An Integrative Review of Strength Milestoning in Mid-Stage Achilles Tendon Rehab
Abstract
1. Introduction
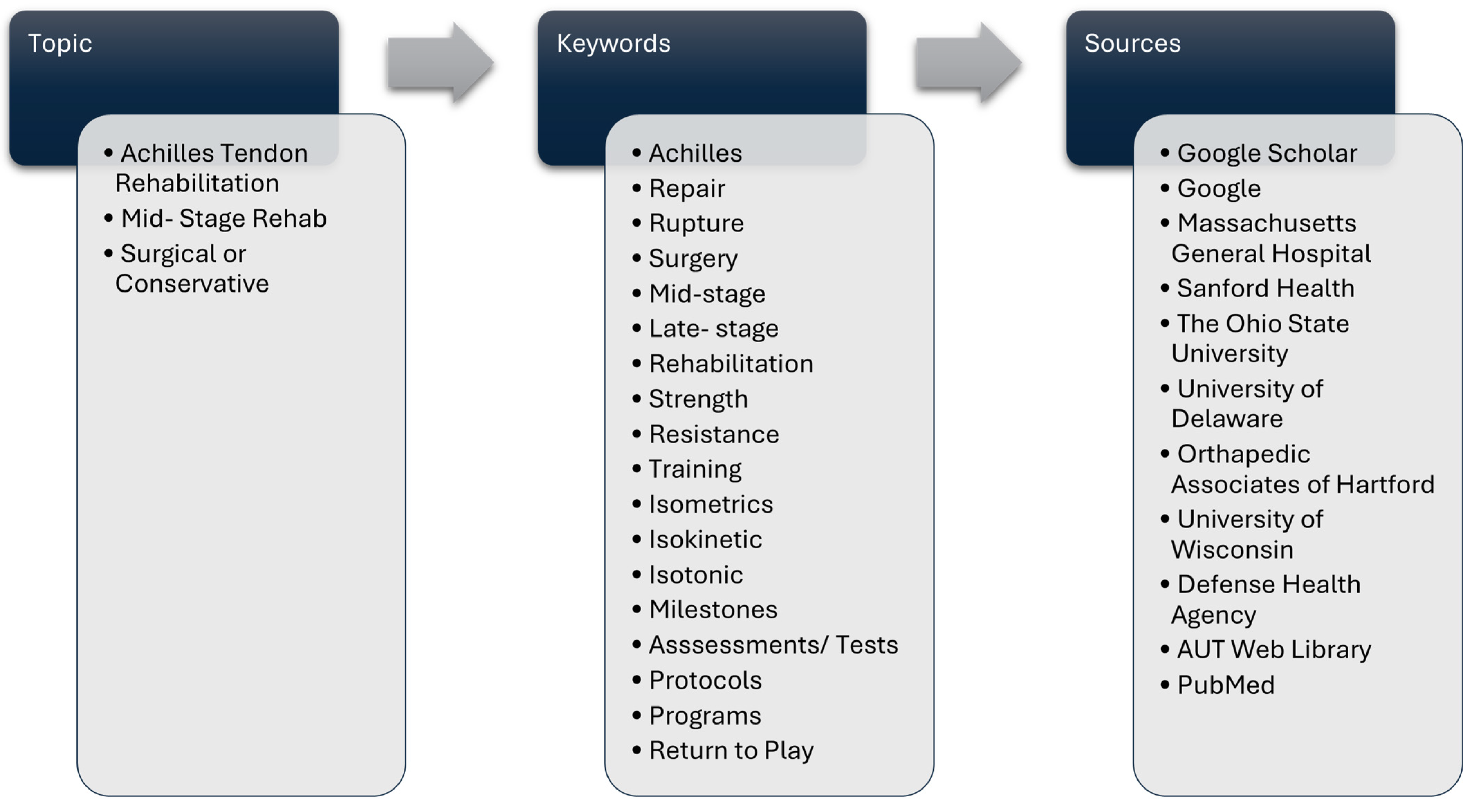
2. Search Strategy
3. Mid-Stage Treatment of ATR
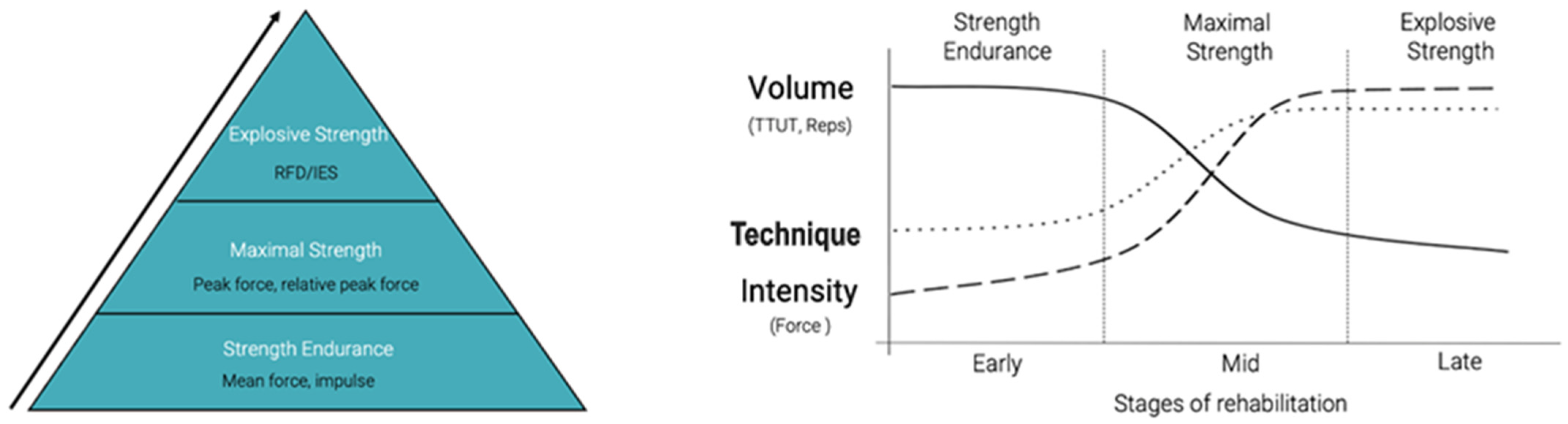
4. Mid-Stage Rehabilitation Strengthening
5. Strength Endurance Protocol Milestones for Clearance into Late-Stage Rehab
6. Maximal-Strength Protocols/Milestones for Clearance into Late-Stage Rehab and/or Clearance for Return to Play
7. Summary and Limitations
8. Mid-Stage Strengthening of the AT: New Perspectives
9. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ballal, M.S.; Walker, C.R.; Molloy, A.P. The anatomical footprint of the Achilles tendon: A cadaveric study. Bone Jt. J. 2014, 96-B, 1344–1348. [Google Scholar] [CrossRef]
- Ekiert, M.; Tomaszewski, K.A.; Mlyniec, A. The differences in viscoelastic properties of subtendons result from the anatomical tripartite structure of human Achilles tendon—Ex vivo experimental study and modeling. Acta Biomater. 2021, 125, 138–153. [Google Scholar] [CrossRef]
- Winnicki, K.; Ochała-Kłos, A.; Rutowicz, B.; Pękala, P.A.; Tomaszewski, K.A. Functional anatomy, histology and biomechanics of the human Achilles tendon—A comprehensive review. Ann. Anat.-Anat. Anz. 2020, 229, 151461. [Google Scholar] [CrossRef] [PubMed]
- Demangeot, Y.; Whiteley, R.; Gremeaux, V.; Degache, F. The load borne by the Achilles tendon during exercise: A systematic review of normative values. Scand. J. Med. Sci. Sports 2023, 33, 110–126. [Google Scholar] [CrossRef]
- Komi, P.V.; Fukashiro, S.; Järvinen, M. Biomechanical Loading of Achilles Tendon During Normal Locomotion. Clin. Sports Med. 1992, 11, 521–531. [Google Scholar] [CrossRef]
- Bonanno, J.; Cheng, J.; Tilley, D.; Abutalib, Z.; Casey, E. Factors Associated With Achilles Tendon Rupture in Women’s Collegiate Gymnastics. Sports Health Multidiscip. Approach 2022, 14, 358–368. [Google Scholar] [CrossRef]
- Hoeffner, R.; Svensson, R.B.; Bjerregaard, N.; Kjær, M.; Magnusson, S.P. Persistent Deficits after an Achilles Tendon Rupture: A Narrative Review. Transl. Sports Med. 2022, 2022, 7445398. [Google Scholar] [CrossRef] [PubMed]
- Eliasson, P.; Agergaard, A.-S.; Couppé, C.; Svensson, R.; Hoeffner, R.; Warming, S.; Warming, N.; Holm, C.; Jensen, M.H.; Krogsgaard, M.; et al. The Ruptured Achilles Tendon Elongates for 6 Months After Surgical Repair Regardless of Early or Late Weightbearing in Combination With Ankle Mobilization: A Randomized Clinical Trial. Am. J. Sports Med. 2018, 46, 2492–2502. [Google Scholar] [CrossRef]
- Rosso, C.; Vavken, P.; Polzer, C.; Buckland, D.M.; Studler, U.; Weisskopf, L.; Lottenbach, M.; Müller, A.M.; Valderrabano, V. Long-term outcomes of muscle volume and Achilles tendon length after Achilles tendon ruptures. Knee Surg. Sports Traumatol. Arthrosc. 2013, 21, 1369–1377. [Google Scholar] [CrossRef] [PubMed]
- Amin, N.H.; Old, A.B.; Tabb, L.P.; Garg, R.; Toossi, N.; Cerynik, D.L. Performance Outcomes After Repair of Complete Achilles Tendon Ruptures in National Basketball Association Players. Am. J. Sports Med. 2013, 41, 1864–1868. [Google Scholar] [CrossRef]
- Barfod, K.W.; Sveen, T.M.; Ganestam, A.; Ebskov, L.B.; Troelsen, A. Severe Functional Debilitations After Complications Associated With Acute Achilles Tendon Rupture With 9 Years of Follow-Up. J. Foot Ankle Surg. 2017, 56, 440–444. [Google Scholar] [CrossRef]
- Suchak, A.A.; Spooner, C.; Reid, D.C.; Jomha, N.M. Postoperative Rehabilitation Protocols for Achilles Tendon Ruptures: A Meta-analysis. Clin. Orthop. 2006, 445, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Petway, A.J.; Jordan, M.J.; Epsley, S.; Anloague, P. Mechanisms of Achilles Tendon Rupture in National Basketball Association Players. J. Appl. Biomech. 2022, 38, 398–403. [Google Scholar] [CrossRef]
- Lantto, I. Acute Achilles Tendon Rupture: Epidemiology and Treatment. 2016. Available online: https://oulurepo.oulu.fi/handle/10024/34866 (accessed on 24 April 2025).
- Egger, A.C.; Berkowitz, M.J. Achilles tendon injuries. Curr. Rev. Musculoskelet. Med. 2017, 10, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Hunt, K.J. Open Reconstructive Strategies for Chronic Achilles Tendon Ruptures. Foot Ankle Clin. 2019, 24, 425–437. [Google Scholar] [CrossRef]
- Cook, J.L.; Khan, K.M.; Purdam, C. Achilles tendinopathy. Man. Ther. 2002, 7, 121–130. [Google Scholar] [CrossRef]
- Hess, G.W. Achilles Tendon Rupture: A Review of Etiology, Population, Anatomy, Risk Factors, and Injury Prevention. Foot Ankle Spec. 2010, 3, 29–32. [Google Scholar] [CrossRef]
- Ganestam, A.; Kallemose, T.; Troelsen, A.; Barfod, K.W. Increasing incidence of acute Achilles tendon rupture and a noticeable decline in surgical treatment from 1994 to 2013. A nationwide registry study of 33,160 patients. Knee Surg. Sports Traumatol. Arthrosc. 2016, 24, 3730–3737. [Google Scholar] [CrossRef]
- Lemme, N.J.; Li, N.Y.; DeFroda, S.F.; Kleiner, J.; Owens, B.D. Epidemiology of Achilles Tendon Ruptures in the United States: Athletic and Nonathletic Injuries from 2012 to 2016. Orthop. J. Sports Med. 2018, 6, 2325967118808238. [Google Scholar] [CrossRef]
- Benzie, M. Achilles ruptures and return to sport in gymnastics: An overview. Sci. Gymnast. J. 2024, 16, 29–41. [Google Scholar] [CrossRef]
- Fenech, M.; Ajjikuttira, A.; Edwards, H. Ultrasound assessment of acute Achilles tendon rupture and measurement of the tendon gap. Australas. J. Ultrasound Med. 2024, 27, 106–119. [Google Scholar] [CrossRef]
- Nilsson-Helander, K.; Silbernagel, G.K.; Thomee, R.; Faxen, E.; Olsson, N.; Eriksson, B.I.; Karlsson, J. Acute Achilles tendon rupture: A randomized, controlled study comparing surgical and nonsurgical treatments using validated outcome measures. Am. J. Sports Med. 2010, 38, 2186–2193. [Google Scholar] [CrossRef]
- Saleh, M.; Marshall, P.D.; Senior, R.; MacFarlane, A. The Sheffield splint for controlled early mobilisation after rupture of the calcaneal tendon. A prospective, randomised comparison with plaster treatment. J. Bone Joint Surg. Br. 1992, 74, 206–209. [Google Scholar] [CrossRef]
- Valkering, K.P.; Aufwerber, S.; Ranuccio, F.; Lunini, E.; Edman, G.; Ackermann, P.W. Functional weight-bearing mobilization after Achilles tendon rupture enhances early healing response: A single-blinded randomized controlled trial. Knee Surg. Sports Traumatol. Arthrosc. 2017, 25, 1795. [Google Scholar] [CrossRef]
- Galloway, M.T.; Lalley, A.L.; Shearn, J.T. The Role of Mechanical Loading in Tendon Development, Maintenance, Injury, and Repair. J. Bone Joint Surg. Am. 2013, 95, 1620–1628. [Google Scholar] [CrossRef]
- Sakai, T.; Kumagai, K. Molecular dissection of tendon development and healing: Insights into tenogenic phenotypes and functions. J. Biol. Chem. 2025, 301, 108353. [Google Scholar] [CrossRef] [PubMed]
- Maquirriain, J. Achilles Tendon Rupture: Avoiding Tendon Lengthening during Surgical Repair and Rehabilitation. Yale J. Biol. Med. 2011, 84, 289–300. [Google Scholar] [PubMed]
- Zellers, J.A.; Marmon, A.R.; Ebrahimi, A.; Grävare Silbernagel, K. Lower extremity work along with triceps surae structure and activation is altered with jumping after Achilles tendon repair. J. Orthop. Res. 2019, 37, 933–941. [Google Scholar] [CrossRef]
- Brorsson, A.; Willy, R.W.; Tranberg, R.; Grävare Silbernagel, K. Heel-Rise Height Deficit 1 Year After Achilles Tendon Rupture Relates to Changes in Ankle Biomechanics 6 Years After Injury. Am. J. Sports Med. 2017, 45, 3060–3068. [Google Scholar] [CrossRef]
- Pečjak, R.; Kozinc, Ž. Long-Term Deficits in Muscle Composition, Performance and Quality of Movement after Achilles Tendon Rupture: A Review. BioMed 2023, 3, 135–151. [Google Scholar] [CrossRef]
- Chartier, C.; ElHawary, H.; Baradaran, A.; Vorstenbosch, J.; Xu, L.; Efanov, J.I. Tendon: Principles of Healing and Repair. Semin. Plast. Surg. 2021, 35, 211–215. [Google Scholar] [CrossRef] [PubMed]
- DiIorio, S.E.; Young, B.; Parker, J.B.; Griffin, M.F.; Longaker, M.T. Understanding Tendon Fibroblast Biology and Heterogeneity. Biomedicines 2024, 12, 859. [Google Scholar] [CrossRef]
- Stańczak, M.; Kacprzak, B.; Gawda, P. Tendon Cell Biology: Effect of Mechanical Loading. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2024, 58, 677–701. [Google Scholar] [CrossRef]
- Heikkinen, J.; Lantto, I.; Piilonen, J.; Flinkkilä, T.; Ohtonen, P.; Siira, P.; Laine, V.; Niinimäki, J.; Pajala, A.; Leppilahti, J. Tendon Length, Calf Muscle Atrophy, and Strength Deficit After Acute Achilles Tendon Rupture: Long-Term Follow-up of Patients in a Previous Study. J. Bone Jt. Surg. 2017, 99, 1509–1515. [Google Scholar] [CrossRef]
- Mullaney, M.; McHugh, M.; Tyler, T.; Nicholas, S.; Lee, S. Weakness in End-Range Plantar Flexion After Achilles Tendon Repair. Am. J. Sports Med. 2006, 34, 1120–1125. [Google Scholar] [CrossRef]
- Tipton, K.D. Nutritional Support for Exercise-Induced Injuries. Sports Med. Auckl. Nz 2015, 45, 93. [Google Scholar] [CrossRef] [PubMed]
- Zellers, J.; Pohlig, R.; Cortes, D.; Grävare, S.K. Achilles Tendon Cross-sectional Area at 12 Weeks Post-rupture Relates to One-year Heel-rise Height. Knee Surg. Sports Traumatol. Arthrosc. Off. J. ESSKA 2020, 28, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Marrone, W.; Andrews, R.; Reynolds, A.; Vignona, P.; Patel, S.; O’Malley, M. Rehabilitation and Return to Sports after Achilles Tendon Repair. Int. J. Sports Phys. Ther. 2024, 19, 1152–1165. [Google Scholar] [CrossRef]
- Hutchison, A.-M.; Topliss, C.; Beard, D.; Evans, R.; Williams, P. The treatment of a rupture of the Achilles tendon using a dedicated management programme. Bone Jt. J. 2015, 97-B, 510–515. [Google Scholar] [CrossRef]
- Wang, K.C.; Cotter, E.J.; Cole, B.J.; Lin, J.L. Rehabilitation and Return to Play Following Achilles Tendon Repair. Oper. Tech. Sports Med. 2017, 25, 214–219. [Google Scholar] [CrossRef]
- Glazebrook, M.; Rubinger, D. Functional Rehabilitation for Nonsurgical Treatment of Acute Achilles Tendon Rupture. Foot Ankle Clin. 2019, 24, 387–398. [Google Scholar] [CrossRef]
- Saxena, A.; Ewen, B.; Maffulli, N. Rehabilitation of the operated achilles tendon: Parameters for predicting return to activity. J. Foot Ankle Surg. Off. Publ. Am. Coll. Foot Ankle Surg. 2011, 50, 37–40. [Google Scholar] [CrossRef]
- Silbernagel, K.G.; Nilsson-Helander, K.; Thomeé, R.; Eriksson, B.I.; Karlsson, J. A new measurement of heel-rise endurance with the ability to detect functional deficits in patients with Achilles tendon rupture. Knee Surg. Sports Traumatol. Arthrosc. 2010, 18, 258–264. [Google Scholar] [CrossRef]
- Silbernagel, K.G.; Crossley, K.M. A proposed return-to-sport program for patients with midportion Achilles tendinopathy: Rationale and implementation. J. Orthop. Sports Phys. Ther. 2015, 45, 876–886. [Google Scholar] [CrossRef]
- Binkley, H.M.; Douglass, D.; Phillips, K.; Wise, S.L. Rehabilitation and Return to Sport After Nonsurgical Treatment of Achilles Tendon Rupture. Strength Cond. J. 2020, 42, 90. [Google Scholar] [CrossRef]
- Kasmar, T.; Groth, A.; Miller, T.; Martin, K.; Marulli, T.; VanEtten, L.; Otto, V. Achilles Tendon Repair Clinical Practice Guideline. The Ohio State University Sports Medicine. Available online: https://medicine.osu.edu/-/media/files/medicine/departments/sports-medicine/medical-professionals/knee-ankle-and-foot/achilles-repair-protocol-2020final-002.pdf (accessed on 24 April 2025).
- William Beaumont Army Medical Center Physical Therapy Section. Achilles Tendon Repair: Rehabilitation Protocol. William Beaumont Army Medical Center. Available online: https://william-beaumont.tricare.mil/Portals/149/Achilles%20Repair%20WB08.pdf (accessed on 24 April 2025).
- Sanford Health Physical Therapy Department. Non-Operative Achilles Rupture Rehabilitation Guide-Line. Sanford Health. Available online: https://www.sanfordhealth.org/-/media/org/files/medical-professionals/resources-and-education/non-operative-achilles-rupture-guidelines.pdf (accessed on 24 April 2025).
- Massachusetts General Hospital Sports Medicine Physical Therapy. Rehabilitation Protocol for Achilles Ten-Don Repair. Norwood, MA: Massachusetts General Hospital. Available online: https://www.massgeneral.org/assets/mgh/pdf/orthopaedics/sports-medicine/physical-therapy/rehabilitation-protocol-for-achilles-tendon-repair.pdf (accessed on 24 April 2025).
- University of Delaware Physical Therapy Clinic. Rehab Practice Guidelines for: Achilles Tendon Repair. University of Delaware. Available online: https://bpb-us-w2.wpmucdn.com/sites.udel.edu/dist/c/3448/files/2017/07/Achilles-Jul-28-2017-284wd86.pdf (accessed on 24 April 2025).
- Zellers, J.A.; Cortes, D.H.; Silbernagel, K.G. From acute Achilles tendon rupture to return to play—A case report evaluating recovery of tendon structure, mechanical properties, clinical and functional outcomes. Int. J. Sports Phys. Ther. 2016, 11, 1150–1159. [Google Scholar]
- Groetelaers, R.; Janssen, L.; Velden, J.; Wieland, A.; Amendt, A.; Geelen, P.; Janzing, H. Functional treatment or cast immobilization after minimally invasive repair of an acute Achilles tendon rupture. Foot Ankle Int. 2014, 35, 771–77835. [Google Scholar] [CrossRef]
- Inglis, A.E.; Sculco, T.P. Surgical repair of ruptures of the tendo Achillis. Clin. Orthop. Relat. Res. (1976–2007) 1981, 156, 160. [Google Scholar] [CrossRef]
- Maffulli, N.; Longo, U.G.; Maffulli, G.D.; Khanna, A.; Denaro, V. Achilles Tendon Ruptures in Elite Athletes. Foot Ankle Int. 2011, 32, 9–15. [Google Scholar] [CrossRef]
- Leppilahti, J.; Lähde, S.; Forsman, K.; Kangas, J.; Kauranen, K.; Orava, S. Relationship between calf muscle size and strength after achilles rupture repair. Foot Ankle Int. 2000, 21, 330–335. [Google Scholar] [CrossRef]
- Don, R.; Ranavolo, A.; Cacchio, A.; Serrao, M.; Costabile, F.; Iachelli, M.; Camerota, F.; Frascarelli, M.; Santilli, V. Relationship between recovery of calf-muscle biomechanical properties and gait pattern following surgery for achilles tendon rupture. Clin. Biomech. 2007, 22, 211–220. [Google Scholar] [CrossRef]
- Bressel, E.; Larsen, B.T.; McNair, P.J.; Cronin, J. Ankle joint proprioception and passive mechanical properties of the calf muscles after an Achilles tendon rupture: A comparison with matched controls. Clin. Biomech. 2004, 19, 284–291. [Google Scholar] [CrossRef]
- Kaniki, N.; Willits, K.; Mohtadi, N.G.H.; Fung, V.; Bryant, D. A Retrospective Comparative Study With Historical Control to Determine the Effectiveness of Platelet-Rich Plasma as Part of Nonoperative Treatment of Acute Achilles Tendon Rupture. Arthrosc. J. Arthrosc. Relat. Surg. 2014, 30, 1139–1145. [Google Scholar] [CrossRef]
- Jallageas, R.; Bordes, J.; Daviet, J.-C.; Mabit, C.; Coste, C. Evaluation of surgical treatment for ruptured Achilles tendon in 31 athletes. Orthop. Traumatol. Surg. Res. 2013, 99, 577–584. [Google Scholar] [CrossRef]
- Willits, K.; Amendola, A.; Bryant, D.; Mohtadi, N.G.; Giffin, J.R.; Fowler, P.; Kean, C.O.; Kirkley, A. Operative versus Nonoperative Treatment of Acute Achilles Tendon Ruptures: A Multicenter Randomized Trial Using Accelerated Functional Rehabilitation. J. Bone Jt. Surg. 2010, 92, 2767–2775. [Google Scholar] [CrossRef]
- Arslan, A.; Cepni, S.K.; Sahinkaya, T.; May, C.; Mutlu, H.; Parmaksızoğlu, A.S. Functional outcomes of repair of Achilles tendon using a biological open surgical method. ACTA Orthop. Traumatol. Turc. 2014, 48, 563–569. [Google Scholar] [CrossRef]
- Möller, M.; Lind, K.; Movin, T.; Karlsson, J. Calf muscle function after Achilles tendon rupture. A prospective, randomised study comparing surgical and non-surgical treatment. Scand. J. Med. Sci. Sports 2002, 12, 9–16. [Google Scholar] [CrossRef]
- Porter, D.A.; Barnes, A.F.; Rund, A.M.; Kaz, A.J.; Tyndall, J.A.; Millis, A.A. Acute achilles tendon repair: Strength outcomes after an acute bout of exercise in recreational athletes. Foot Ankle Int. 2014, 35, 123–130. [Google Scholar] [CrossRef]
- Öhberg, L.; Lorentzon, R.; Alfredson, H. Good clinical results but persisting side-to-side differences in calf muscle strength after surgical treatment of chronic Achilles tendinosis: A 5-year follow-up. Scand. J. Med. Sci. Sports 2001, 11, 207–212. [Google Scholar] [CrossRef]
- Silbernagel, K.G.; Gustavsson, A.; Thomeé, R.; Karlsson, J. Evaluation of lower leg function in patients with Achilles tendinopathy. Knee Surg. Sports Traumatol. Arthrosc. 2006, 14, 1207–1217. [Google Scholar] [CrossRef]
- Fernandez, M.R.; Hébert-Losier, K. Devices to measure calf raise test outcomes: A narrative review. Physiother. Res. Int. 2023, 28, e2039. [Google Scholar] [CrossRef]
- Fernandez, M.R.; Athens, J.; Balsalobre-Fernandez, C.; Kubo, M.; Hébert-Losier, K. Concurrent validity and reliability of a mobile iOS application used to assess calf raise test kinematics. Musculoskelet. Sci. Pract. 2023, 63, 102711. [Google Scholar] [CrossRef]
- Schrefl, A.; Kolokythas, N.; Stamm, M.; Erlacher, D.; Schärli, A. Reliability of a standardized protocol of the Single Leg Heel Rise Test. Curr. Issues Sport Sci. CISS 2024, 9, 009. [Google Scholar] [CrossRef]
- Sman, A.D.; Hiller, C.E.; Imer, A.; Ocsing, A.; Burns, J.; Refshauge, K.M. Design and Reliability of a Novel Heel Rise Test Measuring Device for Plantarflexion Endurance. BioMed Res. Int. 2014, 2014, 391646. [Google Scholar] [CrossRef]
- Hébert-Losier, K.; Wessman, C.; Alricsson, M.; Svantesson, U. Updated reliability and normative values for the standing heel-rise test in healthy adults. Physiotherapy 2017, 103, 446–452. [Google Scholar] [CrossRef]
- UW Health Sports Rehabilitation. Achilles Tendon Rehabilitation Guidelines. Available online: https://www.tigerortho.com/pdfs/uw-health/achilles-tendon-rehab-final.pdf (accessed on 24 April 2025).
- Orthopedic Associates of Hartford. Achilles Tendon Rupture Repair Return-to-Sport Protocol. Available online: https://oahct.com/wp-content/uploads/2020/08/OAH-RTS-Achilles-Tendon-Rupture2-0820.pdf (accessed on 24 April 2025).
- Avers, D.; Lott, D.J.; Brown, M.B. Daniels and Worthingham’s Muscle Testing: Techniques of Manual Examination and Performance Testing, 11th ed.; Saunders: Rhodes, NSW, Australia, 2024. [Google Scholar]
- Feiring, D.C.; Ellenbecker, T.S. Single versus multiple joint isokinetic testing with ACL reconstructed patients. Isokinet. Exerc. Sci. 1996, 6, 109–115. [Google Scholar] [CrossRef]
- Draovitch, P.; Patel, S.; Marrone, W.; Grundstein, M.J.; Grant, R.; Virgile, A.; Myslinski, T.; Bedi, A.; Bradley, J.P.; Williams, R.J.; et al. The Return-to-Sport Clearance Continuum Is a Novel Approach Toward Return to Sport and Performance for the Professional Athlete. Arthrosc. Sports Med. Rehabil. 2022, 4, e93–e101. [Google Scholar] [CrossRef]
- Hébert-Losier, K.; Newsham-West, R.J.; Schneiders, A.G.; Sullivan, S.J. Raising the standards of the calf-raise test: A systematic review. J. Sci. Med. Sport 2009, 12, 594–602. [Google Scholar] [CrossRef]
- Sullivan, K.J.; Wallace, J.G., Jr.; O’Neil, M.E.; Musolino, G.M.; Mandich, M.; Studer, M.T.; Bottomley, J.M.; Cormack, J.C.; Nicholson, S.K.; Jensen, G.M.A. Vision for Society: Physical Therapy as Partners in the National Health Agenda. Phys. Ther. 2011, 91, 1664–1672. [Google Scholar] [CrossRef] [PubMed]
- Khan, K.M.; Scott, A. Mechanotherapy: How physical therapists’ prescription of exercise promotes tissue repair. Br. J. Sports Med. 2009, 43, 247–252. [Google Scholar] [CrossRef]
- Baxter, J.R.; Corrigan, P.; Hullfish, T.J.; O’Rourke, P.; Silbernagel, K.G. Exercise Progression to Incrementally Load the Achilles Tendon. Med. Sci. Sports Exerc. 2021, 53, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Hagley, P.; Ryan, C.; Ye-Lee, D.; Cronin, J. Reliability and Asymmetry Associated with Different Plantar Flexion Assessments Using a Portable Fixed Dynamometer. Personal Communication. 2024. Available online: https://www.researchsquare.com/article/rs-4676750/v1 (accessed on 24 April 2025).
- Chester, R.; Costa, M.L.; Shepstone, L.; Donell, S.T. Reliability of isokinetic dynamometry in assessing plantarflexion torque following Achilles tendon rupture. Foot Ankle Int. 2003, 24, 909–915. [Google Scholar] [CrossRef]

| Abbreviation—Full Term | Definition |
|---|---|
| AT—Achilles Tendon | The strong tendon that connects the gastrocnemius and soleus muscles (triceps surae) to the calcaneus; crucial for plantarflexion and propulsion. |
| ATR—Achilles Tendon Rupture | A complete or partial tear of the Achilles tendon, commonly occurring during explosive or forceful movements. |
| BFR—Blood Flow Restriction | A technique using external compression to reduce blood flow during low-load resistance training to enhance hypertrophy and strength. |
| BW—Body Weight | The weight of an individual; often used to standardize strength tests or exercise prescriptions (e.g., heel raise at %BW). |
| CAM—Controlled Ankle Motion (boot) | A medical walking boot designed to immobilize and support the ankle while allowing limited, protected mobility during recovery. |
| Con—Concentric | A type of muscle contraction where the muscle shortens while producing force (e.g., lifting phase of a heel raise). |
| CL—Contralateral Limb | The limb opposite to the one injured; often used as a reference for performance symmetry or baseline measures. |
| CMJ—Countermovement Jump | A vertical jump test involving a preparatory dip (eccentric phase); used to evaluate lower-body power and explosiveness. |
| DF—Dorsiflexion | Movement of the foot upwards toward the shin; key for ankle mobility and functional tasks like walking or squatting. |
| DL—Double Limb | Describes activities involving both legs simultaneously, such as DL squats or DL heel raises; often precedes SL work in rehab progression. |
| Ecc—Eccentric | A muscle contraction where the muscle lengthens under tension (e.g., lowering phase of a heel raise); essential in tendon loading and strength training. |
| IES—Index of Explosive Strength | A performance metric: peak force divided by time to peak force; assesses an individual’s ability to produce force rapidly. |
| Ht—Height | Typically refers to vertical height achieved (e.g., calf raise heel height) or to anthropometric measurements in assessments. |
| HRT—Heel Raise Test | Plantar flexor strength/strength endurance test. |
| LSI—Limb Symmetry Index | A ratio used to assess the recovery of the injured limb compared to the healthy side: (injured/uninjured × 100); ≥90% is often a return-to-play benchmark. |
| MD—Mean Difference | A statistical value indicating the average difference between two data points or groups (e.g., pre-vs. post-intervention). |
| MTU—Muscle–Tendon Unit | The functional unit combining muscle fibers and tendon that transmits force and coordinates movement. |
| PF—Plantarflexion | Movement of the foot downward, away from the leg; crucial for gait, jumping, and push-off actions. |
| PWB—Partial Weight Bearing | A prescribed rehab phase allowing the patient to apply a limited percentage of BW through the injured limb. |
| RFD—Rate of Force Development | A key performance indicator describing how quickly force can be generated; used to assess explosive strength and tendon recovery. |
| ROM—Range of Motion | The full arc of joint movement, measured in degrees; essential for evaluating mobility and recovery progress. |
| RTP—Return to Play | The final stage of rehab when an athlete is cleared to resume sport-specific training and competitive activities. |
| SL—Single Limb | Refers to movements or assessments involving one leg at a time (e.g., SL hop, SL heel raise); used for strength and symmetry testing. |
| TTUT—Total Time Under Tension | The total duration a muscle is loaded during a set; used to manipulate training intensity and promote adaptation. |
| WBAT—Weight Bearing As Tolerated | A rehab status that allows patients to place as much weight as they comfortably can through the affected limb. |
| Phase | Goals | Interventions | Milestones for Progression | Precautions |
|---|---|---|---|---|
| Immediate Post-Operative/ Immobilization (0–2 weeks) | Protect surgical wound Maintain muscle strength Minimize pain/swelling | Immobilization in short cast or CAM boot (2–3 heel wedges) Proximal Strengthening Ankle active ROM (Not exceed 0-degree DF) | Wound, healing, cast removed and MD clearance | Allow incision to heal Monitor for signs of complications No passive DF No active ankle DF past 0 deg or a surgeon discretion) |
| Early Rehab/Controlled Mobilization (2–6 weeks) | Minimal pain Commence Isometric PF Achieve ankle ROM within phase limits Maintain lower limb, core and CV fitness | Progressive ambulation, initially PWB (50%) w/CAM boot w/heel lifts Active ROM dorsiflexion to 0 deg w/knee in 90 deg flexion Sub-maximal isometrics in shortened position (in boot or in heel wedges) Proximal strengthening w/or w/o BFR (after suture removal and MD clearance) | WBAT in CAM boot Ankle DF AROM to neutral Minimal pain, decreased swelling | No passive DF w/knee flexed past neutral for 4 weeks No passive DF w/knee extended for 10 weeks Monitor resting DF tension in prone w/knee flexed |
| Intermediate Phase (Mid-stage) (6–12 weeks) | Improved ankle DF ROM w/o excessive elongation stress Normalize gait mechanics Improved ankle PF strength | Double leg to single leg heel raise progression Heel raises on heel wedge to end range Continued BFR | Double leg heel raise through ROM | Monitor resting DF tension prone w/knee flexed |
| Late Stage Strengthening (12–24 weeks) | Improve single leg PF strength and endurance throughout full ROM | Eccentric overload SL calf raises Isometric overcoming isometric calf raises | SL standing calf raises-work at ≥90% of CL Seated or standing isometric peak and avg. force ≥ 90% of CL | Monitor loading and intensity |
| Late Stage (24–36 weeks) | DL jump progression Pogo jumping w/band assistance | Gradual progression of plyometrics from extensive to intensive and progress from sagittal-frontal-transverse planes of motion | Monitor loading and intensity | |
| Return to Sports (36+ weeks) | Isokinetic/Isometric strength > 90 LSI | Continued plyometric progression Continued local and global strength progression Initiate and progress on field/court progression | Strength > 80% CL side Strength > 2.5–3× BW w/seated calf isometric DL CMJ asymmetries < 20% SL jump symmetries < 20% ROM: 95% symmetry ROM (DF/PF) compared to uninvolved limb Weight Bearing: Normalized gait and jogging mechanics Strength: <10% plantarflexor asymmetry at 0° DF and <25% asymmetry at 20° PF with handheld dynamometer compared to uninvolved limb Neuromuscular Control: 90% symmetry between limbs on Y-balance test with appropriate lower extremity mechanics Functional Hop Testing: 90% symmetry SL hop testing Physician Clearance Timeframe: Expected time frame between 6 and 9 months | Monitor loading, fatigue, intensity and sport specific high velocity movement biomechanics |
| Physiological Quality | Contraction Type | Protocol | Metric |
|---|---|---|---|
| Strength/Strength Endurance | Isoinertial | Perform 5 sets of 25 concentric SL heel raises [43] | # of reps |
| Heel-raise height test for height and endurance—SL calf raise performed on 10° incline board. Linear transducer to calculate heel height. Tempo every 2 s. Frequency 30 heel raises per minute [44] | Reps per minute, # of reps, displacement, LSI, work (J) | ||
| Heel-raise test for endurance ≥ 90% LSI (of repetitions considered normal) [44] | # of reps, displacement, LSI | ||
| SL heel raises on edge of step—3 × 15 [45,46] | # of reps | ||
| 25 SL raises with heel height 20% of uninvolved limb [47] | # of reps, LSI | ||
| Perform 20+ LS heel raises to ≥75% ht. of contralateral limb [48] | # of reps, displacement | ||
| 25 SL heel raises [49] | # of reps | ||
| 25 SL heel raises to 20% of unaffected | # of reps, LSI | ||
| SL rebounding calf raises—3 × 15 [50] | # of reps | ||
| 5 heel raises at 90% of height [51] | # of reps, Height, LSI | ||
| Ability to perform unilateral leg heel raise [52] | # of reps | ||
| Not Specified | Strength measures 70–80% LSI [39] | LSI | |
| Maximal/Strength and Power | Zero velocity (Isometric) | Isometric ≥ 85% for PF [48] | Peak Force |
| Seated calf raise (Soleus)—1.5–2× BW, soleus strength test 90% LSI [39] | Peak Force | ||
| Seated bent knee 0° (neutral) [53] | Peak Force | ||
| ≤10% plantar flexor asymmetry at 0° DF—seated [47] | Peak Force | ||
| ≤25% asymmetry at 20° PF with HHD compared to uninvolved limb-seated [47] | Peak Force | ||
| 10° DF. 0° (neutral), 20° PF [54] | Peak Force | ||
| 20°, 10° of DF, 0 (neutral), 10, 20° of PF, seated—3 × 3 s [36] | Peak Torque | ||
| 0° (neutral)—seated-2 × 3–5 s [55] | Peak Force | ||
| 10° DF, O°, and 20° PF [56] | Peak Force | ||
|
Slow Velocity
(Isokinetic) | Angular velocity—5°/s; position—prone—2 × 5 [57,58] |
Passive Ankle
Stiffness | |
| Angular velocity—30°/s; position—seated—3 × 4 [59] supine—1 × 3 [60], 1 × 5 [61] |
Peak Torque
LSI | ||
| Angular velocity—30°/s × 4—prone [62] | Peak Torque | ||
| Angular velocity—30°/s—Ecc and Con; seated and seated closed chain—1 × 3 [63] | Peak Torque | ||
| Angular velocity 60°/s; position—seated [59] | Peak Torque | ||
|
Higher Velocity
(Isokinetic) Strength Endurance | Angular velocity 90°/s [54] | Peak Torque and Mean Total Work | |
| Angular velocity—24°/s, 48°/s, and 96° [54] | Peak Torque | ||
| Angular velocity—120°/s, supine—1 × 20 [60] | LSI | ||
| Angular velocity—60°/s, 120°/s, 180°/s, prone position 1 × 6 ea [64] | Peak Torque | ||
| Angular velocity—180°/s—Ecc and Con; Seated, Seated closed chain, and supine—1 × 3 | Peak Torque | ||
| Angular velocity—225°/s, seated—1 × 10 [65] | Peak Torque | ||
| 192°/s [54] | |||
| Angular velocity-240°/s, seated-3 × 10 [59] | Peak Torque | ||
| Angular velocity—30°/s, 90°/s, and 240°/s—supine 1 × 4ea [56] | Peak Torque | ||
| Angular velocity—Ecc and Con at 90°/s × 5 and 225° × 10, Ecc at 90° × 5—seated [65] | Peak Torque | ||
| Angular velocity—120° × 15—prone [62] | Total Work | ||
|
Power
(Isoinertial/Isotonic) | SL loaded concentric only × 3 at 4 different weights | Peak Power | |
| SL loaded eccentric-concentric × 3 at 4 different weights [66] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toland, C.; Cronin, J.; Reid, D.; Laughlin, M.S.; Fleeks, J.L. An Integrative Review of Strength Milestoning in Mid-Stage Achilles Tendon Rehab. Biomechanics 2025, 5, 59. https://doi.org/10.3390/biomechanics5030059
Toland C, Cronin J, Reid D, Laughlin MS, Fleeks JL. An Integrative Review of Strength Milestoning in Mid-Stage Achilles Tendon Rehab. Biomechanics. 2025; 5(3):59. https://doi.org/10.3390/biomechanics5030059
Chicago/Turabian StyleToland, Chris, John Cronin, Duncan Reid, Mitzi S. Laughlin, and Jeremy L. Fleeks. 2025. "An Integrative Review of Strength Milestoning in Mid-Stage Achilles Tendon Rehab" Biomechanics 5, no. 3: 59. https://doi.org/10.3390/biomechanics5030059
APA StyleToland, C., Cronin, J., Reid, D., Laughlin, M. S., & Fleeks, J. L. (2025). An Integrative Review of Strength Milestoning in Mid-Stage Achilles Tendon Rehab. Biomechanics, 5(3), 59. https://doi.org/10.3390/biomechanics5030059







