Calculations of pKa Values for a Series of Fluorescent Nucleobase Analogues
Abstract
1. Introduction
2. Materials and Methods
3. Results
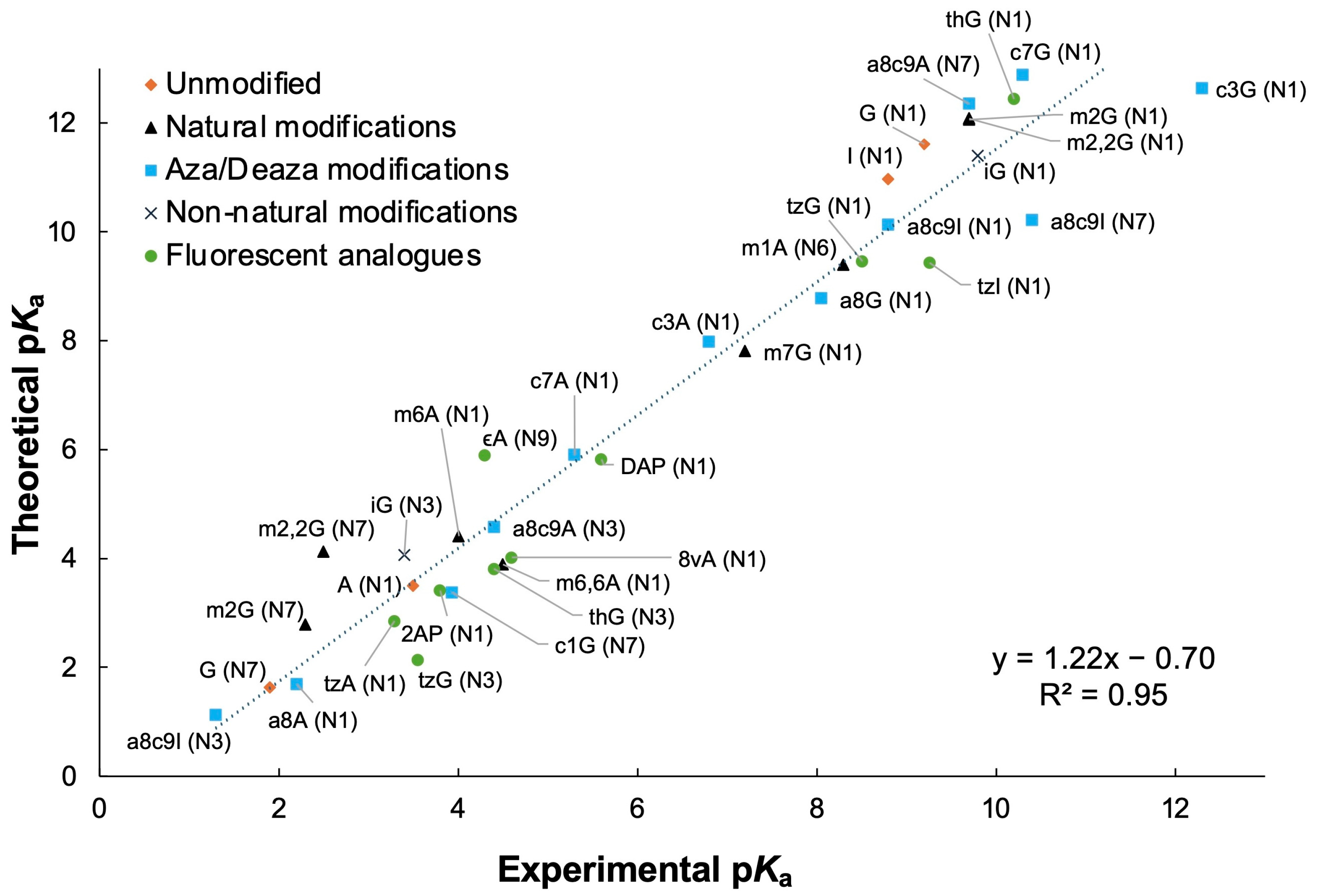
3.1. Determination of Nucleobase pKa Values
3.2. Purines
3.3. Adenosine Fluorescent Analogues
3.4. Guanosine Fluorescent Analogues
3.5. Pyrimidines
3.6. Cytidine Fluorescent Analogues
3.7. Uridine Fluorescent Analogues
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| A | Adenosine |
| C | Cytidine |
| DFT | Density functional theory |
| DNA | Deoxyribonucleic acid |
| G | Guanosine |
| i-motif | Intercalated motif |
| MAE | Mean absolute error |
| pKa | Negative logarithm of the acid dissociation constant |
| ΔpKa | Difference in pKa values |
| RNA | Ribonucleic acid |
| SNP | Single nucleotide polymorphism |
| T | Thymidine |
| th | Thiophene |
| tz | Isothiazole |
| U | Uridine |
References
- Saenger, W. Principles of Nucleic Acid Structure; Springer: New York, NY, USA, 1984. [Google Scholar]
- Šponer, J.; Leszczynski, J.; Hobza, P. Electronic Properties, Hydrogen Bonding, Stacking, and Cation Binding of DNA and RNA Bases. Biopolymers 2001, 61, 3–31. [Google Scholar] [CrossRef]
- Shan, S.O.; Loh, S.; Herschlag, D. The Energetics of Hydrogen Bonds in Model Systems: Implications for Enzymatic Catalysis. Science 1996, 272, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Tor, Y. Isomorphic Fluorescent Nucleosides. Acc. Chem. Res. 2024, 57, 1325–1335. [Google Scholar] [CrossRef] [PubMed]
- Ward, D.C.; Reich, E.; Stryer, L. Fluorescence Studies of Nucleotides and Polynucleotides: I. Formycin, 2-Aminopurine Riboside, 2,6-Diaminopurine Riboside, and Their Derivatives. J. Biol. Chem. 1969, 244, 1228–1237. [Google Scholar] [CrossRef] [PubMed]
- Secrist, J.A.; Barrio, J.R.; Leonard, N.J. A Fluorescent Modification of Adenosine Triphosphate with Activity in Enzyme Systems: 1,N6-Ethenoadenosine Triphosphate. Science 1972, 175, 646–647. [Google Scholar] [CrossRef]
- Wu, P.; Nordlund, T.M.; Gildea, B.; McLaughlin, L.W. Base Stacking and Unstacking as Determined from a DNA Decamer Containing a Fluorescent Base. Biochemistry 1990, 29, 6508–6514. [Google Scholar] [CrossRef]
- Hurley, D.J.; Seaman, S.E.; Mazura, J.C.; Tor, Y. Fluorescent 1,10-Phenanthroline-Containing Oligonucleotides Distinguish between Perfect and Mismatched Base Pairing. Org. Lett. 2002, 4, 2305–2308. [Google Scholar] [CrossRef]
- Wojciechowski, F.; Hudson, R.H.E. Fluorescence and Hybridization Properties of Peptide Nucleic Acid Containing a Substituted Phenylpyrrolocytosine Designed to Engage Guanine with an Additional H-Bond. J. Am. Chem. Soc. 2008, 130, 12574–12575. [Google Scholar] [CrossRef]
- Stolar, T.; Pearce, B.K.D.; Etter, M.; Truong, K.N.; Ostojić, T.; Krajnc, A.; Mali, G.; Rossi, B.; Molčanov, K.; Lončarić, I.; et al. Base-Pairing of Uracil and 2,6-Diaminopurine: From Cocrystals to Photoreactivity. iScience 2024, 27, 109894. [Google Scholar] [CrossRef]
- Mandal, S.; Hebenbrock, M.; Müller, J. A Dinuclear Silver(I)-Mediated Base Pair in DNA Formed from 1,N6-Ethenoadenine and Thymine. Inorg. Chim. Acta 2018, 472, 229–233. [Google Scholar] [CrossRef]
- Sinkeldam, R.W.; Greco, N.J.; Tor, Y. Fluorescent Analogs of Biomolecular Building Blocks: Design, Properties, and Applications. Chem. Rev. 2010, 110, 2579–2619. [Google Scholar] [CrossRef] [PubMed]
- Soulière, M.F.; Haller, A.; Rieder, R.; Micura, R. A Powerful Approach for the Selection of 2-Aminopurine Substitution Sites to Investigate RNA Folding. J. Am. Chem. Soc. 2011, 133, 16161–16167. [Google Scholar] [CrossRef]
- Datta, M.; Liu, J. A DNA Aptamer for 2-Aminopurine: Binding-Induced Fluorescence Quenching. Chem. Asian J. 2024, 19, e202400817. [Google Scholar] [CrossRef]
- Matarazzo, A.; Hudson, R.H.E. Fluorescent Adenosine Analogs: A Comprehensive Survey. Tetrahedron 2015, 71, 1627–1657. [Google Scholar] [CrossRef]
- Serrano-Andrés, L.; Merchán, M.; Borin, A.C. Adenine and 2-Aminopurine: Paradigms of Modern Theoretical Photochemistry. Proc. Natl. Acad. Sci. USA 2006, 103, 8691–8696. [Google Scholar] [CrossRef]
- Nordlund, T.M.; Andersson, S.; Nilsson, L.; Rigler, R.; Graeslund, A.; McLaughlin, L.W. Structure and Dynamics of a Fluorescent DNA Oligomer Containing the EcoRI Recognition Sequence: Fluorescence, Molecular Dynamics, and NMR Studies. Biochemistry 1989, 28, 9095–9103. [Google Scholar] [CrossRef]
- Sowers, L.C.; Fazakerley, G.V.; Eritja, R.; Kaplan, B.E.; Goodman, M.F. Base Pairing and Mutagenesis: Observation of a Protonated Base Pair between 2-Aminopurine and Cytosine in an Oligonucleotide by Proton NMR. Proc. Nat. Acad. Sci. USA 1986, 83, 5434–5438. [Google Scholar] [CrossRef]
- Guest, C.R.; Hochstrasser, R.A.; Sowers, L.C.; Millar, D.P. Dynamics of Mismatched Base Pairs in DNA. Biochemistry 1991, 30, 3271–3279. [Google Scholar] [CrossRef]
- Shin, D.; Sinkeldam, R.W.; Tor, Y. Emissive RNA Alphabet. J. Am. Chem. Soc. 2011, 133, 14912–14915. [Google Scholar] [CrossRef]
- Srivatsan, S.G.; Weizman, H.; Tor, Y. A Highly Fluorescent Nucleoside Analog Based on Thieno[3,4-d]Pyrimidine Senses Mismatched Pairing. Org. Biomol. Chem. 2008, 6, 1334–1338. [Google Scholar] [CrossRef] [PubMed]
- Shields, G.C.; Seybold, P.G. Computational Approaches for the Prediction of pKa Values; CRC Press: Boca Raton, FL, USA; London, UK; New York, NY, USA, 2013. [Google Scholar]
- Ho, J.; Coote, M.L. A Universal Approach for Continuum Solvent pKa Calculations: Are We There Yet? Theor. Chem. Acc. 2010, 125, 3–21. [Google Scholar] [CrossRef]
- Thapa, B.; Raghavachari, K. Accurate pKa Evaluations for Complex Bio-Organic Molecules in Aqueous Media. J. Chem. Theory Comput. 2019, 15, 6025–6035. [Google Scholar] [CrossRef] [PubMed]
- Thapa, B.; Schlegel, H.B. Calculations of pKa’s and Redox Potentials of Nucleobases with Explicit Waters and Polarizable Continuum Solvation. J. Phys. Chem. A 2015, 119, 5134–5144. [Google Scholar] [CrossRef] [PubMed]
- Jones, E.L.; Mlotkowski, A.J.; Hebert, S.P.; Schlegel, H.B.; Chow, C.S. Calculations of pKa Values for a Series of Naturally Occurring Modified Nucleobases. J. Phys. Chem. A 2022, 126, 1518–1529. [Google Scholar] [CrossRef] [PubMed]
- Mlotkowski, A.J.; Schlegel, H.B.; Chow, C.S. Calculated pKa Values for a Series of Aza- and Deaza-Modified Nucleobases. J. Phys. Chem. A 2023, 127, 3526–3534. [Google Scholar] [CrossRef]
- Thapa, B.; Schlegel, H.B. Improved pKa Prediction of Substituted Alcohols, Phenols, and Hydroperoxides in Aqueous Medium Using Density Functional Theory and a Cluster-Continuum Solvation Model. J. Phys. Chem. A 2017, 121, 4698–4706. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 09, Revision D.01; Gaussian Inc.: Wallingford, CT, USA, 2014. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16 Rev. B.01; Gaussian Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti Correlation-Energy Formula into a Functional of the Electron Density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Becke, A.D. Density-Functional Thermochemistry. III. The Role of Exact Exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Hehre, W.J.; Ditchfield, R.; Pople, J.A. Self-Consistent Molecular Orbital Methods. XII. Further Extensions of Gaussian-Type Basis Sets for Use in Molecular Orbital Studies of Organic Molecules. J. Chem. Phys. 1972, 56, 2257–2261. [Google Scholar] [CrossRef]
- Hariharan, P.C.; Pople, J.A. The Influence of Polarization Functions on Molecular Orbital Hydrogenation Energies. Theor. Chim. Acta 1973, 28, 213–222. [Google Scholar] [CrossRef]
- Francl, M.M.; Pietro, W.J.; Hehre, W.J.; Binkley, J.S.; Gordon, M.S.; DeFrees, D.J.; Pople, J.A. Self-Consistent Molecular Orbital Methods. XXIII. A Polarization-Type Basis Set for Second-Row Elements. J. Chem. Phys. 1982, 77, 3654–3665. [Google Scholar] [CrossRef]
- Bryantsev, V.S.; Diallo, M.S.; Goddard, W.A. Calculation of Solvation Free Energies of Charged Solutes Using Mixed Cluster/Continuum Models. J. Phys. Chem. B 2008, 112, 9709–9719. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S. A Reliable and Efficient First Principles-Based Method for Predicting pKa Values. III. Adding Explicit Water Molecules: Can the Theoretical Slope be Reproduced and pKa Values Predicted More Accurately? J. Comput. Chem. 2012, 33, 517–526. [Google Scholar] [CrossRef]
- da Silva, E.F.; Svendsen, H.F.; Merz, K.M. Explicitly Representing the Solvation Shell in Continuum Solvent Calculations. J. Phys. Chem. A 2009, 113, 6404–6409. [Google Scholar] [CrossRef]
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Universal Solvation Model Based on Solute Electron Density and on a Continuum Model of the Solvent Defined by the Bulk Dielectric Constant and Atomic Surface Tensions. J. Phys. Chem. B 2009, 113, 6378–6396. [Google Scholar] [CrossRef] [PubMed]
- Camaioni, D.M.; Schwerdtfeger, C.A. Comment on “Accurate Experimental Values for the Free Energies of Hydration of H+, OH−, and H3O+”. J. Phys. Chem. A 2005, 109, 10795–10797. [Google Scholar] [CrossRef] [PubMed]
- Kelly, C.P.; Cramer, C.J.; Truhlar, D.G. Aqueous Solvation Free Energies of Ions and Ion-Water Clusters Based on an Accurate Value for the Absolute Aqueous Solvation Free Energy of the Proton. J. Phys. Chem. B 2006, 110, 16066–16081. [Google Scholar] [CrossRef]
- Mlotkowski, A.J. The Impacts of Modification on Acid Dissociation Constants of Nucleobases and Structure of Helix 69 RNA. Ph.D. Dissertation, Wayne State University, Detroit, MI, USA, 2023. [Google Scholar]
- Sowers, L.C.; Mhaskar, D.N.; Khwaja, T.A.; Goodman, M.F. Preparation of Imino and Amino N-15 Enriched 2-Aminopurine Deoxynucleoside. Nucleosides Nucleotides 1989, 8, 23–34. [Google Scholar] [CrossRef]
- Harkins, T.R.; Freiser, H. Adenine-Metal Complexes1,2. J. Am. Chem. Soc. 1958, 80, 1132–1135. [Google Scholar] [CrossRef]
- Ma, L.; Kartik, S.; Liu, B.; Liu, J. From General Base to General Acid Catalysis in a Sodium-Specific DNAzyme by a Guanine-to-Adenine Mutation. Nucleic Acids Res. 2019, 47, 8154–8162. [Google Scholar] [CrossRef]
- Penzer, G.R. The Solution Conformation and Some Spectroscopic Properties of 1, N6-Ethenoadenosine Monophosphate, a Fluorescent Analogue of AMP. Eur. J. Biochem. 1973, 34, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Rovira, A.R.; Fin, A.; Tor, Y. Chemical Mutagenesis of an Emissive RNA Alphabet. J. Am. Chem. Soc. 2015, 137, 14602–14605. [Google Scholar] [CrossRef]
- Roday, S.; Saen-oon, S.; Schramm, V.L. Vinyldeoxyadenosine in a Sarcin-Ricin RNA Loop and Its Binding to Ricin Toxin A-Chain. Biochemistry 2007, 46, 6169–6182. [Google Scholar] [CrossRef]
- Levene, P.A.; Bass, L.W. Nucleic Acids; Chemical Catalog Company: New York, NY, USA, 1931. [Google Scholar]
- Dziuba, D.; Didier, P.; Ciaco, S.; Barth, A.; Seidel, C.A.M.; Mély, Y. Fundamental Photophysics of Isomorphic and Expanded Fluorescent Nucleoside Analogues. Chem. Soc. Rev. 2021, 50, 7062–7107. [Google Scholar] [CrossRef]
- Fagan, P.A.; Fàbrega, C.; Eritja, R.; Goodman, M.F.; Wemmer, D.E. NMR Study of the Conformation of the 2-Aminopurine:Cytosine Mismatch in DNA. Biochemistry 1996, 35, 4026–4033. [Google Scholar] [CrossRef]
- Law, S.M.; Eritja, R.; Goodman, M.F.; Breslauer, K.J. Spectroscopic and Calorimetric Characterizations of DNA Duplexes Containing 2-Aminopurine. Biochemistry 1996, 35, 12329–12337. [Google Scholar] [CrossRef]
- Lamm, G.M.; Blencowe, B.J.; Sparoat, B.S.; Iribarren, A.M.; Ryder, U.; Lamond, A.I. Antisense Probes Containing 2-Aminoadenosine Allow Efficient Depletion of U5 snRNP from HeLa Splicing Extracts. Nucleic Acids Res. 1991, 19, 3193–3198. [Google Scholar] [CrossRef]
- Hoheisel, J.D.; Lehrach, H. Quantitative Measurements on the Duplex Stability of 2,6-Diaminopurine and 5-Chloro-Uracil Nucleotides Using Enzymatically Synthesized Oligomers. FEBS Lett. 1990, 274, 103–106. [Google Scholar]
- Bailly, C.; Waring, M.J. The Use of Diaminopurine to Investigate Structural Properties of Nucleic Acids and Molecular Recognition between Ligands and DNA. Nucleic Acids Res. 1998, 26, 4309–4314. [Google Scholar] [CrossRef] [PubMed]
- Kodali, G.; Kistler, K.A.; Narayanan, M.; Matsika, S.; Stanley, R.J. Change in Electronic Structure upon Optical Excitation of 8-Vinyladenosine: An Experimental and Theoretical Study. J. Phys. Chem. A 2010, 114, 256–267. [Google Scholar] [CrossRef] [PubMed]
- Gaied, N.B.; Glasser, N.; Ramalanjaona, N.; Beltz, H.; Wolff, P.; Marquet, R.; Burger, A.; Mély, Y. 8-Vinyl-Deoxyadenosine, an Alternative Fluorescent Nucleoside Analog to 2′-Deoxyribosyl-2-Aminopurine with Improved Properties. Nucleic Acids Res. 2005, 33, 1031–1039. [Google Scholar] [CrossRef]
- Seela, F.; Zulauf, M. 7-Deazaadenine-DNA: Bulky 7-Iodo Substituents or Hydrophobic 7-Hexynyl Chains Are Well Accommodated in the Major Groove of Oligonucleotide Duplexes. Chem. Eur. J. 1998, 4, 1781–1790. [Google Scholar] [CrossRef]
- Booth, J.; Cummins, W.J.; Brown, T. An Analogue of Adenine that Forms an “A:T” Bse Pair of Comparable Stability to G:C. Chem. Commun. 2004, 19, 2208–2209. [Google Scholar] [CrossRef]
- Xu, W.; Chan, K.M.; Kool, E.T. Fluorescent Nucleobases as Tools for Studying DNA and RNA. Nat. Chem. 2017, 9, 1043–1055. [Google Scholar] [CrossRef]
- Thames, K.E.; Cheung, H.C.; Harvey, S.C. Binding of 1,N6-Ethanoadenosine Triphosphate to Actin. Biochem. Biophys. Res. Commun. 1974, 60, 1252–1261. [Google Scholar] [CrossRef]
- McCubbin, W.D.; Willick, G.E.; Kay, C.M. Enzymatic Studies on the Interaction of Myosin and Heavy Meromyosin with 1,N6-Ethenoadenosine Triphosphate (εATP), a Fluorescent Analog of ATP. Biochem. Biophys. Res. Commun. 1973, 50, 926–933. [Google Scholar] [CrossRef]
- Willick, G.E.; Oikawa, K.; McCubbin, W.D.; Kay, C.M. Equilibrium Dialysis Binding Studies of 1,N6-Ethenoadenosine Diphosphate (εADP) to Myosin, Heavy Meromyosin, and Subfragment One. Biochem. Biophys. Res. Commun. 1973, 53, 923–928. [Google Scholar] [CrossRef]
- Miki, M.; Mihashi, K. Fluorescence and Flow Dichroism of F-Actin-ϵ-ADP; the Orientation of the Adenine Plane Relative to the Long Axis of F-Actin. Biophys. Chem. 1976, 6, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Harvey, S.C.; Cheung, H.C. Fluorescence Studies of 1,N6-Ethenoadenosine Triphosphate Bound to G-Actin: The Nucleotide Base is Inaccessible to Water. Biochem. Biophys. Res. Commun. 1976, 73, 865–868. [Google Scholar] [CrossRef] [PubMed]
- Perkins, W.J.; Wells, J.A.; Yount, R.G. Characterization of the Properties of Ethenoadenosine Nucleotides Bound or Trapped at the Active Site of Myosin Subfragment 1. Biochemistry 1984, 23, 3994–4002. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, A.; Tanaka, K.; Saito, I. DNA Logic Gates. J. Am. Chem. Soc. 2004, 126, 9458–9463. [Google Scholar] [CrossRef]
- Nadler, A.; Strohmeier, J.; Diederichsen, U. 8-Vinyl-2′-Deoxyguanosine as a Fluorescent 2′-Deoxyguanosine Mimic for Investigating DNA Hybridization and Topology. Angew. Chem. Int. Ed. 2011, 50, 5392–5396. [Google Scholar] [CrossRef] [PubMed]
- Sholokh, M.; Improta, R.; Mori, M.; Sharma, R.; Kenfack, C.; Shin, D.; Voltz, K.; Stote, R.H.; Zaporozhets, O.A.; Botta, M.; et al. Tautomers of a Fluorescent G Surrogate and Their Distinct Photophysics Provide Additional Information Channels. Angew. Chem. Int. Ed. 2016, 55, 7974–7978. [Google Scholar] [CrossRef] [PubMed]
- Shugar, D.; Fox, J.J. Spectrophotometric Studies of Nucleic Acid Derivatives and Related Compounds as a Function of pH. I. Pyrimidines. Biochim. Biophys. Acta 1952, 9, 199–218. [Google Scholar] [CrossRef]
- Tinsley, R.A.; Walter, N.G. Pyrrolo-C as a Fluorescent Probe for Monitoring RNA Secondary Structure Formation. RNA 2006, 12, 522–529. [Google Scholar] [CrossRef] [PubMed]
- Mata, G.; Schmidt, O.P.; Luedtke, N.W. A Fluorescent Surrogate of Thymidine in Duplex DNA. Chem. Commun. 2016, 52, 4718–4721. [Google Scholar] [CrossRef]
- Hall, R. The Modified Nucleosides in Nucleic Acids; Columbia University Press: New York, NY, USA, 1971. [Google Scholar]
- Singleton, S.F.; Shan, F.; Kanan, M.W.; McIntosh, C.M.; Stearman, C.J.; Helm, J.S.; Webb, K.J. Facile Synthesis of a Fluorescent Deoxycytidine Analogue Suitable for Probing the RecA Nucleoprotein Filament. Org. Lett. 2001, 3, 3919–3922. [Google Scholar] [CrossRef]
- Wada, T.; Kobori, A.; Kawahara, S.I.; Sekine, M. Synthesis and Properties of Oligodeoxyribonucleotides Containing 4-N-Acetylcytosine Bases. Tetrahedron Lett. 1998, 39, 6907–6910. [Google Scholar] [CrossRef]
- Datta, K.; Johnson, N.P.; Villani, G.; Marcus, A.H.; von Hippel, P.H. Characterization of the 6-Methyl Isoxanthopterin (6-MI) Base Analog Dimer, a Spectroscopic Probe for Monitoring Guanine Base Conformations at Specific Sites in Nucleic Acids. Nucleic Acids Res. 2012, 40, 1191–1202. [Google Scholar] [CrossRef]
- Dash, C.; Rausch, J.W.; Le Grice, S.F. Using Pyrrolo-Deoxycytosine to Probe RNA/DNA Hybrids Containing the Human Immunodeficiency Virus Type-1 3’ Polypurine Tract. Nucleic Acids Res. 2004, 32, 1539–1547. [Google Scholar] [CrossRef]
- Wilhelmsson, L.M.; Holmén, A.; Lincoln, P.; Nielsen, P.E.; Nordén, B. A Highly Fluorescent DNA Base Analogue that Forms Watson−Crick Base Pairs with Guanine. J. Am. Chem. Soc. 2001, 123, 2434–2435. [Google Scholar] [CrossRef]
- Reilly, S.M.; Lyons, D.F.; Wingate, S.E.; Wright, R.T.; Correia, J.J.; Jameson, D.M.; Wadkins, R.M. Folding and Hydrodynamics of a DNA i-Motif from the c-MYC Promoter Determined by Fluorescent Cytidine Analogs. Biophys. J. 2014, 107, 1703–1711. [Google Scholar] [CrossRef] [PubMed]
- Mir, B.; Serrano-Chacón, I.; Medina, P.; Macaluso, V.; Terrazas, M.; Gandioso, A.; Garavís, M.; Orozco, M.; Escaja, N.; González, C. Site-Specific Incorporation of a Fluorescent Nucleobase Analog Enhances i-Motif Stability and Allows Monitoring of i-Motif Folding Inside Cells. Nucleic Acids Res. 2024, 52, 3375–3389. [Google Scholar] [CrossRef] [PubMed]
- Noé, M.S.; Sinkeldam, R.W.; Tor, Y. Oligodeoxynucleotides Containing Multiple Thiophene-Modified Isomorphic Fluorescent Nucleosides. J. Org. Chem. 2013, 78, 8123–8128. [Google Scholar] [CrossRef] [PubMed]
- Abou Assi, H.; Garavís, M.; González, C.; Damha, M.J. i-Motif DNA: Structural Features and Significance to Cell Biology. Nucleic Acids Res. 2018, 46, 8038–8056. [Google Scholar] [CrossRef]
- Modi, S.; Nizak, C.; Surana, S.; Halder, S.; Krishnan, Y. Two DNA Nanomachines Map pH Changes Along Intersecting Endocytic Pathways Inside the Same Cell. Nat. Nanotechnol. 2013, 8, 459–467. [Google Scholar] [CrossRef]
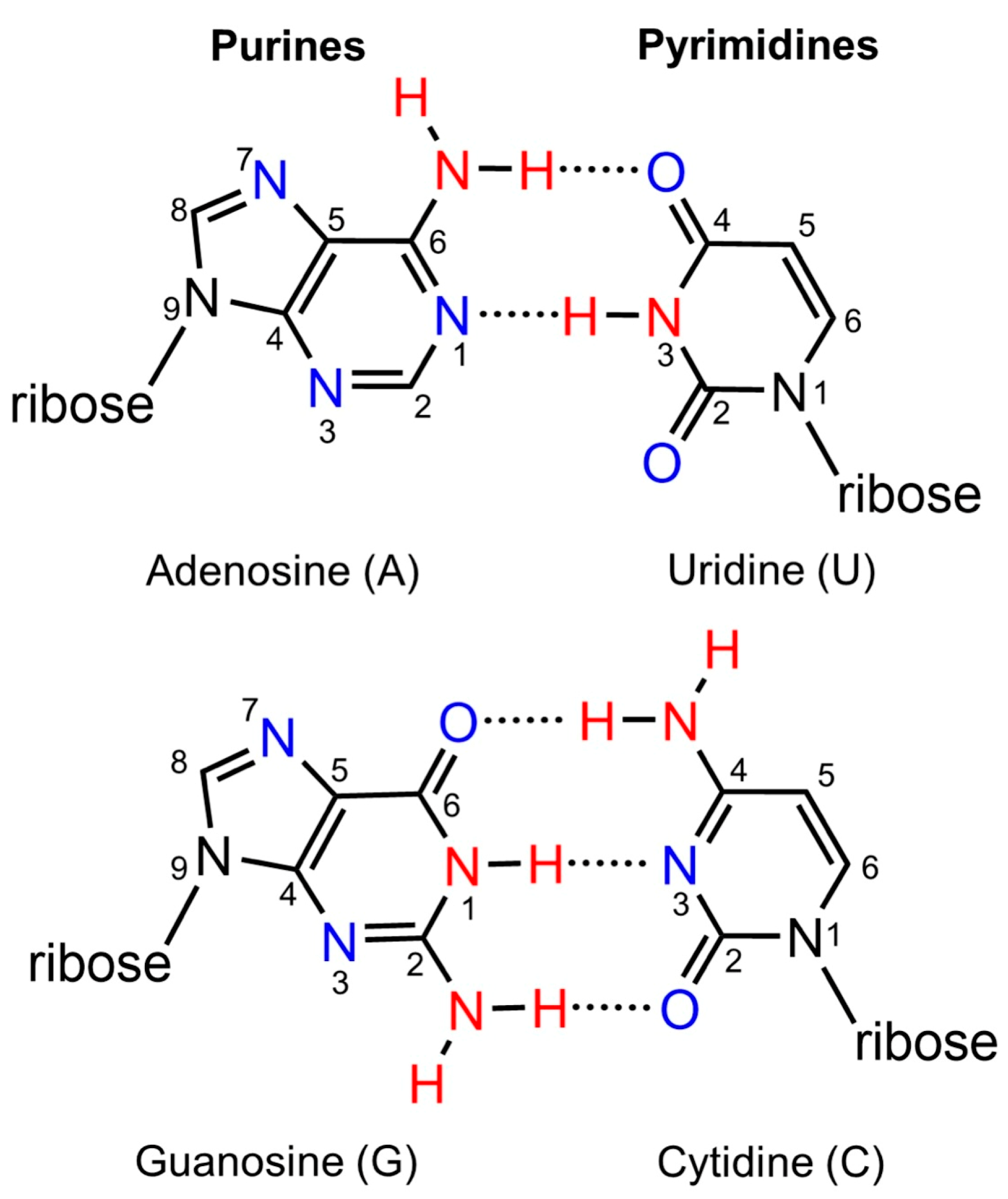


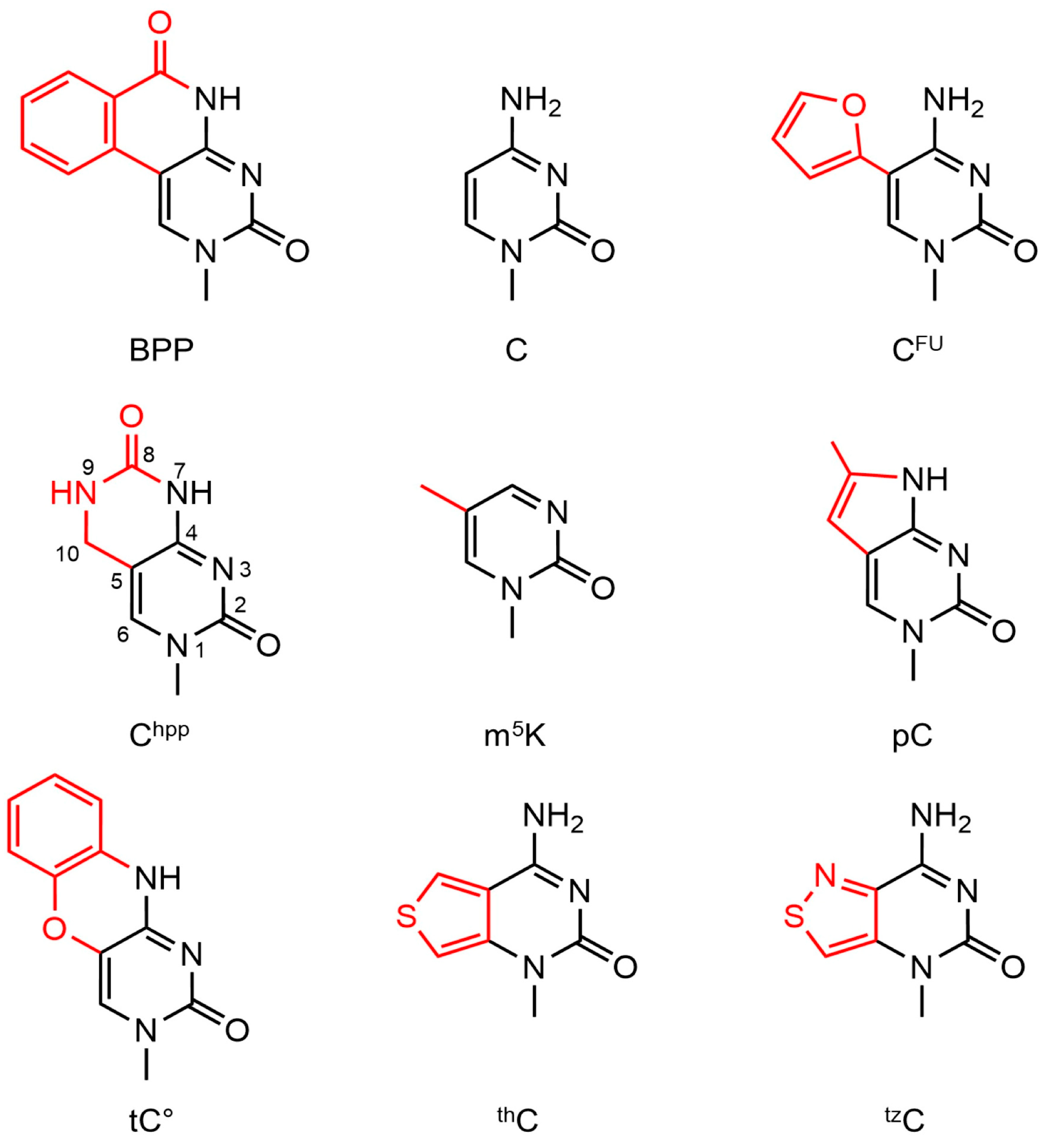
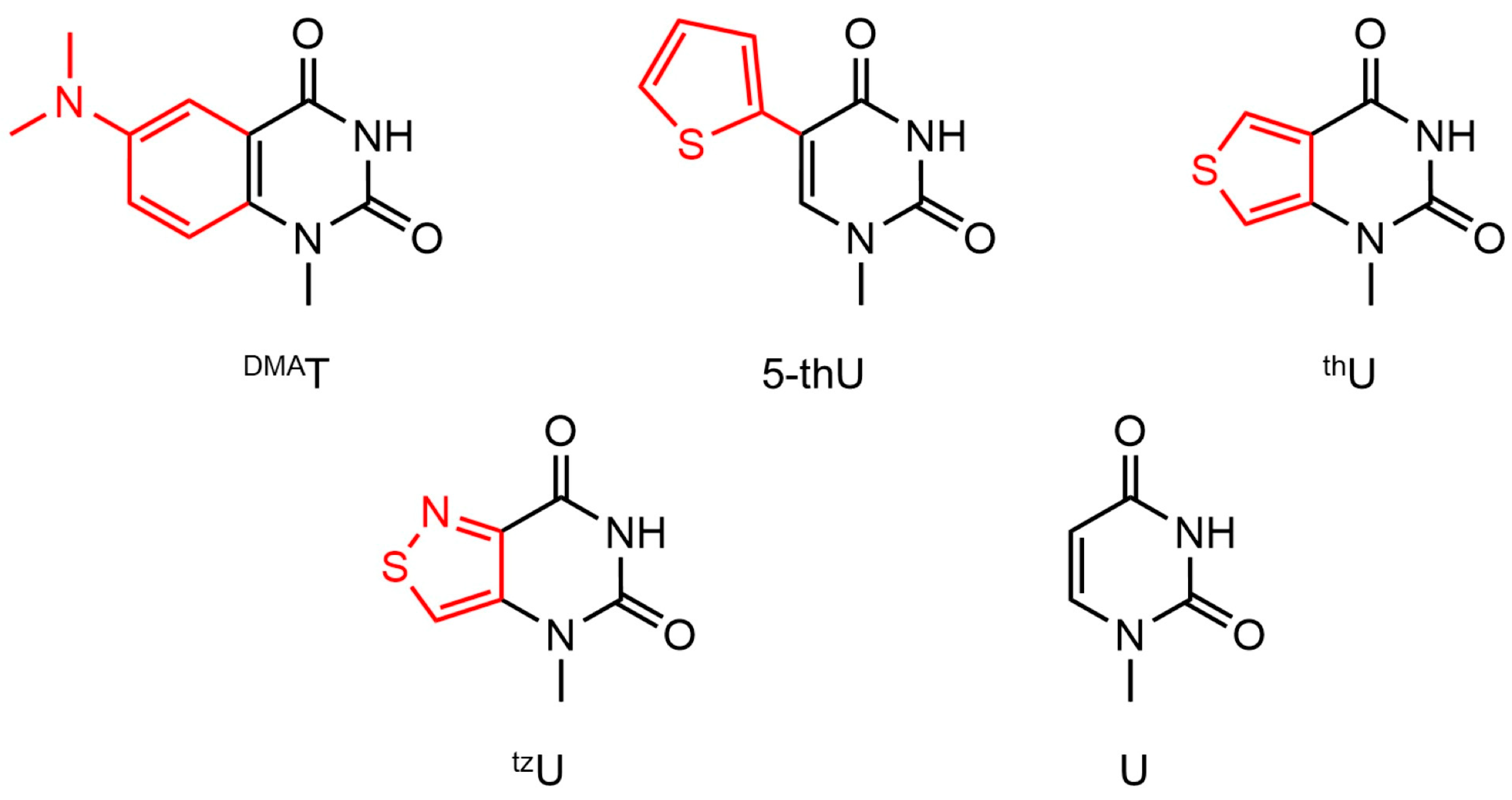
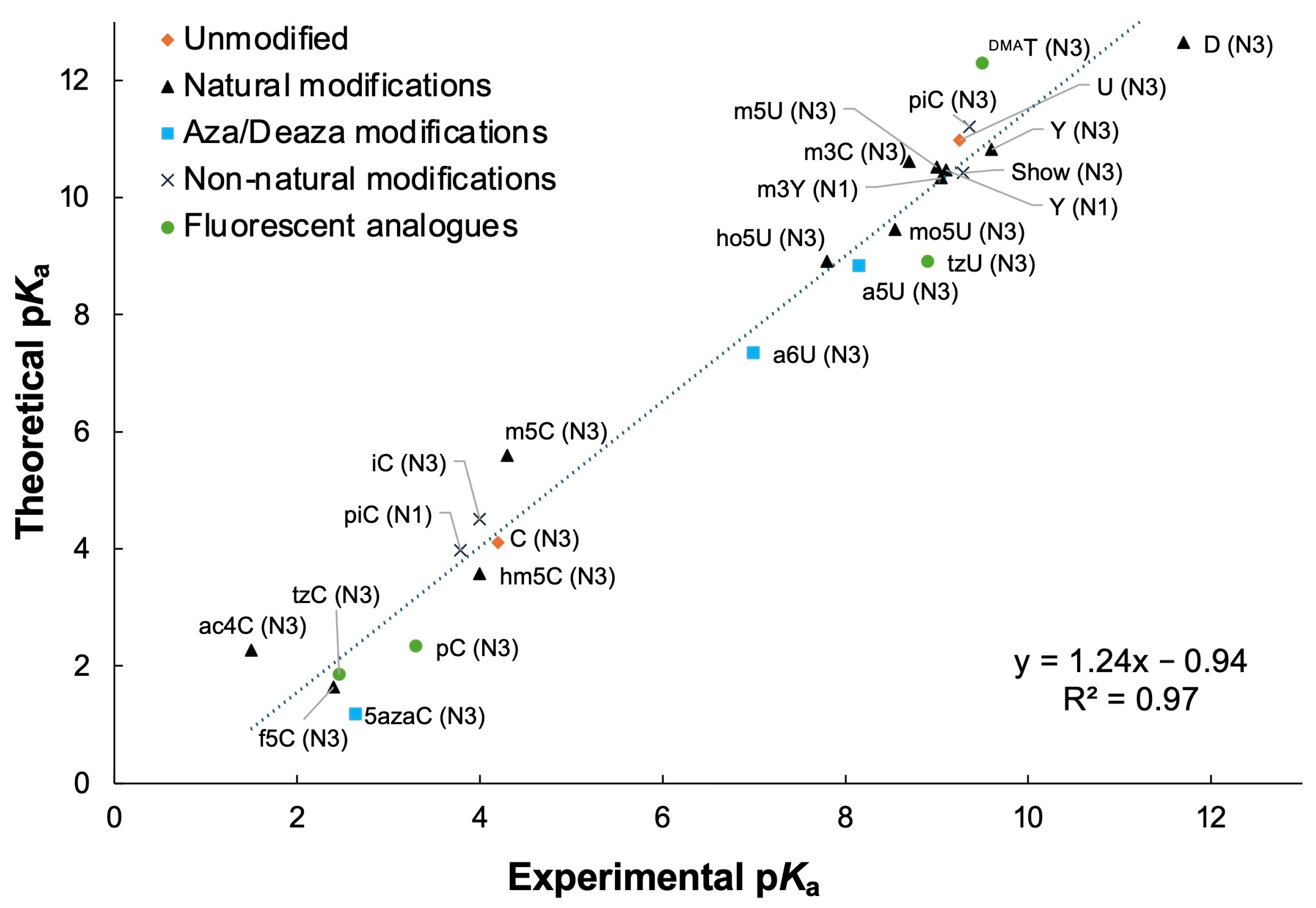
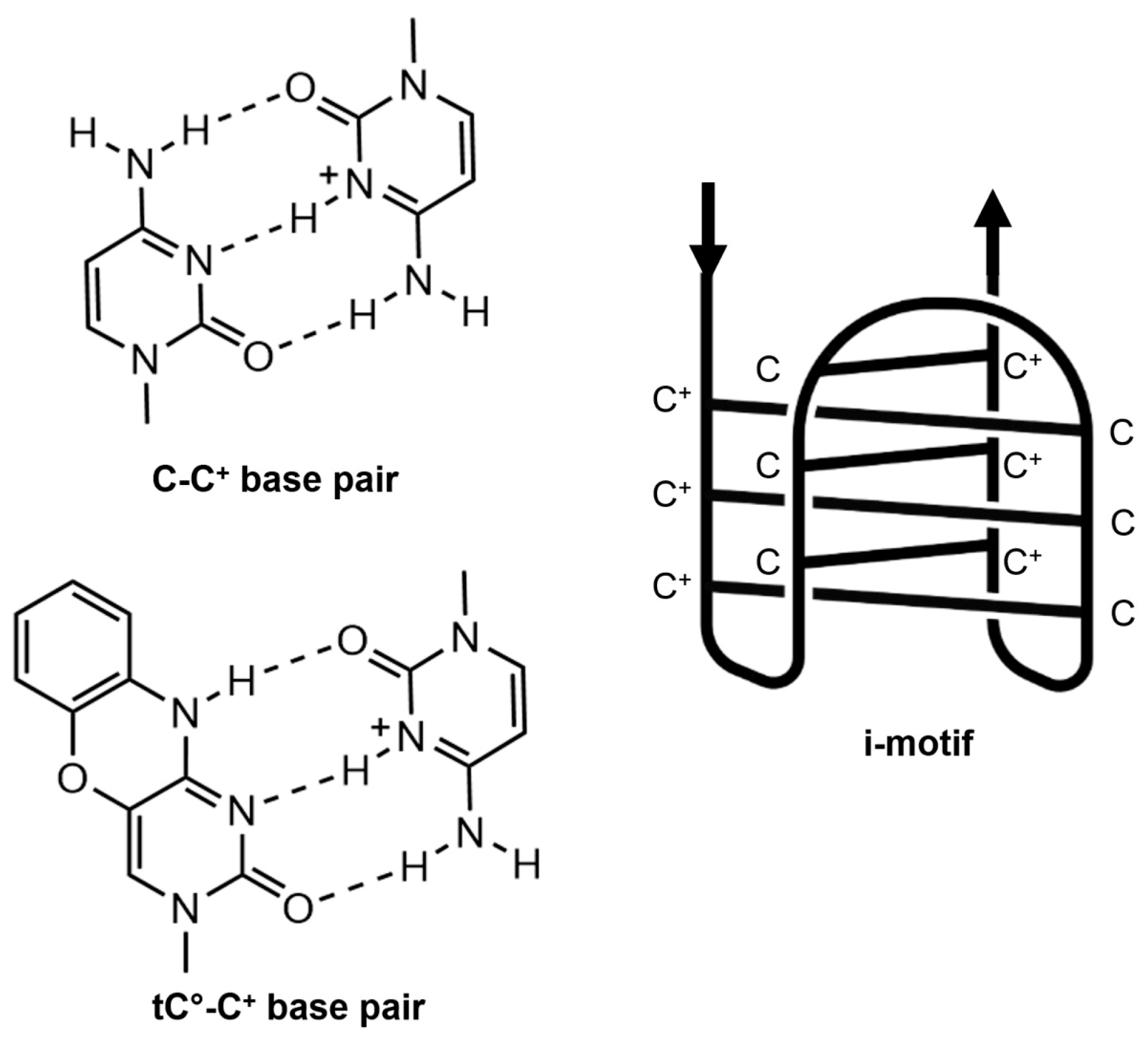
| Modification | Abbreviation | Position | Experimental pKa Value a | Adjusted Theoretical pKa Value b |
|---|---|---|---|---|
| 2-aminopurine | 2AP | N1 | 3.8 [43] | 3.4 |
| N3 | 0.6 | |||
| N7 | 2.5 | |||
| adenosine | A | N1 | 3.5 [44] | 3.4 |
| N3 | 1.3 | |||
| N7 | 1.3 | |||
| 2,6-diaminopurine | DAP | N1 | 5.6 [45] | 5.3 |
| N3 | 3.4 | |||
| N7 | 3.5 | |||
| 1,N6-ethenoadenosine | εA | N1 (N7 c) | 2.6 | |
| N4 (N3 c) | unfavorable | |||
| N9 (N6 c) | 4.3 [46] | 5.4 | ||
| 8-furan-adenosine | 8-Furan-A | N1 | 3.9 | |
| N3 | 2.0 | |||
| N7 | unfavorable | |||
| methoxybenzodeazaadenosine | MDA | N1 | 10.3 | |
| N3 | 9.2 | |||
| thieno[3,4,d]adenosine | thA | N1 | 4.8 | |
| N3 | 5.0 | |||
| isothiazolo[4,3,d]adenosine | tzA | N1 | 3.3 [47] | 2.9 |
| N3 | 3.0 | |||
| N7 | unfavorable | |||
| 8-vinyladenosine | 8vA | N1 | 4.6 [48] | 3.9 |
| N3 | 1.3 | |||
| N7 | 2.5 |
| Modification | Abbreviation | Position | Experimental pKa Value a | Adjusted Theoretical pKa Value b |
|---|---|---|---|---|
| guanosine | G | N1 | 9.2 [49] | 10.1 |
| N3 | 1.0 | |||
| N7 | 1.9 [49] | 1.9 | ||
| 8-phenol-guanosine | 8-Phenol-G | N1 | 9.4 | |
| N3 | 0.2 | |||
| N7 | unfavorable | |||
| thieno[3,4,d]guanosine | thG | N1 | 10.2 [50] | 10.7 |
| N3 | 4.4 [50] | 3.7 | ||
| isothiazolo[4,3,d]guanosine | tzG | N1 | 8.5 [47] | 8.3 |
| N3 | 3.6 [47] c | 2.3 | ||
| N7 | unfavorable | |||
| isothiazolo[4,3,d]inosine | tzI | N1 | 9.3 [47] | 8.3 |
| N3 | −0.1 | |||
| N7 | unfavorable | |||
| 8-vinylguanosine | 8vG | N1 | 9.2 | |
| N3 | 0.03 | |||
| N7 | 3.2 |
| Modification | Abbreviation | Position | Experimental pKa Value a | Adjusted Theoretical pKa Value b |
|---|---|---|---|---|
| benzopyridopyrimidine | BPP | N3 | 0.9 | |
| N7 | 9.4 | |||
| cytidine | C | N3 | 4.2 [70] | 4.0 |
| 5-furan-cytidine | CFU | N3 | 2.1 | |
| locked bicyclic 4-N-carbamoylcytidine | Chpp | N3 | 0.6 | |
| N7 | 5.7 | |||
| N9 | 11.4 | |||
| 5-methyl-2-pyrimidinone | m5K | N3 | 3.5 | |
| pyrrolocytidine | pC | N3 | 3.3 [71] | 2.6 |
| N7 | 13.2 | |||
| 1,3-diaza-2-oxophenoxazine | tC° | N3 | 2.1 | |
| N7 | 11.6 | |||
| thieno[3,4,d]cytidine | thC | N3 | 4.2 | |
| isothiazolo[4,3,d]cytidine | tzC | N3 | 2.5 [47] | 2.2 |
| N7 | unfavorable |
| Modification | Abbreviation | Position | Experimental pKa Value a | Adjusted Theoretical pKa Value b |
|---|---|---|---|---|
| N,N-dimethylaniline-2-deoxythymidine | DMAT | N3 | 9.5 [72] | 10.6 |
| 5-(thien-2-yl)uridine | 5-thU | N3 | 9.3 | |
| thieno[3,4,d]uridine | thU | N3 | 9.8 | |
| isothiazolo[4,3,d]uridine | tzU | N3 | 8.9 [47] | 7.9 |
| N7 | unfavorable | |||
| uridine | U | N3 | 9.2 [73] | 9.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Im, S.J.; Mlotkowski, A.J.; Schlegel, H.B.; Chow, C.S. Calculations of pKa Values for a Series of Fluorescent Nucleobase Analogues. Compounds 2025, 5, 44. https://doi.org/10.3390/compounds5040044
Im SJ, Mlotkowski AJ, Schlegel HB, Chow CS. Calculations of pKa Values for a Series of Fluorescent Nucleobase Analogues. Compounds. 2025; 5(4):44. https://doi.org/10.3390/compounds5040044
Chicago/Turabian StyleIm, Sun Jeong, Alan J. Mlotkowski, H. Bernhard Schlegel, and Christine S. Chow. 2025. "Calculations of pKa Values for a Series of Fluorescent Nucleobase Analogues" Compounds 5, no. 4: 44. https://doi.org/10.3390/compounds5040044
APA StyleIm, S. J., Mlotkowski, A. J., Schlegel, H. B., & Chow, C. S. (2025). Calculations of pKa Values for a Series of Fluorescent Nucleobase Analogues. Compounds, 5(4), 44. https://doi.org/10.3390/compounds5040044







