Chemical Functionalization of Camelina, Hemp, and Rapeseed Oils for Sustainable Resin Applications: Strategies for Tailoring Structure and Performance
Abstract
1. Introduction
1.1. Agricultural Benefits of Camelina, Hemp, and Rapeseed
1.1.1. Camelina
1.1.2. Hemp
1.1.3. Rapeseed
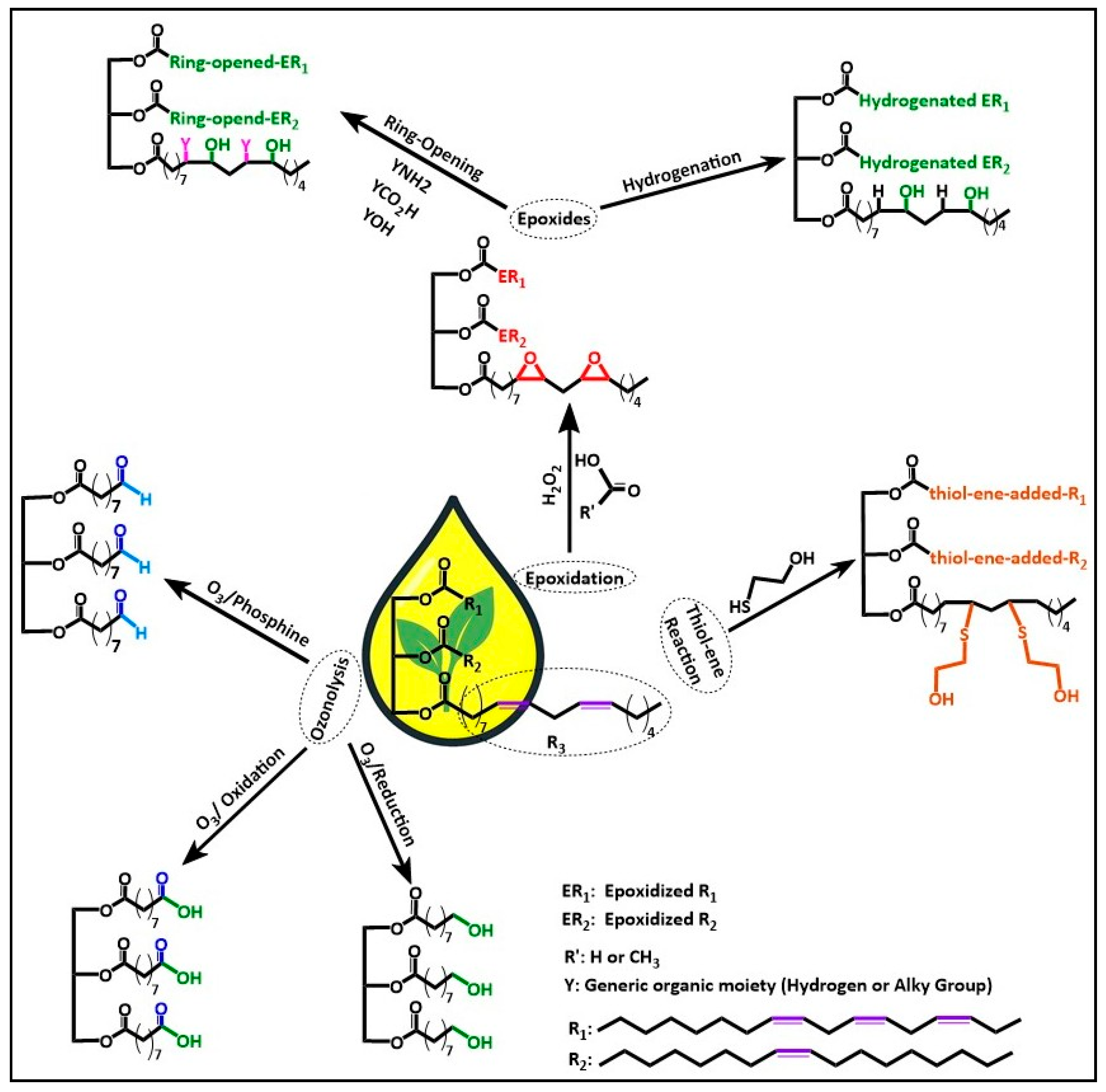
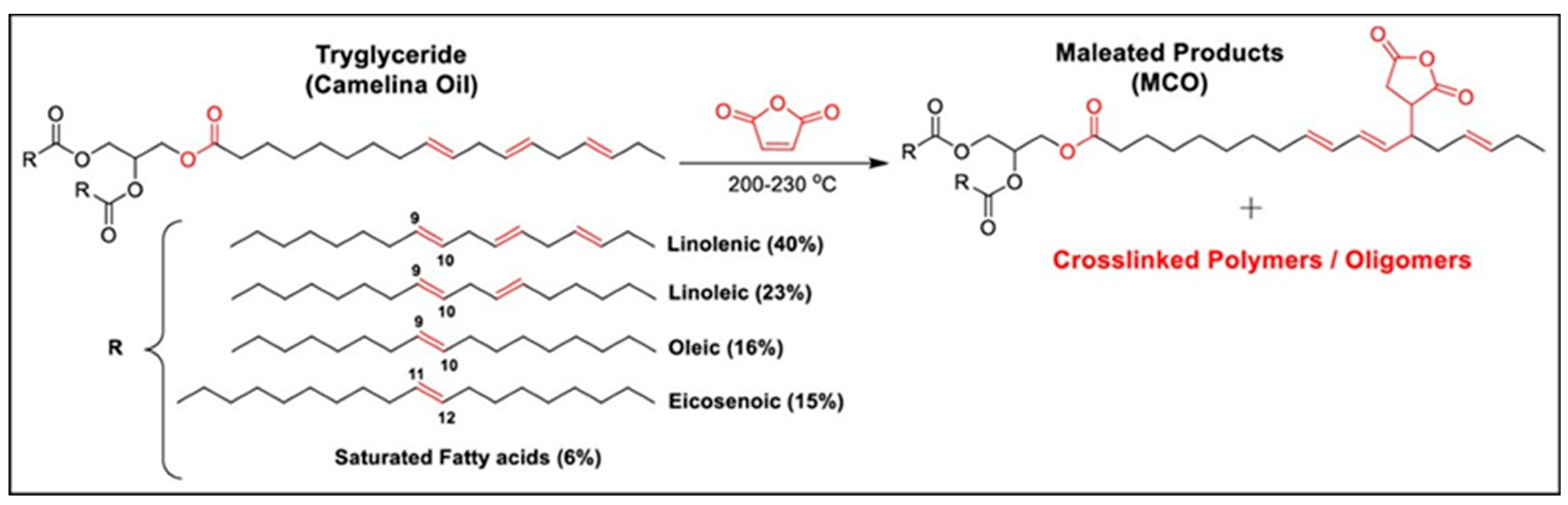
2. Functionalization Strategies of Camelina, Hemp, and Rapeseed Oils for Bio-Based Resin Development
3. Application-Oriented Polymerization Pathways Using Modified Camelina, Hemp, and Rapeseed Oils
3.1. Polyamides
3.2. Polyesterification
3.2.1. Alkyd Resins
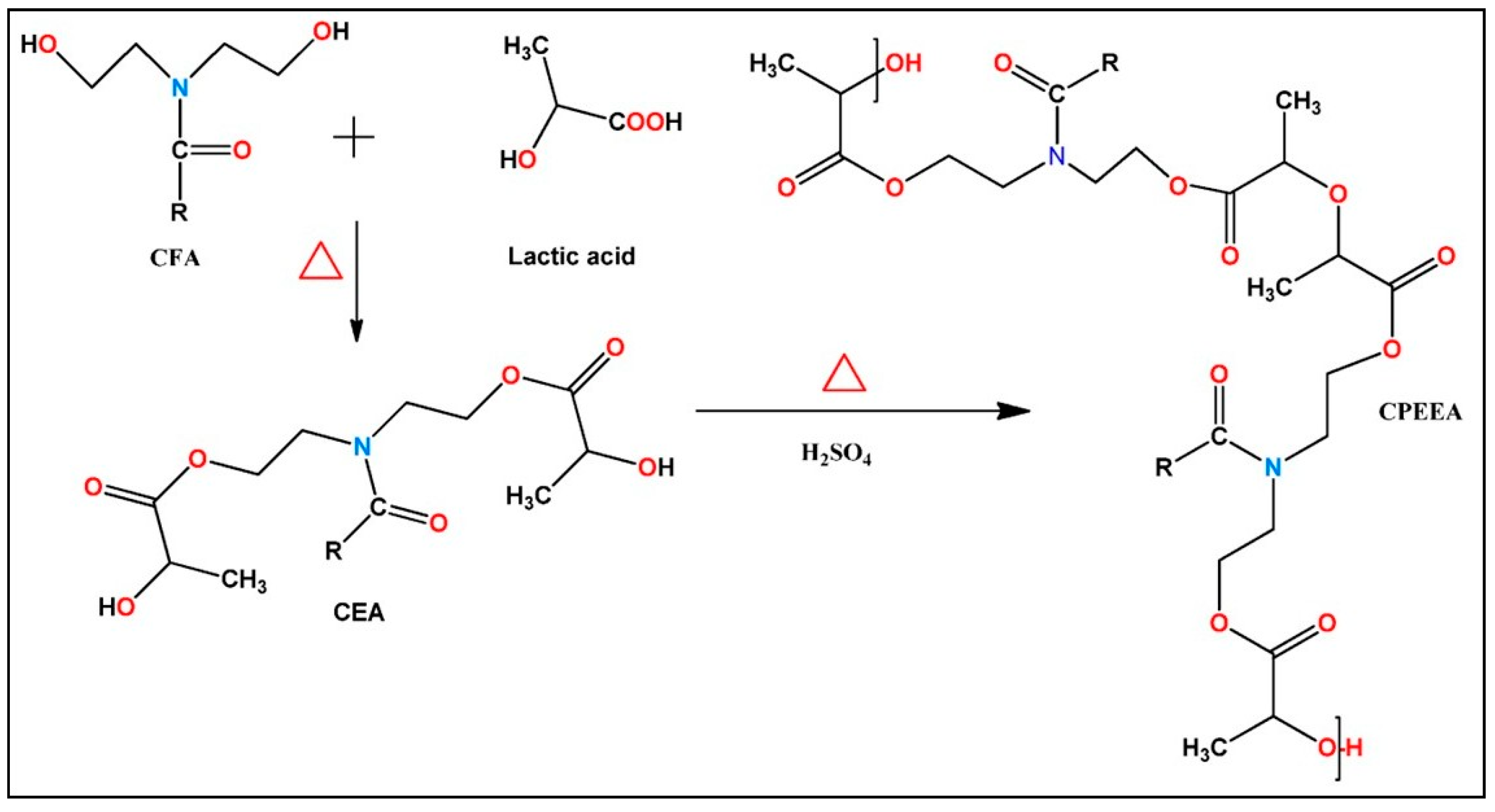
3.2.2. Polyesteramides
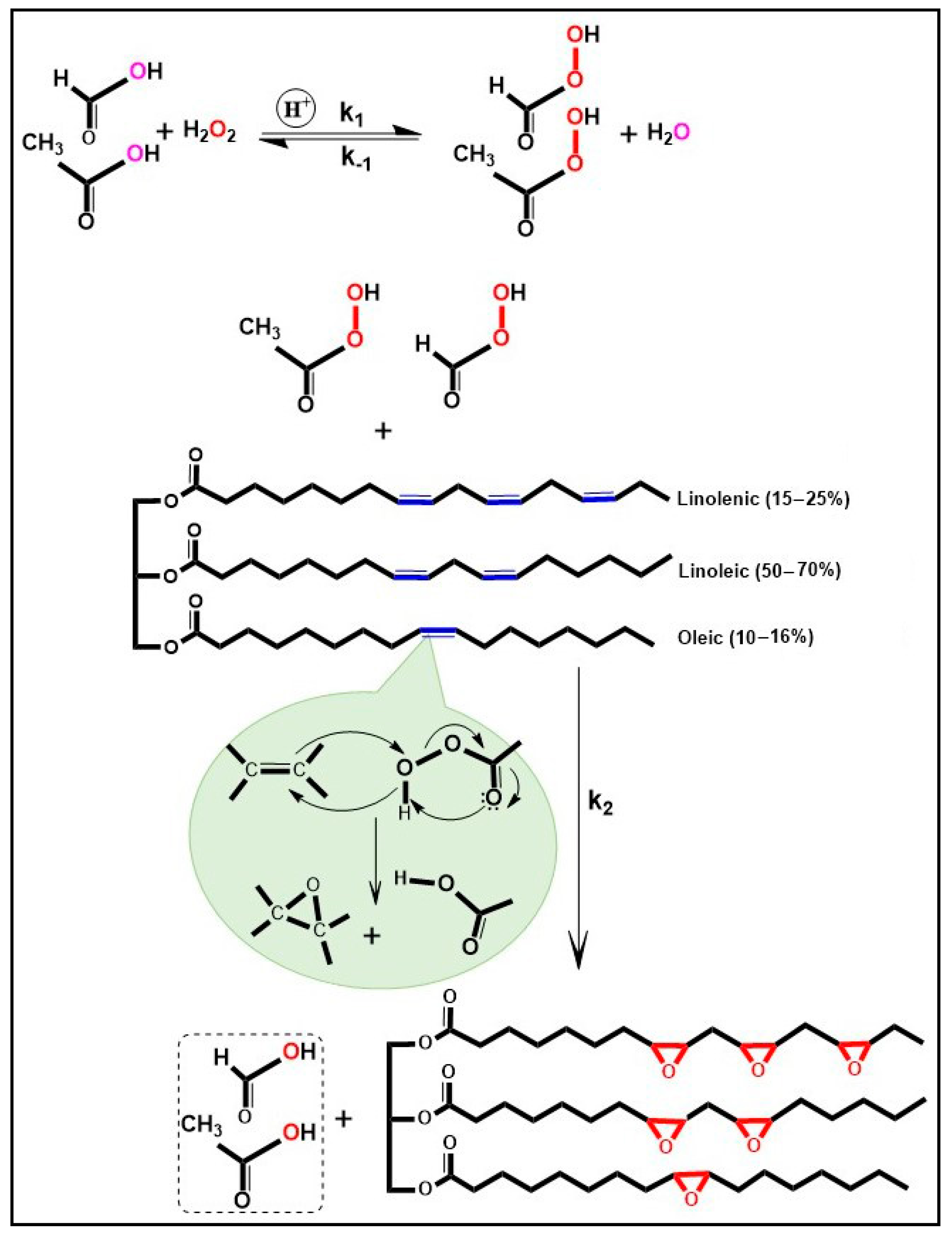
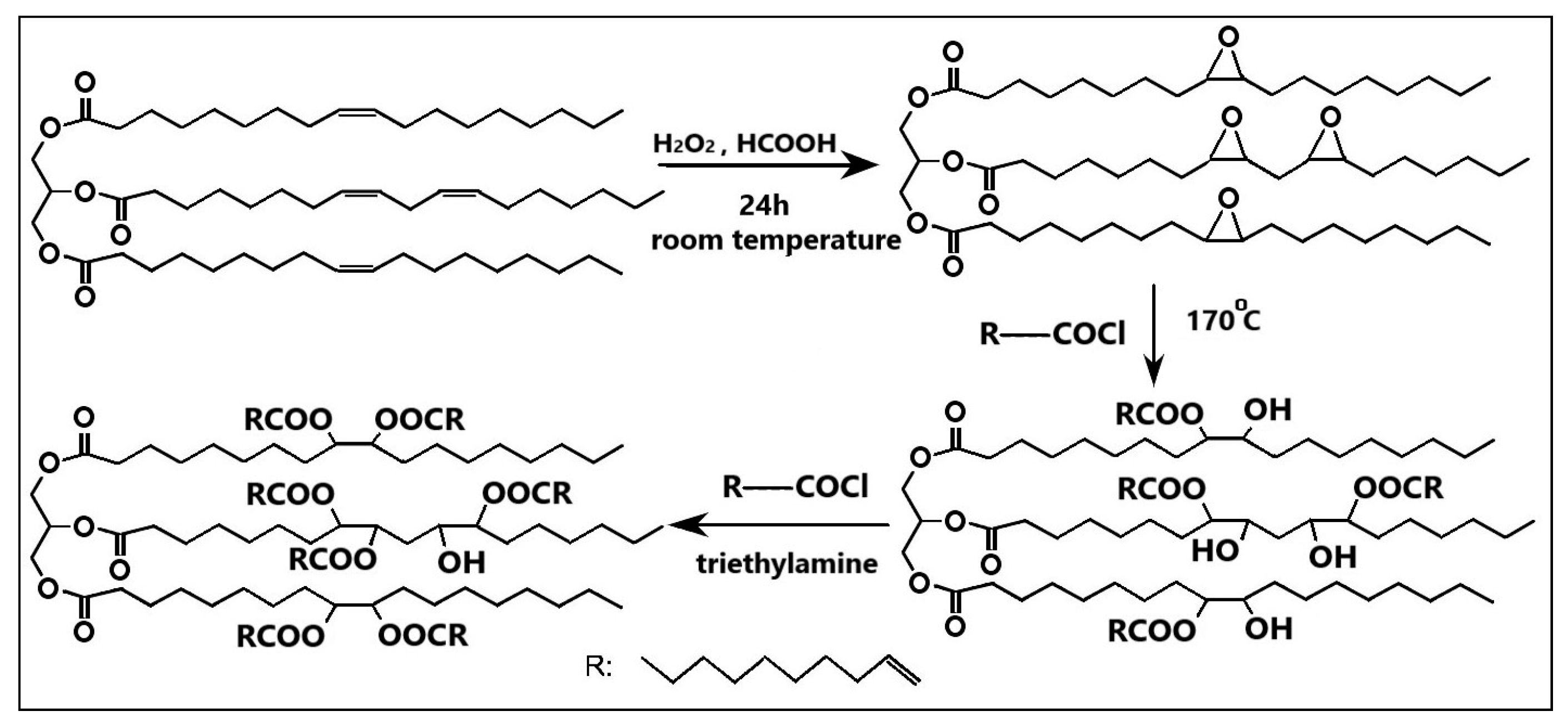
3.3. Epoxidation
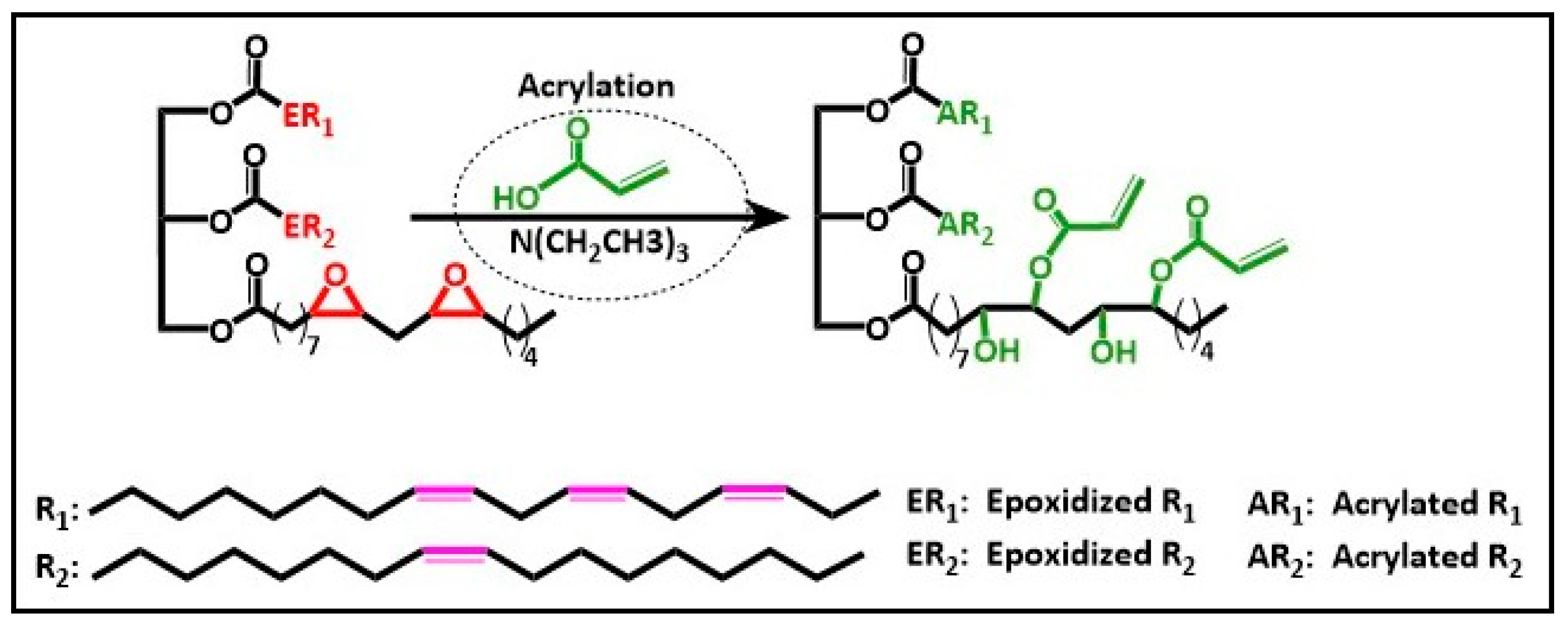
3.4. Acrylation and Vinylation
3.5. Polyurethanes
3.5.1. Polyol Synthesis Routes
3.5.2. Performance and Application Examples
3.6. Click Chemistry
3.7. Comparative Overview and Auxilary Methods
4. Research Gaps, Outlook, and Conclusion
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| CEA | Canola Oil Esteramide |
| CPEEA | Canola Oil Polyesteramide |
| CN | Carbon Number |
| CO2 | Carbon Dioxide |
| DB | Number of Double Bonds |
| DFT | Density Functional Theory |
| IPN | Interpenetrating Polymer Network |
| n.d. | Not Determined |
| UV | Ultraviolet |
| ZnO | Zinc Oxide |
References
- Adekunle, K.F. A Review of Vegetable Oil-Based Polymers: Synthesis and Applications. OJPChem 2015, 05, 34–40. [Google Scholar] [CrossRef]
- Wang, R.; Schuman, T. Towards Green: A Review of Recent Developments in Bio-Renewable Epoxy Resins from Vegetable Oils. In Green Materials from Plant Oils; Liu, Z., Kraus, G., Eds.; The Royal Society of Chemistry: London, UK, 2014; pp. 202–241. ISBN 978-1-84973-901-6. [Google Scholar]
- Baskar, C.; Ramakrishna, S.; Rosa, A.D.L. (Eds.) Encyclopedia of Green Materials; Bio-Based Adhesives from Plant Oils; Springer Nature: Singapore, 2025; pp. 123–134. ISBN 978-981-9746-17-0. [Google Scholar]
- Shahidi, F. Rapeseed and Canola: Global Production and Distribution. In Canola and Rapeseed; Shahidi, F., Ed.; Springer: Boston, MA, USA, 1990; pp. 3–13. ISBN 978-1-4613-6744-4. [Google Scholar]
- Zanetti, F.; Peroni, P.; Pagani, E.; Von Cossel, M.; Greiner, B.E.; Krzyżaniak, M.; Stolarski, M.J.; Lewandowski, I.; Alexopoulou, E.; Stefanoni, W.; et al. The Opportunities and Potential of Camelina in Marginal Land in Europe. Ind. Crops Prod. 2024, 211, 118224–118233. [Google Scholar] [CrossRef]
- Amaducci, S.; Scordia, D.; Liu, F.H.; Zhang, Q.; Guo, H.; Testa, G.; Cosentino, S.L. Key Cultivation Techniques for Hemp in Europe and China. Ind. Crops Prod. 2015, 68, 2–16. [Google Scholar] [CrossRef]
- Poth, U. Drying Oils and Related Products. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH, Ed.; Wiley: Hoboken, NJ, USA, 2001; ISBN 978-3-527-30385-4. [Google Scholar]
- Hrastar, R.; Abramovič, H.; Košir, I.J. In Situ Quality Evaluation of Camelina sativa Landrace. Eur. J. Lipid Sci. Technol. 2012, 114, 343–351. [Google Scholar] [CrossRef]
- Gunstone, F. Rapeseed and Canola Oil, 1st ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2009; ISBN 978-1-4051-4792-7. [Google Scholar]
- Nadim, E.; Paraskar, P.; Moradi, H.; Hesabi, M.; Qiao, Y.; Murphy, E.J.; Major, I. Kinetic and Thermodynamic Analysis of Hemp Oil Epoxidation with Density Functional Theory Insights into Unsaturated Fatty Acid Epoxidation and Ring-Opening Reactions. Chem. Eng. J. Adv. 2025, 22, 100749–100760. [Google Scholar] [CrossRef]
- Lerma-Canto, A.; Samper, M.D.; Dominguez-Candela, I.; Garcia-Garcia, D.; Fombuena, V. Epoxidized and Maleinized Hemp Oil to Develop Fully Bio-Based Epoxy Resin Based on Anhydride Hardeners. Polymers 2023, 15, 1404. [Google Scholar] [CrossRef]
- Moser, B.R. Camelina (Camelina sativa L.) Oil as a Biofuels Feedstock: Golden Opportunity or False Hope? Lipid Technol. 2010, 22, 270–273. [Google Scholar] [CrossRef]
- Da Porto, C.; Decorti, D.; Tubaro, F. Fatty Acid Composition and Oxidation Stability of Hemp (Cannabis sativa L.) Seed Oil Extracted by Supercritical Carbon Dioxide. Ind. Crops Prod. 2012, 36, 401–404. [Google Scholar] [CrossRef]
- Farahmandfar, R.; Asnaashari, M.; Sayyad, R. Comparison Antioxidant Activity of Tarom Mahali Rice Bran Extracted from Different Extraction Methods and Its Effect on Canola Oil Stabilization. J. Food Sci. Technol. 2015, 52, 6385–6394. [Google Scholar] [CrossRef]
- Mikulcová, V.; Kašpárková, V.; Humpolíček, P.; Buňková, L. Formulation, Characterization and Properties of Hemp Seed Oil and Its Emulsions. Molecules 2017, 22, 700. [Google Scholar] [CrossRef]
- Ditrói, K.; Kleiner, D.; Böszörményi, A.; Szentmihályi, K.; Fébel, H. The Alimentary Impact of the Hemp Seed. Acta Aliment. 2013, 42, 410–416. [Google Scholar] [CrossRef]
- Tiefenbacher, K.F. Technology of Main Ingredients—Sweeteners and Lipids. In Wafer and Waffle; Elsevier: San Diego, California, 2017; pp. 123–225. ISBN 978-0-12-809438-9. [Google Scholar]
- Zubr, J.; Matzen, R. Mixed Vegetable and Diesel Oil as Fuel. In Biomass for Energy and the Environment; Elsevier: Oxford, UK, 1996; pp. 1644–1653. ISBN 978-0-08-042849-9. [Google Scholar]
- Anwar, F.; Latif, S.; Ashraf, M. Analytical Characterization of Hemp (Cannabis Sativa) Seed Oil from Different Agro-ecological Zones of Pakistan. J. Americ. Oil Chem. Soc. 2006, 83, 323–329. [Google Scholar] [CrossRef]
- Zubr, J. Oil-Seed Crop: Camelina sativa. Ind. Crops Prod. 1997, 6, 113–119. [Google Scholar] [CrossRef]
- Masarovičová, E.; Kráľová, K.; Malovcová, Ľ. Production of Some Rapeseed Cultivars Production of Some Rapeseed Cultivars Slovakia. Ekológia 2011, 30, 360–386. [Google Scholar] [CrossRef]
- Kandaramath Hari, T.; Yaakob, Z.; Binitha, N.N. Aviation Biofuel from Renewable Resources: Routes, Opportunities and Challenges. Renew. Sustain. Energy Rev. 2015, 42, 1234–1244. [Google Scholar] [CrossRef]
- Sarv, V. A Comparative Study of Camelina, Canola and Hemp Seed Processing and Products. Master’s Thesis, University of Toronto, Toronto, ON, Canada, June 2017. [Google Scholar]
- Ussetti Mohottalalage, S. A Comparative Study of the Structural and Physicochemical Properties of the Major Proteins from Camelina sativa (L.) Crantz and Brassica napus L. Master’s Thesis, University of Saskatchewan, Saskatoon, SK, Canada, September 2016. [Google Scholar]
- Li, Y.; Sun, X.S. Camelina Oil Derivatives and Adhesion Properties. Ind. Crops. Prod. 2015, 73, 73–80. [Google Scholar] [CrossRef]
- Omonov, T.S.; Kharraz, E.; Curtis, J.M. Camelina (Camelina sativa) Oil Polyols as an Alternative to Castor Oil. Ind. Crops. Prod. 2017, 107, 378–385. [Google Scholar] [CrossRef]
- Shuttleworth, P.S.; Díez-Pascual, A.M.; Marco, C.; Ellis, G. Flexible Bionanocomposites from Epoxidized Hemp Seed Oil Thermosetting Resin Reinforced with Halloysite Nanotubes. J. Phys. Chem. B 2017, 121, 2454–2467. [Google Scholar] [CrossRef]
- Afif, M.K.; Biradar, C.H. Production of Biodiesel from Cannabis sativa (Hemp) Seed Oil and Its Performance and Emission Characteristics on DI Engine Fueled with Biodiesel Blends. Int. Res. J. Eng. Technol. 2019, 6, 246–253. [Google Scholar]
- Malabadi, R.B.; Kolkar, K.P.; Chalannavar, R.K.; Vassanthini, R.; Mudigoudra, B.S. Industrial Cannabis Sativa: Hemp Plastic-Updates. World J. Adv. Res. Rev. 2023, 20, 715–725. [Google Scholar] [CrossRef]
- Arthey, T. Challenges and Perspectives in Global Rapeseed Production; 2020. Available online: www.agribenchmark.org/cash-crop (accessed on 15 April 2025).
- Carré, P.; Pouzet, A. Rapeseed Market, Worldwide and in Europe. OCL 2014, 21, D102. [Google Scholar] [CrossRef]
- Fridrihsone, A.; Romagnoli, F.; Cabulis, U. Life Cycle Inventory for Winter and Spring Rapeseed Production in Northern Europe. J. Clean. Prod. 2018, 177, 79–88. [Google Scholar] [CrossRef]
- Maulana, S.; Wibowo, E.S.; Mardawati, E.; Iswanto, A.H.; Papadopoulos, A.; Lubis, M.A.R. Eco-Friendly and High-Performance Bio-Polyurethane Adhesives from Vegetable Oils: A Review. Polymers 2024, 16, 1613. [Google Scholar] [CrossRef]
- Ciastowicz, Ż.; Pamuła, R.; Białowiec, A. Utilization of Plant Oils for Sustainable Polyurethane Adhesives: A Review. Materials 2024, 17, 1738. [Google Scholar] [CrossRef] [PubMed]
- Maisonneuve, L.; Chollet, G.; Grau, E.; Cramail, H. Vegetable Oils: A Source of Polyols for Polyurethane Materials. OCL 2016, 23, D508. [Google Scholar] [CrossRef]
- Desroches, M.; Escouvois, M.; Auvergne, R.; Caillol, S.; Boutevin, B. From Vegetable Oils to Polyurethanes: Synthetic Routes to Polyols and Main Industrial Products. Polym. Rev. 2012, 52, 38–79. [Google Scholar] [CrossRef]
- Nosal, H.; Nowicki, J.; Warzała, M.; Semeniuk, I.; Sabura, E. Synthesis and Characterization of Alkyd Resins Based on Camelina sativa Oil, Glycerol and Selected Epoxidized Vegetable Oils as Functional Modifiers. Prog. Org. Coat. 2016, 101, 553–568. [Google Scholar] [CrossRef]
- Islam, M.R.; Beg, M.D.H.; Jamari, S.S. Development of Vegetable-oil-based Polymers. J. Appl. Polym. Sci. 2014, 131, 40787–40789. [Google Scholar] [CrossRef]
- Nadim, E.; Paraskar, P.; Hesabi, M.; Yahyaei, H.; Murphy, E.J.; Major, I. Sustainable Polyurethane Coatings Derived from Alkyds of Camelina Oil Monoglycerides. J Appl. Polym. Sci. 2024, 141, e56079. [Google Scholar] [CrossRef]
- Paraskar, P.M.; Prabhudesai, M.S.; Hatkar, V.M.; Kulkarni, R.D. Vegetable Oil Based Polyurethane Coatings—A Sustainable Approach: A Review. Prog. Org. Coat. 2021, 156, 106267–106285. [Google Scholar] [CrossRef]
- Sabin, P.; Benjelloun-Mlayah, B.; Delmas, M. Offset Printing Inks Based on Rapeseed and Sunflower Oil. Part I: Synthesis and Characterization of Rapeseed Oil-and Sunflower Oil-modified Alkyd Resins. J. Am. Oil Chem. Soc. 1997, 74, 481–489. [Google Scholar] [CrossRef]
- Seniha Güner, F.; Yağcı, Y.; Tuncer Erciyes, A. Polymers from Triglyceride Oils. Prog. Polym. Sci. 2006, 31, 633–670. [Google Scholar] [CrossRef]
- Nimbalkar, R.V.; Athawale, V.D. Synthesis and Characterization of Canola Oil Alkyd Resins Based on Novel Acrylic Monomer (ATBS). J. Am. Oil Chem. Soc. 2010, 87, 947–954. [Google Scholar] [CrossRef]
- Nosal, H.; Nowicki, J.; Warzała, M.; Nowakowska-Bogdan, E.; Zarębska, M. Synthesis and Characterization of Alkyd Resins Based on Camelina Sativa Oil and Polyglycerol. Prog. Org. Coat. 2015, 86, 59–70. [Google Scholar] [CrossRef]
- Nadim, E.; Paraskar, P.; Murphy, E.J.; Hesabi, M.; Major, I. Sustainable Polyurethane Coatings Based on Functional Camelina Oil-Based Polyols. Ind. Crops. Prod. 2023, 204, 117274. [Google Scholar] [CrossRef]
- Alam, M.; Altaf, M.; Ahmad, N. Canola Oil Based Poly(Ester–Ether–Amide–Urethane) Nanocomposite and Its Anti-Corrosive Coatings. Polymers 2021, 13, 3325. [Google Scholar] [CrossRef]
- Kim, N.; Li, Y.; Sun, X.S. Epoxidation of Camelina sativa Oil and Peel Adhesion Properties. Ind. Crops Prod. 2015, 64, 1–8. [Google Scholar] [CrossRef]
- Jalil, M.J.; Hadi, A.; Azmi, I.S. Catalytic Epoxidation of Palm Oleic Acid Using In Situ Generated Performic Acid—Optimization and Kinetic Studies. Mater. Chem. Phys. 2021, 270, 124754. [Google Scholar] [CrossRef]
- Omonov, T.S.; Curtis, J.M. Plant Oil-Based Epoxy Intermediates for Polymers. In Bio-Based Plant Oil Polymers and Composites; Elsevier: Amsterdam, The Netherlands, 2016; pp. 99–125. ISBN 978-0-323-35833-0. [Google Scholar]
- Karmakar, G.; Ghosh, P.; Sharma, B. Chemically Modifying Vegetable Oils to Prepare Green Lubricants. Lubricants 2017, 5, 44. [Google Scholar] [CrossRef]
- Martinez Villadiego, K.; Arias Tapia, M.J.; Useche, J.; Escobar Macías, D. Thermoplastic Starch (TPS)/Polylactic Acid (PLA) Blending Methodologies: A Review. J. Polym. Environ. 2022, 30, 75–91. [Google Scholar] [CrossRef]
- Llevot, A. Sustainable Synthetic Approaches for the Preparation of Plant Oil-Based Thermosets. J. Am. Oil Chem. Soc. 2017, 94, 169–186. [Google Scholar] [CrossRef]
- Kolář, M.; Honzíček, J.; Podzimek, Š.; Knotek, P.; Hájek, M.; Zárybnická, L.; Machotová, J. Derivatives of Linseed Oil and Camelina Oil as Monomers for Emulsion Polymerization. J. Mater. Sci. 2023, 58, 15558–15575. [Google Scholar] [CrossRef]
- Balanuca, B.; Lungu, A.; Hanganu, A.; Stan, L.R.; Vasile, E.; Iovu, H. Hybrid Nanocomposites Based on POSS and Networks of Methacrylated Camelina Oil and Various PEG Derivatives. Eur. J. Lipid Sci. Technol. 2014, 116, 458–469. [Google Scholar] [CrossRef]
- Malburet, S.; Di Mauro, C.; Noè, C.; Mija, A.; Sangermano, M.; Graillot, A. Sustainable Access to Fully Biobased Epoxidized Vegetable Oil Thermoset Materials Prepared by Thermal or UV-Cationic Processes. RSC Adv. 2020, 10, 41954–41966. [Google Scholar] [CrossRef]
- Devansh; Patil, P.; Pinjari, D.V. Oil-based Epoxy and Their Composites: A Sustainable Alternative to Traditional Epoxy. J. Appl. Polym. Sci. 2024, 141, e55560. [Google Scholar] [CrossRef]
- Omonov, T.S.; Patel, V.; Curtis, J.M. The Development of Epoxidized Hemp Oil Prepolymers for the Preparation of Thermoset Networks. J. Am. Oil Chem. Soc. 2019, 96, 1389–1403. [Google Scholar] [CrossRef]
- Oktay, B.; Türkcan, J.H.; Özdemir, O.K.; Kayaman-Apohan, N. Vegetable Oil-Based Epoxy Coating Materials for Self-Healing and Anticorrosive Applications. Macromol. Res. 2023, 31, 1077–1086. [Google Scholar] [CrossRef]
- Gupta, R.N.; Harsha, A.P.; Singh, S. Tribological Study on Rapeseed Oil with Nano-Additives in Close Contact Sliding Situation. Appl. Nanosci. 2018, 8, 567–580. [Google Scholar] [CrossRef]
- Ho, Y.H.; Parthiban, A.; Thian, M.C.; Ban, Z.H.; Siwayanan, P. Acrylated Biopolymers Derived via Epoxidation and Subsequent Acrylation of Vegetable Oils. Int. J. Polym. Sci. 2022, 2022, 6210128. [Google Scholar] [CrossRef]
- Sung, J.; Sun, X.S. Cardanol Modified Fatty Acids from Camelina Oils for Flexible Bio-Based Acrylates Coatings. Prog. Org. Coat. 2018, 123, 242–253. [Google Scholar] [CrossRef]
- Kalita, D.J.; Tarnavchyk, I.; Sibi, M.; Moser, B.R.; Webster, D.C.; Chisholm, B.J. Biobased Poly(Vinyl Ether)s Derived from Soybean Oil, Linseed Oil, and Camelina Oil: Synthesis, Characterization, and Properties of Crosslinked Networks and Surface Coatings. Prog. Org. Coat. 2018, 125, 453–462. [Google Scholar] [CrossRef]
- Fu, C.; Wang, J.; Chen And Liang Shen, L. Synthesis of a Fully Biobased Polyfunctional Vinyl Oligomer and Their UV Cured Films Prepared via Thiol-Ene Coupling. J. Renew. Mater. 2019, 7, 795–805. [Google Scholar] [CrossRef]
- Zanetti, F.; Alberghini, B.; Marjanović Jeromela, A.; Grahovac, N.; Rajković, D.; Kiprovski, B.; Monti, A. Camelina, an Ancient Oilseed Crop Actively Contributing to the Rural Renaissance in Europe. A Review. Agron. Sustain. Dev. 2021, 41, 2–20. [Google Scholar] [CrossRef]
- Arshad, M.; Mohanty, A.K.; Van Acker, R.; Riddle, R.; Todd, J.; Khalil, H.; Misra, M. Valorization of Camelina Oil to Biobased Materials and Biofuels for New Industrial Uses: A Review. RSC Adv. 2022, 12, 27230–27245. [Google Scholar] [CrossRef]
- Briede, S.; Platnieks, O.; Barkane, A.; Sivacovs, I.; Leitans, A.; Lungevics, J.; Gaidukovs, S. Tailored Biobased Resins from Acrylated Vegetable Oils for Application in Wood Coatings. Coatings 2023, 13, 657. [Google Scholar] [CrossRef]
- Jašek, V.; Fučík, J.; Bartoš, O.; Figalla, S.; Přikryl, R. Photocurable Oil-Based Thermosets Containing Modifiers from Renewable Sources for Coating Applications. ACS Polym. Au 2024, 4, 527–539. [Google Scholar] [CrossRef] [PubMed]
- Jašek, V.; Fučík, J.; Melcova, V.; Figalla, S.; Mravcova, L.; Krobot, Š.; Přikryl, R. Synthesis of Bio-Based Thermoset Mixture Composed of Methacrylated Rapeseed Oil and Methacrylated Methyl Lactate: One-Pot Synthesis Using Formed Methacrylic Acid as a Continual Reactant. Polymers 2023, 15, 1811. [Google Scholar] [CrossRef]
- William Manthey, N. Development of Hemp Oil Based Bioresins. Ph.D. Thesis, University of Southern Queensland: Toowoomba, Australia, 2013. [Google Scholar]
- Platnieks, O.; Briede, S.; Grase, L.; Thakur, V.K.; Gaidukovs, S. Fully Bio-based Thermoset Composites from UV Curable Prepregs: Vegetable Oil Acrylate Impregnated Hemp Nanopaper. Polym. Compos. 2023, 44, 5721–5733. [Google Scholar] [CrossRef]
- Manthey, N.W.; Cardona, F.; Francucci, G.; Aravinthan, T. Thermo-Mechanical Properties of Epoxidized Hemp Oil-Based Bioresins and Biocomposites. J. Reinf. Plast. Compos. 2013, 32, 1444–1456. [Google Scholar] [CrossRef]
- Gómez-de-Miranda-Jiménez-de-Aberast, O.; Perez-Arce, J. Efficient Epoxidation of Vegetable Oils through the Employment of Acidic Ion Exchange Resins. Can. J. Chem. Eng. 2019, 97, 1785–1791. [Google Scholar] [CrossRef]
- Ciubota-Rosie, C.; Díaz-Medino, A.; Ramos, M.J.; Pérez, A.; Carmona, M.; Rodríguez, J.F. The Role of Epoxidation on Camelina Sativa Biodiesel Properties. Glob. NEST J. 2014, 16, 1076–1084. [Google Scholar] [CrossRef]
- Liu, Z.; Erhan, S.Z. Ring-Opening Polymerization of Epoxidized Soybean Oil. J. Am. Oil Chem. Soc. 2010, 87, 437–444. [Google Scholar] [CrossRef]
- Chen, Y.; Xi, Z.; Zhao, L. New Bio-Based Polymeric Thermosets Synthesized by Ring-Opening Polymerization of Epoxidized Soybean Oil with a Green Curing Agent. Eur. Polym. J. 2016, 84, 435–447. [Google Scholar] [CrossRef]
- Slabu, A.I.; Banu, I.; Pavel, O.D.; Teodorescu, F.; Stan, R. Sustainable Ring-Opening Reactions of Epoxidized Linseed Oil in Heterogeneous Catalysis. Sustainability 2023, 15, 4197. [Google Scholar] [CrossRef]
- Guo, Y.; Hardesty, J.H.; Mannari, V.M.; Massingill, J.L. Hydrolysis of Epoxidized Soybean Oil in the Presence of Phosphoric Acid. J. Am. Oil Chem. Soc. 2007, 84, 929–935. [Google Scholar] [CrossRef]
- Kim, N. Epoxidation and Di-Hydroxylation of Camelina sativa Oil. Master’s Thesis, Kansas State University, Manhattan, KS, USA, 2012. [Google Scholar]
- Petrovic, Z. Polyurethanes from Vegetable Oils. Polym. Rev. 2008, 48, 109–155. [Google Scholar] [CrossRef]
- Dumont, M.-J.; Kharraz, E.; Qi, H. Production of Polyols and Mono-Ols from 10 North-American Vegetable Oils by Ozonolysis and Hydrogenation: A Characterization Study. Ind. Crops Prod. 2013, 49, 830–836. [Google Scholar] [CrossRef]
- Jariwala, S.; Desai, Y.N.; Sahu, P.; Gupta, R.K. Hemp Seed Oil Derived Rigid Polyurethane Foams and Their Underlying Flame Retardancy Properties. J. Polym. Environ. 2024, 32, 3822–3834. [Google Scholar] [CrossRef]
- Fridrihsone, A.; Stirna, U.; Lazdiņa, B.; Misāne, M.; Vilsone, D. Characterization of Polyurethane Networks Structure and Properties Based on Rapeseed Oil Derived Polyol. Eur. Polym. J. 2013, 49, 1204–1214. [Google Scholar] [CrossRef]
- Rojek, P.; Prociak, A. Effect of Different Rapeseed-oil-based Polyols on Mechanical Properties of Flexible Polyurethane Foams. J Appl. Polym. Sci. 2012, 125, 2936–2945. [Google Scholar] [CrossRef]
- Desroches, M.; Caillol, S.; Lapinte, V.; Auvergne, R.; Boutevin, B. Synthesis of Biobased Polyols by Thiol−Ene Coupling from Vegetable Oils. Macromolecules 2011, 44, 2489–2500. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, T.; Hao, C.; Mikkelsen, A.; Zhao, B.; Zhang, J. Hempseed Oil-Based Covalent Adaptable Epoxy-Amine Network and Its Potential Use for Room-Temperature Curable Coatings. ACS Sustain. Chem. Eng. 2020, 8, 14964–14974. [Google Scholar] [CrossRef]
- Dworakowska, S.; Bogdal, D.; Prociak, A. Microwave-Assisted Synthesis of Polyols from Rapeseed Oil and Properties of Flexible Polyurethane Foams. Polymers 2012, 4, 1462–1477. [Google Scholar] [CrossRef]
- Tremblay-Parrado, K.-K.; García-Astrain, C.; Avérous, L. Click Chemistry for the Synthesis of Biobased Polymers and Networks Derived from Vegetable Oils. Green Chem. 2021, 23, 4296–4327. [Google Scholar] [CrossRef]
- Arshad, M.; Shankar, S.; Mohanty, A.K.; Todd, J.; Riddle, R.; Van Acker, R.; Taylor, G.W.; Misra, M. Improving the Barrier and Mechanical Properties of Paper Used for Packing Applications with Renewable Hydrophobic Coatings Derived from Camelina Oil. ACS Omega 2024, 9, 19786–19795. [Google Scholar] [CrossRef]
- Mosiewicki, M.A.; Aranguren, M.I. Recent Developments in Plant Oil Based Functional Materials. Polym. Int. 2016, 65, 28–38. [Google Scholar] [CrossRef]
- Sancheti, S.V.; Gogate, P.R. Ultrasound Assisted Selective Catalytic Transfer Hydrogenation of Soybean Oil Using 5% Pd/C as Catalyst under Ambient Conditions in Water. Ultrason. Sonochem. 2017, 38, 161–167. [Google Scholar] [CrossRef]
- Cai, Y.; Liang, Y.; Shi, H.; Cui, J.; Prakash, S.; Zhang, J.; Anaokar, S.; Chai, J.; Schwender, J.; Lu, C.; et al. Creating Yellow Seed Camelina sativa with Enhanced Oil Accumulation by CRISPR-mediated Disruption of Transparent Testa 8. Plant Biotechnol. J. 2024, 22, 2773–2784. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, K.; Huang, S.; Cui, Z.; Chen, B. Choline-Based Deep Eutectic Solvents for Enzymatic Preparation of Epoxy Linseed Oil. Eng. Life Sci. 2025, 25, e70016. [Google Scholar] [CrossRef]
- Cárdenas, J.; Katryniok, B.; Araque-Marin, M.; Hsu, W.-H.; Seeberger, P.H.; Danglad-Flores, J.; Orjuela, A. Continuous Epoxidation of Used Cooking Oils Using an Automated Slug-Flow Millireactor. Chem. Eng. J. 2025, 506, 159907. [Google Scholar] [CrossRef]
- Freites Aguilera, A.; Rahkila, J.; Hemming, J.; Nurmi, M.; Torres, G.; Razat, T.; Tolvanen, P.; Eränen, K.; Leveneur, S.; Salmi, T. Epoxidation of Tall Oil Catalyzed by an Ion Exchange Resin under Conventional Heating and Microwave Irradiation. Ind. Eng. Chem. Res. 2020, 59, 10397–10406. [Google Scholar] [CrossRef]
- European Bioplastics e.V. EUBP STATEMENT on the EU Policy Framework on Biobased, Biodegradable and Compostable Plastics; European Bioplastics e.V.: Berlin, Germany, 2025. [Google Scholar]
- Krzyżaniak, M.; Warmiński, K.; Olba-Zięty, E.; Białoskórski, P.; Stolarski, M.J. Life Cycle Assessment of Camelina and Spelt Wheat in Organic Intercropping Systems. Ind. Crops Prod. 2025, 231, 121198. [Google Scholar] [CrossRef]
- Jašek, V.; Figalla, S. Vegetable Oils for Material Applications—Available Biobased Compounds Seeking Their Utilities. ACS Polym. Au 2025, 5, 105–128. [Google Scholar] [CrossRef] [PubMed]
- Monteserin, C.; Blanco, M.; Uranga, N.; Sanchez, J.; Laza, J.M.; Vilas, J.L.; Aranzabe, E. Sustainable Biobased Epoxy Thermosets with Covalent Dynamic Imine Bonds for Green Composite Development. Polymer 2023, 285, 126339. [Google Scholar] [CrossRef]




| Fatty Acid (%) (CN a:DB b) | Camelina [12] | Hemp [13] | Rapeseed [14] |
|---|---|---|---|
| Palmitic (16:0) | 5.3–6.8 | 7.3–7.7 | 3.4–5.1 |
| Palmitoleic (16:1) | n.d. c | 0.1–0.2 | 0.2–0.3 |
| Stearic (18:0) | 2.5–2.7 | 2.4–2.6 | 2.5–2.6 |
| Oleic (18:1) | 12.6–18.6 | 12.8–13.3 | 64.7–66.0 |
| Linoleic (18:2) | 14.3–19.6 | 56.7–57.7 | 16.3–16.3 |
| α-Linolenic (18:3) | 32.6–38.4 | 17.8–18.7 | 7.5–7.6 |
| γ-Linolenic (18:3) | n.d. | 3.2–3.6 | n.d. |
| Arachidic (20:0) | 1.2–1.4 | n.d. | 0.9–1.01 |
| Gondoinic (20:1) | 12.4–16.8 | 0.7–0.81 | n.d. |
| Eicosadienoic (20:2) | 1.3–2.0 | n.d. | n.d. |
| Eicosatrienoic (20: 3) | 0.8–1.7 | n.d. | n.d. |
| Behenic (22:0) | 0.2–0.3 | 0.1–0.2 | n.d. |
| Erucic (22:1) | 2.3–2.9 | n.d. | 1.0–2.0 |
| Seed | Yield (t ha−1) [6,16] | Oil Content (Wt%) [4,20,21,22] | Sustainability Metrics |
|---|---|---|---|
| Camelina | 1.27–3.3 | 30–45 | Low water and nutrient input Short season (85–100 days) Tolerates salinity, frost, and marginal soils |
| Rapeseed | 2.5–3.5 | 40–48 | Fast biomass gain High carbon-sequestration potential Phytoremediates heavy metals and radionuclides Adaptable to diverse European soils |
| Hemp | 1–1.5 | 26–42 | Improves soil structure and nutrient cycling Low pesticide requirement Weed suppression |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nadim, E.; Paraskar, P.; Murphy, E.J.; Hesabi, M.; Major, I. Chemical Functionalization of Camelina, Hemp, and Rapeseed Oils for Sustainable Resin Applications: Strategies for Tailoring Structure and Performance. Compounds 2025, 5, 26. https://doi.org/10.3390/compounds5030026
Nadim E, Paraskar P, Murphy EJ, Hesabi M, Major I. Chemical Functionalization of Camelina, Hemp, and Rapeseed Oils for Sustainable Resin Applications: Strategies for Tailoring Structure and Performance. Compounds. 2025; 5(3):26. https://doi.org/10.3390/compounds5030026
Chicago/Turabian StyleNadim, Elham, Pavan Paraskar, Emma J. Murphy, Mohammadnabi Hesabi, and Ian Major. 2025. "Chemical Functionalization of Camelina, Hemp, and Rapeseed Oils for Sustainable Resin Applications: Strategies for Tailoring Structure and Performance" Compounds 5, no. 3: 26. https://doi.org/10.3390/compounds5030026
APA StyleNadim, E., Paraskar, P., Murphy, E. J., Hesabi, M., & Major, I. (2025). Chemical Functionalization of Camelina, Hemp, and Rapeseed Oils for Sustainable Resin Applications: Strategies for Tailoring Structure and Performance. Compounds, 5(3), 26. https://doi.org/10.3390/compounds5030026







