Fertilisation with Potassium Silicate Exerted Little Effect on Production Parameters of Cucumbers Exposed to UV and Drought
Abstract
:1. Introduction
2. Results
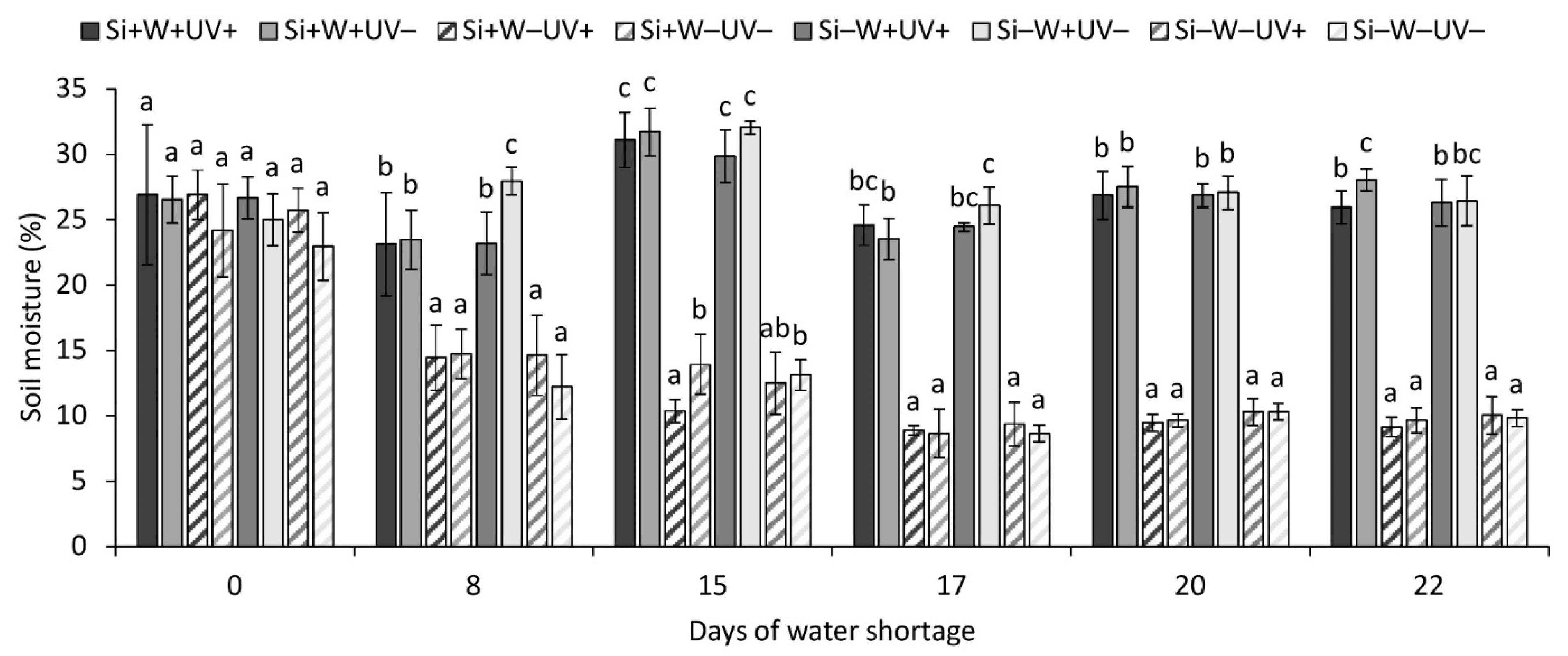
2.1. Environmental Parameters
2.2. General Observations
2.3. Leaf Biochemical Traits
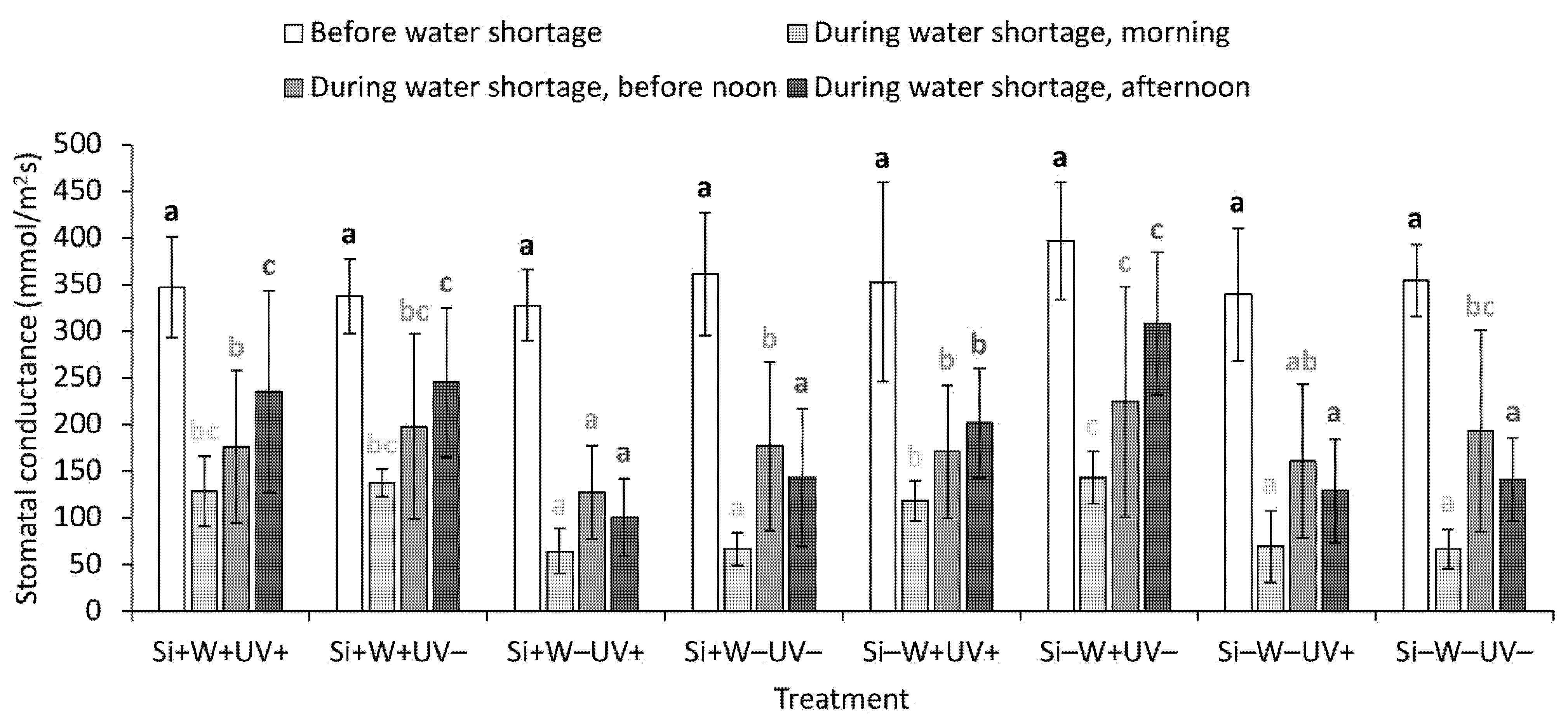
2.4. Leaf Physiological Traits
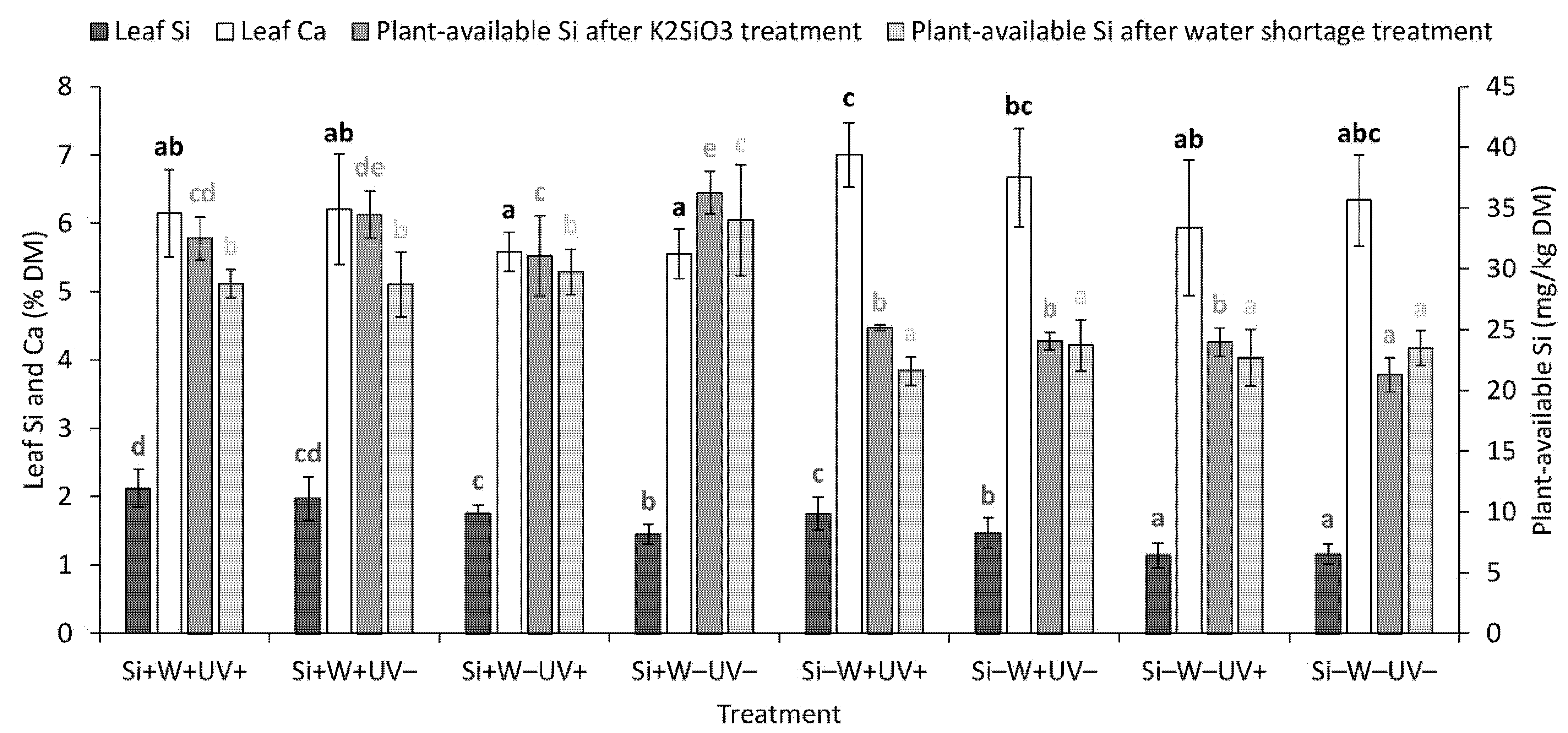
2.5. Leaf and Soil Element Composition and Plant-Available Si Level in the Soil
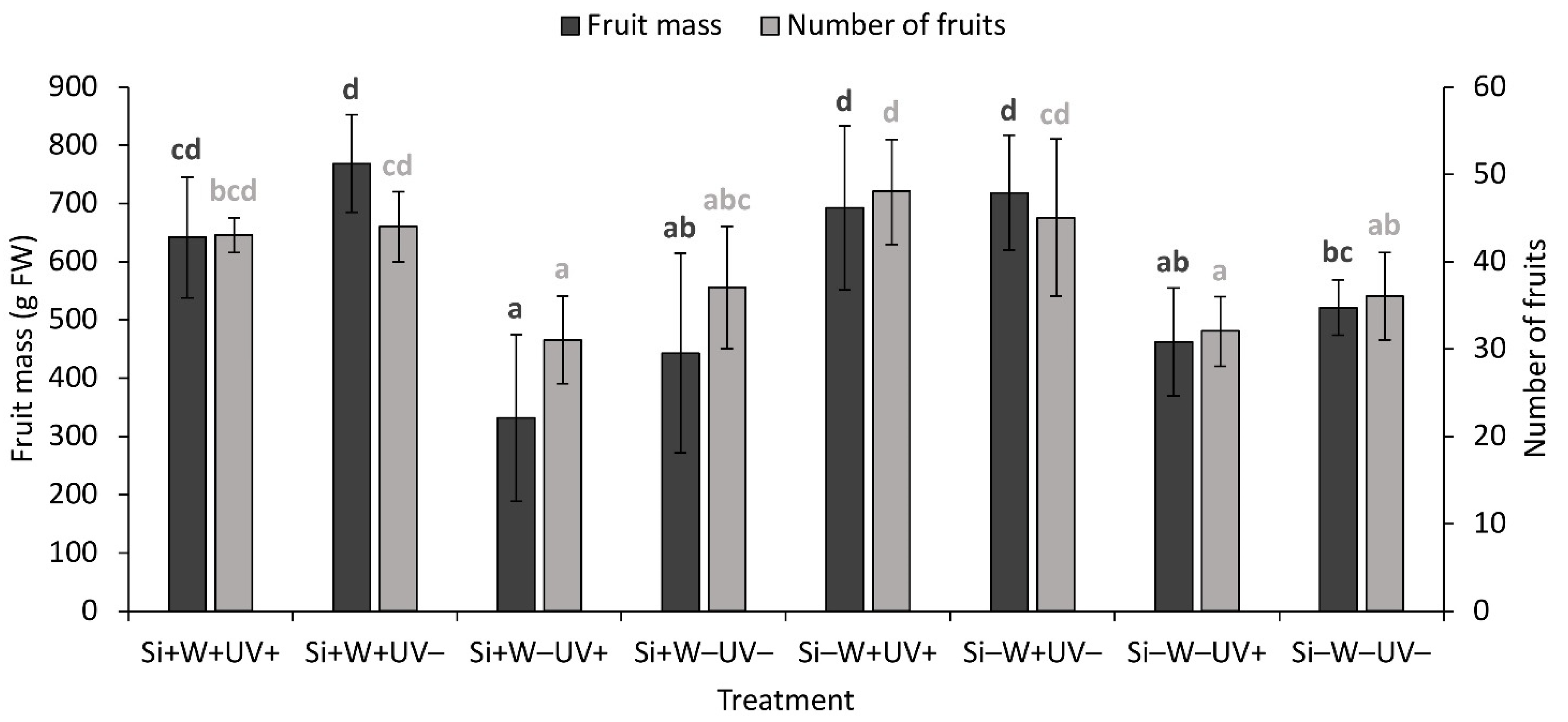
2.6. Plant Biomass and Growth Parameters
2.7. Relationships between Selected Parameters
3. Discussion
4. Materials and Methods
4.1. Experimental Design
4.2. Environmental Conditions
4.3. Plant Analyses
4.3.1. Leaf Biochemical Traits
4.3.2. Leaf Physiological Traits
4.3.3. Leaf Bulk Element Analysis
4.4. Soil Analyses
4.5. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mitani, N.; Ma, J.F. Uptake system of silicon in different plant species. J. Exp. Bot. 2005, 56, 1255–1261. [Google Scholar] [CrossRef] [Green Version]
- Epstein, E. Silicon: Its manifold roles in plants. Ann. Appl. Biol. 2009, 155, 155–160. [Google Scholar] [CrossRef]
- Rogalla, H.; Römheld, V. Role of leaf apoplast in silicon-mediated manganese tolerance of Cucumis sativus L. Plant Cell Environ. 2002, 25, 549–555. [Google Scholar] [CrossRef]
- Maksimović, J.D.; Mojović, M.; Maksimović, V.; Römheld, V.; Nikolic, M. Silicon ameliorates manganese toxicity in cucumber by decreasing hydroxyl radical accumulation in the leaf apoplast. J. Exp. Bot. 2012, 63, 2411–2420. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Han, Y.; Li, P.; Li, F.; Ali, S.; Hou, M. Silicon amendment is involved in the induction of plant defense responses to a phloem feeder. Sci. Rep. 2017, 7, 4232. [Google Scholar] [CrossRef] [PubMed]
- Jadhao, K.R.; Bansal, A.; Rout, G.R. Silicon amendment induces synergistic plant defense mechanism against pink stem borer (Sesamia inferens Walker.) in finger millet (Eleusine coracana Gaertn.). Sci. Rep. 2020, 10, 4229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tripathi, D.; Dwivedi, M.M.; Tripathi, D.K.; Chauhan, D.K. Silicon bioavailability in exocarp of Cucumis sativus Linn. Biotech 2017, 7, 386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pavlovic, P.; Samardzic, J.; Maksimović, V.; Timotijevic, G.; Stevic, N.; Laursen, K.H.; Hansen, T.H.; Husted, S.; Schjoerring, J.K.; Liang, Y.; et al. Silicon alleviates iron deficiency in cucumber by promoting mobilization of iron in the root apoplast. New Phytol. 2013, 198, 1096–1107. [Google Scholar] [CrossRef] [PubMed]
- Neu, S.; Schaller, J.; Dudel, E.G. Silicon availability modifies nutrient use efficiency and content, C:N:P stoichiometry, and productivity of winter wheat (Triticum aestivum L.). Sci. Rep. 2017, 7, 40829. [Google Scholar] [CrossRef]
- Klančnik, K.; Vogel-Mikuš, K.; Gaberščik, A. Silicified structures affect leaf optical properties in grasses and sedge. J. Photochem. Photobiol. B 2014, 130, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Klančnik, K.; Vogel-Mikuš, K.; Kelemen, M.; Vavpetič, P.; Pelicon, P.; Kump, P.; Jezeršek, D.; Gianoncelli, A.; Gaberščik, A. Leaf optical properties are affected by the location and type of deposited biominerals. J. Photochem. Photobiol. B 2014, 140, 276–285. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.F.; Miyake, Y.; Takahashi, E. Silicon as a beneficial element for crop plants. In Silicon in Agriculture; Datnoff, L.E., Snyder, G.H., Korndörfer, G.H., Eds.; Elsevier Science: Amsterdam, The Netherlands, 2001; pp. 17–39. [Google Scholar]
- Meunier, J.D.; Sandhya, K.; Prakash, N.B.; Borschneck, D.; Dussouillez, P. pH as a proxy for estimating plant-available Si? A case study in rice fields in Karnataka (South India). Plant Soil 2018, 432, 143–155. [Google Scholar] [CrossRef]
- Grašič, M.; Sakovič, T.; Abram, D.; Vogel-Mikuš, K.; Gaberščik, A. Do soil and leaf silicon content affect leaf functional traits in Deschampsia caespitosa from different habitats? Biol. Plant 2020, 64, 234–243. [Google Scholar] [CrossRef]
- Tubaña, B.S.; Babu, T.; Datnoff, L.E. A review of silicon in soils and plants and its role in US agriculture: History and future perspectives. Soil Sci. 2016, 181, 393–411. [Google Scholar] [CrossRef] [Green Version]
- Schaller, J.; Puppe, D.; Kaczorek, D.; Ellerbrock, R.; Sommer, M. Silicon cycling in soils revisited. Plants 2021, 10, 295. [Google Scholar] [CrossRef]
- Schaller, J.; Cramer, A.; Carminati, A.; Zarebanadkouki, M. Biogenic amorphous silica as main driver for plant available water in soils. Sci. Rep. 2020, 10, 2424. [Google Scholar] [CrossRef] [Green Version]
- Tubaña, B.S.; Heckman, J.R. Silicon in soils and plants. In Silicon and Plant Diseases; Rodrigues, F.A., Datnoff, L.E., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 7–51. [Google Scholar]
- Ouellette, S.; Goyette, M.-H.; Labbé, C.; Laur, J.; Gaudreau, L.; Gosselin, A.; Dorais, M.; Deshmukh, R.K.; Bélanger, R.R. Silicon transporters and effects of silicon amendments in strawberry under high tunnel and field conditions. Front. Plant Sci. 2017, 8, 949. [Google Scholar] [CrossRef]
- Coskun, D.; Deshmukh, R.; Sonah, H.; Menzies, J.G.; Reynolds, O.; Ma, J.F.; Kronzucker, H.J.; Bélanger, R.R. The controversies of silicon’s role in plant biology. New Phytol. 2019, 221, 67–85. [Google Scholar] [CrossRef]
- Frick, D.A.; Remus, R.; Sommer, M.; Augustin, J.; von Blanckenburg, F. Silicon isotope fractionation and uptake dynamics of three crop plants: Laboratory studies with transient silicon concentrations. Biogeosciences 2020, 17, 6475–6490. [Google Scholar] [CrossRef]
- Marschner, H.; Oberle, H.; Cakmak, I.; Römheld, V. Growth enhancement by silicon in cucumber (Cucumis sativus) plants depends on imbalance in phosphorus and zinc supply. Plant Soil 1990, 124, 211–219. [Google Scholar] [CrossRef]
- Grašič, M.; Dobravc, M.; Golob, A.; Vogel-Mikuš, K.; Gaberščik, A. Water shortage reduces silicon uptake in barley leaves. Agr. Water Manag. 2019, 217, 47–56. [Google Scholar] [CrossRef]
- Grašič, M.; Malovrh, U.; Golob, A.; Vogel-Mikuš, K.; Gaberščik, A. Effects of water availability and UV radiation on silicon accumulation in the C4 crop proso millet. Photochem. Photobiol. Sci. 2019, 18, 375–386. [Google Scholar] [CrossRef] [PubMed]
- Grašič, M.; Golob, A.; Vogel-Mikuš, K.; Gaberščik, A. Severe water deficiency during the mid-vegetative and reproductive phase has little effect on proso millet performance. Water 2019, 11, 2155. [Google Scholar] [CrossRef] [Green Version]
- Golob, A.; Kavčič, J.; Stibilj, V.; Gaberščik, A.; Vogel-Mikuš, K.; Germ, M. The effect of selenium and UV radiation on leaf traits and biomass production in Triticum aestivum L. Ecotox. Environ. Saf. 2017, 136, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Golob, A.; Stibilj, V.; Kreft, I.; Vogel-Mikuš, K.; Gaberščik, A.; Germ, M. Selenium treatment alters the effects of UV radiation on chemical and production parameters in hybrid buckwheat. Acta Agric. Scand. B 2018, 68, 5–15. [Google Scholar] [CrossRef]
- Venuprasad, R.; Lafitte, H.; Atlin, G. Response to direct selection for grain yield under drought stress in rice. Crop. Sci. 2007, 47, 285–293. [Google Scholar] [CrossRef]
- Li, Y.; Ye, W.; Wang, M.; Yan, X. Climate change and drought: A risk assessment of crop yield impacts. Clim. Res. 2009, 39, 31–46. [Google Scholar] [CrossRef]
- Taiz, L.; Zeiger, E. Plant Physiology, 4th ed.; Sinauer Associates: Sunderland, MA, USA, 2006. [Google Scholar]
- Takahashi, S.; Badger, M.R. Photoprotection in plants: A new light on photosystem II damage. Trends Plant Sci. 2011, 16, 53–60. [Google Scholar] [CrossRef]
- Alexieva, V.; Sergiev, I.; Mapelli, S.; Karanov, E. The effect of drought and ultraviolet radiation on growth and stress markers in pea and wheat. Plant Cell Environ. 2001, 24, 1337–1344. [Google Scholar] [CrossRef]
- Björn, L.-O. On the history of phyto-photo UV science (not to be left in skoto toto and silence). Plant Physiol. Biochem. 2015, 93, 3–8. [Google Scholar] [CrossRef]
- Savant, N.K.; Datnoff, L.E.; Snyder, G.H. Depletion of plant-available silicon in soils: A possible cause of declining rice yields. Commun. Soil Sci. Plant Anal. 1997, 28, 1145–1152. [Google Scholar] [CrossRef]
- Kaushik, P.; Saini, D.K. Silicon as a vegetable crops modulator—A review. Plants 2019, 8, 148. [Google Scholar] [CrossRef] [Green Version]
- Leroy, N.; de Tombeur, F.; Walgra, Y.; Cornélis, J.-T.; Verheggen, F.J. Silicon and plant natural defenses against insect pests: Impact on plant volatile organic compounds and cascade effects on multitrophic interactions. Plants 2019, 8, 444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nikolic, M.; Nikolic, N.; Liang, Y.; Kirkby, E.A.; Romheld, V. Germanium-68 as an adequate tracer for silicon transport in plants. Characterization of silicon uptake in different crop species. Plant Physiol. 2007, 143, 495–503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gou, T.; Yang, L.; Hu, W.; Chen, X.; Zhu, Y.; Guo, J.; Gong, H. Silicon improves the growth of cucumber under excess nitrate stress by enhancing nitrogen assimilation and chlorophyll synthesis. Plant Physiol. Biochem. 2020, 152, 53–61. [Google Scholar] [CrossRef]
- Liang, Y.; Si, J.; Römheld, V. Si uptake and transport is an active process in Cucumis sativus. New Phytol. 2005, 167, 797–804. [Google Scholar] [CrossRef] [PubMed]
- Fawe, A.; Abou-Zaid, M.; Menzies, J.G.; Bélanger, R.R. Silicon-mediated accumulation of flavonoid phytoalexins in cucumber. Phytopathology 1998, 88, 396–401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harizanova, A.; Zlatev, Z.; Koleva, L. Effect of silicon on activity of antioxidant enzymes and photosynthesis in leaves of cucumber plants (Cucumis sativus L.). Turk. J. Agric. Nat. Sci. 2014, 1, 1812–1817. [Google Scholar]
- Zhu, Y.-X.; Xu, X.-B.; Hu, Y.-H.; Han, W.-H.; Yin, J.-L.; Li, H.-L.; Gong, H.-J. Silicon improves salt tolerance by increasing root water uptake in Cucumis sativus L. Plant Cell Rep. 2015, 34, 1629–1646. [Google Scholar] [CrossRef]
- Liu, S.; Li, H.; Lv, X.; Ahammed, G.J.; Xia, X.; Zhou, J.; Shi, K.; Asami, T.; Yu, J.; Zhou, Y. Grafting cucumber onto luffa improves drought tolerance by increasing ABA biosynthesis and sensitivity. Sci. Rep. 2016, 6, 20212. [Google Scholar] [CrossRef] [Green Version]
- Tevini, M.; Iwanzik, W.; Teramura, A. Effects of UV-B radiation on plants during mild water stress II. Effects on growth, protein and flavonoid content. Z. Pflanzenphysiol. 1983, 110, 459–467. [Google Scholar] [CrossRef]
- Kanungo, M.; Dubey, A.; Kataria, S. Solar UV-B and UV-A/B exclusion affects growth and antioxidant enzymes in cucumber and wheat. Indian J. Plant Sci. 2013, 2, 63–72. [Google Scholar]
- Kubiš, J.; Rybus-Zając, M. Drought and excess UV-B irradiation differentially alter the antioxidant system in cucumber leaves. Acta Biol. Crac. Ser. Bot. 2008, 50, 35–41. [Google Scholar]
- Shen, X.; Zhou, Y.; Duan, L.; Li, Z.; Eneji, A.E.; Li, J. Silicon effects on photosynthesis and antioxidant parameters of soybean seedlings under drought and ultraviolet-B radiation. J. Plant Physiol. 2010, 167, 1248–1252. [Google Scholar] [CrossRef]
- Nyachiro, J.M.; Briggs, K.G.; Hoddinott, J.; Johnson-Flanagan, A.M. Chlorophyll content, chlorophyll fluorescence and water deficit in spring wheat. Cereal Res. Commun. 2001, 29, 135–142. [Google Scholar] [CrossRef]
- Larsson, E.H.; Bornman, J.F.; Asp, H. Influence of UV-B radiation and Cd2+ on chlorophyll fluorescence, growth and nutrient content in Brassica napus. J. Exp. Bot. 1998, 49, 1031–1039. [Google Scholar] [CrossRef]
- Silva, O.N.; Lobato, A.K.S.; Ávila, F.W.; Costa, R.C.L.; Oliveira Neto, C.F.; Santos Filho, B.G.; Martins Filho, A.P.; Lemos, R.P.; Pinho, J.M.; Medeiros, M.B.C.L.; et al. Silicon-induced increase in chlorophyll is modulated by the leaf water potential in two water-deficient tomato cultivars. Plant Soil Environ. 2012, 58, 481–486. [Google Scholar] [CrossRef] [Green Version]
- Demmig-Adams, B. Carotenoids and photoprotection in plants: A role for the xanthophyll zeaxanthin. Biochim. Biophys. Acta Bioenerg. 1990, 1020, 1–24. [Google Scholar] [CrossRef]
- Larcher, W. Physiological Plant Ecology: Ecophysiology and Stress Physiology of Functional Groups, 4th ed.; Springer: Berlin, Germany, 2003. [Google Scholar]
- Jamila, F.; Fouad, A.; Zakia, B.; Rachid, B.; Nabil, S.; Rachid, M. Effect of silicon application on Coriandrum sativum (L) under salt stress. Int. J. Sci. Eng. Res. 2019, 10, 26–33. [Google Scholar]
- Zhu, Y.; Gong, H. Beneficial effects of silicon on salt and drought tolerance in plants. Agron. Sustain. Dev. 2014, 34, 455–472. [Google Scholar] [CrossRef] [Green Version]
- Cirillo, V.; D’Amelia, V.; Esposito, M.; Amitrano, C.; Carillo, P.; Carputo, D.; Maggio, A. Anthocyanins are key regulators of drought stress tolerance in tobacco. Biology 2021, 10, 139. [Google Scholar] [CrossRef] [PubMed]
- Gitz III, D.C.; Liu-Gitz, L.; Britz, S.J.; Sullivan, J.H. Ultraviolet-B effects on stomatal density, water-use efficiency, and stable carbon isotope discrimination in four glasshouse-grown soybean (Glyicine max) cultivars. Environ. Exp. Bot. 2005, 53, 343–355. [Google Scholar] [CrossRef]
- Zhao, H.; Zhao, Z.; An, L.; Chen, T.; Wang, X.; Feng, H. The effects of enhanced ultraviolet-B radiation and soil drought on water use efficiency of spring wheat. J. Photochem. Photobiol. B 2009, 94, 54–58. [Google Scholar] [CrossRef] [PubMed]
- Nogués, S.; Allen, D.J.; Morison, J.I.L.; Baker, N.R. Ultraviolet-B radiation effects on water relations, leaf development, and photosynthesis in droughted pea plants. Plant Physiol. 1998, 117, 173–181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saud, S.; Li, X.; Chen, Y.; Zhang, L.; Fahad, S.; Hussain, S.; Sadiq, A.; Chen, Y. Silicon application increases drought tolerance of Kentucky bluegrass by improving plant water relations and morphophysiological functions. Sci. World J. 2014, 2014, 368694. [Google Scholar] [CrossRef]
- Hatfield, J.L.; Dold, C. Water-use efficiency: Advances and challenges in a changing climate. Front. Plant Sci. 2019, 10, 103. [Google Scholar] [CrossRef] [Green Version]
- Greger, M.; Kaur, H. A review on Si uptake and transport system. Plants 2019, 8, 81. [Google Scholar]
- Palma, C.F.F.; Castro-Alves, V.; Morales, L.O.; Rosenqvist, E.; Ottosen, C.-O.; Strid, Å. Spectral composition of light affects sensitivity to UV-B and photoinhibition in cucumber. Front. Plant Sci. 2021, 11, 610011. [Google Scholar] [CrossRef]
- Qian, M.; Rosenqvist, E.; Flygare, A.-M.; Kalbina, I.; Teng, Y.; Jansen, M.A.K.; Strid, Å. UV-A light induces a robust and dwarfed phenotype in cucumber plants (Cucumis sativus L.) without affecting fruit yield. Sci. Hortic. 2020, 263, 109110. [Google Scholar] [CrossRef]
- Currie, H.A.; Perry, C.C. Silica in plants: Biological, biochemical and chemical studies. Ann. Bot. 2007, 100, 1383–1389. [Google Scholar] [CrossRef]
- Schaller, J.; Heimes, R.; Ma, J.F.; Meunier, J.-D.; Feng Shao, J.; Fujii-Kashino, M.; Holger Knorr, K. Silicon accumulation in rice plant aboveground biomass affects leaf carbon quality. Plant Soil 2019, 444, 399–407. [Google Scholar] [CrossRef] [Green Version]
- Dallagnol, L.J.; Román Ramos, A.E.; da Rosa Dorneles, K. Silicon use in the integrated disease management of wheat: Current knowledge. In Current Trends in Wheat Research; IntechOpen: London, UK, 2020. [Google Scholar]
- Lichtenthaler, H.K.; Buschmann, C. Extraction of photosynthetic tissues: Chlorophylls and carotenoids. Curr. Protoc. Food Anal. Chem. 2001, 1, 165–170. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Buschmann, C. Chlorophylls and carotenoids: Measurement and characterization by UV-VIS spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, 1, 171–178. [Google Scholar] [CrossRef]
- Caldwell, M.M. Solar ultraviolet radiation as an ecological factor for alpine plants. Ecol. Monogr. 1968, 38, 243–268. [Google Scholar] [CrossRef]
- Drumm, H.; Mohr, H. The mode of interaction between blue (UV) light photoreceptor and phytochrome in anthocyanin formation of the Sorghum seedling. Photochem. Photobiol. 1978, 27, 241–248. [Google Scholar] [CrossRef]
- Schreiber, U.; Kühl, M.; Klimant, I.; Reising, H. Measurement of chlorophyll fluorescence within leaves using a modified PAM fluorometer with a fiber-optic microprobe. Photosynth. Res. 1996, 47, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Nečemer, M.; Kump, P.; Ščančar, J.; Jaćimović, R.; Simčič, J.; Pelicon, P.; Budnar, M.; Jeran, Z.; Pongrac, P.; Regvar, M.; et al. Application of X-ray fluorescence analytical techniques in phytoremediation and plant biology studies. Spectrochim. Acta Part B At. Spectrosc. 2008, 63, 1240–1247. [Google Scholar] [CrossRef]
- Vekemans, B.; Janssens, K.; Vincze, L.; Adams, F.; Vanespen, P. Analysis of X-ray spectra by iterative least squares (AXIL): New developments. X-ray Spectrom. 1994, 23, 278–285. [Google Scholar] [CrossRef]
- Kump, P.; Nečemer, M.; Rupnik, Z.; Pelicon, P.; Ponikvar, D.; Vogel-Mikuš, K.; Regvar, M.; Pongrac, P. Improvement of the XRF quantification and enhancement of the combined applications by EDXRF and micro-PIXE. In Integration of Nuclear Spectrometry Methods as a New Approach to Material Research; IAEA: Vienna, Austria, 2011; pp. 101–109. [Google Scholar]
- Korndörfer, G.H.; Coelho, M.N.; Snyder, G.H.; Mizutani, C.T. Evaluation of soil extractants for silicon availability in upland rice. Rev. Bras. Ciência Solo 1999, 23, 101–106. [Google Scholar] [CrossRef]
- Ter Braak, C.J.F.; Šmilauer, P. CANOCO Reference Manual and CanoDraw for Windows User’s Guide: Software for Canonical Community Ordination (Version 4.5); Microcomputer Power: Ithaca, NY, USA, 2002. [Google Scholar]
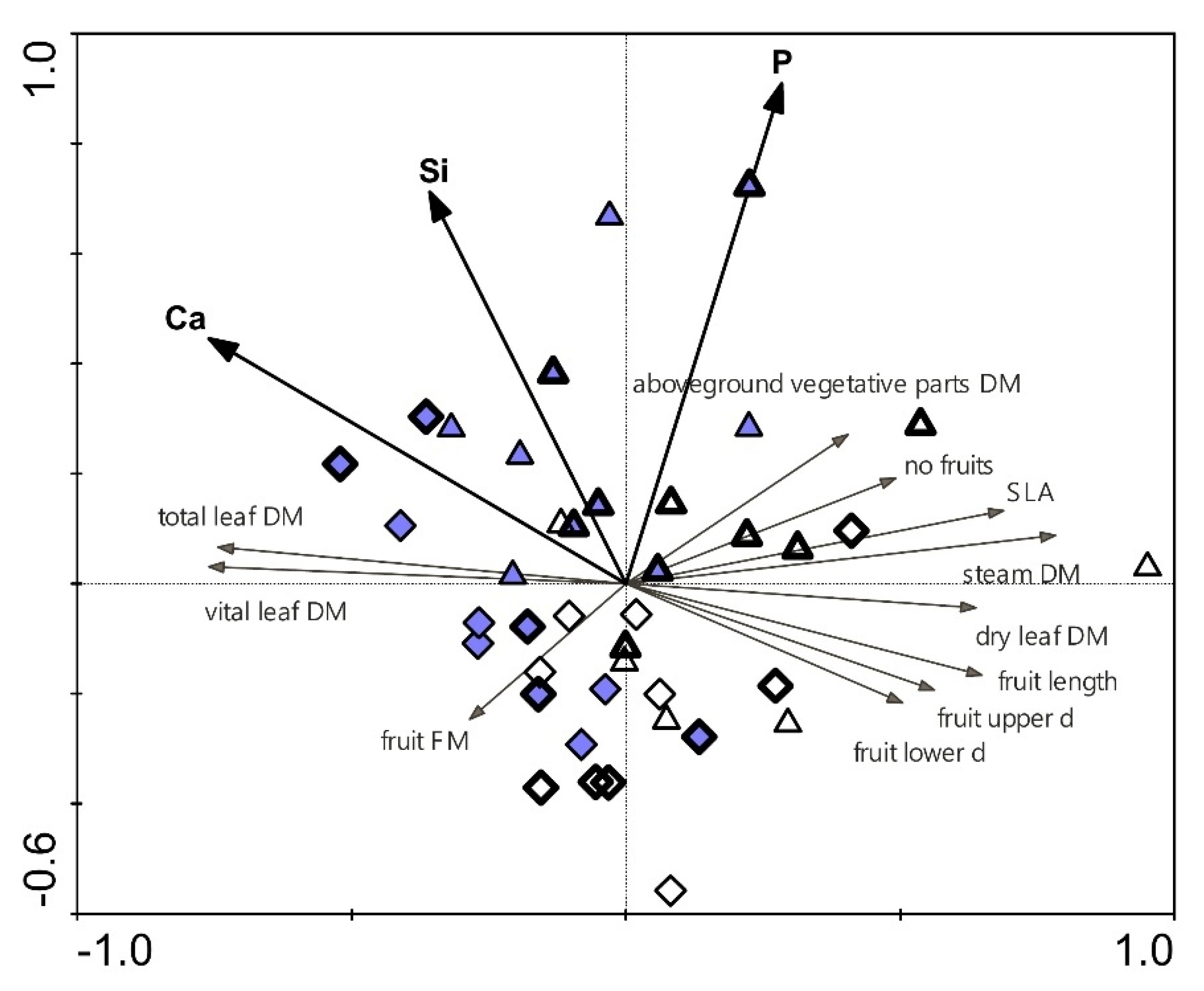
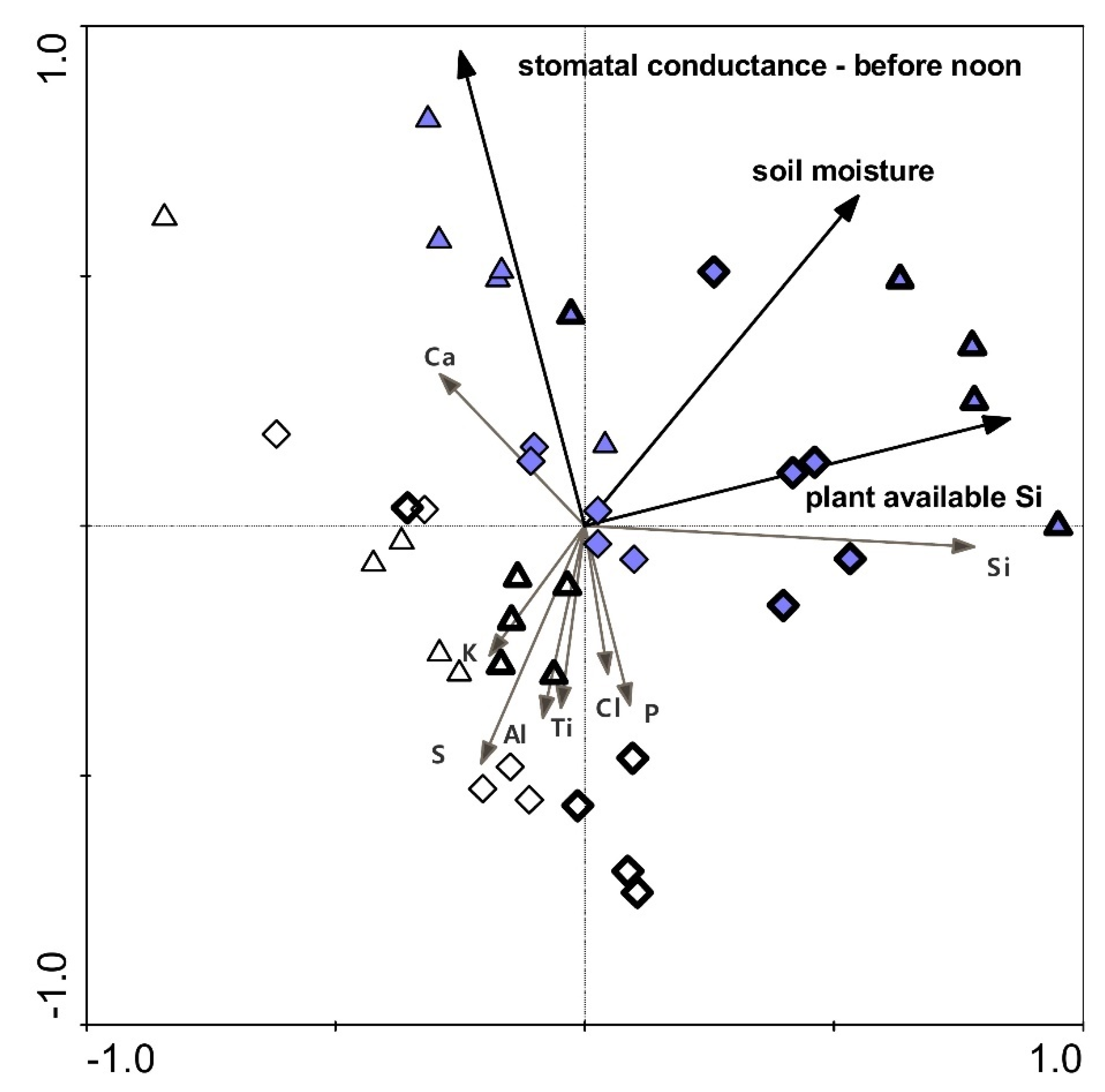
| Plant Parameters | Units | Treatment | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Si+W+UV+ | Si+W+UV– | Si+W–UV+ | Si+W–UV– | Si–W+UV+ | Si–W+UV– | Si–W–UV+ | Si–W–UV– | ||
| Biochemical | |||||||||
| UV-B-AS | a.u. g−1 | 280 ± 38 abc | 242 ± 448 a | 352 ± 60 d | 331.5 ± 76.2 cd | 311 ± 50 bcd | 248 ± 39 ab | 350± 40 d | 328 ± 90 cd |
| UV-A-AS | a.u. g−1 | 470 ± 65 ab | 426 ± 73 a | 611 ± 112 c | 587 ± 133 c | 514 ± 94.0 abc | 424 ± 69.9 a | 576 ± 74 bc | 538 ± 137 bc |
| Chl a/chl b | 1.28 ± 0.27 ab | 1.50 ± 0.17 bc | 1.17 ± 0.18 a | 1.40 ± 0.20 abc | 1.53 ± 0.23 bc | 1.69 ± 0.18 c | 1.12 ± 0.22 a | 1.15 ± 0.21 a | |
| Total chl/car | 6.92 ± 0.73 abc | 5.54 ± 0.41 a | 6.97 ± 1.35 abc | 6.07 ± 0.92 abc | 5.98 ± 0.56 ab | 5.06 ± 0.79 a | 7.74 ± 2.62 bc | 8.02 ± 1.97 c | |
| Element composition | |||||||||
| P content | % of DM | 0.61 ± 0.18 bc | 0.41 ± 0.11 ab | 0.56 ± 0.13 abc | 0.36 ± 0.18 a | 0.70 ± 0.15 c | 0.45 ± 0.09 ab | 0.54 ± 0.15 abc | 0.45 ± 0.15 ab |
| S content | % of DM | 0.26 ± 0.04 ab | 0.24 ± 0.05 a | 0.30 ± 0.05 ab | 0.27 ± 0.08 ab | 0.32 ± 0.08 ab | 0.28 ± 0.05 ab | 0.33 ± 0.08 b | 0.28 ± 0.06 ab |
| Cl content | % of DM | 0.35 ± 0.07 c | 0.18 ± 0.04 a | 0.29 ± 0.07 bc | 0.23 ± 0.07 ab | 0.34 ± 0.08 c | 0.27 ± 0.04 bc | 0.27 ± 0.06 bc | 0.22 ± 0.06 ab |
| K content | % of DM | 2.00 ± 0.35 b | 1.57 ± 0.16 a | 1.88 ± 0.14 ab | 1.95 ± 0.47 ab | 1.91 ± 0.26 ab | 1.89 ± 0.26 ab | 1.98 ± 0.30 b | 1.74 ± 0.21 ab |
| Other | |||||||||
| Vital leaves | g of DM | 249.7 ± 37.8 c | 262.7 ± 29.2 c | 44.6 ± 17.5 a | 123.5 ± 73.6 b | 259.5 ± 31.0 c | 286.0 ± 18.4 c | 111.5 ± 56.6 b | 140.3 ± 24.2 b |
| Dead leaves | g of DM | 16.4 ± 7.6 ab | 15.7 ± 2.7 ab | 29.1 ± 5.4 c | 20.7 ± 13.2 abc | 10.9 ± 2.6 a | 10.7 ± 7.0 a | 21.3 ± 5.2 bc | 19.1 ± 6.9 ab |
| All leaves | g of DM | 266.1 ± 32.7 c | 278.4 ± 30.6 c | 73.7 ± 16.2 a | 144.1 ± 61.1 b | 270.4 ± 29.1 c | 296.7 ± 15.5 c | 132.8 ± 51.7 b | 159.4 ± 19.1 b |
| Stems | g of DM | 264.4 ± 23.9 d | 256.5 ± 18.3 d | 174.9 ± 19.6 a | 216.7 ± 31.6 bc | 232.5 ± 42.2 cd | 238.0 ± 21.7 cd | 205.3 ± 12.6 abc | 197.5 ± 13.5 ab |
| Aboveground vegetative parts | g of DM | 530.5 ± 23.6 c | 535.0 ± 45.3 c | 248.6 ± 27.2 a | 360.9 ± 55.8 b | 502.9 ± 57.6 c | 534.8 ± 32.8 c | 338.0 ± 59.1 b | 356.8 ± 20.7 b |
| Fruits, length | mm | 73.6 ± 12.5 b | 73.7 ± 12.8 b | 61.6 ± 10.4 a | 73.5 ± 14.4 b | 69.5 ± 14.2 ab | 72.9 ± 12.1 b | 72.0 ± 11.3 b | 70.6 ± 11.8 b |
| Fruits, upper width | mm | 23.8 ± 5.8 ab | 26.6 ± 5.1 b | 21.4 ± 4.9 a | 26.7 ± 7.9 b | 22.5 ± 5.4 ab | 24.6 ± 5.2 ab | 24.7 ± 5.3 ab | 25.0 ± 5.7 ab |
| Fruits, lower width | mm | 14.5 ± 3.7 a | 14.9 ± 4.7 a | 11.5 ± 2.6 a | 15.2 ± 4.9 a | 13.3 ± 3.8 a | 13.6 ± 3.8 a | 15.4 ± 3.9 a | 13.3 ± 3.8 a |
| Aboveground vegetative parts/fruit mass | 0.85 ± 0.19 a | 0.70 ± 0.08 a | 0.86 ± 0.34 a | 0.90 ± 0.29 a | 0.75 ± 0.18 a | 0.76 ± 0.13 a | 0.74 ± 0.05 a | 0.69 ± 0.07 a | |
| SLA | cm2 mg−1 | 0.18 ± 0.03 ab | 0.16 ± 0.02 a | 0.22 ± 0.03 b | 0.19 ± 0.04 ab | 0.21 ± 0.05 b | 0.19 ± 0.04 ab | 0.23 ± 0.04 b | 0.20 ± 0.05 b |
| Parameter | Factorial ANOVA | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Si | W | UV | Si × W | Si × UV | W × UV | Si × W × UV | ||||
| Stomatal conductance, morning § | ns | *** | + | ns | ns | ns | ns | ns | ||
| Stomatal conductance, before noon § | ns | ** | + | *** | − | ns | ns | ns | ns | |
| Stomatal conductance, afternoon § | ns | *** | + | ** | − | ns | ns | ns | * | |
| Vital leaves | * | − | *** | + | ** | − | ns | ns | ns | ns |
| Dead leaves | * | + | *** | − | ns | ns | ns | ns | ns | |
| All leaves | * | − | *** | + | ** | − | ns | ns | ns | ns |
| Stems | ns | *** | + | ns | ns | ns | ns | ns | ||
| Aboveground vegetative parts | ns | *** | + | ** | − | * | ns | ns | * | |
| Fruit mass | ns | *** | + | * | − | ns | ns | ns | ns | |
| Number of fruits | ns | *** | + | ns | ns | ns | ns | ns | ||
| Fruits, length | ns | * | + | ns | ns | ns | ns | ns | ||
| Fruits, upper width | ns | ns | ** | − | ns | ns | ns | ns | ||
| Fruits, lower width | ns | ns | ns | ns | ns | ns | ns | |||
| Aboveground vegetative parts/fruit mass | ns | ns | ns | ns | ns | ns | ns | |||
| SLA | ns | * | − | * | + | ns | ns | ns | ns | |
| UV-B-AS | ns | *** | − | * | + | ns | ns | ns | ns | |
| UV-A-AS | ns | *** | − | ns | ns | ns | ns | ns | ||
| Chl a/chl b | ns | *** | + | * | − | ** | ns | ns | ns | |
| Total chl/car | ns | ** | − | ns | * | ns | ns | ns | ||
| Leaf Si | *** | + | *** | + | ** | + | ns | ns | ns | ns |
| Leaf P | ns | ns | *** | + | ns | ns | ns | ns | ||
| Leaf S | ns | ns | ns | ns | ns | ns | ns | |||
| Leaf Cl | ns | ns | *** | + | ns | ns | ns | ns | ||
| Leaf K | ns | ns | ns | ns | ns | ns | * | |||
| Leaf Ca | ** | − | *** | + | ns | ns | ns | ns | ns | |
| Plant-available Si, after K2SiO3 treatment | *** | + | ns | ns | ns | *** | ns | * | ||
| Plant-available Si, after water shortage treatment | *** | + | * | − | * | − | ns | ns | ns | ns |
| Soil Parameter | Units | Treatment | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Si+W+UV+ | Si+W+UV– | Si+W–UV+ | Si+W–UV– | Si–W+UV+ | Si–W+UV– | Si–W–UV+ | Si–W–UV– | ||
| Total soil Si 1 | % of DM | 11.12 ± 1.20 a | 10.07 ± 0.82 a | 11.00 ± 1.21 a | 10.43 ± 1.45 a | 9.93 ± 1.75 a | 9.76 ± 1.68 a | 9.59 ± 1.06 a | 9.98 ± 1.68 a |
| Total soil Si 2 | % of DM | 10.92 ± 0.29 c | 9.54 ± 1.01 ab | 9.84 ± 1.00 abc | 9.91 ± 0.88 abc | 10.60 ± 0.53 bc | 10.44 ± 0.99 bc | 9.91 ± 1.28 abc | 9.00 ± 0.95 a |
| Total soil Ca 1 | % of DM | 4.29 ± 0.16 ab | 4.47 ± 0.24 ab | 4.70 ± 0.56 b | 4.28 ± 0.62 ab | 4.40 ± 0.79 ab | 4.02 ± 0.25 ab | 4.44 ± 0.42 ab | 3.99 ± 0.36 a |
| Total soil Ca 2 | % of DM | 4.34 ± 0.19 b | 4.20 ± 0.25 ab | 4.43 ± 0.40 b | 4.17 ± 0.08 ab | 4.21 ± 0.34 ab | 4.39 ± 0.23 b | 3.97 ± 0.18 a | 4.14 ± 0.16 ab |
| Total soil K 1 | % of DM | 1.14 ± 0.06 a | 1.15 ± 0.08 a | 1.10 ± 0.04 a | 1.06 ± 0.16 a | 1.07 ± 0.16 a | 1.00 ± 0.11 a | 1.04 ± 0.10 a | 1.04 ± 0.11 a |
| Total soil K 2 | % of DM | 1.02 ± 0.04 a | 1.04 ± 0.07 a | 1.08 ± 0.08 a | 1.08 ± 0.05 a | 1.02 ± 0.06 a | 1.06 ± 0.13 a | 1.03 ± 0.06 a | 1.00 ± 0.08 a |
| Plant Biomass and Growth Parameters | Environmental and Leaf Physiological Parameters | |||||||
|---|---|---|---|---|---|---|---|---|
| Soil Moisture # | Fv/Fm, Morning § | Fv/Fm, Noon § | Fv/Fm, Day § | Fq’/Fm’ § | Stomatal Conductance, Morning § | Stomatal Conductance, Before Noon § | Stomatal Conductance, Afternoon § | |
| Vital leaves | 0.874 ** | 0.623 ** | 0.589 ** | −0.262 | 0.123 | 0.833 ** | 0.553 ** | 0.858 ** |
| Dead leaves | −0.546 ** | −0.607 ** | −0.522 ** | 0.074 | −0.104 | −0.568 ** | −0.540 ** | −0.668 ** |
| All leaves | 0.887 ** | 0.610 ** | 0.583 ** | −0.274 | 0.122 | 0.840 ** | 0.542 ** | 0.857 ** |
| Stems | 0.699 ** | 0.239 | 0.369 * | −0.489 ** | 0.110 | 0.618 ** | 0.305 | 0.553 ** |
| Aboveground vegetative parts | 0.898 ** | 0.541 ** | 0.561 ** | −0.366 * | 0.128 | 0.837 ** | 0.510** | 0.829 ** |
| Fruit mass | 0.757 ** | 0.552 ** | 0.495 ** | −0.191 | 0.250 | 0.702 ** | 0.436 ** | 0.750 ** |
| Number of fruits | 0.712 ** | 0.326 * | 0.532 ** | −0.260 | 0.167 | 0.605 ** | 0.178 | 0.597 ** |
| Fruits, length | 0.335 * | 0.340 * | 0.343 * | −0.079 | 0.023 | 0.317 * | 0.412 ** | 0.428 ** |
| Fruits, upper width | 0.079 | 0.113 | 0.380 * | −0.077 | 0.027 | 0.104 | 0.312 | 0.224 |
| Fruits, lower width | 0.159 | 0.286 | 0.155 | −0.077 | −0.023 | 0.114 | 0.194 | 0.172 |
| Aboveground vegetative parts/fruit mass | −0.092 | −0.310 | −0.164 | −0.226 | −0.238 | −0.082 | −0.054 | −0.215 |
| SLA | −0.334 * | −0.378 * | −0.369 * | 0.059 | −0.290 | −0.386 * | −0.331 * | −0.450 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grašič, M.; Germ, M.; Vogel-Mikuš, K.; Ojdanič, N.; Gaberščik, A.; Golob, A. Fertilisation with Potassium Silicate Exerted Little Effect on Production Parameters of Cucumbers Exposed to UV and Drought. Stresses 2021, 1, 142-161. https://doi.org/10.3390/stresses1030012
Grašič M, Germ M, Vogel-Mikuš K, Ojdanič N, Gaberščik A, Golob A. Fertilisation with Potassium Silicate Exerted Little Effect on Production Parameters of Cucumbers Exposed to UV and Drought. Stresses. 2021; 1(3):142-161. https://doi.org/10.3390/stresses1030012
Chicago/Turabian StyleGrašič, Mateja, Mateja Germ, Katarina Vogel-Mikuš, Nik Ojdanič, Alenka Gaberščik, and Aleksandra Golob. 2021. "Fertilisation with Potassium Silicate Exerted Little Effect on Production Parameters of Cucumbers Exposed to UV and Drought" Stresses 1, no. 3: 142-161. https://doi.org/10.3390/stresses1030012
APA StyleGrašič, M., Germ, M., Vogel-Mikuš, K., Ojdanič, N., Gaberščik, A., & Golob, A. (2021). Fertilisation with Potassium Silicate Exerted Little Effect on Production Parameters of Cucumbers Exposed to UV and Drought. Stresses, 1(3), 142-161. https://doi.org/10.3390/stresses1030012








