Emerging Technologies for Extracting Antioxidant Compounds from Edible and Medicinal Mushrooms: An Efficient and Sustainable Approach
Abstract
1. Introduction
2. General Characteristics of Edible Mushrooms
3. Bioactive Compounds in Edible Mushrooms
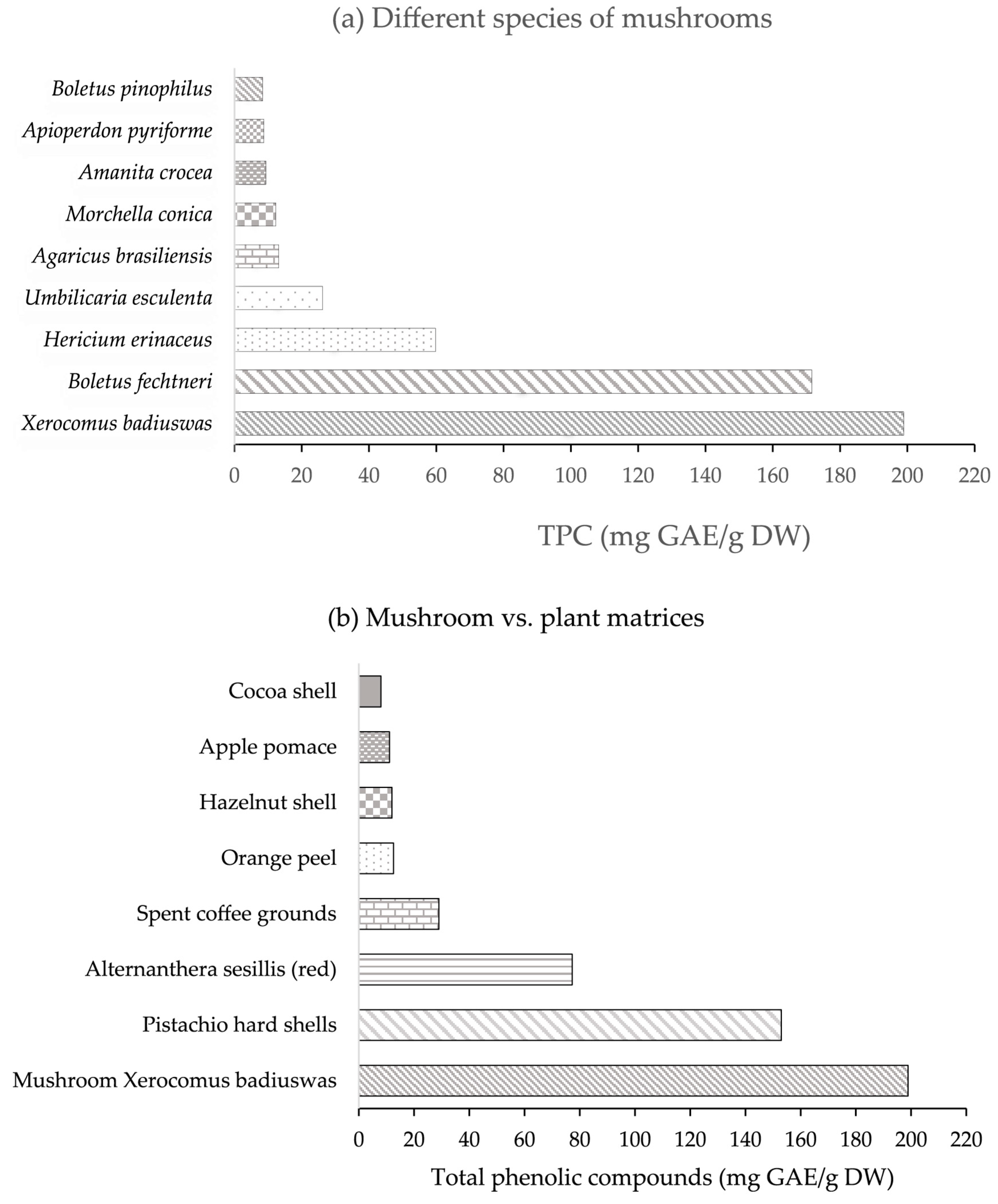
3.1. Phenolic Compounds in Edible Mushrooms
3.2. Specific Polyphenols
4. Extraction Methods
4.1. Conventional Extraction Methods
4.1.1. Soxhlet
4.1.2. Maceration
| Edible Mushroom | Conventional Extraction Method | Parameters | Optimal Conditions | Yield of Phenolic Compounds | Reference |
|---|---|---|---|---|---|
| Hericium erinaceus | Soxhlet | Temperature: 40–70 °C Extraction time: 3–9 h Solid–solvent ratio: 0.5–2 mg/mL | 60.67 °C, 7.83 h, 1.98 mg/mL, and ethanol as extraction solvent | 59.75 ± 1.82 mg GAE/g | [12] |
| Pleutorus abalonus, Auriculata auricula-judae, and Pleutorus sajor-caju | Soxhlet | – | 20 g with 400 mL of absolute ethanol for 11 h | Pleurotus sajorcaju: 12.21 ± 0.14 mg GAE/g | [84] |
| Pleurotus ostreatus and Pleurotus djamor | Soxhlet and maceration | In the Soxhlet procedure, 10 g of sample in 100 mL of the solvent was boiled for 2 h For the maceration method, 10 g of sample was mixed in 100 mL of solvent and kept under stirring at 110 rpm and 25 °C for 48 h Ethanol/water ratios: 100/0, 80/20, 50/50, 20/0, and 0/100 (v/v) Mushroom conditions: fresh, oven-dried, and sun-dried | Pleurotus ostreatus: maceration using pure water and fresh samples Pleurotus djamar: maceration using pure water and fresh samples | Pleurotus ostreatus fresco: 41.6 mg GAE/g Pleurotus djamar fresco: 11.02 mg GAE/g | [20] |
| Agaricus bisporus (white), Agaricus bisporus (brown), Lentinula edodes, Pholiota nameko, Pleurotus eryngii, and Pleurotus ostreatus. | Maceration | 80% (v/v) ethanol at 50 °C, 160 RPM, and solid–solvent ratio of 1:30 g/mL | Stem of P. ostreatus: 1.09 ± 0.09 mg QE/g Stem of white A. bisporus: 4.02 ± 0.20 mg QE/g | [82] | |
| Boletus edulis and Cantharellus cibarius | Maceration | Acid water (10% CH3COOH); ethanol/water/acetic acid (15:76.5:8.5, v/v/v), hexane, and diethyl ether | 5 g of mushroom in 150 mL of 10% acidic water | B. edulis: 3.72 mg GAE/g C. cibarius: 0.79 mg GAE/g | [6] |
| Neolentinus lepideus | Maceration | The sample (1 g) was macerated for two days in 10 mL of 80% methanol | 1.648 mg GAE/g | [85] | |
| Agaricus bisporus (mushroom), Agaricus bisporus (Portobello), Agaricus brasiliensis, F. velutipes, and Lentinus edodes | Maceration | Temperature: 25–55 °C Solvent–solid ratio: 30–70 mL Ethanol concentration: 25–75% | A. bisporus (mushroom): 50 °C/70 mL/g/75% ethanol A. bisporus (Portobello): 55 °C/70 mL/g/75% ethanol A. brasiliensis: 55 °C/70 mL/g/75% ethanol F. Velutipes: 25 °C/60.34 mL/g/25% ethanol L. edodes: 25 °C/59 mL/g/25% ethanol | A. bisporus (mushroom): 9.53 ± 0.16 mg GAE/g A. bisporus (Portobello): 9.97 ± 0.21 mg GAE/g A. brasiliensis: 13.16 ± 0.06 mg GAE/g F. Velutipes: 8.38 ± 0.13 mg GAE/g L. edodes: 5.66 ± 0.10 mg GAE/g | [49] |
| Melanoleuca cognata and Melanoleuca stridula | Maceration | Extraction solvents: ethyl acetate, methanol, and pure water | 5 g of mushroom powder in 100 mL of pure water (solvent) for 48 h | M. cognata: 43.38 ± 1.36 mg GAE/g M. stridula: 34.02 ± 1.19 mg GAE/g | [61] |
| Ganoderma lucidum | Maceration | Extraction time (days): 1, 15, and 30 Particle size (mm): 10 and 0.13 | 40 g of mushroom was extracted with 60% ethanol (1000 mL), 25 °C, 1 day, and 0.13 mm particle size | 0.013971.77 ± 0.3 mg GAE/g | [62] |
| Pleurotus djamor | Maceration | Water (100%) and methanol (100%) | 10 g of mushroom in 100 mL of water (100%) for 2 h | 5.95 mg GAE/g | [79] |
| Lentinus edodes, Volvariella volvácea, Pleurotus eous, Pleurotus sajor-caju, and Auricularia auricular | Maceration | Extraction solvents: water, 50% (v/v) ethanol, and diethyl ether, and 1.5 h | Volvariella volvácea: 50% (v/v) ethanol For the mushrooms Lentinus edodes, Pleurotus eous, Pleurotus sajor-caju Auricularia auricula: pure water | Lentinus edodes: 36.19 ± 0.59 mg GAE/g Volvariella volvácea: 27.89 ± 0.23 mg GAE/g Pleurotus eous: 20.31 ± 0.56 mg GAE/g Pleurotus sajor-caju: 16.46 ± 0.67 mg GAE/g Auricularia auricula: 2.90 ± 0.40 mg GAE/g | [86] |
| Pleurotus ostreatus, Pleurotus pulmonarius, Schizophyllum commune, Volvariella volvácea, and Lentinus edodes | Maceration | Methanol: 10 g of each mushroom powder in 100 mL of methanol at 150 rpm for 1 h at room temperature Hot water: 10 g of each mushroom powder with 100 mL of boiling water (100 °C) and stirring at 150 rpm for 30 min | Volvariella volvácea presented the highest content of phenolic compounds in both cases | 14.56 mg GAE/g | [80] |
| Pleurotus citrinopileatus | Maceration with a bath | With cold water for 24 h Hot water: 1 or 2 h | 5 g of mushroom in 100 mL of cold water for 24 h | 27.5 mg GAE/g | [81] |
| Pleurotus ostreatus (gray mushroom) | Maceration with a bath | Extraction time: 240–420 min Temperature: 40–60 °C | 5 g of sample in 50 mL, 347.6 min at 49.7 °C | 8.26 mg GAE/g | [78] |
4.2. Alternative Methods (Green Technologies)
4.2.1. Pressurized Liquid Extraction
4.2.2. Microwave-Assisted Extraction
4.2.3. Ultrasound-Assisted Extraction
| Extraction Method | Edible Mushroom | Type | Parameters | Optimal Conditions | Yield of Phenolic Compounds | Reference |
|---|---|---|---|---|---|---|
| Pressurized Liquid Extraction | Inonotus obliquus | Subcritical water extraction (SWE) | Temperature: 120 and 200 °C | Solid–liquid ratio (1:10 g/mL), 20 min extraction at 200 °C | 89.94 ± 1.58 mg GAE/g | [18] |
| Gray oyster mushroom (Pleurotus sajor-caju (Fr.) Singer) | Pressurized hot water (PHW) | Temperature (°C): 100, 120, and 140 Pressure (bar): 4, 7, and 10 Time (min): 20, 40, and 60 | 10 g of mushroom in 300 mL at 140 °C, 9.2 bar, and 20 min | 8.49 ± 0.66 mg GAE/100 g DW | [19] | |
| Lentinula edodes | Accelerated solvent extraction (ASE) | Temperature: 40 to 160 °C Ethanol concentration: 0 to 100% | 3 g of mushroom in 100 mL of 23% ethanol at 160 °C | 5.78 mg GAE/g DW | [105] | |
| Pleurotus sajur cajur | Temperature: 40 and 80 °C Mode: dynamic and static Time: 25 min for the static mode and 45 min in a dynamic mode | 6 g of mushroom, pure ethanol (99.8%), 10 MPa pressure for 45 min at 80 °C | 14.1 ± 04 mg GAE/g | [88] | ||
| Chaga mushroom (Inonotus obliquus) | Accelerated solvent extraction (ASE) | Ionized water at different pH values: 2.5, 7.0, 9.5, and 11.5 Temperature: 60 and 100 °C | Extraction solvent at pH 11.5 at 100 °C | 82.53 ± 0.66 mg QE/g | [21] | |
| Microwave-Assisted Extraction | White mushrooms (Agaricus bisporus) | - | Ethanol concentration: 10–90% Time: 1–30 min Solvent–solid ratio: 5, 8, 13, 17, and 20 mL in 0.2 g | 58% ethanol, 16 min, and 12.9 mL/0.2 g | 14.82 mg GAE/g | [96] |
| Lentinula edodes (shiitake) | - | Particle size: 1.75, 3.35, and 4.75 mm Solid to liquid ratio: 1/40, 1/50, 1/60, and 1/70 g/mL Microwave power: 200, 300, 400, 500, and 600 W Time: 2.5, 5, 10, 15, and 17.5 min | 1.75 mm, 1/40 g/mL, 600 W, and 15 min | 11.23 ± 1.05 mg GAE/g | [66] | |
| Wild edible mushrooms (Terfezia boudieri Chatin, Boletus edulis, Lactarius volemus) | - | Methanol/water: 100, 80, 50, 20, and 0% Time: 5–25 min Temperature: 20–140 °C Microwave power: 0–1500 W | Dry sample (0.2 g) in 20 mL (80% methanol) at 80 °C, 1500 W, and 5 min extraction | Terfezia boudieri: 45.60 mg TR/g Boletus edulis: 89.53 mg TR/g and Lactarius volemus: 57.62 mg TR/g | [109] | |
| Coriolus versicolor | - | Extraction time: 1–5 min Ethanol concentration: 0–100% Microwave power: 0–200 W | 2 g of mushroom in 20 mL of 40% ethanol, 125 W and 3.8 min | 4.70 mg GAE/g | [97] | |
| White oyster mushrooms (Pleurotus ostreatus var. Florida and Pleurotus ostreatus (Jacq.) P. Kumm); gray oyster mushrooms (Pleurotus sajor-caju); pink oyster mushrooms (Pleurotus flabellatus); brown oyster mushrooms (Pleurotus cystidiosus); Pleurotus eryngii and Pleurotus pulmonarius from Indonesia; Pleurotus ostreatus samples from Spain and Germany and Pleurotus eryngii from Portugal | - | Solvent composition (0–30% methanol in water), solvent to sample ratio (10:1–20:1 mL/g), and temperature (40–70 °C) | Pure water as solvent, a 17.5:1 mL/g, and a 44 °C, with a 10 min extraction time | Pleurotus pulmonarius exhibiting the highest level of gallic acid at 0.43 ± 0.007 mg/g and Pleurotus ostreatus var. Florida showing the highest concentration of ρ-hydroxybenzaldehyde at 0.199 ± 0.001 mg/g | [93] | |
| Ultrasound-Assisted Extraction | Suillus bovinus | Ultrasonic bath | % MeOH (50–100%), temperature (10–60 °C), amplitude (10–40%), cycle (0.2–1 s–1), and solid–solvent ratio (0.25 g:10 mL–0.25 g:20 mL) | 0.2 g of sample extracted with 15.3 mL of solvent (93.6% MeOH) at 60 °C for 5 min and using 16.86% amplitude and 0.71 s−1 cycles | 11.33 mg GAE/g | [22] |
| Agaricus biphorus | Ultrasonic bath | Influence of different ultrasound frequencies: 25, 33, and 45 kHz | A frequency of 25 kHz | [110] | ||
| Pleurotus. ostreatus (PeruMyc2412 and PeruMyc2475) | Ultrasonic bath | Solvent (% water in ethanol): 0–50% Solvent–solid ratio (mL/g): 10, 35, and 60 Time (min): 10, 35, and 60 Temperature (°C): 3, 45, and 60 | 60 °C for 10 min with a solvent–solid ratio of 60 mL/g and 50% ethanol | 4.24 mg GAE/g | [106] | |
| Pleurotus citrinopileatus | Ultrasonic bath | Temperature of 30–55 °C; treatment time: 8–20 min; and solvent–solid 20–50 mL/g | For the aqueous extract: 44 °C, 14 min, 20 mL/g For the ethanolic extract: 39 °C, 13 min, and 20 mL/g | For the aqueous extract: 6.87 mg GAE/g For the ethanolic extract: 9.9 mg GAE/g | [74] | |
| Agaricus bisporus and Pleurotus ostreatus | Ultrasonic bath | Extraction solvents: ethanol, methanol, and acetone Mushrooms dried in a conventional oven (60, 70, and 80 °C) or in a microwave oven (180, 360, and 600 W) | 1 g of Agaricus bisporus, methanol (80%), conventional drying at 80 °C Pleurotus ostreatus: conventional drying at 80 °C using methanol (80%) | Agaricus bisporus: 31.680 mg GAE/g Pleurotus ostreatus: 30.58 mg GAE/g | [103] | |
| Thelephora ganbajun | Ultrasonic bath | Ethanol concentration: 10–80%; solvent–solid ratio: 10–80 mL/g Extraction time: 0–30 min Extraction temperature: 30–80 °C Ultrasonic power: 300–800 W | 57.38% ethanol, 70.15 mL/g, 10.58 min at 40 °C, and 500 W ultrasound power | 91.51 ± 4.38 mg GAE/g | [104] | |
| Ganoderma lucidum, Morchella esculenta, Lentinula edodes, and Hericium erinaceus | Ultrasonic bath | 10 g of dried mushrooms in 70 mL of methanol at 60 °C for 3 h | The Ganoderma lucidum mushroom was shown to have the highest content of phenolic compounds | 26.40 ± 0.33 mg GAE/g | [111] | |
| Pleurotus pulmonarius (Fr.) | Probe ultrasound | Extraction power and time: E0 (300 W, 40 min), E1 (460 W, 30 min), E2 (140 W, 50 min), E3 (140 W, 30 min), and E4 (460 W, 50 min) The sample-to-solvent ratio (1:10 g/mL) | E0: 40 min and 300 W | 11.07 mg GAE/g | [102] | |
| Stalks of Agaricus bisporus | Probe ultrasound | Ethanol: 70% and 96% Time: 2, 4, 6, 8, 10, 12, 15, 20, 25, 30, and 60 min | The solid–solvent ratio was 1:5 (w/v, g/mL), 30 min using 70% ethanol at 25 °C | 6.00 ± 0.43 mg GAE/g | [73] | |
| Inonotus hispidus | Probe ultrasound | Extraction solvent: 80% methanol and 40% ethanol (v/v) Time: 20 to 68.28 min Solvent-to-solid ratio (mL/g): 14.64 to 85.36 | Ethanol at 40% (v/v), 75 mL/g DW, during 20 min of sonication | 104.68 mg GAE/g DW | [101] | |
| Ganoderma lucidum | Probe ultrasound | Time: 20, 40, 60, and 80 min | Ultrasonic power of 600 W for 1 h of extraction; 10 g of mushroom was mixed with 250 mL of distilled water | 9.14 ± 0.002 mg GAE/g | [99] | |
| Ear mushrooms (Auricularia auricula-judae) | Probe ultrasound | Solvent-to-sample ratios (10:1, 20:1, 30:1 mL/g); pulse duty cycles (0.2, 0.6, 1.0 s−1) and temperatures (10, 40, 70 °C) | 1 g sample was 18 mL of methanol at 59 °C, with a pulse duty cycle of 0.7 s−1 | 0.386 mg GAE/g | [112] | |
| Boletus bicolor | Probe ultrasound | Ethanol at 10, 20, 30, 40, 50, 60, 70, and 80% Liquid-to-solid ratios: 10,1, 15:1, 20:1, 25:1, 30:1, 35:1, 40:1, and 45:1 mL/g Ultrasonic times: 20, 25, 30, 35, 40, and 45 min Temperatures: 30, 35, 40, 45, 50, and 55 °C | Ethanol concentration 42%; solvent to solid ratio 34:1 mL/g; ultrasonic time 41 min; and temperature 40 °C. | 13.69 ± 0.13 mg GAE/g DW | [27] | |
| Hericium erinaceus | Probe ultrasound | Ethanol concentration: 40, 60, and 80% Time: 20, 30, and 45 min Solvent–material ratio: 10, 20, and 30 mL/g | 80% ethanol, extraction time of 45 min, and solvent-to-material ratio of 1:30 (g/mL) | 23.2 mg GAE/g MS | [113] | |
| Pulsed Electric Field-Assisted Extraction | Agaricus bisporus | Field intensity (kV/cm): 2, 2.5, and 3 Specific energy (kJ/kg): 50, 51, 125, and 200 Time (h): 0, 3, and 6 | 20 g of fresh mushroom in 200 mL of distilled water, 2.5 kV/cm, 50 kJ/kg for 5 h | 28.80 ± 2.86 mg GAE/g | [114] | |
| White mushroom (Agaricus bisporus) | Electric field intensity: 12.4 to 38.4 kV/cm Bipolar square pulses: 136 μs and 272 μs | 38.4 kV/cm and 272 μs at 85 °C | 1.6 mg GAE/g | [13] | ||
| Enzyme-Assisted Extraction | Oyster mushrooms (Pleurotus sajor-caju) | Cellulose | pH: 4.5, 5.0, 5.5, and 6.0 Temperature: 40, 45, 50, and 55 °C Time: 4, 6, 8, and 10 h. | 5 g of mushroom in 100 mL of water, 50 °C, pH 5.5, and 8 h of extraction | 0.626 mg TAE/g | [115] |
| Inonotus obliquus | Viscozyme L | 10 g was dispersed in 400 mL water. The enzyme was added at 5% (v/w) | 72.0 ± 1.2 mg GAE/g | [107] | ||
| Supercritical Fluid-Assisted Extraction | Mushroom melena of leon (Hericium erinaceus) | Temperature: 40 °C to 60 °C Pressure: 100 bar to 300 bar Ethanol flow rate: 0 a 1 mL/min | 3 g de polvo de hongo, 46.38 °C, 100 bar, and 0.99 mL/min de caudal de EtOH. | 0.816 mg GAE/g | [108] | |
| Pleurotus ostreatus | Pressure: 15–25 MPa Temperature: 40–60 °C Amount of co-solvent (ethanol): 100–200 mL | 5 g of mushroom, 21 MPa, 48 °C, and 133 mL ethanol as co-solvent | 5.48 mg de GAE/g | [116] |
4.2.4. Pulsed Electric Field Extraction
4.2.5. Enzyme-Assisted Extraction
4.2.6. Supercritical Fluid Extraction
4.2.7. Green Solvents
4.2.8. Economic Evaluation
5. Bioactivity of Specific Phenolic Compounds
| Bioactive Properties | Bioactivity | Reference | |
|---|---|---|---|
| Gallic acid | Antibacterial activity | Inhibits the proliferation of bacteria such as Stenotrophomonas maltophilia, Achromobacter xylosoxidans, and Burkholderia cenocepacia | [133] |
| Antioxidant activity | Relieves paclitaxel-induced neuropathic pain symptoms by inhibiting oxidative stress | [139] | |
| Antioxidant activity | Prevents oxidative damage to the brain from environmental toxins such as heavy metals, aflatoxins, and dust | [134] | |
| Anticancer activity | Significantly inhibits cell proliferation and induction of apoptosis in human melanoma A375S2 cells | [140] | |
| Neuroprotective activity | In a rat model of hypoxic–ischemic brain damage, gallic acid demonstrated neuroprotective effects by reducing neuroinflammation and neuronal loss through the inhibition of reactive oxygen species (ROS) and proinflammatory cytokines | [141] | |
| Antioxidant activity | In streptozotocin-induced diabetic rats, oral administration of gallic acid significantly reduced high levels of lipid peroxidation and improved reduced glutathione levels | [142] | |
| Cardioprotective | In rats intoxicated with sodium arsenite, administering gallic acid reduced histological damage in cardiac tissue | [143] | |
| Protocatechuic acid | Antioxidant activity | Inhibits lipid oxidation of meat | [135] |
| It attenuates liver injury induced by the intraperitoneal administration of high doses of cisplatin, as evidenced by decreased levels of AST, ALT, GGT, ALP, and bilirubin, as well as increased serum albumin | [136] | ||
| Cinnamic acid | Anti-inflammatory activity | Inhibits the production of basal levels of nitric oxide | [144] |
| Inhibits the release of IL-6 at the cellular level, reducing the risk of developing acute lung injury | [145] | ||
| Fumaric acid | Functional activity in the food industry | It acts as an acidifying, preservative, and flavoring agent in food and feed | [146] |
| Chlorogenic acid | Anticancer activity | Inhibits the proliferation, migration, and invasion of cancer cells | [147] |
| Antibacterial activity | Inhibits the growth of Gram-positive bacteria such as Bacillus cereus, Bacillus subtilis, Enterococcus faecalis, and Enterococcus faecium | [148] | |
| Anti-inflammatory activity | In a rat model of acute myocardial infarction, intravenous administration of extracts enriched with chlorogenic acid significantly reduced the inflammatory response, as well as inhibited the activation of NF-KappaB and JNK | [149] | |
| Homogentisic acid | Cytoprotective activity | Counteracts the cytotoxic and genotoxic effects of the antineoplastic drug irinotecan in vitro | [150] |
| Ellagic acid | Antioxidant and antimutagenic activity | Protects cells from DNA damage caused by free radicals | [151] |
| Kaempferol | Antioxidant activity | Reduces lipid oxidation in the human body, preventing the deterioration of organs and cellular structure and protecting their functional integrity | [152] |
| Quercetin | Antioxidant and antidiabetic activity | Protects the pancreas from hyperglycemia mediated by oxidative stress | [137] |
| Antiglycemic activity | Regulates glucose levels and insulin sensitivity in the blood | [16] | |
| Antidiabetic activity | In diabetic mice, quercetin administration lowers blood glucose levels, maintains islet cell function, and increases the number of β-cells | [153] | |
| Antimicrobial activity | In rats, quercetin provides a protective effect against catheter-associated infections caused by Staphylococcus aureus by inhibiting coagulase activity | [154] | |
| Neuroprotective activity | Administering quercetin at 100 mg/kg in rats enhanced the effects of sitagliptin and improved cognitive performance and memory | [155] |
6. Future Perspectives
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hussain, S.; Sher, H.; Ullah, Z.; Elshikh, M.S.; Al Farraj, D.A.; Ali, A.; Abbasi, A.M. Traditional Uses of Wild Edible Mushrooms among the Local Communities of Swat, Pakistan. Foods 2023, 12, 1705. [Google Scholar] [CrossRef] [PubMed]
- Kaliyaperumal, M.; Kezo, K.; Gunaseelan, S. A Global Overview of Edible Mushrooms. In Biology of Macrofungi; Springer: Cham, Switzerland, 2018; pp. 15–56. [Google Scholar] [CrossRef]
- Effiong, M.E.; Umeokwochi, C.P.; Afolabi, I.S.; Chinedu, S.N. Comparative Antioxidant Activity and Phytochemical Content of Five Extracts of Pleurotus ostreatus (Oyster mushroom). Sci. Rep. 2024, 14, 3794. [Google Scholar] [CrossRef] [PubMed]
- Erbiai, E.H.; Pinto da Silva, L.; Saidi, R.; Lamrani, Z.; Esteves da Silva, J.C.G.; Maouni, A. Chemical Composition, Bioactive Compounds and Antioxidant Activity of Two Wild Edible Mushrooms Armillaria mellea and Macrolepiota procera from Two Countries (Morocco and Portugal). Biomolecules 2021, 11, 575. [Google Scholar] [CrossRef] [PubMed]
- Dimopoulou, M.; Kolonas, A.; Mourtakos, S.; Androutsos, O.; Gortzi, O. Nutritional Composition and Biological Properties of Sixteen Edible Mushroom Species. Appl. Sci. 2022, 12, 8074. [Google Scholar] [CrossRef]
- Fogarasi, M.; Socaciu, M.I.; Sălăgean, C.D.; Ranga, F.; Fărcaş, A.C.; Socaci, S.A.; Socaciu, C.; Țibulcă, D.; Fogarasi, S.; Semeniuc, C.A. Comparison of Different Extraction Solvents for Characterization of Antioxidant Potential and Polyphenolic Composition in Boletus edulis and Cantharellus cibarius Mushrooms from Romania. Molecules 2021, 26, 7508. [Google Scholar] [CrossRef]
- Valverde, M.E.; Hernández-Pérez, T.; Paredes-López, O. Edible Mushrooms: Improving Human Health and Promoting Quality Life. Int. J. Microbiol. 2015, 2015, 376387. [Google Scholar] [CrossRef]
- Asghar, A.; Randhawa, M.A.; Masood, M.M.; Abdullah, M.; Irshad, M.A. Nutraceutical Formulation Strategies to Enhance the Bioavailability and Efficiency: An Overview. In Role of Materials Science in Food Bioengineering; Academic Press: Cambridge, MA, USA, 2018; pp. 329–352. [Google Scholar] [CrossRef]
- Chu, M.; Khan, R.D.; Zhou, Y.; Agar, O.T.; Barrow, C.J.; Dunshea, F.R.; Suleria, H.A.R.; Bao, Z.; Xu, Q.; Chu, M.; et al. LC-ESI-QTOF-MS/MS Characterization of Phenolic Compounds in Common Commercial Mushrooms and Their Potential Antioxidant Activities. Processes 2023, 11, 1711. [Google Scholar] [CrossRef]
- Albuquerque, B.R.; Heleno, S.A.; Oliveira, M.B.P.P.; Barros, L.; Ferreira, I.C.F.R. Phenolic Compounds: Current Industrial Applications, Limitations and Future Challenges. Food Funct. 2021, 12, 14–29. [Google Scholar] [CrossRef]
- Huamán-Castilla, N.L.; Mariotti-Celis, M.S.; Martínez-Cifuentes, M.; Pérez-Correa, J.R. Glycerol as Alternative Co-Solvent for Water Extraction of Polyphenols from Carménère Pomace: Hot Pressurized Liquid Extraction and Computational Chemistry Calculations. Biomolecules 2020, 10, 474. [Google Scholar] [CrossRef]
- Sevindik, M.; Gürgen, A.; Khassanov, V.T.; Bal, C. Biological Activities of Ethanol Extracts of Hericium erinaceus Obtained as a Result of Optimization Analysis. Foods 2024, 13, 1560. [Google Scholar] [CrossRef]
- Xue, D.; Farid, M.M. Pulsed Electric Field Extraction of Valuable Compounds from White Button Mushroom (Agaricus bisporus). Innov. Food Sci. Emerg. Technol. 2015, 29, 178–186. [Google Scholar] [CrossRef]
- Abdelshafy, A.M.; Belwal, T.; Liang, Z.; Wang, L.; Li, D.; Luo, Z.; Li, L. A Comprehensive Review on Phenolic Compounds from Edible Mushrooms: Occurrence, Biological Activity, Application and Future Prospective. Crit. Rev. Food Sci. Nutr. 2022, 62, 6204–6224. [Google Scholar] [CrossRef]
- Xiong, Y.; Kamboj, M.; Ajlouni, S.; Fang, Z. Incorporation of Salmon Bone Gelatine with Chitosan, Gallic Acid and Clove Oil as Edible Coating for the Cold Storage of Fresh Salmon Fillet. Food Control 2021, 125, 107994. [Google Scholar] [CrossRef]
- Trivedi, R.; Upadhyay, T.K. Preparation, Characterization and Antioxidant and Anticancerous Potential of Quercetin Loaded β-Glucan Particles Derived from Mushroom and Yeast. Sci. Rep. 2024, 14, 16047. [Google Scholar] [CrossRef]
- Miklasińska-Majdanik, M.; Kępa, M.; Wojtyczka, R.D.; Idzik, D.; Wąsik, T.J. Phenolic Compounds Diminish Antibiotic Resistance of Staphylococcus aureus Clinical Strains. Int. J. Environ. Res. Public Health 2018, 15, 2321. [Google Scholar] [CrossRef] [PubMed]
- Lazić, V.; Klaus, A.; Kozarski, M.; Doroški, A.; Tosti, T.; Simić, S.; Vunduk, J. The Effect of Green Extraction Technologies on the Chemical Composition of Medicinal Chaga Mushroom Extracts. J. Fungi 2024, 10, 225. [Google Scholar] [CrossRef] [PubMed]
- Sakdasri, W.; Arnutpongchai, P.; Phonsavat, S.; Bumrungthaichaichan, E.; Sawangkeaw, R. Pressurized Hot Water Extraction of Crude Polysaccharides, β-Glucan, and Phenolic Compounds from Dried Gray Oyster Mushroom. LWT 2022, 168, 113895. [Google Scholar] [CrossRef]
- Martínez-Flores, H.E.; Contreras-Chávez, R.; Garnica-Romo, M.G. Effect of Extraction Processes on Bioactive Compounds from Pleurotus ostreatus and Pleurotus djamor: Their Applications in the Synthesis of Silver Nanoparticles. J. Inorg. Organomet. Polym. Mater. 2021, 31, 1406–1418. [Google Scholar] [CrossRef]
- Abu-Reidah, I.M.; Critch, A.L.; Manful, C.F.; Rajakaruna, A.; Vidal, N.P.; Pham, T.H.; Cheema, M.; Thomas, R. Effects of Ph and Temperature on Water under Pressurized Conditions in the Extraction of Nutraceuticals from Chaga (Inonotus obliquus) Mushroom. Antioxidants 2021, 10, 1322. [Google Scholar] [CrossRef]
- Aliaño-González, M.J.; Barea-Sepúlveda, M.; Espada-Bellido, E.; Ferreiro-González, M.; López-Castillo, J.G.; Palma, M.; Barbero, G.F.; Carrera, C. Ultrasound-Assisted Extraction of Total Phenolic Compounds and Antioxidant Activity in Mushrooms. Agronomy 2022, 12, 1812. [Google Scholar] [CrossRef]
- Dias, A.L.B.; de Aguiar, A.C.; Rostagno, M.A. Extraction of Natural Products Using Supercritical Fluids and Pressurized Liquids Assisted by Ultrasound: Current Status and Trends. Ultrason. Sonochem. 2021, 74, 105584. [Google Scholar] [CrossRef]
- Nzekoue, F.K.; Sun, Y.; Caprioli, G.; Vittori, S.; Sagratini, G. Effect of the Ultrasound-Assisted Extraction Parameters on the Determination of Ergosterol and Vitamin D2 in Agaricus bisporus, A. bisporus Portobello, and Pleurotus ostreatus Mushrooms. J. Food Compos. Anal. 2022, 109, 104476. [Google Scholar] [CrossRef]
- Thikham, S.; Jeenpitak, T.; Shoji, K.; Phongthai, S.; Therdtatha, P.; Yawootti, A.; Klangpetch, W. Pulsed Electric Field-Assisted Extraction of Mushroom ß-Glucan from Pleurotus pulmonarius by-Product and Study of Prebiotic Properties. Int. J. Food Sci. Technol. 2024, 59, 3939–3949. [Google Scholar] [CrossRef]
- Zhang, C.; Xie, Y.; Liu, D.; Liu, R.; Han, J. Effects of Drying Process and High Hydrostatic Pressure on Extraction of Antioxidant Ergothioneine from Pleurotus citrinopileatus Singer. Foods 2024, 13, 878. [Google Scholar] [CrossRef] [PubMed]
- Dong-Bao, H.; Xue, R.; Zhuang, X.C.; Zhang, X.S.; Shi, S.L. Ultrasound-Assisted Extraction Optimization of Polyphenols from Boletus Bicolor and Evaluation of Its Antioxidant Activity. Front. Nutr. 2023, 10, 1135712. [Google Scholar] [CrossRef]
- Contato, A.G.; Conte-Junior, C.A. Mushrooms in Innovative Food Products: Challenges and Potential Opportunities as Meat Substitutes, Snacks and Functional Beverages. Trends Food Sci. Technol. 2025, 156, 104868. [Google Scholar] [CrossRef]
- Wong, K.M.; Decker, E.A.; Autio, W.R.; Toong, K.; DiStefano, G.; Kinchla, A.J. Utilizing Mushrooms to Reduce Overall Sodium in Taco Filling Using Physical and Sensory Evaluation. J. Food Sci. 2017, 82, 2379–2386. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Ma, H.; Guo, X.; Weng, Y.; Hu, X.; Ren, L. Cultivation and Utilization of Edible Mushrooms: From Extraction of Active Components to Effective Substrate Utilization. J. Food Compos. Anal. 2025, 140, 107224. [Google Scholar] [CrossRef]
- Ramos, M.; Burgos, N.; Barnard, A.; Evans, G.; Preece, J.; Graz, M.; Ruthes, A.C.; Jiménez-Quero, A.; Martínez-Abad, A.; Vilaplana, F.; et al. Agaricus bisporus and Its By-Products as a Source of Valuable Extracts and Bioactive Compounds. Food Chem. 2019, 292, 176–187. [Google Scholar] [CrossRef] [PubMed]
- Bhambri, A.; Srivastava, M.; Mahale, V.G.; Mahale, S.; Karn, S.K. Mushrooms as Potential Sources of Active Metabolites and Medicines. Front. Microbiol. 2022, 13, 837266. [Google Scholar] [CrossRef]
- FAOSTAT. Cultivos Y Productos Ganaderos. Available online: https://www.fao.org/faostat/es/#data/QCL (accessed on 2 March 2025).
- Singh, I.; Rani Rajpal, V.; Navi, S.S. Fungal Resources for Sustainable Economy; Springer Nature: Singapore, 2023. [Google Scholar]
- Sun, M.; Zhuang, Y.; Gu, Y.; Zhang, G.; Fan, X.; Ding, Y. A Comprehensive Review of the Application of Ultrasonication in the Production and Processing of Edible Mushrooms: Drying, Extraction of Bioactive Compounds, and Post-Harvest Preservation. Ultrason. Sonochem 2024, 102, 106763. [Google Scholar] [CrossRef]
- Thakur, M.P. Advances in Mushroom Production: Key to Food, Nutritional and Employment Security: A Review. Indian Phytopathol. 2020, 73, 377–395. [Google Scholar] [CrossRef]
- De Ita, M.A.V.; Aranda, D.P.B.; Lezama, C.P.; Reyes, J.R.T.; Martinez, A.I.; Romero-Arenas, O. Evaluation of Substrates in the Elaboration of Secondary Inoculum for the Cultivation of Pleurotus ostreatus. J. Pure Appl. Microbiol. 2018, 12, 679–686. [Google Scholar] [CrossRef]
- Barua, R.C.; Coniglio, R.O.; Molina, M.A.; Díaz, G.V.; Fonseca, M.I. Fungi as Biotechnological Allies: Exploring Contributions of Edible and Medicinal Mushrooms. J. Food Sci. 2024, 89, 6888–6915. [Google Scholar] [CrossRef]
- Bell, V.; Silva, C.R.P.G.; Guina, J.; Fernandes, T.H. Mushrooms as Future Generation Healthy Foods. Front. Nutr. 2022, 9, 1050099. [Google Scholar] [CrossRef]
- Haro, A.; Trescastro, A.; Lara, L.; Fernández-Fígares, I.; Nieto, R.; Seiquer, I. Mineral Elements Content of Wild Growing Edible Mushrooms from the Southeast of Spain. J. Food Compos. Anal. 2020, 91, 103504. [Google Scholar] [CrossRef]
- Sánchez, C. Bioactives from Mushroom and Their Application. In Food Bioactives: Extraction and Biotechnology Applications; Springer International Publishing: Cham, Switzerland, 2017; pp. 23–57. ISBN 9783319516394. [Google Scholar]
- El-Baba, C.; Baassiri, A.; Kiriako, G.; Dia, B.; Fadlallah, S.; Moodad, S.; Darwiche, N. Terpenoids’ Anti-Cancer Effects: Focus on Autophagy. Apoptosis 2021, 26, 491–511. [Google Scholar] [CrossRef] [PubMed]
- Morales, D.; Gil-Ramirez, A.; Smiderle, F.R.; Piris, A.J.; Ruiz-Rodriguez, A.; Soler-Rivas, C. Vitamin D-Enriched Extracts Obtained from Shiitake Mushrooms (Lentinula edodes) by Supercritical Fluid Extraction and UV-Irradiation. Innov. Food Sci. Emerg. Technol. 2017, 41, 330–336. [Google Scholar] [CrossRef]
- Aranha, G.M.; Contato, A.G.; Salgado, J.C.D.S.; de Oliveira, T.B.; Retamiro, K.M.; Ortolan, G.G.; Crevelin, E.J.; Nakamura, C.V.; de Moraes, L.A.B.; Peralta, R.M.; et al. Biochemical Characterization and Biological Properties of Mycelium Extracts from Lepista sordida GMA-05 and Trametes hirsuta GMA-01: New Mushroom Strains Isolated in Brazil. Braz. J. Microbiol. 2022, 53, 349–358. [Google Scholar] [CrossRef]
- Zeng, Y.; Zhou, W.; Yu, J.; Zhao, L.; Wang, K.; Hu, Z.; Liu, X. By-Products of Fruit and Vegetables: Antioxidant Properties of Extractable and Non-Extractable Phenolic Compounds. Antioxidants 2023, 12, 418. [Google Scholar] [CrossRef] [PubMed]
- Santos-Buelga, C.; González-Paramás, A.M.; Oludemi, T.; Ayuda-Durán, B.; González-Manzano, S. Plant Phenolics as Functional Food Ingredients. In Advances in Food and Nutrition Research; Academic Press Inc.: Cambridge, MA, USA, 2019; Volume 90, pp. 183–257. ISBN 9780128165676. [Google Scholar]
- Alkan, S.; Uysal, A.; Kasik, G.; Vlaisavljevic, S.; Berežni, S.; Zengin, G. Chemical Characterization, Antioxidant, Enzyme Inhibition and Antimutagenic Properties of Eight Mushroom Species: A Comparative Study. J. Fungi 2020, 6, 166. [Google Scholar] [CrossRef]
- Altaf, U.; Lalotra, P.; Sharma, Y.P. Nutritional and Mineral Composition of Four Wild Edible Mushrooms from Jammu and Kashmir, India. Indian. Phytopathol. 2020, 73, 313–320. [Google Scholar] [CrossRef]
- Bach, F.; Zielinski, A.A.F.; Helm, C.V.; Maciel, G.M.; Pedro, A.C.; Stafussa, A.P.; Ávila, S.; Haminiuk, C.W.I. Bio Compounds of Edible Mushrooms: In Vitro Antioxidant and Antimicrobial Activities. LWT 2019, 107, 214–220. [Google Scholar] [CrossRef]
- Dimitrijević, M.; Stankov Jovanović, V.; Cvetković, J.; Mitić, M.; Petrović, G.; Đorđević, A.; Mitić, V. Phenolics, Antioxidant Potentials, and Antimicrobial Activities of Six Wild Boletaceae Mushrooms. Anal. Lett. 2017, 50, 1691–1709. [Google Scholar] [CrossRef]
- Islam, T.; Yu, X.; Xu, B. Phenolic Profiles, Antioxidant Capacities and Metal Chelating Ability of Edible Mushrooms Commonly Consumed in China. LWT Food Sci. Technol. 2016, 72, 423–431. [Google Scholar] [CrossRef]
- Biondi, F.; Balducci, F.; Capocasa, F.; Visciglio, M.; Mei, E.; Vagnoni, M.; Mezzetti, B.; Mazzoni, L. Environmental Conditions and Agronomical Factors Influencing the Levels of Phytochemicals in Brassica Vegetables Responsible for Nutritional and Sensorial Properties. Appl. Sci. 2021, 11, 1927. [Google Scholar] [CrossRef]
- Alonso-Vázquez, P.; Lujan-Facundo, M.J.; Sánchez-Arévalo, C.M.; Cuartas-Uribe, B.; Vincent-Vela, M.C.; Álvarez-Blanco, S. Recovery of Phenolic Compounds from Orange Juice Solid Waste by Solid-Liquid Extraction. LWT 2024, 203, 116355. [Google Scholar] [CrossRef]
- Campos, L.; Seixas, L.; Henriques, M.H.F.; Peres, A.M.; Veloso, A.C.A. Pomegranate Peels and Seeds as a Source of Phenolic Compounds: Effect of Cultivar, By-Product, and Extraction Solvent. Int. J. Food Sci. 2022, 2022, 9189575. [Google Scholar] [CrossRef]
- Cardullo, N.; Leanza, M.; Muccilli, V.; Tringali, C. Valorization of Agri-Food Waste from Pistachio Hard Shells: Extraction of Polyphenols as Natural Antioxidants. Resources 2021, 10, 45. [Google Scholar] [CrossRef]
- Hobbi, P.; Okoro, O.V.; Delporte, C.; Alimoradi, H.; Podstawczyk, D.; Nie, L.; Bernaerts, K.V.; Shavandi, A. Kinetic Modelling of the Solid–Liquid Extraction Process of Polyphenolic Compounds from Apple Pomace: Influence of Solvent Composition and Temperature. Bioresour. Bioprocess. 2021, 8, 114. [Google Scholar] [CrossRef] [PubMed]
- Mohd Hazli, U.H.A.; Abdul-Aziz, A.; Mat-Junit, S.; Chee, C.F.; Kong, K.W. Solid-Liquid Extraction of Bioactive Compounds with Antioxidant Potential from Alternanthera Sesillis (Red) and Identification of the Polyphenols Using UHPLC-QqQ-MS/MS. Food Res. Int. 2019, 115, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Rebollo-Hernanz, M.; Cañas, S.; Taladrid, D.; Segovia, Á.; Bartolomé, B.; Aguilera, Y.; Martín-Cabrejas, M.A. Extraction of Phenolic Compounds from Cocoa Shell: Modeling Using Response Surface Methodology and Artificial Neural Networks. Sep. Purif. Technol. 2021, 270, 118779. [Google Scholar] [CrossRef]
- Yuan, B.; Lu, M.; Eskridge, K.M.; Isom, L.D.; Hanna, M.A. Extraction, Identification, and Quantification of Antioxidant Phenolics from Hazelnut (Corylus avellana L.) Shells. Food Chem. 2018, 244, 7–15. [Google Scholar] [CrossRef]
- Zhu, B.; Shang, B.; Li, Y.; Zhen, Y. Inhibition of Histone Deacetylases by Trans-Cinnamic Acid and Its Antitumor Effect against Colon Cancer Xenografts in Athymic Mice. Mol. Med. Rep. 2016, 13, 4159–4166. [Google Scholar] [CrossRef]
- Bahadori, M.B.; Sarikurkcu, C.; Yalcin, O.U.; Cengiz, M.; Gungor, H. Metal Concentration, Phenolics Profiling, and Antioxidant Activity of Two Wild Edible Melanoleuca Mushrooms (M. cognata and M. stridula). Microchem. J. 2019, 150, 104172. [Google Scholar] [CrossRef]
- Veljović, S.; Veljović, M.; Nikićević, N.; Despotović, S.; Radulović, S.; Nikšić, M.; Filipović, L. Chemical Composition, Antiproliferative and Antioxidant Activity of Differently Processed Ganoderma Lucidum Ethanol Extracts. J. Food Sci. Technol. 2017, 54, 1312–1320. [Google Scholar] [CrossRef]
- Çayan, F.; Deveci, E.; Tel-Çayan, G.; Duru, M.E. Identification and Quantification of Phenolic Acid Compounds of Twenty-Six Mushrooms by HPLC–DAD. J. Food Meas. Charact. 2020, 14, 1690–1698. [Google Scholar] [CrossRef]
- Çol Ayvaz, M.; Aksu, F.; Kır, F. Phenolic Profile of Three Wild Edible Mushroom Extracts from Ordu, Turkey and Their Antioxidant Properties, Enzyme Inhibitory Activities. Br. Food J. 2019, 121, 1248–1260. [Google Scholar] [CrossRef]
- de Souza Campos Junior, F.A.; Petrarca, M.H.; Meinhart, A.D.; de Jesus Filho, M.; Godoy, H.T. Multivariate Optimization of Extraction and Validation of Phenolic Acids in Edible Mushrooms by Capillary Electrophoresis. Food Res. Int. 2019, 126, 108685. [Google Scholar] [CrossRef]
- Xiaokang, W.; Lyng, J.G.; Brunton, N.P.; Cody, L.; Jacquier, J.C.; Harrison, S.M.; Papoutsis, K. Monitoring the Effect of Different Microwave Extraction Parameters on the Recovery of Polyphenols from Shiitake Mushrooms: Comparison with Hot-Water and Organic-Solvent Extractions. Biotechnol. Rep. 2020, 27, e00504. [Google Scholar] [CrossRef] [PubMed]
- Yahia, E.M.; Gutiérrez-Orozco, F.; Moreno-Pérez, M.A. Identification of Phenolic Compounds by Liquid Chromatography-Mass Spectrometry in Seventeen Species of Wild Mushrooms in Central Mexico and Determination of Their Antioxidant Activity and Bioactive Compounds. Food Chem. 2017, 226, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Alam, N.; Yoon, K.N.; Lee, T.S. Evaluation of the Antioxidant and Antityrosinase Activities of Three Extracts from Pleurotus nebrodensis Fruiting Bodies. Afr. J. Biotechnol. 2011, 10, 2978–2986. [Google Scholar] [CrossRef]
- Ameer, K.; Shahbaz, H.M.; Kwon, J.H. Green Extraction Methods for Polyphenols from Plant Matrices and Their Byproducts: A Review. Compr. Rev. Food Sci. Food Saf. 2017, 16, 295–315. [Google Scholar] [CrossRef] [PubMed]
- Brglez Mojzer, E.; Knez Hrnčič, M.; Škerget, M.; Knez, Ž.; Bren, U. Polyphenols: Extraction Methods, Antioxidative Action, Bioavailability and Anticarcinogenic Effects. Molecules 2016, 21, 901. [Google Scholar] [CrossRef]
- Carvalho, V.S.D.; Gómez-Delgado, L.; Curto, M.Á.; Moreno, M.B.; Pérez, P.; Ribas, J.C.; Cortés, J.C.G. Analysis and Application of a Suite of Recombinant Endo-β(1,3)-d-Glucanases for Studying Fungal Cell Walls. Microb. Cell Factories 2021, 20, 126. [Google Scholar] [CrossRef]
- Majumdar, S.; Negi, P.S. Extraction of Chitin-Glucan Complex from Shiitake (Lentinula edodes) Fruiting Bodies Using Natural Deep Eutectic Solvents and Its Prebiotic Potential. Int. J. Biol. Macromol. 2024, 273, 133046. [Google Scholar] [CrossRef]
- Umaña, M.; Eim, V.; Garau, C.; Rosselló, C.; Simal, S. Ultrasound-Assisted Extraction of Ergosterol and Antioxidant Components from Mushroom by-Products and the Attainment of a β-Glucan Rich Residue. Food Chem. 2020, 332, 127390. [Google Scholar] [CrossRef]
- Gogoi, P.; Chutia, P.; Singh, P.; Mahanta, C.L. Effect of Optimized Ultrasound-Assisted Aqueous and Ethanolic Extraction of Pleurotus Citrinopileatus Mushroom on Total Phenol, Flavonoids and Antioxidant Properties. J. Food Process Eng. 2019, 42, e13172. [Google Scholar] [CrossRef]
- Sunkar, S.; Ranimol, G.; Nellore, J.; Nachiyar, C.V.; Namasivayam, S.K.R.; Renugadevi, K. Extraction and Purification of Bioactive Ingredients from Natural Products. Handb. Nutraceuticals Nat. Prod. Biol. Med. Nutr. Prop. Appl. 2022, 2, 221–257. [Google Scholar] [CrossRef]
- Ocampo, R.; Ríos, L.A.; Betancur, L.A.; Ocampo, D.M. Curso Práctico de Química Orgánica: Enfocado a Biología Y Alimentos; Universidad de Caldas: Caldas, Colombia, 2008; ISBN 9789588319186. [Google Scholar]
- Naviglio, D.; Scarano, P.; Ciaravolo, M.; Gallo, M. Rapid Solid-Liquid Dynamic Extraction (RSLDE): A Powerful and Greener Alternative to the Latest Solid-Liquid Extraction Techniques. Foods 2019, 8, 245. [Google Scholar] [CrossRef]
- Lim, S.M.; Yim, H.S. Determination of Optimal Extraction Time and Temperature by Response Surface Methodology to Obtain High-Level Antioxidant Activity in Culinary-Medicinal Oyster Mushroom, Pleurotus ostreatus (Jacq.: Fr.) P. Kumm. (Higher Basidiomycetes). Int. J. Med. Mushrooms 2012, 14, 593–602. [Google Scholar] [CrossRef]
- Sudha, G.; Janardhanan, A.; Moorthy, A.; Chinnasamy, M.; Gunasekaran, S.; Thimmaraju, A.; Gopalan, J. Comparative Study on the Antioxidant Activity of Methanolic and Aqueous Extracts from the Fruiting Bodies of an Edible Mushroom Pleurotus djamor. Food Sci. Biotechnol. 2016, 25, 371–377. [Google Scholar] [CrossRef]
- Tepsongkroh, B.; Jangchud, K.; Trakoontivakorn, G. Antioxidant Properties and Selected Phenolic Acids of Five Different Tray-Dried and Freeze-Dried Mushrooms Using Methanol and Hot Water Extraction. J. Food Meas. Charact. 2019, 13, 3097–3105. [Google Scholar] [CrossRef]
- Chen, P.H.; Weng, Y.M.; Yu, Z.R.; Koo, M.; Wang, B.J. Extraction Temperature Affects the Activities of Antioxidation, Carbohydrate-Digestion Enzymes, and Angiotensin-Converting Enzyme of Pleurotus citrinopileatus Extract. J. Food Drug Anal. 2016, 24, 548–555. [Google Scholar] [CrossRef]
- Radzki, W.; Tutaj, K.; Skrzypczak, K.; Michalak-Majewska, M.; Gustaw, W. Ethanolic Extracts of Six Cultivated Mushrooms as a Source of Bioactive Compounds. Appl. Sci. 2024, 14, 66. [Google Scholar] [CrossRef]
- Vorobiev, E.; Lebovka, N. Solid/Liquid Extraction and Expression. In Processing of Foods and Biomass Feedstocks by Pulsed Electric Energy; Springer International Publishing: Cham, Switzerland, 2020; pp. 113–148. [Google Scholar] [CrossRef]
- Panthong, S.; Boonsathorn, N.; Chuchawankul, S. Antioxidant Activity, Anti-Proliferative Activity, and Amino Acid Profiles of Ethanolic Extracts of Edible Mushrooms. Genet. Mol. Res. 2016, 15, 1–14. [Google Scholar] [CrossRef]
- Quintero-Cabello, K.P.; Palafox-Rivera, P.; Lugo-Flores, M.A.; Gaitán-Hernández, R.; González-Aguilar, G.A.; Silva-Espinoza, B.A.; Tortoledo-Ortiz, O.; Ayala-Zavala, J.F.; Monribot-Villanueva, J.L.; Guerrero-Analco, J.A. Contribution of Bioactive Compounds to the Antioxidant Capacity of the Edible Mushroom Neolentinus lepideus. Chem. Biodivers. 2021, 18, e2100085. [Google Scholar] [CrossRef]
- Boonsong, S.; Klaypradit, W.; Wilaipun, P. Antioxidant Activities of Extracts from Five Edible Mushrooms Using Different Extractants. Agric. Nat. Resour. 2016, 50, 89–97. [Google Scholar] [CrossRef]
- de OXMachado, T.; Portugal, I.; de ACKodel, H.; Fathi, A.; Fathi, F.; Oliveira, M.B.P.; Dariva, C.; Souto, E.B. Pressurized Liquid Extraction as an Innovative High-Yield Greener Technique for Phenolic Compounds Recovery from Grape Pomace. Sustain. Chem. Pharm. 2024, 40, 101635. [Google Scholar] [CrossRef]
- Krümmel, A.; Gonçalves Rodrigues, L.G.; Vitali, L.; Ferreira, S.R.S. Bioactive Compounds from Pleurotus Sajor-Caju Mushroom Recovered by Sustainable High-Pressure Methods. LWT 2022, 160, 113316. [Google Scholar] [CrossRef]
- Tejedor-Calvo, E.; Morales, D.; Marco, P.; Sánchez, S.; Garcia-Barreda, S.; Smiderle, F.R.; Iacomini, M.; Villalva, M.; Santoyo, S.; Soler-Rivas, C. Screening of Bioactive Compounds in Truffles and Evaluation of Pressurized Liquid Extractions (PLE) to Obtain Fractions with Biological Activities. Food Res. Int. 2020, 132, 109054. [Google Scholar] [CrossRef] [PubMed]
- Plaza, M.; Turner, C. Pressurized Hot Water Extraction of Bioactives. TrAC Trends Anal. Chem. 2015, 71, 39–54. [Google Scholar] [CrossRef]
- Alvarez-Rivera, G.; Bueno, M.; Ballesteros-Vivas, D.; Mendiola, J.A.; Ibañez, E. Pressurized Liquid Extraction. In Liquid-Phase Extraction; Elsevier: Amsterdam, The Netherlands, 2020; pp. 375–398. [Google Scholar] [CrossRef]
- Morales, D.; Smiderle, F.R.; Villalva, M.; Abreu, H.; Rico, C.; Santoyo, S.; Iacomini, M.; Soler-Rivas, C. Testing the Effect of Combining Innovative Extraction Technologies on the Biological Activities of Obtained β-Glucan-Enriched Fractions from Lentinula edodes. J. Funct. Foods 2019, 60, 103446. [Google Scholar] [CrossRef]
- Harun, M.U.; Palma, M.; Setyaningsih, W. Development and Validation of Microwave-Assisted Extraction for Phenolic Compound Profiling in Diverse Oyster Mushrooms (Pleurotus spp.) Sourced from Various Geographical Regions. J. Agric. Food Res. 2025, 20, 101754. [Google Scholar] [CrossRef]
- López-Salazar, H.; Camacho-Díaz, B.H.; Arenas Ocampo, M.L.; Jiménez-Aparicio, A.R. Microwave-Assisted Extraction of Functional Compounds from Plants: A Review. Bioresources 2023, 18, 6614–6638. [Google Scholar] [CrossRef]
- Bouchez, A.; Vauchel, P.; Périno, S.; Dimitrov, K. Multi-Criteria Optimization Including Environmental Impacts of a Microwave-Assisted Extraction of Polyphenols and Comparison with an Ultrasound-Assisted Extraction Process. Foods 2023, 12, 1750. [Google Scholar] [CrossRef] [PubMed]
- Latif, A.; Khan, M.I.; Latif, A.; Khan, U.M.; Mousavi Khaneghah, A.; Aadil, R.M. Enhancing Polyphenol Extraction from White Button Mushrooms Using Microwave-Assisted Extraction: A Response Surface Methodology Optimization Approach. Microchem. J. 2024, 203, 110876. [Google Scholar] [CrossRef]
- Maeng, J.H.; Muhammad Shahbaz, H.; Ameer, K.; Jo, Y.; Kwon, J.H. Optimization of Microwave-Assisted Extraction of Bioactive Compounds from Coriolus Versicolor Mushroom Using Response Surface Methodology. J. Food Process Eng. 2017, 40, e12421. [Google Scholar] [CrossRef]
- Papoutsis, K.; Pristijono, P.; Golding, J.B.; Stathopoulos, C.E.; Bowyer, M.C.; Scarlett, C.J.; Vuong, Q.V. Screening the Effect of Four Ultrasound-Assisted Extraction Parameters on Hesperidin and Phenolic Acid Content of Aqueous Citrus Pomace Extracts. Food Biosci. 2018, 21, 20–26. [Google Scholar] [CrossRef]
- Alzorqi, I.; Sudheer, S.; Lu, T.J.; Manickam, S. Ultrasonically Extracted β-d-Glucan from Artificially Cultivated Mushroom, Characteristic Properties and Antioxidant Activity. Ultrason. Sonochem. 2017, 35, 531–540. [Google Scholar] [CrossRef] [PubMed]
- Klausen, S.J.; Falck-Ytter, A.B.; Strætkvern, K.O.; Martin, C. Evaluation of the Extraction of Bioactive Compounds and the Saccharification of Cellulose as a Route for the Valorization of Spent Mushroom Substrate. Molecules 2023, 28, 5140. [Google Scholar] [CrossRef]
- Machado-Carvalho, L.; Martins, T.; Aires, A.; Marques, G. Optimization of Phenolic Compounds Extraction and Antioxidant Activity from Inonotus hispidus Using Ultrasound-Assisted Extraction Technology. Metabolites 2023, 13, 524. [Google Scholar] [CrossRef]
- Amirullah, N.A.; Zainal Abidin, N.; Abdullah, N.; Manickam, S. Application of Ultrasound towards Improving the Composition of Phenolic Compounds and Enhancing in Vitro Bioactivities of Pleurotus pulmonarius (Fr.) Quél Extracts. Biocatal. Agric. Biotechnol. 2021, 31, 101881. [Google Scholar] [CrossRef]
- Sezer, Y.Ç.; Süfer, Ö.; Sezer, G. Extraction of Phenolic Compounds from Oven and Microwave Dried Mushrooms (Agaricus bisporus and Pleurotus ostreatus) by Using Methanol, Ethanol and Aceton as Solvents. Indian J. Pharm. Educ. Res. 2017, 51, S393–S397. [Google Scholar] [CrossRef]
- Xu, D.P.; Zheng, J.; Zhou, Y.; Li, Y.; Li, S.; Li, H. Bin Extraction of Natural Antioxidants from the Thelephora ganbajun Mushroom by an Ultrasound-Assisted Extraction Technique and Evaluation of Antiproliferative Activity of the Extract against Human Cancer Cells. Int. J. Mol. Sci. 2016, 17, 1664. [Google Scholar] [CrossRef] [PubMed]
- Kaya, M.; Cam, M. Eritadenine: Pressurized Liquid Extraction from Lentinula Edodes and Thermal Degradation Kinetics. Sustain. Chem. Pharm. 2022, 29, 100809. [Google Scholar] [CrossRef]
- Ianni, F.; Blasi, F.; Angelini, P.; Di Simone, S.C.; Flores, G.A.; Cossignani, L.; Venanzoni, R. Extraction Optimization by Experimental Design of Bioactives from Pleurotus ostreatus and Evaluation of Antioxidant and Antimicrobial Activities. Processes 2021, 9, 743. [Google Scholar] [CrossRef]
- Hwang, A.Y.; Yang, S.C.; Kim, J.; Lim, T.; Cho, H.; Hwang, K.T. Effects of Non-Traditional Extraction Methods on Extracting Bioactive Compounds from Chaga Mushroom (Inonotus obliquus) Compared with Hot Water Extraction. LWT 2019, 110, 80–84. [Google Scholar] [CrossRef]
- Joradon, P.; Rungsardthong, V.; Ruktanonchai, U.; Suttisintong, K.; Thumthanaruk, B.; Vatanyoopaisarn, S.; Uttapap, D.; Mendes, A.C. Extraction of Bioactive Compounds from Lion’s Mane Mushroom by-Product Using Supercritical CO2 Extraction. J. Supercrit. Fluids 2024, 206, 106162. [Google Scholar] [CrossRef]
- Özyürek, M.; Bener, M.; Güçlü, K.; Apak, R. Antioxidant/Antiradical Properties of Microwave-Assisted Extracts of Three Wild Edible Mushrooms. Food Chem. 2014, 157, 323–331. [Google Scholar] [CrossRef]
- Alves Filho, E.G.; Lima, M.; Silva, L.; Ribeiro, P.; Tiwari, B.K.; Fernandes, F.N.; Brito, E.S. Green Ultrasound-Assisted Extraction of Bioactive Compounds from Button Mushrooms, Potatoes, and Onion Peels. ACS Food Sci. Technol. 2021, 1, 1274–1284. [Google Scholar] [CrossRef]
- Yildiz, O.; Can, Z.; Laghari, A.Q.; Şahin, H.; Malkoç, M. Wild Edible Mushrooms as a Natural Source of Phenolics and Antioxidants. J. Food Biochem. 2015, 39, 148–154. [Google Scholar] [CrossRef]
- Nugraha, A.; Briliantama, A.; Harun, M.U.; Sing-Chung, L.; Tan, C.X.; Lim, V.; Husni, A.; Setyaningsih, W. Ultrasound-Assisted Extraction of Phenolic Compounds from Ear Mushrooms (Auricularia auricula-judae): Assessing Composition and Antioxidant Activity during Fruiting Body Development. AIMS Agric. Food 2024, 9, 1134–1150. [Google Scholar] [CrossRef]
- Valu, M.V.; Soare, L.C.; Sutan, N.A.; Ducu, C.; Moga, S.; Hritcu, L.; Boiangiu, R.S.; Carradori, S. Optimization of Ultrasonic Extraction to Obtain Erinacine A and Polyphenols with Antioxidant Activity from the Fungal Biomass of Hericium erinaceus. Foods 2020, 9, 1889. [Google Scholar] [CrossRef]
- Calleja-Gómez, M.; Castagnini, J.M.; Carbó, E.; Ferrer, E.; Berrada, H.; Barba, F.J. Evaluation of Pulsed Electric Field-Assisted Extraction on the Microstructure and Recovery of Nutrients and Bioactive Compounds from Mushroom (Agaricus bisporus). Separations 2022, 9, 302. [Google Scholar] [CrossRef]
- Nguyen, G.T.N.; Nguyen, T.M. Effect of Extraction Conditions (Temperature, Ph and Time) by Cellulase on Chemical Properties of Dried Oyster Mushroom (Pleurotus sajor-caju) Extract. Food Res. 2021, 5, 351–358. [Google Scholar] [CrossRef]
- Bhattacharya, M.; Srivastav, P.P.; Mishra, H.N. Optimization of Process Variables for Supercritical Fluid Extraction of Ergothioneine and Polyphenols from Pleurotus ostreatus and Correlation to Free-Radical Scavenging Activity. J. Supercrit. Fluids 2014, 95, 51–59. [Google Scholar] [CrossRef]
- El Kantar, S.; Boussetta, N.; Lebovka, N.; Foucart, F.; Rajha, H.N.; Maroun, R.G.; Louka, N.; Vorobiev, E. Pulsed Electric Field Treatment of Citrus Fruits: Improvement of Juice and Polyphenols Extraction. Innov. Food Sci. Emerg. Technol. 2018, 46, 153–161. [Google Scholar] [CrossRef]
- Mahnič-Kalamiza, S.; Miklavčič, D. The Phenomenon of Electroporation. In Pulsed Electric Fields Technology for the Food Industry: Fundamentals and Applications; Springer International Publishing: Cham, Switzerland, 2022; pp. 107–141. [Google Scholar] [CrossRef]
- Ahmed, M.; Ranjan, H.; Roy, S. Pulsed Electric Fields for Sustainable Meat, Poultry, and Fish Processing: Recent Advances, Prospects, and Industry Challenges. Innov. Food Sci. Emerg. Technol. 2025, 102, 104008. [Google Scholar] [CrossRef]
- Soto-Maldonado, C.; Zúñiga-Hansen, M.E. Enzyme-Assisted Extraction of Phenolic Compounds. In Water Extraction of Bioactive Compounds; Elsevier: Amsterdam, The Netherlands, 2017; pp. 369–384. [Google Scholar] [CrossRef]
- Huang, Z.; Foo, S.C.; Choo, W.S. A Review on the Extraction of Polyphenols from Pomegranate Peel for Punicalagin Purification: Techniques, Applications, and Future Prospects. Sustain. Food Technol. 2025, 3, 396–413. [Google Scholar] [CrossRef]
- Arumugham, T.; Rambabu, K.; Hasan, S.W.; Show, P.L.; Rinklebe, J.; Banat, F. Supercritical Carbon Dioxide Extraction of Plant Phytochemicals for Biological and Environmental Applications—A Review. Chemosphere 2021, 271, 129525. [Google Scholar] [CrossRef]
- Sökmen, M.; Demir, E.; Alomar, S.Y. Optimization of Sequential Supercritical Fluid Extraction (SFE) of Caffeine and Catechins from Green Tea. J. Supercrit. Fluids 2018, 133, 171–176. [Google Scholar] [CrossRef]
- Torres-Valenzuela, L.S.; Ballesteros-Gómez, A.; Rubio, S. Green Solvents for the Extraction of High Added-Value Compounds from Agri-Food Waste. Food Eng. Rev. 2019, 12, 83–100. [Google Scholar] [CrossRef]
- Gil-Martín, E.; Forbes-Hernández, T.; Romero, A.; Cianciosi, D.; Giampieri, F.; Battino, M. Influence of the Extraction Method on the Recovery of Bioactive Phenolic Compounds from Food Industry By-Products. Food Chem. 2022, 378, 131918. [Google Scholar] [CrossRef] [PubMed]
- Galanakis, C.M.; Goulas, V.; Tsakona, S.; Manganaris, G.A.; Gekas, V. A Knowledge Base for The Recovery of Natural Phenols with Different Solvents. Int. J. Food Prop. 2013, 16, 382–396. [Google Scholar] [CrossRef]
- Jessop, P.G.; Jessop, D.A.; Fu, D.; Phan, L. Solvatochromic Parameters for Solvents of Interest in Green Chemistry. Green Chem. 2012, 14, 1245–1259. [Google Scholar] [CrossRef]
- Huaman-Castilla, N.L.; Martínez-Cifuentes, M.; Camilo, C.; Pedreschi, F.; Mariotti-Celis, M.; Pérez-Correa, J.R. The Impact of Temperature and Ethanol Concentration on the Global Recovery of Specific Polyphenols in an Integrated HPLE/RP Process on Carménère Pomace Extracts. Molecules 2019, 24, 3145. [Google Scholar] [CrossRef]
- Jessop, P.G. Searching for Green Solvents. Green Chem. 2011, 13, 1391–1398. [Google Scholar] [CrossRef]
- Alibaba Manufacturers, Suppliers, Exporters & Importers. Available online: https://www.alibaba.com/trade/search?spm=a2700.product_home_newuser.home_new_user_first_screen_fy23_pc_search_bar.keydown__Enter&tab=all&SearchText=polyphenols+extracts (accessed on 4 July 2025).
- de Aguiar, A.C.; Osorio-Tobón, J.F.; Viganó, J.; Martínez, J. Economic Evaluation of Supercritical Fluid and Pressurized Liquid Extraction to Obtain Phytonutrients from Biquinho Pepper: Analysis of Single and Sequential-Stage Processes. J. Supercrit. Fluids 2020, 165, 104935. [Google Scholar] [CrossRef]
- Solana, M.; Mirofci, S.; Bertucco, A. Production of Phenolic and Glucosinolate Extracts from Rocket Salad by Supercritical Fluid Extraction: Process Design and Cost Benefits Analysis. J. Food Eng. 2016, 168, 35–41. [Google Scholar] [CrossRef]
- Flores-Maldonado, O.; Dávila-Aviña, J.; González, G.M.; Becerril-García, M.A.; Ríos-López, A.L. Antibacterial Activity of Gallic Acid and Methyl Gallate against Emerging Non-Fermenting Bacilli. Folia Microbiol. 2024, 70, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Ojo, O.A.; Rotimi, D.E.; Ojo, A.B.; Ogunlakin, A.D.; Ajiboye, B.O. Gallic Acid Abates Cadmium Chloride Toxicity via Alteration of Neurotransmitters and Modulation of Inflammatory Markers in Wistar Rats. Sci. Rep. 2023, 13, 1577. [Google Scholar] [CrossRef]
- Deuchande, T.; Fundo, J.F.; Pintado, M.E.; Amaro, A.L. Protocatechuic Acid as an Inhibitor of Lipid Oxidation in Meat. Meat Sci. 2024, 213, 109519. [Google Scholar] [CrossRef]
- Habib, S.A.; Suddek, G.M.; Abdel Rahim, M.; Abdelrahman, R.S. The Protective Effect of Protocatechuic Acid on Hepatotoxicity Induced by Cisplatin in Mice. Life Sci. 2021, 277, 119485. [Google Scholar] [CrossRef]
- Shabir, I.; Kumar Pandey, V.; Shams, R.; Dar, A.H.; Dash, K.K.; Khan, S.A.; Bashir, I.; Jeevarathinam, G.; Rusu, A.V.; Esatbeyoglu, T.; et al. Promising Bioactive Properties of Quercetin for Potential Food Applications and Health Benefits: A Review. Front. Nutr. 2022, 9, 999752. [Google Scholar] [CrossRef]
- Xue, H.; Sang, Y.; Gao, Y.; Zeng, Y.; Liao, J.; Tan, J. Research Progress on Absorption, Metabolism, and Biological Activities of Anthocyanins in Berries: A Review. Antioxidants 2022, 12, 3. [Google Scholar] [CrossRef] [PubMed]
- Kaur, S.; Muthuraman, A. Ameliorative Effect of Gallic Acid in Paclitaxel-Induced Neuropathic Pain in Mice. Toxicol. Rep. 2019, 6, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Lo, C.; Lai, T.Y.; Yang, J.S.; Yang, J.H.; Ma, Y.S.; Weng, S.W.; Lin, H.Y.; Chen, H.Y.; Lin, J.G.; Chung, J.G. Gallic Acid Inhibits the Migration and Invasion of A375.S2 Human Melanoma Cells through the Inhibition of Matrix Metalloproteinase-2 and Ras. Melanoma Res. 2011, 21, 267–273. [Google Scholar] [CrossRef]
- Hadidi, M.; Liñán-Atero, R.; Tarahi, M.; Christodoulou, M.C.; Aghababaei, F. The Potential Health Benefits of Gallic Acid: Therapeutic and Food Applications. Antioxidants 2024, 13, 1001. [Google Scholar] [CrossRef]
- Pereira, M.M.; de Morais, H.; dos Santos Silva, E.; Corso, C.R.; Adami, E.R.; Carlos, R.M.; Acco, A.; Zanoveli, J.M. The Antioxidant Gallic Acid Induces Anxiolytic-, but Not Antidepressant-like Effect, in Streptozotocin-Induced Diabetes. Metab. Brain Dis. 2018, 33, 1573–1584. [Google Scholar] [CrossRef]
- Hosseinzadeh, A.; Houshmand, G.; Goudarzi, M.; Sezavar, S.H.; Mehrzadi, S.; Mansouri, E.; Kalantar, M. Ameliorative Effect of Gallic Acid on Sodium Arsenite-Induced Spleno-, Cardio- and Hemato-Toxicity in Rats. Life Sci. 2019, 217, 91–100. [Google Scholar] [CrossRef]
- Taofiq, O.; Heleno, S.A.; Calhelha, R.C.; Fernandes, I.P.; Alves, M.J.; Barros, L.; González-Paramás, A.M.; Ferreira, I.C.F.R.; Barreiro, M.F. Phenolic Acids, Cinnamic Acid, and Ergosterol as Cosmeceutical Ingredients: Stabilization by Microencapsulation to Ensure Sustained Bioactivity. Microchem. J. 2019, 147, 469–477. [Google Scholar] [CrossRef]
- Chen, P.; Chen, P.; Wang, X. Design, Synthesis and Bioactivity Evaluation of Cinnamic Acid Derivatives as Potential Anti-Inflammatory Agents against LPS-Induced Acute Lung Injury. Bioorg. Med. Chem. Lett. 2025, 116, 130036. [Google Scholar] [CrossRef]
- Martin-Dominguez, V.; Estevez, J.; De Borja Ojembarrena, F.; Santos, V.E.; Ladero, M. Fumaric Acid Production: A Biorefinery Perspective. Fermentation 2018, 4, 33. [Google Scholar] [CrossRef]
- Refolo, M.G.; Lippolis, C.; Carella, N.; Cavallini, A.; Messa, C.; D’Alessandro, R. Chlorogenic Acid Improves the Regorafenib Effects in Human Hepatocellular Carcinoma Cells. Int. J. Mol. Sci. 2018, 19, 1518. [Google Scholar] [CrossRef] [PubMed]
- Santana-Gálvez, J.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. Chlorogenic Acid: Recent Advances on Its Dual Role as a Food Additive and a Nutraceutical against Metabolic Syndrome. Molecules 2017, 22, 358. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, Y.; Ren, X.; Zhou, H.; Wang, K.; Zhang, H.; Luo, P. Caffeoylquinic Acid Derivatives Extract of Erigeron Multiradiatus Alleviated Acute Myocardial Ischemia Reperfusion Injury in Rats through Inhibiting NF-KappaB and JNK Activations. Mediat. Inflamm. 2016, 2016, 7961940. [Google Scholar] [CrossRef]
- Jurič, A.; Brčić Karačonji, I.; Kopjar, N. Homogentisic Acid, a Main Phenolic Constituent of Strawberry Tree Honey, Protects Human Peripheral Blood Lymphocytes against Irinotecan-Induced Cytogenetic Damage in Vitro. Chem. Biol. Interact. 2021, 349, 109672. [Google Scholar] [CrossRef] [PubMed]
- García-Niño, W.R.; Zazueta, C. Ellagic Acid: Pharmacological Activities and Molecular Mechanisms Involved in Liver Protection. Pharmacol. Res. 2015, 97, 84–103. [Google Scholar] [CrossRef]
- Bangar, S.P.; Chaudhary, V.; Sharma, N.; Bansal, V.; Ozogul, F.; Lorenzo, J.M. Kaempferol: A Flavonoid with Wider Biological Activities and Its Applications. Crit. Rev. Food Sci. Nutr. 2023, 63, 9580–9604. [Google Scholar] [CrossRef]
- Zu, G.; Sun, K.; Li, L.; Zu, X.; Han, T.; Huang, H. Mechanism of Quercetin Therapeutic Targets for Alzheimer Disease and Type 2 Diabetes Mellitus. Sci. Rep. 2021, 11, 22959. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, B.B.; Si, X.; Liu, X.; Deng, X.; Niu, X.; Jin, Y.; Wang, D.; Wang, J. Quercetin Protects Rats from Catheter-Related Staphylococcus Aureus Infections by Inhibiting Coagulase Activity. J. Cell Mol. Med. 2019, 23, 4808–4818. [Google Scholar] [CrossRef] [PubMed]
- Thiruvengadam, M.; Venkidasamy, B.; Subramanian, U.; Samynathan, R.; Shariati, M.A.; Rebezov, M.; Girish, S.; Thangavel, S.; Dhanapal, A.R.; Fedoseeva, N.; et al. Bioactive Compounds in Oxidative Stress-Mediated Diseases: Targeting the NRF2/ARE Signaling Pathway and Epigenetic Regulation. Antioxidants 2021, 10, 1859. [Google Scholar] [CrossRef] [PubMed]



| Mushroom Species | Specific Polyphenols | Reference |
|---|---|---|
| Morchella elata | Gallic acid (1.17 µg/g), protocatechuic acid (1.98 µg/g), catechin (10.24 µg/g), ellagic acid (0.39 µg/g), rosmarinic acid (0.04 µg/g) | [63] |
| Russula vinosa Lindblad | Fumaric acid (52 µg/g), gallic acid (2.5 µg/g), catechin hydrate (3.65 µg/g) | |
| Russula azurea Bres | Gallic acid (1.45 µg/g), fumaric acid (41.76 µg/g), ellagic acid (0.73 µg/g), rosmarinic acid (0.09 µg/g), trans-cinnamic acid (0.35 µg/g) | |
| Cantharellus cibarius | Pyrogallol (187.28 µg/g), benzoic acid (6.08 µg/g), resveratrol (1.65 µg/g), homogentisic acid (3.75 µg/g) | [64] |
| Agaricus bisporus (white mushroom) | Cinnamic acid (216.67 µg/g) | [65] |
| Pleurotus ostreatus (oyster mushroom) | Homogentisic acid (317 µg/g), cinnamic acid (131.73 µg/g), p-coumaric acid (13 µg/g) | |
| Lentinula edodes (shiitake) | Rutin (2100 µg/g) and quercetin (91 µg/g) | [66] |
| Ganoderma lucidum | Gallic acid (1.016 µg/g), trans-cinnamic acid (0.104 µg/g), quercetin (0.968 µg/g), kaempferol (0.918 µg/g), herretin (3.22 µg/g), and naringenin (2.18 µg/g) | [62] |
| Sarcodon imbricatus | Protocatechuic acid (7.48 µg/g), myricetin (34 µg/g), chlorogenic acid (20.7 µg/g), quercetin (65 µg/g) | [67] |
| Pleurotus nebrodensis | Protocatechuic acid (105 µg/g) and hesperetin and biochanin-A (12 µg/g) | [68] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parí, S.M.; Saldaña, E.; Rios-Mera, J.D.; Quispe Angulo, M.F.; Huaman-Castilla, N.L. Emerging Technologies for Extracting Antioxidant Compounds from Edible and Medicinal Mushrooms: An Efficient and Sustainable Approach. Compounds 2025, 5, 29. https://doi.org/10.3390/compounds5030029
Parí SM, Saldaña E, Rios-Mera JD, Quispe Angulo MF, Huaman-Castilla NL. Emerging Technologies for Extracting Antioxidant Compounds from Edible and Medicinal Mushrooms: An Efficient and Sustainable Approach. Compounds. 2025; 5(3):29. https://doi.org/10.3390/compounds5030029
Chicago/Turabian StyleParí, Salome Mamani, Erick Saldaña, Juan D. Rios-Mera, María Fernanda Quispe Angulo, and Nils Leander Huaman-Castilla. 2025. "Emerging Technologies for Extracting Antioxidant Compounds from Edible and Medicinal Mushrooms: An Efficient and Sustainable Approach" Compounds 5, no. 3: 29. https://doi.org/10.3390/compounds5030029
APA StyleParí, S. M., Saldaña, E., Rios-Mera, J. D., Quispe Angulo, M. F., & Huaman-Castilla, N. L. (2025). Emerging Technologies for Extracting Antioxidant Compounds from Edible and Medicinal Mushrooms: An Efficient and Sustainable Approach. Compounds, 5(3), 29. https://doi.org/10.3390/compounds5030029







