Growing Up with MS: The Adolescent Experience of Pediatric-Onset Multiple Sclerosis
Abstract
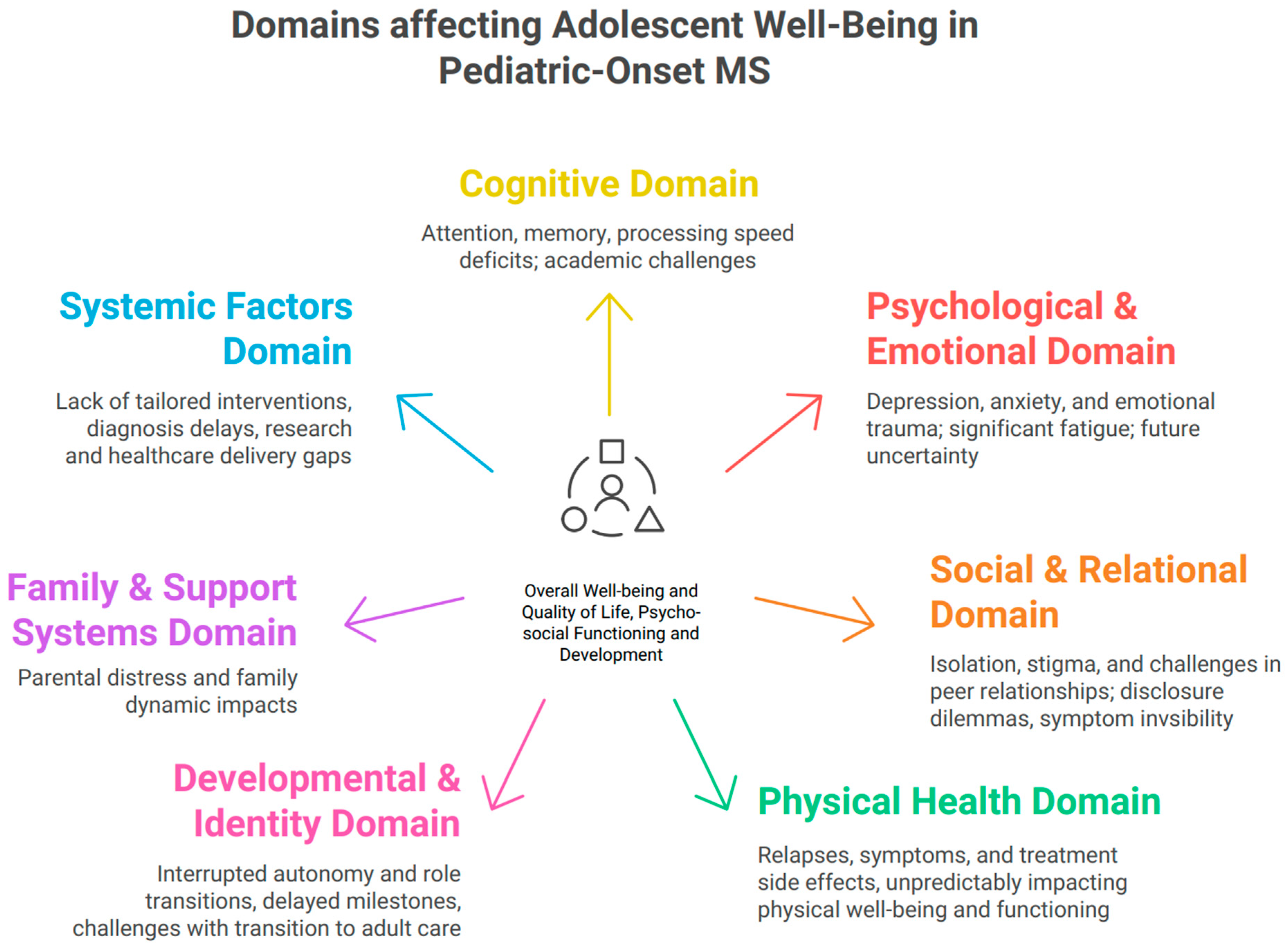
1. Introduction
1.1. Methodological Note
1.2. The Experience of Diagnosis
1.3. Interruption of Autonomy and Identity Formation
1.4. Cognitive Impairment and Academic Disruption
1.5. Emotional and Mental Health Risks
1.6. Impact on Future Planning and Role Transitions
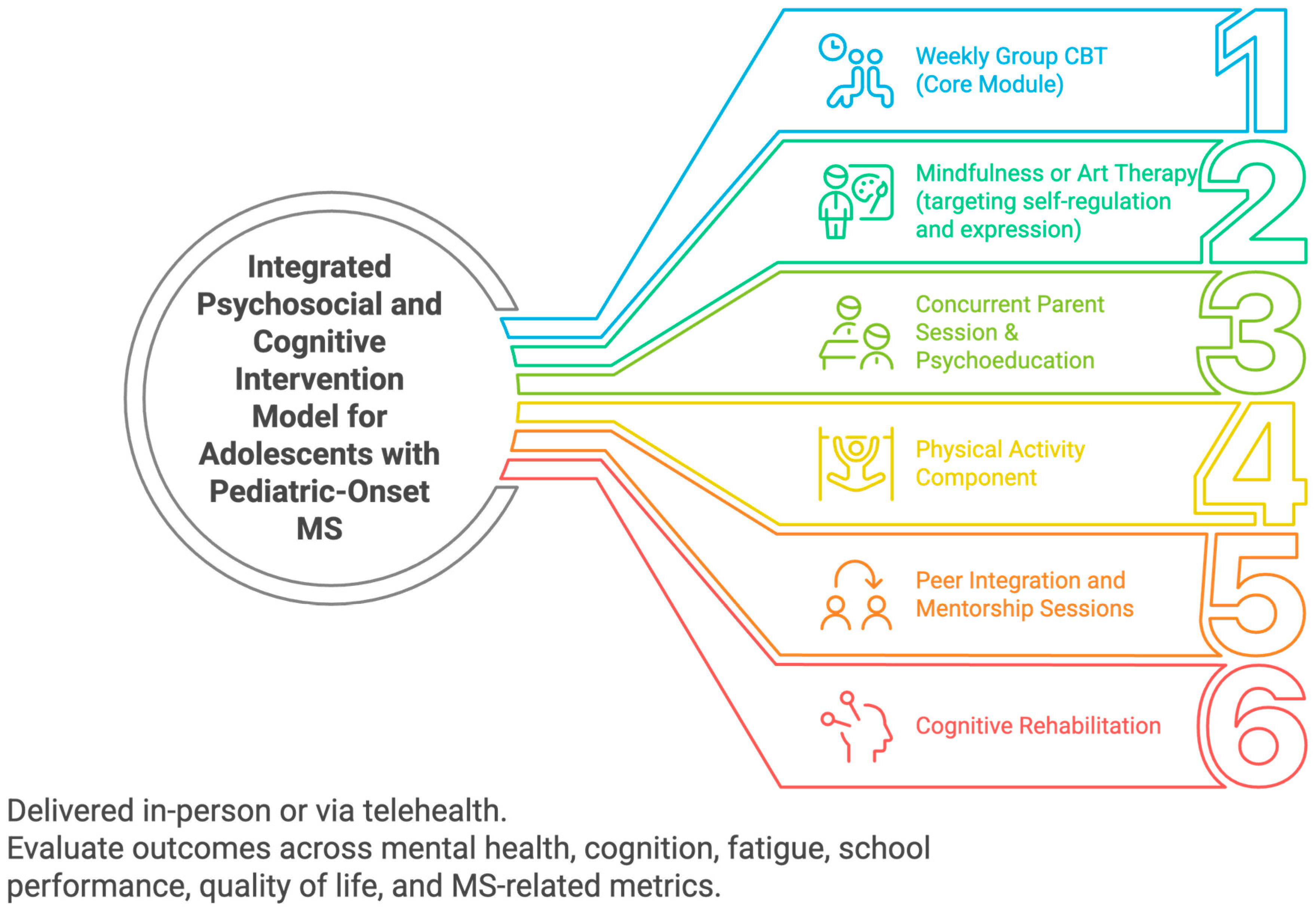
2. Discussion
2.1. Future Directions
2.2. Conclusions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| MS | Multiple Sclerosis |
| POMS | Pediatric-onset Multiple Sclerosis |
| CIS | Clinically Isolated Syndrome |
| ADHD | Attention Deficit Hyperactivity Disorder |
| CBT | Cognitive Behavioral Therapy |
| MBI/MBSR | Mindfulness-Based Intervention/Stress Reduction |
| SDMT | Symbol Digit Modalities Test |
References
- Yan, K.; Balijepalli, C.; Desai, K.; Gullapalli, L.; Druyts, E. Epidemiology of pediatric multiple sclerosis: A systematic literature review and meta-analysis. Mult. Scler. Relat. Disord. 2020, 44, 102260. [Google Scholar] [CrossRef] [PubMed]
- Waldman, A.; Ness, J.; Pohl, D.; Simone, I.L.; Anlar, B.; Amato, M.P.; Ghezzi, A. Pediatric multiple sclerosis: Clinical features and outcome. Neurology 2016, 87, S74–S81. [Google Scholar] [CrossRef]
- Chou, I.-J.; Whitehouse, W.P.; Wang, H.-S.; Tanasescu, R.; Constantinescu, C.S. Diagnostic modalities in multiple sclerosis: Perspectives in children. Biomed. J. 2014, 37, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Wallach, A.I.; Waltz, M.; Casper, T.C.; Aaen, G.; Belman, A.; Benson, L.; Chitnis, T.; Gorman, M.; Graves, J.; Harris, Y.; et al. Cognitive processing speed in pediatric-onset multiple sclerosis: Baseline characteristics of impairment and prediction of decline. Mult. Scler. 2020, 26, 1938–1947. [Google Scholar] [CrossRef]
- Barlow-Krelina, E.; Fabri, T.L.; O’Mahony, J.; Gur, R.C.; Gur, R.E.; De Somma, E.; Bolongaita, L.; Dunn, C.L.; Bacchus, M.; Yeh, E.A.; et al. Examining cognitive speed and accuracy dysfunction in youth and young adults with pediatric-onset multiple sclerosis using a computerized neurocognitive battery. Neuropsychology 2021, 35, 388–398. [Google Scholar] [CrossRef] [PubMed]
- Novak, A.M.; Lev-Ari, S. Resilience, Stress, Well-Being, and Sleep Quality in Multiple Sclerosis. J. Clin. Med. 2023, 12, 716. [Google Scholar] [CrossRef]
- Weisbrot, D.; Charvet, L.; Serafin, D.; Milazzo, M.; Preston, T.; Cleary, R.; Moadel, T.; Seibert, M.; Belman, A.; Krupp, L. Psychiatric diagnoses and cognitive impairment in pediatric multiple sclerosis. Mult. Scler. 2014, 20, 588–593. [Google Scholar] [CrossRef]
- Kang, W. Understanding the Effect of Multiple Sclerosis on General and Dimensions of Mental Health. J. Clin. Med. 2022, 11, 7483. [Google Scholar] [CrossRef]
- MacAllister, W.S.; Boyd, J.R.; Holland, N.J.; Milazzo, M.C.; Krupp, L.B. International Pediatric MS Study Group The psychosocial consequences of pediatric multiple sclerosis. Neurology 2007, 68, S66–S69. [Google Scholar] [CrossRef]
- Tarantino, S.; Proietti Checchi, M.; Papetti, L.; Monte, G.; Ferilli, M.A.N.; Valeriani, M. Neuropsychological performances, quality of life, and psychological issues in pediatric onset multiple sclerosis: A narrative review. Neurol. Sci. 2024, 45, 1913–1930. [Google Scholar] [CrossRef]
- Boyd, J.R.; MacMillan, L.J. Experiences of children and adolescents living with multiple sclerosis. J. Neurosci. Nurs. 2005, 37, 334–342. [Google Scholar] [CrossRef]
- Ghai, S.; Kasilingam, E.; Lanzillo, R.; Malenica, M.; Van Pesch, V.; Burke, N.C.; Carotenuto, A.; Maguire, R. Needs and Experiences of Children and Adolescents with Pediatric Multiple Sclerosis and Their Caregivers: A Systematic Review. Children 2021, 8, 445. [Google Scholar] [CrossRef]
- Martino, M.L.; De Luca Picione, R.; Lemmo, D.; Boursier, V.; Freda, M.F. Meaning-Making Trajectories of Resilience in Narratives of Adolescents with MS. Mediterr. J. Clin. Psychol. 2019, 7. [Google Scholar] [CrossRef]
- McCabe, M.P.; Ebacioni, K.J.; Simmons, R.; McDonald, E.; Melton, L. Unmet education, psychological and peer support needs of people with multiple sclerosis. J. Psychosom. Res. 2015, 78, 82–87. [Google Scholar] [CrossRef]
- Hanghøj, S.; Boisen, K.A.; Schmiegelow, K.; Hølge-Hazelton, B. A photo elicitation study on chronically ill adolescents’ identity constructions during transition. Glob. Qual. Nurs. Res. 2016, 3, 2333393616631678. [Google Scholar] [CrossRef] [PubMed]
- Mah, J.K.; Thannhauser, J.E. Management of multiple sclerosis in adolescents-current treatment options and related adherence issues. Adolesc. Health Med. Ther. 2010, 1, 31–43. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Akre, C.; Suris, J.-C. From controlling to letting go: What are the psychosocial needs of parents of adolescents with a chronic illness? Health Educ. Res. 2014, 29, 764–772. [Google Scholar] [CrossRef]
- Benson, P.; Bundick, M. Erikson and adolescent development: Contemporary views on an enduring legacy. J. Child Youth Care Work 2020, 25, 195–205. [Google Scholar] [CrossRef]
- Wicks, S.; Berger, Z.; Camic, P.M. It’s how I am... it’s what I am... it’s a part of who I am: A narrative exploration of the impact of adolescent-onset chronic illness on identity formation in young people. Clin. Child Psychol. Psychiatry 2019, 24, 40–52. [Google Scholar] [CrossRef] [PubMed]
- Thannhauser, J.E. Navigating life and loss in pediatric multiple sclerosis. Qual. Health Res. 2014, 24, 1198–1211. [Google Scholar] [CrossRef]
- Garey, J.; Latella, L.; Howard, H. Mental Health in Kids with Chronic Illness-Child Mind Institute. Available online: https://childmind.org/article/mental-health-in-children-with-chronic-illness/ (accessed on 17 May 2025).
- Jordan, A.; Noel, M.; Caes, L.; Connell, H.; Gauntlett-Gilbert, J. A developmental arrest? Interruption and identity in adolescent chronic pain. Pain Rep. 2018, 3, e678. [Google Scholar] [CrossRef] [PubMed]
- Narula, S. Transition of care to adult neuroimmunology. Semin. Pediatr. Neurol. 2023, 46, 101052. [Google Scholar] [CrossRef] [PubMed]
- Thoby, E.; Veras, J.; Nallapati, S.; Jimenez, M.E.; Bhise, V. No one really plans to have multiple sclerosis: Transition readiness and quality of life in paediatric multiple sclerosis. Child Care Health Dev. 2024, 50, e13304. [Google Scholar] [CrossRef]
- Deibel, F.; Edwards, M.; Edwards, A. Patients’, carers’ and providers’ experiences and requirements for support in self-management of multiple sclerosis: A qualitative study. Eur. J. Pers. Centered Healthcare 2013, 1, 457–467. [Google Scholar] [CrossRef][Green Version]
- Ghezzi, A.; Goretti, B.; Portaccio, E.; Roscio, M.; Amato, M.P. Cognitive impairment in pediatric multiple sclerosis. Neurol. Sci. 2010, 31, S215–S218. [Google Scholar] [CrossRef] [PubMed]
- Julian, L.; Serafin, D.; Charvet, L.; Ackerson, J.; Benedict, R.; Braaten, E.; Brown, T.; O’Donnell, E.; Parrish, J.; Preston, T.; et al. Network of Pediatric MSCenters of Excellence Cognitive impairment occurs in children adolescents with multiple sclerosis: Results from a United States, network. J. Child Neurol. 2013, 28, 102–107. [Google Scholar] [CrossRef]
- Ceyhun, H.A.; Bilge, N.; Değirmencioğlu Gök, D. Impulsivity and attention deficit-hyperactivity symptoms among patients with relapsing-remitting multiple sclerosis: A case-control study. Neurol. Res. 2024, 46, 243–252. [Google Scholar] [CrossRef]
- McKay, K.A.; Manouchehrinia, A.; Berrigan, L.; Fisk, J.D.; Olsson, T.; Hillert, J. Long-term Cognitive Outcomes in Patients with Pediatric-Onset vs Adult-Onset Multiple Sclerosis. JAMA Neurol. 2019, 76, 1028–1034. [Google Scholar] [CrossRef]
- Hosseini, B.; Flora, D.B.; Banwell, B.L.; Till, C. Age of onset as a moderator of cognitive decline in pediatric-onset multiple sclerosis. J. Int. Neuropsychol. Soc. 2014, 20, 796–804. [Google Scholar] [CrossRef]
- Gur, N.; Hoofien, D.; Pilowsky Peleg, T.; Ganelin-Cohen, E. Detecting cognitive decline in pediatric MS: The significance of personal measures for high-achievers. Mult. Scler. Relat. Disord. 2025, 97, 106385. [Google Scholar] [CrossRef]
- Portaccio, E.; Goretti, B.; Zipoli, V.; Hakiki, B.; Giannini, M.; Pastò, L.; Razzolini, L.; Amato, M.P. Cognitive rehabilitation in children and adolescents with multiple sclerosis. Neurol. Sci. 2010, 31, S275–S278. [Google Scholar] [CrossRef] [PubMed]
- Boesen, M.S.; Thygesen, L.C.; Uldall, P.V.; Eriksson, F.; Born, A.P.; Blinkenberg, M.; Koch-Henriksen, N.; Greisen, G.; Magyari, M. Psychiatric morbidity develops after onset of pediatric multiple sclerosis: A Danish nationwide population-based study. Mult. Scler. Relat. Disord. 2018, 19, 30–34. [Google Scholar] [CrossRef]
- Bigi, S.; Banwell, B. Pediatric multiple sclerosis. J. Child Neurol. 2012, 27, 1378–1383. [Google Scholar] [CrossRef]
- Charvet, L.; Cersosimo, B.; Schwarz, C.; Belman, A.; Krupp, L.B. Behavioral symptoms in pediatric multiple sclerosis: Relation to fatigue and cognitive impairment. J. Child Neurol. 2016, 31, 1062–1067. [Google Scholar] [CrossRef]
- Goretti, B.; Ghezzi, A.; Portaccio, E.; Lori, S.; Zipoli, V.; Razzolini, L.; Moiola, L.; Falautano, M.; De Caro, M.F.; Viterbo, R.; et al. Study Group of the Italian Neurological Society Psychosocial issue in children and adolescents with multiple sclerosis. Neurol. Sci. 2010, 31, 467–470. [Google Scholar] [CrossRef]
- King, E. Chronic Illness and Functionality: How It Affects Adolescents Academically and Socially and How They Can Cope. Intuit. BYU Undergrad. J. Psychol. 2017, 12, 94–106. [Google Scholar]
- Colasanti, A.; Guo, Q.; Giannetti, P.; Wall, M.B.; Newbould, R.D.; Bishop, C.; Onega, M.; Nicholas, R.; Ciccarelli, O.; Muraro, P.A.; et al. Hippocampal neuroinflammation, functional connectivity, and depressive symptoms in multiple sclerosis. Biol. Psychiatry 2016, 80, 62–72. [Google Scholar] [CrossRef]
- Green, R.; Adler, A.; Banwell, B.L.; Fabri, T.L.; Yeh, E.A.; Collins, D.L.; Sled, J.G.; Narayanan, S.; Till, C. Involvement of the Amygdala in Memory and Psychosocial Functioning in Pediatric-Onset Multiple Sclerosis. Dev. Neuropsychol. 2018, 43, 524–534. [Google Scholar] [CrossRef]
- Storm Van’s Gravesande, K.; Blaschek, A.; Calabrese, P.; Rostásy, K.; Huppke, P.; Kessler, J.J.; Kalbe, E.; Mall, V.; MUSICADO Study group. Fatigue and depression predict health-related quality of life in patients with pediatric-onset multiple sclerosis. Mult. Scler. Relat. Disord. 2019, 36, 101368. [Google Scholar] [CrossRef] [PubMed]
- Lulu, S.; Julian, L.; Shapiro, E.; Hudson, K.; Waubant, E. Treatment adherence and transitioning youth in pediatric multiple sclerosis. Mult. Scler. Relat. Disord. 2014, 3, 689–695. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Carbonell, C.; Charvet, L.E.; Krupp, L.B. Enhancing mood, cognition, and quality of life in pediatric multiple sclerosis. Paediatr. Drugs 2021, 23, 317–329. [Google Scholar] [CrossRef] [PubMed]
- Zeltzer, L. Chronic illness and disability in adolescents. Int. J. Adolesc. Med. Health 2011, 1, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Wainwright, E.; Jordan, A.; Fisher, E.; Wilson, C.; Mullen, D.; Madhavakkannan, H. Beliefs about worry and pain amongst adolescents with and without chronic pain. J. Pediatr. Psychol. 2022, 47, 432–445. [Google Scholar] [CrossRef]
- Allen, D.A.; Affleck, G.; Tennen, H.; McGrade, B.J.; Ratzan, S. Concerns of children with a chronic illness: A cognitive-developmental study of juvenile diabetes. Child Care Health Dev. 1984, 10, 211–218. [Google Scholar] [CrossRef]
- Carroll, S.; Chalder, T.; Hemingway, C.; Heyman, I.; Bear, H.; Sweeney, L.; Moss-Morris, R. Adolescent and parent factors related to fatigue in paediatric multiple sclerosis and chronic fatigue syndrome: A comparative study. Eur. J. Paediatr. Neurol. 2019, 23, 70–80. [Google Scholar] [CrossRef]
- Menzies, V.; Kelly, D.L.; Yang, G.S.; Starkweather, A.; Lyon, D.E. A systematic review of the association between fatigue and cognition in chronic noncommunicable diseases. Chronic Illn. 2021, 17, 129–150. [Google Scholar] [CrossRef]
- Targum, S.D.; Fava, M. Fatigue as a residual symptom of depression. Innov. Clin. Neurosci. 2011, 8, 40–43. [Google Scholar] [PubMed]
- Billones, R.R.; Kumar, S.; Saligan, L.N. Disentangling fatigue from anhedonia: A scoping review. Transl. Psychiatry 2020, 10, 273. [Google Scholar] [CrossRef]
- Tarasiuk, J.; Kapica-Topczewska, K.; Czarnowska, A.; Chorąży, M.; Kochanowicz, J.; Kułakowska, A. Co-occurrence of Fatigue and Depression in People with Multiple Sclerosis: A Mini-Review. Front. Neurol. 2021, 12, 817256. [Google Scholar] [CrossRef]
- McIntosh, G.E.; Liu, E.S.; Allan, M.; Grech, L.B. Clinical practice guidelines for the detection and treatment of depression in multiple sclerosis: A systematic review. Neurol. Clin. Pract. 2023, 13, e200154. [Google Scholar] [CrossRef]
- McKay, K.A.; Friberg, E.; Razaz, N.; Alexanderson, K.; Hillert, J. Long-term Socioeconomic Outcomes Associated with Pediatric-Onset Multiple Sclerosis. JAMA Neurol. 2021, 78, 478–482. [Google Scholar] [CrossRef]
- Hamama, L.; Hamama-Raz, Y.; Lebowitz-Sokolover, K.; Ganelin-Cohen, E. Well-being among parents of youth with multiple sclerosis: A preliminary longitudinal study. Front. Psychol. 2024, 15, 1308141. [Google Scholar] [CrossRef]
- Cooley, W.C. Adolescent health care transition in transition. JAMA Pediatr. 2013, 167, 897–899. [Google Scholar] [CrossRef]
- Marani, H.; Fujioka, J.; Tabatabavakili, S.; Bollegala, N. Systematic narrative review of pediatric-to-adult care transition models for youth with pediatric-onset chronic conditions. Child. Youth Serv. Rev. 2020, 118, 105415. [Google Scholar] [CrossRef]
- Masciulli, C.; Portaccio, E.; Goretti, B.; Niccolai, C.; Simone, M.; Viterbo, R.G.; Zaffaroni, M.; Pippolo, L.; Cocco, E.; Fenu, G.; et al. Home-based, computer-assisted cognitive rehabilitation for attention in pediatric onset multiple sclerosis: A randomized, multicenter pilot study. Neurol. Sci. 2025, 46, 1013–1017. [Google Scholar] [CrossRef]
- Malti, T.; Noam, G.G.; Beelmann, A.; Sommer, S. Toward dynamic adaptation of psychological interventions for child and adolescent development and mental health. J. Clin. Child Adolesc. Psychol. 2016, 45, 827–836. [Google Scholar] [CrossRef]
- Lucien, A.; Francis, H.; Wu, W.; Woldhuis, T.; Gandy, M. The efficacy of cognitive behavioural therapy for depression and anxiety in multiple sclerosis: A systematic review and meta-analysis. Mult. Scler. Relat. Disord. 2024, 91, 105858. [Google Scholar] [CrossRef]
- Gold, S.M.; Friede, T.; Meyer, B.; Moss-Morris, R.; Hudson, J.; Asseyer, S.; Bellmann-Strobl, J.; Leisdon, A.; Ißels, L.; Ritter, K.; et al. Internet-delivered cognitive behavioural therapy programme to reduce depressive symptoms in patients with multiple sclerosis: A multicentre, randomised, controlled, phase 3 trial. Lancet Digit. Health 2023, 5, e668–e678. [Google Scholar] [CrossRef] [PubMed]
- Rechenberg, K.; Koerner, R. Cognitive Behavioral Therapy in Adolescents with Type 1 Diabetes: An Integrative Review. J. Pediatr. Nurs. 2021, 60, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Morey, A.; Loades, M.E. Review: How has cognitive behaviour therapy been adapted for adolescents with comorbid depression and chronic illness? A scoping review. Child Adolesc. Ment. Health 2021, 26, 252–264. [Google Scholar] [CrossRef]
- Thompson, R.D.; Delaney, P.; Flores, I.; Szigethy, E. Cognitive-behavioral therapy for children with comorbid physical illness. Child Adolesc. Psychiatr. Clin. N. Am. 2011, 20, 329–348. [Google Scholar] [CrossRef]
- Sesel, A.-L.; Sharpe, L.; Beadnall, H.N.; Barnett, M.H.; Szabo, M.; Naismith, S.L. A randomized controlled trial of a web-based mindfulness programme for people with MS with and without a history of recurrent depression. Mult. Scler. 2022, 28, 1392–1401. [Google Scholar] [CrossRef]
- Simpson, R.; Simpson, S.; Ramparsad, N.; Lawrence, M.; Booth, J.; Mercer, S.W. Effects of Mindfulness-based interventions on physical symptoms in people with multiple sclerosis-a systematic review and meta-analysis. Mult. Scler. Relat. Disord. 2020, 38, 101493. [Google Scholar] [CrossRef]
- Nauta, I.M.; Bertens, D.; Fasotti, L.; Fieldhouse, J.; Uitdehaag, B.M.J.; Kessels, R.P.C.; Speckens, A.E.M.; de Jong, B.A. Cognitive rehabilitation and mindfulness reduce cognitive complaints in multiple sclerosis (REMIND-MS): A randomized controlled trial. Mult. Scler. Relat. Disord. 2023, 71, 104529. [Google Scholar] [CrossRef]
- Ahola Kohut, S.; Stinson, J.; Davies-Chalmers, C.; Ruskin, D.; van Wyk, M. Mindfulness-Based Interventions in Clinical Samples of Adolescents with Chronic Illness: A Systematic Review. J. Altern. Complement. Med. 2017, 23, 581–589. [Google Scholar] [CrossRef]
- Ng, L.; Amatya, B.; Khan, F. Outcomes of a peer support program in multiple sclerosis in an Australian community cohort: A prospective study. J. Neurodegener. Dis. 2013, 1, 429171. [Google Scholar] [CrossRef]
- Join a Support Group or Program|National MS Society. Available online: https://www.nationalmssociety.org/resources/get-support/find-support-groups-and-programs (accessed on 25 June 2025).
- Clark, H.B.; Ichinose, C.K.; Meseck-Bushey, S.; Perez, K.R.; Hall, M.S.; Gibertini, M.; Crowe, T. Peer support group for adolescents with chronic illness. Child. Health Care 1992, 21, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Shafran, R.; Bennett, S.D.; Jolly, A.; Morant, N. The impact of therapeutic recreation camps in the United Kingdom on the wellbeing of youth with serious illness and disability: A qualitative investigation. J. Pediatr. Nurs. 2022, 67, e31–e37. [Google Scholar] [CrossRef]
- Hasan, I.; Chowdhury, A.A.; Haque, M.I.; Patterson, C.C. Changes in glycated hemoglobin, diabetes knowledge, quality of life, and anxiety in children and adolescents with type 1 diabetes attending summer camps: A systematic review and meta-analysis. Pediatr. Diabetes 2021, 22, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Torres-Costoso, A.; Martínez-Vizcaíno, V.; Reina-Gutiérrez, S.; Álvarez-Bueno, C.; Guzmán-Pavón, M.J.; Pozuelo-Carrascosa, D.P.; Fernández-Rodríguez, R.; Sanchez-López, M.; Cavero-Redondo, I. Effect of Exercise on Fatigue in Multiple Sclerosis: A Network Meta-analysis Comparing Different Types of Exercise. Arch. Phys. Med. Rehabil. 2022, 103, 970–987.e18. [Google Scholar] [CrossRef] [PubMed]
- Sesel, A.-L.; Sharpe, L.; Naismith, S.L. Efficacy of Psychosocial Interventions for People with Multiple Sclerosis: A Meta-Analysis of Specific Treatment Effects. Psychother. Psychosom. 2018, 87, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Hugos, C.L.; Copperman, L.F.; Fuller, B.E.; Yadav, V.; Lovera, J.; Bourdette, D.N. Clinical trial of a formal group fatigue program in multiple sclerosis. Mult. Scler. 2010, 16, 724–732. [Google Scholar] [CrossRef] [PubMed]
- Mulligan, H.; Wilkinson, A.; Barclay, A.; Whiting, H.; Heynike, C.; Snowdon, J. Evaluation of a Fatigue Self-Management Program for People with Multiple Sclerosis. Int. J. MS Care 2016, 18, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Field, T. Exercise research on children and adolescents. Complement. Ther. Clin. Pract. 2012, 18, 54–59. [Google Scholar] [CrossRef]
- Wang, T.; Li, W.; Deng, J.; Zhang, Q.; Liu, Y. The influence of physical exercise on negative emotions in adolescents: A meta-analysis. Front. Psychiatry 2024, 15, 1457931. [Google Scholar] [CrossRef]
- Lampit, A.; Heine, J.; Finke, C.; Barnett, M.H.; Valenzuela, M.; Wolf, A.; Leung, I.H.K.; Hill, N.T.M. Computerized Cognitive Training in Multiple Sclerosis: A Systematic Review and Meta-analysis. Neurorehabil. Neural Repair 2019, 33, 695–706. [Google Scholar] [CrossRef]
- Rezvani, S.; Sharifi, A.A.; Zare, H. The effectiveness of cognitive rehabilitation on improving working memory of adolescents with brain injury. J. Cogn. Psychol. 2021, 9, 1–15. [Google Scholar] [CrossRef]
- Resch, C.; Rosema, S.; Hurks, P.; de Kloet, A.; van Heugten, C. Searching for effective components of cognitive rehabilitation for children and adolescents with acquired brain injury: A systematic review. Brain Inj. 2018, 32, 679–692. [Google Scholar] [CrossRef]
- Azimian, M.; Arian, M.; Shojaei, S.F.; Doostian, Y.; Ebrahimi Barmi, B.; Khanjani, M.S. The Effectiveness of Group Hope Therapy Training on the Quality of Life and Meaning of Life in Patients with Multiple Sclerosis and Their Family Caregivers. Iran. J. Psychiatry 2021, 16, 260–270. [Google Scholar] [CrossRef]
- Oz, H.S.; Oz, F. A psychoeducation program for stress management and psychosocial problems in multiple sclerosis. Niger. J. Clin. Pract. 2020, 23, 1598–1606. [Google Scholar] [CrossRef]
- Law, E.; Fisher, E.; Eccleston, C.; Palermo, T.M. Psychological interventions for parents of children and adolescents with chronic illness. Cochrane Database Syst. Rev. 2019, 3, CD009660. [Google Scholar] [CrossRef]
- Bergmame, L.; Shaw, S.R. Psychoeducational interventions to improve adolescents’ medical management of diabetes: A comprehensive review. Health Psychol. Rep. 2017, 6, 10–39. [Google Scholar] [CrossRef]
- Gallagher, L.M.; Bethoux, F. Multiple Sclerosis Therapeutic use of the Arts for Patients with Multiple Sclerosis. Touch Neurol. 2017, 13, 82–89. [Google Scholar]
- Adelwöhrer, C.; Nausner, A.; Stieglbauer, K.; Bibl, D.; Engleder, C.; Schimetta, W.; Pölz, W.; Ransmayr, G.; Tölk, A.; Aichner, F. Kunsttherapie bei Patienten mit schubförmiger Multipler Sklerose. Psychiatrie 2008, 4, 92–99. [Google Scholar] [CrossRef]
- Newland, P.; Miller, R.; Bettencourt, B.A.; Hendricks-Ferguson, V. Pilot Study of Videos to Deliver Mindfulness-Based Art Therapy for Adults with Multiple Sclerosis. J. Neurosci. Nurs. 2020, 52, E19–E23. [Google Scholar] [CrossRef] [PubMed]
- Basli, E.; Özmen, S.; Demirci, E.; Kendirci, M.; Tatli, Z.; Kondolot, M. The effect of art therapy techniques on depression, anxiety levels and quality of life in the adolescent with type 1 diabetes mellitus: Preliminary study. Erciyes Med. J. 2020, 42, 431–435. [Google Scholar] [CrossRef]
- Golubowski, E. How Can Art Therapy Be Utilized to Improve the Mental Health and Quality of life of Pediatric Oncology Patients? Expressive Therapies Capstone Theses 2020, 354. Available online: https://digitalcommons.lesley.edu/expressive_theses/354 (accessed on 15 September 2025).
- Poulsen, V.R. Enhancing health literacy among disadvantaged youth: A participatory approach in school settings. Eur. J. Public Health 2024, 34, 144–693. [Google Scholar] [CrossRef]
- Gonsalves, P.P.; Ansari, S.; Berry, C.; Gonsalves, F.; Iyengar, S.; Kashyap, P.; Mittal, D.; Pal, S.; Razdan, E.; Michelson, D. Co-designing digital mental health interventions with young people: 10 recommendations from lessons learned in low-and-middle-income countries. Health Educ. J. 2025, 84, 373–384. [Google Scholar] [CrossRef]
- Abujaradeh, H.; Safadi, R.; Sereika, S.M.; Kahle, C.T.; Cohen, S.M. Mindfulness-Based Interventions Among Adolescents with Chronic Diseases in Clinical Settings: A Systematic Review. J. Pediatr. Health Care 2018, 32, 455–472. [Google Scholar] [CrossRef]
- Moola, F.J.; Faulkner, G.E.J.; White, L.; Kirsh, J.A. The psychological and social impact of camp for children with chronic illnesses: A systematic review update. Child Care Health Dev. 2014, 40, 615–631. [Google Scholar] [CrossRef]
- Day, S.; Laver, K.; Jeon, Y.-H.; Radford, K.; Low, L.-F. Frameworks for cultural adaptation of psychosocial interventions: A systematic review with narrative synthesis. Dementia 2023, 22, 1921–1949. [Google Scholar] [CrossRef] [PubMed]
- Mesa, A.; Anderson, K.H.; Askey-Jones, S.; Gray, R.; Silber, E. The mental health needs of individuals living with multiple sclerosis: Implications for occupational therapy practice and research. Ment. Health Spec. Interest Sect. Q. 2012, 35, 1–4. [Google Scholar]
| Study | Study Design | Sample | Main Findings | CEBM Level * |
|---|---|---|---|---|
| Barlow-Krelina et al., 2021 [5] | Cross-sectional case–control (computerized neurocognitive battery vs. healthy controls) | POMS n = 65 (8–29 y); Controls n = 76 | • Lower overall performance in POMS vs. controls; impairments in accuracy (executive, episodic memory, complex cognition) even after adjusting for response speed • Slower overall response time in POMS | 3b (individual case-control study) |
| Boesen et al., 2018 [33] | Nationwide population-based cohort with nested case–control using Danish registers | POMS n = 212; age- and sex-matched controls 5:1; followed till 20y | • No association with psychiatric morbidity before POMS onset • After onset, hazard of psychiatric morbidity approximately doubled (HR ≈ 2.0, 95% CI 1.3–3.1). | 2b (individual cohort study) |
| Boyd & Macmillan, 2005 [11] | Qualitative study (interviews) | n = 12; ages 8–18 | • Explores lived experiences of children/adolescents with POMS | Qualitative |
| Charvet et al., 2016 [35] | Cross-sectional clinical sample | n = 140; ages 5–18 (MS or Clinically Isolated Syndrome (CIS)) | • 33.1% had ≥1 clinically significant BASC-2 scale • Most common: attention problems, somatization, anxiety • Cognitive functioning predicted a clinical problem. | 4 (cross-sectional study) |
| Gur et al., 2025 [31] | Cohort observational (cross-sectional neuropsychological and mood assessment) | POMS n = 31 (20F); mean age 15.8 y | • Cognitive Impairment in 26%; Personal-Cognitive-Decline (Personal-CD) in 45% (73% among high-achievers) • Cognitive Impairment associated with disability; Personal-CD associated with depression, not disease severity. | 4 (cross-sectional study) |
| Hamama et al., 2024 [53] | Preliminary longitudinal cohort (3 waves; multilevel causal mediation) | Parents of youth with POMS: n = 36 (clinic sample) | • Perceived social support (PSS) from friends at T1 → ↑ coping flexibility at T2 → ↓ psychological distress at T3 • Mothers reported higher PSS from friends than fathers • Overall levels of PSS, coping flexibility, life satisfaction, and distress were stable over 12 months. | 2b (individual cohort study) |
| Hosseini et al., 2014 [30] | Longitudinal growth-curve analysis | POMS n = 35; repeated cognitive testing | • Younger age at onset predicted steeper decline on SDMT and Trail-making test over time • Baseline IQ and social status did not moderate decline. | 2b (individual cohort study) |
| Julian et al., 2013 [27] | Multicenter cross-sectional analysis (US Pediatric MS Centers) | MS n = 187; CIS n = 44; mean age 14.8 y; disease duration ≈1.9 y | • Cognitive impairment in 35% of MS and 18% of CIS • Most frequent deficits: fine motor/pegboard, visuomotor integration, speeded processing • Independent predictors: MS diagnosis (OR ≈ 3.6) and higher disability. | 4 (cross-sectional study) |
| Lulu et al., 2014 [41] | Cross-sectional study: prospectively enrolled POMS patients (self/parent surveys, clinical data) | n = 30; ages 12–23 (mean 15.8); 53% female; 47% Hispanic | • Non-adherence (missing ≥20% DMT doses in past month) in 37%; most common reason was forgetting (50%). • Higher disability associated with lower QoL and healthcare skills; higher SDMT associated with greater transition readiness and healthcare skills. | 4 (cross-sectional study) |
| Masciulli et al., 2025 [56] | Randomized, double-blind, multicenter pilot trial (specific vs. non-specific home-based attention training) | 22 POMS (9–18 y) | • Primary outcome: SDMT improved in the specific-training group (31.2→42.4 at ~3 months; p = 0.043). • No benefit on other cognitive measures; feasibility demonstrated. | 2b (individual randomized pilot trial) |
| Mckay et al., 2019 [29] | Nationwide longitudinal cohort (Swedish MS Registry); mixed-effects models, repeated SDMT | 5704 adults with MS (300 POMS); 46,429 SDMTs; analyzed ages 18–55; median follow-up 3.0 y | • POMS had lower SDMT than AOMS (β −3.59) and faster decline (β −0.30). • Higher odds of ever meeting cognitive impairment vs. Adult-Onset MS (OR 1.44). | 2b (individual cohort study) |
| Mckay et al., 2021 [52] | Register-based matched cohort (10:1) of socioeconomic outcomes | POMS n = 485 vs. matched references n = 4850; outcomes analyzed across ages 19–54 | • Lower odds of university attendance in POMS (OR 0.80, 95% CI 0.66–0.97). • Lower annual earnings (−$1618 to −$10,683 across age bands). • Higher disability benefits: sickness-absence RR 3.06 (ages 19–24); disability-pension RR 1.43 (ages 45–54). | 2b (individual cohort study) |
| Wallach et al., 2020 [4] | Multicenter cohort (US Network of Pediatric MS Centers); baseline + serial SDMT | Assessed: 955; included: POMS n = 500, CIS n = 116; mean disease duration 3.0 y; follow-up mean 1.8 y | • Impaired processing speed at baseline in 23.4% (POMS) and 16.4% (CIS). • Clinically meaningful SDMT decline in 14.1% over ~1.8 y; older age at onset and male sex predicted decline. • Relapse or recent steroids → transient SDMT worsening. | 2b (individual cohort study) |
| Weisbrot et al., 2014 [7] | Clinic-based cross-sectional case series with structured psychiatric interviews and neuropsychological testing | n = 45; ages 8–17; non-consecutive referrals for psychiatric evaluation | • Most frequent diagnoses: anxiety (n = 15), ADHD (n = 12), mood disorders (n = 11). • Cognitive impairment in 80% with a psychiatric diagnosis vs. 55% without (p = 0.08); highest with mood/anxiety (p = 0.05). | 4 (cross-sectional study/case series) |
| Intervention Modality | Evidence in Adult ms | Evidence in Adolescents with Chronic Illness |
|---|---|---|
| Cognitive-Behavioral Therapy (CBT) | - Efficacious for depression & anxiety in MS [58] - MS-specific internet CBT reduced depressive symptoms in a trial [59] | - Feasible and effective for teens with diabetes; improved psychosocial outcomes including stress and self-efficacy as well as quality of life [60] - Shown to reduce depressive symptoms in chronic conditions (e.g., asthma, chronic pain) [61,62] |
| Mindfulness-Based Interventions (MBI) | - Web-based MBI significantly improved depression and quality of life in MS [63] - MB Stress Reduction (MBSR) pilot in MS showed benefits for fatigue, sleep, and subjective cognitive symptoms [63,64,65] | - Promising for coping with emotional distress and chronic illness symptoms in teens [66] - Found to be acceptable in the adolescent population [66] |
| Peer Support (Support Groups, Camps, Retreats) | - Reports of improved coping, psychological well-being, and quality of life in adult MS support group participants [67] - MS societies facilitate peer mentor and support programs, communities, meet-ups and camps, though these have not been rigorously evaluated [68] | - Peer group interventions improved coping and QoL in teens with chronic illness [69] - Disease-specific camps (e.g., diabetes camp) increase self-esteem, social skills, and illness knowledge [70,71] |
| Exercise and Fatigue Management | - Regular exercise in MS improves fatigue and mood [72] - Group or individual fatigue management programs reduced fatigue impact in trials [73,74,75] | - Exercise programs for teens with chronic illnesses show enhanced mood, psychological symptoms, and energy [76,77] |
| Cognitive Rehabilitation | - Memory and attention training in MS can yield cognitive improvements including in memory, attention, and processing speed [78] - Cognitive rehabilitation therapy showed alleviation of subjective cognitive complaints [65] | - In POMS specifically, a pilot computer-assisted attention training showed preliminary benefits [56] - There exist protocols for cognitive rehabilitation for adolescents who suffer from brain injury but published work remains limited [79,80] |
| Family Programs and Psychoeducation | - Family therapy is not well-studied in adult MS [81] - Psychoeducational programs increase patient knowledge and may reduce anxiety and stress [82] | - Parent-focused psychological interventions in pediatric chronic illness improve parent mental health and indirectly child outcomes, though quality of evidence is low [83] - While outcomes are mixed, psychoeducational interventions, especially those using family-focused or tech-supported delivery, can improve disease management and psychosocial outcomes in chronically ill adolescents [84] |
| Art Therapy and Mindfulness-Based Art Therapy | - Small RCTs and qualitative studies show improved mood and self-efficacy, reduced fatigue, and enhanced emotional expression [85,86] - May be effectively delivered via video-conference tools [87] | - Used in adolescent cancer, diabetes, and arthritis to reduce anxiety and improve coping and quality of life [88] - Enhances emotional expression, communication, and self-esteem [89] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Novak, A.M. Growing Up with MS: The Adolescent Experience of Pediatric-Onset Multiple Sclerosis. Adolescents 2025, 5, 66. https://doi.org/10.3390/adolescents5040066
Novak AM. Growing Up with MS: The Adolescent Experience of Pediatric-Onset Multiple Sclerosis. Adolescents. 2025; 5(4):66. https://doi.org/10.3390/adolescents5040066
Chicago/Turabian StyleNovak, Anne Marie. 2025. "Growing Up with MS: The Adolescent Experience of Pediatric-Onset Multiple Sclerosis" Adolescents 5, no. 4: 66. https://doi.org/10.3390/adolescents5040066
APA StyleNovak, A. M. (2025). Growing Up with MS: The Adolescent Experience of Pediatric-Onset Multiple Sclerosis. Adolescents, 5(4), 66. https://doi.org/10.3390/adolescents5040066






