 56.4 | |
| PI 358018 | 6.9 | ± | 1.3 | de | 5.6 | ± | 3 | de | −19 | |
| PI 601339 | 6.7 | ± | 4.1 | de | 5.9 | ± | 4.6 | de | −12 | |
| PI 601488 | 13.9 | ± | 2.9 | b | 10 | ± | 6 | cde | −28 | |
| Ruby | 5.9 | ± | 2.1 | de | 3.6 | ± | 1.9 | e | −39 | |
| Romaine | Burgundy Delight | 8.1 | ± | 1.8 | de | 9.4 | ± | 1.8 | de | 16 |
| Green Forest | 12.4 | ± | 1.4 | bcd | 9.5 | ± | 0.8 | de | −23 | |
| PI 278066 | 7.2 | ± | 3.4 | de | 4 | ± | 2.3 | e | −44 | |
| PI 289023 | 7.3 | ± | 4.4 | de | 5 | ± | 2.3 | de | −32 | |
| PI 536708 | 12.5 | ± | 3.3 | bcd | 11.6 | ± | 6.4 | bcd | −7.2 | |
| PI 612664 | 8 | ± | 6.2 | de | 11 | ± | 5.4 | bcde | 37.5 | |
| PI 613577 | 6.5 | ± | 3.8 | de | 6.3 | ± | 0.7 | de | −3.1 | |
| PI 274366 | 3.5 | ± | 1.9 | e | 2.9 | ± | 2.5 | e | −17 | |
| Type | Variety | AI 1 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Control | Salt | % 3 | ||||||||
| Butterhead | Burpee Bibb | 0.1 | ± | 0.1 | klm 2 | 0.2 | ± | 0.1 | jk | 100 |
| Buttercrunch-1 | 0.2 | ± | 0.1 | lm | 0.2 | ± | 0 | m | 0 | |
| Buttercrunch-2 | 0.2 | ± | 0.2 | lm | 0.2 | ± | 0 | lm | 0 | |
| Hybrid Bibb | 0.2 | ± | 0 | m | 0.2 | ± | 0 | m | 0 | |
| PI 273606 | 0.2 | ± | 0 | klm | 0.2 | ± | 0 | klm | 0 | |
| PI 342448 | 0.4 | ± | 0 | hij | 0.3 | ± | 0.1 | j | −25 | |
| PI 342515 | 0.2 | ± | 0.2 | klm | 0.3 | ± | 0.2 | jk | 50 | |
| PI 358020 | 0.2 | ± | 0 | jk | 0.2 | ± | 0 | jk | 0 | |
| PI 372908 | 0.3 | ± | 0 | jk | 0.2 | ± | 0 | jk | −33.3 | |
| PI 615052 | 0.2 | ± | 0.1 | j | 0.2 | ± | 0.1 | j | 0 | |
| PI 634671 | 0.2 | ± | 0.1 | jkl | 0.2 | ± | 0.1 | klm | 0 | |
| Crisphead | Crispino | 0.2 | ± | 0.1 | klm | 0.2 | ± | 0.1 | klm | 0 |
| Iceberg | 0.4 | ± | 0 | ij | 0.3 | ± | 0.1 | j | −25 | |
| Parris Island | 0.2 | ± | 0.1 | klm | 0.1 | ± | 0.1 | m | −50 | |
| PI 177423 | 0.3 | ± | 0 | jk | 0.2 | ± | 0 | jk | −33 | |
| PI 536803 | 0.2 | ± | 0.1 | klm | 0.2 | ± | 0.1 | klm | 0 | |
| PI 536822 | 0.4 | ± | 0 | hi | 0.4 | ± | 0 | ij | 0 | |
| PI 593426 | 0.2 | ± | 0 | klm | 0.2 | ± | 0 | klm | 0 | |
| PI 600773 | 0.1 | ± | 0.1 | m | 0.2 | ± | 0 | m | 100 | |
| PI 635075 | 0.1 | ± | 0 | m | 0.2 | ± | 0.1 | lm | 100 | |
| PI 635077 | 0.2 | ± | 0.1 | m | 0.2 | ± | 0.1 | klm | 0 | |
| Prizehead | 0.6 | ± | 0 | e | 0.5 | ± | 0 | g | −17 | |
| Type | Variety | AI 1 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Control | Salt | % 3 | ||||||||
| Leaf | PI 171675 | 0.2 | ± | 0.1 | klm 2 | 0.3 | ± | 0 | jk | 50 |
| PI 171676 | 0.2 | ± | 0.3 | klm | 0.2 | ± | 0 | klm | 0 | |
| PI 175737 | 0.2 | ± | 0 | klm | 0.3 | ± | 0 | jk | 50 | |
| PI 212099 | 0.2 | ± | 0 | klm | 0.2 | ± | 0.1 | jk | 0 | |
| PI 358018 | 0.2 | ± | 0 | klm | 0.2 | ± | 0 | jk | 0 | |
| PI 601339 | 0.5 | ± | 0 | fg | 0.4 | ± | 0 | hi | −20 | |
| PI 601488 | 0.2 | ± | 0 | m | 0.2 | ± | 0 | klm | 0 | |
| Ruby | 0.5 | ± | 0 | fg | 0.6 | ± | 0 | ef | 20 | |
| Romaine | Burgundy Delight | 1.1 | ± | 0 | a | 1 | ± | 0 | b | −9.1 |
| Green Forest | 0.1 | ± | 0.1 | m | 0.2 | ± | 0.1 | klm | 100 | |
| PI 278066 | 0.2 | ± | 0 | klm | 0.2 | ± | 0 | jk | 0 | |
| PI 289023 | 0.2 | ± | 0 | klm | 0.2 | ± | 0 | klm | 0 | |
| PI 536708 | 0.1 | ± | 0 | m | 0.1 | ± | 0 | m | 0 | |
| PI 612664 | 0.3 | ± | 0 | j | 0.3 | ± | 0 | jk | 0 | |
| PI 613577 | 0.8 | ± | 0.1 | c | 0.7 | ± | 0 | d | −13 | |
| PI 274366 | 0.3 | ± | 0 | jk | 0.3 | ± | 0 | jk | 0 | |
| Tolerance Classification | Salt Tolerance Coefficient Index Value | Cultivars |
|---|---|---|
| Highly salt-sensitive | (≤0.89) | PI 171675, PI 536822, Ruby, PI 601339, Green Forest, PI 601488, PI 613577, PI 278066, Hybrid Bibb, PI 273606, Burpee Bibb |
| Low salt tolerance | (0.90–1.03) | PI 372908, PI 358018, PI 274366, Prizehead, PI 177423, PI 289023, Iceberg, PI 635075, PI 342515, PI 358020, PI 536708, Buttercrunch-2, Crispino, PI 600773, PI 536803 |
| Moderate salt tolerance | (1.04–1.17) | PI 342448, PI 615052, PI 175737, PI 593426, PI 634671, PI 612664, Parris Island, Burgundy Delight, PI 635077 |
| High salt tolerance | (≥1.18) | PI 171676, Buttercrunch-1, PI 212099 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adhikari, B.; Olorunwa, O.J.; Wilson, J.C.; Barickman, T.C. Morphological and Physiological Response of Different Lettuce Genotypes to Salt Stress. Stresses 2021, 1, 285-304. https://doi.org/10.3390/stresses1040021
Adhikari B, Olorunwa OJ, Wilson JC, Barickman TC. Morphological and Physiological Response of Different Lettuce Genotypes to Salt Stress. Stresses. 2021; 1(4):285-304. https://doi.org/10.3390/stresses1040021
Chicago/Turabian StyleAdhikari, Bikash, Omolayo J. Olorunwa, Jeff C. Wilson, and T. Casey Barickman. 2021. "Morphological and Physiological Response of Different Lettuce Genotypes to Salt Stress" Stresses 1, no. 4: 285-304. https://doi.org/10.3390/stresses1040021
APA StyleAdhikari, B., Olorunwa, O. J., Wilson, J. C., & Barickman, T. C. (2021). Morphological and Physiological Response of Different Lettuce Genotypes to Salt Stress. Stresses, 1(4), 285-304. https://doi.org/10.3390/stresses1040021








