Abstract
This investigation was done to assess the role of abscisic acid (ABA; 25 µM) and/or nitrogen (N; 10 mM) in the alleviation of salinity (NaCl; 100 mM)-induced reduction in photosynthetic activity and growth, N and sulfur (S) assimilation of mustard (Brassica juncea L.) cv. RH0-749. Salinity treatment caused oxidative stress and significantly elevated the content of both H2O2 and thiobarbituric acid reactive substances (TBARS), and impaired photosynthetic activity and growth, but increased the content of nitrogenous osmolyte proline and the activity of antioxidant enzymes involved in the metabolism of reactive oxygen species. The application of 25 µM ABA under a controlled condition negatively affected photosynthesis and growth. However, ABA, when combined with N, minimized oxidative stress and mitigated the salinity-inhibited effects by increasing the activity of antioxidant enzymes (superoxide dismutase, SOD; glutathione reductase, GR; ascorbate peroxidase, APX) and proline content. Overall, the supplementation of 10 mM N combined with 25 µM ABA provides an important strategy for enhancing the photosynthetic potential of B. juncea under saline conditions.
1. Introduction
Oilseed crops play a prominent role in agricultural industries and trade throughout the world [1]. The edible oilseed crop, Indian mustard (Brassica juncea L.), stands second to peanuts; accounts for 27.8% of the country’s oilseed economy; and is cultivated throughout the northern Indian plains [2]. Unfortunately, most of its cultivation depends on groundwater sources varying from medium to high salinity levels [3]. Salinity is a serious soilconstraint that negatively affects agriculture production, particularly in arid and semi-arid regions of the world [4]. Salinity affects about 2% (6.74 Mha) of Indian land [5,6].
In plants, salt stress mainly causes ionic stress by increasing Na+ and Cl− ions concentrations and thereby prevents essential elements attainment, homeostasis and metabolism [7,8]. In addition to causing cellular water imbalance, osmotic stress and abscission, salinity stress may also significantly impact all phases of photosynthesis and other gas exchange traits [9]. Notably, the accumulation of Na+ and Cl− ions (ionic toxicity) causes oxidative stress due to the physiological imbalance between generation and antioxidants-mediated scavenging of reactive oxygen species (ROS), such as H2O2, O2− and ˙OH [10]. Elevated or non-metabolized ROS may cause the oxidation of proteins and membrane lipids and also impair cellular redox homeostasis [11].
Plants tolerance to salinity stress mainly involve the maintenance of ionic homeostasis; metabolic adjustments that lead to the accumulation of compounds containing nitrogen (N; such as an organic solute proline) or sulfur (S; such as cysteine, Cys; glutathione, GSH); induction of phytohormones (such as abscisic acid, ABA) and/or the scavenging of ROS through antioxidant enzymes (such as superoxide dismutase, SOD; glutathione reductase, GR; ascorbate peroxidase, APX) and antioxidant metabolites/non-enzymes (such as ascorbate, AsA; glutathione, GSH) [12,13,14]. Osmotic adjustment can be maintained by the endogenous production of ABA and/or nitrogenous compatible solutes, such as proline. Interestingly, both ABA and proline are closely linked, where exogenously applied ABA-mediated salinity tolerance has been linked to the increased production of proline [15,16]). On the one hand, ABA can effectively reduce Na+ and Cl− content and Na+/K+ ratio, increase K+ and Ca2+ content and the accumulation of proline [17]. On the other hand, in addition to osmotic adjustment, proline scavenges varied ROS and thereby contributes to the strengthening of the antioxidant defense system and the eventual alleviation of stressimpacts in plants [16]. Proline metabolism in salinity-exposed plants is also closely linked with N nutrition, where N-availability regulates proline production and plant salinity tolerance [14,18]. Notably, S-assimilatory products in plant salinity tolerance are also of great significance, where one of the S-assimilation products and S-containing amino acid Cys acts as a precursor or donor of reduced S for a range of S-compounds including GSH [19]. Interestingly, coordination occurs between the assimilatory pathways of N and S, where nitrate reductase (NR) is involved in NO3− reduction and ATP-sulfurylase (ATP-S) activates the metabolically inert SO42−; and makes it usable in Cys and GSH [14,18,19]. In turn, besides acting as ROS-scavengers, GSH (and also proline) can modulate the photosynthesis functions by influencing the activity of ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco) [20,21,22]. It has also been shown that exogenous supplementation of ABA or N enhances salinity tolerance in plants [23,24,25]. Additionally, N-supply was reported to improve salinity tolerance by maintaining GSH production [26].
Despite the above facts, the study on the interaction of ABA and N in the regulation of photosynthetic activity via the control on the antioxidant defense system under salt stress has not been done. Additionally, the projected increase in salinity-affected areas to 16.2 Mha by 2050 due to climate change can further aggravate the situation of the cultivation of oilseed crops [27]. Hence, it is hypothesized that a coordination between N-containing osmolyte (proline) and phytohormone (ABA) can help tomaintain the cellular ionic (Na+ and Cl−) homeostasis; reduced S-containing compounds (Cys and GSH); and a fine-tuning among antioxidant enzymes, and thereby can combat elevated ROS-accrued oxidative stress and improve cellular redox homeostasis, and eventually improve growth and stomatal behavior; photosynthesis in salinity-exposed plants supplied with optimum concentrations of ABA and N. To test the highlighted hypothesis, the present work investigated the interactive effects of ABA and N in the regulation of photosynthetic activity, growth and N and S-assimilation, antioxidant activity and proline in B. juncea plants under NaCl stressed conditions.
2. Results
In the first experiment, where the screening of ABA concentrations was performed, exogenously supplied ABA (>5 μM) significantly decreased photosynthetic and growth attributes in the absence of NaCl but did not influence the status of oxidative stress parameters (H2O2 and TBARS contents) in comparison to the control. However, in the presence of NaCl (100 mM), photosynthetic and growth parameters were favorably influenced by exogenously supplied ABA when compared to the NaCl-stressed plants. Under 100 mM NaCl stress, a lesser effect of 50 μM ABA on the studied parameters was observed when compared to 25 μM ABA. However, among the ABA concentrations screened, 25 μM ABA most efficiently increased chlorophyll content (+56.6%), net photosynthesis (+108.1%), stomatal conductance (+55.6%), intercellular CO2 concentration (+59%), leaf area (+50.2%), plant dry mass (+60.3%) and reduced the contents of H2O2 (−59.3%) and TBARS (−54.0%) in comparison to the NaCl-stressed plants (Table 1).

Table 1.
Selection of ABA concentration. Chlorophyll content (SPAD value), net photosynthesis (μmol CO2 m−2 s−1), stomatal conductance (mmol CO2 m−2 s−1), intercellular CO2 concentration (µmol CO2 mol−1), leaf area (cm2 plant−1), plant dry mass (g plant−1), Na+ and Cl− content (mg g−1 leaf dry mass) and H2O2 and TBARS content (nmol g−1 fresh weight) of Indian mustard (Brassica juncea L.) at 30 days after sowing. Plants were grown individually with 0, 5, 10, 25 and 50 μM ABA in the presence or absence of 100 mM NaCl. Data are presented as treatments mean ± SE (n = 4). The same letter in columns indicates that data did not differ significantly by Duncan’s multiple range test at p < 0.05. ABA, Abscisic acid.
2.1. Impact of ABA and/or N on Accumulation of Na+ and Cl− Ions and Oxidative Stress
The content of Na+ and Cl− in both the leaves and roots of plants was analyzed to determine the potential of 25 μM ABA along with 10 mM N in modulating the ion accumulation NaCl (100 mM)-exposed B. juncea plants. The content of Na+ and Cl− significantly increased in NaCl-treated B. juncea roots and leaves; however, higher contents of Na+ and Cl− were observed in roots than in leaves (Figure 1A–D). Exogenously applied ABA or N individually lowered Na+ and Cl− ion accumulation in comparison with both the control and salt-stressed plants. Additionally, the combined treatment of ABA and N further reduced the accumulation of these ions in both roots and leaves when compared to the control plants. In particular, under salt stress conditions, N reduced leaf and root Na+ accumulation by 33.1% and 35.2% and Cl− accumulation by 38.0% and 28.0%, respectively; whereas ABA reduced leaf and root Na+ accumulation by 26.6% and 21.1% and Cl− accumulation by 32.1% and 20.9%, respectively, in comparison to the NaCl-stressed plants. Moreover, the application of ABA together with N reduced root Na+ by 43.7%, root Cl− by 36.4%, leaf Na+ by 43.1% and leaf Cl− by 46.2% in the presence of salt stress compared to the NaCl alone treated plants (Figure 1A–D).
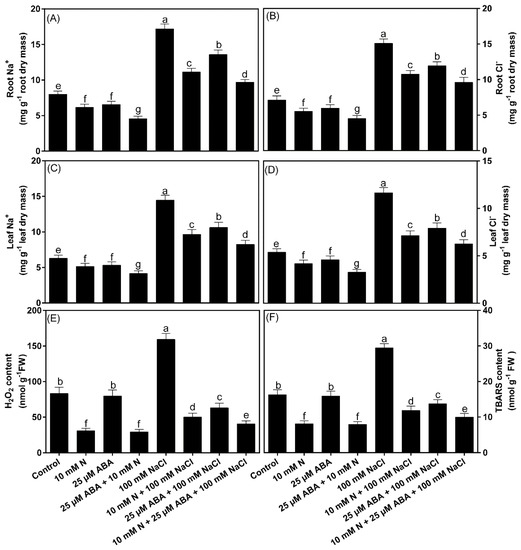
Figure 1.
Contents of leaf and root Na+ and Cl− (A–D), H2O2 (E), thiobarbituric acid reactive substances (TBARS; (F)) in Indian mustard (Brassica juncea L.) at 30 days after sowing. Plants were grown individually with 0 or 10 mM N and treated with 25 μM ABA in the presence or absence of 100 mM NaCl. Data are presented as treatments mean ± SE (n = 4). The same letter above bars indicates that data did not differ significantly by Duncan’s multiple range test at p < 0.05. ABA, Abscisic acid; N, Nitrogen.
Contents of H2O2 and TBARS were quantified in order to evaluate the role of ABA and/or N in the mitigation of oxidative stress. NaCl (100 mM) stress alone increased the content of H2O2 (+99.5%) and TBARS (+81.1%) in comparison to the control. Plants treated with ABA or N without NaCl showed N-mediated reduction in H2O2 and TBARS content compared to control; whereas ABA treated plants had values equivalent to control plants. In the presence of NaCl, both ABA and N independently reduced H2O2 by 69.1% and 59.1% and TBARS by 62.0% and 53.1%, respectively. However, ABA combined with N reduced oxidative stress more conspicuously and reduced the content of H2O2 by 75.6% and TBARS by 66.0% in comparison to NaCl-stressed plants (Figure 1E,F).
2.2. Visualization of the Status of O2− and H2O2 in Leaves Using Histochemical Staining and Confocal Laser Scanning Microscopy
Histochemical staining method was employed to visualize the status of oxidative stress parameters, such as O2− generation (as shown by blue staining of leaves) and H2O2 (as shown by brown staining of leaves) using NBT and DAB staining methods, respectively. The staining spots were more pronounced in NaCl treated leaf discs compared to the control. However, restricted staining spots were observed under salt stress in the leaves of plants thatreceived ABA or N alone in comparison with NaCl treated leaves. Moreover, ABA, together with N, more prominently reduced the staining spots in the presence of NaCl stress (Figure 2A–J).
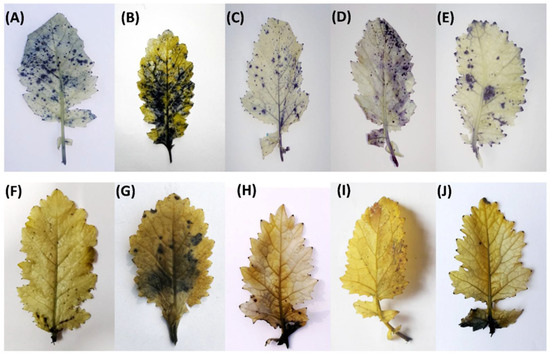
Figure 2.
Representative images showing the level of in situ generation of superoxide ions (O2−) by nitro blue tetrazolium (NBT) staining of the leaves (A–E) and generation of H2O2 by 3,3′-diaminobenzidine (DAB) staining of the leaves (F–J) in Indian mustard (Brassica juncea L.) at 30 days after sowing. Plants were grown with 0 (A,F), 100 mM NaCl (B,G), 10 mM N + 100 mM NaCl (C,H), 25 μM ABA + 100 mM NaCl (D,I) and 25 μM ABA + 10 mM N + 100 mM NaCl (E,J). NaCl, Sodium chloride; ABA, Abscisic acid; N, Nitrogen.
Further, the analysis with 2’,7’dichlorofluorescein (H2DCFDA) fluorescence revealed the status of the accumulation of H2O2 in roots. The roots grown with NaCl alone showed a higher intensity of green fluorescence. In contrast, the roots of plants supplemented with ABA or N individually exhibited a lesser intensity of green fluorescence, though the result was more conspicuous with N than ABA supply. The combined supplementation of ABA and N under NaCl stress most effectively reduced the H2O2 content, which was revealed through a lesser green fluorescence, almost similar to the green fluorescence observed in the roots of the control plants (Figure 3A–E).
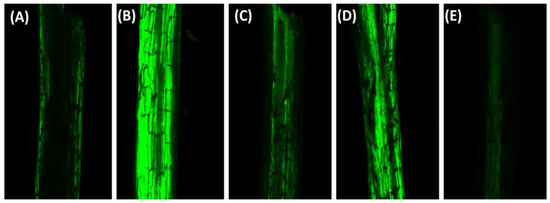
Figure 3.
Representative confocal microscopic images of H2O2 formation in roots of Indian mustard (Brassica juncea L.) using H2DCFDA staining (A–E) at 30 days after sowing. Plants were grown with 0 (A), 100 mM NaCl (B), 10 mM N + 100 mM NaCl (C), 25 μM ABA + 100 mM NaCl (D) and 25 μM ABA + 10 mM N + 100 mM NaCl (E). NaCl, Sodium chloride; ABA, Abscisic acid; N, Nitrogen.
2.3. Influence of ABA and/or N on Photosynthetic Characteristics under NaCl Stress
Salt stress severely inhibited photosynthetic characteristics (PN, gs, Ci and chlorophyll). Plants receiving N showed higher values of the photosynthetic characteristics in comparison to the control, both under stress and non-stress conditions. Under control conditions (without stress), ABA treatment decreased the values of photosynthetic parameters. However, in NaCl stressed plants, supplementation of ABA or N independently improved PN by 109% and 181%, gs by 63% and 101%, Ci by 52% and 98% and Chl content by 55% and 96%, respectively, in comparison to NaCl alone treated plants. The combined supplementation of ABA and N resulted in prominent increases in PN by 238%, gs by 136%, Ci by 133% and Chl content by 137% in comparison to NaCl-stressed plants (Table 2). NaCl stress severely inhibited Rubisco activity in comparison to the control plants. The plants supplemented with N showed higher Rubisco activity both under control and stressed conditions. Under non-stress conditions, plants treated with ABA showed reduced Rubisco activity. However, under stress conditions, plants supplemented with ABA or N individually resulted in enhanced Rubisco activity by 173.1% and 247.0%, respectively, in comparison to the NaCl-treated plants. Further, supplementation of ABA and N together maximally increased Rubisco activity by about three-fold in comparison to the NaCl-treated plants (Table 2).

Table 2.
Net photosynthetic rate (μmol CO2 m−2 s−1), stomatal conductance (mmol CO2 m−2 s−1), intercellular CO2 (µmol CO2 mol−1), chlorophyll content (SPAD value), Rubisco activity (µmol CO2 mg−1 protein m−1), proline content (mg g−1 fresh mass), leaf area (cm2 plant−1) and plant dry mass (g plant−1) in Indian mustard (Brassica juncea L.) at 30 days after sowing. Plants were grown individually with 0 or 10 mM N, treated with 25 μM ABA in the presence or absence of 100 mM NaCl. Data are presented as treatments mean ± SE (n = 4). The same letter in rows indicates that data did not differ significantly by Duncan’s multiple range test at p < 0.05. ABA, Abscisic acid; N, Nitrogen.
2.4. Impact of ABA and/or N on Growth under NaCl Stress
Salt stress significantly decreased leaf area and plant dry mass. In contrast, N supplementation increased leaf area by 52.0% and plant dry mass by 66.2% under non-stressed conditions in comparison to control plants. ABA application in the absence of NaCl resulted in reduced leaf area by 30.2% and plant dry mass by 28.8% in comparison to the control plants. Under NaCl stress conditions, leaf area and plant dry mass were improved by 55.8% and 97.1% with ABA and by 112.0% and 166.1% with N application. A higher increase of 167.2% in leaf area and 222.1% in plant dry mass was noted with the combined application of ABA and N in comparison to salt-stressed plants (Table 2).
2.5. Impact of ABA and/or N on Proline Content under NaCl Stress
NaCl treatment induced proline accumulation in plants. Exogenous supplementation of either N or ABA individually increased proline content compared to the control plants. However, ABA supplementation with N increased proline accumulation by 80% under salt stress and 36.9% under non-stress compared to salt-stressed plants (Table 2).
2.6. Impact ofABA and/or N on Stomatal Behavior under NaCl Stress
The leaf samples of NaCl treated plants showed closed stomata, while stomata were open in the leaf samples of control plants. The treatment of ABA in the presence of NaCl resulted in stomatal closure. Plants treated with N alone or N + ABA in the presence of NaCl showed that stomata were partially opened (Figure 4).
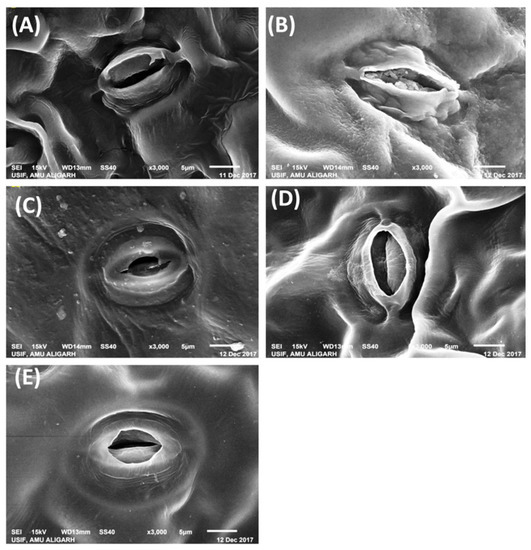
Figure 4.
Representative images showing the leaf stomatal behavior in Indian mustard (Brassica juncea L.) under 0 (A), 100 mM NaCl (B), 10 mM N + 100 mM NaCl (C), 25 μM ABA + 100 mM NaCl (D) and 25 μM ABA + 10 mM N + 100 mM NaCl (E). The opening and closing of stomata were studied under the scanning electron microscope at 3000× g magnifications at 30 days after sowing. NaCl, Sodium chloride; ABA, Abscisic acid; N, Nitrogen.
2.7. Impact of ABA and/or N on Antioxidant Metabolism under NaCl Stress
The activity of APX, GR and SOD was measured for assessing the involvement of these enzymes in the antioxidant defense system when treated with ABA and/or N under NaCl-induced oxidative stress. Under NaCl stress, the activity of APX, GR, and SOD increased by 33.3%, 53.6% and 42.2%, respectively, in comparison to the control plants. Individual supplementation of N increased the activity of these enzymes both under stress and control conditions. On the contrary individual supplementation of ABA under control conditions did not show any significant change in the activity of these enzymes. However, under stress conditions, plants supplemented with ABA exhibited an increase in the activity of APX by 120.3%, GR by 117.0% and SOD by 74.2%; whereas, plants receiving N exhibited an increase in APX by 120.2%, GR by 117.5% and SOD by 78.2% in comparison to the control plants. A comparatively more pronounced increase in the activity of these enzymes was observed when ABA + N was supplemented together under NaCl stress conditions. The combined application of ABA + N increased activity of APX, GR and SOD by 182.0%, 175.1% and 92.2%, respectively, in comparison to the plants exposed to NaCl stress (Table 3).

Table 3.
Activity of ascorbate peroxidase (APX), glutathione reductase (GR) and superoxide dismutase (SOD) in Indian mustard (Brassica juncea L.) at 30 days after sowing. Plants were grown individually with 0 or 10 mM N and treated with 25 μM ABA in the presence or absence of 100 mM NaCl. Data are presented as treatments mean ± SE (n = 4). The same letter in rows indicates that data did not differ significantly by Duncan’s multiple range test at p < 0.05. ABA, Abscisic acid; N, Nitrogen.
2.8. Impact of ABA and/or N on Cysteine and GSH Content under NaCl Stress
Under NaCl stress, the contents of Cys and GSH increased by 14.6% and 39.2%, respectively, in comparison to the control plants. Individual supplementation of N showed increased Cys and GSH content both under stress and in control conditions. ABA alone could not induce Cys and GSH content under control conditions, while it showed a significant increase in the contents of these parameters in N-supplemented plants. Under control conditions, N increased GSH content by 27.3% and Cys content by 128.1% in comparison to the control. Under stress conditions, plants supplemented with ABA exhibited an increase in GSH content by 26.1% and Cys content by 98.0%, while plants receiving N exhibited an increase in GSH content by 34.0% and Cys content by 109.2% in comparison to the NaCl-treated plants. A comparatively more pronounced increase was found under stress conditions when ABA was supplemented together with N. The combined application of ABA plus N under salt stress increased GSH by 45.2% and Cys by 126.1% in comparison to the NaCl-treated plants (Figure 5).
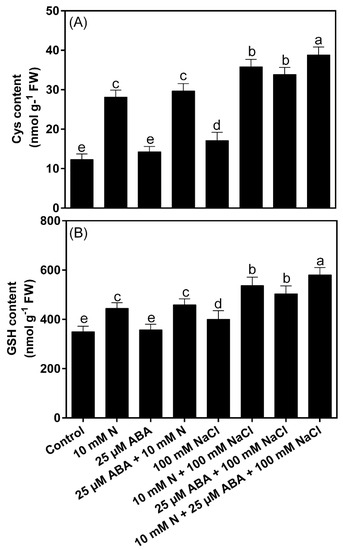
Figure 5.
Content of cysteine (Cys; (A)) and reduced glutathione (GSH; (B)) in Indian mustard (Brassica juncea L.) at 30 days after sowing. Plants were grown individually with 0 or 10 mM N and treated with 25 μM ABA in the presence or absence of 100 mM NaCl. Data are presented as treatments mean ± SE (n = 4). The same letter above bars indicates that data did not differ significantly by Duncan’s multiple range test at p < 0.05. ABA, Abscisic acid; N, Nitrogen; FW, Fresh weight.
2.9. Effect of ABA and/or N on Contents of N, S and Activity of NR and ATP-S under NaCl
NaCl treatment reduced N content by 48.1% and NR activity by 23.6% in comparison to the control plants. Under control conditions, the supplementation of N alone or N together with ABA increased N content and NR activity equally by about 62.2% and 46.3%, respectively, while individual supplementation of ABA did not result in any significant change in both the parameters. However, under NaCl stress, plants supplemented with ABA or N alone showed an increase in the N content (25.2% or 49.1%) and NR activity (49.2% or 57.1%), respectively. The combined treatment of ABA and N showed a more prominent increase in N content and NR activity by 130.5% and 116.0%, respectively, in comparison to the NaCl-treated plants (Figure 6A,B). Under NaCl stress, S content decreased by 44.0%, while ATP-S activity increased by 33.4% in comparison to the control plants. Under non-stress conditions, N supplementation increased S content by 43.1% and ATP-S activity by 76.0%, while ABA supplementation alone had no significant effect in comparison to the control plants. Plants grown with salt stress and N showed increased S content and ATP-S activity by 50.1% and 48.2%, respectively, while plantsreceiving ABA in the presence of salt stress increased S content and ATP-S activity by 26.3% and 8.2%, respectively, in comparison to the NaCl-treated plants. However, a more prominent increase was noted with combined treatment of ABA and N; where the increase of 122.2% and 60.0% in S content and ATP-S activity, respectively, was observed in comparison to the NaCl-treated plants (Figure 6C,D).
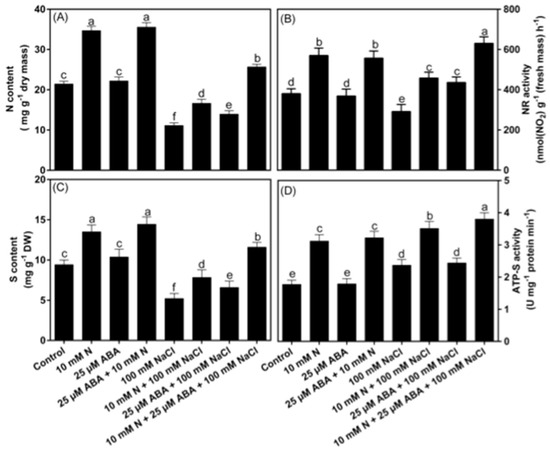
Figure 6.
Content of nitrogen (N; (A)), sulfur (S; (C)) and the activity of nitrate reductase (NR; (B)) and ATP−sulfurylase (ATP−S; (D)) in Indian mustard (Brassica juncea L.) at 30 days after sowing. Plants were grown individually with 0 or 10 mM N and treated with 25 μM ABA in the presence or absence of 100 mM NaCl. Data are presented as treatments mean ± SE (n = 4). The same letter above bars indicates that data did not differ significantly by Duncan’s multiple range test at p < 0.05. ABA, Abscisic acid; N, Nitrogen; DW, Dry weight.
3. Discussion
To date, most studies on salinity, ABA and N have focused either on salinity as a limiting factor for plant health and productivity [10] or on the ABA [28] or N [29] influence on plant health and productivity, while their interactive effects are still not fully understood. Hence, understanding the interaction between salinity, ABA and N supply could be of great agronomic importance. Given this, the effort was made herein to interpret the results and discuss the same in detail the ABA, N and their interactive significance taking into account three major aspects: (i) modulation of ionic toxicity (Na+ and Cl−) and mineral elements (N and S)-accumulation, and the status of oxidative stress and antioxidant metabolism; (ii) control of the contents of N and S; N-containing osmolyte proline and S-containing compounds (Cys and GSH), and activity of NR and ATP-S; and (iii) the significance of the cumulative outcome of the aspects on the overall improvement of the photosynthetic and growth parameters.
3.1. Modulation of Ionic Toxicity (Na+ and Cl−) and Mineral Elements (N and S)-Accumulation, and the Status of Oxidative Stress and Antioxidant Metabolism
As observed in the present study, the elevated accumulation of Na+ and Cl− ions in plant tissues was considered an obvious response in plants exposed to soils with high NaCl concentrations [30]. Upon entry into the cells, these Na+ and Cl− may cause osmotic stress (causing cellular water deficit), imbalance of the uptake of essential nutrients (such as N and S) and/or elevated or non-metabolized ROS-accrued oxidative stress [4]. Plants tend to acquire nutrients from the environment surrounding their root system. However, the presence of soil salinity and excess Na+ and Cl− ions can disrupt the uptake of most nutrients in glycophytes (such as B. juncea) through competitive interactions or by affecting the membrane selectivity for ions [13]. In context with the salinityimpact on N and S contents, salinity (50 mM NaCl)-mediated decreases in the contents of both N and S were reported in B. juncea [31]. In particular, enhancing the supply of N or its sources (such as NO3−) in salinity-exposed crop plants was reported to significantly reduce Na+ and Cl− contents, increase N level in leaves and thereby alleviate crop salt injury or enhance its salt tolerance [26,32]. On the other hand, the occurrence of the positive relationship between ABA accumulation and salinity tolerance, and the ABA-mediated accumulation of K+, Ca2+ and compatible solutes, such as proline (and also sugars), in root vacuoles were argued to counteract the uptake of Na+ and Cl− [17,33].
Elevated ions (Na+ and Cl−) in plant organs, including roots and leaves, can cause oxidative stress by hampering the balance between the generation and scavenging of ROS, such as studied herein O2− and H2O2 [34]. Excess or non-metabolized ROS may cause the oxidation of macromolecules, including membrane lipids [11]. Measured herein in terms of TBARS content, the highest lipid peroxidation was observed in NaCl (100 mM)-alone treated B. juncea, which coincides with recent reports [34,35]. However, the contents of both oxidative stress parameters (O2− and H2O2) and lipid peroxidation indicator (TBARS) were least in NaCl (100 mM)-exposed B. juncea supplied with 25 μM ABA and 10 mM N, which could be attributed to ABA- and/or N-mediated decreases in the toxicity of ions (Na+ and Cl−) and eventual minimization of oxidative stress and its consequences.
As one of the defense strategies, the increased oxidative stressinduced the antioxidant defense system in order to protect B. juncea against salt stress-caused damages. This study revealed ABA (25 μM) and N (10 mM)-mediated maximal enhancements in the activity of SOD, an enzyme involved in O2− dismutation to H2O2; APX, an enzyme involved in H2O2-metabolism; and also the activity of GR, an enzyme involved in the regeneration of reduced GSH that as a major component of the Halliwell–Asada pathway is directly or indirectly involved in ROS-metabolism [36,37,38,39,40]. Earlier, the supply of N [41] and ABA [42,43,44,45] has been reported to enhance ROS-metabolizing enzymes in different stressed test plants.
3.2. Control of the Status of N-Containing Osmolyte Proline and S-Containing Compounds (Cys and GSH), and Activity of NR and ATP-S
Plants tend to metabolically adapt to salinity stress by mainly enhancing the synthesis of S-rich compounds (such as Cys and GSH) and N-containing proteogenic amino acids/osmolytes (such as proline) [22]. Therefore, in relation toABA and N supplies in NaCl-exposed B. juncea, it is imperative to discuss the status of tuning between N- and S-assimilatory enzymes, NR and ATP-S, respectively; and N-and S-assimilatory products, proline, and Cys and GSH, respectively. On the one hand, exogenously supplied N triggers the synthesis of compatible solutes, such as proline (a nitrogenous osmolyte), and the solute played a vital role in osmotic adjustment in NaCl-exposed plants [46]. In the study presented here, the maximal increase in proline was observed when 10 mM N and 25 μM ABA were supplemented together under both stressed and non-stressed conditions. Notably, besides acting as a compatible solute, proline also played an important role in scavenging free radicals and protecting redox potential under NaCl stress conditions [47]. The plant status of proline and other compatible solutes was regulated by phytohormones (including ABA) and mineral nutrients (including N) [16]. Further, exogenously supplied proline was also reported to mediate the upregulation of genes associated with the antioxidant defense system in salinity-stressed plants [48]. Interestingly, proline biosynthesis is regulated by the crosstalk of ABA signaling and phosphate homeostasis through activation of the P5CS1 gene [49]. On the other hand, the assimilatory pathways of N and S are well coordinated in plants; and the S metabolism is strongly affected by the plant’s N status [50]. Hence, N (10 mM)-supply mediated enhancements in the contents of N and S, the activity of NR and APT-S and increases in Cys and GSH contents in NaCl (100 mM)-exposed B. juncea are obvious. NR is a rate-limiting enzyme for NO3− reduction, where its sustained activity is crucial for N assimilation; whereas, ATP-S activates the metabolically inert SO42− and makes it usable in Cys and GSH during S assimilation [18,19]. Earlier, exogenous supplementation of N was reported to restore the ATP-S activity in N-deficient mediums [51]. Additionally, an exogenous supply of S enhanced the ATP-sulfurylase and NR activitywhen compared with plants grown without S [52]. In earlier studies, the exogenous supplementation of GSH improved salt stress tolerance and thereby growth and development in plants under NaCl stress [53,54,55]. With an improved S assimilation pathway, the plants’ potential to survive under oxidative stress conditions has been reported in various studies [56,57].
3.3. Overall Improvement in the Photosynthetic and Growth Parameters
Photosynthetic and growth parameters are among the major parameters at the whole plant level that can reflect the cell-level metabolic processes in plants growing under varying conditions. The reduction in growth and photosynthesis under salt stress may be due to the overproduction of ROS, as increased TBARS and H2O2 content were observed. ROS interferes with the proper functioning of cell membrane lipids, proteins and other important enzymes of metabolic pathways, and thus, resulted in reduced growth and photosynthetic attributes. Several studies showed a decrease in growth under NaCl stress [58,59,60]. The reduction in leaf area under salt stress might be due to the toxicity of Na+ and Cl− in the shoot cells. If finely coordinated, various components of the antioxidant defense system (such as GSH, proline, SOD, APX and GR) can efficiently metabolize varied ROS, such as H2O2 and O2− and ˙OH; and thereby protect photosynthetic and growth parameters. As discussed above in Section 4.1 and Section 4.2, the combined supply of ABA (25 μM) and N (10 mM) significantly improved primarily: (i) NR activity that contributed to an increased cellular level of proline, which in turn maintained osmotic adjustment; and secondarily (ii) enhanced ATP-S activity contributed in yielding first Cys and thereafter the reduced pool of GSH; and thereby cumulatively scavenged varied ROS and protected photosynthetic and growth parameters of B. juncea against NaCl (100 mM)-impacts. The increased leaf area and plant dry mass with N has been attributed to the increased GSH level [61]. This is in confirmation of the earlier findings that exogenously supplied N improves growth and development by increasing photosynthesis, chlorophyll content, proline production and nitrogen metabolism [62,63]. Fatma et al. [64] reported that increased ATP-S activity in B. juncea indicated its higher sulfate accumulation capacity with increased photosynthetic attributes and plant dry mass.Notably, GSH and proline can influence the activity of Rubisco and modulate the photosynthetic functions and eventually growth parameters [20,21,22].
Notably, the scanning electron microscopic study revealed the potential of ABA andN on the stomatal behavioral response of plants. In our study, comparatively closed stomata were found in NaCl treated plants compared tothe control plants. Treatment with N reduced the closing effect of NaCl stress on stomata, and combined treatment of ABA and N maximally reduced the effect of NaCl on stomatal width aperture. Generally, it has been shown that ABA induces stomatal closure, but it has also been found that an increase in intracellular GSH suppresses stomatal closure [65]. The combined treatment of ABA and N prominently influenced GSH production, which resulted in the opening of stomata when compared with salt-treated plants. It is interesting to note that stomatal closure occurs on the reduction of intracellular GSH and that intracellular GSH regulates stomatal behavior. Hence, this study confirmed the role of intracellular GSH in N and ABA-mediated regulation of NaCl-induced ROS production and stomatal closure.
4. Methodology
4.1. Experimental Design and Growth Conditions
Seeds of Indian mustard (Brassica juncea L. cv. RH0-749) obtained from the Indian Agricultural Research Institute (IARI), New Delhi, were sterilized using HgCl2 solution (0.01%) and were washed repeatedly with double distilled water (DDW). Sterilized seeds were sown in clay pots filled with 5 kg of acid-washed sand, purified according to the method given by Hewitt [66]. The pots were kept in the greenhouse of the Department of Botany, Aligarh Muslim University, Aligarh, India, with average day/night temperatures of 23/14 ± 3 °C, 620 μmol m−2 s−1 photosynthetically active radiation (PAR) and the relative humidity was 62 ± 3%. After germination, three plants per pot were maintained. Two experiments were performed. In the first experiment, a concentration of ABA exhibiting the highest alleviation of salinity stress-impact was worked out by applying 0, 5, 10, 25 and 50 μM ABA to plants grown with 0 or 100 mM NaCl and studying photosynthesis, growth and oxidative stress. ABA was dissolved in ethanol to prepare the stock solution (100 mM), which was then diluted with DDW to obtain the desired concentrations of the solution. The ABA solution was sprayed on the foliage evenly at 15 days after germination using a hand sprayer. Based on the results obtained from this first experiment, 25 μM ABA was found to maximally alleviate the impact of 100 mM NaCl on photosynthesis, growth and oxidative stress. Additionally, based on an earlier report, 10 mM was considered as the optimal concentration for N in alleviating the salinity impact on B. juncea [18]. Hence, 25 μM ABA and 10 mM N were considered in the second experiment, where 0 or 25 μM ABA was applied to the leaf of plants grown with 10 mM N in the absence or presence of 100 mM NaCl at 20 days after sowing (DAS). Salt and N treatments were given at 10 DAS. KNO3 was used as the source of N, K+ concentration was maintained in all treatments by the addition of KCl. The control set of plants was supplemented with 250 mL of Hoagland nutrient solution at alternate days and 250 mL of distilled water daily. Sampling was done at 30 DAS. The design of the experiment was a randomized complete block design, and the number of replicates for each treatment was four.
4.2. Analyses of Na+ and Cl− Content
Content of Na+ and Cl− was measured in roots and leaves. One gram of oven-dried plant tissue was dissolved in 4 mL concentrated HNO3 (68%) in a 100 mL glass beaker. The beaker containing the digested sample was heated on a water bath until brown effervescence was observed. Afterward, 38 mL of tri acid mixture solution was added drop-wise till a clear solution was obtained. The solution containing plant samples was dried on the hot plate, and after that, dried samples were diluted with DDW to make a final volume of 100 mL. The Na+ content was measured using a flame photometer (Khera-391: Khera Instruments, New Delhi), and Cl− content by titration against the 0.02 N silver nitrate using 5% potassium chromate solution as an indicator.
4.3. Content of H2O2 and TBARS
The H2O2 content was measured in leaves by following the method of Okuda et al. [67]. Leaves (1.0 g) were grounded using 200 mM perchloric acid (ice-cold) and centrifuged at 1200× g for 10 min, and the supernatant was neutralized by adding 4 M potassium hydroxide. For measuring optical density (OD) at 590 nm, 2 mL of the eluate was mixed with 1 mL solution containing 160 µL of 3- methyl-2-benzothiazoline hydrazone, 40 µL of peroxidase and 800 µL of 12.5 mM 3-(dimethylamino) benzoic acid.
The TBARS content was measured in leaves by following the method of Dhindsa et al. [68]. Leaves (1.0 g) grounded in 0.25% thiobarbituric acid (TBA) were heated for 30 min in a water bath and rapidly cooled in cold water, followed by centrifugation (10,000× g) for 15 min. For measuring OD at 532 nm, 2 mL of supernatant was mixed with 8 mL 20% TCA containing 0.5% TBA. The content of TBARS was calculated using an extinction coefficient of 155 mM−1 cm−1.
4.4. Superoxide Ion (O2−) and H2O2 by a Histochemical Staining Method
In situ determination of the level of superoxide ion (O2−) and H2O2 generation were visually detected by following the method of Kumar et al. [69] with slight modification. Freshly prepared nitro blue tetrazolium (NBT) solution and 3,3-diaminobenzidine (DAB) solution were used for detecting O2− and H2O2, respectively. NBT solution was formed by dissolving 0.2 g of NBT in 100 mL of sodium phosphate buffer (50 mM; pH 7.5). The DAB solution (pH 3.8) was formed by dissolving 100 mg of DAB in DDW in an amber-colored bottle. For NBT and DAB staining, leaf samples were soaked in NBT solution and DAB solutions, respectively, and incubated overnight at room temperature. The samples were then boiled for 20 min in absolute ethanol, and then photographs were taken.
4.5. Analysis of H2O2 in Roots by Confocal Laser Scanning Microscopy
The content, as well as visualization of H2O2, was done in leaves. In order to know the status of ROS (such as H2O2) at the root level, confocal microscopy was employed to visualize H2O2 formation in roots of B. juncea using 2′,7′ dichlorofluorescein (H2DCFDA) staining. In brief, root samples were immersed for 15 min in a freshly prepared 12.5 µM H2DCFDA solution. After repeated washing with DDW, temporary slides of stained samples were prepared, and fluorescence was monitored using a confocal laser scanning microscope (Model LSM 780; Carl Zeiss, Oberkochen, Germany) at excitation 400–490 nm and emission ≥ 520 nm.
4.6. Photosynthetic Parameters and Rubisco Activity
Net photosynthetic rate (PN), intercellular CO2 concentration (Ci) and stomatal conductance (gs) were measured using an Infrared Gas Analyzer (IRGA, Model CID-340). The parameters were measured between 11.00 a.m. and 1.00 p.m. at light saturating intensity (PAR: 760 μmol m−2 s−1), temperature 24 ± 2 °C and at 385 ± 15 μmol mol−1 atmospheric CO2 concentration. Chlorophyll content was measured in fully expanded young leaves using the SPAD chlorophyll meter (Model 502 DL PLUS, Konica Minolta, Inc., Tokyo, Japan). The Rubisco activity was measured in leaves by following the method of Usuda [70].
4.7. Growth Parameters
The plants were dried in the oven at 80 °C, and the dried material was weighed on an electrical balance. Leaf area (LA) was calculated with a leaf area meter (Model LA 211, Systronics, New Delhi, India).
4.8. Assay of Antioxidant Enzymes
Fresh leaves (0.2 g) were homogenized with an extraction buffer using a chilled mortar and pestle. The extraction buffer was prepared by dissolving 0.05% Triton X-100 and 1% polyvinylpyrrolidone in 100 mM potassium phosphate buffer (pH 7.0). The homogenate was centrifuged (15,000× g) at 4 °C for 20 min, and the supernatant was used for the assay of superoxide dismutase (SOD) and glutathione reductase (GR). For the ascorbate peroxidase (APX) assay, 2 mM ascorbate was added to the extraction buffer. The activity of APX and SOD was calculated by following the method of Asada [71] and Beyer and Fridovich [72], respectively. The activity of GR was measured according to the method of Foyer and Halliwell [73].
4.9. Determination of Nitrate Reductase (NR) Activity and N Content
The NR activity was measured according to the method of Kuo et al. [74]. One gram of leaves was frozen in liquid nitrogen, ground to a powder and then homogenized in 250 mM Tris-HCl buffer (pH 8.5) using a chilled mortar and pestle. The buffer was prepared by dissolving 1 mM EDTA, 10 mM Cys, 1 mM DTT and 20 µM FAD in 10% glycerol. The homogenate was centrifuged (10,000× g) at 4 °C for 30 min. NR activity was measured as the rate of nitrite production at 28 °C following the method of Nakagawa et al. [75]. In the reaction mixture, NADH was used for initiating the reaction. After 20 min, the reaction was ended by adding 1 N HCl containing 1 mL of 1% sulphanilamide solution. Subsequently, 1 mL of 0.02% aqueous NED was added. The reaction mixture (1.5 mL) containing enzyme extract, 10 mM KNO3, 0.065 M HEPES (pH 7.0) and 0.5 mM NADH in 0.04 mM phosphate buffer (pH 7.2) was used for measuring absorbance at 540 nm using a spectrophotometer after 10 min.
The content of N was measured by the Kjeldahl digestion method, as described by Lindner [76]. A 20 mL aliquot of the digested leaf sample was taken in a 100 mL volumetric flask. To this flask, 10% sodium silicate (2 mL) and 4 mL of 2.5 N sodium hydroxide solutions were added to prevent turbidity and neutralize the excess of acid, respectively. The volume was made up to 100 mL with DDW. In a 20 mL test tube, 10 mL aliquot was taken, and 1 mL Nessler’s reagent was added. The final volume was maintained with DDW. The OD was recorded on a spectrophotometer at 525 nm.
4.10. Determination of Proline Content
The proline content was calculated according to the method of Bates et al. [77]. Fresh leaf tissues (1.0 g) were homogenized in 10 mL of 3% sulphosalicylic acid, and 4 mL each of acid ninhydrin and glacial acetic acid was added to the filtrate. The test tubes containing the homogenate filtrate were heated in a water bath for one hour, followed by immediate cooling of the test tubes in ice-cold water. Afterward, the mixture was extracted with toluene, and the OD was measured on a spectrophotometer at 520 nm using L-proline as a standard.
4.11. Analyses of Content of S, Cysteine, GSH, and ATP-S Activity
For measuring S content, 0.2 g oven-dried leaves were ground and dissolved in a solution containing 70% HNO3 and 60% HClO4 (85:15, v/v). The sulfur content was measured following the turbidimetric method. For turbidity development, 2.5 mL gum acacia solution (0.25%) and 1.0 g BaCl2 were added to a 5 mL aliquot, and the final volume was made using DDW. Within 10 min of the turbidity development, the OD was measured at 415 nm.
The content of Cys and GSH was measured according to the method of Gaitonde [78] and Anderson [79], respectively. In fresh leaves, the activity of ATP-sulfurylase (ATP-S) was measured according to the method of Lappartient and Touraine [80].
4.12. Analysis of Stomatal Behavior
Fresh leaves were fixed with 2.5% glutaraldehyde solution for 4–5 h at room temperature. Following repeated washing steps using phosphate buffer (15 min at each step), the samples were dehydrated through a graded series of ethanol solutions (60%, 70%, 80% and 95%) for about 20 min at each step. After that, the samples were placed in absolute ethanol. The small sections of dehydrated samples were coated with gold-palladium and observed under the scanning electron microscope (SEM; Carl Zeiss EVO 40, Carl Zeiss AG, Oberkochen, Germany) at a magnification of 3000× g. The stomata were visualized using SEM images.
4.13. Statistical Analysis
The data were subjected to statistical analysis using analysis of variance (ANOVA), and Duncan’s multiple range test was used to compare means of different treatments by IBM SPSS software (version 22.0).The number of replicates for each treatment was four, and data are presented as a mean ± SE. The least significant difference (LSD) at p < 0.05 was considered as significant.
5. Conclusions
Overall, the results indicated that NaCl stress severely induced oxidative stress through overproducing ROS and eventually adversely affected the photosynthesis and plant growth of B. juncea. Exogenously supplied N enhanced photosynthesis and growth parameters under no stress and protected these parameters against salt stress. However, ABA supplementation improved these parameters only under stressed conditions. Compared to the individual influence of N and ABA, their combined application proved to be the most effective in combating NaCl−-induced toxic effects on photosynthesis and growth. The more positive influence of the combined application of ABA and N was through their effect on nitrogenous osmolyte (proline), antioxidant enzymes (SOD, APX and GR), N and S-assimilation and production of Cys and GSH. Therefore, the supplementation of 10 mM N in combination with 25 µM ABA prominently enhanced the photosynthetic potential of oleiferous brassica under saline conditions. However, the precise regulatory molecular mechanism of ABA and/or N-induced NaCl stress tolerance opens the avenue for future research.
Author Contributions
Conceptualization, A.M. (Arif Majid), N.A.A. and N.A.K.; investigation and data curation, A.M. (Asim Masood), B.A.R. and A.M. (Arif Majid); microscopic analysis, biochemical analysis and physiological analysis, A.M. (Asim Massod), B.A.R. and Z.S.; original draft preparation, A.M. (Asim Masood); editing and improvement, A.M. (Arif Majid), N.A.A. and N.A.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not Applicable.
Informed Consent Statement
Not Applicable.
Data Availability Statement
The data presented in this study are available in the graphs and tables provided in the manuscript.
Acknowledgments
The author A.M. (Asim Masood) is thankful for the necessary research facility developed under the Department of Science and Technology SERB (Project code:SB/YS/LS-108/2014), and UGC START-UP Project (Code: 100057/B30493) New Delhi, India. The first author would also like to thank the University Grant Commission (UGC), New Delhi, for the student scholarship.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Abrol, D.P.; Shankar, U. Integrated pest management. In Breeding Oilseed Crops for Sustainable Production; Academic Press: Cambridge, MA, USA, 2016; pp. 523–549. [Google Scholar]
- Shekhawat, K.; Rathore, S.S.; Premi, O.P.; Kandpal, B.K.; Chauhan, J.S. Advances in agronomic management of Indian mustard (Brassicajuncea (L.) Czern & Coss.): An overview. Int. J. Agron. 2012, 2012, 408284. [Google Scholar] [CrossRef]
- Singh, K.H.; Shakya, R.; Mahawar, R.K. Genetic diversity and patterns of variation among Indian mustard (Brassica juncea (L.) Czern. & Coss.) varieties. J. Breed. Genet. 2014, 46, 329–339. [Google Scholar]
- Shrivastava, P.; Kumar, R. Soil salinity: A serious environmental issue and plant growth promoting bacteria as one of the tools for its alleviation. Saudi J. Biol. Sci. 2015, 22, 123–131. [Google Scholar] [CrossRef]
- Management of Salt Affected Soils. FAO Soil Portal. Food and Agriculture Organization of the United Nations. 2021. Available online: http://www.fao.org/soils-portal/soil-management/management-of-some-problem-soils/salt-affected-soils/en/ (accessed on 16 August 2021).
- Sehgal, J.L.; Abrol, I.P. Soil Degradation in India: Status and Impact; Oxford & IBH Publishing Co.: Delhi, India, 1994. [Google Scholar]
- Khan, M.I.R.; Asgher, M.; Khan, N.A. Alleviation of salt-induced photosynthesis and growth inhibition by salicylic acid involves glycine betaine and ethylene in mungbean (Vigna radiata L.). Plant Physiol. Biochem. 2014, 80, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Ahanger, M.A.; Agarwal, R.M. Salinity stress induced alterations in antioxidant metabolism and nitrogen assimilation in wheat (Triticum aestivum L) as influenced by potassium supplementation. Plant Physiol. Biochem. 2017, 115, 449–460. [Google Scholar] [CrossRef]
- Rasheed, F.; Anjum, N.A.; Masood, A.; Sofo, A.; Khan, N.A. The key roles of salicylic acid and sulfur in plant salinity stress tolerance. J. Plant Growth Regul. 2020, 1–14. [Google Scholar] [CrossRef]
- Isayenkov, S.V.; Maathuis, F.J.M. Plant Salinity Stress: Many Unanswered Questions Remain. Front. Plant Sci. 2019, 10, 80. [Google Scholar] [CrossRef] [PubMed]
- Anjum, N.A.; Sofo, A.; Scopa, A.; Roychoudhury, A.; Gill, S.S.; Iqbal, M.; Lukatkin, A.S.; Pereira, E.; Duarte, A.C.; Ahmad, I. Lipids and proteins—Major targets of oxidative modifications in abiotic stressed plants. Environ. Sci. Pollut. Res. 2015, 22, 4099–4121. [Google Scholar] [CrossRef]
- Tuteja, N. Mechansims of high salinity tolerance in plants. Methods Enzymol. 2007, 428, 419–438. [Google Scholar] [PubMed]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef]
- Anjum, N.A.; Gill, S.S.; Gill, R. Cysteine—Jack of all glutathione-based plant stress defense trades. In Plant Adaptation to Environmental Change: Significance of Amino Acids and Their Derivatives; Anjum, N.A., Gill, S.S., Gill, R., Eds.; CABI: Wallingford, UK, 2014; pp. 35–52. [Google Scholar]
- Iqbal, N.; Umar, S.; Khan, N.A.; Khan, M.I.R. A new perspective of phytohormones in salinity tolerance: Regulation of proline metabolism. Environ. Exp. Bot. 2014, 100, 34–42. [Google Scholar] [CrossRef]
- Per, T.S.; Khan, N.A.; Reddy, P.S.; Masood, A.; Hasanuzzaman, M.; Khan, M.I.R.; Anjum, N.A. Approaches in modulating proline metabolism in plants for salt and drought stress tolerance: Phytohormones, mineral nutrients and transgenics. Plant Physiol. Biochem. 2017, 115, 126–140. [Google Scholar] [CrossRef]
- Gurmani, A.R.; Bano, A.; Ullah, N.; Khan, H.; Jahangir, M.; Flowers, T.J. Exogenous abscisic acid (ABA) and silicon (Si) promote salinity tolerance by reducing sodium (Na+) transport and bypass flow in rice (Oryza sativa indica). Aust. J. Crop Sci. 2013, 7, 1219–1226. [Google Scholar]
- Iqbal, N.; Umar, S.; Khan, N.A. Nitrogen availability regulates proline and ethylene production and alleviates salinity stress in mustard (Brassica juncea). J. Plant Physiol. 2015, 178, 84–89. [Google Scholar] [CrossRef]
- Anjum, N.A.; Gill, R.; Kaushik, M.; Hasanuzzaman, M.; Pereira, E.; Ahmad, I.; Tuteja, N.; Gill, S.S. ATP-sulfurylase, sulfur-compounds, and plant stress tolerance. Front. Plant Sci. 2015, 6, 210. [Google Scholar] [CrossRef] [PubMed]
- Sivakumar, P.; Sharmila, P.; Saradhi, P.P. Proline suppresses rubisco activity by dissociating small subunits from holoenzyme. Biochem. Biophys. Res. Commun. 2001, 282, 236–241. [Google Scholar] [CrossRef] [PubMed]
- Son, J.A.; Narayanankutty, D.P.; Roh, K.S. Influence of exogenous application of glutathione on rubisco and Rubiscoactivase in heavy metal-stressed tobacco plant grown in vitro. Saudi J. Biol. Sci. 2014, 21, 89–97. [Google Scholar] [CrossRef]
- Anjum, N.A.; Aref, I.M.; Duarte, A.C.; Pereira, E.; Ahmad, I.; Iqbal, M. Glutathione and proline can coordinately make plants withstand the joint attack of metal(loid) and salinity stresses. Front. Plant Sci. 2014, 5, 662. [Google Scholar] [CrossRef]
- Sripinyowanich, S.; Klomsakul, P.; Boonburapong, B.; Bangyeekhun, T.; Asami, T.; Gu, H.; Chadchawan, S. Exogenous ABA induces salt tolerance in Indica rice (Oryza sativa L.): The role of OsP5CS1 and OsP5CR gene expression during salt stress. Environ. Exp. Bot. 2013, 86, 94–105. [Google Scholar] [CrossRef]
- Gao, S.; Sun, W.; Li, Y.; Shi, Y.; Qi, X. Physiological and biochemical effects of exogenous salicylic acid (SA) and abscisic acid (ABA) on maize seedlings under salt stress. Mol. Breed. 2017, 15, 4159–4164. [Google Scholar]
- Sikder, R.K.; Wang, X.; Zhang, H.; Gui, H.; Dong, Q.; Jin, D.; Song, M. Nitrogen enhances salt tolerance by modulating the antioxidant defense system and osmoregulation substance content in Gossypiumhirsutum. Plants 2020, 9, 450. [Google Scholar] [CrossRef]
- Ahanger, M.A.; Qin, C.; Begum, N.; Maodong, Q.; Dong, X.X.; El-Esawi, M.; El-Sheikh, M.A.; Alatar, A.A.; Zhang, L. Nitrogen availability prevents oxidative effects of salinity on wheat growth and photosynthesis by up-regulating the antioxidants and osmolytes metabolism, and secondary metabolite accumulation. BMC Plant Biol. 2019, 19, 479. [Google Scholar] [CrossRef]
- Mandal, S.; Raju, R.; Kumar, A.; Kumar, P.; Sharma, P.C. Current status of research, technology response and policy needs of salt-affected soils in India–A Review. J. Indian Soc. Coast. Agric. Res. 2018, 36, 40–53. [Google Scholar]
- Sah, S.K.; Reddy, K.R.; Li, J. Abscisic acid and abiotic stress tolerance in crop plants. Front. Plant Sci. 2016, 7, 571. [Google Scholar] [CrossRef] [PubMed]
- Leghari, S.J.; Wahocho, N.A.; Laghari, G.M.; Hafeez Laghari, A.; Mustafa Bhabhan, G.; Hussain Talpur, K.; Bhutto, T.A.; Wahocho, S.A.; Lashari, A.A. Role of nitrogen for plant growth and development: A review. Adv. Environ. Biol. 2016, 10, 209–219. [Google Scholar]
- Gupta, B.; Huang, B. Mechanism of salinity tolerance in plants: Physiological, biochemical, and molecular characterization. Int. J. Genom. 2014, 2014, 701596. [Google Scholar] [CrossRef] [PubMed]
- Nazar, R.; Iqbal, N.; Syeed, S.; Khan, N.A. Salicylic acid alleviates decreases in photosynthesis under salt stress by enhancing nitrogen and sulfur assimilation and antioxidant metabolism differentially in two mungbean cultivars. J. Plant Physiol. 2011, 168, 807–815. [Google Scholar] [CrossRef] [PubMed]
- Abdolzadeh, A.; Shima, K.; Lambers, H.; Chiba, K. Change in uptake, transport and accumulation of ions in Nerium oleander (Rosebay) as affected by different nitrogen sources and salinity. Ann. Bot. 2008, 102, 735–746. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Li, J.; Wang, S.; Hüttermann, A.; Altman, A. Salt, nutrient uptake and transport, and ABA of Populuseuphratica; a hybrid in response to increasing soil NaCl. Trees 2001, 15, 186–194. [Google Scholar] [CrossRef]
- Hussain, S.J.; Khan, N.A.; Anjum, N.A.; Masood, A.; Khan, M.I.R. Mechanistic elucidation of salicylic acid and sulphur-induced defence systems, nitrogen metabolism, photosynthetic, and growth potential of mung-bean (Vignaradiata) under salt stress. J. Plant Growth Regul. 2021, 40, 1000–1016. [Google Scholar] [CrossRef]
- Syeed, S.; Sehar, Z.; Masood, A.; Anjum, N.A.; Khan, N.A. Control of Elevated Ion Accumulation, Oxidative Stress, and Lipid Peroxidation with Salicylic Acid-Induced Accumulation of Glycine Betaine in Salinity Exposed Vignaradiata L. Appl. Biochem. Biotechnol. 2021, 1–20. [Google Scholar] [CrossRef]
- Alscher, R.G.; Erturk, N.; Heath, L.S. Role of superoxide dismutases (SODs) in controlling oxidative stress in plants. J. Exp. Bot. 2002, 53, 1331–1341. [Google Scholar] [CrossRef] [PubMed]
- Bor, M.; Özdemir, F.; Türkan, I. The effect of salt stress on lipid peroxidation and antioxidants in leaves of sugar beet Beta vulgaris L. and wild beet Beta maritima L. Plant Sci. 2003, 164, 77–84. [Google Scholar] [CrossRef]
- Rather, B.A.; Mir, I.R.; Sehar, Z.; Anjum, N.A.; Masood, A.; Khan, N.A. The outcomes of the functional interplay of nitric oxide and hydrogen sulfide in metal stress tolerance in plants. Plant Physiol. Biochem. 2020, 155, 523–534. [Google Scholar] [CrossRef]
- Kaya, C.; Ashraf, M.; Alyemeni, M.N.; Ahmad, P. The role of endogenous nitric oxide in salicylic acid-induced up-regulation of ascorbate-glutathione cycle involved in salinity tolerance of pepper (Capsicum annuum L.) plants. Plant Physiol. Biochem. 2020, 147, 10–20. [Google Scholar] [CrossRef]
- Bray, E.A. Response to abiotic stress. Physiol. Mol. Biol. Plants 2000, 1158–1203. [Google Scholar]
- Borella, J.; Becker, R.; Lima, M.C.; Oliveira, D.D.S.C.D.; Braga, E.J.B.; Oliveira, A.C.B.D.; Amarante, L.D. Nitrogen source influences the antioxidative system of soybean plants under hypoxia and re-oxygenation. Sci. Agric. 2019, 76, 51–62. [Google Scholar] [CrossRef]
- Giraudat, J.; Parcy, F.; Bertauche, N.; Gosti, F.; Leung, J.; Morris, P.C.; Vartanian, N. Current advances in abscisic acid action and signalling. Plant Mol. Biol. 1994, 26, 1557–1577. [Google Scholar] [CrossRef]
- Merlot, S.; Giraudat, J. Genetic analysis of abscisic acid signal transduction. Plant Physiol. 1997, 114, 751. [Google Scholar] [CrossRef][Green Version]
- Leung, J.; Giraudat, J. Abscisic acid signal transduction. Annu. Rev. Plant Biol. 1998, 49, 199–222. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.S.; Anjum, N.A.; Gill, R.; Yadav, S.; Hassanuzzaman, M.; Fujita, M.; Mishra, P.; Sabat, S.C.; Tuteja, N. Superoxide dismutase–mentor of abiotic stress olerace in crop plants. Environ. Sci. Pollut. Res. 2015, 22, 10375–10394. [Google Scholar]
- Wangeline, A.L.; Burkhead, J.L.; Hale, K.L.; Lindblom, S.D.; Terry, N.; Pilon, M.; Pilon-Smits, E.A. Overexpression of ATP sulfurylase in Indian mustard: Effects on tolerance and accumulation of twelve metals. J. Environ. Qual. 2004, 33, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Kumar, J.; Singh, S.; Singh, V.P.; Prasad, S.M. Roles of osmoprotectants in improving salinity and drought tolerance in plants: A review. Rev. Environ. Sci. Biotechnol. 2015, 14, 407–426. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Alam, M.; Rahman, A.; Hasanuzzaman, M.; Nahar, K.; Fujita, M. Exogenous proline and glycine betaine mediated upregulation of antioxidant defense and glyoxalase systems provides better protection against salt-induced oxidative stress in two rice (Oryza sativa L.) varieties. BioMed Res. Int. 2014, 2014, 757219. [Google Scholar] [CrossRef] [PubMed]
- Aleksza, D.; Horváth, G.V.; Sándor, G.; Szabados, L. Proline accumulation is regulated by transcription factors associated with phosphate starvation. Plant Physiol. 2017, 175, 555–567. [Google Scholar] [CrossRef]
- Hawkesford, M.; Hoefgen, R.; Galili, G.; Amir, R.; Angenon, G.; Hesse, H.; Rentsch, D.; Schaller, J.; Van der Meer, I.; Rouster, J.; et al. Optimising nutritional quality of crops. In Plant Genetic Engineering; Jaiwal, P.K., Ed.; Plant Metabolic Engineering and Molecular Farming; Studium Press LLC: Houston, TX, USA, 2006; Volume 7, pp. 85–116. [Google Scholar]
- Brunold, C.; Suter, M. Regulation of sulfate assimilation by nitrogen nutrition in the duckweed Lemna minor L. Plant Physiol. 1984, 76, 579–583. [Google Scholar] [CrossRef]
- Jamal, A.; Fazli, I.S.; Ahmad, S.; Kim, K.T.; Oh, D.G.; Abdin, M.Z. Effect of sulfur on nitrate reductase and ATP sulfurylase activities in groundnut (Arachis hypogea L.). J. Plant Biol. 2006, 49, 513–517. [Google Scholar] [CrossRef]
- Akram, S.; Siddiqui, M.N.; Hussain, B.N.; Al Bari, M.A.; Mostofa, M.G.; Hossain, M.A.; Tran, L.S.P. Exogenous glutathione modulates salinity tolerance of soybean [Glycine max (L.) Merrill] at reproductive stage. J. Plant Growth Regul. 2017, 36, 877–888. [Google Scholar] [CrossRef]
- Cao, F.; Cai, Y.; Liu, L.; Zhang, M.; He, X.; Zhang, G.; Wu, F. Differences in photosynthesis, yield and grain cadmium accumulation as affected by exogenous cadmium and glutathione in the two rice genotypes. Plant Growth Regul. 2015, 75, 715–723. [Google Scholar] [CrossRef]
- Ding, X.; Jiang, Y.; He, L.; Zhou, Q.; Yu, J.; Hui, D.; Huang, D. Exogenous glutathione improves high root-zone temperature tolerance by modulating photosynthesis, antioxidant and osmolytes systems in cucumber seedlings. Sci. Rep. 2016, 6, 35424. [Google Scholar] [CrossRef] [PubMed]
- Pilon-Smits, E.A.; Hwang, S.; Mel Lytle, C.; Zhu, Y.; Tai, J.C.; Bravo, R.C.; Chen, Y.; Leustek, T.; Terry, N. Overexpression of ATP sulfurylase in Indian mustard leads to increased selenate uptake, reduction, and tolerance. Plant Physiol. 1999, 119, 123–132. [Google Scholar] [CrossRef]
- Bashir, H.; Ahmad, J.; Bagheri, R.; Nauman, M.; Qureshi, M.I. Limited sulfur resource forces Arabidopsis thaliana to shift towards non-sulfur tolerance under cadmium stress. Environ. Exp. Bot. 2013, 94, 19–32. [Google Scholar] [CrossRef]
- Turan, M.A.; Katkat, V.; Taban, S. Salinity-Induced stomatal resistance, proline, chlorophyll and. Int. J. Agric. Res. 2007, 2, 483–488. [Google Scholar]
- Taffouo, V.D.; Kouamou, J.K.; Ngalangue, L.M.T.; Ndjeudji, B.A.N.; Akoa, A. Effects of salinity stress on growth, ions partitioning and yield of some cowpea (Vigna unguiculata L. Walp.) cultivars. Int. J. Bot. 2009, 5, 135–143. [Google Scholar]
- Memon, S.A.; Hou, X.; Wang, L.J. Morphlogical analysis of salt stress response of pakchoi. Electron. J. Environ. Agric. Food Chem. 2010, 9, 248–254. [Google Scholar]
- Kumar, V.; Shriram, V.; Kishor, P.K.; Jawali, N.; Shitole, M.G. Enhanced proline accumulation and salt stress tolerance of transgenic Indica rice by over-expressing P5CSF129A gene. Plant Biotechnol. Rep. 2010, 4, 37–48. [Google Scholar] [CrossRef]
- Singh, M.; Singh, V.P.; Prasad, S.M. Responses of photosynthesis, nitrogen and proline metabolism to salinity stress in Solanum lycopersicum under different levels of nitrogen supplementation. Plant Physiol. Biochem. 2016, 109, 72–83. [Google Scholar] [CrossRef] [PubMed]
- Sudmalis, D.; Millah, S.K.; Gagliano, M.C.; Butré, C.I.; Plugge, C.M.; Rijnaarts, H.H.M.; Temmink, H. The potential of osmolytes and their precursors to alleviate osmotic stress of anaerobic granular sludge. Water Res. 2018, 147, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Fatma, M.; Asgher, M.; Masood, A.; Khan, N.A. Excess sulfur supplementation improves photosynthesis and growth in mustard under salt stress through increased production of glutathione. Environ. Exp. Bot. 2014, 107, 55–63. [Google Scholar] [CrossRef]
- Schwartz, S.H.; Zeevaart, J.A. Abscisic acid biosynthesis and metabolism. In Plant Hormones; Springer: Dordrecht, The Netherlands, 2010; pp. 137–155. [Google Scholar]
- Hewitt, E.J. Sand and water culture methods used in the study of plant nutrition. In Commonwealth Agricultural Bureaux, 2nd ed.; Farnham Royal: Bucks, UK, 1966; p. 547. [Google Scholar]
- Okuda, T.; Matsuda, Y.; Yamanaka, A.; Sagisaka, S. Abrupt increase in the level of hydrogen peroxide in leaves of winter wheat is caused by cold treatment. Plant Physiol. 1991, 97, 1265–1267. [Google Scholar] [CrossRef] [PubMed]
- Dhindsa, R.S.; Plumb-Dhindsa, P.; Thorpe, T.A. Leaf senescence: Correlated with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. J. Exp. Bot. 1981, 32, 93–101. [Google Scholar] [CrossRef]
- Kumar, D.; Yusuf, M.A.; Singh, P.; Sardar, M.; Sarin, N.B.; Biosciences, J.M.I. Histochemical detection of superoxide and H2O2 accumulation in Brassica juncea seedlings. Bio-Protocol 2014, 4, e1108. [Google Scholar] [CrossRef]
- Usuda, H. The activation state of ribulose 1, 5-bisphosphate carboxylase in maize leaves in dark and light. Plant Cell Physiol. 1985, 26, 1455–1463. [Google Scholar]
- Asada, K. Ascorbate peroxidase–a hydrogen peroxide-scavenging enzyme in plants. Physiol. Plant 1992, 85, 235–241. [Google Scholar] [CrossRef]
- Beyer, W.F., Jr.; Fridovich, I. Assaying for superoxide dismutase activity: Some large consequences of minor changes in conditions. Anal. Biochem. 1987, 161, 559–566. [Google Scholar] [CrossRef]
- Foyer, C.H.; Halliwell, B. The presence of glutathione and glutathione reductase in chloroplasts: A proposed role in ascorbic acid metabolism. Planta 1976, 133, 21–25. [Google Scholar] [CrossRef]
- Kuo, T.M.; Warner, R.L.; Kleinhofs, A. In vitro stability of nitrate reductase from barley leaves. Phytochemistry 1982, 21, 531–533. [Google Scholar] [CrossRef]
- Nakagawa, H.; Poulle, M.; Oaks, A. Characterization of nitrate reductase from corn leaves (Zea mays cv W64A × W182E): Two molecular forms of the enzyme. Plant Physiol. 1984, 75, 285–289. [Google Scholar] [CrossRef]
- Lindner, R.C. Rapid analytical methods for some of the more common inorganic constituents of plant tissues. Plant Physiol. 1944, 19, 76. [Google Scholar] [CrossRef] [PubMed]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Gaitonde, M.K. A spectrophotometric method for the direct determination of cysteine in the presence of other naturally occurring amino acids. Biochem. J. 1967, 104, 627–633. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.E. Determination of glutathione and glutathione disulfide in biological samples. Methods Enzymol. 1985, 113, 548–555. [Google Scholar] [PubMed]
- Lappartient, A.G.; Touraine, B. Demand-driven control of root ATP sulfurylase activity and SO42− uptake in intact canola (the role of phloem-translocated glutathione). Plant Physiol. 1996, 111, 147–157. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).